Abstract
BACKGROUND
Paroxysmal and permanent atrial fibrillation (AF) are common in heart failure with preserved ejection fraction (HFpEF).
OBJECTIVES
The study sought to determine the implications of left atrial (LA) myopathy and dysrhythmia across the spectrum of AF burden in HFpEF.
METHODS
Consecutive patients with HFpEF (n = 285) and control subjects (n = 146) underwent invasive exercise testing and echocardiographic assessment of cardiac structure, function, and pericardial restraint.
RESULTS
Patients with HFpEF were categorized into stages of AF progression: 181 (65%) had no history of AF, 49 (18%) had paroxysmal AF, and 48 (17%) had permanent AF. Patients with permanent AF stage were more congested with greater pulmonary vascular disease and lower cardiac output. LA volumes increased, while LA compliance, LA reservoir strain, and right ventricular function decreased with increasing AF burden. The presence of permanent AF was characterized by a distinct pathophysiology, with greater total heart volume caused by atrial dilatation, leading to elevated filling pressures through heightened pericardial restraint. Survival decreased with increasing AF burden. Ten-year progression to permanent AF was common, particularly in paroxysmal AF (52%), and the likelihood of AF progression increased with higher AF stage, poorer LA compliance, and lower LA strain.
CONCLUSIONS
LA compliance and mechanics progressively decline with increasing AF burden in HFpEF, increasing risk for new onset AF. These changes promote development of a unique phenotype of HFpEF characterized by heightened ventricular interaction, right heart failure, and worsening pulmonary vascular disease. Further study is required to identify therapeutic interventions targeting LA myopathy to improve outcomes in HFpEF.
Keywords: atrial fibrillation, exercise hemodynamics, HFpEF, left atrial strain, pericardial restraint
Atrial fibrillation (AF) is both common and associated with adverse outcomes in heart failure with preserved ejection fraction (HFpEF) (1–4). When AF becomes permanent, afflicted patients display severe atrial dysfunction and abnormal right ventricular (RV)-pulmonary vascular coupling as compared with patients with HFpEF in sinus rhythm (HFpEFno-AF) (5–16). These changes develop gradually, just as permanent AF typically evolves insidiously from an earlier stage in which rhythm disturbances are intermittent. When sustained chronically, increasing exposure to both the dysrhythmia itself and the metabolic and inflammatory stresses associated with AF may lead to a unique clinical AF phenotype in HFpEF that differs from other phenotypes.
The optimal approaches for treatment and prevention of AF in HFpEF are unknown (17). The adverse functional and hemodynamic consequences of permanent AF suggest that efforts to restore sinus rhythm might improve clinical status (18,19). However, there is also concern that patients with AF may display atrial remodeling that is less reversible, in which treatment efficacy may become limited, or potentially even detrimental, worsening LA hypertension to even greater extent (20).
To provide greater insight into the pathophysiologic progression across the spectrum of AF burden in HFpEF, we performed a detailed characterization combining direct measures of cardiac structure and function in control subjects free of AF and HFpEF patients with and without AF. We hypothesized that abnormalities in LA structure, function, pericardial restraint, and hemodynamics in HFpEF would progress along a phenotypic continuum related to the exposure to AF burden, and that increasing severity of LA myopathy would identify patients at higher risk for AF progression.
METHODS
GROUP DEFINITIONS.
Consecutive outpatient subjects undergoing elective invasive hemodynamic exercise testing at the Mayo Clinic catheterization laboratory for exertional dyspnea between 2000 and 2015 were identified with HFpEF defined by gold standard invasive criteria (Supplemental Appendix). Control subjects were identified as those with normal rest and exercise pulmonary capillary wedge pressure (PCWP), no history of AF, and left ventricular ejection fraction (LVEF) ≥50%. The Mayo Clinic Institutional Review Board approved the study protocol.
All electrocardiograms were manually reviewed to determine if AF was present at any time, and charts were reviewed in detail to establish any history of AF episodes prior to and following index evaluation. Patients in AF at the time of evaluation were classified as permanent AF (HFpEFperm-AF), those in sinus rhythm at the time of evaluation but with a history of documented AF were classified paroxysmal AF (HFpEFparox-AF), and patients with HFpEF but no prior AF were classified as no AF (HFpEFno-AF). Patients with persistent AF (duration <1 year) and permanent AF (>1 year) were both included in the permanent AF group for this analysis.
ASSESSMENT OF CARDIAC STRUCTURE AND FUNCTION.
Details of LV and RV assessment are provided in the Supplemental Appendix. LA volume-based indices were calculated as previously described (details in Supplemental Appendix) (6,21). LA strain was calculated as the average of strain in 6 segments in the 4-chamber and 2-chamber views to calculate reservoir, conduit, and contractile strain, taken as the average of 3 beats (Supplemental Figure 1) (8,9). Atrial strain was measured using the QRS interval as fiducial point because of the absence of p waves in AF. We have demonstrated high inter- and intra-observer reproducibility using these methods in our laboratory (9).
HEART VOLUMES, VENTRICULAR INTERACTION,AND PERICARDIAL RESTRAINT.
Total epicardial heart volume (atrial and ventricular volume) was estimated from 2 hemi-ellipsoids containing both atria and ventricles using the apical 4-chamber view. This allowed calculation of not only total epicardial volume, but also its individual components—total atrial volume and total ventricular volume (5). If total heart volume increases out of proportion to pericardial volume, there is increased pericardial restraint and ventricular interaction (22–24). As previously described, an increase in pericardial restraint is connoted by increases in right atrial pressure (RAP), RAP/PCWP ratio, eccentricity index, and ideal-to-actual radius on short-axis echocardiography.
CATHETERIZATION PROTOCOL.
Right heart catheterization was performed as previously described at rest and during exercise to volitional exhaustion (details in the Supplemental Appendix) (24–26). End-expiratory mean PCWP pressure, a-wave, v-wave, and x- and y-descents were manually measured at rest and peak exercise as the average of 3 measurements by a single observer. The rise in PCWP from the x nadir to the v-wave peak represents the pressure change during the LA reservoir phase (reservoir pressure) (Supplemental Figure 1), and difference from the y nadir to peak a-wave represents the rise in pressure with LA contraction (booster pressure). The fall in pressure from the v-wave peak to the y nadir represented the LA pressure change during the conduit phase.
LV transmural pressure (LVTMP), which reflects LV preload independent of pericardial restraint, was estimated as PCWP minus RAP. Details regarding calculation of the LV stiffness constant β (27) and pulmonary vascular load indices (26,28) are provided in the Supplemental Appendix.
INTEGRATED ASSESSMENT OF LA FUNCTION.
Because imaging does not account for pressure loading, LA strain and volumetric measures from echocardiography were combined with invasive pressure measurements to provide an integrated pressure-volume index. LA compliance was calculated from the quotient of LA deformation (strain) or LA volume change indexed to PCWP x-v pressure rise (Supplemental Figure 1).
OUTCOMES.
Patient follow-up was initiated on the day of cardiac catheterization. Vital status was determined from manual review of the electronic medical records, the Mayo Clinic registration database, and the Rochester Epidemiology Project death database, which uses data ascertained from other medical records, death certificates, obituaries, and notices of death in local newspapers. Data on all Minnesota deaths were obtained from the State of Minnesota annually. Progression of AF stage was ascertained by detailed review of all medical records following cardiac catheterization assessment, including all patient encounters at Mayo Clinic as well as other facilities as part of the “Care Everywhere” application in the Epic electronic health record platform (Epic Systems, Verona, Wisconsin). AF progression was defined as any worsening of AF grade: specifically development of permanent AF for patients with HFpEFparox-AF, or the development of any AF in control subjects and patients with HFpEFno-AF. Censoring was performed at last date of known follow-up for vital status and AF progression.
STATISTICAL ANALYSIS.
Data are reported as mean ± SD, median and interquartile range, or number (%). Between-group differences were compared by analysis of variance, Kruskal-Wallis test, or chi-square test, as appropriate. The Tukey honestly significant difference test or Steel-Dwass test was used to adjust for multiple testing between subgroups. Linear regression and Pearson’s correlation were used to assess associations between variables. For non-normally distributed variables entered into regression models, the assumption of normally distributed residuals was verified by quantile plots. Mortality and risk of progressive AF burden were compared using the log-rank test to account for censoring and varying time of follow-up. All tests were 2-sided, with a p value <0.05 considered significant. Given the mechanistic basis of this analyses, and to minimize risk of type II error, correction for multiple hypothesis testing was not performed. All analyses were performed by JMP 13.0.0 (SAS Institute, Cary, North Carolina).
RESULTS
The study cohort includes 146 control subjects with no history of AF and 285 HFpEF patients, of whom 181 (65%) were HFpEFno-AF, 49 (18%) were HFpEFparox-AF, and 48 (17%) were HFpEFperm-AF. As compared with other groups, AF patients were older, and patients with permanent AF displayed lower body mass index than others (Table 1). HFpEFperm-AF patients displayed greater congestion than other groups, evidenced by more radiographic cardiomegaly and pulmonary edema, higher N-terminal pro-B-type natriuretic peptide levels and E/e′ ratio, lower hemoglobin, and the greatest LA volumes (Tables 1 and 2).
TABLE 1.
