Abstract
A challenge of chronic diseases that remains to be solved is how to liberate patients and medical resources from the burdens of long-term monitoring and periodic visits. Precise management based on artificial intelligence (AI) holds great promise; however, a clinical application that fully integrates prediction and telehealth computing has not been achieved, and further efforts are required to validate its real-world benefits. Taking congenital cataract as a representative, we used Bayesian and deep-learning algorithms to create CC-Guardian, an AI agent that incorporates individualized prediction and scheduling, and intelligent telehealth follow-up computing. Our agent exhibits high sensitivity and specificity in both internal and multi-resource validation. We integrate our agent with a web-based smartphone app and prototype a prediction-telehealth cloud platform to support our intelligent follow-up system. We then conduct a retrospective self-controlled test validating that our system not only accurately detects and addresses complications at earlier stages, but also reduces the socioeconomic burdens compared to conventional methods. This study represents a pioneering step in applying AI to achieve real medical benefits and demonstrates a novel strategy for the effective management of chronic diseases.
Subject terms: Lens diseases, Translational research, Computer science, Health care economics
Introduction
A medical revolution driven by artificial intelligence (AI) is expected in the near future1–3. Individualized prediction using machine learning promises to be transformative and indispensable for complex medical situations4, including nephrology5, cardiology6, and ophthalmology7. Moreover, intelligent telehealth computing has shown great potential in enabling cost-effective applications of medical AI8. Although the promise of these technologies is broad, a full integration of prediction and telehealth has not been achieved, and the actual benefits of AI regarding healthcare quality and socioeconomic burden remain to be validated.
Chronic diseases and conditions are the leading causes of death and disability worldwide9. Follow-up management of chronic-disease patients remains one of the most intractable healthcare problems, and solutions are urgently needed10. Presently, conventional follow-up plans scheduled by clinicians are one-size-fits-all and are primarily based on personal experience or limited clinical evidence, resulting in the delayed detection of complications11. Furthermore, among developing countries, only a few specialized care centers are capable of effective examinations and accurate interventions12,13. The sparse distribution of these specialized centers creates significant difficulty and economic pressure for patients, resulting in low follow-up rates14. Therefore, a new strategy for precise and effective follow-up management leveraging AI is highly desirable.
The study focuses on the follow-up management of patients with congenital cataract (CC), a typical chronic condition characterized by high long-term risk of two main complications: high intraocular pressure (IOP)15 and visual axis opacification (VAO)16. Rigorous follow-up care and timely intervention are necessary to prevent the irreversible and permanent loss of vision caused by these complications17. Therefore, CC is an ideal test case for exploring follow-up management strategies for chronic conditions.
To explore the feasibility of applying AI to improve the quality of follow-up care, we applied Bayesian and deep-learning algorithms to create CC-Guardian, an intelligent agent that consists of three functional modules: (i) a prediction module that identifies potential high-risk CC patients who are likely to suffer complications, (ii) a dispatching module that schedules individual follow-up based on the prediction results, and (iii) a telehealth module that makes intervention decisions in each follow-up examination. We verified CC-Guardian’s performance using internal and multi-resource validation. Using CC-Guardian, we implemented a web-based smartphone app and proposed an operating mechanism for our intelligent follow-up system. We conducted a retrospective self-controlled test to investigate the real-world efficiency of our system in terms of complication prediction, telehealth detection, and cost-efficient benefits. More broadly, this study takes a pioneering step in translating AI into actual medical benefits and provides a novel approach to the effective management of chronic conditions.
Results
Dataset collection and preparation for agent training
The pipeline for our study is shown in Fig. 1. The training dataset included clinical records of 594 CC patients before and after surgery, and 4881 of their follow-up images from January 2011 to December 2016. The eligible criteria of patients included diagnosis of CC, informed consent, and completed records of baseline information, lesion condition, comorbidities, surgical procedures, and complications in the first follow-up year. The eligible images for training were defined as the anterior segment images covering the posterior lens capsule by retro-illumination using slit-lamp photography. All these records were derived from routine examinations at the Childhood Cataract Program of the Chinese Ministry of Health (CCPMOH)18.
Fig. 1. Study pipeline for agent training, validation, application, and testing.
a Multidimensional clinical records of 594 congenital cataract patients were collected for prediction module training (279 VAO: 315 non-VAO; 341 high IOP: 253 normal). For telehealth module training, a total of 4881 postoperative retro-illumination images were obtained (2615 follow-up: 2266 intervention). Each image was independently described and labeled by an expert panel. b Two datasets were used for the validation of the trained agent, including internal validation dataset (clinical records of 142 patients, and 1220 follow-up images) and multi-resource dataset (clinical records of 79 patients, 214 follow-up images). c A web-based smartphone app was implemented, and a prediction-telehealth cloud platform was prototyped for the clinical application of our intelligent follow-up system. d A retrospective self-controlled test was conducted to investigate the real-world efficiency of our follow-up system in complication prediction, telehealth detection, and cost-effect benefit. VAO visual axis opacification, IOP intraocular pressure.
