Abstract
Ephemeral microbial communities usually undergo priority effect and result in higher diversity with a few representatives of each species. Community structure of yeasts in bromeliad tanks was compared between two rupestrian savanna (Cerrado) areas in Brazil and to yeasts isolated from water holes in the same areas. Water samples were collected from 60 tanks of bromeliads Bromelia karatas and Encholirium sp. and rock holes at the Karstic Area of Aurora, Tocantins State and 60 tanks of Vriesea minarum (Bromeliaceae) and Paepalanthus bromelioides (Eriocaulaceae) at Serra do Cipó National Park, Minas Gerais State in Brazil. The yeast diversity comprised 90 species from which 60% are basidiomycetous yeasts usually associated with phylloplane, soils, and aquatic habitats. The species Papiliotrema laurentii, Rhodotorula mucilaginosa, Pa. nemorosus, and Pseudozyma hubeiensis were the most frequent species associated with bromeliads. Eighteen yeast species, two ascomycetous and 16 basidiomycetous, were consistently isolated from the substrates in both areas and may represent a core community in bromeliads in rupestrian fields. Singlets occurred in 38 to 69% of samples, and 32 species were isolated only once. Our findings reinforce the ephemeral nature of the yeast communities associated with tank-forming plants in which individual phytotelmata act as patches or aquatic islands prone to rapid colonization-extinction rates receiving inocula from plant and soil debris. Ephemeral rock holes also represent a transitory habitat for yeast species associated with plants and soil.
Keywords: Yeast biogeography, Bromeliaceae, Eriocaulaceae, Priority effect, Diversity, Cerrado ecosystem
Introduction
The bromeliads are plants that belong to the family Bromeliaceae and comprise nearly 3140 species belonging to 58 genera and eight subfamilies [1]. These plants constitute one of the most morphologically distinctive, ecologically diverse, and species-rich clades of flowering plants native to tropical ecosystems [1, 2]. Bromeliad leaves are spiral with ample and flexible sheath that frequently form a receptacle in which they accumulate organic debris and water. The tank-forming bromeliads in particular possess foliage arranged into a compact rosette (known as phytotelmata), which is capable of retaining water [1, 3]. These phytotelmata can be rich in insects and other arthropods, as well as frogs, salamanders, and small snakes [1]. Microorganisms can colonize these central tanks with accumulated water [3–5], and they are the main group responsible for detritus decomposition in bromeliads and provide particulate organic matter (POM) to filter feeders [6]. According to Brandt et al. [7], every bromeliad tank is a unique island with respect to its resident microbial community. Klann et al. [8] affirm that the tank is a basin for water storage that homes an array of microorganisms, among which are algae, rotifers, viruses, bacteria, and fungi [6], including yeasts [3, 4, 9].
The yeast communities associated with phytotelmata of bromeliads comprise both basidiomycetous and ascomycetous species [3, 9–12]. Most frequent species comprise Aureobasidium pullulans, Papiliotrema spp., Saytozyma podzolica, Occultifur brasiliensis, and Saturnispora silvae [3]. Possible geographic and host specificities are yet to be clarified. The objective of this work is to describe the yeast communities found in plant phytotelmata water in two different locations of the rupestrian savannas of Brazil at an average distance of 1.200 km. In this work, our predicted hypothesis is that yeasts colonizing water tanks of plants present geographical and host plant specificity in rupestrian savannas. We expect that they will differ greatly in different host plants in the same area, and they will also differ between distant areas with similar physiognomies. Finally, we expect that they differ from those yeasts isolated from surrounding ephemeral water habitats such as rock holes. To study this, we isolated yeasts from two bromeliad species in Northeastern Cerrado ecosystem, a bromeliad and an everlasting species of Eriocaulaceae in the Southeastern Cerrado area. In addition, we investigated the presence of yeasts in water accumulated in shallow rock holes that accumulate rainwater surrounding bromeliads insertion in rocks in search of characterizing the specificity of the bromeliad habitat for yeasts and the biogeographical component of yeast diversity in this ephemeral habitat.
When discussing if fungi present biogeographical patterns, it is clear that major fungal lineages arose sufficiently early and dispersed well enough to have nearly global distributions [13]. Pre-molecular studies tended to emphasize alpha diversity as the most important component of fungal biodiversity, but molecular studies have shown that high beta diversity contributes to macroecological patterns such as strong species-area relationships and geographical differentiation of fungal communities. Studies show that most major genera of fungi, such as the ectomycorrhizal genera Russula, Boletus, Inocybe, Cortinarius, and Amanita, seem to be present on all habitable continents [14–16]. Some of the deepest biogeographical splits in the fungal kingdom are between taxa from the Northern and Southern hemispheres in a manner similar to plant and animal groups. Although genera and other higher-level taxa are broadly distributed, it has become increasingly evident in recent years that most fungal species do not generally have cosmopolitan distributions and that patterns of fungal diversity at the species level are strongly influenced by biogeographical factors, such as climate [14] and isolation [17]. Latitudinal gradients vary according to taxon and functional guilds. In fact, regional endemism is one of the most consistent results from sequence-based continental-scale and global-scale studies of fungal communities [18]. In addition to continental endemism, patterns of geographical clustering—in which spatially adjacent fungal communities share a larger number of taxa in common than spatially non-adjacent fungal communities—can be observed at local and regional scales. Also, dispersal has an important role in shaping fungal communities [19] as exemplified by post-glacial co-migration of ectomycorrhizal fungi and host tree species and also across Behring land bridge into North America [13].
The biogeographical component of diversity was defined by Whittaker [20] and reviewed by Tuomisto [21], among others. Beta diversity is an important concept used in its broadest sense to describe variation in species identities from site to site [22]. It was originally conceived in order to bridge the gap between local (alpha) and regional (gamma) measures of diversity [20] and has since become a multifaceted concept with a large number of verbal and mathematical definitions [21, 22]. Tuomisto [21] argues that Whittaker when first presenting the concept of beta diversity (β) used the term in a rather vague sense to refer to compositional heterogeneity among places. Both alpha diversity (α) and gamma diversity (γ) represent species diversity, but α is the mean species diversity at the local, within-site, or within-habitat scale, whereas γ is the total species diversity at the regional or landscape scale. Cody [23] redefined β-diversity as the rate of compositional turnover along a habitat gradient within one geographical region. Bratton [24] also used the term β-diversity to refer to the rate of species turnover along a gradient. In the present work, we define α-diversity as Simpson’s Index of Diversity (1-D) which has a meaningful and logical interpretation as the effective number of species, and it has a sensitivity to rare vs abundant species [21], the high occurrence of rare species being a feature common to yeast communities. There is a yet no general framework for describing the spatial scaling of β-diversity, but two main approaches can be used to conceptualize spatial variation in β-diversity: (i) the distance decay of community similarity and (ii) the partitioning of species diversity into α and β components [21]. This study will discuss the partitioning of gamma diversity in the two components beta and alpha. Diversity partitioning studies derive aggregate measures of β-diversity (e.g., Whittaker’s multiplicative β [20] or Lande’s additive β [25]) from the relationship between mean α-diversity in a sample unit versus gamma diversity from all sampling units and indicates the average diversity not found in any one sampling unit [26]. Thus, it can give information about variation in species composition among sampling units at different spatial scales. For example, bacterial [27] and soil faunal communities [28] are often quantified in sampling units of square centimeters or as in the case of the present study individual phytotelmata.
