Abstract
The antimicrobial peptide PMAP-36 is a cationic peptide derived from porcine myeloid. The N-terminally paired lysine of PMAP-36 was substituted with tryptophan, and the C-terminal hydrophobic tail was deleted, thereby obtaining the antimicrobial peptide PRW4. PRW4 is a α-helical antimicrobial peptide with broad-spectrum antimicrobial activity. In this study, PRW4 was fused to the 6× His-Trx, and the fusion protein was successfully expressed in Pichia pastoris GS115 from the vector pPICZαA. The maximal induction of recombinant protein occurred in the presence of 1% methanol after 96 h at pH 6.0. After purification by a Ni-NTA resin column and digestion by enterokinase protease, 15 mg of recombinant PRW4 with a purity of 90% was obtained from 1 L of fermentation culture. The results indicated that recombinant PRW4 had similar antimicrobial activity as synthetic PRW4 against bacteria such as Escherichia coli ATCC 25922, Escherichia coli UB 1005, Salmonella typhimurium C7731, Salmonella typhimurium 7913, Salmonella typhimurium ATCC 14028, Staphylococcus aureus ATCC 29213, Staphylococcus epidermidis ATCC 12228, and Streptococcus faecalis ATCC 29212. We have successfully expressed PRW4 in P. pastoris, and this work provides a reference for the production of modified antimicrobial peptides in P. pastoris.
Electronic supplementary material
The online version of this article (10.1007/s42770-020-00291-4) contains supplementary material, which is available to authorized users.
Keywords: Antimicrobial peptides, PRW4, Pichia pastoris, Antimicrobial activity, Gene expression
Introduction
For decades, edible antibiotics have been used in the livestock industry. However, the long-term use of antibiotics, as well as the abuse of these compounds, has contributed to the increase in bacterial resistance, threatening public health [1, 2]. Therefore, it is necessary to find new types of antibiotic substitutes that can be applied to livestock production [3].
In recent decades, antimicrobial peptides (AMPs) have drawn increasing attention due to their rapid and broad-spectrum activities against gram-negative and gram-positive bacteria, fungi, viruses, etc. In addition, the antimicrobial mechanism of AMPs is different from that of antibiotics. AMPs physically destroy the cell membrane structure of bacteria and leak the contents of cells to kill cells, so it is difficult to acquire drug resistance [4–6]. Therefore, AMPs are an effective alternative to antibiotics [6].
However, the expensive synthesis cost of AMPs greatly limits their application in livestock production. Recombinant DNA technology as a low-cost, high-efficiency method for large-scale protein production provides a possible opportunity for AMPs to be used in livestock production [7]. For decades, many protein expression systems, such as E. coli, Bacillus subtilis, and P. pastoris, have been widely used to generate recombinant proteins [8, 9]. As a eukaryotic expression system, P. pastoris can provide suitable environment and conditions for the folding, glycosylation, and other post-translational modifications of foreign proteins to ensure the activity of the proteins, which are not available in prokaryotic expression systems such as E. coli and B. subtilis [10]. In addition, the P. pastoris expression system can secrete the expressed foreign protein extracellularly, so it is beneficial for later separation and purification. Therefore, the P. pastoris expression system is more convenient, practical, and efficient for the expression of AMPs and has more industrial development potential than other expression systems. Many natural AMPs have been successfully expressed in the P. pastoris expression system, such as plectasin, PMAP-36, fowlicidin-2, snakin-1, and abaecin [11–17].
The antimicrobial peptide PRW4, designed and engineered by our laboratory, is a short peptide derived from amino acids 2–17 of the porcine antimicrobial peptide PMAP-36, and lysines at positions 7 and 11 are substituted with tryptophan to obtain better stability. PRW4 (RFRRLRWKTRWRLKKI-NH2), with a molecular weight of 2299.8 Da, shows broad-spectrum antimicrobial activity; therefore, PRW4 has the potential to be a new class of antimicrobial drugs [18]. However, there are no means to produce PRW4 in large quantities at a low cost, which is the most important factor limiting its application in production.
In this study, we established an efficient method to express the antimicrobial peptide PRW4 in the P. pastoris expression system.
Materials and methods
Strains, vectors, culture medium, and reagents
E. coli DH5α used to construct the recombinant vector was purchased from Invitrogen (Carlsbad, USA). P. pastoris GS115 used as the expression host was kept by our laboratory. E. coli ATCC 25922, E. coli UB 1005, S. typhimurium C7731, S. pullorum 7913, S. typhimurium ATCC 14028, S. aureus ATCC 29213, S. epidermidis ATCC 12228, and S. faecalis ATCC 29212 were kept by our laboratory and were used for the antimicrobial activity assay. The expression vector pPICZαA was purchased from Invitrogen (Carlsbad, USA). All of the above strains were stored in 30% glycerol at − 80 °C.
Luria-Bertani (LB) medium, yeast extract peptone dextrose medium (YPD), and Mueller-Hinton broth medium (MHB) were used to cultivate E. coli DH5α, P. pastoris GS115, E. coli ATCC 25922, E.coli UB 1005, S. typhimurium C7731, S. pullorum 7913, S. typhimurium ATCC 14028, S. aureus ATCC 29213, S. epidermidis ATCC 12228, and S. faecalis ATCC 29212.
The P. pastoris containing the plasmid pPICZαA was cultured in buffered glycerol complex (BMGY) medium, and for the inducible expression of 6× His-Trx-PRW4, cells were grown in buffered methanol complex (BMMY) medium. The restriction enzymes XhoI, XbaI and SacI, T4 DNA ligase, Taq DNA polymerase, and low range protein ladder were purchased from Fermentas (Carlsbad, USA). DNA markers (5000 bp) were purchased from Takara Biomedical Technology (Dalian, China). Ni sepharose™ 6 fast flow resin and recombinant enterokinase were purchased from Sangon Biotech Co., Ltd. (Shanghai, China). Zeocin™ and ampicillin were purchased from Invitrogen (Carlsbad, USA). The gel extraction kit and plasmid mini kit were purchased from GenStar Co., Ltd. (Beijing, China). Synthetic PRW4 was synthesized by Sangon Biotech Co., Ltd. (Shanghai, China). All other chemicals and reagents used in this study were of analytical grade or higher.
Construction of the recombinant plasmid
A gene sequence encoding the N-terminal 6× His-Trx-PRW4 fusion with a stop codon containing XhoI and XbaI restriction enzyme sites at its 5′- and 3′-ends, respectively, was synthesized by Shanghai Sangon Biotechnology Co., Ltd. and was cloned into the Pichia expression vector pPICZαA, which resulted in the expression vector pPICZαA-PRW4. Based on the codon preference of P. pastoris, the online software Condon Usage Database (http://www.kazusa.or.jp/codon/) was used to codon optimize the genes. pPICZαA-PRW4 was then transformed into E. coli DH5α and selected on LB plates with Zeocin (25 μg/mL). Restriction enzyme analysis and DNA sequencing analysis were used to ensure that the plasmid harbored the expected nucleotide sequence and the sequence was in the correct in-frame orientation.