Demographics and Clinical Characteristics
| Age, yrs | 56 ± 15* | 66 ± 11* | 71 ± 7 | 75 ± 6 | <0.0001 |
| Female | 60 | 62 | 61 | 60 | 0.9 |
| BMI, kg/m2 | 28.3 ± 5.6 | 33.8 ± 7.4†‡ | 32.9 ± 7.9† | 30.7 ± 5.7 | <0.0001 |
| Hemoglobin, g/dl | 12.9 ± 1.3* | 12.3 ± 1.5 | 12.2 ± 1.5 | 11.8 ± 1.6 | <0.0001 |
| Diabetes | 14 | 30 | 35 | 21 | <0.0001 |
| Hypertension | 84 | 94 | 100 | 98 | 0.0001 |
| COPD | 8 | 12 | 12 | 10 | 0.7 |
| Laboratories and chest radiography | |||||
| NT-proBNP, pg/ml | 69 (31–127)* | 195 (68–557)* | 613 (296–1,061)* | 1,859 (969–3,051)* | <0.0001 |
| Creatinine, mg/dl | 0.9 ± 0.2* | 1.1 ± 0.4 | 1.2 ± 0.4 | 1.2 ± 0.4 | <0.0001 |
| eGFR, ml/min/1.73 m2 | 73 ± 18* | 61 ± 17 | 58 ± 20 | 55 ± 20 | <0.0001 |
| Cardiomegaly | 4 | 16 | 31 | 62 | <0.0001 |
| Lung congestion | 0 | 3 | 4 | 19 | <0.0001 |
| Medication | |||||
| Beta-blocker | 29 | 51 | 47 | 75 | <0.0001 |
| ACE inhibitor or ARB | 24 | 41 | 39 | 58 | <0.0001 |
| Diuretic | 23 | 43 | 55 | 65 | <0.0001 |
| MRA | 6 | 10 | 16 | 27 | 0.0008 |
| Digoxin | 2 | 2 | 23 | <0.0001 | |
| Anticoagulation | 5 | 7 | 33 | 75 | <0.0001 |
Values are mean ± SD, %, or median (interquartile range). Correction for multiple hypothesis testing was not performed.
p < 0.05 vs. all by Tukey’s test.
p < 0.05 vs. control subjects by Tukey’s test.
p < 0.05 vs. HFpEFperm-AF by Tukey’s test.
ACE = angiotensin-converting enzyme; ARB, angiotensin receptor blocker; BMI = body mass index; COPD = chronic obstructive pulmonary disease; eGFR = estimated glomerular filtration rate; HFpEFno-AF = heart failure with preserved ejection fraction in sinus rhythm; HFpEFparox-AF = heart failure with preserved ejection fraction with paroxysmal atrial fibrillation; HFpEFperm-AF = heart failure with preserved ejection fraction in permanent atrial fibrillation; MRA = mineralocorticoid receptor antagonist; NT-proBNP = N-terminal pro-B-type natriuretic peptide.
TABLE 2.
Cardiac Structure and Function
| LV structure and function | |||||
| LA volume index, ml/m2 | 28 ± 8* | 33 ± 9* | 41 ±12* | 56 ± 15* | <0.0001 |
| LVEDD, mm | 48 ± 5 | 48 ± 5 | 49 ± 6 | 49 ± 6 | 0.2 |
| EF, % | 63 ± 5 | 64 ± 6 | 62 ± 7 | 61 ± 6† | 0.005 |
| LV mass index, g/m2 | 84 ± 19* | 90 ± 21 | 98 ± 26 | 97 ± 27 | <0.0001 |
| LV GLS, % | 16 ± 3 | 16 ± 3 | 15 ± 3‡ | 14 ± 4†‡ | <0.0001 |
| LV stiffness b, mm Hg/ml | 0.42 ± 0.11 | 0.47 ± 0.15‡ | 0.46 ± 0.14 | 0.47 ± 0.12‡ | 0.009 |
| E/e′ | 9 ± 4* | 13 ± 7 | 14 ± 6 | 16 ± 6‡§ | <0.0001 |
| Septal a′, cm/s | 10 ± 2 | 9 ± 3 | 7 ± 3* | – | <0.0001 |
| RV structure and function | |||||
| RV basal diameter | 31 ± 6 | 32 ± 6 | 34 ± 6 †‡ | 35 ± 8 †‡ | <0.0001 |
| RV mid diameter | 24 ± 5 | 25 ± 6 | 26 ± 6 | 27 ± 7‡ | 0.02 |
| RV dilation∥ | 11 | 22 | 30 | 66 | <0.0001 |
| RV diastolic area, cm2 | 13.1 ± 4.1 | 13.7 ± 4.0 | 14.8 ± 3.9 | 15.0 ± 5.3 | 0.04 |
| RV systolic area, cm2 | 6.3 ± 2.3 | 6.8 ± 2.7 | 7.6 ± 3.0 | 8.9 ± 4.5†‡ | <0.0001 |
| RV FAC, % | 53 ± 6 | 51 ± 8 | 49 ± 9 | 43 ± 11* | <0.0001 |
| TAPSE, mm | 23 ± 6 | 23 ± 6 | 19 ± 7 | 17 ± 4†‡ | 0.0001 |
| TV s′, cm/s | 14 ± 3 | 13 ± 3 | 13 ± 3 | 11 ± 2†‡ | 0.0002 |
| Ventriculoatrial remodeling and interdependence | |||||
| Total heart volume, ml | 664 ± 216* | 857 ± 251 | 901 ± 235 | 1212 ± 400* | <0.0001 |
| Biatrial volume, ml | 158 ± 72* | 255 ±128 | 311 ± 125 | 658 ± 291* | <0.0001 |
| Biventricular volume, ml | 506 ±198 | 602 ±190‡ | 590 ± 223 | 555 ±196 | 0.02 |
| Biatrial volume/total heart volume, % | 25 ± 10* | 30 ± 10* | 35 ± 13* | 53 ± 11* | <0.0001 |
| Eccentricity index diastole | 0.97 ± 0.09 | 1.02 ± 0.12# | 1.00 ± 0.13 | 1.03 ± 0.13‡ | 0.004 |
| Eccentricity index systole | 0.97 ± 0.11 | 1.03 ± 0.12# | 1.00 ± 0.09 | 1.08 ± 0.23‡§ | <0.0001 |
| Ideal radius index diastole | 1.04 ± 0.24 | 1.16 ± 0.29‡ | 1.11 ± 0.22 | 1.18 ± 0.31‡ | 0.009 |
| Ideal radius index systole | 1.06 ± 0.28 | 1.14 ± 0.31 | 1.08 ± 0.22 | 1.22 ± 0.54‡ | 0.05 |
Values are mean ± SD or %. Correction for multiple hypothesis testing was not performed.
p < 0.05 vs. all by Tukey’s test.
p < 0.05 vs. HFpEFno-AF by Tukey’s test.
p < 0.05 vs. control subjects by Tukey’s test.
p < 0.05 vs. HFpEFparox-AF by Tukey’s test.
Based on qualitative assessment.
a′ = •••; E/e′ = •••; FAC = fractional area change; GLS = global longitudinal strain; LA = left atrial; LV = left ventricular; LVEDD = left ventricular end-diastolic dimension; RV = right ventricular; TAPSE = tricuspid annular plane systolic excursion; s′ = •••; TV = tricuspid valve; other abbreviations as in Table 1.
BIVENTRICULAR STRUCTURE AND FUNCTION.
Compared with control subjects, HFpEF patients displayed greater LV mass and worse LV diastolic dysfunction, with higher estimated LV diastolic stiffness (β) (Table 2). However, within the HFpEF groups, there were no differences in LV chamber stiffness or mass when stratifying by AF (Table 2). In contrast, systolic function in both ventricles decreased in a graded fashion with increasing AF burden (evidenced by lower LVEF and global longitudinal strain, increased RV size, and lower tricuspid annular plane systolic excursion), RV fractional area change, and RV s′ velocities with increasing AF status (Table 2).
HEART VOLUMES AND PERICARDIAL RESTRAINT.
HFpEFperm-AF patients displayed the largest total heart volume of all patient groups, both qualitatively by chest roentgenogram and quantitatively by echocardiography (Table 2, Figure 1). Cardiomegaly in HFpEFperm-AF was exclusively caused by atrial dilation: biventricular volumes were equivalent, but there were 2-fold greater atrial volumes in HFpEFperm-AF than the other HFpEF groups, and 4-fold greater atrial volumes than in control subjects (Figure 1).
FIGURE 1. Pericardial and Atrial Size Interaction Across the Spectrum of AF Risk in HFpEF.
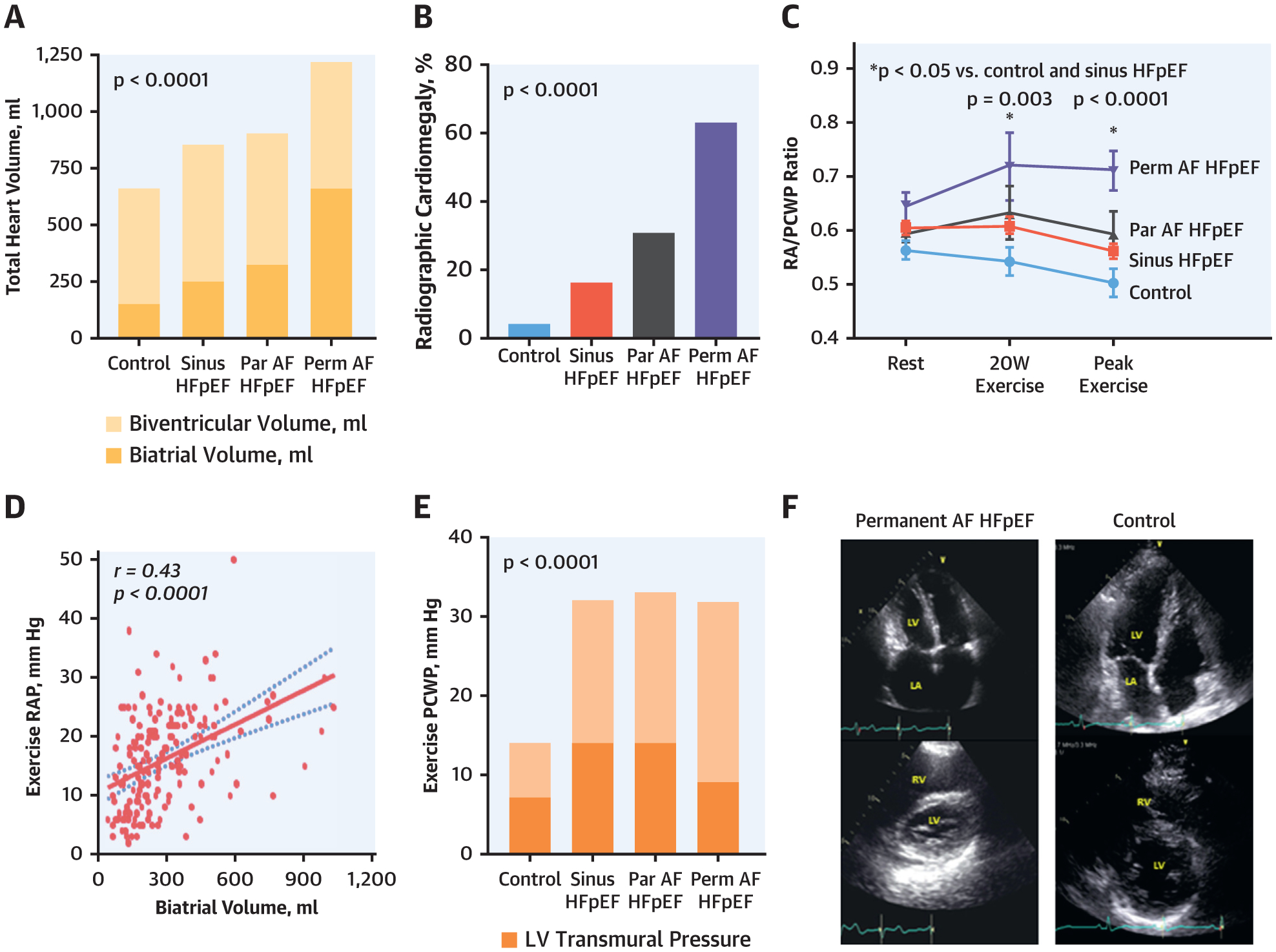
(A, B) Increasing total heart volume by both echocardiography and chest radiography was driven by atrial enlargement with increasing atrial fibrillation (AF) burden in heart failure with preserved ejection fraction (HFpEF). (C to F) Patients with greater AF burden displayed increased pericardial restraint with a higher exertional right atrial pressure (RAP), RAP/pulmonary capillary wedge pressure (PCWP) ratio, and lower left ventricular (LV) transmural pressure and enhanced diastolic ventricular interaction in the permanent AF HFpEF group. Par = paroxysmal; Perm = permanent.