Materials and methods
Geographical locations of the sampling sites
The Southeastern area of sampling is situated in Serra do Cipó National Park, in central Minas Gerais state, South Espinhaço Mountain Range, between 19 and 20° S and 43 e 44° W. The complexity of climate and geological features creates a heterogeneous vegetation landscape of Cerrado vegetation and rupestrian fields of high floristic diversity [29]. Rupestrian fields are the predominant landscapes in Serra do Cipó National Park and vicinities [30, 31].
The Northeastern site is located at the Tocantins State, with dry sub-humid climate with moderate hydric deficiency during winter [32]. It is geologically situated in the Sedimentary San Franciscan basin [33] in the Karstic Area of Aurora do Tocantins between parallels 12°10′00″S and 13°05′00″S and meridian 46°10′00″W and 46°50′00″W, composed of karren, dolines, poljes, and limestone mastiffs [34]. According to Haidar et al. [35], the vegetation mosaic comprises seasonally semi-deciduous and deciduous forests in the zone of ecological tension between Cerrado and Caatinga biomes, with patches of rupestrian fields in the karst areas. Vegetation of rupestrian fields presents adaptations for water reservation such as foliar sheaths of the genus Vellozia, life cycle synchronous to climate such as species of Eriocaulaceae, and structures to minimize water loss such as in Melastomataceae e Asteraceae [36].
Sampling
Sampling was undertaken in two expeditions to the rupestrian fields of the Northeastern area of Cerrado biome and one expedition to Serra do Cipó Park area in the Southeastern Cerrado. Expeditions to the Northeastern area took place in the rainy season of 2011 and 2012, whereas the expeditions to Southeastern area took place in the dry season of 2013 and 2014. Water samples from phytotelmata were collected from 30 individuals, each of the bromeliads Bromelia karatas and Encholirium sp., and also from 30 water samples collected in rock holes (total samples of each bromeliad species were 60). The rock holes are shallow with different geometrical irregular forms and sharp edges that were in the neighborhood of the bromeliads and contained small amounts of water (volume was not quantified), at each sampling expedition in the Northeastern area. Water samples were collected aseptically with a sterile pipette and transferred to sterile flasks that were transported to the laboratory for analysis in the same day. No physicochemical measures were taken. In the Southeastern area, phytotelmata water samples were obtained from 60 individuals of Vriesea minarum and Paepalanthus bromelioides. The latter species belongs to the flowering plant family Eriocaulaceae native to Cerrado. This family is close to the Bromeliaceae and shares its particular morphology. The plant individuals were located approximately 5 m from each other.
Isolation of yeasts
Aliquots of 0.2 ml of appropriate decimal dilutions of the samples were spread on YM agar (glucose 1%, yeast extract 0.3%, malt extract 0.3%, peptone 0.5%, agar 2%, and chloramphenicol 0.02%). The plates were incubated at 25 °C for 3 to 15 days, and colonies of each different yeast morphotype were counted, purified, and preserved at − 80 °C for later identification.
Identification of the strains
Yeasts were morphological and physiologically characterized as described by Kurtzman et al. [37]. Isolates with identical morphological and physiological characteristics were grouped together and subjected to PCR fingerprinting with primer (GTG)5 [38]. Yeast strains with identical PCR fingerprinting patterns were grouped and putatively considered to belong to the same species [3, 37, 39]. At least half of the yeast isolates of each molecular group were identified by sequencing. Species identifications were performed by analysis of the sequences of the ITS-5.8S region and the D1/D2 variable domains of the large subunit rRNA gene [40–44]. The amplified DNA was cleaned and sequenced in an ABI 3130 Genetic Analyzer automated sequencing system (Life Technologies, CA, USA) using BigDye v3.1 and POP7 polymer. The sequences obtained were compared with those included in the GenBank database using the Basic Local Alignment Search Tool (BLAST at http://www.ncbi.nlm.nih.gov).
Statistics
Yeast counts were made on basis of detection of the yeast species i in the sample unit corresponding to an individual plant from plant species j. Frequency of occurrence (Fo) is the percentage of plant/rock hole individuals in which the yeast species i was isolated, in relation to the total number of plant or rock hole sampled. The index S means the actual number of species occurring in the samples; we have discussed it as an index of species richness in each and all sampled plants and rock holes in order to diagnose the number of yeast species occurring in each substrate and area. Simpson index (E1/D) was used as a diversity index in each substrate in each area, and we discussed it as an estimate of equitability [45]. We also discussed community structure by comparing species composition by Simpson’s Index of Diversity (1-D). We calculated β-diversity by the index of Whittaker [20] that measures the substitution in species composition between substrates in order to discuss a possible core yeast community in bromeliads and differences in communities among substrates and between geographic regions. Values of β-diversity vary from 0 to 1 where 0 indicates the greater similarity in species composition and 1 the smaller one [45]. All statistical analyses were made using the R “vegan” package [46].
Results
Occurrence of yeasts
A total of 534 isolates belonging to 90 yeast species were isolated from phytotelmata and rock holes (Table 1). Species richness varied from 43 species in bromeliad tanks of B. karatas and 28 of Encholirium sp. and 26 from water accumulated in rock holes in the Northeastern rupestrian Cerrado area. In the Southeastern rupestrian Cerrado in Minas Gerais, 23 species were isolated from V. minarum phytotelmata, and 39 species were isolated from P. bromelioides. Among yeast species, Papiliotrema laurentii and Rhodotorula mucilaginosa were observed in all phytotelmata and rock holes. Papiliotrema nemorosus and Pseudozyma hubeiensis also occurred in all plants but not in rock holes. Eighteen yeast species were isolated from the substrates in both areas, Northeastern and Southeastern Cerrado. Among these, two species are ascomycetous: Aureobasidium pullulans and Candida orthopsilosis, and the other 16 are basidiomycetes.
