Abstract
Background
Quality of life is a multidimensional concept that includes perceptions of one’s physical, psychological, social, and spiritual functioning, all of which are theorized to be interdependent. The focus of this study is social functioning, which itself is a multidimensional concept that includes social support and social constraint among other things. In cancer survivors, social support receives most of the research attention, but social constraint may have a stronger influence on quality of life.
Purpose
This systematic literature review evaluates which aspect of social functioning—social support or social constraint—has a stronger relationship with the psychological functioning of cancer survivors.
Methods
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines were followed in the identification and review of 32 independent records. Multiple measures of social support and social constraint were used across studies, with most having adequate psychometric properties. Psychological outcomes were divided into (a) general distress, (b) cancer-specific distress, (c) general well-being, and (d) cancer-specific well-being.
Results
For general and cancer-specific distress, social constraint exhibited a larger association with distress than social support. Similarly, for general well-being, most studies reported a stronger association with social constraint than social support. For cancer-specific well-being, the opposite was true such that associations were stronger for social support than social constraint.
Conclusions
Results highlight the importance of considering social constraint when examining quality-of-life outcomes like psychological distress and well-being. Findings support social constraint as a target in interventions to reduce cancer survivors’ distress, while social support could be considered in attempts to promote cancer-specific well-being.
Keywords: Cancer, Social constraint, Social support, Psychological functioning, Systematic review
In cancer survivors, social constraint (a negative form of social interaction) has stronger ties to general distress, cancer-specific distress, and general wellbeing than social support (a positive form of social interaction), yet it has relatively weaker ties to cancer-specific wellbeing
Introduction
Quality of life is comprised of an individual’s perception of her or his physical, psychological, social, and spiritual functioning [1]. All domains of functioning are understood to be interdependent, such that changes in one domain affect the other domains [1, 2]. Although it is not always the case, quality of life can be impaired in the short and long term by stressful life events like disease occurrence. As individuals living with a chronic disease, cancer survivors often report worse overall quality of life than individuals without a cancer history [3–6]. However, the underlying aspects of quality of life can be differentially impacted by cancer diagnosis.
One of the more commonly studied quality-of-life domains is psychological functioning. Psychological functioning is broadly divided into two dimensions: distress and well-being. Distress is a common, if not normal, reaction to the stressful life event of a cancer diagnosis (for reviews, see [7,8,9]), and it can range from modest feelings of sadness to disabling problems like major depression [10]. Furthermore, as the distress many cancer survivors experience may or may not improve with time [8], it is important to note that distress is related to cancer care outcomes like treatment compliance [11], hospital stay [12], and medical costs [13]. Well-being is the positively valenced counterpart to distress, and it includes the experience of happiness, gratitude, benefit finding, and posttraumatic growth. Although both are important quality-of-life dimensions, distress is studied far more frequently in cancer survivors than well-being. With that caveat, it is known that cancer diagnosis can elicit increases in well-being [8, 14], although this experience is far from universal or easily explained.
Cancer diagnosis is a stressful life event that reaches beyond the cancer survivor to impact the lives of individuals close to the cancer survivor (e.g., family members and romantic partners). Likewise, a cancer survivor’s social network can have a significant bearing on her or his ability to cope with the stress of cancer and its sequelae. Poor social functioning, which is broadly understood as an inadequate or unhealthy interaction with one’s social environment, can decrease opportunities to talk about one’s cancer experience. According to the social-cognitive processing model, insufficient and/or negative social experiences would hinder cancer survivors’ ability to emotionally and cognitively process their cancer experiences [15], which is necessary for proper psychological adjustment. In this way, social functioning may predict or explain psychological functioning, which would support the interdependence of quality-of-life domains [1, 2]. However, the nature and strength of the association between cancer survivors’ psychological and social functioning are inconsistent across studies [16–21]. This inconsistency in the literature could be explained by social functioning also being a multidimensional construct that encompasses distinct elements. If this is indeed the case, then one would expect the overarching relationship between cancer survivors’ psychological and social functioning to vary as a function of the underlying dimensions at play.
Like psychological functioning, the various aspects of social functioning can be divided into positive and negative dimensions, only two of which are the focus here. First, social support is positively valenced, and it is defined as the emotional, instrumental, and informational support provided by an individual’s social network [22]. Second, social constraint is negatively valenced, and it is defined as “the objective social conditions and individuals’ construal of those conditions that lead individuals to refrain from or modify their disclosure of stress- and trauma- related thoughts, feelings, or concerns” [15] (p. 315). As an illustration, social support and social constraint might arise when a cancer survivor starts telling a coworker about her fears of recurrence and the response elicited is “Don’t worry. You will be fine!” With this statement, the coworker is trying to be emotionally supportive but, in doing so, may inadvertently shut down the cancer survivor’s attempts to share her worries and ask for precisely what she needs to cope. In general, social support and social constraint are inversely related, where higher levels of social support are associated with lower levels of social constraint [19, 23–25]. However, like in the example above, because social support is generally planned and purposeful, while social constraint can occur unintentionally, social support and social constraint can co-occur within any given dyad or social relationship [26]. The fact that social support is typically intentional, while social constraint is not, may explain why social support tends to increase in response to cancer diagnosis but then fade within the first year [27], while social constraint remains stable over time, at least in the short term [28]. In terms of their association with psychological functioning, social support is generally related to less distress and more well-being [9, 29–34], while the opposite is typically true of social constraint [26, 35]. It is important to appreciate, though, that these associations are neither universal nor unidirectional. For instance, the provision of unwanted or inappropriate social support can increase distress [36], and significant or persistent distress can eventually degrade or exhaust one’s social network, thereby harming social functioning [37]. Finally, similar to how much research attention is given to distress versus well-being, there is a clear difference in the amount of research on social support versus social constraint, with the former being studied far more frequently.