P. pastoris transformation and positive transformant selection
pPICZαA-PRW4 and the empty pPICZαA plasmid were linearized by SacI. Then, the linearized pPICZαA-PRW4 plasmid and the linearized empty pPICZαA plasmid were transformed into GS115 competent cells at 1500 V, 25 μF capacitance, and 200 Ω resistance. Colonies containing the resistance gene were screened on YPDS plates (YPD medium + 1.5% agar powder) containing 100 μg/mL Zeocin. Positive transformants were confirmed by PCR using the following primers.
Primer 5′AOX1:5′-GACTGGTTCCAATTGACAAGC-3′
Primer 3′AOX1: 5′-GCAAATGGCATTCTGACATCC-3′
Expression of recombinant proteins in P. pastoris GS115 and shake-flask cultivation optimization
The 6× His-Trx-PRW4 positive transformants were selected and cultured in 10 mL of YPD for 24 h at 30 °C with a rotation speed of 250 rpm. The cells were then grown in 50 mL of BMGY medium until the OD600 of the culture was 2–6. The cells were collected by centrifugation at 10,000×g for 5 min to remove the supernatant. Cell pellets obtained in the previous step were then re-suspended in BMMY medium, and the OD600 of the culture was adjusted to 1.0 to induce the expression of the recombinant protein. The positive transformants were expressed at 30 °C with a rotation speed of 250 rpm and the methanol was added every 24 h during the 120 h induction period. The culture supernatants were harvested by centrifugation at different time intervals. The molecular weight of the target protein was determined by Tricine-SDS-PAGE and the supernatants were collected and assessed by Bradford assay to determine the quantity of total extracellular protein.
To optimize the induction time for total protein yield at initial pH 6.0 under the induction by 1.0% methanol, the culture was continued for up to 120 h, and fermentation supernatants were collected at 24, 48, 72, 96, and 120 h post-induction. The changes in the amount of total proteins secreted into the culture supernatants were determined by Bradford assay as described above. When the initial pH of the fermentation medium was 6.0, fermentation supernatants were collected for 96 h after the addition of 0.5%, 1.0%, 1.5%, 2.0%, and 2.5% (v/v) methanol to optimize the methanol concentration. In order to explore the effect of the initial pH of the medium on the expression of protein, fermentation cultures were performed at different initial pH values of the medium (5.5, 6.0, 6.5, 7.0, and 7.5) under the 1.0% methanol induction and we collected the fermentation supernatants at 96 h post-induction. The concentrations of protein secreted into the supernatant were evaluated by Bradford assay as described above.
Purification of 6× His-Trx-PRW4 and enterokinase cleavage
The 6× His-Trx-PRW4 was purified with a Ni-NTA resin column and the yield of 6× His-Trx-PRW4 was assessed by Bradford assay. The column was pre-equilibrated with 4 column volumes of binding buffer (20 mM Tris-HCl, 500 mM NaCl, 20 mM imidazol; pH 8.0). The culture supernatant was mixed with 5× binding buffer (0.1 M Tris-HCl, 2.5 M NaCl, 0.1 M imidazole; pH 8.0). The mixture was passed through a 0.22-μm filter and then applied to the column. The 6× His-Trx-PRW4 was eluted with 5 column volumes of elution buffer containing 20, 50, 80, 200, 300, or 500 mM imidazole at a flow rate of 1 mL/min.
The 6× His-Trx-PRW4 obtained by affinity chromatography with Ni-NTA was subjected to overnight dialysis in enterokinase buffer (150 mM Tris-HCl, 15 mM NaCl, 2.5 mM CaCl2; pH 7.4). The 6× His-Trx-PRW4 was digested with enterokinase and incubated at 25 °C for 16 h. The cleaved recombinant PRW4 (rPRW4) was then re-purified by affinity chromatography on a Ni-NTA column.
The purified and cleaved rPRW4 was detected using Tricine-SDS-PAGE followed by Coomassie Blue staining. The concentration of cleaved rPRW4 was determined by Bradford assay as described above. The purity of the target protein bands was evaluated with Quantity One Software (BioRad, USA).
Antimicrobial activity assay of rPRW4 in vitro
The antimicrobial activity of rPRW4 in vitro was determined against several bacteria. The minimum inhibitory concentrations (MICs) were determined using a modified standard microtiter dilution method as described previously [19]. The bacteria were incubated overnight at 37 °C until the logarithmic growth phase was reached. The bacteria were then diluted to 105 CFU/mL. A 50-μL volume of bacteria in Mueller-Hilton broth (MHB) was mixed with 50 μL of a 2-fold serial dilution of the peptides that were dissolved in 0.01% (v/v) acetic acid and 0.2% (w/v) bovine serum albumin (BSA). The mixtures were incubated at 37 °C for 16–24 h. All of the experiments were performed in triplicate.
Statistical analysis
The statistical means and standard deviation (SD) were computed with the SAS 9.3 software (SAS Institute, Inc., Cary, NC, USA). The minimum inhibitory concentrations (MICs) were presented as the means ± SD.
