Abstract
Cardiac arrhythmia is a known manifestation of novel coronavirus 2019 (COVID-19) infection. Herein, we describe the clinical course of an otherwise healthy patient who experienced persistent ventricular tachycardia and fibrillation which is believed to be directly related to inflammation, as opposed to acute myocardial injury or medications that can prolong the QT interval.
In addition to acute respiratory complications, coronavirus disease 2019 (COVID-19) also causes significant consequences for the cardiovascular system, particularly arrhythmia.1 , 2 Due to the panoply of precipitating factors that can cause cardiac arrhythmia in COVID-19 patients, identifying the underlying culprit is crucial for proper management. Herein, we describe the course of a COVID-19 patient who experienced an electrical storm of ventricular fibrillation (VF).
Case Presentation
A 55-year-old woman with history significant for ischemic cerebral vascular infarction (6 years prior) presented with expressive aphasia and stroke-like symptoms. Computed tomography head scan ruled out acute cerebral stroke. Vital signs, laboratory data, and electrocardiogram are in Table 1 . Urine analysis was positive for bacteriuria. At screening, she tested positive for COVID-19. At that time she was not complaining of any constitutional or respiratory symptoms. She was admitted for cerebral vascular stroke workup and was started on intravenous ceftriaxone for urinary tract infection. Following admission, she became severely hypotensive and experienced worsening leukocytosis and renal function, causing concern for septic shock. Further laboratory workup revealed high interleuken 6 (16.4 pg/ml, upper limit normal = 1.8 pg/ml). She was transferred to the intensive care unit, where circulatory support and corticosteroids were initiated. She experienced sinus bradycardia with a heart rate of 40 beats per minute and was subsequently started on a dopamine infusion. Later that day, she went into cardiac arrest due to Torsades de Pointe (TdP) (Figure 1 ). A 200 joules shock was delivered, the patient regained sinus rhythm, and was subsequently intubated; her QTc interval was 535 ms. A lidocaine infusion and aggressive empiric magnesium supplementation were initiated. Although receiving dopamine and lidocaine infusions, she remained bradycardic and experienced another VF and TdP cardiac arrest requiring defibrillation; transcutaneous pacing was started. Convalescent serum was administered as her inflammatory markers continued to rise along with worsening circulatory and respiratory failure. Despite dopamine, lidocaine, and transcutaneous pacing, she had multiple VF arrests requiring more than 60 defibrillator shocks. Her echocardiogram revealed normal left ventricular wall motion with normal function. Serial troponin levels were assessed, peaking at 0.064 ng/ml. At that point, the family signed comfort care only, the patient was extubated, and infusions stopped. Miraculously, she dramatically improved, with QTc improving from > 700 ms to 500 ms. The repeat echocardiogram did not reveal significant changes. The patient was never treated with hydroxychloroquine or azithromycin. She did not have any additional arrhythmias for the duration of her hospital stay. QTc interval returned to baseline. She had a full recovery and was discharged home on a life vest with plans for outpatient genetic tests for QTc prolongation. On a 4-week follow-up, her EKG showed normal QRS/QTc and she felt great without palpitations, syncope, or life vest shocks.
Table 1.
Vital signs, laboratory data and electrocardiogram parameters
| Time of Observation |
|||
|---|---|---|---|
| Variable | Presentation | Ventricular Fibrillation | Discharge |
| Blood pressure (mm Hg) | 112/69 | 95/50 | 98/63 |
| Heart rate (beats per minute) | 94 | 40–50 | 80–90 |
| Temperature (Fahrenheit) | 99.7 | 98.1 | 98 |
| Respiratory rate (breaths per minute) | 16–20 | 28–28 | 16–18 |
| Oxygen saturation (%) | 99 | 97 | 98 |
| Sodium (meq/L) | 133 | 143 | 136 |
| Potassium (meq/L) | 3.1 | 3.2 | 3.9 |
| Magnesium (meq/L) | 2.0 | 1.5 | 1.8 |
| Serum creatinine (mg/dl) | 1.5 | 0.67 | 0.74 |
| Aspartate transaminase (H/L) | 107 | 33 | 70 |
| Alanine transaminase (U/L) | 74 | 26 | 80 |
| Alkaline phosphatase (U/L) | 122 | 99 | 78 |
| Total bilirubin (mg/dl) | 0.9 | 0.3 | 0.3 |
| Troponins (ng/ml) | <0.012 | <0.012 | <0.012 |
| D-dimer (mg/L FEU) | 21 | 14 | 10 |
| C-reactive protein (mg/dl) | >20 | 5.4 | 1.3 |
| Interleukin-6 (pg/ml) | - | 16.4 | - |
| Lactate dehydrogenase (U/L) | 324 | 496 | 220 |
| Ferritin (ng/ml) | 872 | >100 | 640 |
| White blood cell count (10*3/uL) | 18.7 | 37 | 10 |
| Hemoglobin (g/dl) | 11.5 | 11 | 11.5 |
| Platelet (K/uL) | 240 | 528 | 407 |
| International normalized ratio | 1.1 | - | 1.2 |
| P-R interval (ms) | 150 | 168 | 150 |
| QRS duration (ms) | 72 | 80 | 62 |
| QTc interval (ms) | 427 | 650 | 467 |
Figure 1.