To the authors’ knowledge, no published literature review has examined psychological functioning in relation to the two aforesaid aspects of social functioning. This systematic literature review aims to evaluate which aspect of social functioning, social support, or social constraint has a stronger association with the distress and well-being of cancer survivors. Based on the application of “domain-specific effects” [38] to quality-of-life models, the strength of relationship between psychological and social functioning should depend upon how closely the underlying dimensions of interest “match” in terms of their valence. Negative social exchanges (e.g., critical comments and invalidating statements) are expected to have a strong association with distress and positive social exchanges (e.g., words of encouragement and expressions of warmth) should have a strong association with well-being [37–41]. Thus, within the context of cancer survivorship, these hypotheses are advanced: (a) distress is more consistently and strongly associated with social constraint than social support, while (b) well-being is more consistently and strongly associated with social support than social constraint. The current systematic literature review will be the first to evaluate the veracity of these hypotheses, the results of which could give greater credibility to quality-of-life models of interdependence and guide development of quality-of-life interventions for cancer survivors and potentially others with chronic diseases. If social support demonstrates a comparably stronger association with psychological functioning, then interventions that focus on selectively increasing or strengthening social networks and teaching individuals how to obtain the support they need are warranted to mitigate distress and promote well-being. However, if social constraint demonstrates the stronger relationship, then interventions that attempt to improve individuals’ interpersonal skills (e.g., creating boundaries and being assertive) are needed to address these same psychological outcomes. Of course, interventions that center on social functioning as the target outcome are equally possible, with the results of this review also potentially informing their design.
Methods
Search Procedures and Data Sources
In accordance with best practices [42], this review was registered on PROSPERO International at the outset (#42018100816; http://www.crd.york.ac.uk/PROSPERO/display_record.php?ID=CRD42018100816), and the additional Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines were followed throughout the process. A systematic search of peer-reviewed, English language publications was conducted in the CINAHL, PsycINFO, Pubmed, SCOPUS, and Web of Science databases by a reference librarian, and all search results were combined in a bibliographic reference management tool [43]. Respectively, two sets of keywords representing the population and constructs of interest were used in each search: (a) cancer (or neoplasm, carcino-, oncol-, tumor and tumour) and (b) social constraint (or negative support, critical responses, holding back, unsupportive behaviors, punishing responses, and negative responses); the keyword search focused on social constraint as opposed to social support or psychological functioning because far fewer publications are published on this topic, making it the least common denominator for this research. A complete description of the Medical Subject Headings (MeSH) and keyword terms and exact search strategy is available upon request.
Inclusion–Exclusion Criteria
Eligibility for this review required the record to meet these criteria: (a) written in English; (b) publication date between January 1, 1996 and December 31, 2017; (c) adult sample comprised of at least 50% cancer patients or survivors; and (d) an empirical article as opposed to a narrative or meta-analytic review, conference abstract, commentary, letter to the editor, or case study. If all criteria were met (Phase 1), the record was considered further to determine whether it also included a quantitative measure of psychological functioning, social support, and social constraint plus a report of the correlation among the variables (Phase 2). Measures of psychological functioning encompassed: distress (e.g., anxiety, depression, fear, posttraumatic stress disorder, and worry) and well-being (e.g., benefit finding, happiness, mental functioning, and posttraumatic growth). Measures of social support included but were not limited to indices of emotional support, instrumental support, social provisions, intimacy, solicitous responses, and support receipt. Finally, measures of social constraint included but were not limited to indices of critical responses, holding back, negative support, punishing responses, and unsupportive behaviors. If a record was otherwise eligible for inclusion but correlations were not reported in it, then the corresponding author was emailed and the missing data requested, with three being the maximum number of contact attempts.