Results
Construction of the P. pastoris expression plasmid pPICZαA-PRW4
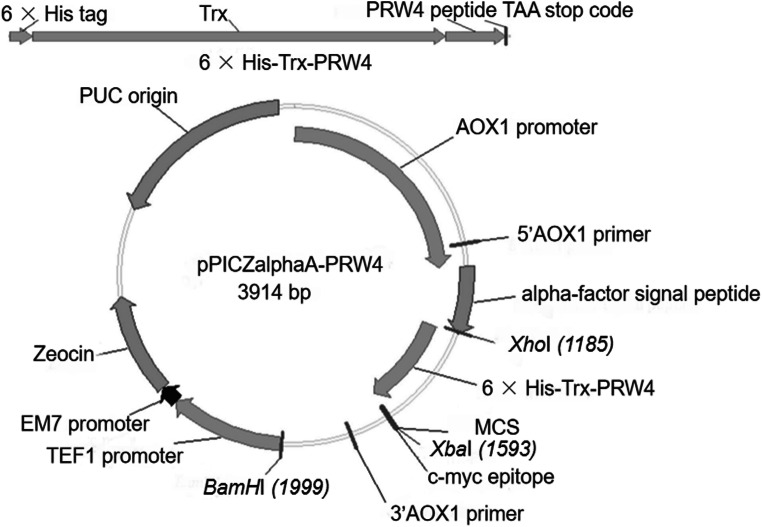
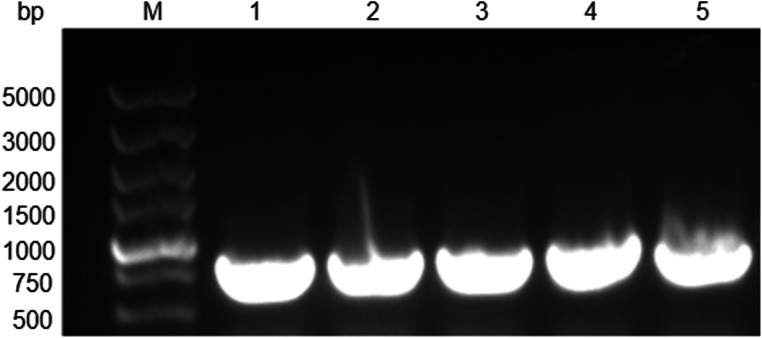
The yeast expression vector pPICZαA-PRW4, containing the N-terminal codon-optimized 6× His-Trx tag and the codon-optimized PRW4 gene, was successfully constructed (Fig. 1; Supplementary Fig. S1). Restriction enzyme analysis showed that the DNA fragments digested by XhoI and XbaI were in accordance with the expected size (Table 1). The constructed vector was sequenced, and the sequenced result was used for alignment by BLAST, which indicated that the synthesized gene was in accordance with the design (data not shown). The pPICZαA-PRW4 plasmid was transformed into P. pastoris GS115 competent cells, and the transformants were selected by colony PCR. The results showed that the 6× His-Trx-PRW4 coding sequence was successfully inserted into the Zeocin-resistant P. pastoris transformants (Fig. 2).
Fig. 1.
Construction of the P. pastoris recombinant plasmid pPICZαA-PRW4
Table 1.
Amino acid sequence and DNA sequences of 6× His-Trx-PRW4
| Sequences | Number of amino acids/gene length (bp) | |
|---|---|---|
| Amino acid sequences | KRHHHHHHMAIVKATDQSFSAETSEGVVLADFWAPWCGPCKMIAPVLEELDQEMGDKLKIVKIDVDENQETAGKYGVMSIPTLLVLKDGEVVETS0VGFKPKEALQELVNKHLDDDDKRFRRLRWKTRWRLKKI | 131 |
| DNA sequences | CTCGAGAAAAGACATCACCACCACCACCACATGGCTATCGTTAAAGCTACAGATCAATCTTTCTCTGCTGAAACATCTGAAGGCGTTGTTCTTGCTGATTTCTGGGCTCCTTGGTGCGGCCCTTGCAAAATGATCGCTCCTGTTCTTGAAGAACTTGATCAAGAAATGGGCGATAAACTTAAAATCGTTAAAATCGATGTTGATGAAAACCAAGAAACAGCTGGCAAATACGGCGTTATGTCTATCCCTACACTTCTTGTTCTTAAAGATGGCGAAGTTGTTGAAACATCTGTTGGCTTCAAACCTAAAGAAGCTCTTCAAGAACTTGTTAACAAACATCTTGATGATGATGATAAACGTTTTCGCCGATTACGGTGAAAACTTAGATGGAGGTTGAAGAAAATTTAATCTAGA | 414 |
The underlined and bold regions are the XhoI and XbaI cleavage site; the underlined regions are the Kex2 cleavage site, enterokinase digestion sequences; the italic region is the 6× His-Trx tag sequence; the shaded region is the target peptide PRW4 sequence; the bold region is the stop codon
Fig. 2.
Identification of recombinant expression vector pPICZαA-PRW4 by PCR amplification. M: low range prestained protein marker; lanes 1–5: the recombinant expression vector pPICZαA-PRW4 electroporation transformation of yeast PCR products
Effects of induction conditions on the expression of the recombinant protein
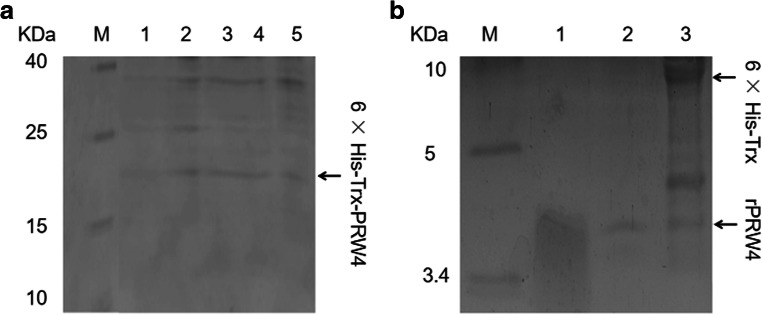
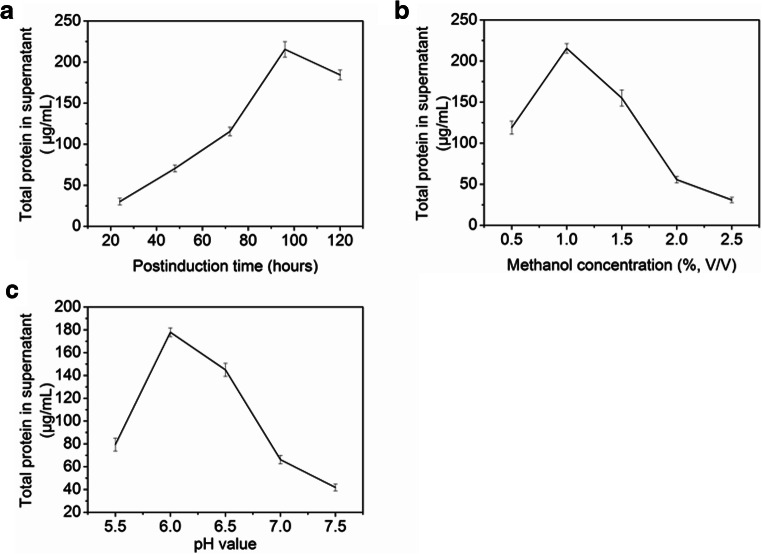
A band at approximately 17 kDa was observed from Tricine-SDS-PAGE and Coomassie Blue staining from samples collected between 24 and 120 h after induction (Fig. 3a). The Bradford assay determined that the concentration of total protein secreted in the culture supernatant after 24 h of induction was 30.2 μg/mL. After 96 h of induction, the maximum yield of total protein was 215.5 μg/mL. The results showed that the 6× His-Trx-PRW4 was expressed in the P. pastoris GS115 cells, and the expression yield of the total protein in yeast was correlated with the induction time (Fig. 4a).