Functional architecture of CC-Guardian
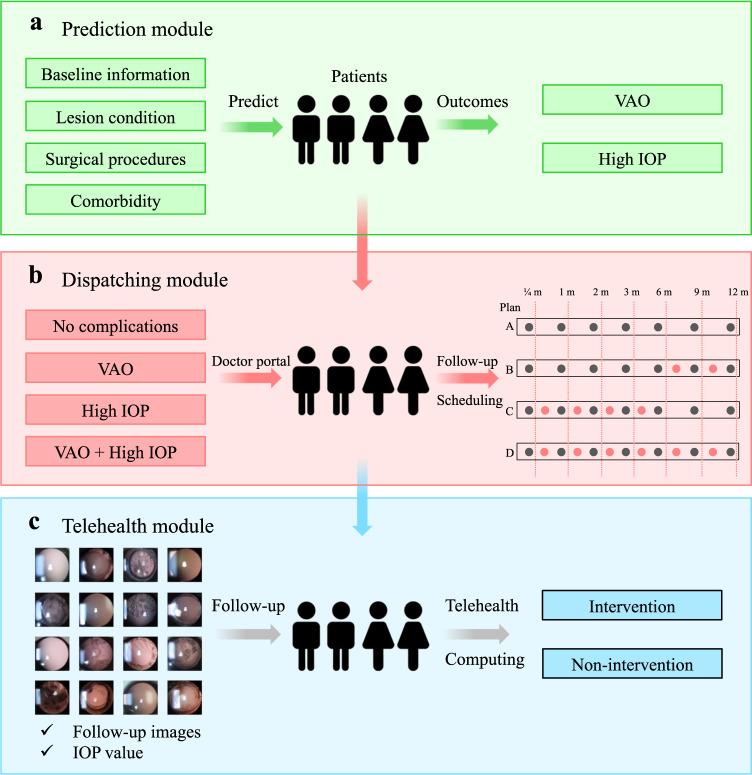
The CC-Guardian incorporates three functional modules: a prediction module designed to identify high-risk patients who are likely to suffer complications (Fig. 2a), a dispatching module responsible for scheduling individual follow-up visits (depending on the results of the prediction module) (Fig. 2b), and a telehealth module that makes a clinical decision regarding additional treatment (intervention or continued follow-up) in each follow-up examination using telehealth computing (Fig. 2c).
Fig. 2. Functional architecture of CC-Guardian.
a The prediction module is trained to identify high-risk patients likely to suffer complications (the occurrence of VAO, happen or not and the occurrence of high IOP, happen or not). b The dispatching module is responsible for scheduling individual follow-up based on the prediction module results. c In the telehealth module, a clinical decision regarding further treatment (intervention or continued follow-up) is made after each telehealth examination based on follow-up images and IOP values. VAO visual axis opacification, IOP intraocular pressure, m months.
Naive Bayes algorithm for prediction module
We selected 12 variables that both can be easily collected in routine clinical practice and were reported to be associated with complications after surgery16,17 as the inputs for the prediction module. These 12 variables can be generally grouped into four categories: (1) baseline information, (2) lesion condition, (3) comorbidities, and (4) surgical procedures (“Methods”).
To find the optimal algorithm, we first performed a comparison among naive Bayes and two classic machine learning algorithms (random forest and neural network, “Methods”). Based on the average performance of the 5-fold cross-validation by the training set of 594 CC patients, the naive Bayes algorithm provided comparative performance with random forest and neural network algorithms (Table 1). We finally selected the naive Bayes algorithm due to its intrinsic simplicity19, which can improve the processing speed and saving computing resource in real-world application. After selecting the naive Bayes, a complete algorithm was then trained on the entire dataset of 594 CC patients prior to later validations.
Table 1.
Performance of methodological comparison among algorithms.
| Outcome | Accuracy | Sensitivity | Specificity | |
|---|---|---|---|---|
| Naïve Bayes | VAO | 0.966 (0.916–0.991) | 0.964 (0.875–0.996) | 0.969 (0.892–0.996) |
| High IOP | 0.975 (0.928–0.995) | 0.972 (0.902–0.997) | 0.979 (0.889–0.999) | |
| Random forest | VAO | 0.950 (0.894–0.981) | 0.946 (0.849–0.989) | 0.953 (0.869–0.990) |
| High IOP | 0.941 (0.883–0.976) | 0.944 (0.862–0.984) | 0.938 (0.828–0.987) | |
| Neural network | VAO | 0.950 (0.894–0.981) | 0.909 (0.801–0.970) | 0.984 (0.916–0.999) |
| High IOP | 0.933 (0.872–0.971) | 0.986 (0.924–0.999) | 0.854 (0.722–0.939) |
VAO visual axis opacification, IOP intraocular pressure, accuracy (TP + TN)/(TP + TN + FP + FN), sensitivity TP/(TP + FN), specificity TN/(TN + FP), TP true positive, TN true negative, FP false positive, FN false negative, CI confidence interval.
A methodological comparison among naive Bayes, random forest, and neural network was performed using the average performance of the 5-fold cross-validation by the training set of 594 patients.
The normalized contributions of all 12 input variables to predict VAO (from 0.01 to 0.2, Supplementary Fig. 1a) and high IOP (from 0.03 to 0.15, Supplementary Fig. 1b) were presented, suggesting their substantial importance to outcome. Both the age at surgery (0.07 to VAO and 0.12 to high IOP) and gender (0.03 to VAO and 0.02 to high IOP) do not show any dominant role, indicating that predictive results will not significantly vary from these two variables.
Follow-up schedules for dispatching module
For patients predicted with no complications, the dispatching module will schedule seven time points (1 week, 1 month, 2 months, 3 months, 6 months, 9 months, and 12 months after surgery), which is the conventional plan in current clinical practice14. If the patients were predicted to have VAO, the module will add two time points (7.5 months and 10.5 months) to the conventional plan, since VAO was inclined to happen in the second half year17,20. If the patients were predicted to have high IOP, the module will add three time points (2.5 weeks, 2.5 months and 4.5 months) to the conventional plan, since high IOP was inclined to happen in the second half year16,20.