Table 1.
Yeast species frequency of occurrence in phytotelmata of Vriesea minarum and Paepalanthus bromelioides in rupestrian Cerrado of Southeast and phytotelmata of Bromelia karatas and Encholirium sp. and temporary rock holes in rupestrian fields of Northeastern Cerrado of Brazil
| Yeasts | P. bromelioides | V. minarum | B. karatas | Encholirium sp. | Rock holes |
|---|---|---|---|---|---|
| (n = 62)a | (n = 62) | (n = 60) | (n = 60) | (n = 37) | |
| Anomalomyces panici | 5 | 11 | – | – | – |
| Anomalomyces yakirrae | 19 | 3 | – | – | – |
| Aureobasidium pullulans | 1 | – | 1 | – | 1 |
| Bullera alba | – | – | – | – | 1 |
| Candida albicans | – | – | 1 | – | – |
| Candida buenavistaensis | – | – | – | 1 | 1 |
| Candida duobushaemulonii | – | – | 3 | – | 1 |
| Candida glabrata | – | – | 10 | 11 | 1 |
| Candida heveicola | 1 | 1 | – | – | – |
| Candida intermedia | – | – | 8 | 3 | 1 |
| Candida leandrae | – | – | 1 | – | – |
| Candida melibiosica | 1 | – | – | – | – |
| Candida nivariensis | – | – | 1 | 2 | – |
| Candida orthopsilosis | 1 | – | 17 | 6 | 4 |
| Candida parapsilosis | – | – | 5 | 1 | – |
| Candida pseudointermedia | – | – | 19 | 5 | 3 |
| Candida ubatubensis | 1 | 4 | – | – | – |
| Candida sp.1 | – | – | 3 | – | – |
| Candida sp.2 | – | – | 1 | – | – |
| Candida sp.3 | – | – | 1 | – | – |
| Colacogloea foliorum | 1 | – | – | – | – |
| Curvibasidium nothofagi | 1 | – | – | 1 | – |
| Cytobasidium calyptogenae | – | – | – | 1 | – |
| Cryptococcus sp. | 3 | – | – | – | – |
| Endosporium aviarium | – | 1 | – | – | – |
| Erythrobasidium elongatus | 3 | – | – | – | 1 |
| Erythrobasidium hasegawianum | – | – | – | 1 | – |
| Exophiala alcalophila | 2 | – | – | – | – |
| Exophiala nigra | 2 | – | – | – | – |
| Exophiala placitae | – | 1 | – | – | – |
| Gjaerumia minor | 4 | 1 | – | – | – |
| Gymnocintractia samanensis | 1 | 1 | – | – | – |
| Hagleromyces aurorensis | – | – | 3 | – | – |
| Hannaella luteola | – | – | 6 | 5 | 2 |
| Hannaella pagnoccae | – | – | 1 | 4 | 3 |
| Hannaella zeae | – | – | 1 | 3 | – |
| Hanseniaspora guilliermondii | – | – | 1 | – | – |
| Hanseniaspora opuntiae | – | – | 1 | – | – |
| Hasegawazyma lactosa | 1 | – | – | – | 1 |
| Kalmanozyma vetiver | 1 | – | – | – | – |
| Kalmanozyma brasiliensis | – | – | – | 1 | 2 |
| Kwoniella dendrophila | – | – | 1 | – | – |
| Kwoniella heveanensis | – | 1 | 16 | 21 | 2 |
| Kwoniella mangroviensis | – | – | 3 | 2 | 1 |
| Langdonia jejuensis | 19 | 35 | – | – | – |
| Meira geulakonigii | 5 | – | – | – | – |
| Meira sp. | 2 | – | – | – | – |
| Meyerozyma caribbica | – | – | 7 | 2 | – |
| Meyerozyma guilliermondii | – | – | 19 | 7 | 7 |
| Microbotryozyma collariae | – | – | 1 | – | – |
| Moesziomyces aphidis | 3 | – | – | – | – |
| Myriangium sp. | 1 | – | – | – | – |
| Naganishia albida | – | – | – | – | 1 |
| Neobulgaria pura | 2 | – | – | – | – |
| Occultifur brasiliensis | – | – | 1 | – | – |
| Occultifur externus | – | 1 | 1 | 1 | – |
| Papiliotrema flavescens | – | 1 | 1 | 2 | – |
| Papiliotrema laurentii | 9 | 2 | 8 | 7 | 3 |
| Papiliotrema nemorosus | 12 | 14 | 5 | 2 | – |
| Papiliotrema rajasthanensis | 1 | – | 1 | 1 | – |
| Papiliotrema terrestris | – | – | 5 | 3 | 1 |
| Phragmotaenium oryzicola | 2 | – | – | – | – |
| Pichia sp. | – | – | 1 | – | – |
| Prototheca wickerhamii | – | – | – | – | 1 |
| Pseudozyma hubeiensis | 4 | 2 | 7 | 3 | – |
| Pseudozyma rugulosa | 1 | – | – | – | – |
| Pseudozyma sp. | – | 2 | – | – | – |
| Rhodosporidiobolus ruineniae | – | – | – | 2 | – |
| Rhodotorula mucilaginosa | 7 | 2 | 1 | 2 | 2 |
| Rhodotorula paludigena | – | 1 | – | – | – |
| Rhodotorula taiwanensis | – | 1 | – | – | – |
| Rhodotorula toruloides | – | – | – | 4 | 2 |
| Rhodotorula sp. | 1 | 1 | – | – | – |
| Rhynchogastrema complexa | – | – | 1 | – | – |
| Saitozyma flava | 1 | 3 | – | – | – |
| Saitozyma podzolica | 4 | – | 1 | – | – |
| Sporisorium graminicola | 1 | – | – | – | – |
| Sporobolomyces sp. | – | – | – | – | 1 |
| Sporisorium andropogonis | 2 | – | – | – | – |
| Sporisorium elionuri | – | – | 1 | – | – |
| Sporisorium everhartii | – | – | 3 | – | – |
| Sporisorium trachypogonis-plumosi | 1 | – | – | – | – |
| Sporisorium japonicus | 1 | – | – | – | – |
| Symmetrospora marina | – | 1 | – | – | – |
| Torulaspora delbrueckii | – | – | 2 | – | 1 |
| Tremella globispora | 5 | 1 | 2 | – | 1 |
| Tremella sp. | – | – | 1 | – | – |
| Trimorphomyces sp. | – | – | – | – | 1 |
| Ustilago sparsa | – | – | 1 | – | – |
| Ustilago spermophora | 1 | – | – | – | – |
| Species richness (S) | 39 | 23 | 43 | 28 | 26 |
| Simpson’s Index of Diversity (1-D) | 0.93 | 0.80 | 0.94 | 0.91 | 0.93 |
anumber of phytotelmata and/or rock holes sampled
In the Northeastern region, C. pseudointermedia, M. guilliermondii, C. orthopsilosis, and K. heveanensis were the most frequent species in B. karatas. Candida glabrata, C. intermedia, and Pa. laurentii were also frequently associated with this plant. These yeast species can be considered prevalent in this microhabitat. Kwoniella heveanensis was the most frequent species in Encholirium sp. phytotelmata. Also, M. guilliermondii, Pa. laurentii, and Ha. luteola were isolated with frequencies above 5% associated with this plant. M. guilliermondii was the most frequent yeast (15%) in rock holes, followed by C. orthopsilosis, C. pseudointermedia, Ha. pagnoccae, and Pa. laurentii, all with 7–9% of occurrence.
