Abstract
Purpose
Baicalin (BA) has a good neuroprotective effect, but it is eliminated quickly in the body and does not easily reach the brain. In this experiment, borneol (BO) was used as an auxiliary drug to prepare borneol-baicalin-liposomes (BO-BA-LP) to prolong the efficacy time of BA, synergistically synergize, introduce drugs into the brain, and better exert the therapeutic effect on cerebral ischemia-reperfusion (I/R) injury.
Methods
Through single-factor inspection and response surface optimization analysis, obtained the best preparation process of BO-BA-LP and characterized by various analytical techniques. Validated the long-term effectiveness of BA-BO-LP through pharmacokinetic studies and conducted pharmacodynamic studies on the middle cerebral artery occlusion (MCAO) rat model to verify the therapeutic effect of BO-BA-LP on cerebral I/R injury.
Results
The optimum preparation conditions of BO-BA-LP were as follows: the dosage of BO was 9.55 mg, the ratio of phospholipid to drug was 4.02:1, the ratio of phospholipid to cholesterol was 7.25:1, the entrapment efficiency (EE) was 41.49%, and the drug loading (DL) was 4.29%. The particle size range of the liposomes was 167.1 nm, and the polydispersity index (PDI) range was 0.113. The results of pharmacokinetic experiments showed that the combination of BA and BO liposomes effectively improved the pharmacokinetic parameters of BA and prolonged the half-life of BA. Pharmacodynamic studies have found that, compared with BA-LP, BO-BA-LP can significantly improve neurological deficits, cerebral infarction volume, and brain pathological states on MCAO rats.
Conclusion
These results demonstrated that BO-BA-LP can improve the circulation of drugs in the blood, and the addition of BO can enhance the therapeutic effect of BA and effectively improve cerebral I/R.
Keywords: borneol-baicalin-liposome, pharmacokinetics, pharmacodynamics, MCAO
Introduction
In recent years, ischemic cerebral stroke has become one of the leading causes of human death and disability worldwide, after cancer and cardiovascular diseases.1 Ischemic stroke is one of the most serious cerebrovascular diseases, affecting the quality of life of patients. Ischemic stroke accounts for 88% of the total number of strokes, with clinical manifestations of rapidly developing brain dysfunction.2 During cerebral I/R, microcirculation obstacles block substrate transport, leading to energy loss, and the beginning of reperfusion after cerebral ischemia will cause greater damage. At the same time, the inflammatory cells in the ischemic area abnormally aggregate, producing excessive free radicals.3,4 Evidence has accumulated that cerebral neuroprotective drugs could be an effective method for treating neurological diseases. Therefore, through targeting inflammatory processes and inhibiting neuronal apoptosis we can achieve neuroprotection of cerebral ischemia.
BA (5,6,7-trihydroxyflavone-7-β-D-glucuronic acid) is a natural flavonoid compound extracted from the rhizome of Scutellaria baicalensis georgi.5 Modern pharmacological studies showed that BA had an obvious protective effect on central nervous system (CNS) diseases, such as Parkinson’s disease, Ischemic stroke and depression.6 For a long time, BA plays a role as a potential treatment for cerebral ischemia, which can protect the ischemic brain injury by inhibiting apoptosis, inhibiting inflammatory factors, and anti-oxidative stress.7
However, the low hydrophobicity, poor stability and low bioavailability of BA limit its further development.8,9 In order to develop new drug delivery systems for BA, we tried many times, in our previous study, we successfully prepared the baicalin-liposomes (BA-LP), which improved the bioavailability and extended the residence time of BA in the body. Compared with BA monomer, BA-LP can enhance the efficacy of BA to a certain extent after i.v. administration, and has a certain brain targeting effect.10 Therefore, how to better exert the curative effect of BA and improve its brain targeting and therapeutic effect has become an urgent problem to be solved.
BO is a bicyclic monoterpene and is widely used in traditional Chinese medicine (TCM) for “waking up” the brain.11 BO shows extensive pharmacological effects including obvious anti-inflammatory, antioxidation and neuroprotective effects.12,13 It was suggested to be protective against brain injury in cerebral I/R and cognitive impairment in cerebral ischemia mice, and can be used as an auxiliary drug for treating cerebral I/R injury.14 Moreover, based on the TCM theories, BO is an upper guiding drug, which has the effect of targeting the drug to the brain to enhance the efficacy.15 Mr. Tang’s team research showed that BO and lactoferrin co-modified nanoparticles encapsulated drug can effectively enhance dopamine delivery into the brain for the treatment of Parkinson’s disease.16 In addition, BO can enhance the ability of other drugs to penetrate the blood-brain barrier (BBB) to improve bioavailability and enhance the distribution of drugs in BBB.17 The above all suggest that borneol as a supplementary drug has potential value for the treatment of brain diseases.
Thus, the purpose of this study is to prepare BO-BA-LP, and optimize the optimal preparation process by response surface-central composite design, then through i.v. administration, pharmacokinetic and pharmacodynamic studies performing in normal rats and MCAO rats, respectively. To the best of our knowledge, this is the first time that combines BO with BA and prepares for liposomes, then we initially discuss the efficacy of pharmacokinetics on MCAO rats. In this study, natural medicine was used as an adjunct medicine, which not only introduced drugs upward, enhanced the efficacy of the main drug, but also produced part of the therapeutic effect, providing new research ideas for improving the efficacy of drugs and enhancing targeting.
Materials and Methods
Chemicals and Reagents
BA (No. MUST-18,010,410) were obtained from China Pharmaceutical and Biological Product Identification Institute. Rutin (L-001-181,216) were obtained from Chengdu Ruifensi Biotechnology Co., Ltd. (Chengdu, PR China). Soybean lecithin (No.SY-SI-160,802) and cholesterol (NO.B40936) were obtained from Shanghai Ivet Medical Technology Co., Ltd. (Shanghai, PR China). BA raw material (weight content >90%) was purchased from Nanjing Zelang Biotechnology Co., Ltd. (Nanjing, PR China). Methanol with High Performance Liquid Chromatography grade was from Thermo Fisher Scientific (Massachusetts, USA). AR-grade chloroform came from Sinopharm Chemical Reagent Co., Ltd. (China). AR-ether came from Taicang Zhou Chemical Co., Ltd. (China). Water used in this paper was from China Resources C’estbon Beverage (China) Co., Ltd. (Shenzhen, PR China).
Investigation of BO-BA-LP Preparation Process
Preparation of BO-BA-LP
Liposomes were prepared by the reverse evaporation method. Briefly, BA (50 mg) was dissolved into PBS (pH7.0) at 5 mg/mL. 200 mg of phospholipid, 35 mg of cholesterol and 15 mg of BO were dissolved into the mixed liquor (24 mL of absolute ether and 12 mL of chloroform), then injected the completely dissolved BA solution into the mixed liquor. Placed it in a 20°C water bath for ultrasonic dispersion for 10 minutes to make it emulsify evenly, and put it in a 250 mL pear-shaped flask; then, at 20°C rotary evaporation to obtain a flowable colloid. The colloid was hydrated with PBS (pH7.0) by rotary evaporation under reduced pressure, and obtain the BO-BA-LP.18
Optimization of BO-BA-LP Preparation by Response Surface-Central Composite Design
The preparation of BA-BO-LP was optimized according to the three-factor central composite design (CCD). The content of BO (A), the ratio of phospholipid to drug (B) and the ratio of phospholipid to cholesterol (C) were determined as independent variables. The range of the three arguments is shown in Table 1. BO-BA-LP were evaluated with EE and DL as optimization indexes.
Table 1.
