Abstract
The growing demand of consumers for synthetic chemical-free foods has increased the search for natural preservatives such as bacteriocins and bacteriocin-like inhibitory substances (BLIS) to give them adequate microbiological safety, sensory characteristics, and shelf life. In this study, the antimicrobial activity of BLIS produced by Pediococcus pentosaceus ATCC 43200 was compared with that of nisin. Lactobacillus sakei ATCC 15521, Listeria seeligeri NCTC 11289, Enterococcus En2052 and En2865, and Listeria monocytogenes CECT 934 and NADC 2045 exhibited larger inhibition halos in BLIS-treated than in Nisaplin-treated samples, unlike Listeria innocua NCTC 11288. In artificially contaminated ready-to-eat pork ham, BLIS was effective in inhibiting the growth of L. seeligeri NCTC 11289 for 6 days (counts from 1.74 to 0.00 log CFU/g) and ensured lower weight loss (2.7%) and lipid peroxidation (0.63 mg MDA/kg) of samples compared with the control (3.0%; 1.25 mg MDA/kg). At the same time, coloration of ham samples in terms of luminosity, redness, and yellowness as well as discoloration throughout cold storage was not influenced by BLIS or Nisaplin taken as a control. These results suggest the potential use of P. pentosaceus BLIS as a biopreservative in meat and other food processing industries.
Keywords: Antimicrobial, BLIS, TBARS, Meat color, Food spoilage
Introduction
In the last years, lifestyle of consumers has changed, and their growing demand for food products with higher quality, nutritional value, and sensory properties has posed major challenges for meat or meat-based food manufacturing industry [1].
According the data from the United States Department of Agriculture [2], pork meat is the most produced meat in the world with global production of 113.1 million tons in 2018. Brazil, the third largest producer worldwide (3.7 million tons/year) after China and European Union, has been recorded as the fourth consumer market (2.9 million tons/year) after them and Russia. Due to the versatility of the use of pork meat as human food, its leadership in world consumption compared to meat from other species is expected to be maintained over the next years [3]. Despite its lower consumption levels when compared with poultry and beef, a growth prospect of pork meat production has been forecast in Brazil up to an annual growth rate of 1.9% until 2023 [4]. Although consumption of fresh (not frozen) pork meat has been increasing in recent years, 67.9% of overall Brazilian consumption is based on processed pork products. Consumers are generally satisfied with the products available in the market, although convenience, price, and health aspects may be improved [5].
Contamination of foods by pathogenic bacteria such as Clostridium botulinum, Staphylococcus aureus, Bacillus cereus, and Listeria monocytogenes is still a problem for the food industry, being responsible for morbidity even in developed countries [6]. Known for its moderate resistance to traditional food preservation methods and higher pathogenicity than several non-spore-forming food spoilage and foodborne pathogens, the genus Listeria has been reported to be associated with several outbreaks linked to the consumption of contaminated ready-to-eat meat products [7].
Among the various species of the genus Listeria [8–10], three have raised special attention due to their hemolytic activity, namely, L. monocytogenes, L. ivanovii, and L. seeligeri. Being responsible for approximately 98% of disease cases in humans and 85% in animals, the first species stimulated researchers to seek additional actions to prevent its presence in foods. Moreover, although seldom associated with diseases in humans and animals, L. seeligeri and L. ivanovii have been also considered as potentially pathogenic [11, 12]. Despite recognized as non-virulent, L. seeligeri has been identified as capable of inducing human diseases such as acute purulent meningitis [13]; therefore, it has been widely used in experimental studies performed with artificially contaminated foods.
In order to solve the problem of food contamination by microorganisms, significant efforts have been devoted by food industry, food regulators, and scientific community to developing new preservation protocols and technologies [14]. Due to their proven antimicrobial action against food spoilage and foodborne pathogens, natural ingredients have been increasingly considered as a suitable tool to address this issue. Among them, innovative food biopreservation strategies using lactic acid bacteria (LAB), recognized by the US Food and Drug Administration (FDA) as Generally Regarded as Safe (GRAS), and/or their metabolites are progressively considered as effective in inhibiting the growth of pathogens in foods [15]. A specific class of amphiphilic compounds called bacteriocins, produced by some LAB strains, have been cited in a large number of scientific works as natural candidates to replace their synthetic counterparts [16–18]. When not completely characterized in terms of amino acid composition or nucleotide sequence of the corresponding gene, these molecules are called bacteriocin-like inhibitory substance (BLIS) [19–22]. In addition to their ability to inhibit the growth of competitive bacterial strains [23], protozoa, fungi, and viruses [24], the preservation of their antimicrobial potential under different processing conditions, such as high hydrostatic pressure [7], high temperature, wide ranges of pH, and NaCl concentration, has made them suitable for a wide variety of products and food processes [25].
In addition to the search for new strategies capable of ensuring food microbiological safety, the permanent quest for higher and adequate product sensory characteristics has been established as another top priority of the food industry [26]. In this sense, besides their safety for human consumption [27], natural compounds used as preservative agents should not induce significant changes in food appearance, texture, odor, and taste [28].