Fig. 3.
a Expression of the 6× His-Trx-PRW4 fusion protein in Pichia pastoris GS115. M: low range prestained protein marker (5 μL); lanes 1–5: supernatant samples taken at 24 h (40 μL, 1.208 μg), 48 h (40 μL, 2.820 μg), 72 h (40 μL, 4.620 μg), 96 h (40 μL, 8.620 μg), and 120 h (40 μL, 7.380 μg) of induction at pH 6.0 by 1.0% (v/v) pure methanol, respectively. b Tricine-SDS-PAGE of purified and cleaved 6× His-Trx-PRW4. M: low range prestained protein marker (5 μL); lane 1: chemically synthesized PRW4 sample (40 μL, 4 μg); lane 2: purified rPRW4 sample (40 μL, 0.6 μg); lane 3: 6× His-Trx-PRW4 digestion system (40 μL)
Fig. 4.
a Effect of post-induction time on the expression of the total protein in supernatant at pH 6.0 under 1.0% methanol induction. b Effect of methanol concentration on the expression of the total protein in supernatant at pH 6.0 for 96 h. c Effect of culture medium pH on the expression of the total protein in supernatant for 96 h under 1.0% methanol induction
To optimize the methanol concentration and increase the expression level of the total protein, we chose five different concentrations of methanol to induce total protein expression, and the other induction conditions and medium remained unchanged. After collecting the culture supernatant, the amounts of secreted total protein were measured. The results showed that the concentration of total protein in the culture supernatant induced by 1.0% methanol was the highest compared to the induction by 0.5%, 1.5%, 2.0%, or 2.5% methanol, which indicated that the 1.0% methanol-induced secretion of the total protein reached the highest yield (Fig. 4b).
To optimize the medium pH for total protein yield, fermentation supernatants were collected after 96 h of induction at different pH values (5.5, 6.0, 6.5, 7.0, and 7.5). Then, we measured the amounts of secreted total protein after the culture supernatants were obtained. It was found that the expression level of total protein in the culture supernatant was the highest when the pH was 6.0, which indicated that different medium pH values might have different effects on the expression yield of secreted total proteins (Fig. 4c).
Expression and antimicrobial activity of purified and cleaved 6× His-Trx-PRW4
The 6× His-Trx-PRW4 was purified with a Ni-NTA resin column and the yield of 6× His-Trx-PRW4 was 160.8 μg/mL. We used enterokinase to cleave the 6× His-Trx-PRW4 to obtain rPRW4 with the molecular weight of 4.0 kDa (Fig. 3b). A yield of 15 mg of rPRW4 with a purity of 90% was obtained from 1 L of fermentation culture. We then tested the antimicrobial activity of PRW4 against E. coli ATCC 25922, E. coli UB 1005, S. typhimurium C7731, S. aureus ATCC 29213, S. epidermidis ATCC 12228, and S. faecalis ATCC 29212. As presented in Table 2, the MICs assay demonstrated that rPRW4 exhibited antimicrobial activity against those strains, which were consistent with previous conclusions.
Table 2.
Antimicrobial activity of recombinant and synthetic PRW4
| Strains | MICs (μM) (means ± SD) | |
|---|---|---|
| Recombinant PRW4 | Synthetic PRW4 | |
| Gram-negative bacteria | ||
| E. coli 25922 | 2.44 ± 0.83 | 2.00 ± 0.00 |
| E. coli UB1005 | 4.00 ± 0.00 | 4.00 ± 0.00 |
| S. typhimurium C7731 | 4.83 ± 1.90 | 2.83 ± 0.98 |
| S. typhimurium ATCC14028 | 4.67 ± 2.49 | 3.25 ± 0.96 |
| S. pullorum 7913 | 2.22 ± 1.31 | 3.66 ± 3.09 |
| Gram-positive bacteria | ||
| S. aureus 29213 | 4.00 ± 0.00 | 4.00 ± 0.00 |
| S. epidermidis 12228 | 7.33 ± 1.49 | 8.00 ± 0.00 |
| S. faecalis 29212 | 10.67 ± 3.77 | 10.67 ± 3.77 |
Data are shown as the means ± SD of three independent tests
Discussion
With the rapid development of genetic engineering technology, different heterologous expression systems, such as P. pastoris and E. coli, are chosen for large-scale preparation of AMPs [20, 21]. However, foreign proteins expressed by the E. coli expression system easily form inclusion bodies. And endotoxins produced by E. coli can contaminate target proteins [22]. In addition, as a eukaryotic expression system, P. pastoris can provide suitable environment and conditions for the folding, glycosylation, and other post-processing of foreign proteins to ensure the activity of the protein. Therefore, compared with the E. coli expression system, the P. pastoris expression system has a higher expression yield and the expression product can show better biological activity [23]. Fan et al. used the E. coli expression system and P. pastoris expression system to express Beauveria bassiana chitinase (Bbchit1), respectively. The results showed that P. pastoris could secrete recombinant Bbchit1 directly into the fermentation broth. However, recombinant Bbchit1 expressed by E. coli existed in the form of inclusion bodies, which was undoubtedly not conducive to later isolation and purification. In addition, the yield and specific activity of recombinant Bbchit1 produced by P. pastoris were higher than those of recombinant Bbchit1 produced by E. coli, respectively, 153 mg/L versus 50 mg/L and 3.9 U/mg versus 2.8 U/mg [23]. Therefore, in recent years, P. pastoris has become a very successful expression system for the production of active heterogeneous proteins [24]. The methanol metabolism promoter alcohol oxidase 1 (AOX1) is one of the most potent promoters. Under the induction of methanol, AOX1 transcripts account for more than 30% of the whole cell transcript [25]. In addition, codon bias of P. pastoris varies from species to species, and the efficiency of foreign genes in the host can affect expression [17, 26]. It has been found that replacing rarely occurring codons according to the preferred codon usage of P. pastoris can increase the expression level of foreign genes [27]. Therefore, we optimized codons for the fusion protein gene 6 × His-Trx-PRW4. The 6× His-Trx-PRW4 could be secreted into the fermentation supernatant with an α-mating factor (α-MF) signal peptide, and endoprotease Kex2 targeted the peptide and was responsible for the cleavage of α-MF [28]. Therefore, we used the methanol-inducible yeast expression vector pPICZαA with the α-MF and AOX1 promoters to produce the fusion protein in P. pastoris. After purification by a Ni-NTA resin column and digestion by enterokinase protease, rPRW4 was obtained and had a similar antimicrobial activity as synthetic PRW4 against bacteria, such as E. coli ATCC 25922, E. coli UB 1005, S. typhimurium C7731, S. pullorum 7913, S. typhimurium ATCC 14028, S. aureus ATCC 29213, S. epidermidis ATCC 12228, and S. faecalis ATCC 29212.