Deep residual network for telehealth module
A total of 4881 postoperative retro-illumination images were included as the training set for telehealth module. These 4881 images were collected from 2175 CC patients. Images from the same child were taken at different follow-up visits. Each image was independently labeled (intervention or continued follow-up) by two licensed ophthalmologists, and a third ophthalmologist was consulted when disagreements occurred. Then, a senior ophthalmologist with over 20 years of clinical CC experience verified the labels for each image. The ophthalmologists were blind and had no access to the outcome classifications. A stacked 101-layer residual network21 was utilized for telehealth module training and classification (“Methods”). The first 100 layers were used to extract multidimensional, high-level features from the input images and a Softmax classifier was applied to the last layer. The architecture of the deep residual network is provided in Supplementary Fig. 2 in the form of a diagram that highlights the arrangement of each layer.
Internal validation
A randomly selected dataset derived from the original CCPMOH records prior to training was used to validate CC-Guardian, which included clinical records of 142 patients (61 VAO, 81 non-VAO; 79 high-IOP, 63 normal) and 1220 follow-up images (671 follow-up, 549 intervention). The 1,220 images were collected from 507 CC patients. This dataset did not overlap with the CCPMOH data for agent training. The trained deep-learning model was frozen prior to any validations. The deep-learning predictions with time stamps were verified and saved by an individual who was blind to the expert-panel labels, thus precluding information leaking or double-dipping when comparing the prediction results with the expert-panel labels. After internal validation, the models trained on all of the training sets were used for following validation.
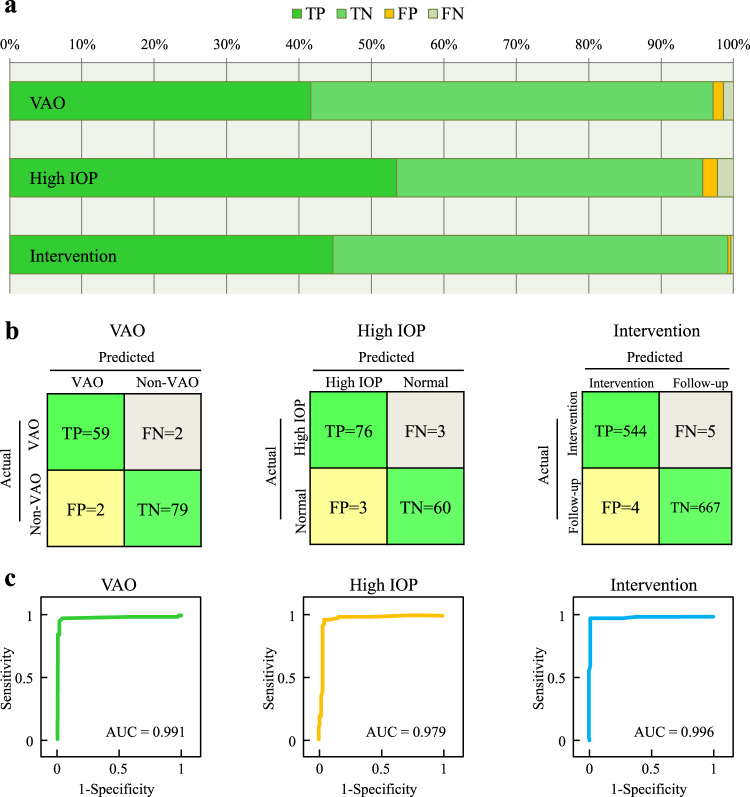
Using the prediction module, our agent predicted VAO with sensitivity of 0.967, specificity of 0.975, and area under curve (AUC) of 0.991; and high IOP with sensitivity of 0.962, specificity of 0.952, and AUC of 0.979. By virtue of its telehealth module, CC-Guardian provides intervention decisions with sensitivity of 0.991, specificity of 0.994, and AUC of 0.996. The detailed distributions for the accurate and mistaken detections are presented in Fig. 3a. Confusion matrices and receiver operating characteristic (ROC) curves are shown in Fig. 3b, c, respectively.
Fig. 3. Highly accurate performance of CC-Guardian in internal validation.
a Using the prediction module, our agent predicted VAO with 96.7% sensitivity and 97.5% specificity, and high IOP with 96.2% sensitivity and 95.2% specificity in internal validation. Using the telehealth module, our agent provided intervention suggestions with 99.1% sensitivity and 99.4% specificity. b Confusion matrices for agent validation. c Our agent had AUCs of 0.991, 0.979, and 0.996 for detecting VAO, high IOP, and intervention, respectively, in internal validation. AUC area under curve, TP true positive, TN true negative, FP false positive, FN false negative.
Multi-resource validation
The multi-resource dataset had two parts. The first part consisted of clinical records of 79 patients (33 VAO, 46 non-VAO; 28 high-IOP, 51 normal) collected from 7 centers, which were used for validating prediction module. Seven centers included Shenzhen Eye Hospital, Beijing Tongren Hospital, People’s Hospital of Guangxi Zhuang Autonomous Region, the Third Affiliated Hospital of Sun Yat-sen University, Puning People’s Hospital, The Central Hospital of Wuhan, and the First Affiliated Hospital of University of South China. These 7 centers were distributed separately in northern, central, and southern China, and thus collectively composed a representative and independent sample for Chinese population. The eligible criteria were the same as the previous datasets, which were diagnosis of CC, informed consent, and completed records of inputs and outcomes required for prediction module. Detailed description of the datasets collected in each center was summarized in Supplementary Table 1.
The second part contained images collected from heterogeneous open-access databases, which can further validate versatility and generalization of telehealth module. We performed image searches using the Google, Baidu and Bing search engines through June 2016, with a combination of key words (e.g., congenital, infantile, pediatric cataract, follow-up, and retro-illumination) in the form of title words or medical subject headings. Two individuals (E.L. and H.L.) independently completed the searches. In addition, these two individuals cross-checked and confirmed that all the collected cases were retro-illumination images of CC during follow-up management. When discrepancies arose, consensus was achieved after further discussion. All confirmed images (91 follow-up and 123 intervention) were subsequently sent to CC-Guardian for validation.