Based on this background, the aim of this work was the study of antimicrobial activity of BLIS produced by Pediococcus pentosaceus ATCC 43200 against different food spoilage and foodborne pathogenic bioindicators, as well as the evaluation of its effectiveness as biopreservative against Listeria seeligeri NCTC 11289 on ready-to-eat pork ham. For this purpose, BLIS effectiveness was compared with that of nisin-based Nisaplin, a well-known industrial food biopreservative, and its effect on ham quality parameters such as weight loss, pH, lipid oxidation, and color was evaluated.
Materials and methods
Microorganisms
The bacterium used to perform fermentations was Pediococcus pentosaceus ATCC 43200, while seven indicator strains were used to determine BLIS activity, namely, Ls (Lactobacillus sakei ATCC 15521), Lse (Listeria seeligeri NCTC 11289), Li (Listeria innocua NCTC 11288), En2052 and En2865 (Enterococcus spp., strains), Lm934 (Listeria monocytogenes CECT 934), and Lm2045 (L. monocytogenes NADC 2045). To carry out artificial contamination of ready-to-eat pork ham, L. seeligeri NCTC 11289 was used.
All strains were cryopreserved after addition of 20% (v/v) glycerol to each culture and stored at − 70 °C.
Inoculum and fermentation conditions
To prepare the inocula, P. pentosaceus and L. seeligeri were grown at 37 °C overnight in MRS broth (Roth®, Karlsruhe, Germany) and Brain Heart Infusion (BHI) (Roth), respectively. Both culture media were previously autoclaved (Tuttnauer 2540 ELV, New York, NY, USA) at 121 °C for 12 min.
Fermentations were started by inoculating 1.0 mL of the above P. pentosaceus suspension into a 500-mL Erlenmeyer flask containing 300 mL of MRS broth and incubating it at 30 °C without agitation.
Nisin solution
Nisaplin® (DuPont Danisco, Grindsted, Denmark), a commercial solution containing 2.5% (w/v) Nisin, a bacteriocin produced by Lactococcus lactis subsp. lactis, was used as a biopreservative control. It was diluted in sterile distilled water up to 1.0% (w/v) and filter sterilized (0.22-μm pore diameter; Millipore, Billerica, MA, USA) prior to use.
Determination of BLIS antimicrobial activity
BLIS antimicrobial activity was determined using cell-free supernatants (CFS) obtained by centrifugation (4470×g, 15 min, 4 °C), after pH correction to 6.0–6.5 with 1.0-M NaOH, thermal treatment for 25 min at 70 °C, and filter sterilization (0.22 μm). Antimicrobial tests were done against each one of the above five bioindicator strains according to the agar well diffusion method [29]. Briefly, an overnight culture of each strain was diluted with medium (MRS for Ls and BHI for the other strains) up to an optical density (OD) at 600 nm (Hitachi U-5100, Tokyo, Japan) of 0.3, and further 1:100 diluted with sterile deionized water up to 8.5 × 106 CFU/mL for Ls, 3.0 × 106 CFU/mL for Lse, 5.0 × 106 CFU/mL for Li, 3.0 × 106 CFU/mL for En2052, 2.0 × 106 CFU/mL for En2865, 5.5 × 106 CFU/mL for Lm 2045, and 3.5 × 106 CFU/mL for Lm 934. Fifteen milliliters of melted soft agar MRS or BHI (0.75% w/v) inseminated with 150 μL of each bacterial suspension were poured into Petri dishes, and after agar solidification, 50 μL of CFS were added into each well. All plates were incubated for 16–18 h at 37 °C in duplicate. The antimicrobial activity (A), taken as a measure of BLIS production, was calculated by the equation [30]:
| 1 |
where dH is the diameter of the clearance zone (mm), dW the diameter of the well (6.5 mm), D the dilution factor, V the sample volume (mL), and expressed in AU/mL.
Preparation of sliced pork ham samples and microbiological analyses
To investigate BLIS efficiency against Lse growth, ready-to-eat pork ham slices, aseptically cut into 25 g pieces and exposed to UV radiation for 30 min on each surface to reduce the presence of possible undesired contaminants, were artificially contaminated by spraying 500 μL of 1:100 water-diluted 0.3 OD Lse suspension on each sample surface.
Samples divided into three groups, namely, control without any antimicrobial, BLIS-treated samples and Nisaplin-treated samples, received 500 μL of cell-free BLIS-containing fermented broth or 1% Nisaplin solution by a spray gun to uniformly distribute them on each surface of artificially contaminated pork ham slices [29]. After vacuum packaging (Vacuboy, Komet Plochingen, Germany) in appropriate plastic bags (Siegelrandbeutel 180 × 225) and weighing, samples were stored at 4 ± 0.5 °C for 10-day shelf life, and aliquots drawn for analyses after 0, 2, 6 and 10 days.
To monitor Lse growth during cold storage, 225 mL of 0.3% sterile saline were added to the plastic bags containing the pork ham slices, homogenized for 2 min in a Lab-Blender 400 (Seward Stomacher, Worthing, UK) and tenfold serially diluted in 0.3% sterile saline. Ten microliters of each dilution (treatment) were spotted in duplicate on the surface of solidified selective medium (10 mL) for Listeria (Oxford Listeria Agar – Roth®, Karlsruhe, Germany) and incubated for 24 h at 37 °C. All counts of bacteria recovered from ham pieces were expressed in CFU/g and converted to logarithmic values before computing their means and standard deviations.