Factors and Responses in Response Surface-Central Composite Design
| Factors | Levels | ||
|---|---|---|---|
| −1 | 0 | 1 | |
| A | 5 | 10 | 15 |
| B | 3 | 4 | 5 |
| C | 4 | 7 | 10 |
Characterization of BO-BA-LP
The particle size of distributions, mean diameter, polydispersity index (PDI) and zeta of BO-BA-LP were measured by a nanoparticle size measuring instrument (PARTICLE SIZING SYSTEMS Santa Barbara, California, USA), and the morphology of BO-BA-LP was examined by the particle size analyzer and the transmission electron microscope (TEM). The EE and DL of liposomes were determined by dialysis. The procedure is as follows: 1 mL liposome was injected into the dialysis bag (molecular interception 10,000), and it was dialyzed in 300mL PBS (pH7.0) for 6 h by dialysis bag method. An appropriate amount of dialysate was taken and the absorbance was measured by UV (Uv-vis-2550 UV spectrophotometer, Shimadzu, Japan) spectrophotometer to calculate the encapsulation rate.
According to the dialysis bag method, the Wf and Wtotal of liposomes were determined. The equations for EE and DL of BO-BA-LP are as follows:
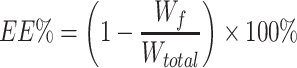 |
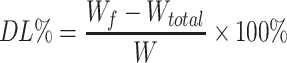 |
Wf is the amount of free BA in the BO-BA-LP sample, Wtotal is the total amount of BA in the BO-BA-LP sample, and W is the total amount of BO, phospholipid, BA and cholesterol.
Study on the Stability and in vitro Release of BO-BA-LP
BO-BA-LP were prepared according to the optimal technology. The EE(%) and DL(%) were determined after the liposomes were placed at 4°C for 0, 5, 10, 15, 20 and 30 days, respectively, and investigated its stability. The in vitro studies of BO-BA-LP solution were performed using the dialysis bag method (molecular retention 1000) with 300 mL of PBS (pH 7.0) as a release medium. At different time point as designated, aliquots PBS (3 mL) were withdrawn from the dialysis and replaced with equal fresh PBS. The release amount of the drug was determined by UV.
In vivo Pharmacokinetic Study
Animals
Healthy adult male Sprague Dawley rats (240–260 g) of clean grade were purchased from Chengdu Dashuo Experimental Animal Center (Chengdu, China). The rats were housed in the animal facility of Chengdu University of Traditional Chinese Medicine until used. Animals (5 per case) were kept at a 12 h (light)–12 h (dark) cycle under controlled temperature (22±2°C) and relative humidity (50%±10%). Before the study begins, all rats got access to food and water freely and isolated to acclimate for 1 week. All studies were conducted with reference to the “Laboratory Animal Care and Use Guide” (NIH Publication # 85–23, revised 1996) published by the National Institutes of Health, and have been approved by the animal and ethics review committee of Chengdu University of Traditional Chinese Medicine, China.
Collection and Preparation of Plasma
Rats were anesthetized with 10% chloral hydrate (0.35 g/kg, i.p.). Rats in the BA group and the BO-BA-LP group were given the corresponding drug 25 mg/kg intravenously (calculated based on the BA content). The blank group and the model group were given an equal amount of saline. Blood was taken from the fundus venous plexus of rats at 2, 7, 15, 30, 60, 90, 120, 180, 360, 480 min after administration. Every blood sample was placed in a 2 mL heparin sodium anticoagulation blood collection tubes. After standing a little, centrifuged at 5000 r/min for 10min. After centrifugation, the supernatant was taken as a plasma sample and stored at −20°C.
Assay of BA in Rat Plasma
Took the sample 150 μL, and added with 30μL hydrochloric acids (1 mol/L), then swirled for 1 min. Rudin (40 μL) was added as internal standard and 0.6 mL acetonitrile was added to precipitate the protein, and then obtained supernatant at 12000r/min for 10 min. All supernatant was blow-dried by nitrogen gas, redissolved with 70% methanol water at 100 μL, vortexed for 4 min, and centrifuged at 15,000 r/min for 10 min to obtain supernatant. Finally, 20μL of supernatant was injected into HPLC system for analysis.
The mobile phase used for the assay of BA in rat plasma consists of methanol and 0.2% phosphoric acid (41:59, v/v). The flow rate of a mobile phase was 1.0 mL/min and the injection volume was 20 μL. The chromatographic analysis was performed with the detection wavelength at 276 nm and the operating temperature at 35°C.
Calibration Curve, Recovery, Precision, Accuracy and Stability
Took 150 μL of rat’s blank plasma, added 80 μL of BA standard solutions of different concentrations, and diluted to plasma mass concentration: 0.109, 2.729, 5.458, 27.2 93, 38.21, 54.58 μg/mL. Injected and measured according to the given method to get the regression equation and correlation coefficient of the standard curve, and the correlation coefficient (R2) should be greater than 0.99. Took samples of high, medium, and low concentrations. Each sample was injected five times a day for five consecutive days, and its recovery and accuracy were determined. High, middle and low three BA plasma samples were stored at 25°C for 0, 2, 4, 8, 12 h to examined the stability at room temperature, and stored at −80°C for 1, 5, 10, 20, 30 days to investigate the stability of freezing. After 3 freeze-thaw cycles (−80°C), the freeze-thaw stability was examined.
In vivo Pharmacodynamics Research
Establishment of MCAO Model
Rats were anesthetized with 10% chloral hydrate (0.35 g/kg, i.p.). Placed the rats on the operating table, the skin was cut in the middle of the neck, and separated right common carotid artery, internal carotid artery and external carotid artery with a blunt instrument. Made a small incision in the common carotid artery (near the bifurcation of the internal and external carotid arteries), and inserted a MCAO plug along the internal carotid, then fixed with sutures, caused focal middle cerebral artery infarction and recorded the infarct time. After 2 h, the suppository line was pulled out about 1cm to achieve reperfusion of ischemic brain tissue. Rats were sacrificed after 24 h of reperfusion. The first administration (20 mg/kg, i.v.) was performed immediately before model establishment, followed by an administration after 8h, and then 12 h thereafter. After sacrificed, all the brains were taken out quickly for TTC staining (n = 5 each group) and histomorphological assays (n = 3 each group).
Neurological Deficit Evaluation
The Zea-Longa MCAO scoring method was used to score neurological.19 The scoring criteria are as follows: 0-No neurological deficits; 1-Slight neuropathological features, raising the tail, knee flexion of left forelimb; 2-Moderate neurological deficit, the rat circling to the left; 3-Severe neurological deficit, hemiplegicing to the left and falling down; 4-Unconscious state of activity. Experimental rats will be scored twice, the first scoring was carried out 2 h after cerebral ischemia, the second scoring after 24 h of reperfusion.
Measurement of the Percentage of Cerebral Infarction Volume
Rats were sacrificed at 2 h after the last administration, and their brain tissue was removed to calculate the infarct size (n = 5). After deep anesthesia, the rats were sacrificed by bloodletting and their brains were isolated immediately and rinsed by NS. After they were placed in a refrigerator at −20°C for 20 min, consecutive coronal sections were spaced 2 mm apart in a brain-cutting mold. Then the brain slices were placed in 2% TTC (2, 3, 5-triph-enylte tolazoline chloride) for 30min under the condition of 37°C, and then fixed in 4% paraformaldehyde at 4°C for 24 h. The normal tissue was dyed pink and the area of the infarct was dyed white. The infarct volume was calculated using the following formula.20
The infarct volume (%) = Weight of white area/Weight of whole brain × 100%
Histopathological Study
Rats' brains were fixed in 4% paraformaldehyde for at least 24 h, the coronal brain tissue was cut into slices of 3 mm thickness followed by routine embedding in paraffin. The thicknesses were stained with hematoxylin and eosin (H&E staining). The histomorphology of neurons was observed under the microscope. The injured neuron cells were dark staining, shrinkage or dysmorphic, and intact were distinct nucleus and nucleolus. On Nissl staining, the sections were mounted on clean glass slides, dewax, added 1% toluidine blue preheated at 60°C for 40 min, washed three times with distilled water, differentiated with 5% ethanol, and decolorized until the background is clear. Finally, placed the coverslip under a microscope to observe the cerebral cortex number of neurons positive for Nissl staining in hippocampal CA1 area.