Electrocardiogram of the patient with ventricular fibrillation.
Discussion
One study reported ventricular tachycardia/VF in 5.9% (11/187) of COVID-19 patients (Table 2 3, 4, 5, 6, 7, 8, 9), with elevated troponin-T increasing risk, suggesting that myocardial injury precipitates arrhythmia.2 However, ventricular tachycardia/VF also occurs in patients with low troponin, suggesting alternative causes, such as QTc prolonging medication (e.g., hydroxychloroquine).2 Another possibility is the fulminant systemic inflammatory state in sick patients with cardiovascular morbidities and metabolic disarray. Inflammatory cardiac channelopathies, induced by inflammatory markers, can prolong the action potential and cause long QT syndrome and TdP.10 , 11 Moreover, systemic inflammation can increase risk for ventricular arrhythmia indirectly by inducing a hyper-sympathetic state or inhibiting cytochrome p450.11 Other inflammatory responses, such as fever, can trigger undiagnosed conduction diseases or channelopathies, like Brugada syndrome.12
Table 2.
Articles describing arrhythmic events in patients with COVID-19
| Number of Patients with Event of Interest |
||||
|---|---|---|---|---|
| First Author Date Sample Size |
Ventricular Tachycardia | Elevated Troponins | Ventricular Tachycardia/Fibrillation Cardiac Arrest | Ventricular Tachycardia Storm |
| Guo March 2020 n = 187 |
11 | 9 | —- | —- |
| Goyal April 2020 n = 393 |
1 | —- | —- | —- |
| Kochav May 2020 n = 1 |
1* | 1 | 1 | 1 |
| Chorin May 2020 n = 251 |
1† | —- | —- | 1 |
| Shao June 2020 n = 761 |
8 | —- | —- | —- |
| Bhatla June 2020 n = 700 |
10 | —- | 1 | —- |
| Mitacchione July 2020 n = 1 |
1‡ | —- | —- | 1 |
The patient had prolonged QTc, heart failure with preserved ejection fraction, diabetes mellitus, and atrial fibrillation.
The patient was in Torsades de Pointe and on hydroxychloroquine + azithromycin.
The patient had heart failure with reduced ejection fraction and coronary artery disease.