Weight loss of samples
Pork ham slices were weighed with a semi-analytical balance, and their weight losses during storage were determined by the equation:
| 2 |
where mi and mf are the sample masses at the start and the end of storage, respectively.
pH measurements
Ten grams of each pork ham sample were grinded using a mixer, model Home Turbo Deluxe 2552 (Leopold Kober, Klagenfurt am Wörthersee, Austria), and the pH was measured with a contact pH meter for solid phase samples (Testo 204, Lenzkirch, Germany).
Lipid oxidation
Lipid oxidation of pork ham samples was assessed in duplicate by the 2-thiobarbituric acid (TBA) method. Five grams of each sample were homogenized with 25 mL of 7.5% (w/v) trichloroacetic acid using the above mixer. After filtration of the homogenate through paper filter MN 619 EH ¼ Ø 185 mm (Macherey-Nagel, Düren, Germany), 5 mL of filtrate were mixed with 5.0 mL of 0.02 M TBA solution and thermally treated for 40 min at 100 °C in a Techne Dri-Block Heater DB-3D, model FDB03DP (Cole-Palmer, Stone, UK). After cooling the solution to room temperature, OD was measured at 532 nm, and the amount of thiobarbituric acid reactive substances (TBARS) in the samples was determined from a standard curve of malonaldehyde (MDA) (R2 = 0.9956) and expressed in mg MDA/kg.
Color determination
To check color stability after treatments, which is a meat quality parameter, subjective percent discoloration (photometric image) was determined on ham samples in vacuum packages using a spectrophotometer, model CM-600d (Konica Minolta, Osaka, Japan), with an 8-mm diameter measurement area, illuminant D65, operated in the CIELAB system. The L* value referred to color lightness, ranging from 0 (black) to 100 (white); the a* value referred to the span of red-green color, ranging from − 100 (greenness) to + 100 (redness); and the b* value referred to the extent of yellow-blue color, ranging from − 100 (blueness) to + 100 (yellowness) [31]. Values of L*, a* and b* were recorded in three different portions of samples.
Chromatographic coordinates such as saturation index (C*) and hue angle (h*) were calculated from a* and b* by the equations [32]:
| 3 |
| 4 |
Statistical analysis
The experimental data were presented as means values while variations with respect to them as standard deviations. Mean values of concentrations were submitted to analysis of variance (ANOVA) by the Statistica Software 13.3 (TIBCO Software Inc., Paolo Alto, CA, USA), compared using the Tukey’s post hoc test and considered significantly different at p < 0.05.
Results and discussion
BLIS antimicrobial activity against different bioindicators
The antimicrobial activity of cell-free BLIS-containing broth fermented by P. pentosaceus was compared with that of 1% Nisaplin solution, the only accepted nisin-based antimicrobial substance used to preserve a wide range of foods [13, 33]. For this purpose, the activity of both antimicrobials was evaluated against Lactobacillus sakei (Ls), Listeria seeligeri (Lse), Listeria innocua (Li), two strains of Enterococcus spp. (En2052 and En2865), and two of Listeria monocytogenes (Lm 2045 and Lm 934) according to the well-diffusion assay. As shown by the results of Table 1, the inhibition halos for all target microorganisms except for Li (p > 0.05) were wider in the presence of BLIS-treated samples than of Nisaplin-treated ones. Among the bioindicators, Ls was the most sensitive to BLIS, showing an inhibition halo diameter (18.00 mm) 6.00 mm longer than that obtained using commercial Nisaplin (p < 0.05).
Table 1.
Antimicrobial activity, determined according to the well-diffusion method and expressed as diameter of inhibition halo (mm), of BLIS-containing medium and 1% Nisaplin after 10 h of fermentation against different target microorganisms
| Strain | BLIS | Nisaplin |
|---|---|---|
| Ls | 18.00 ± 0.12e | 12.00 ± 0.42c |
| En2052 | 16.20 ± 0.28d | 12.50 ± 0.11c |
| En2865 | 16.45 ± 0.21d | 12.30 ± 0.09c |
| Lse | 15.50 ± 0.51d | 12.50 ± 0.13c |
| Li | 9.70 ± 0.15a | 9.50 ± 0.14a |
| Lm 2045 | 11.50 ± 0.22c | 10.10 ± 0.32b |
| Lm 934 | 10.70 ± 0.41b | 9.90 ± 0.39a |
BLIS = bacteriocin-like inhibitory substance, Ls = Lactobacillus sakei ATCC 15521, En2052 = Enterococcus 2052, En2865 = Enterococcus 2865, Lse = Listeria seeligeri NCTC11289, Li = Listeria innocua NCTC 11288, Lm 2045 = Listeria monocytogenes NADC 2045, and Lm 934 = Listeria monocytogenes CECT 934. Values are the means of duplicates ± standard deviation. Different letters in the same column mean statistically significant difference among the values for the same antimicrobial, according to the test of Tukey (p < 0.05). Well diameter = 6.5 mm
The corresponding results of antimicrobial activity calculated by Eq. (1) are illustrated in Fig. 1. It is noteworthy that, even though both antimicrobial substances were very effective against Ls, the inhibitory activity of BLIS (2077 AU/mL) was more than fourfold that of Nisaplin (p < 0.05). On the other hand, the activities against Enterococcus strains (1477–1555 AU/mL) and Lse (1272 AU/mL) were about 2.8- and 2.2-fold those of nisin solution, and the ones against Lm 2045 (393 AU/mL) and Lm 934 (277 AU/mL) only 1.9-fold and 1.5-fold higher.