Statistical Analysis
The data were expressed as meaning ± standard deviation (S.D.). DAS2.0 (Mathematical Pharmacology Professional Committee of China, Shanghai, China) was used to calculate the in vivo pharmacokinetics parameters by a non-compartmental model. The statistical package was performed on SPSS 22.0 (SPSS Inc., Chicago, IL, USA) Software, Statistical analyzed by one-way variance (ANOVA) and Student’s t-test. A value of P<0.05 or P<0.01 was considered statistically significant.
Results
Preparation of BO-BA-LP
The liposomes prepared by reverse evaporation method had higher EE and DL (Table 2), so reverse evaporation method was selected to prepare BO-BA-LP. It was found that the film dispersion method and the ethanol injection method were difficult to produce stable and uniform BO-BA-LP, and the entrapment efficiency and drug loading of the liposomes were low, it is possible that BA and BO are less soluble in the corresponding organic solvents. Reverse evaporation method was used to prepare BO-BA-LP, because the liposomes were uniform and the entrapment efficiency and drug loading were high.
Table 2.
Screening Results of Preparation Methods
| Results | EE (%) | DL (%) |
|---|---|---|
| Thin film dispersion | 31.55 | 6.13 |
| Reverse evaporation | 42.28 | 5.65 |
| Ethanol injection | 38.97 | 3.90 |
Based on the experimental basis of single-factor investigation, 17 experimental runs were used for optimizing the three main factors in a central composite design (Table 3), which was also used to maximize the EE and DL.21
Table 3.
Box–Behnken Design and Results
| No. | Independent Variables | Dependent Variables | |||
|---|---|---|---|---|---|
| A | B | C | Y1 | Y2 | |
| 1 | 5 | 5:1 | 7:1 | 30.41 | 5.61 |
| 2 | 15 | 4:1 | 10:1 | 32.64 | 3.17 |
| 3 | 5 | 4:1 | 10:1 | 31.51 | 6.51 |
| 4 | 10 | 4:1 | 7:1 | 40.78 | 3.84 |
| 5 | 15 | 3:1 | 7:1 | 31.98 | 8.36 |
| 6 | 15 | 5:1 | 7:1 | 28.32 | 4.51 |
| 7 | 5 | 3:1 | 7:1 | 38.82 | 6.77 |
| 8 | 10 | 5:1 | 10:1 | 38.48 | 4.89 |
| 9 | 10 | 5:1 | 4:1 | 32.7 | 3.56 |
| 10 | 5 | 4:1 | 4:1 | 34.05 | 7.03 |
| 11 | 10 | 3:1 | 10:1 | 31.38 | 6.49 |
| 12 | 10 | 4:1 | 7:1 | 43.69 | 6.72 |
| 13 | 10 | 3:1 | 4:1 | 25.54 | 4.10 |
| 14 | 15 | 4:1 | 4:1 | 36.69 | 5.04 |
| 15 | 10 | 4:1 | 7:1 | 40.78 | 3.79 |
| 16 | 10 | 4:1 | 7:1 | 41.88 | 3.25 |
| 17 | 10 | 4:1 | 7:1 | 40.08 | 3.52 |
Notes: Independent variables: A, the dosage of BO (mg); B, the ratio of phospholipid to drug (w/w); C, the ratio of phospholipid to cholesterol (w/w). Dependent variables: Y1: EE(%); Y2: DL(%).
The model equation of EE is as follows:
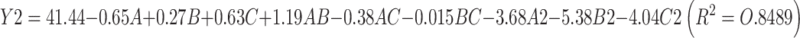 |
The model equation of DL is as follows:
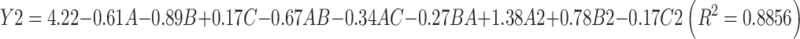 |
The statistical significance of the regression model was checked by P value (Tables 4 and 5). The results showed that C2 had a significant effect on EE (P<0.05), while B, C and A2 had a significant effect on the DL (P<0.05).
Table 4.
Variance Analysis of EE Regression Model
| Source | Sum of Squares | df | Mean Square | F Value | P value Prob>F |
|---|---|---|---|---|---|
| Model | 288.82 | 9 | 32.09 | 1.44 | 0.0048 |
| A | 3.33 | 1 | 3.33 | 0.15 | 0.7109 |
| B | 0.6 | 1 | 0.6 | 0.027 | 0.8745 |
| C | 3.16 | 1 | 3.16 | 0.14 | 0.7178 |
| AB | 5.64 | 1 | 5.64 | 2.60 | 0.6306 |
| AC | 5.70E-01 | 1 | 5.70E-01 | 2.60E-02 | 0.8775 |
| BC | 9.00E-04 | 1 | 9.00E-04 | 4.03E-05 | 0.9951 |
| A2 | 57.05 | 1 | 57.05 | 2.56 | 0.1539 |
| B2 | 121.8 | 1 | 121.8 | 5.46 | 0.0522 |
| C2 | 68.67 | 1 | 68.67 | 3.08 | 0.0229 |
| Residual | 156.25 | 7 | 22.32 | ||
| Lack of Fit | 148.27 | 3 | 49.42 | 24.78 | 0.3233 |
| Pure Error | 7.98 | 4 | 1.99 | ||
| Cor Total | 445.07 | 16 | 445.07 |
Table 5.