The increases in heart volume in HFpEFperm-AF were coupled with findings indicating greater ventricular interdependence and pericardial restraint, as demonstrated by higher eccentricity index and ideal-to-actual radius index, and higher RA pressure and RA/PCWP ratios (Table 2, Figure 1). Notably, heart volumes were not significantly different comparing HFpEFno-AF patients with HFpEFparox-AF patients, indicating that this change was more specific to HFpEFperm-AF patients.
INVASIVE HEMODYNAMICS.
HFpEFperm-AF patients displayed the highest biventricular filling pressures at rest, with lowest CO and stroke volume (Figure 2, Table 3). The pressures at the v-wave peak and y nadir were both highest in HFpEFperm-AF patients, consistent with LA reservoir dysfunction and reduced operating LA compliance. There were no differences in systemic arterial load at rest, but patients with HFpEFperm-AF displayed more severe pulmonary vascular disease, with higher pulmonary vascular resistance, lower pulmonary arterial (PA) compliance, higher PA elastance, and the most severe pulmonary hypertension (Table 3, Figure 2, Supplemental Table 1).
FIGURE 2. Left Heart Filling Pressures, Pulmonary Vascular Load, and Cardiac Output Reserve Across AF Grades in HFpEF.
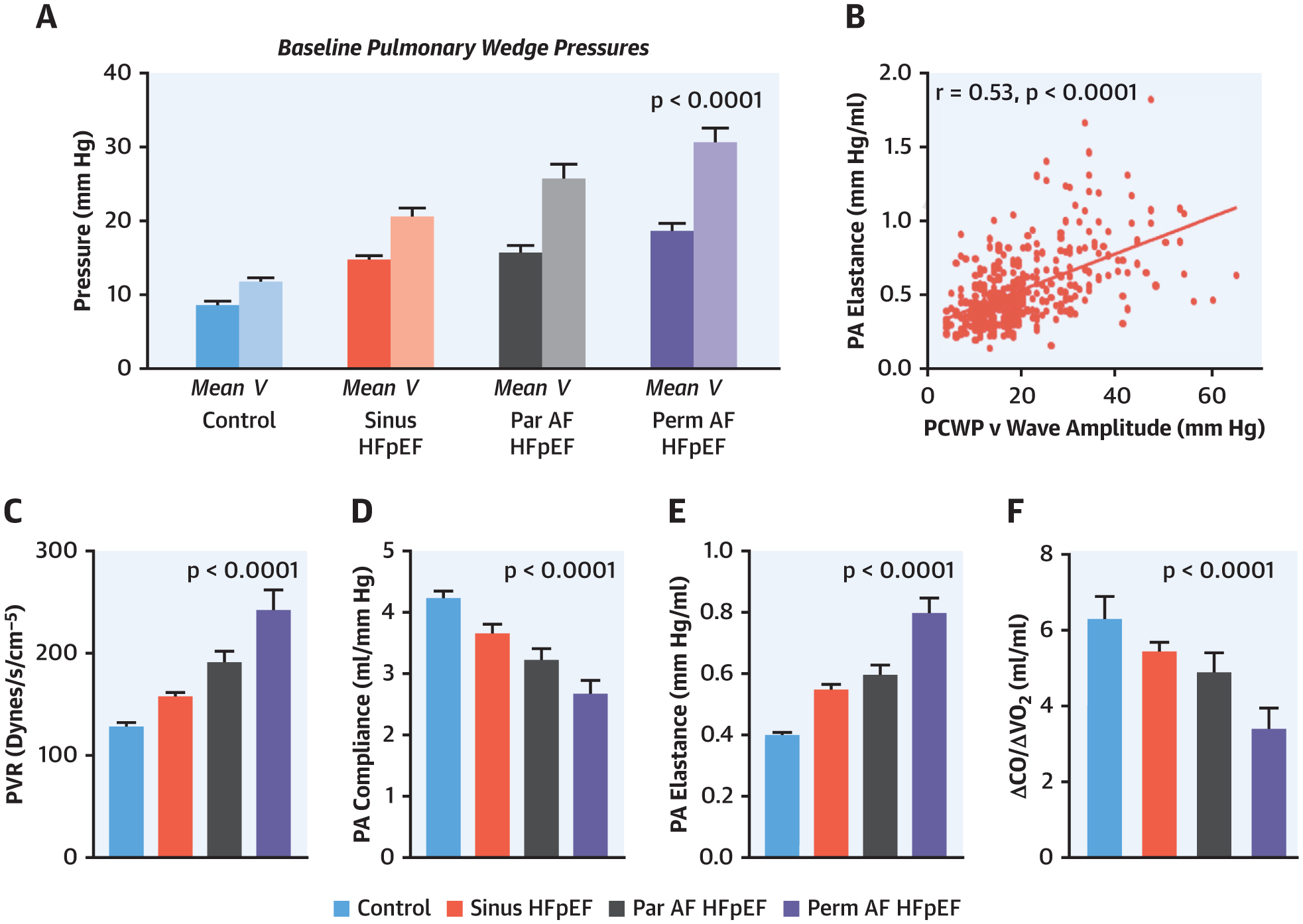
Baseline (resting) PCWP was highest in the permanent AF HFpEF group, and the corresponding PCWP v-wave height (A) increased progressively across groups and (B) was associated with worse pulmonary arterial (PA) elastance. (C to E) There was progressive worsening of pulmonary vascular load measured at rest with increasing AF stages along with (F) decreased cardiac output (CO) reserve during exercise. PVR = pulmonary vascular resistance; Vo2 = oxygen consumption; other abbreviations as in Figure 1.
TABLE 3.
Resting Hemodynamics
| Pressure data, mm Hg | |||||
| PCWP, mean | 9 ± 3* | 15 ± 5 | 16 ± 6 | 19 ± 6 †‡ | <0.0001 |
| Peak a-wave | 12 ± 4* | 19 ± 6 | 20 ± 6 | – | <0.0001 |
| Nadir x-descent | 8 ± 3* | 14 ± 5* | 16 ± 6* | 18 ± 5* | <0.0001 |
| Peak v-wave | 12 ± 5* | 21 ± 10* | 26 ± 12* | 31 ± 11* | <0.0001 |
| Nadir y-descent | 8 ± 3* | 14 ± 5* | 16 ± 6 | 18 ± 5†‡ | <0.0001 |
| RA mean | 5 ± 2* | 9 ± 4 | 10 ± 4 | 12 ± 5* | <0.0001 |
| PA mean | 17 ± 4* | 25 ± 7* | 28 ± 10 | 31 ± 9 | <0.0001 |
| PA systolic | 28 ± 7* | 38 ± 11§∥ | 43 ± 15 | 47 ± 14 | <0.0001 |
| LVTMP | 4 ± 2* | 6 ± 4 | 7 ± 4 | 7 ± 4 | <0.0001 |
| RA/PCWP | 0.57 ± 0.21 | 0.61 ± 0.20 | 0.59 ± 0.15 | 0.64 ± 0.17 | 0.08 |
| PCWP/LVEDV | 0.08 ± 0.04* | 0.14 ± 0.06 | 0.15 ± 0.06 | 0.17 ± 0.07†‡ | <0.0001 |
| LVTMP/LVEDV | 0.04 ± 0.03* | 0.06 ± 0.04 | 0.07 ± 0.05 | 0.06 ± 0.04 | <0.0001 |
| o2 transport | |||||
| Vo2, ml/min | 217 ± 56 | 229 ± 62 | 220 ± 55 | 224 ± 53 | 0.4 |
| Vo2, ml/min/kg | 2.7 ± 0.6‡§ | 2.5 ± 0.6 | 2.4 ± 0.6 | 2.6 ± 0.5 | 0.002 |
| CO, l/min | 5.5 ± 1.6 | 5.4 ± 1.5 | 4.9 ± 1.2 | 4.6 ± 1.2†‡ | 0.0007 |
| HR, beats/min | 73 ± 13 | 70 ± 11 | 67 ± 11# | 71 ± 9 | 0.003 |
| CI, l/min/m2 | 2.86 ± 0.78§∥ | 2.68 ± 0.66 | 2.43 ± 0.53 | 2.30 ± 0.58†‡ | <0.0001 |
| SV, ml | 76 ± 23 | 78 ± 25 | 76 ± 22 | 65 ± 18†‡ | 0.005 |
| SVI, ml/m2 | 39 ± 10 | 39 ± 11 | 37 ± 10 | 33 ± 9†‡ | 0.0008 |
| Afterload | |||||
| PVR, dynes/s/cm−5 | 128 ± 63* | 157 ± 82 | 191 ± 83 | 241 ± 135* | <0.0001 |
| TPG, mm Hg | 8 ± 3* | 10 ± 5 | 11 ± 5 | 13 ± 6†‡ | <0.0001 |
| PAC, ml/mm Hg | 4.2 ± 1.6* | 3.7 ± 1.9 | 3.2 ± 1.1 | 2.7 ± 1.5†‡ | <0.0001 |
| PA Ea, mm Hg/ml | 0.4 ± 0.1* | 0.5 ± 0.2* | 0.6 ± 0.2§∥ | 0.8 ± 0.3* | <0.0001 |
Values are mean ± SD. Correction for multiple hypothesis testing was not performed.