The substrates from the Southeastern region presented Langdonia jejuensis (39% in V. minarum and 14% in P. bromelioides), P. nemorosus (16% in V. minarum and 9% in P. bromelioides), and Anomalomyces yakirrae (12% in V. minarum and 14% in P. bromelioides) as most frequent species. In P. bromelioides, also Pa. laurentii (7%) and Rh. mucilaginosa (5%) were frequent.
Singlets occurred in 69% of samples of V. minarum, 56% of B. karatas, 39% of Encholirium sp., 38% of P. bromelioides, and 61% of rock waters samples. Since most of the yeast communities of the phytotelmata were composed of singlets, this resulted in high species richness but low diversity of species in each habitat type (Table 1). Among those singlets, 13 species were isolated solely from one sample of B. karatas: six from one sample each of V. minarum and P. bromelioides, four from one sample each of rock holes, and three from one sample each of Encholirium sp. That is to say, 32 species were isolated only once from the samples.
Yeast biodistribution in substrates and geographical regions
A Venn diagram shows the distribution of the yeast species among substrates and areas (Fig. 1). Among the 90 species of yeasts, 37 were found solely in the Northeastern area and 34 only in the Southeastern area, being 18 species found in both areas. Papiliotrema nemorosus and P. hubeiensis were particular to plant substrates in both geographical areas and did not occur in rock holes. Papiliotrema flavescens and O. externus occurred only in bromeliads of both areas, but not in the P. bromelioides phytotelmata. On the other hand, the rock holes presented five species not isolated from plants: Trimorphomyces sp., Sporobolomyces sp., Bullera alba, Naganishia albida, and the colorless alga Prototheca wickerhamii. Besides the geographical effect, characteristics of the substrates do act on the distribution of yeasts, and this indicates that each particular substrate provides a selective pressure to colonization. Especially, rock holes differ highly as habitat to yeasts from plant phytotelmata.
Fig. 1.
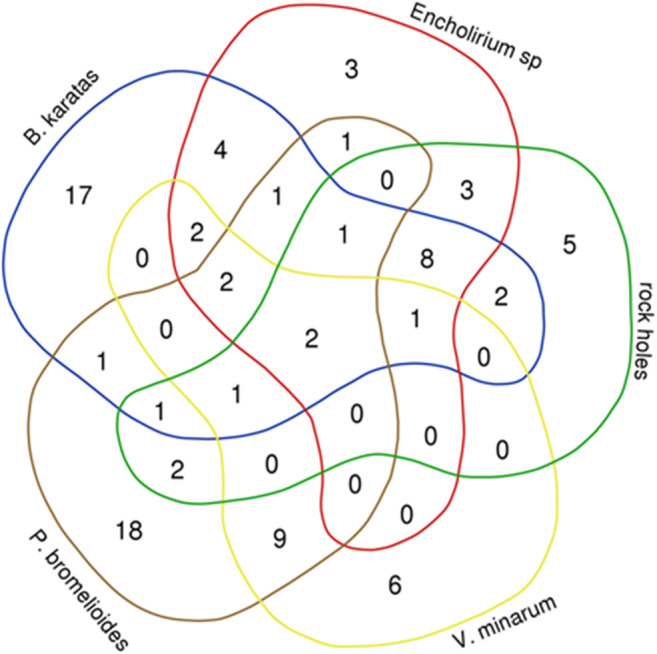
Venn diagram representing the biodistribution of yeast species among the phytotelmata of four plant species and rock holes in two different regions of rupestrian Cerrado of Brazil. The numbers represent the yeasts occurring in each subset of samples
The three substrates from the Northeastern area shared eight species not isolated in the Southeastern area: C. pseudointermedia, Ha. luteola, Me. guilliermondii, C. intermedia, Pa. terrestris, Ha. pagnoccae, K. mangroviensis, and C. glabrata. The two bromeliads shared another four species not isolated from rock holes in this area: C. parapsilosis, C. nivariensis, Ha. zeae, and M. caribbica. Phytotelmata of B. karatas presented 17 species, and Encholirium sp. presented three yeasts not isolated from other substrates.
In the Southeastern area, phytotelmata of V. minarum presented six yeasts particular to this substrate and P. bromelioides 18 other species not isolated elsewhere. They shared nine species particular to these substrates in the Southeastern area: Anomalomyces yakirrae, Rhodotorula sp., A. panici, L. jejuensis, C. ubatubensis, Sa. flava, G. minor, C. heveicola, and Gy. samanensis. Two species occurring in both areas (Pa. laurentii and Rh. mucilaginosa) and also other ten species occurring in the bromeliads and rock holes of the Cerrado area in Tocantins State were also isolated from the bromeliad V. minarum in a rupestrian Cerrado area in Minas Gerais State by Gomes et al. [3].
All substrates presented similar values of diversity measured by Simpson 1-D index although V. minarum showed the lowest value explained by the dominance of three species that occurred with 12% (A. panici), 16% (Pa. nemorosus), and 39% (L. jejuensis) (67% of total occurrences). β-diversity was higher between P. bromelioides and other substrates which is expected since it is not a bromeliad species. Lower β-diversity was obtained between bromeliads. Values of β-diversity were lower between bromeliads and rock holes than bromeliads and P. bromelioides, a plant of a different family (Table 2).
Table 2.