Fusion expression is commonly used in the production of genetically engineered peptides in P. pastoris [29]. Fusion expression is an effective strategy to protect small molecule AMPs from proteolysis and reduce the toxicity of AMPs to host cells. To date, many fusion protein tags, such as His [30], GST [31], thioredoxin (Trx) [32], and SUMO [33], are often used in the expression of AMPs in P. pastoris, E. coli, and B. subtilis. These tags, to a large extent, make AMPs convenient for analysis and separation.
Among these fusion protein tags, Trx has been used to express AMPs existing in nature, such as plectasin, cecropin A, and LL-37 [34–36]. Trx is a non-toxic, small, and highly soluble protein [32]. Trx is employed as a fusion tag with the ability of increasing the solubility of recombinant proteins [37]. Hlavac et al. used the E. coli expression system to express the tyrosine kinase Lck and found that the solubility of Trx-Lck fusion protein was significantly better than that of GST-Lck fusion protein. Moreover, the solubilization technology would lead to high degradation of GST-Lck, but for Trx-Lck fusion protein, even if there was no protease inhibitor, there was no sign of degradation of Trx-Lck fusion protein [38]. In addition, its small size makes it particularly suitable for peptide production because it allows the target peptide to account for a relatively large percentage of the fusion. An earlier study showed that compared to other tags tested, the target peptide had the highest relative yield, although the overall expression level of the fusion protein was not the highest [39]. Moreover, the positive charge of AMPs may interfere with the interaction between peptide and the host cell DNA and RNA, which may result in unstable transcription and translation [40]. Trx (PI 4.67) can be used as an acidic fusion tag to neutralize the highly purified positive charge of AMPs to ensure smooth transcription and translation processes [36]. Therefore, Trx is recognized as a suitable tag for fusion expression of AMPs [32, 37]. In this study, we used the 6× His-Trx fusion tag to express a novel antimicrobial peptide PRW4, and this work provided a reference for the large-scale and safe production of AMPs in P. pastoris. Moreover, in this experiment, we added an enterokinase cleavage site (DDDDK) so that the recombinant protein obtained after cleavage had no additional amino acid residues, and the structure and antimicrobial activity were protected to the greatest extent.
There are many factors that affect the production of recombinant proteins in P. pastoris, and the main factors are the concentration of methanol inducer, the induction time, and the pH of the medium [41, 42]. Methanol concentration is a critical factor to determine because AOX1 promoter activity responds to methanol induction, thus regulating the expression levels of foreign genes contained within the pPICZαA plasmid. However, excessively high methanol concentration increases the accumulation of methanol metabolites such as formaldehyde and hydrogen peroxide. The methanol metabolites will have a toxic effect on Pichia pastoris, thereby increasing cell death and lysis and affecting heterologous protein expression [26, 43]. Indeed, our results showed that when the concentration of methanol was too high, it could not increase the protein expression of recombinant proteins but reduced the expression yield.
In addition to the concentration of methanol inducer, the induction time is also a factor affecting the expression level. Too long fermentation time will undoubtedly increase costs, and too short fermentation time may affect fermentation efficiency. Therefore, it is important to choose a suitable fermentation time. With the extension of the induction time, the expression products in the culture medium accumulate continuously. The nutrients contained in the medium continue to decrease until they are completely consumed, which prevents the microorganisms from continuing to survive, eventually leading to the death of the microbial cells [44–46]. Our results showed that the protein expression level continued to increase after induction, reaching a maximum at 96 h, and then the protein expression level began to decrease. The reason for this condition might be that the medium at the initial stage of induction could provide a large amount of nutrients, when P. pastoris grew vigorously and had a relatively strong ability to secrete proteins. However, as time went by, the nutrients in the medium were gradually consumed, and P. pastoris could not obtain enough nutrients, which resulted in a decline in its ability to secrete proteins. Meanwhile, the metabolites of P. pastoris were relatively complex, and the secreted proteases might degrade the expressed heterologous proteins [47].
In addition to the above two factors, the pH of the medium also affects the expression of the protein, and too high or too low are not conducive to protein expression [41, 48]. P. pastoris can express foreign proteins in a wide range of pH (3.0–7.0). This range will have minimal effect on the growth rate of P. pastoris but will significantly affect protease activity and the stability of expressed proteins [49, 50]. In our experiments, the expression level of the recombinant protein was highest at the optimum pH of 6.0. This result was consistent with the result of Lee and co-workers using the P. pastoris expression system to express ice-binding proteins [41]. This might be because the protease activity was low and the stability of the recombinant protein was high at pH 6.0, thereby ensuring the yield of the recombinant protein to the greatest extent. High pH had been reported to reduce cell viability and might reduce the stability or activity of recombinant products [51]. In our experiment, when the pH of the medium was increased to 7.5, the protein expression level continued to decrease. This might be because the cell viability and the stability of the recombinant protein were decreased affected by pH. Therefore, the optimal conditions for the expression of recombinant proteins in Pichia pastoris were 96 h with 1.0% (v/v) methanol and pH 6.0.
The use of Ni-NTA affinity chromatography to separate the 6× His-tagged proteins is a simple and efficient method for protein purification. Moreover, the 6× His tag does not affect the secretion, folding, or function of the recombinant protein [52]. In this study, rPRW4 with a purity of 90% was obtained. This purity value was similar to the results of a large number of studies using this purification method [53, 54]. Approximately 56.1% rPRW4 was successfully released from the 6× His-Trx-PRW4 after enterokinase cleavage. This result was ideal compared to other studies using similar purification strategies. For example, Xu and co-workers used E. coli expression system to express the fusion protein Trx-6× His-Mdmcec. The soluble fusion protein (48.0 mg/L) was obtained. The fusion protein was treated with enterokinase and recombinant Mdmcec (11.2 mg/L) was recovered and purified. In their study, approximately 55% recombinant Mdmcec was successfully released after enterokinase cleavage [55].
Antimicrobial peptide activity is generally evaluated with the MICs. The rPRW4 in this experiment had antimicrobial activity against E. coli ATCC 25922, E. coli UB 1005, S. typhimurium C7731, S. pullorum 7913, S. typhimurium ATCC 14028, S. aureus ATCC 29213, S. epidermidis ATCC 12228, and S. faecalis ATCC 29212, which was consistent with the results of chemically synthetic PRW4 in vitro [18]. This was likely due to the ability of the P. pastoris expression system to express the foreign protein and carry out protein folding and processing [56], thus ensuring the integrity of the original structure of the peptides and maintaining the original biological activity of the peptides. Although the antimicrobial activity of rPRW4 has been verified, other biological activities such as the antimicrobial mechanism of rPRW4 still require further study.