Fig. 1.
Antimicrobial activity, determined according to the welldiffusion assay and expressed in AU/mL, of ( ) cell-free BLIScontaining broth fermented by P. pentosaceus ATCC 43200 after 10 h of fermentation and of (
) cell-free BLIScontaining broth fermented by P. pentosaceus ATCC 43200 after 10 h of fermentation and of ( ) 1% Nisaplin solution
) 1% Nisaplin solution
Finally, the activities against Li were up to an order of magnitude lower and almost the same for the two antimicrobials (141 and 161 AU/mL). These results taken together pointed out a decreasing sensitivity of the target strains to BLIS in the order Ls > En > Lse > Lm > Li.
Treatment of BLIS on ready-to-eat pork ham
In order to evaluate the P. pentosaceus BLIS antimicrobial effect against Lse on artificially contaminated ready-to-eat pork ham, the growth of this strain was followed throughout 10 days of vacuum package storage at 4.0 °C and compared with those in Nisaplin and control (ham without any preservative) treatments (Fig. 2a).
Fig. 2.
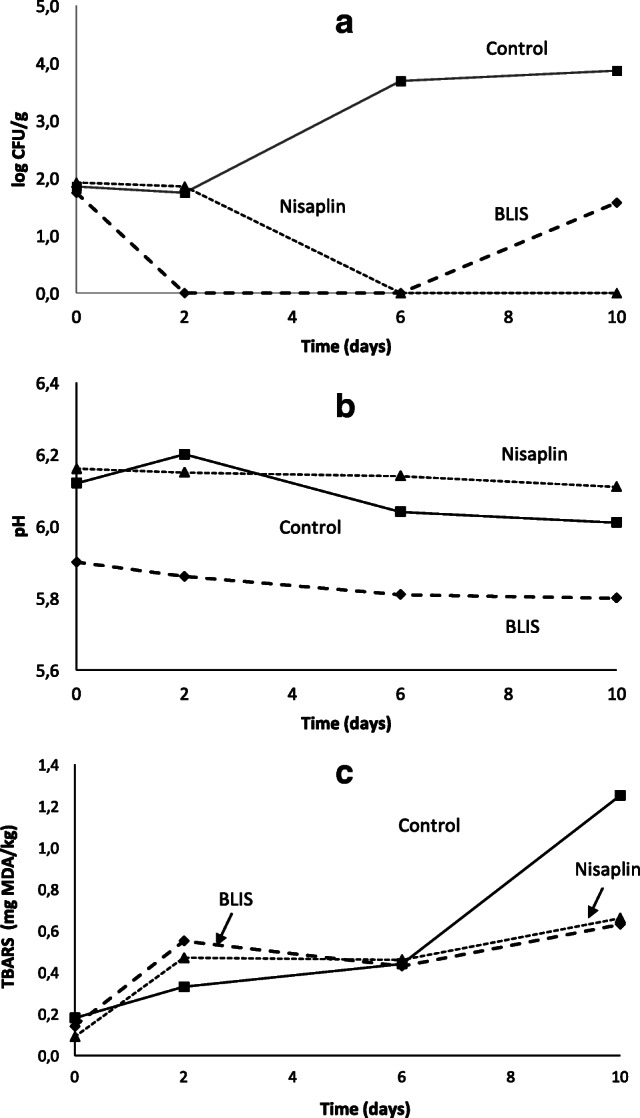
a Growth of Listeria seeligeri NCTC11289, b pH, and c thiobarbituric acid reactive substances (TBARS) in artificially contaminated ready-to-eat pork ham during 10-day shelf life at 4 °C. Sample: ■, control (meat with no treatment); ▲, Nisaplin-treated meat; ◆, BLIS-treated meat
Between the 2nd and 6th days of cold storage, the average viable Lse population in BLIS-treated pork ham samples was almost completely suppressed (˂ 1 log CFU/g), thereby providing a strong indication of the typical bactericidal effect of bacteriocins. On the other hand, Nisaplin did not exert any bactericidal effect up to the 2nd day, being Lse growth (1.85 ± 0.26 log CFU/g) statistically coincident with that of the control treatment (1.74 ± 0.24 log CFU/g). Only after this time, Nisaplin started to induce a decay in Lse count throughout the shelf life (˂ 1 log CFU/g after both 6 and 10 days), confirming the weaker antimicrobial activity of nisin compared with P. pentosaceus BLIS [34]. The slight recovery of Lse proliferation observed in BLIS-treated samples from the 6th day onward, up to a maximum count of 1.57 log CFU/g at the end of storage, might have been due to higher sensitivity of BLIS to ham proteases compared to nisin, thereby impairing its antimicrobial effect. In this scenario, the initial BLIS attachment to the majority of Lse cells may have caused such a reduction of functional BLIS (undigested) available at the pork ham surface that the few survived cells may have proliferated in the final period of storage. Nonetheless, counts of viable Lse cells in the BLIS treatment were always less than half those in the control (2 log CFU/g increase) throughout the whole storage period. These results are very promising considering that BLIS was contained in a crude fermented broth, in that a great increase in its activity is expected from its total or partial purification.