Reducing this inflammatory state in COVID-19 patients can mitigate arrhythmic events, as well as other morbidity and mortality. Tocilizumab, an anti-interleuken 6 receptor monoclonal-antibody, yielded a survival benefit in COVID-19 patients.13, 14, 15 Tocilizumab was shown to have a robust shortening of the QTc prolongation induced by abundant inflammatory cytokines in patients with acute rheumatoid arthritis.16 Additionally, preliminary reports from ongoing trials indicate toclizumab may reduce adverse myocardial injury in acute inflammatory cardiac injury.2 , 13
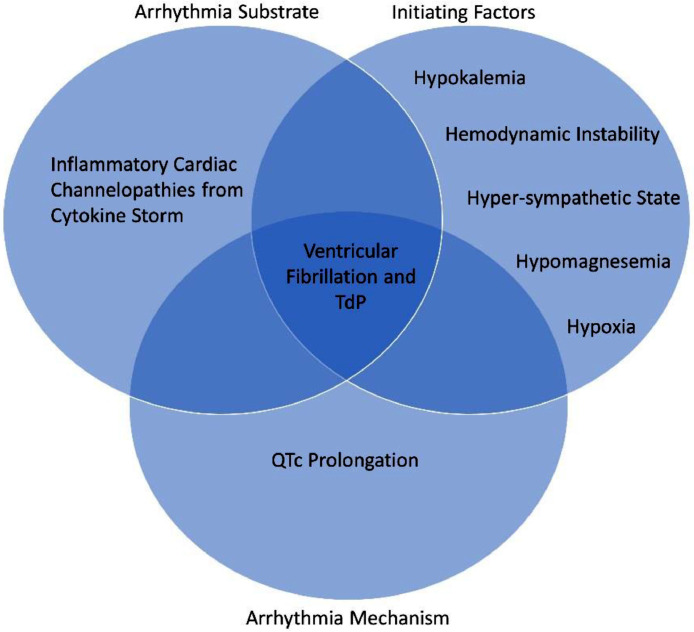
The initial hypothesis regarding our patient's VF storm etiology was acute myocarditis; however, with consistently normal troponins, the explanation is likely a constellation of factors, including hypokalemia, hypomagnesemia, bradycardia and long QTc, in the setting of hyper-inflammation (Figure 2 ). This hypothesis is supported by significantly elevated inflammatory markers. As such, this report suggests that the hyper-inflammatory state in COVID-19 patients can induce ventricular arrhythmias, which may cease abruptly following a reduction in inflammation, in our case from convalescent serum and/or hydrocortisone therapy.17 , 18 Hence, dampening this fulminant inflammatory condition may prevent or decrease cardiac arrhythmias.
Figure 2.
A Venn diagram illustrating the hypothesis that this patient's ventricular tachycardia resulted from a scenario involving a multitude of factors (abnormal labs, renal injury, long QTC, and inflammation). In order for the ventricular tachycardia to occur, these factors need to happen together. Conversely, a normal patient who happens to have a high degree of inflammation will not have ventricular tachycardia if other variables in the Venn diagram are absent. TdP = Torsades de Pointe.
Footnotes
This work was partially funded by the Baylor Health Care System Foundation.
References
- 1.World Health Organization. Coronavirus Disease (COVID-19) Situation Reports. Available at:https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200404-sitrep-75-covid-19.pdf?sfvrsn=99251b2b_2. Accessed on June 5, 2020.
- 2.Driggin E, Madhavan MV, Bikdeli B, Chuich T, Laracy J, Biondi-Zoccai G, Brown TS, Der Nigoghossian C, Zidar DA, Haythe J, Brodie D, Beckman JA, Kirtane AJ, Stone GW, Krumholz HM, Parikh SA. Cardiovascular Considerations for patients, health care workers, and health systems during the coronavirus disease 2019 (COVID-19) pandemic. J Am Coll Cardiol. 2020;75:2352–2371. doi: 10.1016/j.jacc.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guo T, Fan Y, Chen M, Wu X, Zhang L, He T, Wang H, Wan J, Wang X, Lu Z. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5:1–8. doi: 10.1001/jamacardio.2020.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goyal P, Choi JJ, Pinheiro LC, Schenck EJ, Chen R, Jabri A, Satlin MJ, Campion TR, Jr, Nahid M, Ringel JB, Hoffman KL, Alshak MN, Li HA, Wehmeyer GT, Rajan M, Reshetnyak E, Hupert N, Horn EM, Martinez FJ, Gulick RM, Safford MM. Clinical characteristics of Covid-19 in New York City. N Engl J Med. 2020;382:2372–2374. doi: 10.1056/NEJMc2010419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kochav SM, Coromilas E, Nalbandian A, Ranard LS, Gupta A, Chung MK, Gopinathannair R, Biviano AB, Garan H, Wan EY. Cardiac arrhythmias in COVID-19 infection. Circ Arrhythm Electrophysiol. 2020;13 doi: 10.1161/CIRCEP.120.008719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chorin E, Wadhwani L, Magnani S, Dai M, Shulman E, Nadeau-Routhier C, Knotts R, Bar-Cohen R, Kogan E, Barbhaiya C, Aizer A, Holmes D, Bernstein S, Spinelli M, Park DS, Stefano C, Chinitz LA, Jankelson L. QT interval prolongation and torsade de pointes in patients with COVID-19 treated with hydroxychloroquine/azithromycin. Heart Rhythm. 