Conclusions
In this study, the PRW4 gene was designed and synthesized, and the fusion gene was inserted into the expression vector pPICZαA after restriction enzyme digestion. The expression vector was successfully constructed and transformed into P. pastoris by electroporation. Recombinant expression plasmids were successfully achieved, and the expression conditions were optimized. The amount of accumulated recombinant protein reached the highest level with the 1% methanol fermentation for 96 h at pH 6.0. The maximum expression was approximately 215.5 mg/L. After purification and digestion with enterokinase protease, 15 mg of rPRW4 with a purity of 90% was obtained from a 1 L fermentation culture. In this study, rPRW4 had antimicrobial activity against E. coli ATCC 25922, E. coli UB 1005, S. typhimurium C7731, S. pullorum 7913, S. typhimurium ATCC 14028, S. aureus ATCC 29213, S. epidermidis ATCC 12228, and S. faecalis ATCC 29212. The results showed that the P. pastoris expression system was a suitable expression system for extracellular expression of biologically active recombinant proteins compared with the E. coli expression system.
Electronic supplementary material
(PNG 2384 kb)
Funding information
This project was supported by the Natural Science Foundation of China (31672434, 31872368, 31472104), the Natural Science Foundation of Heilongjiang Province (TD2019C001), and the China Agriculture Research System [CARS-35].
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Na Zhan and Tianyu Wang contributed to the work equally and should be regarded as co-first authors.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Wanmakok M, Orrapin S, Santhasiri I, et al. Expression in Escherichia coli of novel recombinant hybrid antimicrobial peptide AL32-P113 with enhanced antimicrobial activity in vitro. Gene. 2018;671:1–9. doi: 10.1016/j.gene.2018.05.106. [DOI] [PubMed] [Google Scholar]
- 2.Wang CH, Feng SL, Qie JK. Polyion complexes of a cationic antimicrobial peptide as a potential systemically administered antibiotic. Int J Pharm. 2019;554:284–291. doi: 10.1016/j.ijpharm.2018.11.029. [DOI] [PubMed] [Google Scholar]
- 3.Lin Q, Deslouches B, Montelaro RC, di YP. Prevention of ESKAPE pathogen biofilm formation by antimicrobial peptides WLBU2 and LL37. Int J Animicrob Ag. 2018;52(5):667–672. doi: 10.1016/j.ijantimicag.2018.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pedron CN, de Oliveira CS, da Silva AF, Andrade GP, da Silva Pinhal MA, Cerchiaro G, da Silva Junior PI, da Silva FD, Torres MDT, Oliveira VX. The effect of lysine substitutions in the biological activities of the scorpion venom peptide VmCT1. Eur J Pharm Sci. 2019;136(1):104952. doi: 10.1016/j.ejps.2019.06.006. [DOI] [PubMed] [Google Scholar]
- 5.Scott RW, Tew GN. Mimics of host defense proteins; strategies for translation to therapeutic applications. Curr Top Med Chem. 2017;17(5):576–589. doi: 10.2174/1568026616666160713130452. [DOI] [PubMed] [Google Scholar]
- 6.Wang JJ, Dou XJ, Song J, Lyu Y, Zhu X, Xu L, Li W, Shan A. Antimicrobial peptides: promising alternatives in the post feeding antibiotic era. Med Res Rev. 2019;39(3):831–859. doi: 10.1002/med.21542. [DOI] [PubMed] [Google Scholar]
- 7.Wang J, Yu H, Tian SY, Yang H, Wang J, Zhu W. Recombinant expression insulin-like growth factor 1 in Bacillus subtilis using a low-cost heat-purification technology. Process Biochem. 2017;63:49–54. doi: 10.1016/j.procbio.2017.08.015. [DOI] [Google Scholar]
- 8.Gao H, Shi MH, Wang RH, Wang C, Shao C, Gu Y, Yu W. A widely compatible expression system for the production of highly O-GlcNAcylated recombinant protein in Escherichia coli. Glycobiology. 2018;28(12):949–957. doi: 10.1093/glycob/cwy077. [DOI] [PubMed] [Google Scholar]
- 9.Zhang LC, Wei DD, Zhan N, Sun T, Shan B, Shan A (2020) Heterologous expression of the novel α-helical hybrid peptide PR-FO in Bacillus subtilis. Bioprocess Biosyst Eng. 10.1007/s00449-020-02353-1 [DOI] [PubMed]
- 10.Zhang LC, Li GQ, Zhan N, Sun T, Cheng B, Li Y, Shan A. Expression of a Pseudomonas aeruginosa-targeted antimicrobial peptide T9W in Bacillus subtilis using a maltose-inducible vector. Process Biochem. 2019;81:22–27. doi: 10.1016/j.procbio.2019.03.008. [DOI] [Google Scholar]
- 11.Kuddus MR, Rumi F, Tsutsumi M, Takahashi R, Yamano M, Kamiya M, Kikukawa T, Demura M, Aizawa T. Expression, purification and characterization of the recombinant cysteine-rich antimicrobial peptide snakin-1 in Pichia pastoris. Protein Expr Purif. 2016;122:15–22. doi: 10.1016/j.pep.2016.02.002. [DOI] [PubMed] [Google Scholar]
- 12.Luiz DP, Almeida JF, Goulart LR, Nicolau-Junior N, Ueira-Vieira C. Heterologous expression of abaecin peptide from Apis mellifera in Pichia pastoris. Microb Cell Factories. 2017;16(76):1–7. doi: 10.1186/s12934-017-0689-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meng DM, Dai HX, Gao XF, Zhao JF, Guo YJ, Ling X, Dong B, Zhang ZQ, Fan ZC. Expression, purification and initial characterization of a novel recombinant antimicrobial peptide Mytichitin-A in Pichia pastoris. Protein Expr Purif. 2016;127:35–43. doi: 10.1016/j.pep.2016.07.001. [DOI] [PubMed] [Google Scholar]
- 14.Sang M, Wei H, Zhang JX, Wei Z, Wu X, Chen Y, Zhuge Q. Expression and characterization of the antimicrobial peptide ABP-dHC-cecropin A in the methylotrophic yeast Pichia pastoris. Protein Expr Purif. 2017;140:44–51. doi: 10.1016/j.pep.2017.08.001. [DOI] [PubMed] [Google Scholar]