β-diversity of the yeast communities in phytotelmata of Bromelia karatas, Encholirium sp., Vriesea minarum, Paepalanthus bromelioides, and rock holes and β-diversity of the yeast communities of the Southeastern and Northeastern locations of rupestrian Cerrado in Brazil
| B. karatas | Encholirium sp. | V. minarum | P. bromelioides | Rock holes | Southeastern plants | |
|---|---|---|---|---|---|---|
| B. karatas | * | 0.49 | 0.41 | 1.2 | 0.4 | – |
| Encholirium sp. | * | 0.3 | 1.4 | 0.48 | – | |
| Vriesea minarum | * | 0.58 | 0.38 | – | ||
| P. bromelioides | * | 1.37 | – | |||
| Rock holes | – | |||||
| Northeastern plants | – | – | – | – | – | 0.7 |
| Northeastern substrates | – | – | – | – | – | 0.68 |
A regional pool of yeasts could be identified in bromeliad tanks of B. karatas and Encholirium sp. and rock holes that may be represented by the highly diverse group of 57 species occurring in any of the three substrates sampled and vicinities, whereas the regional pool in V. minarum and P. bromelioides comprises 42 species associated with those bromeliads and their ecosystems. There is a potential for the discovery of new yeasts, such as Hagleromyces aurorensis, a new genus, and Hanaella pagnoccae, a new species first isolated in the present work.
Discussion
Basidiomycetous yeasts were prevalent in bromeliad substrates (67% of the species isolated) and also in rock waters (62%). Large populations of basidiomycetous yeasts have been frequently observed in bromeliad phytotelmata and phylloplane [3, 4, 10, 47], and they may characterize the core group of yeasts belonging to bromeliad water tanks. According to Lachance et al. [47], the predominance of basidiomycetes in aquatic oligotrophic ecosystems shows these yeasts are more nutritionally versatile and tolerate harsher environmental conditions than ascomycetous yeasts. The yeast species Papiliotrema laurentii, Rhodotorula mucilaginosa, Papiliotrema nemorosus, and Pseudozyma hubeiensis which were found in all or most substrates sampled are ubiquitously distributed throughout all environments, such as soil, plant, aquatic environment, and air [37, 47–57]. Also, these four yeasts are basidiomycetous species that prevail in more oligotrophic substrates when compared with ascomycetous yeasts [55]. As successful isolation of these yeasts in aquatic environments elsewhere indicates [49], they have the ability to occupy aquatic environments that may correlate with the colonization of phytotelmata and also of the waters accumulated in rock holes.
Goffredi et al. [5] suggest that bromeliad tanks provide important habitats for a diverse microbial community, distinct from the surrounding environment. The probable origin of the yeast communities in bromeliad phytotelmata may be as epiphytes in the phylloplane and as endophytes in neighboring bromeliads and other plants and inhabiting soil particles. According to Brouard et al. [6], rosettes of bromeliads form aquatic islands in a terrestrial matrix. They store precipitated water from the forest canopy, and thus they accumulate forest debris that may be an important source of microbial inoculum. This would explain the high frequency of plant-associated yeasts in the tank water of all four plants, such as species of the genera Papiliotrema and Pseudozyma and Rh. mucilaginosa.
Dézerald et al. [57], when studying microbial communities in bromeliads in a range of open to forested habitats, noticed that there was a core group of detritivores that was maintained constant across environments. This was particularly true for detritivores that shared traits that determine responses to environmental changes. Yeasts are a saprobic group which nutritional basis is carbohydrates, especially simple sugars. It is probably that those 16 species are part of the core yeast community of ephemeral aquatic habitats from rupestrian Cerrado habitats. As shown in Venn diagram, this indicates a strong effect of geography in the distribution of yeasts associated with phytotelmata.
Yeast biodistribution showed a pattern of differentiation due to geography and habitat type. A regional pool of yeast species can be inferred by the high β-diversity between Northeastern and Southeastern areas. According to [13], biogeographical patterns of regional diversity of fungi are best understood in the light of fungal biology—the unique ecological roles of fungi and the modes of fungal dispersal. Yeasts are prevalently unicellular non-motile fungi that need dispersers [56]. Most evidence points for a role of insects as dispersers of yeasts—especially Ascomycetous species among plant substrates [58]. Cactophilic and ephemeral flower yeast communities and also mushroom-beetle-associated yeasts are more closely associated with dispersers than to substrates [50, 59, 60]. The insects visiting phytotelmata in each geographical region may be the drivers of regional differences in yeast composition. Dominant terrestrial arthropods with aquatic larvae inhabiting bromeliad phytotelmata are typically larvae of Diptera, of which at least 16 families have been reported, but in some circumstances are Coleoptera, of which only three families have been reported. Other groups include crabs and the insect orders Odonata, Plecoptera, and Trichoptera, plus Hemiptera with adults active on the water surface [61]. Among those, Diptera and Coleoptera especially may act as vectors of yeasts among substrates.
As pointed by Fukami [62], there must be a regional pool containing species that can together cause priority effects, where the order in which species arrive at local sites dictates the effect of species on one another, and local dynamics rapid enough for early-arriving species to preempt or modify niches before other species arrive. In nectar yeasts, just as early-arriving species with high negative impact cause strong inhibitory priority effects, those with high positive impact cause strong facilitative priority effects [63]. The bromeliad habitat selects basidiomycetous yeasts due to its aquatic characteristic and because the probable source is neighboring vegetation. Thus, there is a strong biogeographical component in diversity of yeasts in this ephemeral habitat in Neotropical savannas of Brazil. Lopez et al. [64] explains that, because of the wide variation of the water conditions in the bromeliad tanks, caused by their exposure to sunlight, the organisms found there are adapted to rapid recolonization. As pointed above, yeasts act as metacommunities when occupying ephemeral habitats, with a high turnover rate and intense colonization and extinction dynamics in each individual habitat [13]. This would lead to strong influence of priority effect on these communities, and it would explain the heterogeneity found in bromeliad yeast communities in our work and also in other rupestrian habitats. Gomes et al. [3], studying yeasts associated with bromeliad from rupestrian sites, showed that there is significant spatial heterogeneity in the composition of the yeast communities within bromeliad tanks.
The high frequency of yeasts species occurring in only one of the three substrates may be accounted to the ephemeral nature of these substrates. Peay et al. [13] explains that yeast communities in ephemeral habitats function as metacommunities with high extinction/colonization rates. As we could consider individual phytotelmata as patches or aquatic islands, it is expected a high occurrence of low frequencies and single isolates.
Permissions for collection
According to ICMBio instructions, no special permission is required for collection of microbiological samples. Document 53301 was voluntarily registered by PB Morais in SISBIO as registry of collection of fungal samples.