The CC-Guardian’s prediction module predicted VAO with sensitivity of 0.940, specificity of 0.935, and area under curve (AUC) of 0.944; and high IOP with sensitivity of 0.964, specificity of 0.941, and AUC of 0.961. The CC-Guardian’s telehealth module provided intervention suggestions with sensitivity of 0.959, specificity of 0.945, and AUC of 0.981, a performance comparable to that obtained from internal validation. The detailed distributions for the accurate and mistaken detections are presented in Fig. 4a. Confusion matrices and ROC curves are shown in Fig. 4b, c, respectively.
Fig. 4. Comparative performance of CC-Guardian in multi-resource validation.
a Using the prediction module, our agent predicted VAO with 94.0% sensitivity and 93.5% specificity, and high IOP with 96.4% sensitivity and 94.1% specificity for the multi-resource dataset. Using the telehealth module, our agent provided intervention suggestions with 95.9% sensitivity and 94.5% specificity for the multi-resource dataset. b Confusion matrices for agent validation. c Our agent had AUCs of 0.944, 0.961, and 0.981 for detecting VAO, high IOP, and intervention, respectively, for the multi-resource dataset. AUC area under curve, TP true positive, TN true negative, FP false positive, FN false negative.
CC-Guardian web-based smartphone app
To establish a telehealth cloud platform for clinical application, we built a CC-Guardian web-based smartphone app. The functions of the website (available at https://www.cc-cruiser.com/cc_guardian) include prediction-metric input, risk-stratification output, follow-up-examination results upload, and intervention decisions reporting. The functions of the smartphone app (available for iOS and Android systems) include a doctor portal (individual follow-up scheduling and updating) and a patient portal (automatic short message service (SMS) and interaction between patients and CCPMOH).
Users login to the website and input the necessary prediction metrics to obtain the risk-stratification output (occurrence of VAO and/or high IOP) (Supplementary Fig. 3a). Doctors can assign an individual follow-up plan using the smartphone app (doctor portal) (Supplementary Fig. 3b). Patients will receive individual follow-up schedules and can check these schedules using the smartphone app (patient portal) (Supplementary Fig. 3c). Interaction between patients and CCPMOH is enabled through either the website or the app. Doctors can also reschedule individual plans if necessary. During the follow-up process, users can upload their follow-up examination results to the website and obtain an intervention decision in a timely manner (Supplementary Fig. 3d). The algorithm embedded in this app is the same as the one in following validations.
CC-Guardian prediction-telehealth cloud platform
A compatible mechanism to promote the real-world clinical application of CC-Guardian to the follow-up management is highly desirable. Therefore, we created a prediction-telehealth cloud platform for the CC-Guardian smartphone app. As shown in Fig. 5, when a potential patient registers at the specialized care center (CCPMOH), their clinical metrics (valuable for the prediction module) are collected with their permission and immediately sent to the CC-Guardian website for complication prediction. Based on the prediction results, the dispatching module individually determines a follow-up schedule and sends an SMS to remind the patient in a timely manner. Patients can complete their regular follow-up examinations in primary care hospitals (telehealth follow-up examination) and upload their examination results to the web-based telehealth module. If the telehealth module recommends intervention, a fast-track notification system is triggered, and an emergency notification is sent to doctors at CCPMOH for immediate confirmation. Such patients will be informed that they should undergo intervention to manage their complications. Instructions for using the prediction-telehealth cloud platform are provided in Supplementary Video 1.
Fig. 5. The prediction-telehealth cloud platform.
When potential patients register at the specialized care center (CCPMOH), their clinical metrics (valuable for the prediction module) are collected with their permission and immediately uploaded to the CC-Guardian cloud platform for complication prediction. Based on the prediction results, the dispatching module designs an individualized follow-up schedule and sends a short message to notify each corresponding patient in a timely manner. Patients can complete their regular follow-up in primary care hospitals and upload their examination results to the web-based telehealth module. If the telehealth module recommends intervention, the fast-track notification system is triggered, and an emergency notification is sent to doctors at the specialized care center (CCPMOH) for immediate confirmation. These patients are informed that they should undergo intervention to manage complications after confirmation.
Retrospective self-controlled test
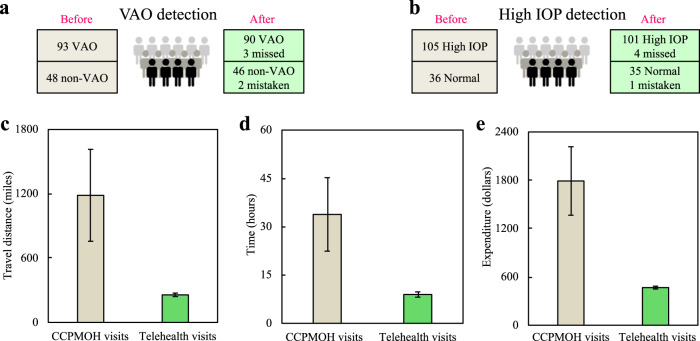
To further investigate the real-world efficiency of our system, we retrospectively used longitudinal follow-up records of 141 CC patients from CCPMOH between January of 2016 and May of 2017 (“Methods”). Before using our system, these 141 CC patients had 987 distant follow-up visits to CCPMOH, including 93 patients who underwent VAO and 105 patients who suffered from high IOP (Fig. 6a, b).
Fig. 6. Real-world efficiency in retrospective self-controlled test.