BLIS effects on weight loss and acidification
Knowing the critical influence of water retention capacity on the aspect and quality of meat products during shelf life [35], measurements of weight loss (WL) were performed on pork ham samples. The obtained results indicate that neither BLIS nor Nisin treatment led to significant changes in the water retention capacity of artificially contaminated pork ham samples. During the 10 days of shelf life in vacuum package storage at 4.0 °C, all samples did in fact show statistically coincident (p > 0.05) weight losses (3.00% ± 0.12 for the control, 2.77% ± 0.23 for BLIS-treated sample, and 3.10% ± 0.21 for Nisaplin-treated sample). Due to the well-known correlation of pH with meat moisture retention (barrier to water diffusion) [36], pH measurements were also performed. It can be seen in Fig. 2 b, that similarly to weight loss, the pH of all samples kept almost constant throughout the storage period, varying in the control only from 6.0 to 6.2 and in Nisaplin- and BLIS-treated samples from 5.9 to 5.8.
BLIS effect on lipid peroxidation and color stability
Figure 2 c shows the level of thiobarbituric acid reactive substances (TBARS) as an index of undesired lipid peroxidation, which is one of the major factors responsible for reduction of shelf life and progressive deterioration of meat products [37]. Although the TBARS level in both samples treated with antimicrobials was higher than in the control at the beginning of storage, it increased more slowly over the whole shelf life. As a result, at the end of storage, the control sample showed the highest TBARS level (1.25 ± 0.01 mg MDA/kg) followed by the Nisaplin-treated (0.66 ± 0.00 mg MDA/kg) and BLIS-treated (0.63 ± 0.00 mg MDA/kg) ones. The statistically significant differences observed between the antimicrobial-treated samples and the control (p < 0.05) suggest a protective effect of both antimicrobial agents against lipid peroxidation. These findings agree with the reduction of lipid and protein oxidation observed during storage of turkey meat sausage using a bacteriocin as biopreservative [28]. Both antimicrobials might have promoted hydrophobic interactions among fatty acids chains, making lipids no longer exposed to oxidation and delaying the oxidative process [28, 38].
Even though taste, healthiness, and price remain the strongest driving forces of food consumption, several deterioration processes such as discoloration, usually occurring at the end of shelf life, are a crucial aspect affecting consumers’ products choice. Therefore, identifying the right moment of change in meat color during storage is an important issue for both meat industry and distribution sector [39]. For this purpose, superficial color of pork ham samples in the presence or absence of antimicrobials was evaluated in terms of color lightness (L*), span of red-green color (a*), and extent of yellow-blue color (b*), an increase of which indicates paler, more red and more yellow appearance of a food product, respectively [40]. The results listed in Table 2 did not show any statistically significant differences (p > 0.05) among the three sample groups, since the above three parameters varied in the ranges 64.66–71.32, 4.28–7.69, and 10.29–12.91, respectively; therefore, similarly to the control, the antimicrobial-treated samples had acceptable visual properties between the start and the end of storage. Since meat discoloration is usually accompanied by an increase in the values of the saturation index (C*) and hue angle (h*) [41], which are parameters often used to check the real browning of meat along the time [32, 41, 42], an indirect evaluation was performed using the L*, a*, and b* data as explained in the Materials and Methods section. Despite the low values of C*, this parameter progressively increased similarly for all samples along the storage time (Table 2), achieving 13.97 ± 0.34 in BLIS-treated, 14.36 ± 0.10 in Nisaplin-treated groups, and 13.39 ± 0.06 in the control. Moreover, all h* angle values were statistically coincident, which means that, similarly to C*, neither BLIS nor nisin exerted any evident effect on the natural progressive discoloration trend throughout pork ham storage.
Table 2.
Effect of antimicrobial treatments on color parameters of artificially contaminated ready-to-eat pork ham slices during 10-day vacuum package storage at 4 °C
| Parameter | Time (days) | Control | BLIS | Nisaplin |
|---|---|---|---|---|
| L* | 0 | 69.40 ± 0.68a | 69.24 ± 3.05a | 71.32 ± 0.85a |
| 2 | 64.67 ± 3.05a | 65.78 ± 3.84a | 67.25 ± 2.31a | |
| 6 | 66.40 ± 1.31a | 66.14 ± 1.24a | 64.55 ± 3.08a | |
| 10 | 66.64 ± 0.82a | 64.66 ± 2.02a | 66.57 ± 1.32a | |
| a* | 0 | 4.99 ± 0.36a,c | 5.55 ± 0.93a,b,c | 4.28 ± 0.40c |
| 2 | 7.23 ± 1.87a,b | 7.68 ± 2.26b | 6.35 ± 0.97a,b,c | |
| 6 | 7.34 ± 0.74a,b,c | 7.16 ± 0.62a,b,c | 7.49 ± 2.11a,b | |
| 10 | 6.73 ± 0.42a,b,c | 7.69 ± 1.36a,b | 6.28 ± 0.73a,b,c | |
| b* | 0 | 10.51 ± 0.27a,b | 10.29 ± 0.45a | 10.46 ± 0.36a,b |
| 2 | 11.92 ± 0.35c,d | 11.10 ± 0.46a,b,c | 11.78 ± 0.66c,d | |