Variance Analysis of DL Regression Model
| Source | Sum of Squares | df | Mean Square | F Value | P value Prob>F |
|---|---|---|---|---|---|
| Model | 22.71 | 9 | 2.52 | 0.98 | 0.0205 |
| A | 2.93 | 1 | 2.93 | 1.44 | 0.3206 |
| B | 6.39 | 1 | 6.39 | 2.49 | 0.1583 |
| C | 0.22 | 1 | 0.22 | 0.086 | 0.7775 |
| AB | 1.81 | 1 | 1.81 | 0.71 | 0.4286 |
| AC | 4.60E-01 | 1 | 4.60E-01 | 1.80E-01 | 0.6859 |
| BC | 2.80E-04 | 1 | 2.80E-04 | 1.10E-01 | 0.0003 |
| A2 | 8.05 | 1 | 8.05 | 3.14 | 0.0196 |
| B2 | 2.1 | 1 | 2.1 | 0.82 | 0.3959 |
| C2 | 0.12 | 1 | 0.12 | 0.047 | 0.8342 |
| Residual | 17.94 | 7 | 2.56 | ||
| Lack of Fit | 9.93 | 3 | 3.31 | 1.65 | 0.3124 |
| Pure Error | 8.01 | 4 | 2 | ||
| Cor Total | 40.65 | 16 |
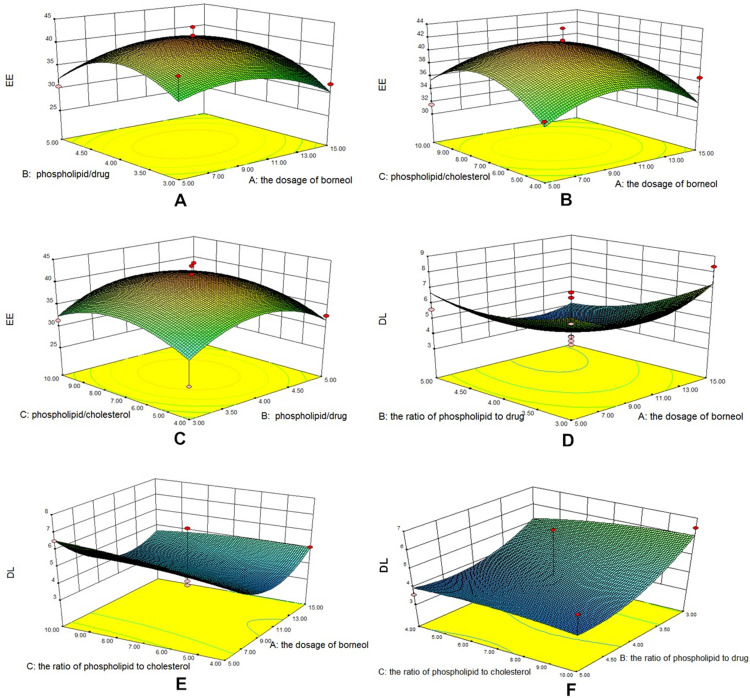
The results showed that the EE increased first and then decreased with the increase of BO dosage (Figure 1), phospholipid–drug ratio and phospholipid–cholesterol ratio. This phenomenon may be related to the degree of saturation of liposomes. Cholesterol acts as a “flow buffer” in the liposome system. Higher than the phase transition temperature, the lipid bilayer can be arranged closely, thus reducing the drug leakage and increasing the EE, the asymmetry, permeability and rigidity of Lipid bilayer membrane increased with the increase of cholesterol dosage, which resulted in drug penetration and decreased EE. In addition, the increase of phospholipid–cholesterol ratio may increase the DL rate to some extent. The optimum conditions were as follows: the dosage of BO was 9.55 mg, the ratio of phospholipid to medicine was 4.02:1, and the ratio of phospholipid to cholesterol was 7.25:1.
Figure 1.
Three-dimensional effect surface diagrams of encapsulation efficiency. (A) BO dosage to phospholipid–drug ratio (EE). (B) BO dosage to phospholipid–cholesterol ratio (EE). (C) Phospholipid–drug ratio to phospholipid–cholesterol ratio (EE). (D) BO dosage to phospholipid–drug ratio (DL). (E) BO dosage to phospholipid–cholesterol ratio (DL). (F) Phospholipid–drug ratio to phospholipid–cholesterol ratio (DL).
Verification of the Predictive Model
The optimized ranges of process conditions by response surface methodology were as follows: phospholipid/drug ratio (A): 4.02:1, phospholipid/cholesterol ratio: 7.25;1, the dosage of BO: 9.55 mg. Three batches of liposomes were prepared according to the optimal formulation. The EE(%) and DL(%) were measured, and the deviation was calculated (Deviation value=(Predicted value-Measured value/Predicted value×100%). The actual EE of BO-BA-LP was 40.93% and the DL was 7.09% (Table 6). Deviations of EE and DL between predicted values and measured values were 1.35% and 0.84%. The results showed that the liposomes prepared by this method had a good fit and a high reliability.
Table 6.
The Results of Verification of the Predictive Model Test
| No. | EE (%) | DL (%) |
|---|---|---|
| 1 | 41.23 | 7.15 |
| 2 | 40.39 | 7.00 |
| 3 | 41.17 | 7.14 |
| Measured average values | 40.39 | 7.09 |
| Predicted value | 41.49 | 7.15 |
| Deviation% | 1.35 | 0.84 |
Characterization of BO-BA-LP
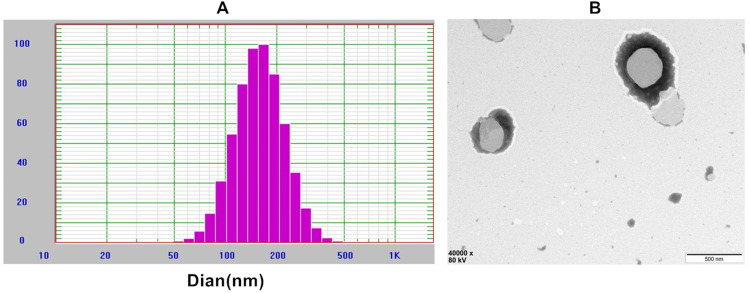
The liposomes were prepared according to the optimal formulation and stored at 4°C for 24 h without delamination. The liposomes were diluted by 30 times with normal saline, and the particle size was determined by Malvern particle size analyzer. The particle size range of the liposomes was 167.1 nm (Figure 2), and the PDI range was 0.113, indicating a relative particle size. Zeta potential was 0.04 mv. The TEM photographs showed that liposomes were spherical and the particle size distribution is narrow, indicating that the BO-BA-LP would have a good dispersion.22
Figure 2.
Characterization of BO-BA-LP. (A) The size distribution of BO-BA-LP. (B) The transmission electron microscope photograph of BO-BA-LP.
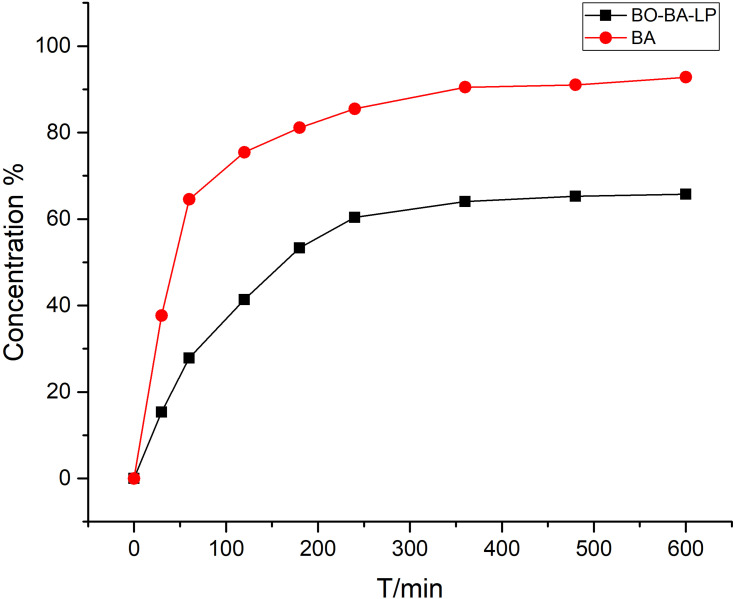
EE and DL of BO-BA-LP decreased after 20 days at 4°C, indicating that the liposomes are stable (Table 7). The release rate of BA in vitro was 90.47% at 6h, which indicates that the drug release through the dialysis bag was not a restrictive release step (Figure 3). After the drug was prepared into liposomes, it can be divided into two release stages: rapid release and slow release. The release rate of BO-BA-LP was 64.06% before 6 h, and was slow after 6 h, which indicating that it had a certain slow release effect.23
Table 7.
The Results of Stability Test
| Time/d | 1 | 2 | 3 | |||
|---|---|---|---|---|---|---|
| EE% | DL% | EE% | DL% | EE% | DL% | |
| 0 | 41.32 | 7.16 | 41.56 | 7.21 | 40.92 | 7.10 |
| 5 | 41.37 | 7.17 | 40.97 | 7.10 | 42.23 | 7.32 |
| 10 | 40.63 | 7.05 | 41.35 | 7.17 | 41.26 | 7.16 |
| 15 | 41.22 | 7.15 | 40.63 | 7.05 | 40.15 | 6.96 |
| 20 | 37.49 | 6.50 | 38.22 | 6.63 | 37.03 | 6.42 |
| 30 | 30.57 | 5.30 | 32.49 | 5.63 | 29.59 | 5.13 |
Figure 3.