p < 0.05 vs. all by Tukey’s test.
p < 0.05 vs. control subjects by Tukey’s test.
p < 0.05 vs. sinus HFpEF by Tukey’s test.
p < 0.05 vs. HFpEFparox-AF by Tukey’s test.
p < 0.05 vs. HFpEFperm-AF by Tukey’s test.
CI = •••; CO = •••; Ea = elastance; HR = heart rate; LVEDV = left ventricular end-diastolic volume; LVTMP = left ventricular transmural pressure; PA = pulmonary artery; PAC = pulmonary arterial compliance; PCWP = pulmonary capillary wedge pressure; PVR = pulmonary vascular resistance; RA = right atrial; SV = stroke volume; SVI = stroke volume index; TPG = transpulmonary gradient; Vo2 = oxygen consumption; other abbreviations as in Table 1.
During exercise, patients with HFpEF displayed lower peak oxygen consumption compared with control subjects (Table 4). Exercise CO reserve was lowest in HFpEFperm-AF patients, with a blunted increase in CO relative to oxygen consumption (Figure 2). Similar to resting values, pulmonary vascular resistance and elastance were highest and PA compliance the lowest during exercise in HFpEFperm-AF patients (Table 4).
TABLE 4.
Exercise Hemodynamics
| Pressure data, mm Hg | |||||
| PCWP mean | 14 ± 5* | 32 ± 6 | 32 ± 6 | 32 ± 5 | <0.0001 |
| Peak a-wave | 18 ± 5* | 36 ± 9 | 35 ± 8 | – | <0.0001 |
| Nadir x-descent | 12 ± 5* | 28 ± 8 | 27 ± 6 | 29 ± 6 | <0.0001 |
| Peak v-wave | 21 ± 8* | 47 ± 13 | 51 ± 14 | 52 ± 13 | <0.0001 |
| Nadir y-descent | 13 ± 5* | 29 ± 8 | 27 ± 7 | 28 ± 5 | <0.0001 |
| RA mean | 7 ± 4* | 18 ± 7 | 19 ± 7 | 23 ± 9† | <0.0001 |
| PA mean | 26 ± 8* | 45 ± 11 | 47 ± 11 | 48 ± 9 | <0.0001 |
| PA systolic | 42 ± 13* | 63 ± 16 | 67 ± 17 | 69 ± 12 | <0.0001 |
| RA/PCWP | 0.50 ± 0.25 | 0.56 ± 0.17 | 0.59 ± 0.22 | 0.71 ± 0.22†‡ | <0.0001 |
| LVTMP | 7 ± 4†§ | 14 ± 6‡∥ | 14 ± 8 | 9 ± 7†§ | <0.0001 |
| o2 transport | |||||
| Vo2, ml/min | 933 ± 323* | 819 ± 266 | 756 ± 233 | 745 ± 252 | 0.0006 |
| Vo2, ml/min/kg | 11.7 ± 4.2* | 9.0 ± 3.1 | 8.3 ± 2.2 | 8.6 ± 3.1 | <0.0001 |
| AVo2diff, ml/dl | 9.2 ± 2.0 | 9.3 ± 2.1 | 10.0 ± 2.2 | 11.1 ± 2.0†‡ | <0.0001 |
| HR, beats/min | 117 ± 25* | 101 ±17‡§ | 92 ± 15 | 94 ± 21 | <0.0001 |
| CO, l/min | 10.3 ± 3.8* | 9.2 ± 2.9* | 7.7 ± 2.7 | 6.5 ± 2.4 | <0.0001 |
| ΔCO/ΔVo2, ml/ml | 6.8 (5.0–7.9)* | 5.9 (4.2–6.8) | 4.7 (2.9–7.4) | 4.4 (2.0–5.1)†‡ | <0.0001 |
| CO, % predicted | 113 (83–131)* | 99 (69–114) | 79 (49–123) | 73 (33–85)†‡ | <0.0001 |
| SV, ml | 89 ± 32 | 91 ± 29 | 86 ± 33 | 71 ± 28†‡ | 0.002 |
| SVI, ml/m2 | 46 ± 14 | 45 ± 12 | 42 ± 13 | 36 ± 14†‡ | 0.0002 |
| CI, l/min*m2 | 5.4 ± 1.9* | 4.6 ± 1.3* | 3.8 ± 1.1 | 3.2 ± 1.1 | <0.0001 |
| Afterload | |||||
| PVR, dynes/s/cm−5 | 105 ± 74 | 134 ± 104 | 182 ± 119#* | 232 ± 132#* | <0.0001 |
| PAC, ml/mm Hg | 3.6 ± 2.1* | 2.6 ± 1.2 | 2.2 ± 1.1 | 1.8 ± 0.8†‡ | <0.0001 |
| PA Ea, mm Hg/ml | 0.5 ± 0.2* | 0.8 ± 0.4* | 0.9 ± 0.4‡∥ | 1.1 ± 0.6* | <0.0001 |
Values are mean ± SD or median (interquartile range). Correction for multiple hypothesis testing was not performed.
p < 0.05 vs. all by Tukey’s test.
p < 0.05 vs. sinus HFpEF by Tukey’s test.
p < 0.05 vs. control subjects by Tukey’s test.
p < 0.05 vs. HFpEFparox-AF by Tukey’s test.
p < 0.05 vs. HFpEFperm-AF by Tukey’s test.
PCWP increased markedly during exercise in all HFpEF groups, reaching similar peak values (Table 4), but the mechanisms by which this occurred differed. In HFpEFno-AF and HFpEFparox-AF patients, LVTMP increased by over 200% compared with baseline, in keeping with a marked increase in LV preload (Figure 1, Table 4) in the setting of a stiff ventricle. However, in the HFpEFperm-AF group, LVTMP failed to increase, meaning that the increase in PCWP during exercise in HFpEFperm-AF patients was exclusively related to increases in pericardial restraint, estimated by the greater elevation in RA pressure. Consistent with this, the ratio of RAP/PCWP increased more dramatically with exercise in the HFpEFperm-AF group as compared with all others. The increase in RA pressure with exercise was directly correlated with biatrial volume measured at rest (Figure 1).
INTEGRATED ASSESSMENT OF LA FUNCTION USING PRESSURE, VOLUME, AND STRAIN.
LA maximum, pre A, and minimum LA volume showed a progressive increase with increasing AF severity (Table 5). Abso-lute LA filling volume (reservoir volume) was not significantly different in the 4 groups, but because HFpEFperm-AF patients displayed the largest mini-mum LA volume (starting volume), they displayed the lowest LA expansion index. The increase in PCWP pressure during LA reservoir filling (x-v wave height) was highest in the HFpEFperm-AF group (Table 5). LA reservoir strain progressively worsened across all groups, with the lowest values in HFpEFperm-AF patients. Together with the higher x-v wave pressure height, this led to a progressive reduction in LA compliance with increasing AF stage (Figure 3). The height of the v-wave correlated with abnormal PA vascular load both at rest and with exercise, but the relationship was stronger between measures reflecting pulsatile load and LA pressure (PA elastance) (r = 0.53, p < 0.0001) (Figure 3) compared with non-pulsatile load (pulmonary vascular resistance) (r = 0.26, p < 0.0001).
TABLE 5.