| 6 | 10.50 ± 0.46a,b,c | 11.24 ± 0.72a,b,c | 11.32 ± 0.37a,b,c | |
| 10 | 11.57 ± 0.50a,b,c,d | 11.67 ± 0.59b,c,d | 12.91 ± 0.59d | |
| C* | 0 | 11.63 ± 0.06a,b | 11.69 ± 0.34a,b | 11.30 ± 0.03a |
| 2 | 13.94 ± 1.07c | 13.50 ± 1.27c | 13.38 ± 0.22b,c | |
| 6 | 12.81 ± 0.20a,b,c | 13.32 ± 0.07b,c | 13.57 ± 1.23c | |
| 10 | 13.39 ± 0.06b | 13.97 ± 0.34c | 14.36 ± 0.10c | |
| h* | 0 | 1.13 ± 0.06a | 1.08 ± 0.34a | 1.18 ± 0.03a |
| 2 | 1.03 ± 0.07a | 0.97 ± 0.27a | 1.08 ± 0.22a | |
| 6 | 0.96 ± 0.20a | 1.00 ± 0.07a | 0.99 ± 0.23a | |
| 10 | 1.04 ± 0.06a | 0.99 ± 0.54a | 1.12 ± 0.10a |
BLIS = bacteriocin-like inhibitory substance; L* = color lightness; a* = span of red-green color; b* = extent of yellow-blue color; C* = saturation index; h* = hue angle. Values are the means of triplicates ± standard deviation. Different letters mean statistically significant difference among the values of the same parameter, according to the test of Tukey (p < 0.05)
Conclusions
Food biopreservation using natural food preservatives such as bacteriocins or bacteriocin-like inhibitory substances (BLIS) has attracted the interest of researches in this field, but only one nisin-based antimicrobial substance has been commercially accepted to this day. In this sense, tests on the biopreservation potential of a new BLIS produced by Pediococcus pentosaceus ATCC 43200 were performed taking into consideration not only its antimicrobial profile but also its effects on pork ham visual characteristics during shelf life. BLIS showed to be effective in suppressing the growth of Listeria seeligeri (Lse) in ready-to-eat pork ham after 2 days (from 1.74 to ˂ 1 log CFU/g). Lower weight loss (2.7%) and lipid peroxidation (0.63 mg MDA/kg) were observed in comparison with control samples (3.0%; 1.25 mg MDA/kg). Tests demonstrated that the application of BLIS or nisin-containing Nisaplin did not change the natural progressive discoloration trend observed throughout cold storage at 4 °C. Based on its antimicrobial profile, it is possible to infer that BLIS might have a stronger antimicrobial activity than Nisaplin. As Nisaplin, BLIS did not have any impact on the visual aspect of the tested food product. Further studies should be undertaken on the use of purified or partially purified BLIS in order to look for stricter correlations between BLIS and Nisaplin antimicrobial action profiles. A comparison with Nisaplin results allows concluding that P. pentosaceus BLIS fulfills the necessary criteria to be regarded as a biopreservative for meat and other products of food processing industries.
Acknowledgments
The authors also would like to make special thanks to Prof. Dr. Martin Wagner and Dr. Luminita Ciolacu from the Institute of Milk Hygiene, Milk Technology and Food Science, Department for Farm Animals and Veterinary Public Health of the University of Veterinary Medicine (Vienna, Austria) for kindly providing the strain L. seeligeri NCTC 11289.
Funding information
The authors are grateful to the financial support by National Council for Scientific and Technological Development – CNPq, by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, Brasil (CAPES), Finance Code 001, and by the São Paulo Research Foundation (FAPESP process n° 2018/04385-8).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Cortés-Zavaleta O, López-Malo A, Hernández-Mendoza A, García HS. Antifungal activity of lactobacilli and its relationship with 3-phenyllactic acid production. Int J Food Microbiol. 2014;173:30–35. doi: 10.1016/j.ijfoodmicro.2013.12.016. [DOI] [PubMed] [Google Scholar]
- 2.USDA-United States Department of Agriculture (2019) Pork production. Available in https://www.fas.usda.gov. Accessed 05 Mar 2019
- 3.Fávero JA (2002) Carne suína de qualidade: Uma exigência do consumidor moderno. An do I Congr Lat Am Suinocultura. I Congr Lat Am Suinocultura, Foz do Iguaçu 56–66
- 4.MAPA (Ministério da Agricultura Pecuária e Abastecimento) (2013) Projeções do Agronegócio Brasil. https://agricultura.gov.br Accessed 04 Oct 19
- 5.de Barcellos MD, Saab MSM, Pérez-Cueto FA, Perin MG, Neves MF, Verbeke W. Pork consumption in Brazil: challenges and opportunities for the Brazilian pork production chain. J Chain Netw Sci. 2011;11:99–113. doi: 10.3920/JCNS2011.Qpork3. [DOI] [Google Scholar]
- 6.EFSA (European Food Safety Authority) (2015) ECDC (European Center for Disease Prevention and Control). The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2013. Eurosurveillance. 10.2903/j.efsa.2015.3991
- 7.Dallagnol AM, Barrio Y, Cap M, Szerman N, Castellano P, Vaudagna SR, Vignolo G. Listeria inactivation by the combination of high hydrostatic pressure and Lactocin AL705 on cured-cooked pork loin slices. Food Bioprocess Technol. 2017;10:1824–1833. doi: 10.1007/s11947-017-1956-6. [DOI] [Google Scholar]
- 8.Rocourt J, Buchrieser C (2007) The genus Listeria and Listeria monocytogenes: Phylogenetic position, taxonomy, and identification. In Ryser ET, Marth EH (eds.), Listeria, Listeriosis and Food Safety, pp. 1-12. CRC Press, New York.