In vitro release profiles of BA and BO-BA-LP.
In vivo Pharmacokinetic Parameters
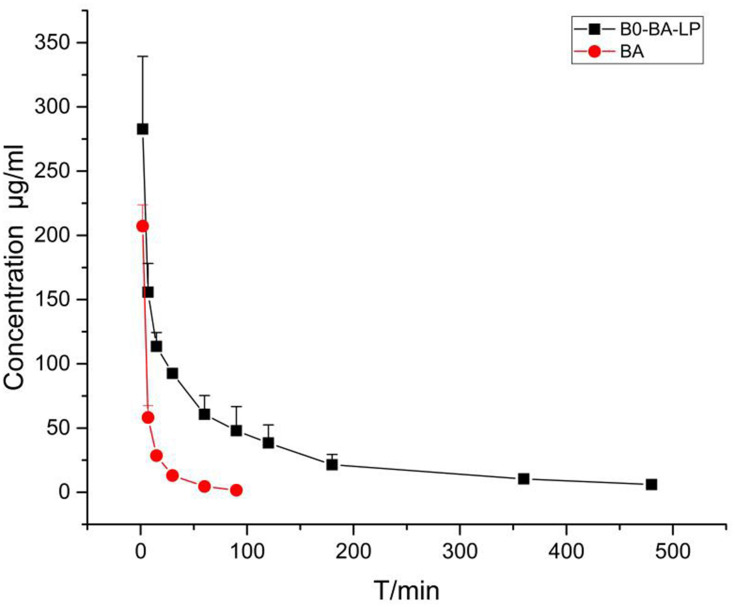
From Table 8 and Figure 4, the t1/2 values of the BA and BO-BA-LP groups were 17.05±1.81 min and 128.81±16.16 min, respectively. The half-life of the BA group was significantly lower than that of the BO-BA-LP group (p <0.01), indicating that BO-BA-LP greatly prolonged the role of BA in vivo and the circulation time. The MRT0-t values were 12.65±0.89 min (BA) and 114.23±16.03 min (BO-BA-LP). This results suggesting that BO-BA-LP can significantly increase the retention time of BA in plasma, thereby enhancing the therapeutic effect. More importantly, the AUC0-t of the BA-LP group is much higher than that of the BA group (p <0.05), indicating that BO-BA-LP has better absorption in the body and reduced the metabolism of BA by tissues.
Table 8.
Main Plasma Pharmacokinetic Parameters of BA and BA-LP After i.v. Administration to MCAO Rats at the Dosage of 25 Mg/Kg (n=5, mean±SD)
| Parameters | Units | BA | BA-BO-LP |
|---|---|---|---|
| Cmax | μg/mL | 230.14±37.58 | 282.72±56.59* |
| t1/2 | min | 17.05±1.81 | 128.81±16.16** |
| AUC0-t | μg/mL*min | 2233.72±44.10 | 14,718.50±2368.97** |
| AUC0-∞ | μg/mL*min | 2264.09±35.78 | 15,674.28±2697.39 |
| MRT0-t | min | 12.65±0.89 | 114.23±16.03** |
| MRT0-∞ | min | 14.03±1.34 | 146.94±25.23 |
Notes: Data were presented as mean±SD. *Significantly different from the BA group at P < 0.05, **Extremely significant different from the I/R group at P < 0.01.
Figure 4.
The plasma concentration-time profile of BA after i.v. administration of BA and BO-BA-LP (25 mg/kg).
Effects of BO-BA-LP on the Neurological Defect Scores in Rats
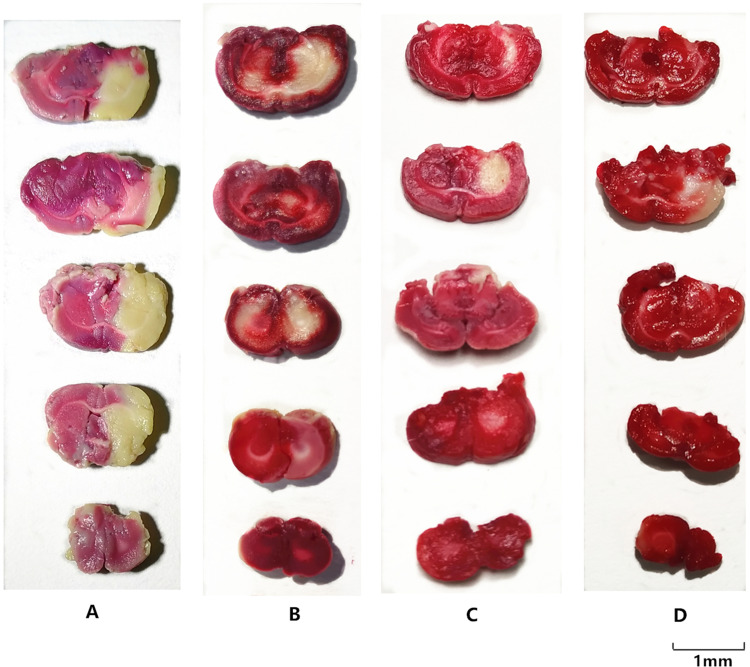
The results of MCAO behavior score and infarction area are shown in Table 9. The neurological defect score showed that rats in the sham group did not show neurological deficits. The I/R group had a neurobehavioral dysfunction value of 3.5, indicating that the MCAO model was successfully prepared. Behavioral score of the animals in each administrative group decreased, and the difference was statistically significant compared with the model group. Table 9 also showed that MCAO significantly elevated brain infarct area (25.08±3.46). The BA, BA-LP, and BO-BA-LP group all significantly reduced the brain infarct area of MCAO rats, and the liposome group had significant differences compared with the monomer group (P < 0.05). Additionally, the BO-BA-LP group showed a better therapeutic effect than the BA-LP group (P < 0.05), suggesting the addition of BO can better protect cerebral ischemia-reperfusion injury.
Table 9.
Neurological Function Score and Infarction Rate in Different Groups (n=5)
| Groups | Neurological Function Score | Average Infarct Volume Ratio (%) |
|---|---|---|
| Sham | – | – |
| I/R | 3.10±0.32 | 25.08±3.46 |
| BA | 2.30±0.46* | 19.14±2.28* |
| BA-LP | 1.70±0.67* | 15.86±1.37* |
| BA/BO-LP | 1.30±0.90* | 12.67±1.48*# |
Notes: Data were presented as mean ± SD. All animals were scored, 10 rats per group, and half of them in the determination of cerebral infarction. Differences were considered significant at P< 0.05. *Significantly different from the I/R group at P < 0.05; #Significantly different from the BA-LP group at P < 0.05.
From Figure 5, in the comparative study with the I/R group, the average infarct volume ratio in the I/R group was significantly higher than in the other groups (p <0.05). After intravenous administration of BA-LP, the average infarct volume ratio of MCAO rats was significantly reduced (p <0.05). The volume of cerebral infarction was significantly reduced in the BO-BA-LP group compared with the BA-LP group (p <0.05), and the effect was the most obvious. It further showed that the combination of BA and BO could effectively reduce cerebral I/R.
Figure 5.
The results of the TTC staining in each groups. (A) I/R group; (B) MCAO model with BA (25mg/kg); (C) MCAO model with BA-LP (25mg/kg); (D) MCAO model with BO-BA-LP (25mg/kg). The infarct area exhibited white, whereas the non-infarct areas were stained red.