Error bars presented the standard deviation. a, b The longitudinal follow-up records of 141 CC patients (987 follow-up visits to CCPMOH) were retrospectively used for real-world testing. Before using CC-Guardian, a total of 93 patients underwent VAO and 105 patients suffered from High IOP. After using our system, 90 cases of VAO (90/93, 96.8%) and 101 cases of high IOP (101/105, 96.2%) are successfully predicted. c–e Each family would have saved an average travel distance of 928.6 miles (1185.4 vs. 256.8 miles, P < 0.001), an average time of 24.9 h (33.8 vs. 8.9 h, P < 0.001), and an average expenditure of $1324.1 ($1791.4 vs. $467.3, P < 0.001) per year. VAO visual axis opacification, IOP intraocular pressure.
After applying our system, 90 cases of VAO (90/93, 96.8%) and 101 cases of high IOP (101/105, 96.2%) were successfully predicted (Fig. 6a, b). A total of 73 patients (73/90, 81.1%) would have benefited from earlier VAO detection (2759 risk days in total, 37.8 risk days earlier per person on average, median 45.0 risk days) and 89 patients (89/101, 88.1%) would have benefited from earlier high-IOP detection (1709 risk days in total, 19.2 risk days earlier per person on average, median 15.0 risk days).
All 141 patients would have had a total of 1579 telehealth visits rather than 987 distant visits to the CCPMOH, which would have, on average, saved each family significant travel of 928.6 miles per year (1185.4 vs. 256.8 miles, P < 0.001, Fig. 6c), time of 24.9 h per year (33.8 vs. 8.9 h, P < 0.001, Fig. 6d), and an expenditure of $1324.1 per year, ($1791.4 vs. $467.3, P < 0.001, Fig. 6e). These results demonstrate that our system has the potentials to detect and address the complications at an earlier stage, and to reduce the socioeconomic burden faced by patients compared to conventional in-person follow-up.
Discussion
In this study, we integrated the benefits of individualized prediction and telehealth computing to create an AI agent and introduced a prediction-telehealth cloud platform to support its clinical application to the management of congenital cataract. With high precision as a prerequisite, our system may effectively translate correct predictions and detections into real advancements in treatment timing and significant savings in travel distance, time, and cost.
Earlier diagnosis and timely interventions in response to serious complications can significantly improve healthcare quality22. For CC patients whose visual development is in a sensitive stage, even short duration form-deprivation due to VAO will significantly increase the risk of amblyopia23. There is a greater risk of glaucoma caused by high IOP, which is one of the leading causes of blindness, progressing rapidly and causing irreversible damage to the optic nerve24. Unfortunately, most symptoms of complications (e.g., decreased visual acuity) are insidious and tend to be neglected, which can cause patients to miss the “treatment window” for saving their remaining vision25. Therefore, the earlier diagnosis (37.8 risk days per person for VAO and 19.2 risk days per person for high IOP) provided by our system may reduce the risk of permanent visual impairment.
Telehealth computing significantly reduces the travel distance, time, and cost to our patients during follow-up visits (928.6 miles, 24.9 h, and $1324.1 per year for each family), which can substantially increase their compliance. Such benefits will be even greater when considering reduced labor costs for clinicians and patients. Moreover, experts in specialized centers will be free to transfer to more first-visit patients. Therefore, from this perspective, our telehealth pattern will provide substantial impetus for creating a more rational allocation of medical resources and expand the coverage of high-quality medical care.
It should be noted that our previous study has developed an automatic diagnosis system for CC patients26. Comparing to our previous work, the current study presented significant advances and novelties. First, our previous study can only be applied in preoperative patients for primary screen and diagnosis. However, the current study focused on follow-up management of CC (high cost of long-term follow-up and delayed detection on postoperative complications), a more general and intractable situation that can be happen in patients with chronic diseases, such as metabolic and cardiovascular diseases. Second, our previous work only analyzed 886 images using a 7-layer convolution network. In comparison, the current work innovatively integrated Bayesian and 101-layer deep residual network to take the advantage of both structured clinical records and 4886 postoperative images, which finally achieved fully automatic flow of prediction and telehealth. Third, although both our previous and current works have achieved highly accurate performance in their specific clinical scenarios, the current work further conducted a retrospective self-controlled test to articulate the exact benefits that our system can provide for patients in real-world settings. These evidence address a matter of greatest concern to both clinicians and patients and provide the direct implications and merit to routine clinical practice.
This study has certain limitations. First, the multi-resource dataset for validating prediction module is relatively small and limited to the Chinese population, due to difficulties for assessing clinical records from centers in other countries27 and limited cohorts tracking congenital cataract worldwide28. It is also essential to validate our system using datasets in other ethnic population or other countries in the future to achieve global generalization, as previously achieved in diabetic retinopathy29. Second, it should be emphasized that the application of our system is limited to CC patients. For other chronic conditions like hypertension and diabetes, it is expected that machine learning can be applied to efficiently manage the ketoacidosis or stroke by prediction-telehealth approach in resource-intensive situations. Third, images used to train the telehealth module are acquired through slit-lamp photography. Therefore, assistances (e.g., strap30) would be required for the uncooperative infants during the photography process. Fourth, the duration of self-controlled test is relatively short (one and four months). The records after May 2018 are not available for this test because of the incomplete 1-year follow-up records when we submitted this work. Fourth, the complications’ prevalence in our study is higher than those reported by previous studies31–33. It may be attributed to difference of measurement and definition, or “severe patient effects” (that is, infants with complications have better compliance and thus are more likely to have complete follow-up records). We further estimated that such prevalence difference will not influence the results on advance detection timing, savings of travel distance, time and expenditure (Supplementary Information, Supplementary Table 2). Fifth, it should be noted that we cannot quantify the effects of earlier detection on improved outcomes due to retrospective design. It is necessary to conduct prospective studies to further investigate the agent’s robustness in performance and benefits in real-world setting.