- 9.Liu D. Molecular approaches to the identification of pathogenic and nonpathogenic Listeriae. Microbiol Insights. 2013;6:MBI.S10880. doi: 10.4137/MBI.S10880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weller D, Andrus A, Wiedmann M, den Bakker HC. Listeria booriae sp. nov. and Listeria newyorkensis sp. nov., from food processing environments in the USA. Int J Syst Evol Microbiol. 2015;65:286–292. doi: 10.1099/ijs.0.070839-0. [DOI] [PubMed] [Google Scholar]
- 11.Jay JM (2005) In: 6th (ed) in Microbiologia de Alimentos, Porto Alegre, pp 517–542
- 12.Lapenda AMVS (2010) Ocorrência de Listeria spp. em embutidos resfriados comercializados na cidade do Recife-PE. Dissertação, Universidade Federal Rural de Pernambuco (UFRPE)
- 13.Rocourt J, Hof H, Schrettenbrunner A, Maluverni R, Bille J (1985) Acute purulent meningitis due to L. seeligeri. 9th Int Symp Probl list. PMID:3082004
- 14.Schulz D, Pereira MA, Bonnelli RR, Nunes MM, Batista CRV. Bacteriocinas: Mecanismo de ação e uso na conservação de alimentos. Alim Nutr, Araraquara. 2003;14:229–235. [Google Scholar]
- 15.Calo-Mata P, Arlindo S, Boehme K, de Miguel T, Pascoal A, Barros-Velzquez J (2008) Current applications and future trends of lactic acid bacteria and their bacteriocins for the biopreservation of aquatic food products. Food Bioprocess Technol 1:43–63. 10.1007/s11947-007-0021-2
- 16.Acuña L, Morero RD, Bellomio A. Development of wide-spectrum hybrid bacteriocins for food biopreservation. Food Bioprocess Technol. 2011;4:1029–1049. doi: 10.1007/s11947-010-0465-7. [DOI] [Google Scholar]
- 17.Zacharof MP, Lovitt RW. Bacteriocins produced by lactic acid bacteria a review article. APCBEE Procedia. 2012;2:50–56. doi: 10.1016/j.apcbee.2012.06.010. [DOI] [Google Scholar]
- 18.Kassaa IA, Belguesmia Y, Chihib NE, Hamze M, Bendali F, Naghmouchi K, Fliss I, Drider D (2015) Applications des bacteriocines et bactéries lactiques dans le contrôle des pathogènes alimentaires. Sécurité Sanit des Aliment:231–258 http://hdl.handle.net/20.500.12210/7836.3
- 19.Jack RW, Tagg JR, Ray B. Bacteriocins of gram-positive bacteria. Microbiol Rev. 1995;59:171–200. doi: 10.1128/MMBR.59.2.171-200.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leite JA, Tulini FL, dos Reis-Teixeira B, Rabinovitch L, Chaves JQ, Rosa NG, Cabal H, De Martinis ECP. Bacteriocin-like inhibitory substances (BLIS) produced by Bacillus cereus: preliminary characterization and application of partially purified extract containing BLIS for inhibiting Listeria monocytogenes in pineapple pulp. LWT Food Sci Technol. 2016;72:261–266. doi: 10.1016/j.lwt.2016.04.058. [DOI] [Google Scholar]
- 21.de Souza de Azevedo PO, Converti A, Domínguez JM, de Souza Oliveira RP. Stimulating effects of sucrose and inulin on growth, lactate, and bacteriocin productions by Pediococcus pentosaceus. Probiotics Antimicrob Proteins. 2017;9:466–472. doi: 10.1007/s12602-017-9292-8. [DOI] [PubMed] [Google Scholar]
- 22.da Silva SS, Pérez-Rodríguez N, Domínguez JM, de Souza Oliveira RP. Inhibitory substances production by Lactobacillus plantarum ST16Pa cultured in hydrolyzed cheese whey supplemented with soybean flour and their antimicrobial efficiency as biopreservatives on fresh chicken meat. Food Res Int. 2017;99:762–769. doi: 10.1016/j.foodres.2017.05.026. [DOI] [PubMed] [Google Scholar]
- 23.Hwanhlem N, Biscola V, El-Ghaish D, Jaffrès E, Dousset X, Haertlé T, H-Kittikun A, Chobert JM. Bacteriocin-producing lactic acid bacteria isolated from mangrove forests in southern Thailand as potential bio-control agents: purification and characterization of bacteriocin produced by Lactococcus lactis subsp. lactis KT2W2L. Probiotics Antimicrob. Proteins. 2013;5:264–278. doi: 10.1007/s12602-013-9150-2. [DOI] [PubMed] [Google Scholar]
- 24.Reddy KVR, Yedery RD, Aranha C. Antimicrobial peptides: premises and promises. Int J Antimicrob Agents. 2004;24:536–547. doi: 10.1016/j.ijantimicag.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 25.Sant’Anna V, Utpott M, Cladera-Olivera F, Brandelli A. Antimicrobial activity of peptide P34 during thermal processing. Food Bioprocess Technol. 2013;6:73–79. doi: 10.1007/s11947-011-0633-4. [DOI] [Google Scholar]