Histopathological Examination
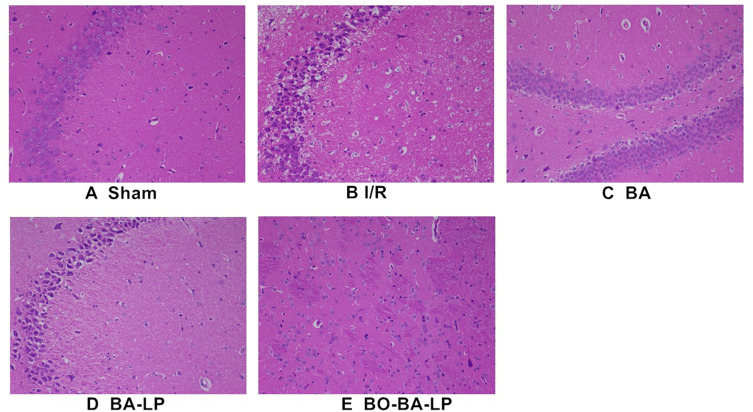
The HE staining results are shown in Figure 6. In the sham operation group, neurons, glial cells and capillaries were normal in shape and nerve fibers were densely arranged. However, in the I/R group, vascularization of the hippocampus neurons and looseness and swelling of the tissue were seen. In addition, the pictures of the I/R group also showed that the neurons were necrotic, the cell nuclei shrank, and some neurons disappeared, suggesting the MCAO operation caused obvious damage to the brain. Compared with the sham operation group, the BA group and the BA-LP group alleviated the degree of injury, but there was still a slight vacuole, and the BO-BA-LP group had a relatively normal tissue structure.
Figure 6.
The HE staining of brain tissue slices with the treatment of sham (A), I/R (B), BA group (25 mg/kg, (C), BA-LP group (25 mg/kg; (D) and BO-BA-LP (25 mg/kg; (E) under 200-fold light microscope.
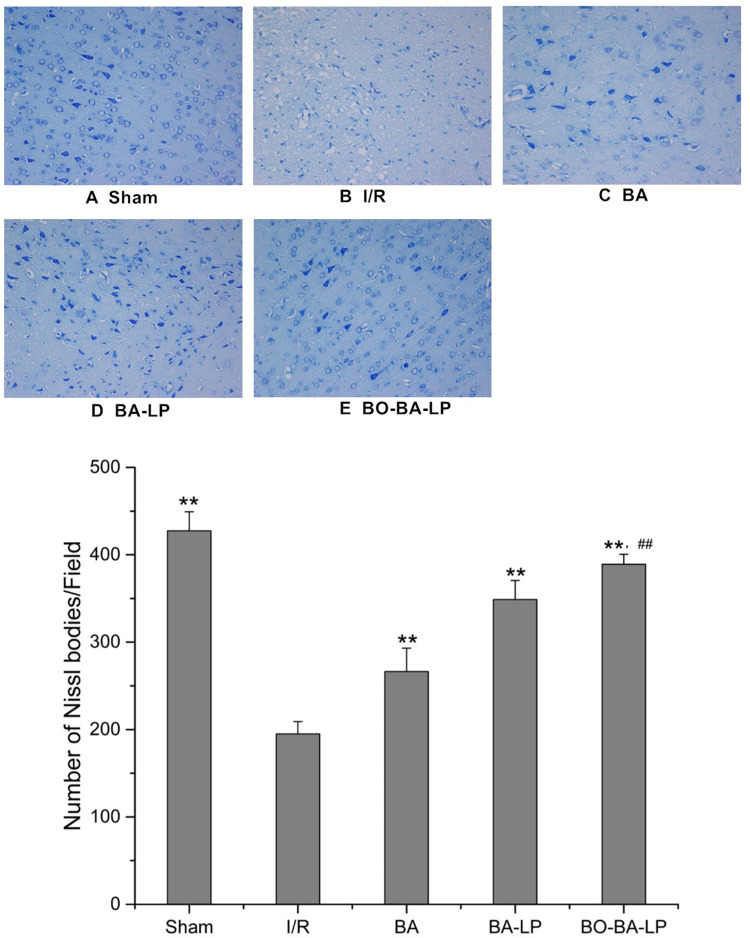
Next, Nissl staining was applied to examine the magnitude of neuronal injury. As shown in Figure 7, compared with the Sham group, the I/R group had more glial cells, disordered and necrotic neurons, and significantly reduced neurons. There was a slight disorder in the arrangement of neurons in the cortex of the BA group and BA-LP group, and the number of neurons increased compared with I/R (P<0.01). There were no obvious pathological changes in the BO-BA-LP group, and the number of neurons increased significantly compared with the BA-LP groups (P<0.01).
Figure 7.
The Nissl staining of brain tissue slices with the treatment of sham (A), I/R (B), BA group (25 mg/kg, (C), BA-LP group (25 mg/kg; (D) and BO-BA-LP group (25 mg/kg; (E) under 200-fold light microscope. ** Extremely significant different from the I/R group at P < 0.01, ## Extremely significant different from the BA-LP group at P < 0.01.
Discussion
Scutellaria baicalensis has a wide range of applications in traditional Chinese medicine and modern preparations. BA has better biological activity, but its solubility and permeability are poor. It belongs to the Class IV drug in the Biopharmaceutical Classification System (BCS). In vivo, the binding rate of BA to plasma proteins is between 86% and 92%, and the high plasma protein binding rate makes BA disappear quickly after absorption in the blood.24 New formulation can be used to extend the residence time of BA in the body, such as liposomes.25 Liposomes are spherical vesicles composed of a bilayer of phospholipids. Because their phospholipids are close to the components of cell membranes, they are extremely suitable for drug delivery.26 The liposome is divided into a hydrophilic shell and a hydrophobic inner core, which can encapsulate hydrophilic and hydrophobic drugs, and is widely used to improve the solubility and bioavailability of drugs.27
The BA-LP we prepared can effectively protect cerebral I/R, but it is difficult to achieve an effective concentration in the brain. BO has a molecular formula of C10H18O and a relative molecular mass of 154.25. There are many types of BO according to its source and composition. It is a small-molecule fat-soluble monoterpenoid compound that can lead drugs upward and has a certain brain targeting effect.28 The pharmacodynamic effect of BO is that it can enhance the activity of antioxidant enzymes in brain tissue and reduce inflammation by improving energy metabolism in ischemic brain regions, thereby reducing brain tissue damage.29,30 Based on this, it can be considered that BO can be combined with other central drugs to promote the latter’s brain concentration. In this paper, we prepared BO and BA into BO-BA-LP to investigate the function of the BO-BA-LP on MCAO model rats via i.v. administration.
Through various aspects of the preparation process of BO-BA-LP, we can see that there are many factors that affect the liposome encapsulation rate and drug loading. After a single-factor investigation, we found that the reverse evaporation method has a higher encapsulation rate than the liposome prepared by other methods. If the film formation is too thick in the film dispersion method, visible particles will be formed when the thin film is eluted, which affects the quality of the liposomes, which showed that the reverse phase evaporation method is suitable for the preparation of liposomes for water-soluble drugs. After response surface optimization design, we produced a BO-BA-LP with uniform particle size, high encapsulation rate and drug loading. The in vitro release test proves that the preparation of the drug into liposomes can enhance the stability of the drug and can also selectively release the drug. The release rate of BO-BA-LP is significantly reduced. It may be due to the dissolution of the dissolved drug in the center of the liposome to the dissolution medium, so that the drug can achieve the purpose of sustained release and continuous treatment, which is more conducive to the absorption and role of the drug in the brain injury site.