- 15.Teng D, Xi D, Zhang J, Wang X, Mao R, Zhang Y, Wang J. Multiple copies of the target gene enhances plectasin secretion in Pichia pastoris X-33. Process Biochem. 2015;50(4):553–560. doi: 10.1016/j.procbio.2015.01.010. [DOI] [Google Scholar]
- 16.Wang L, Zhang H, Jia ZG, Ma Q, Dong N, Shan A. In vitro and in vivo activity of the dimer of PMAP-36 expressed in Pichia pastoris. J Mol Microbiol Biotechnol. 2014;24(4):234–240. doi: 10.1159/000365572. [DOI] [PubMed] [Google Scholar]
- 17.Xing LW, Tian SX, Gao W, Yang N, Qu P, Liu D, Jiao J, Wang J, Feng XJ. Recombinant expression and biological characterization of the antimicrobial peptide fowlicidin-2 in Pichia pastoris. Exp Ther Med. 2016;12(4):2324–2330. doi: 10.3892/etm.2016.3578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhu X, Dong N, Wang ZY, Ma Z, Zhang L, Ma Q, Shan A. Design of imperfectly amphipathic alpha-helical antimicrobial peptides with enhanced cell selectivity. Acta Biomater. 2014;10(1):244–257. doi: 10.1016/j.actbio.2013.08.043. [DOI] [PubMed] [Google Scholar]
- 19.Dong N, Zhu X, Chou SL, Shan A, Li W, Jiang J. Antimicrobial potency and selectivity of simplified symmetric-end peptides. Biomaterials. 2014;35(27):8028–8039. doi: 10.1016/j.biomaterials.2014.06.005. [DOI] [PubMed] [Google Scholar]
- 20.Liu RZ, Zhao B, Zhang YL, Gu J, Yu M, Song H, Yu M, Mo W. High-level expression, purification, and enzymatic characterization of truncated human plasminogen (Lys531-Asn791) in the methylotrophic yeast Pichia pastoris. BMC Biotechnol. 2015;15(50):1–8. doi: 10.1186/s12896-015-0179-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.See PT, Iagallo EM, Oliver RP, Moffat CS (2019) Heterologous expression of the Pyrenophora tritici-repentis effector proteins ToxA and ToxB, and the prevalence of effector sensitivity in Australian cereal crops. Front Microbiol 10. 10.3389/fmicb.2019.00182 [DOI] [PMC free article] [PubMed]
- 22.Dong N, Li XR, Xue CY, Zhang L, Wang C, Xu X, Shan A. Astragalus polysaccharides alleviates LPS-induced inflammation via the NF-kB/MAPK signaling pathway. J Cell Physiol. 2020;235(7–8):5525–5540. doi: 10.1002/jcp.29452. [DOI] [PubMed] [Google Scholar]
- 23.Fan YH, Zhang YJ, Yang XY, Pei X, Guo S, Pei Y. Expression of a Beauveria bassiana chitinase (Bbchit1) in Escherichia coli and Pichia pastoris. Protein Expr Purif. 2007;56(1):93–99. doi: 10.1016/j.pep.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 24.Cregg JM, Cereghino JL, Shi JY, Higgins DR. Recombinant protein expression in Pichia pastoris. Mol Biotechnol. 2000;16(1):23–52. doi: 10.1385/mb:16:1:23. [DOI] [PubMed] [Google Scholar]
- 25.Yang ZL, Zhang ZS. Engineering strategies for enhanced production of protein and bio-products in Pichia pastoris: a review. Biotechnol Adv. 2018;36(1):182–195. doi: 10.1016/j.biotechadv.2017.11.002. [DOI] [PubMed] [Google Scholar]
- 26.Werten MWT, Eggink G, Cohen MAC, et al. Production of protein-based polymers in Pichia pastoris. Biotechnol Adv. 2019;37(5):642–666. doi: 10.1016/j.biotechadv.2019.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Perumal PS, Wilson S, Santhiagu A. Optimization of codon usage of the envelope protein E2gene from various genotypes of hepatitis C virus to predict the expression level in Pichia pastoris. Genes Genome. 2016;38(10):977–984. doi: 10.1007/s13258-016-0442-2. [DOI] [Google Scholar]
- 28.Ahmad M, Hirz M, Pichler H, Schwab H. Protein expression in Pichia pastoris: recent achievements and perspectives for heterologous protein production. Appl Microbiol Biotechnol. 2014;98(12):5301–5317. doi: 10.1007/s00253-014-5732-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bell MR, Engleka MJ, Malik A, Strickler JE. To fuse or not to fuse: what is your purpose? Protein Sci. 2013;22(11):1466–1477. doi: 10.1002/pro.2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Niu MF, Chai SM, You XY, et al. Expression of porcine protegrin-1 in Pichia pastoris and its anticancer activity in vitro. Exp Ther Med. 2015;9(3):1075–1079. doi: 10.3892/etm.2015.2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Do BH, Park SS, Kwon GG, Nguyen MT, Kang HJ, Song JA, Yoo J, Nguyen AN, Jang J, Jang M, Lee S, So S, Sim S, Jin J, Lee KJ, Osborn MJ, Choe H. Soluble expression and purification of bioactive interleukin 33 in E-coli. Biotechnol Bioproc Eng. 2017;22(3):256–264. doi: 10.1007/s12257-017-0060-0. [DOI] [Google Scholar]
- 32.Spagnoli G, Bolchi A, Cavazzini D, Pouyanfard S, Müller M, Ottonello S. Secretory production of designed multipeptides displayed on a thermostable bacterial thioredoxin scaffold in Pichia pastoris. Protein Expr Purif. 2017;129:150–157. doi: 10.1016/j.pep.2016.04.012. [DOI] [PubMed] [Google Scholar]
- 33.Zhang LC, Li XD, Wei DD, Wang J, Shan A, Li Z. Expression of plectasin in Bacillus subtilis using SUMO technology by a maltose-inducible vector. J Ind Microbiol Biotechnol. 2015;42(10):1369–1376. doi: 10.1007/s10295-015-1673-y. [DOI] [PubMed] [Google Scholar]
- 34.Jing XL, Luo XG, Tian WJ, Lv LH, Jiang Y, Wang N, Zhang TC. High-level expression of the antimicrobial peptide plectasin in Escherichia coli. Curr Microbiol. 2010;61(3):197–202. doi: 10.1007/s00284-010-9596-3. [DOI] [PubMed] [Google Scholar]
- 35.Zheng XL, Wang W. High-level expression of housefly cecropin A in Escherichia coli using a fusion protein. Asian Pac J Trop Med. 2010;3(6):421–426. doi: 10.1016/S1995-7645(10)60102-2. [DOI] [Google Scholar]
- 36.Krahulec J, Hyrsova M, Pepeliaev S, et al. High level expression and purification of antimicrobial human cathelicidin LL-37 in Escherichia coli. Appl Microbiol Biotechnol. 2010;88(1):167–175. doi: 10.1007/s00253-010-2736-7. [DOI] [PubMed] [Google Scholar]