- 26.Borges CD, Moreira ÂN, Moreira A d S, Del Pino FAB, Vendruscolo CT. Caracterização de biopolímeros produzidos por Beijerinckia sp. 7070 em diferentes tempos de cultivo TT - Characterization of biopolymers produced by Beijerinckia sp 7070 at different culture times. Food Sci Technol. 2004;24:327–332. doi: 10.1590/S0101-20612004000300004. [DOI] [Google Scholar]
- 27.Giraffa G, Chanishvili N, Widyastuti Y. Importance of lactobacilli in food and feed biotechnology. Res Microbiol. 2010;161:480–487. doi: 10.1016/j.resmic.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 28.Smaoui S, Ennouri K, Chakchouk-Mtibaa A, Karray-Rebai I, Hmidi M, Boucchaala K, Mellouli L. Relationships between textural modifications, lipid and protein oxidation and sensory attributes of refrigerated turkey meat sausage treated with bacteriocin BacTN635. Food Bioprocess Technol. 2017;10:1655–1667. doi: 10.1007/s11947-017-1933-0. [DOI] [Google Scholar]
- 29.Azevedo POS, Converti A, Gierus M, Oliveira RPS. Application of nisin as biopreservative of pork meat by dipping and spraying methods. Braz J Microbiol. 2019;50:523–526. doi: 10.1007/s42770-019-00080-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Md Sidek NL, Tan JS, Abbasiliasi S, Wong FW, Mustafa S, Ariff AB. Aqueous two-phase flotation for primary recovery of bacteriocin-like inhibitory substance (BLIS) from Pediococcus acidilactici Kp10. J Chromatogr B Anal Technol Biomed Life Sci. 2016;1027:81–87. doi: 10.1016/j.jchromb.2016.05.024. [DOI] [PubMed] [Google Scholar]
- 31.Elias MG (2013) Caracterização de presuntos artesanais e industriais de suíno alentejano. Modificações introduzidas pela embalagem sob vácuo. Tese, Universidade Federal de Lavras (UFLA)
- 32.Ledward DA. Quality attributes and their measurement in meat, poultry and fish products. Food Control. 2003;6:181. doi: 10.1016/0956-7135(95)90004-7. [DOI] [Google Scholar]
- 33.Khajehali E, Shekarforoush SS, Nazer AHK, Hoseinzadeh S. Effects of nisin and modified atmosphere packaging (map) on the quality of emulsion-type sausage. J Food Qual. 2012;35:119–126. doi: 10.1111/j.1745-4557.2012.00438.x. [DOI] [Google Scholar]
- 34.Leroy F, De Vuyst L (2000) Sakacins. In: Naidu AS (ed) Natural food antimicrobial systems, Boca Raton, pp 589–610. 10.1201/9781420039368.ch21
- 35.Da Silva Sobrinho AG, Purchas RW, Kadim IT, Yamamoto SM. Meat quality in lambs of different genotypes and ages at slaughter. Rev Bras Zootec. 2005;34:1070–1078. doi: 10.1590/S1516-35982005000300040. [DOI] [Google Scholar]
- 36.Toldrá F (2002) Dry-cured meat products. Food & Nutrition Press, Inc, pp 224. Online ISBN: 9780470385111. 10.1002/9780470385111.fmatter
- 37.Chakchouk-Mtibaa A, Smaoui S, Ktari N, Sellem I, Najah S, Karray-Rebai I, Mellouli L. Biopreservative efficacy of bacteriocin BacFL31 in raw ground turkey meat in terms of microbiological, physicochemical, and sensory qualities. Biocontrol Sci. 2017;22:67–77. doi: 10.4265/bio.22.67. [DOI] [PubMed] [Google Scholar]
- 38.Berset C, Cuvelier ME (1996) Revue: méthodes d’évaluation du degré d’oxydation des lipides et mesure du pouvoir antioxydant. Sci Aliment 16:219–245 https://hal.archives-ouvertes.fr/hal-01199692. Accessed 18 Feb 2020
- 39.McMillin KW. Advancements in meat packaging. Meat Sci. 2017;132:153–162. doi: 10.1016/j.meatsci.2017.04.015. [DOI] [PubMed] [Google Scholar]
- 40.Monte ALS, Selaive-Villarroel AB, Garruti DS, Zapata JF, Borges AS. Physical and sensory quality parameters of the meat of crossbred goat kids of different genetic groups. Food Sci Technol. 2005;27:233–238. doi: 10.1590/S0101-20612007000200004. [DOI] [Google Scholar]
- 41.Lee S, Decker EA, Faustman C, Mancini RA. The effects of antioxidant combinations on color and lipid oxidation in n-3 oil fortified ground beef patties. Meat Sci. 2005;70:683–689. doi: 10.1016/j.meatsci.2005.02.017. [DOI] [PubMed] [Google Scholar]
- 42.Luciano G, Monahan FJ, Vasta V, Pennisi P, Bella M, Priolo A. Lipid and colour stability of meat from lambs fed fresh herbage or concentrate. Meat Sci. 2009;82:193–199. doi: 10.1016/j.meatsci.2009.01.010. [DOI] [PubMed] [Google Scholar]