In the future, the performance of CC-Guardian will be enhanced though the collection of larger datasets using our platform. Furthermore, CC-Guardian can be combined with wearable devices (e.g., smartphone-based slit-lamp photography) to implement real-time eye condition monitoring, which will provide a foundation for the development of a home-based telehealth platform.
Methods
Predictive indicators and outcomes for prediction module
The baseline information included gender, age at surgery, and laterality. The lesion condition included area, density, and location. The area was defined as “extensive” when the opacity covered more than 50% of the pupillary area; otherwise, it was “limited”. The density was defined as “dense” when the opacity fully disrupted viewing of the fundus; otherwise, it was “non-dense”. The location was defined as “central” when the opacity fully covered the visual-axis area; otherwise, it was “peripheral”. For comorbidities, nystagmus, microphthalmia, microcornea, and persistent hyperplastic primary vitreous were defined according to the 11th Revision of the International Classification of Diseases (ICD-11) Beta Draft34. For surgical procedures, three standardized cataract extraction methods were classified, including lens aspiration (I/A), I/A with posterior continuous curvilinear capsulorhexis (I/A + PCCC), I/A with PCCC and anterior vitrectomy (A-Vit)20. Intraocular lens (IOL) implantation was classified as primary or secondary IOL implantation.
These 12 inputs aim to predict two high-risk complications after one-year of follow-up after surgery: (1) the occurrence of VAO (happen or not) and (2) the occurrence of high IOP (happen or not). VAO was defined as lens epithelial cells proliferation that extends into the pupillary area and covers the visual axis. High IOP was defined as IOP > 21 mmHg. Supplementary Table 3 summarizes the distribution of all inputs and outcomes.
Methodological comparison for prediction module
To find the optimal algorithm, we first performed a comparison among naive Bayes and two classic machine learning algorithms (random forest and neural network). Briefly, we developed the naive Bayes by constructing a scoring function to perform binary classifications19. Random forest was developed by constructing a multitude of decision trees at training time and outputting the mode of the classes for classification7. Neural network was developed by systematic training of interlinked simple processing elements and artificial neurons for classification35. The methodological comparison was conducted using the toolbox in MatLab 2016 (MathWorks, Inc., Natick, MA, USA). The optimal algorithm was selected based on the average performance of the 5-fold cross-validation by the training set of 594 CC patients. After selection, a complete algorithm was then trained on the entire 594 CC patients prior to later validations.
Deep residual network for telehealth module
A stacked 101-layer residual network21 was utilized for telehealth module training and classification. Specifically, several techniques, including convolution, max pooling, residual blocks, batch-normalizing transform36, non-saturating rectified linear units, and data augmentation were integrated into this algorithm. The residual block was employed to avoid the degradation, further enhance recognition rates, and accelerate convergence. The entire network included thirty-three residual blocks, each of which consists of three convolutions and a nonlinear transformation function (i.e., the residual blocks together comprise 99 layers). Batch normalization was employed to address the problem of vanishing and exploding gradients to help convergence. Transfer learning technology was also employed by pre-training on the large-scale 1000-class datasets from ImageNet37. We then optimized the learning process on our ocular images by transfer learning an existing model that was. All codes were executed in the Convolutional Architecture for Fast Feature Embedding (Caffe) framework running on an Ubuntu 14.04 64 bit operating system and the Compute Unified Device Architecture (CUDA) 8.038.
Retrospective self-controlled test
To further investigate the real-world efficiency of the prediction-telehealth cloud platform, we retrospectively used longitudinal follow-up records from CCPMOH between January 2017 and May 2018. Informed consent was obtained from all subjects. Among 172 CC patients, 141 of which were finally included because they had complete follow-up records in all visits. Specifically, we included 141 patients’ clinical records (93 VAO, 48 non-VAO; 105 high-IOP, 36 normal) for prediction module testing and 987 images (394 intervention and 593 follow-up) for telehealth module testing. The mean age at surgery of the included patients were 28.32 months, and 74 participants (52.5%) were male. Supplementary Table 4 summarizes the clinical characteristics of all 141 included patients. All images are eligible as the inputs for the telehealth module.
In the self-controlled analysis, we retrospectively investigated the efficiency (advanced complication detection) and cost-efficient benefits (time, travel distance, and expenditure) before and after the use of our system. For complication detection, true prediction with an advanced schedule and accurate detection was considered a timelier intervention, and the time difference of intervention before and after the use of our system was calculated. For example, an individual had no VAO in 9-month visit but had VAO in 12-month visit, which is considered as 90 risk days before using our system. If our system can predict and detect successfully, this individual will have an additional visit at 10.5 month and be detected at this visit, which is considered as 45 risk days (45 risk days in advance).
The cost-efficient benefits were calculated based on the assumption that all follow-up visits using our agent were conducted via telehealth computing. Travel distance savings was defined as the round-trip distance savings between the distance traveled from the patient’s home address to the telehealth site and the distance the patient would have traveled at CCPMOH. Amap 7.8.6 (Alibaba Group, Hangzhou, China) was used to geocode the patients’ addresses, the telehealth sites (hospitals with ophthalmological departments) and CCPMOH and to calculate the travel distances. Distances were calculated by doubling one-way distances and the “quickest route” option was selected rather than the “shortest route” or “straight line” options.
Time savings was defined as the round-trip time savings for travel to the telehealth sites compared to travel to an in-person consultation at CCPMOH. The following travel speeds were used to calculate travel time: highways, 65 miles per hour (mph); major roads, 50 mph; arterial roads, 30 mph; and streets, 20 mph.