LA Structure and Function
| LA reservoir function | |||||
| Volume/strain | |||||
| LA maximal volume, ml | 44 ± 16* | 52 ± 19* | 71 ± 30* | 97 ± 41* | <0.0001 |
| LA minimum volume, ml | 18 ± 9* | 24 ± 15* | 44 ± 24* | 74 ± 33* | <0.0001 |
| LA reservoir volume, ml | 27 ± 9 | 28 ± 10 | 28 ± 15 | 23 ± 18 | 0.06 |
| LA expansion index, % | 163 (122–209) | 147 (89–200)†‡ | 72 (39–104)* | 34 (15–54)* | <0.0001 |
| LA total EF, % | 62 ± 11* | 57 ± 15* | 40 ± 16* | 24 ± 14* | <0.0001 |
| LA reservoir strain, % | 40 ± 13* | 35 ± 15* | 23 ± 11* | 12 ± 6* | <0.0001 |
| Pressure, mm Hg | |||||
| LA x-v height rest | 5 ± 3* | 8 ± 6* | 10 ± 7 | 13 ± 7§∥ | <0.0001 |
| LA x-v height exercise | 9 ± 5* | 19 ± 10†§ | 24 ± 12 | 22 ± 11 | <0.0001 |
| Pressure-volume relation | |||||
| LA volume compliance, ml/mm Hg | 5.5 (3.9–9.5)* | 4.3 (2.4– 8.5)* | 2.2 (1.4–4.2) | 1.7 (0.9–3.5)§∥ | <0.0001 |
| LA strain compliance, %/mm Hg | 9.3 (5.7–13.7)* | 5.7 (2.8–9.6)* | 1.7 (1.1–4.0)* | 1.2 (0.7–1.4)* | <0.0001 |
| LA conduit function | |||||
| Volume/strain | |||||
| LA pre A volume, ml | 30 ± 13* | 37 ± 16* | 56 ± 25* | – | <0.0001 |
| LA passive volume, ml | 14 ± 7 | 15 ± 8 | 16 ± 12 | – | 0.5 |
| LA passive EF, % | 33 ± 13 | 30 ± 14 | 22 ± 13* | – | <0.0001 |
| LA conduit strain, % | 22 ± 10* | 19 ± 11 | 14 ± 6 | – | <0.0001 |
| Pressure, mm Hg | |||||
| LA v-y height rest | 4 ± 3* | 7 ± 7* | 10 ± 7* | 14 ± 8 * | <0.0001 |
| LA v-y height exercise | 8 ± 5* | 18 ± 10* | 24 ± 12 | 23 ± 11§∥ | <0.0001 |
| LA booster function | |||||
| Volume/strain | |||||
| LA booster volume, ml | 13 ± 6 | 14 ± 7 | 13 ± 10 | – | 0.5 |
| LA active EF, % | 42 ± 11 | 39 ± 14 | 25 ± 17* | – | <0.0001 |
| LA booster strain, % | 17 ± 8 | 17 ± 9 | 10 ± 6* | – | <0.0001 |
| Pressure, mm Hg | |||||
| LA y-a height rest | 3.2 ± 1.9 | 5.0 ± 2.6§ | 4.3 ± 3.0§ | – | <0.0001 |
| LA y-a height exercise | 5.7 ± 3.8 | 7.6 ± 4.8§ | 8.0 ± 6.6§ | – | 0.0007 |
Values are mean ± SD or median (interquartile range). Correction for multiple hypothesis testing was not performed.
p < 0.05 vs. all by Tukey’s test.
p < 0.05 vs. HFpEFparox-AF by Tukey’s test.
p < 0.05 vs. HFpEFperm-AF by Tukey’s test.
p < 0.05 vs. control subjects by Tukey’s test.
p < 0.05 vs. sinus HFpEF by Tukey’s test.
FIGURE 3. LA Reservoir Dysfunction With Increasing AF Burden in HFpEF.
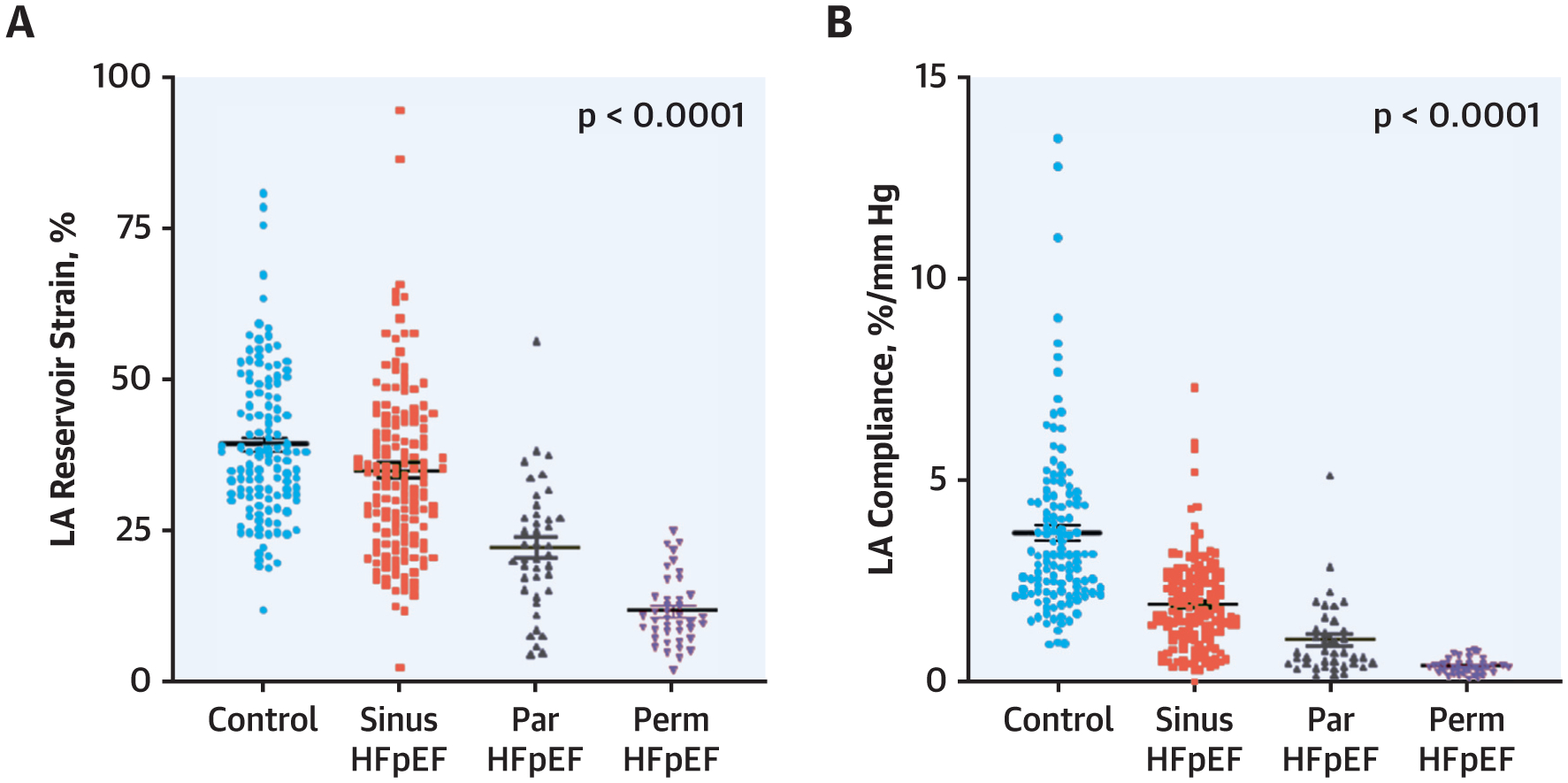
Progressive impairment in (A) LA reservoir strain and (B) LA compliance with worsening AF stage. Abbreviations as in Figure 1.
Similar to LA reservoir strain, LA conduit strain was impaired in HFpEF, with lower passive EF in the HFpEFparox-AF as a result of a higher maximum LA volume prior to mitral valve opening (Table 5). LA booster function was impaired in HFpEFparox-AF patients, as evidenced by a smaller LA active EF and LA booster strain compared with control subjects and HFpEFno-AF patients.
OUTCOMES.
Ten-year survival was lower in HFpEFperm-AF patients (38%) than in HFpEFparox-AF patients (62%), HFpEFno-AF patients (73%), and control subjects (94%) (p < 0.001 by log-rank test; hazard ratio [HR]: 1.95; 95% confidence interval [CI]: 1.56 to 2.45 per increase in AF stage). Ten-year progression from paroxysmal to permanent AF was common in HFpEFparox-AF patients (52%), and a higher AF stage was associated with an increased risk for progression to greater AF stages over 10 years (31% in HFpEFno-AF patients vs. 1% sinus control subjects) (p < 0.001 by log-rank test; HR: 4.95; 95% CI: 3.07 to 8.20 per increasing AF stage). Progression of AF stage was also associated with impaired LA reservoir strain (HR: 6.8; 95% CI: 3.3 to 14.1; p < 0.0001), poorer LA compliance (HR: 6.0; 95% CI: 2.9 to 12.7; p < 0.0001), and greater elevation in PCWP at baseline (HR: 5.3; 95% CI: 2.8 to 10.3; p < 0.0001) (Figure 4, Supplemental Figure 2).
FIGURE 4. Risk of Progression of AF Stratified by Baseline AF Burden.