The pharmacokinetic profile of BA following the i.v. administration of BO-BA-LP was evaluated in healthy rats. Within 90min after the administration, the BA concentration in the BA monomer solution decreased and was quickly cleared from the bloodstream, while the liposomes prepared by the BO and BA co-prepared could resist the clearance (>480 min). Our previous research and other studies have consistently accepted manuscripts that showed that nanoencapsulation can improve the pharmacokinetics and biodistribution of BA.31 Our results clearly showed that compared with conventional BA, the co-encapsulated liposomes of BA and BO significantly increased the exposure of BA in the blood. Compared with BA monomers, including these, the plasma half-life was prolonged by t1/2 (p <0.01), and the AUC increased significantly high (p <0.01), MRT increased (p <0.01), thus improved the treatment effect of BA.
A large number of data proved that ischemic brain I/R is a multi-reaction and multi-link process.32,33 The pathological changes of brain injury after cerebral ischemic injury are mainly necrosis in the central area of ischemia and apoptosis in the penumbra area. Neuronal necrosis is an irreversible death process, and apoptosis is a reversible process, so neuron apoptosis plays an important role in brain I/R.34,35 Its morphological characteristics are: cell shrinkage, encapsulation concentration, and cell lysis. Microcirculation disorders can induce angioedema and hemorrhage, leading to damage to brain neurons and other pathological conditions. Dr Liu team found that in cerebral ischemia the area of infarction was significantly elevated.36 After treatment with BA-LP, it can improve neurological deficits, infarct size and pathological morphology of striatum and hippocampus of MCAO rats. The results showed that BA-LP can reduce ischemia-induced neuronal injury, which is consistent with previous studies. However, after adding BO, the treatment effect is more significant and it can effectively improve neuronal damage. This showed that the addition of BO can enhance the therapeutic effect of BA, which is related to the ability of BO to attract drugs, improve inflammation and reduce vascular endothelial damage. The combination of the two can play a synergistic role.30,37 At the same time, some studies have shown that BO can increase the permeability of the blood-brain barrier and increase the amount of drugs into the brain. Therefore, the improvement of curative effect may also be related to this, which needs further research to confirm.
Conclusion
In this paper, we have optimized the optimal preparation process of BO-BA-LP by response surface methodology. Pharmacological studies have shown that BO-BA-LP can improve circulation and extend the half-life of BA. The addition of BO can lead drugs upward and play a synergistic effect, the results of measuring the infarct area show that BO helps the BA to promote the recovery of the brain, and the results of histopathological studies show that BO-BA-LP compares with BA-LP can more effectively inhibit neuronal cell damage and apoptosis. The successful preparation and good curative effect of BO-BA-LP can provide new ideas for the development of other drugs for treating brain diseases and lay the foundation for further research.
Funding Statement
This research was fund by National Natural Science Foundation of China, NO: 81673615; the Sichuan Provincial Administration of Traditional Chinese Medicine Research Project, NO: 2018QN009; the Xinglin Scholar Discipline Promotion Talent Program of Chengdu University of Traditional Chinese Medicine, NO: QNXZ2018018; the Sichuan Human Resources and Social SecurityBureau, Grant Number: 00809503; and National training program for talents of traditional Chinese medicine characteristic technology inheritance, NO: T20194828003; the Key Laboratory of Modern Chinese Medicine Preparation of Ministry of Education of Jiangxi University of Traditional Chinese Medicine, NO: TCM-201908.
Abbreviations
Baicalin, BA; borneol, BO; Borneol-baicalin-liposomes, BO-BA-LP; ischemia-reperfusion (I/R); middle cerebral artery occlusion, MCAO; entrapment efficiency, EE; drug loading, DL; polydispersity index, PDI; ischemia-reperfusion injury, I/R; blood-brain barrier, BBB; Central nervous system, CNS; BA liposomes, BA-LP; traditional Chinese medicine, TCM; blood-brain barrier, BBB; central composite design, CCD; transmission electron microscope, TEM; Biopharmaceutical Classification System, BCS.
Disclosure
The authors declare no competing interests and no financial interests. The authors alone are responsible for the content and writing of this article.
References
- 1.Benjamin EJ, Virani SS, Callaway CW, et al. Heart disease and stroke statistics-2018 update a report from the American Heart Association. Circulation. 2018;137(12):143–152. doi: 10.1161/cir.0000000000000558 [DOI] [PubMed] [Google Scholar]
- 2.Wan L, Cheng YF, Luo ZY, et al. Neuroprotection, learning and memory improvement of a standardized extract from Renshen Shouwu against neuronal injury and vascular dementia in rats with brain ischemia. J Ethnopharmacol. 2015;165:118–126. doi: 10.1016/j.jep.2015.02.027 [DOI] [PubMed] [Google Scholar]
- 3.CK N, RT L, MD B. Inflammation and neuroprotection in traumatic brain injury. JAMA Neurol. 2015;72:3. doi: 10.1001/jamaneurol.2014.3558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dirnagl U, Lindauer U, Them A, et al. Global cerebral-ischemia in the rat – online monitoring of oxygen-free radical production using chemiluminescence in-vivo. J Cereb Blood Flow Metab. 1995;15(6):929–940. doi: 10.1038/jcbfm.1995.118 [DOI] [PubMed] [Google Scholar]
- 5.Cao YG, Mao XY, Sun CY, et al. BA attenuates global cerebral ischemia/reperfusion injury in gerbils via anti-oxidative and anti-apoptotic pathways. Brain Res Bull. 2011;85(6):396–402. doi: 10.1016/j.brainresbull.2011.05.002 [DOI] [PubMed] [Google Scholar]
- 6.Wang PQ, Liu Q, Xu WJ, et al. Pure mechanistic analysis of additive neuroprotective effects between BA and jasminoidin in ischemic stroke mice. Acta Pharmacol Sin. 2018;39(6):961–974. doi: 10.1038/aps.2017.145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dai J, Chen L, Qiu YM, et al. Activations of gabaergic signaling, HSP70 and MAPK cascades are involved in BA’s neuroprotection against gerbil global ischemia/reperfusion injury. Brain Res Bull. 2013;90:1–9. doi: 10.1016/j.brainresbull.2012.09.014 [DOI] [PubMed] [Google Scholar]
- 8.Grossi C, Guccione C, Isacchi B, et al. Development of blood-brain barrier permeable nanoparticles as potential carriers for salvianolic acid b to CNS. Planta Med. 2017;83(5):382–391. doi: 10.1055/s-0042-101945 [DOI] [PubMed] [Google Scholar]
- 9.Li JX, Zhang M, Chao JB, et al. Preparation and characterization of the inclusion complex of BA (bg) with beta-cd and hp-beta-cd in solution: an antioxidant ability study. Spectroc Acta Pt A-Molec Biomolec Spectr. 2009;73(4):752–756. doi: 10.1016/j.saa.2009.03.025 [DOI] [PubMed] [Google Scholar]