Authors’ contribution
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Francisca Maria Sousa. The first draft of the manuscript was written by Paula Benevides de Morais, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Data availability
The genomic datasets generated during and/or analyzed during the current study are available in the GenBank repository.
The ecological datasets on yeast population counts in the current study are available from the corresponding author on reasonable request.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Paula B. Morais and Francisca M. P. de Sousa contributed equally to this work.
References
- 1.Givnish TJ, Barfuss MH, Van Ee B, Riina R, Schulte K, Horres R, Gonsiska PA, Jabaily RS, Crayn DM, Smith JA, Winter K, Brown GK, Evans TM, Holst BK, Luther H, Till W, Zizka G, Berry PE, Sytsma KJ. Phylogeny, adaptive radiation, and historical biogeography in Bromeliaceae: insights from an eight-locus plastid phylogeny. Am J Bot. 2011;98:872–895. doi: 10.3732/ajb.1000059. [DOI] [PubMed] [Google Scholar]
- 2.Luther HE. An alphabetical list of bromeliad binomials. 11. Sarasota, Fl: The Marie Selby Botanical Gardens; 2008. [Google Scholar]
- 3.Gomes FCO, Safar SVB, Marques AR, Medeiros AO, Santos ARO, Carvalho C, Lachance M-A, Sampaio JP, Rosa CA (2015) The diversity and extracellular enzymatic activities of yeasts isolated from water tanks of Vriesea minarum, an endangered bromeliad species in Brazil, and the description of Occultifur brasiliensis f.a., sp. nov. Ant van Leeu Int J Gen Mol Microbiol. 10.1007/s10482-014-0356-4 [DOI] [PubMed]
- 4.Landell MF, Mautone JN, Valente P (2006) Biodiversity of yeasts associated to bromeliads in Itapuã Park , Viamão / Rs. Biociencias 14:144–149
- 5.Goffredi SK, Jang GE, Woodside WT, Ussler W III (2011) Bromeliad catchments as habitats for methanogenesis in tropical rainforest canopies. Front Microbiol 2. 10.3389/fmicb.2011.00256 [DOI] [PMC free article] [PubMed]
- 6.Brouard O, Céréghino R, Corbara B, Leroy C, Pelozuelo L, Dejean A, Carrias J-F. Understorey environments influence functional diversity in tank-bromeliad ecosystems. Freshw Biol. 2012;57:815–823. doi: 10.1111/j.1365-2427.2012.02749.x. [DOI] [Google Scholar]
- 7.Brandt FB, Martinson GO, Conrad R. Bromeliad tanks are unique habitats for microbial communities involved in methane turnover. Plant Soil. 2017;410:167–179. doi: 10.1007/s11104-016-2988-9. [DOI] [Google Scholar]
- 8.Klann J, McHenry A, Montelongo C, Goffredi SK. Decomposition of plant-sourced carbon compounds by heterotrophic betaproteobacteria isolated from a tropical Costa Rican bromeliad. Microbiology Open. 2016;5:479–489. doi: 10.1002/mbo3.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Safar, SVB, Gomes, FCO, Marques, AR, Lachance, MA, Rosa, CA (2013) Kazachstania rupicola sp. nov., a yeast species isolated from water tanks of a bromeliad in Brazil. Int J Syst Evol Microbiol doi:10.1099/ijs.0.048462-0, 2013 [DOI] [PubMed]
- 10.Hagler AN, Rosa CA, Morais PB, Mendonça-Hagler LC, Franco GM, Araujo FV, Soares CA. Yeasts and coliform bacteria of water accumulated in bromeliads of mangrove and sand dune ecosystems of Southeast Brazil. Can J Microbiol. 1993;39:9373–9977. doi: 10.1139/m93-146. [DOI] [PubMed] [Google Scholar]
- 11.Landell MF, Billodre R, Ramos JP, Leoncini O, Vainstein MH, Valente P. Candida aechmeae sp. nov. and Candida vrieseae sp. nov., novel yeast species isolated from the phylloplane of bromeliads in Southeastern Brazil. Int J Syst Evol Microbiol. 2010;60:244–248. doi: 10.1099/ijs.0.011577-0. [DOI] [PubMed] [Google Scholar]
- 12.Piątek, M, Lutz, M, Sousa, FMP, Santos, ARO, Félix, CR, Landell, MF, Gomes, FCO, Rosa, CA (2017) Pattersoniomyces tillandsiae gen. et comb. nov.: linking sexual and asexual morphs of the only known smut fungus associated with Bromeliaceae. Org Divers Evol 17:531. doi:10.1007/s13127-017-0340-8
- 13.Peay KG, Kennedy PG, Talbot JM. Dimensions of biodiversity in Earth mycobiome. Nature Rev: microbiol. 2016;14:434–447. doi: 10.1038/nrmicro.2016.59. [DOI] [PubMed] [Google Scholar]
- 14.Tedersoo L, Bahram M, Polme S, Koljag U, Yourou NS, Wijesundera R, et al. Global diversity and geography of soil fungi. Science. 2014;346:1256688. doi: 10.1126/science.1256688. [DOI] [PubMed] [Google Scholar]
- 15.Smith ME, Henkel TW, Uehling JK, Fremier AK, Clarke HD, Vilgalys R. The ectomycorrhizal fungal community in a Neotropical forest dominated by the endemic dipterocarp Pakaraimaea dipterocarpacea. PLoS One. 2013;8:e55160. doi: 10.1371/journal.pone.0055160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peay KG, Russo SE, McGuire KL, Lim Z, Chan JP, Tan S, Davies SJ. Lack of host specificity leads to independent assortment of dipterocarps and ectomycorrhizal fungi across a soil fertility gradient. Ecol Lett. 2015;18:807–816. doi: 10.1111/ele.12459. [DOI] [PubMed] [Google Scholar]
- 17.Peay KG, Belisle M, Fukami T. Phylogenetic relatedness predicts priority effects in nectar yeast communities. Proc Royal Soc B: Biol Sci. 2012;279:749–758. doi: 10.1098/rspb.2011.1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meiser A, Balint M, Schmitt I. Meta-analysis of deep-sequenced fungal communities indicates limited taxon sharing between studies and the presence of biogeographic patterns. New Phytol. 2014;201:623–635. doi: 10.1111/nph.12532. [DOI] [PubMed] [Google Scholar]