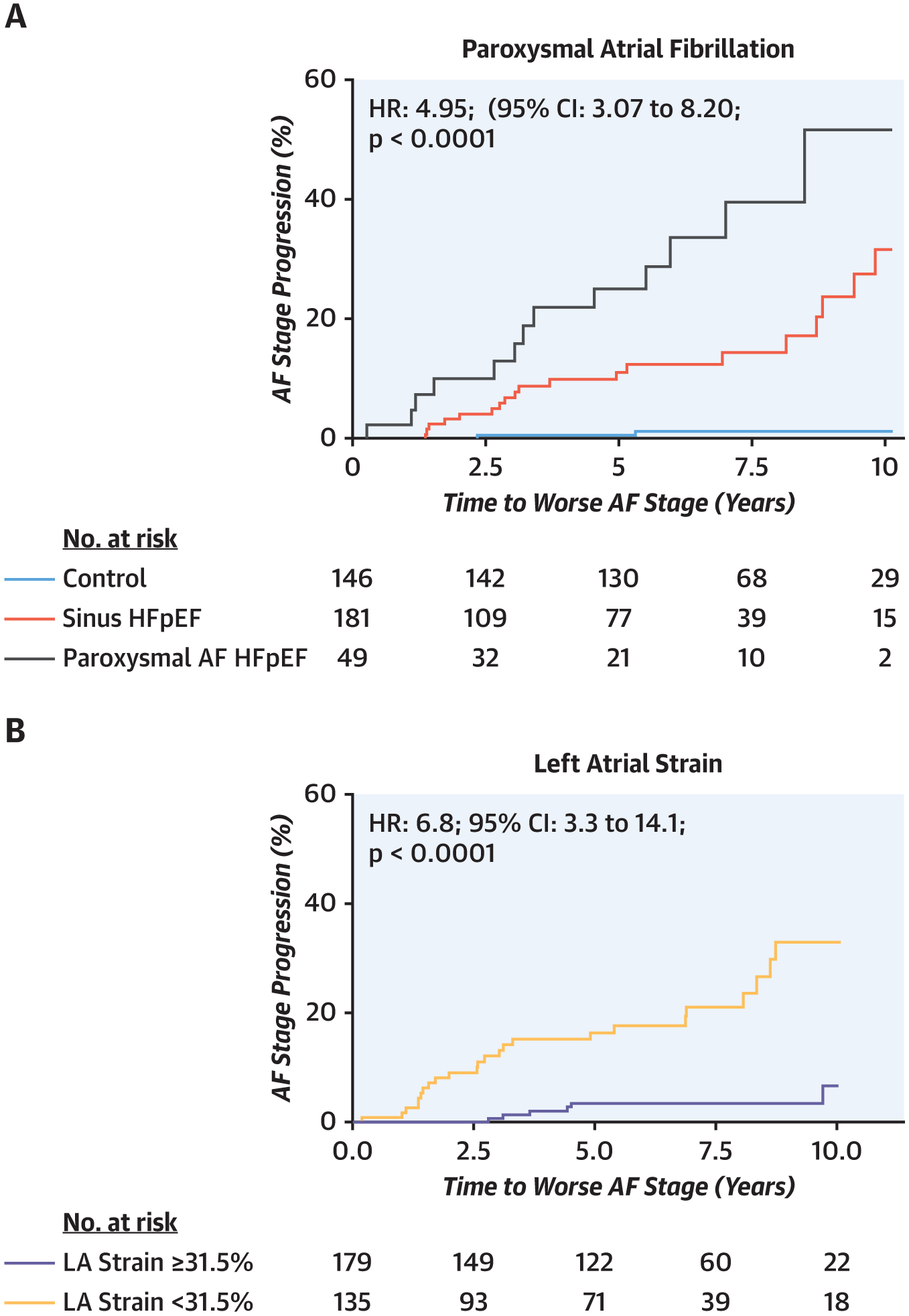
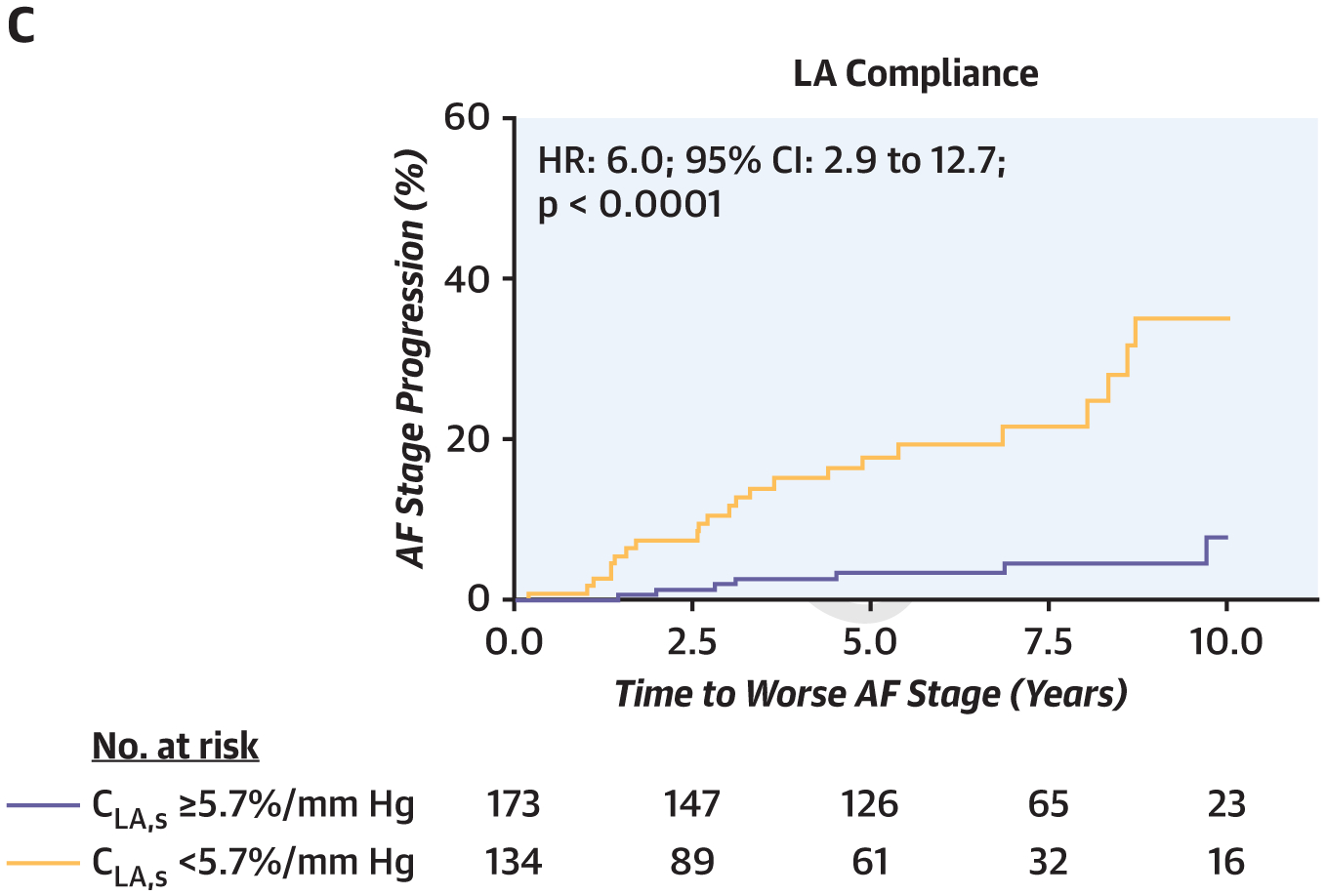
The risk of progressing to higher AF stage increased (A) with higher AF burden at index assessment and (B, C) in patients with more severe LA myopathy as evidenced by lower LA reservoir strain and compliance. For panels B and C, results are presented stratified by median LA reservoir strain and compliance. CI = confidence interval; HR = hazard ratio; other abbreviations as in Figure 1.
DISCUSSION
In this study, we present a comprehensive analysis of atrial and ventricular structure, function, and hemodynamics utilizing a combination of invasive and noninvasive assessments across the spectrum of AF burden, in patients with and without HFpEF. We observed that LA remodeling, compliance, and contractile function progressively worsen as AF burden increases, while LV diastolic stiffness was similarly abnormal in all HFpEF groups. The presence of AF, particularly permanent AF, was associated with more biventricular systolic dysfunction, the poorest cardiac output reserve, higher filling pressures, more severe pulmonary vascular disease, reduced LA reservoir function, and increased LA stiffness. Although most functional and hemodynamic abnormalities worsened across the AF stages, there were important differences between paroxysmal and permanent AF. Patients with permanent AF demonstrated greater cardiomegaly because of atrial dilatation, such that a greater proportion of LA hypertension was driven by ventricular interdependence and pericardial restraint. Increasing AF burden was associated with graded increases in mortality. Progression to permanent AF was common in patients with paroxysmal AF, and risk was greater in patients with more severe reductions in LA strain and compliance. These data identify important and unique pathophysiologic mechanisms by which AF contributes to morbidity and mortality in HFpEF, emphasizing the relevance of a distinct atrial fibrillation or LA myopathy phenotype, as well as the central role of LA reservoir function in progression of AF in HFpEF (Central Illustration). Further study of interventions to mitigate or prevent progression of LA myopathy in patients with HFpEF is urgently required, particularly in patients with paroxysmal AF.
CENTRAL ILLUSTRATION. Progressive LA Myopathy and AF Burden in Heart Failure With Preserved Ejection Fraction.
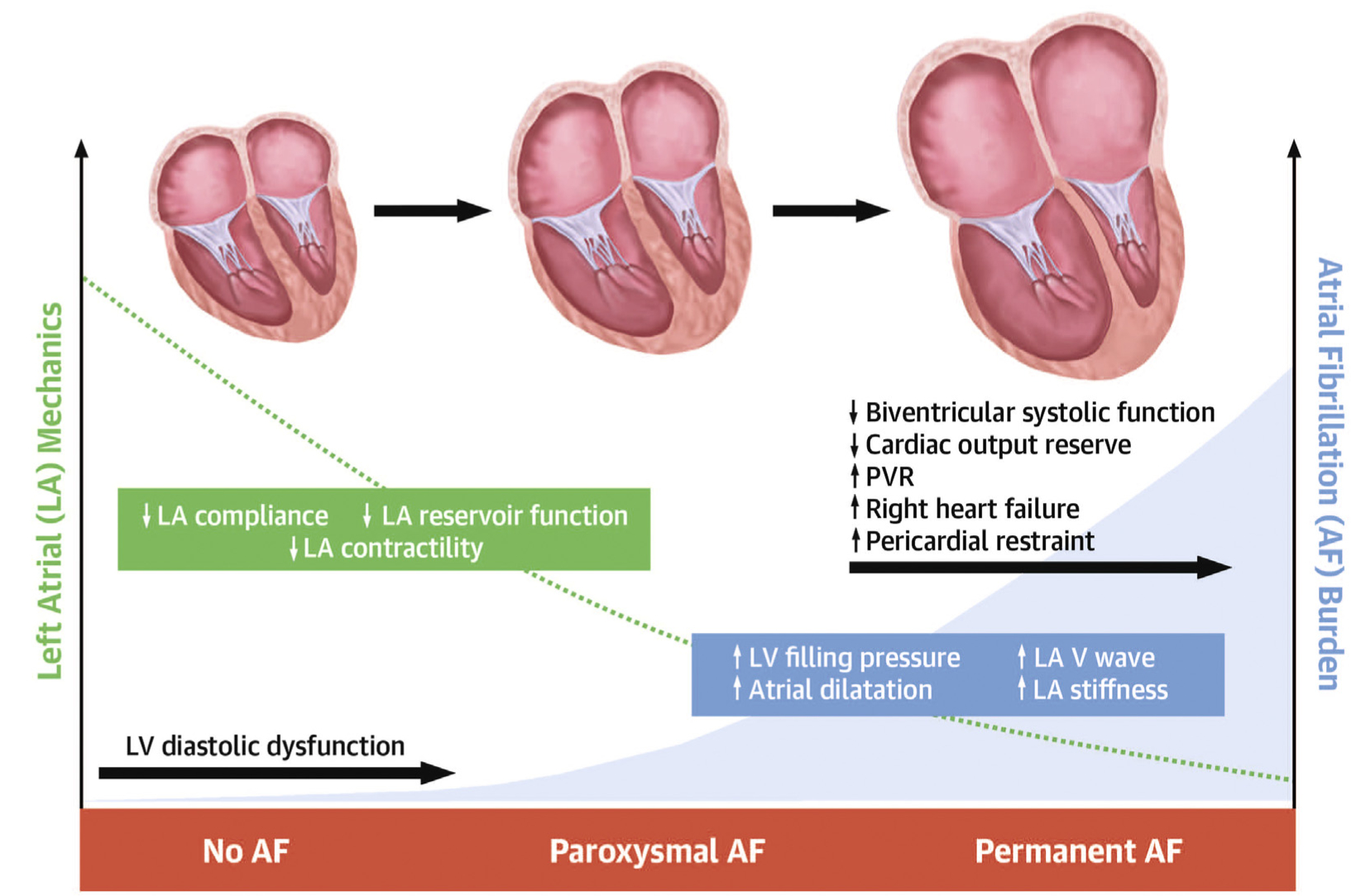
Increasing atrial fibrillation (AF) burden in heart failure with preserved ejection fraction was associated with worsening left atrial (LA) mechanics, LA remodeling, central hemodynamics, right ventricular-pulmonary artery coupling, and pericardial restraint. LV = left ventricular; PVR = pulmonary vascular resistance.