Expenditure savings was defined as the round-trip cost savings between the travel costs associated with traveling to the telehealth sites and those associated with traveling to CCPMOH. The estimated cost savings were calculated according to the latest standard of travel and traffic expenses from the Ministry of Finance of the People’s Republic of China in 2016 (file identifier: 2016–71), which reflects the cost of vehicular travel including insurance, fuel, and vehicle maintenance for miles driven.
Statistical analysis
Due to the skewed distributions of variables difference, Wilcoxon paired test was used to compare the differences of distance, time, and cost before and after the use of CC-Guardian. The Yonden index method was used to decide the optimal operating threshold for calculating the sensitivity and specificity values39. The 95% confidence interval of sensitivity, specificity was calculated using binomial proportion confidence intervals. All the statistical tests were two-tailed, and a P value below 0.05 was considered statistically significant. All tests were conducted using the statistical package R, version 3.2.4.
Ethics approval
Our study was approved by the centralized institutional review board (IRB) of Sun Yat-sen University. It was conducted in accordance with the Declaration of Helsinki. All included datasets were anonymized according to the Health Insurance Portability and Accountability Act Safe Harbor guidelines prior to their transfer to the study investigators.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Supplementary information
Acknowledgements
This study was funded by the National Key R&D Program of China (2018YFC0116500), Natural Science Foundation of China (81822010), Guangzhou Key Laboratory Project (202002010006) and the Guangdong Science and Technology Innovation Leading Talents (2017TX04R031). The sponsors played no role in this study.
Author contributions
H.L. and E.L. designed the research; E.L., J.-J.C., X.W., Z.Liu, W.L., Z.Lin, J.Li, X.L., H.C., C.G., L.Z., W.C., H.L. and Y.L. collected the CCPMOH data; H.L. organized the multicenter validation and D.N., X.-H.L., X.L., Z.D., B.Y., W.W., F.X., J.Lv., M.L., S.L., L.Z., J.-H.C., Q.Z., L.Z., Y.X. and G.T. provided the multicenter data. E.L., L.W., J.J., D.L., X.Z., M.D., D.W. and X.-Y.L. conducted the study; E.L., Y.-F.X., and W.L. analyzed the data; H.L. and E.L. co-wrote the paper; K.H., Y.Z., and C.C. critically revise the paper; all authors discussed the results and commented on the paper. E.L., J.-J.C., X.W., Z.Liu, and L.W. are considered the co-first author.
Data availability
The authors declare that the main data supporting the results in this study are available within the paper and its Supplementary Information. The datasets generated during the study are available for research purposes from the corresponding authors on reasonable request.
Code availability
The source code for the AI agent CC-Guardian is available in Github at https://github.com/longerping/cc-guardian.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Erping Long, Jingjing Chen, Xiaohang Wu, Zhenzhen Liu, Liming Wang.
These authors jointly supervised this work: Weirong Chen, Xiyang Liu, Haotian Lin, Yizhi Liu.
Supplementary information
Supplementary information is available for this paper at 10.1038/s41746-020-00319-x.
References
- 1.He J, et al. The practical implementation of artificial intelligence technologies in medicine. Nat. Med. 2019;25:30–36. doi: 10.1038/s41591-018-0307-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yu KH, Beam AL, Kohane IS. Artificial intelligence in healthcare. Nat. Biomed. Eng. 2018;2:719–731. doi: 10.1038/s41551-018-0305-z. [DOI] [PubMed] [Google Scholar]
- 3.Kermany DS, et al. Identifying medical diagnoses and treatable diseases by image-based deep learning. Cell. 2018;172:1122–1131.e9. doi: 10.1016/j.cell.2018.02.010. [DOI] [PubMed] [Google Scholar]
- 4.Obermeyer Z, Emanuel EJ. Predicting the future—big data, machine learning, and clinical medicine. N. Engl. J. Med. 2016;375:1216–1219. doi: 10.1056/NEJMp1606181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thottakkara P, et al. Application of machine learning techniques to high-dimensional clinical data to forecast postoperative complications. PLoS ONE. 2016;11:e0155705. doi: 10.1371/journal.pone.0155705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mohammadzadeh N, Safdari R. Chronic heart failure follow-up management based on agent technology. Health. Inf. Res. 2015;21:307–314. doi: 10.4258/hir.2015.21.4.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lin H, Long E, et al. Prediction of myopia development among Chinese school-aged children using refraction data from electronic medical records: A retrospective, multicentre machine learning study. PLoS Med. 2018;15:e1002674. doi: 10.1371/journal.pmed.1002674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Car J, Huckvale K, Hermens H. Telehealth for long term conditions. BMJ. 2012;344:e4201. doi: 10.1136/bmj.e4201. [DOI] [PubMed] [Google Scholar]
- 9.GBD 2013 Mortality and Causes of Death Collaborators. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1736–1788. doi: 10.1016/S0140-6736(18)32203-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang HH, et al. Epidemiology of multimorbidity in China and implications for the healthcare system: cross-sectional survey among 162,464 community household residents in southern China. BMC Med. 2014;12:188. doi: 10.1186/s12916-014-0188-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jin J, et al. Factors affecting therapeutic compliance: a review from the patient’s perspective. Ther. Clin. Risk Manag. 2008;4:269–286. doi: 10.2147/TCRM.S1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kalia A, et al. Assessing the impact of a program for late surgical intervention in early-blind children. Public Health. 2017;146:15–23. doi: 10.1016/j.puhe.2016.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lester RT, et al. Effects of a mobile phone short message service on antiretroviral treatment adherence in Kenya (WelTel Kenya1): a randomised trial. Lancet. 2010;376:1838–1845. doi: 10.1016/S0140-6736(10)61997-6. [DOI] [PubMed] [Google Scholar]
- 14.Lin H, et al. Effectiveness of a short message reminder in increasing compliance with pediatric cataract treatment: a randomized trial. Ophthalmology. 2012;119:2463–2470. doi: 10.1016/j.ophtha.2012.06.046. [DOI] [PubMed] [Google Scholar]
- 15.Lambert SR, et al. Long-term risk of glaucoma after congenital cataract surgery. Am. J. Ophthalmol. 2013;156:355–361. doi: 10.1016/j.ajo.2013.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Plager DA, et al. Complications, adverse events, and additional intraocular surgery 1 year after cataract surgery in the infant Aphakia Treatment Study. Ophthalmology. 2011;118:2330–2334. doi: 10.1016/j.ophtha.2011.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang L, et al. Visual outcome and related factors in bilateral total congenital cataract patients: a prospective cohort study. Sci. Rep. 2016;6:31307. doi: 10.1038/srep31307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin H, et al. Documenting rare disease data in China. Science. 2015;349:1064. doi: 10.1126/science.349.6252.1064-b. [DOI] [PubMed] [Google Scholar]
- 19.Han J, Kamber M. Data mining: concepts and techniques. The Morgan Kaufmann series in data management systems. Antimicrobial Agents Chemother. 2015;59:1435–1440. doi: 10.1128/AAC.04251-14. [DOI] [Google Scholar]
- 20.Lin HT, et al. Timing and approaches in congenital cataract surgery: a four-year, two-layer randomized controlled trial. Int J. Ophthalmol. 2017;10:1835–1843. doi: 10.18240/ijo.2017.12.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.He K. et al. Deep Residual Learning for Image Recognition. In Proc. IEEE Conference on Computer Vision and Pattern Recognition. 770–778 (2016).