2020 doi: 10.1016/j.hrthm.2020.05.014. S1547-5271(20)30435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shao F, Xu S, Ma X, Xu Z, Lyu J, Ng M, Cui H, Yu C, Zhang Q, Sun P, Tang Z. In-hospital cardiac arrest outcomes among patients with COVID-19 pneumonia in Wuhan, China. Resuscitation. 2020;151:18–23. doi: 10.1016/j.resuscitation.2020.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bhatla A, Mayer MM, Adusumalli S, Hyman MC, Oh E, Tierney A, Moss J, Chahal AA, Anesi G, Denduluri S, Domenico CM, Arkles J, Abella BS, Bullinga JR, Callans DJ, Dixit S, Epstein AE, Frankel DS, Garcia FC, Kumareswaram R, Nazarian S, Riley MP, Santangeli P, Schaller RD, Supple GE, Lin D, Marchlinski F, Deo R. COVID-19 and cardiac arrhythmias. Heart Rhythm. 2020 doi: 10.1016/j.hrthm.2020.06.016. S1547-5271(20)30594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mitacchione G, Schiavone M, Gasperetti A, Forleo GB. Ventricular tachycardia storm management in a COVID-19 patient: a case report. Eur Heart J - Case Rep. 2020;ytaa217:1–6. doi: 10.1093/ehjcr/ytaa217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lazzerini PE, Capecchi PL, Laghi-Pasini F. Systemic inflammation and arrhythmic risk: lessons from rheumatoid arthritis. Eur Heart J. 2017;38:1717–1727. doi: 10.1093/eurheartj/ehw208. [DOI] [PubMed] [Google Scholar]
- 11.Lazzerini PE, Laghi-Pasini F, Boutjdir M, Capecchi PL. Cardioimmunology of arrhythmias: the role of autoimmune and inflammatory cardiac channelopathies. Nat Rev Immunol. 2019;19:63–64. doi: 10.1038/s41577-018-0098-z. [DOI] [PubMed] [Google Scholar]
- 12.Chang D, Saleh M, Garcia-Bengo Y, Choi E, Epstein L, Willner J. COVID-19 infection unmasking brugada syndrome. HeartRhythm Case Rep. 2020;6:237–240. doi: 10.1016/j.hrcr.2020.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coyle J, Igbinomwanhia E, Sanchez-Nadales A, Danciu S, Chu C, Shah N. A recovered case of COVID-19 myocarditis and ARDS treated with corticosteroids, tocilizumab, and experimental AT-001 [published online ahead of print, 2020 May 3] JACC Case Rep. 2020 doi: 10.1016/j.jaccas.2020.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lazzerini PE, Boutjdir M, Capecchi PL. COVID-19, arrhythmic risk, and inflammation: mind the gap! Circulation. 2020;142:7–9. doi: 10.1161/CIRCULATIONAHA.120.047293. [DOI] [PubMed] [Google Scholar]
- 15.Xu X, Han M, Li T, Sun W, Wang D, Fu B, Zhou Y, Zheng X, Yang Y, Li X, Zhang X, Pan A, Wei H. Effective treatment of severe COVID-19 patients with tocilizumab. Proc Natl Acad Sci U S A. 2020;117:10970–10975. doi: 10.1073/pnas.2005615117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lazzerini PE, Acampa M, Capecchi PL, Fineschi I, Selvi E, Moscadelli V, Zimbone S, Gentile D, Galeazzi M, Laghi-Pasini F. Antiarrhythmic potential of anticytokine therapy in rheumatoid arthritis: tocilizumab reduces corrected QT interval by controlling systemic inflammation. Arthritis Care Res (Hoboken) 2015;67:332–339. doi: 10.1002/acr.22455. [DOI] [PubMed] [Google Scholar]
- 17.Yang SS, Lipes J. Corticosteroids for critically ill COVID-19 patients with cytokine release syndrome: a limited case series. Can J Anaesth. 2020;11:1–3. doi: 10.1007/s12630-020-01700-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Man SF, Sin DD. Effects of corticosteroids on systemic inflammation in chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2005;2:78–82. doi: 10.1513/pats.200406-034MS. [DOI] [PubMed] [Google Scholar]