- 19.Talbot JM, Bruns TD, Taylor JW, Smith DP, Branco S, Glassman SI, Erlandson S, Vilgalys R, Liao HL, Smith ME, Peay KG. Endemism and functional convergence across the North American soil mycobiome. Proc Natl Acad Sci U S A. 2014;111:6431–6346. doi: 10.1073/pnas.1402584111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Whittaker RH. Evolution and measurement of species diversity. Taxon. 1972;21:213251. doi: 10.2307/1218190. [DOI] [Google Scholar]
- 21.Tuomisto H. A diversity of beta-diversities: straightening up a concept gone awry. Part 1. Defining beta-diversity as a function of alpha and gamma diversity. Ecography. 2010;33:2–22. doi: 10.1111/j.1600-0587.2009.05880.x. [DOI] [Google Scholar]
- 22.Anderson MJ, Crist TO. Chase JM, Vellend M, Inouye BD, Freestone AL, Sanders NJ, Cornell HV, Comita LS, Davies KF, Harrison SP, Kraft NJ, Stegen JC, Swenson NG. Navigating the multiple meanings of β diversity: a roadmap for the practicing ecologist. Ecol Lett. 2011;14:19–28. doi: 10.1111/j.1461-0248.2010.01552.x. [DOI] [PubMed] [Google Scholar]
- 23.Cody ML. Towards a theory of continental species diversities: bird distributions over Mediterranean habitat gradients. In: Cody ML, Diamond JM, editors. Ecology and evolution of communities. Press: Harvard Univ; 1975. p. 214257. [Google Scholar]
- 24.Bratton SP. A comparison of the beta diversity functions of the overstory and herbaceous understory of a deciduous forest Bull. Torrey Bot Club. 1975;102:5560. doi: 10.2307/2484413. [DOI] [Google Scholar]
- 25.Lande R (1996) Statistics and partitioning of species diversity, and similarity among multiple communities Oikos 76: 513
- 26.Martiny JBH, Eisen JA, Penn K, Allison SD, HornerDevine MC. Drivers of bacterial beta-diversity depend on spatial scale. Proc Natl Acad Sci U S A. 2011;108:7850–7854. doi: 10.1073/pnas.1016308108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nielsen UN, Osler GHR, Campbell CD, Neilson R, Burslem D, Van Der Wal R. The enigma of soil animal species diversity revisited: the role of small-scale heterogeneity. PLoS One. 2010;5(7):e11567. doi: 10.1371/journal.pone.0011567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Veech JA. Crist, TO. Toward a unified view of diversity partitioning Ecology. 2010;91:1988–1992. doi: 10.1890/09-1140.1. [DOI] [PubMed] [Google Scholar]
- 29.Almeida-Abreu PA (1995) O Supergrupo Espinhaço da Serra do Espinhaço Meridional (Minas Gerais): O rifte, a bacia e o orógeno. Geonomos 3: 1–18. 10.18285/geonomos.v3i1.211
- 30.Giulietti, AM, Menezes, NL, Pirani, JR, Meguro, M, Wanderley, MGL (1987) Flora da Serra do Cipó, MG: caracterização e lista das espécies. Bol Bot Univ SP 9:1–151. 10.11606/issn.2316-9052.v9i0p1-151
- 31.Madeira JA, Ribeiro KT, Oliveira MJR, Nascimento JS, Paiva CL. Distribuição espacial do esforço de pesquisa biológica na Serra do Cipó. Minas Gerais: subsídios ao manejo das unidades de conservação da região Megadiversidade. 2008;175:385–394. doi: 10.1007/s10909-013-0933-3. [DOI] [Google Scholar]
- 32.Tocantins (2012) Secretariat of Planning. Superintendence of planning and management of public policies: executive board of directors. Ecological Economic Zoning. Atlas do Tocantins: Subsidies to Territorial Management Planning. 5. Ed. Palmas: Seplan. http://www.sefaz.to.gov.br/zoneamento/zoneamento/atlas-do-tocantins/. Accessed on: 16 July, 2019
- 33.Morais F (2009) Contexto geológico das cavernas em arenito do Estado do Tocantins. An XXX Cong Bras Espeleol:139–144
- 34.Morais F. Caracterização geomorfológica da região de Aurora do Tocantins, Brasil. Rev Bras Geomorfol. 2013;14:163–170. [Google Scholar]
- 35.Haidar RF, Fagg JMF, Pinto JRR, Dias RR, Silva LCR, Fagg CW. Florestas estacionais e áreas de ecótono no Estado do Tocantins, Brasil: parâmetros estruturais, classificação das fitofisionomias florestais e subsídios para Conservação. acta Amazon. 2013;43:261–290. doi: 10.1590/s0044-59672013000300003. [DOI] [Google Scholar]
- 36.Joly AB. Conheça a vegetação brasileira. Polígono, São Paulo: Edusp; 1970. [Google Scholar]
- 37.Kurtzman CP, Fell JW, Boekhout T. The yeasts: a taxonomic study. 5. Amsterdam: Elsevier; 2011. [Google Scholar]
- 38.Libkind D, Brizzio S, Ruffini A, Gadanho M, van Broock M, Sampaio P. Molecular characterization of carotenogenic yeasts from aquatic environments in Patagonia, Argentina. Ant van Leeuw. 2003;84:313–322. doi: 10.1023/A:1026058116545. [DOI] [PubMed] [Google Scholar]
- 39.Lopes MR, Lara CA, Moura MEF, Uetanabaro APT, Morais PB, Vital MJS, Rosa CA. Characterization of the diversity and physiology of cellobiose-fermenting yeasts isolated from rotting wood in Brazilian ecosystems. Fung Biol. 2018;122:668–676. doi: 10.1016/j.funbio.2018.03.008. [DOI] [PubMed] [Google Scholar]
- 40.Sampaio JP, Gadanho M, Santos S, Duarte, FL, et al. Polyphasic taxonomy of the basidiomycetous yeast genus Rhodosporidium: Rhodosporidium kratochvilovae and related anamorphic species. Int J Syst Evol Microbiol doi:10.1099/00207713-51-2-687, 2001 [DOI] [PubMed]
- 41.White TJ, Bruns T, Lee SJWT, Taylor JW. Amplification and direct sequencing 661 of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ, editors. PCR protocols: a guide to methods and applications. San Diego: Academic Press; 1990. pp. 315–322. [Google Scholar]