The prevalence of permanent AF in this study was lower than other series, likely related to the fact that the patient population was ambulatory not hospitalized. Community-based studies have shown that two-thirds of patients with HFpEF develop AF at some point during their lifespan (1). Compared with HFpEFno-AF patients, HFpEFperm-AF patients display poorer exercise capacity, greater burden of RV dysfunction, and increased risk of death (1–16). The findings from the current study, the largest and most comprehensive to date, confirm and importantly extend multiple studies from smaller cohorts focused on permanent AF, extending understandings to include patients across the entire AF or LA phenotypic spectrum in a graded spectrum, including paroxysmal AF. Ventricular systolic function as well as LA systolic and diastolic performance progressively worsened across HFpEF patients as AF burden worsened. These data point to the presence of progressive underlying cardiomyopathic process as AF burden worsens in HFpEF, especially in the atrium, and may help in staging HFpEF patients for targeted LA-specific interventions.
IMPLICATIONS FOR TREATMENT.
The vast majority of abnormalities in atrial function and hemodynamics identified were found to exist along a continuous spectrum mirroring the burden of AF. Therefore, interventions targeting LA myopathy such as unloading the LA may also be useful to mitigate progression. One such therapy is atrial septostomy, which lowers LA pressure and may elicit reverse remodeling of the LA (29,30), and improve pulmonary vascular function in HFpEF (28), consistent with the observed relationship between the height of the LA v-wave (an indicator of LA stiffness) and PA elastance in the present study (Figure 1). Therapies targeting LA myopathy and AF incidence such as sodium-glucose cotransporter-2 inhibitors (31) and risk factor interventions may have a role in these patients, particularly in patients with paroxysmal AF in whom progression rates to permanent AF are very high (32).
ROLE OF PERICARDIAL RESTRAINT IN PERMANENT AF.
Although PCWP was highest in the permanent AF cohort, LV mass, diastolic function, and chamber stiffness were similar across HFpEF groups regardless of rhythm status, suggesting that the higher PCWP in HFpEFperm-AF is not caused by differences in LV diastolic properties. Left heart filling pressure is determined by the vector sum of the chamber distending pressure (LVTMP) and the external pressure applied by the right heart and pericardium, which is estimated by the RA pressure (22,23). Using multiple separate indices, we found that this component related to extrinsic restraint was increased and explained the higher pulmonary capillary pressures in permanent AF (Figure 1).
In animal preparations, slight increases in atrial volume induced by loss of atrial contraction acutely shift the ventricular diastolic pressure-volume relationship upward (33). This effect is completely abrogated by pericardiectomy, proving that the effect is due to extrinsic restraint from atrial enlargement rather than due to LV properties. In the present study, LVTMP was similar in HFpEFperm-AF patients at rest but did not increase during exercise nearly as much in the permanent AF group as in others, even as PCWP was equally elevated during stress (Figure 1). This indicates that the increase in PCWP during exercise in HFpEFperm-AF was strongly related to extrinsic restraint during the volume loading induced by exercise, rather than to an isolated increase in LV distending pressures resulting from LV myocardial pathology, similar to an animal study (33). HFpEFperm-AF patients displayed greater increases in RA pressure at rest and during exercise, which promotes greater pulmonary congestion (34), as noted radiographically in the HFpEFperm-AF group in the present study. Increased pericardial constraint also promotes underdistention of the LV, contributing to the observed limitations in cardiac output at rest and during exercise, in part through impaired Frank-Starling reserve (Figure 2) (35). Novel therapies are being developed to release pericardial restraint in HFpEF (22,36,37), and the current data suggest that patients with permanent AF may particularly stand to benefit from this intervention.
AF AS AN INDICATOR OF LA MYOPATHY.
With reductions in LA compliance, there is an increase in the LA v-wave out of proportion to mean LA pressure, which increases the pulmonary capillary pressure out of proportion to the increase in LV end-diastolic pressure (38). This may explain the stronger association between pulmonary capillary pressure and outcome in HFpEF, as compared with LV end-diastolic pressure (39). In the present study, increases in PCWP and impairments in LA strain and compliance were all strongly associated with increased risk for development of AF. This strongly supports the importance of LA myopathy as a risk factor for AF and important therapeutic target.
One finding of caution from this study is the demonstration that atrial reservoir function was poor at baseline in HFpEF and worsened with greater AF burden. The reduction in atrial distensibility with AF likely contributed in an important way to the elevation in pulmonary capillary pressures observed. Given the potential for catheter ablation to further reduce LA compliance through scar formation (20), catheter ablation must be performed with careful attention to minimizing atrial injury, especially because the benefit of more extensive ablation remains uncertain (40). In addition to considering paroxysmal versus permanent AF, the current data suggest that consideration of LA reservoir strain may also be important in clinical trials to identify patients in whom ablation may be safer and more effective.
STUDY LIMITATIONS.
Echocardiography was not performed simultaneously with catheterization, and was only obtained during rest. However, this limitation applies similarly to all patient groups, so this does not affect the internal validity of the results, as this variability would similarly apply to all patient groups. The pressure-volume relationships for LA filling were assumed to be linear but actually represent a curvilinear pressure-volume relationship. However, the linear approximation avoids the complexities of curvilinear estimates of atrial pressure-volume relationships and represents a reasonable assumption of atrial operating compliance. The control group were not truly normal healthy volunteers, but had dyspnea of noncardiac origin, but this would only be expected to bias our results toward the null. The control group was also younger, which may have contributed to differences in hemodynamics compared with the HFpEF groups. Assignment of paroxysmal AF was based on meticulous review of clinical charts and prior electrocardiograms, but despite this, it is possible that some of the patients in the HFpEFno-AF group might have had an episode of AF at some time point prior to evaluation. However, this too would only be expected only to bias our results toward the null. A number of baseline differences were present between patient groups, and these were not adjusted for in multivariable analyses. Given the multiple hypotheses tested, this does increase the risk for type I error, and further validation of our results is needed. However, the differences in atrial mechanics and function persisted independent of the use of either echocardiographic or catheterization based methodologies, supporting the robustness of our findings
CONCLUSIONS
Left atrial myopathy in HFpEF progressively worsens with increasing burden of atrial fibrillation and is strongly associated with hemodynamic abnormalities that contribute to morbidity and mortality. HFpEFperm-AF patients display more pulmonary vascular dysfunction and right heart failure, with increases in pericardial restraint because of atrial dilation that adversely impacts exertional cardiac output and filling pressures. These unique pathophysiologic differences suggest that patients with permanent AF may respond differently to treatment and ought to be considered as a separate phenotype. Progression to permanent AF is strongly associated with baseline LA myopathy and compliance, suggesting that therapies targeting LA function may have a role in preventing this transition.
Supplementary Material
PERSPECTIVES.
COMPETENCY IN MEDICAL KNOWLEDGE:
In patients with HFpEF, LA compliance and mechanics worsen as the burden of AF increases, leading to LA enlargement and often to permanent AF.
TRANSLATIONAL OUTLOOK:
Additional research is needed to develop strategies that prevent adverse atrial remodeling in patients with HFpEF and AF.
Acknowledgments
Dr. Verbrugge is supported by a Fellowship of the Belgian American Educational Foundation and by the Special Research Fund of Hasselt University (BOF19PD04). Dr. Borlaug is supported by National Institutes of Health grants RO1 HL128526 and U10 HL110262. The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
ABBREVIATIONS AND ACRONYMS
- AF
atrial fibrillation
- CI
confidence interval
- EF
ejection fraction
- HFpEF
heart failure with preserved ejection fraction
- HFpEFno-AF
heart failure with preserved ejection fraction with no atrial fibrillation
- HFpEFparox-AF
heart failure with preserved ejection fraction with paroxysmal atrial fibrillation
- HFpEFperm-AF
heart failure with preserved ejection fraction with permanent atrial fibrillation
- HR
hazard ratio
- LA
left atrium/atrial
- LV
left ventricular
- LVTMP
left ventricular transmural pressure
- PA
pulmonary arterial
- PCWP
pulmonary capillary wedge pressure
- RAP
right atrial pressure
- RV
right ventricular
Footnotes
APPENDIX For expanded Methods and References sections as well as a supplemental table and figures, please see the online version of this paper.