- 37.Wang AP, Su YP, Wang S, et al. High efficiency preparation of bioactive human alpha-defensin 6 in Escherichia coli Origami (DE3) pLysS by soluble fusion expression. Appl Microbiol Biotechnol. 2010;87(5):1935–1942. doi: 10.1007/s00253-010-2688-y. [DOI] [PubMed] [Google Scholar]
- 38.Hlavac F, Rouer E. Expression of the protein–tyrosine kinase p56lckby the pTRX vector yields a highly soluble protein recovered by mild sonication. Protein Expr Purif. 1997;11(3):227–232. doi: 10.1006/prep.1997.0791. [DOI] [PubMed] [Google Scholar]
- 39.Li Y. Carrier proteins for fusion expression of antimicrobial peptides in Escherichia coli. Biotechnol Appl Biochem. 2009;54(1):1–9. doi: 10.1042/ba20090087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee JH, Minn I, Park CB, Kim SC. Acidic peptide-mediated expression of the antimicrobial peptide buforin II as tandem repeats in Escherichia coli. Protein Expr Purif. 1998;12(1):53–60. doi: 10.1006/prep.1997.081420090087. [DOI] [PubMed] [Google Scholar]
- 41.Lee JH, Lee SG, Do H, et al. Optimization of the pilot-scale production of an ice-binding protein by fed-batch culture of Pichia pastoris. Appl Microbiol Biotechnol. 2013;97(8):3383–3393. doi: 10.1007/s00253-012-4594-y. [DOI] [PubMed] [Google Scholar]
- 42.Li HB, Zhang TQ, Li J, Li H, Xu Y, Yu J. Expression of Zea mays transglutaminase in Pichia pastoris under different promoters and its impact on properties of acidified milk protein concentration (MPC) gel. J Sci Food Agric. 2019;99(10):4518–4523. doi: 10.1002/jsfa.9688. [DOI] [PubMed] [Google Scholar]
- 43.Luo G, Tian JH, Huang HQ, Lei An Improving heterologous expression of porcine follicle-stimulating hormone in Pichia pastoris by integrating molecular strategies and culture condition optimization. Appl Microbiol Biotechnol. 2018;102(20):8867–8882. doi: 10.1007/s00253-018-9260-6. [DOI] [PubMed] [Google Scholar]
- 44.Gasser B, Prielhofer R, Marx H, Maurer M, Nocon J, Steiger M, Puxbaum V, Sauer M, Mattanovich D. Pichia pastoris: protein production host and model organism for biomedical research. Future Microbiol. 2013;8(2):191–208. doi: 10.2217/fmb.12.133. [DOI] [PubMed] [Google Scholar]
- 45.Wei Y-C, Braun-Galleani S, Henriquez MJ, et al. Biotransformation of β-hydroxypyruvate and glycolaldehyde to l-erythrulose by Pichia pastoris strain GS115 overexpressing native transketolase. Biotechnol Prog. 2018;34(1):99–106. doi: 10.1002/btpr.2577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Amorim FG, Boldrini-Franca J, Figueiredo Bordon K, et al. Heterologous expression of rTsHyal-1: the first recombinant hyaluronidase of scorpion venom produced in Pichia pastoris system. Appl Microbiol Biotechnol. 2018;102(1):1–14. doi: 10.1007/s00253-018-8821-z. [DOI] [PubMed] [Google Scholar]
- 47.Liu WH, Zhao WB, Lai J, Shen Q, Xu Y, Pan L, Chen S. RSM optimization of HSA/IL1Ra in Pichia pastoris overexpression strain and study of its in vivo activity in reducing hyperglycemia of GK rats. Biotechnol Appl Biochem. 2016;64(5):627–637. doi: 10.1002/bab.1532. [DOI] [PubMed] [Google Scholar]
- 48.Tab MM, Hashim NHF, Najimudin N, Mahadi NM, Bakar FDA, Murad AMA. Large-scale production of glaciozyma antarctica Antifreeze Protein 1 (afp1) by fed-batch fermentation of Pichia pastoris. Arab J Sci Eng. 2017;43(1):133–141. doi: 10.1007/s13369-017-2738-1. [DOI] [Google Scholar]
- 49.Macauley-Patrick S, Fazenda ML, McNeil B, Harvey LM. Heterologous protein production using the Pichia pastoris expression system. Yeast. 2005;22(4):249–270. doi: 10.1002/yea.1208. [DOI] [PubMed] [Google Scholar]
- 50.Peng LS, Zhong XF, Ou JX, Zheng S, Liao J, Wang L, Xu A. High-level secretory production of recombinant bovine enterokinase light chain by Pichia pastoris. J Biotechnol. 2004;108(2):185–192. doi: 10.1016/j.jbiotec.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 51.Potvin G, Ahmad A, Zhang ZS. Bioprocess engineering aspects of heterologous protein production in Pichia pastoris: a review. Biochem Eng J. 2012;64:91–105. doi: 10.1016/j.bej.2010.07.017. [DOI] [Google Scholar]
- 52.Liu YC, Wang ZK, Yin YP, Cao Y, Zhao H, Xia Y. Expression, purification, and characterization of recombinant Metarhizium anisopliae acid trehalase in Pichia pastoris. Protein Expr Purif. 2007;54(1):66–72. doi: 10.1016/j.pep.2007.02.016. [DOI] [PubMed] [Google Scholar]
- 53.Wu Q, Li B, Wu F, Yang L, Li S, Li H, Wu D, Cui T, Tang D. High level expression, efficient purification, and bioactivity of recombinant human metallothionein 3 (rhMT3) from methylotrophic yeast Pichia pastoris. Protein Expr Purif. 2014;101:121–126. doi: 10.1016/j.pep.2014.06.009. [DOI] [PubMed] [Google Scholar]
- 54.Anikeeva N, Lebedeva T, Sumaroka M, Kalams SA, Sykulev Y. Soluble HIV-specific T cell receptor: expression, purification and analysis of the specificity. J Immunol Methods. 2003;277(1–2):75–86. doi: 10.1016/s0022-1759(03)00179-0. [DOI] [PubMed] [Google Scholar]
- 55.Xu XX, Jin FL, Yu XQ, Ji S, Wang J, Cheng H, Wang C, Zhang W. Expression and purification of a recombinant antibacterial peptide, cecropin, from Escherichia coli. Protein Expr Purif. 2007;53(2):293–301. doi: 10.1016/j.pep.2006.12.020. [DOI] [PubMed] [Google Scholar]
- 56.Tai HM, Li CC, Hung CY, Yin LJ. Production of functional peptides with inhibition ability against angiotensin I-converting enzyme using Pichia pastoris expression system. J Food Drug Anal. 2018;26(3):1097–1104. doi: 10.1016/j.jfda.2018.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PNG 2384 kb)