- 22.Ryu J, Lee TH. The waiting game—why providers may fail to reduce wait times. N. Engl. J. Med. 2017;376:2309–2311. doi: 10.1056/NEJMp1704478. [DOI] [PubMed] [Google Scholar]
- 23.Lewis TL, Maurer D. Effects of early pattern deprivation on visual development. Optom. Vis. Sci. 2009;86:640–646. doi: 10.1097/OPX.0b013e3181a7296b. [DOI] [PubMed] [Google Scholar]
- 24.Calkins DJ. Critical pathogenic events underlying progression of neurodegeneration in glaucoma. Prog. Retin Eye Res. 2012;31:702–719. doi: 10.1016/j.preteyeres.2012.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ledolter J, Kardon RH. Does testing more frequently shorten the time to detect disease progression? Transl. Vis. Sci. Technol. 2017;6:1. doi: 10.1167/tvst.6.3.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Long E, et al. An artificial intelligence platform for the multihospital collaborative management of congenital cataracts. Nat. Biomed. Eng. 2017;1:0032. doi: 10.1038/s41551-016-0024. [DOI] [Google Scholar]
- 27.Malin B, et al. Between access and privacy: challenges in sharing health data. Yearb. Med Inform. 2018;27:55–59. doi: 10.1055/s-0038-1641216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lenhart PD, et al. Global challenges in the management of congenital cataract: proceedings of the 4th International Congenital Cataract Symposium held on March 7, 2014, New York. J. Aapos. 2015;19:e1–e8. doi: 10.1016/j.jaapos.2015.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ting DSW, et al. Development and validation of a deep learning system for diabetic retinopathy and related eye diseases using retinal images from multiethnic populations with diabetes. JAMA. 2017;318:2211–2223. doi: 10.1001/jama.2017.18152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Long E, et al. Monitoring and morphologic classification of pediatric cataract using slit-lamp-adapted photography. Transl. Vis. Sci. Technol. 2017;6:2. doi: 10.1167/tvst.6.6.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Beck AD, et al. Glaucoma-related adverse events in the infant aphakia treatment study: 1-year results. Arch. Ophthalmol. 2012;130:300–305. doi: 10.1001/archophthalmol.2011.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Solebo, AL, et al. Visual axis opacity after intraocular lens implantation in children in the first 2 years of life: findings from the IoLunder2 Cohort Study. Ophthalmology. pii: S0161-6420 30226-8 (2020). [DOI] [PubMed]
- 33.Bothun ED, et al. Outcomes of unilateral cataracts in infants and toddlers 7 to 24 months of Age: Toddler Aphakia and Pseudophakia Study (TAPS) Ophthalmology. 2019;126:1189–1195. doi: 10.1016/j.ophtha.2019.03.011. [DOI] [PubMed] [Google Scholar]
- 34.WHO. The International Classification of Diseases. http://www.who.int/classifications/icd/en (2019).
- 35.Arvind V, et al. Predicting surgical complications in adult patients undergoing anterior cervical discectomy and fusion using machine learning. Neurospine. 2018;15:329–337. doi: 10.14245/ns.1836248.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ioffe, S. & Szegedy, C. Batch normalization: accelerating deep network training by reducing internal covariate shift. Preprint at https://arxiv.org/abs/1502.0316 (2015)
- 37.Russakovsky O, et al. Imagenet large scale visual recognition challenges. Int. J. Comput. Vis. 2015;115:211–252. doi: 10.1007/s11263-015-0816-y. [DOI] [Google Scholar]
- 38.Jia, Y. et al. Caffe: convolutional architecture for fast feature embedding. In Proc. 22nd ACM Int. Conf. Multimedia. 675–678 (2014).
- 39.Youden WJ. Index for rating diagnostic tests. Cancer. 1950;3:32–35. doi: 10.1002/1097-0142(1950)3:1<32::AID-CNCR2820030106>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors declare that the main data supporting the results in this study are available within the paper and its Supplementary Information. The datasets generated during the study are available for research purposes from the corresponding authors on reasonable request.
The source code for the AI agent CC-Guardian is available in Github at https://github.com/longerping/cc-guardian.