REFERENCES
- 1.Zakeri R, Chamberlain AM, Roger VL, Redfield MM. Temporal relationship and prognostic significance of atrial fibrillation in heart failure patients with preserved ejection fraction: a community-based study. Circulation 2013;128: 1085–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zakeri R, Borlaug BA, McNulty SE, et al. Impact of atrial fibrillation on exercise capacity in heart failure with preserved ejection fraction: a RELAX trial ancillary study. Circ Heart Fail 2014;7:123–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zafrir B, Lund LH, Laroche C, et al. Prognostic implications of atrial fibrillation in heart failure with reduced, mid-range, and preserved ejection fraction: a report from 14 964 patients in the European Society of Cardiology Heart Failure Long-Term Registry. Eur Heart J 2018;39: 4277–84. [DOI] [PubMed] [Google Scholar]
- 4.Kotecha D, Lam CS, Van Veldhuisen DJ, Van Gelder IC, Voors AA, Rienstra M. Heart failure with preserved ejection fraction and atrial fibrillation: vicious twins. J Am Coll Cardiol 2016;68:2217–28. [DOI] [PubMed] [Google Scholar]
- 5.Melenovsky V, Borlaug BA, Rosen B, et al. Cardiovascular features of heart failure with preserved ejection fraction versus nonfailing hypertensive left ventricular hypertrophy in the urban Baltimore community: the role of atrial remodeling/dysfunction. J Am Coll Cardiol 2007;49: 198–207. [DOI] [PubMed] [Google Scholar]
- 6.Melenovsky V, Hwang SJ, Redfield MM, Zakeri R, Lin G, Borlaug BA. Left atrial remodeling and function in advanced heart failure with preserved or reduced ejection fraction. Circ Heart Fail 2015;8:295–303. [DOI] [PubMed] [Google Scholar]
- 7.Tamargo M, Obokata M, Reddy YNV, et al. Functional mitral regurgitation and left atrial myopathy in heart failure with preserved ejection fraction. Eur J Heart Fail 2020;22:489–98. [DOI] [PubMed] [Google Scholar]
- 8.Freed BH, Daruwalla V, Cheng JY, et al. Prognostic utility and clinical significance of cardiac mechanics in heart failure with preserved ejection fraction: importance of left atrial strain. Circ Cardiovasc Imaging 2016;9:e003754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reddy YNV, Obokata M, Egbe A, et al. Left atrial strain and compliance in the diagnostic evaluation of heart failure with preserved ejection fraction. Eur J Heart Fail 2019;21:891–900. [DOI] [PubMed] [Google Scholar]
- 10.von Roeder M, Rommel KP, Kowallick JT, et al. Influence of left atrial function on exercise capacity and left ventricular function in patients with heart failure and preserved ejection fraction. Circ Cardiovasc Imaging 2017;10:e005467. [DOI] [PubMed] [Google Scholar]
- 11.Sugimoto T, Bandera F, Generati G, Alfonzetti E, Bussadori C, Guazzi M. Left atrial function dynamics during exercise in heart failure: pathophysiological implications on the right heart and exercise ventilation inefficiency. J Am Coll Cardiol Img 2017;10:1253–64. [DOI] [PubMed] [Google Scholar]
- 12.Obokata M, Reddy YNV, Melenovsky V, Pislaru S, Borlaug BA. Deterioration in right ventricular structure and function over time in patients with heart failure and preserved ejection fraction. Eur Heart J 2019;40:689–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaye DM, Silvestry FE, Gustafsson F, et al. Impact of atrial fibrillation on rest and exercise haemodynamics in heart failure with mid-range and preserved ejection fraction. Eur J Heart Fail 2017;19:1690–7. [DOI] [PubMed] [Google Scholar]
- 14.Lam CS, Rienstra M, Tay WT, et al. Atrial fibrillation in heart failure with preserved ejection fraction: association with exercise capacity, left ventricular filling pressures, natriuretic peptides, and left atrial volume. J Am Coll Cardiol HF 2017; 5:92–8. [DOI] [PubMed] [Google Scholar]
- 15.Gorter TM, van Melle JP, Rienstra M, et al. Right heart dysfunction in heart failure with preserved ejection fraction: the impact of atrial fibrillation. J Card Fail 2018;24:177–85. [DOI] [PubMed] [Google Scholar]
- 16.Telles F, Nanayakkara S, Evans S, et al. Impaired left atrial strain predicts abnormal exercise haemodynamics in heart failure with preserved ejection fraction. Eur J Heart Fail 2019;21: 495–505. [DOI] [PubMed] [Google Scholar]
- 17.Borlaug BA. Evaluation and management of heart failure with preserved ejection fraction. Nat Rev Cardiol 2020. March 30 [E-pub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 18.Packer DL, Mark DB, Robb RA, et al. Effect of catheter ablation vs. antiarrhythmic drug therapy on mortality, stroke, bleeding, and cardiac arrest among patients with atrial fibrillation: the CABANA randomized clinical trial. JAMA 2019;321: 1261–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mark DB, Anstrom KJ, Sheng S, et al. Effect of catheter ablation vs. medical therapy on quality of life among patients with atrial fibrillation: the CABANA randomized clinical trial. JAMA 2019;321: 1275–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Packer M Effect of catheter ablation on pre-existing abnormalities of left atrial systolic, diastolic, and neurohormonal functions in patients with chronic heart failure and atrial fibrillation. Eur Heart J 2019;40:1873–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoit BD. Left atrial size and function: role in prognosis. J Am Coll Cardiol 2014;63:493–505. [DOI] [PubMed] [Google Scholar]
- 22.Borlaug BA, Reddy YNV. The role of the pericardium in heart failure: implications for pathophysiology and treatment. J Am Coll Cardiol HF 2019;7:574–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tyberg JV, Taichman GC, Smith ER, Douglas NW, Smiseth OA, Keon WJ. The relationship between pericardial pressure and right atrial pressure: an intraoperative study. Circulation 1986;73:428–32. [DOI] [PubMed] [Google Scholar]
- 24.Obokata M, Reddy YN, Pislaru SV, Melenovsky V, Borlaug BA. Evidence supporting the existence of a distinct obese phenotype of heart failure with preserved ejection fraction. Circulation 2017;136:6–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Obokata M, Kane GC, Reddy YN, Olson TP, Melenovsky V, Borlaug BA. Role of diastolic stress testing in the evaluation for heart failure with preserved ejection fraction: a simultaneous invasive-echocardiographic study. Circulation 2017;135:825–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reddy YNV, Andersen MJ, Obokata M, et al. Arterial stiffening with exercise in patients with heart failure and preserved ejection fraction. J Am Coll Cardiol 2017;70:136–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Klotz S, Hay I, Dickstein ML, et al. Single-beat estimation of end-diastolic pressure-volume relationship: a novel method with potential for noninvasive application. Am J Physiol Heart Circ Physiol 2006;291:H403–12. [DOI] [PubMed] [Google Scholar]
- 28.Obokata M, Reddy YNV, Shah SJ, et al. Effects of interatrial shunt on pulmonary vascular function in heart failure with preserved ejection fraction. J Am Coll Cardiol 2019;74:2539–50. [DOI] [PubMed] [Google Scholar]
- 29.Feldman T, Mauri L, Kahwash R, et al. Transcatheter interatrial shunt device for the treatment of heart failure with preserved ejection fraction (REDUCE LAP-HF I [Reduce Elevated Left Atrial Pressure in Patients With Heart Failure]): a phase 2, randomized, sham-controlled trial. Circulation 2018;137:364–75. [DOI] [PubMed] [Google Scholar]
- 30.Hanff TC, Kaye DM, Hayward CS, et al. Assessment of predictors of left atrial volume response to a transcatheter interatrial shunt device (from the REDUCE LAP-HF Trial). Am J Cardiol 2019;124:1912–7. [DOI] [PubMed] [Google Scholar]
- 31.Zelniker TA, Bonaca MP, Furtado R, et al. Effect of dapagliflozin on atrial fibrillation in patients with type 2 diabetes mellitus: insights from the DECLARE-TIMI 58 trial. Circulation 2020; 141:1227–34. [DOI] [PubMed] [Google Scholar]
- 32.Chung MK, Eckhardt LL, Chen LY, et al. Lifestyle and risk factor modification for reduction of atrial fibrillation: a scientific statement from the American Heart Association. Circulation 2020;141: e750–72. [DOI] [PubMed] [Google Scholar]
- 33.Linderer T, Chatterjee K, Parmley WW, Sievers RE, Glantz SA, Tyberg JV. Influence of atrial systole on the Frank-Starling relation and the end-diastolic pressure-diameter relation of the left ventricle. Circulation 1983;67:1045–53. [DOI] [PubMed] [Google Scholar]
- 34.Reddy YNV, Obokata M, Wiley B, et al. The haemodynamic basis of lung congestion during exercise in heart failure with preserved ejection fraction. Eur Heart J 2019;40:3721–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gorter TM, Obokata M, Reddy YNV, Melenovsky V, Borlaug BA. Exercise unmasks distinct pathophysiologic features in heart failure with preserved ejection fraction and pulmonary vascular disease. Eur Heart J 2018;39:2825–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Borlaug BA, Schaff HV, Pochettino A, et al. Pericardiotomy enhances left ventricular diastolic reserve with volume loading in humans. Circulation 2018;138:2295–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Borlaug BA, Carter RE, Melenovsky V, et al. Percutaneous pericardial resection: a novel potential treatment for heart failure with preserved ejection fraction. Circ Heart Fail 2017;10: e003612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dickinson MG, Lam CS, Rienstra M, et al. Atrial fibrillation modifies the association between pulmonary artery wedge pressure and left ventricular end-diastolic pressure. Eur J Heart Fail 2017;19: 1483–90. [DOI] [PubMed] [Google Scholar]
- 39.Mascherbauer J, Zotter-Tufaro C, Duca F, et al. Wedge pressure rather than left ventricular end-diastolic pressure predicts outcome in heart failure with preserved ejection fraction. J Am Coll Cardiol HF 2017;5:795–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Verma A, Jiang CY, Betts TR, et al. Approaches to catheter ablation for persistent atrial fibrillation. N Engl J Med 2015;372:1812–22. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