- 10.Li N, Ye YJ, Yang M, et al. Pharmacokinetics of BA-phospholipid complex in rat plasma and brain tissues after intranasal and intravenous administration. Pharmazie. 2011;66(5):374–377. doi: 10.1691/ph.2011.0783 [DOI] [PubMed] [Google Scholar]
- 11.Bhatia SP, Letizia CS, Api AM. Fragrance material review on borneol. Food Chem Toxicol. 2008;46(11):77–80. doi: 10.1016/j.fct.2008.06.031 [DOI] [PubMed] [Google Scholar]
- 12.Chen ZX, Xu QQ, Shan CS, et al. Borneol for regulating the permeability of the blood-brain barrier in experimental ischemic stroke: preclinical evidence and possible mechanism. Oxidative Med Cell Longev. 2019;2019:15. doi: 10.1155/2019/2936737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tambe R, Jain P, Patil S, et al. Antiepileptogenic effects of borneol in pentylenetetrazole-induced kindling in mice. Naunyn-Schmiedebergs Arch Pharmacol. 2016;389(5):467–475. doi: 10.1007/s00210-016-1220-z [DOI] [PubMed] [Google Scholar]
- 14.Yu B, Ruan M, Zhang ZN, et al. Synergic effect of borneol and ligustrazine on the neuroprotection in global cerebral ischemia/reperfusion injury: a region-specificity study. Evid-Based Complement Altern Med. 2016;8:1–8. doi: 10.1155/2016/4072809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cai Z, Hou SX, Li YB, et al. Effect of borneol on the distribution of gastrodin to the brain in mice via oral administration. J Drug Target. 2008;16(2):178–184. doi: 10.1080/10611860701794395 [DOI] [PubMed] [Google Scholar]
- 16.Tang SN, Wang AP, Yan XJ, et al. Brain-targeted intranasal delivery of dopamine with borneol and lactoferrin co-modified nanoparticles for treating parkinson’s disease. Drug Deliv. 2019;26(1):700–707. doi: 10.1002/bmc.655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu JY, Li YJ, Yang L, et al. Borneol and asarone as adjuvant agents for improving blood-brain barrier permeability of puerarin and tetramethylpyrazine by activating adenosine receptors. Drug Deliv. 2018;25(1):1858–1864. doi: 10.1080/10717544.2018.1516005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li M-H, Ruan L-Y, Chen C, et al. Protective effects of polygonum multiflorum on ischemic stroke rat model analysed by 1 H NMR metabolic profiling. J Pharm Biomed Anal. 2018;155:91–103. doi: 10.1016/j.jpba.2018.03.049 [DOI] [PubMed] [Google Scholar]
- 19.Longa EZ, Weinstein PR, Carlson S, et al. Reversible middle cerebral-artery occlusion without craniectomy in rats. Stroke. 1989;20(1):84–91. doi: 10.1161/01.Str.20.1.84 [DOI] [PubMed] [Google Scholar]
- 20.Li N, Feng LL, Tan YJ, et al. Preparation, characterization, pharmacokinetics and biodistribution of BA-loaded liposome on cerebral ischemia-reperfusion after i.v. administration in rats. Molecules. 2018;23(7):15. doi: 10.3390/molecules23071747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lv ZY, Yang YW, Wang J, et al. Optimization of the preparation conditions of borneol-modified ginkgolide liposomes by response surface methodology and study of their blood brain barrier permeability. Molecules. 2018;23(2):15. doi: 10.3390/molecules23020303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu Y, Wang SJ, Shang L, et al. Effect of borneol as a penetration enhancer on brain targeting of nanoliposomes: facilitate direct delivery to neurons. Nanomedicine. 2018;13(21):2709–2727. doi: 10.2217/nnm-2018-0282 [DOI] [PubMed] [Google Scholar]
- 23.Ahmad N, Al-Subaiec AM, Ahmad R, et al. Brain-targeted glycyrrhizic-acid-loaded surface decorated nanoparticles for treatment of cerebral ischaemia and its toxicity assessment. Artif Cell Nanomed Biotechnol. 2019;47(1):475–490. doi: 10.1080/21691401.2018.1561458 [DOI] [PubMed] [Google Scholar]
- 24.Tang YH, Zhang HY, Zhao YY, et al. Determination of human plasma protein binding of BA by ultrafiltration and high-performance liquid chromatography. Biomed Chromatography. 2006;20(10):1116–1119. doi: 10.1002/bmc.655 [DOI] [PubMed] [Google Scholar]
- 25.Huang T, Liu YA, Zhang CL. Pharmacokinetics and bioavailability enhancement of BA: a review. Eur J Drug Metabol Pharmacokinet. 2019;44(2):159–168. doi: 10.1007/s13318-018-0509-3 [DOI] [PubMed] [Google Scholar]
- 26.Mandpe P, Prabhakar B, Shende P. Role of liposomes-based stem cell for multimodal cancer therapy. Stem Cell Rev Rep. 2020;16(1):103–117. doi: 10.1007/s12015-019-09933-z [DOI] [PubMed] [Google Scholar]
- 27.Sharma A, Sharma US. Liposomes in drug delivery: progress and limitations. Int J Pharm. 1997;154(2):123–140. doi: 10.1016/s0378-5173(97)00135-x [DOI] [Google Scholar]
- 28.Almeida J, Souza GR, Silva JC, et al. Borneol, a bicyclic monoterpene alcohol, reduces nociceptive behavior and inflammatory response in mice. Sci World J. 2013:5. doi: 10.1155/2013/808460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bin Y, Ming R, Zhen-Nian Z, et al. Synergic effect of borneol and ligustrazine on the neuroprotection in global cerebral ischemia/reperfusion injury: a region-specificity study. Evid Based Complement Alternat Med. 2016;2016:1–8. doi: 10.1155/2016/4072809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kong Q, Wu Z, Chu X, et al. Study on the anti-cerebral ischemia effect of borneol and its mechanism. African J Traditional Complementary Alternative Med Ajtcam. 2013;11:1. doi: 10.4314/ajtcam.v11i1.25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang HY, Lai Q, Li Y, et al. Learning and memory improvement and neuroprotection of Gardenia jasminoides (Fructus gardenia) extract on ischemic brain injury rats. J Ethnopharmacol. 2017;196:225–235. doi: 10.1016/j.jep.2016.11.042 [DOI] [PubMed] [Google Scholar]
- 32.SM S, AP N, MD K, et al. Brain hypoxia secondary to diffusion limitation in hypoxic ischemic brain injury postcardiac arrest. Crit Care Med. 2020;48:3. doi: 10.1097/CCM.0000000000004138 [DOI] [PubMed] [Google Scholar]
- 33.Guan QH, Pei DS, Liu XM, et al. Neuroprotection against ischemic brain injury by sp600125 via suppressing the extrinsic and intrinsic pathways of apoptosis. Brain Res. 2006;1092:36–46. doi: 10.1016/j.brainres.2006.03.086 [DOI] [PubMed] [Google Scholar]
- 34.Zhi Z, Mengdi G, Ying L, et al. RNPS1 inhibition aggravates ischemic brain injury and promotes neuronal death. Biochem Biophys Res Commun. 2020;523(1):39–45. doi: 10.1016/j.bbrc.2019.11.185 [DOI] [PubMed] [Google Scholar]
- 35.Zeng CS, Wang DS, Chen C, et al. Zafirlukast protects blood-brain barrier integrity from ischemic brain injury. Chem-Biol Interact. 2020;316:6. doi: 10.1016/j.cbi.2019.108915 [DOI] [PubMed] [Google Scholar]
- 36.Lin RH, Lin YK, Tao J, et al. Electroacupuncture ameliorates learning and memory in rats with cerebral ischemia-reperfusion injury by inhibiting oxidative stress and promoting p-CREB expression in the hippocampus. Mol Med Rep. 2015;12(5):6807–6814. doi: 10.3892/mmr.2015.4321 [DOI] [PubMed] [Google Scholar]
- 37.Dong TW, Chen N, Ma X, et al. The protective roles of l-borneolum, d-borneolum and synthetic borneol in cerebral ischaemia via modulation of the neurovascular unit. Biomed Pharmacother. 2018;102:874–883. doi: 10.1016/j.biopha.2018.03.087 [DOI] [PubMed] [Google Scholar]