- 42.O'Donnell K. Fusarium and its near relatives. In: Reynolds DR, Taylor JW, editors. The fungal holomorph: mitotic, meiotic and pleomorphic speciation in fungal systematics. Oregon: CAB International; 1993. pp. 225–233. [Google Scholar]
- 43.Kurtzman CP, Robnett CJ. Identification and phylogeny of ascomycetous yeasts from analysis of nuclear large subunit (26S) ribosomal DNA partial sequences. Ant van Leeuw Int J Gen Mol Microbiol. 1998;1998:331–371. doi: 10.1023/A:1001761008817. [DOI] [PubMed] [Google Scholar]
- 44.Lachance M-A, Bowles JM, Starmer WT, Barker JSF. Kodamaea kakaduensis and Candida tolerans, two new ascomycetous yeast species from Australian Hibiscus flowers. Can J Microbiol. 1999;45:172–177. doi: 10.1139/cjm-45-2-172. [DOI] [PubMed] [Google Scholar]
- 45.Magurran AE (2004) Measuring biological diversity. Blackwell Publ, 577 p
- 46.Oksanen, J, Kindt, R, Blanchet, FG, Legendre, P et al (2012) Package vegan – community ecology package, v. 2.0-4
- 47.Araújo FV, Medeiros RJ, Mendonça-Hagler LC, Hagler AN. A preliminary note on yeast communities of bromeliad-tank waters of Rio de Janeiro, Brazil. Rev Microbiol. 1998;29:118–121. [Google Scholar]
- 48.Boekhout T, Fonseca A, Sampaio JP, Bandoni RJ, Fell JW, Kwon-Chung KJ. Kurtzman, CP, Fell, JW, Boekhout, T (Edn) the yeasts: a taxonomic study. 5. Amsterdam: Elsevier; 2011. Discussion of teleomorphic and anamorphic basidiomycetous yeasts; pp. 1339–1372. [Google Scholar]
- 49.Brandão LR, Vaz ABM, Espírito-Santo LC, Pimenta RS, Morais PB, Libkind D, Rosa LH, Rosa CA. Diversity and biogeographical patterns of yeast communities in Antarctic, Patagonian and tropical lakes. Fungal Ecol. 2017;28:33–43. doi: 10.1016/j.funeco.2017.04.003. [DOI] [Google Scholar]
- 50.Ganter PF, Morais PB, Rosa CA. Yeasts in cacti and tropical fruit. In: Buzzini P, Lachance MA, Yurkov A, editors. Yeasts in natural ecosystems: diversity. New York: Springer; 2017. pp. 225–264. [Google Scholar]
- 51.Fonseca A, Boekhout T, Fell JW. Kurtzman, CP, Fell, JW, Boekhout, T (Edn) the yeasts: a taxonomic study. 5. Amsterdam: Elsevier; 2011. Cryptococcus Vuillemin (1901) pp. 1661–1739. [Google Scholar]
- 52.Sampaio JP (2011) Rhodotorula Harrison (1928). In: Kurtzman, CP, Fell, JW, Boekhout, T (Edn) the yeasts: a taxonomic study. 5th edition. Elsevier, Amsterdam, pp 1873–1927. 10.1016/B978-0-444-52149-1.00155-5
- 53.Statzell-Tallman A, Fell JW. Kwoniella Statzell-Tallman & Fell (2007). In: Kurtzman, CP, Fell, JW, Boekhout, T (Edn) the yeasts: a taxonomic study. 5th edition. Elsevier, Amsterdam, pp 1481–1484. 10.1016/B978-0-444-52149-1.00119-1
- 54.Wang SA, Bay FY. Saccharomyces arboricolus sp. nov., a yeast species from tree bark. Int J Syst Evol Microbiol. 2008;58:510–514. doi: 10.1099/ijs.0.65331-0. [DOI] [PubMed] [Google Scholar]
- 55.Brandão LR, Libkind D, Vaz ABM, Espirito Santo LC, Moliné M, de Garcia V, van Brook M, Rosa CA. Yeasts from an oligotrophic lake in Patagonia (Argentina): diversity, distribution and synthesis of photoprotective compounds and extracellular enzymes. FEMS Microbiol Ecol. 2011;73:1–13. doi: 10.1111/j.1574-6941.2010.01030.x. [DOI] [PubMed] [Google Scholar]
- 56.Lachance MA (2013) The biodiversity, ecology, and biogeography of ascomycetous yeasts. In: Martin, F (Edn) The Ecological Genomics of Fungi pp 355–370 10.1002/9781118735893.ch16
- 57.Dézerald O, Talaga S, Leroy C, Carrias J-F, Corbara B, Dejean A, Céréghino R. Environmental determinants of macroinvertebrate diversity in small water bodies: insights from tank-bromeliads. Hydrobiologia. 2014;723:77–83. doi: 10.1007/s10750-013-1464-2. [DOI] [Google Scholar]
- 58.Blackwell M (2017) Made for each other: ascomycete yeasts and insects. Microbiology Spectrum 5, FUNK-0081-2016. 10.1128/microbiolspec.FUNK-0081-2016 [DOI] [PMC free article] [PubMed]
- 59.Lachance M-A, Starmer WT, Rosa CA, Bowles JM, Barker JSF, Janzen DH. Biogeography of the yeasts of ephemeral flowers and their insects. FEMS Yeast Res. 2001;1:1–8. doi: 10.1111/j.1567-1364.2001.tb00007.x. [DOI] [PubMed] [Google Scholar]
- 60.Aanen DK, Boomsma JJ. Evolutionary dynamics of the mutualistic symbiosis between fungus-growing termites and Termitomyces fungi in Vega FE, Blackwell M (ed), insect-fungal associations: ecology and evolution. New York, NY: Oxford University Press; 2005. pp. 191–210. [Google Scholar]
- 61.Greeny HF. The insects of plant-held waters: a review and bibliography. J Trop Ecol. 2001;17:241. doi: 10.1017/S026646740100116X. [DOI] [Google Scholar]
- 62.Fukami T. Historical contingency in community assembly: integrating niches, species pools, and priority effects. Ann Rev Ecol Evol Syst. 2015;43:1–23. doi: 10.1146/annurev-ecolsys-110411-160340. [DOI] [Google Scholar]
- 63.Vannette RL, Fukami T. Historical contingency in species interactions: towards niche-based predictions. Ecol Lett. 2014;17:115–124. doi: 10.1111/ele.12204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lopez LCS, Madeira JA, Torres KR, Rios RI (1993) Composição e dinâmica hídrica de phytotelmata de Aechmea nudicaulis e Neoregelia cruenta (Bromeliaceae Bromeloideae) de Restinga de
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The genomic datasets generated during and/or analyzed during the current study are available in the GenBank repository.
The ecological datasets on yeast population counts in the current study are available from the corresponding author on reasonable request.