Search Results
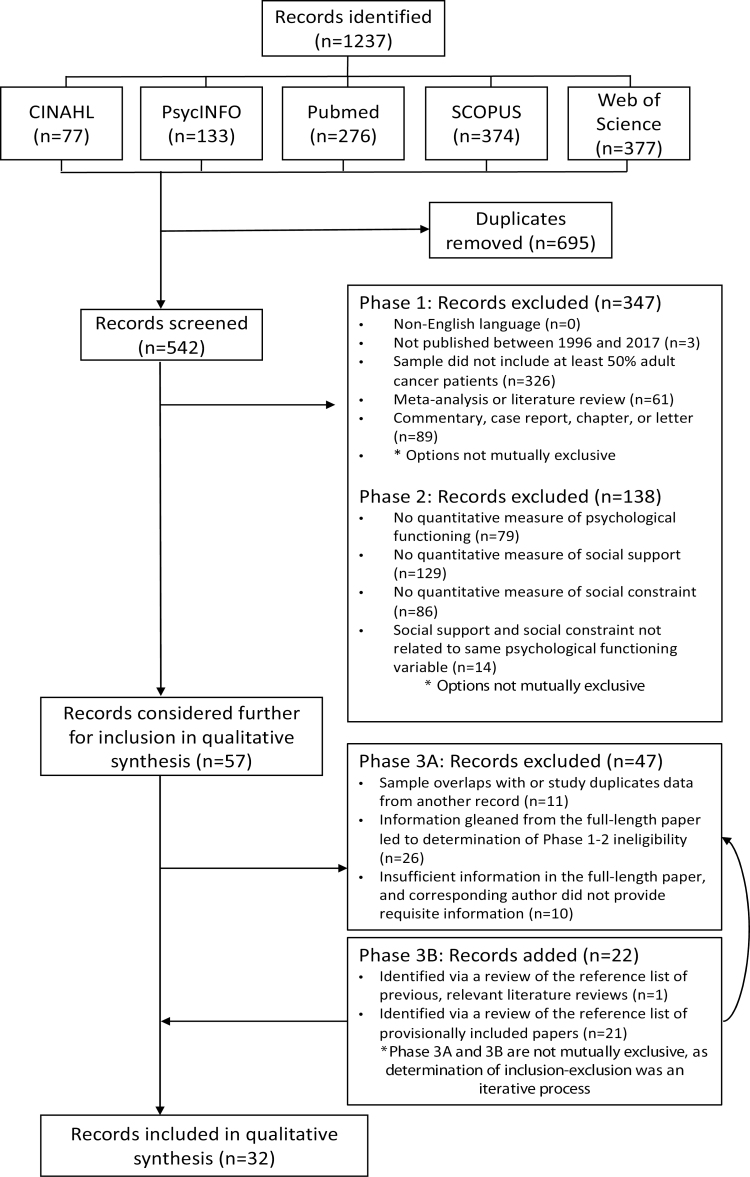
Fig. 1 is a flow diagram of the search results and inclusion–exclusion review process. The initial search of the five databases resulted in 1,237 records, of which 695 (56.18%) were duplicates. Thus, 542 unique records (i.e., titles, abstracts, and full-length articles) were identified and screened for inclusion–exclusion by two independent coders. In the case of discrepancies between coders, consensus was reached through facilitated discussion by a third coder. Of the 542 unique records, 57 (10.52%) were considered further for inclusion in the review. At this point (Phase 3), the full-length article was thoroughly reviewed to ensure it met all inclusion–exclusion criteria and did not duplicate data from another record. Corresponding authors were also contacted at this point (see comment above), with some furnishing the necessary information. Finally, to identify any “missing records” that should be included in this review, a manual search of the reference list of a prior review on social constraint in cancer survivors [35] as well as the reference list of all records provisionally included in this review was conducted. In the end, 32 independent records yielded data for this review.
Figure 1.
Flow diagram depicting the systematic review process
Data Extraction and Qualitative Synthesis
All data extracted from the aforesaid 32 records were captured and stored securely in a Research Electronic Data Capture (REDCap) database [44]. One coder served as the primary coder, reviewing and extracting data from each record, while a second reviewer provided quality control by independently coding 20% of the 32 records; no disagreement between these two coders was found, but discussions about those records guided the primary reviewer’s approach to the remainder of the coding. Extracted data included key features of the study described in the record, including the author(s), year of publication, study location, study design, sample size, and sample characteristics. Information about measures of psychological functioning, social support, and social constraint were also extracted. Descriptive information about the variables of interests (e.g., means [Ms] and standard deviation [SD]) and relationships among them (e.g., bivariate correlations) were then extracted. Finally, information about the methodological quality of each study, as judged by the completeness and transparency of the record was extracted via completion of the appropriate STROBE checklist [45]. In a few cases, a record would reference a prior publication for requisite information and, whenever necessary, the requisite information in that publication was then incorporated in the review. Additionally, the information from corresponding authors was included. In total, all relevant data extracted from the 32 records [19, 21, 23, 24, 26, 46–72] and other, complementary sources [25, 52, 73–76] were coded and are summarized below.
Vast heterogeneity in measures of psychological functioning, social support, and social constraint across studies prevented a quantitative meta-analysis, so a descriptive synthesis approach was used. The data extracted were organized into four broad categories of psychological functioning: (a) general distress, (b) cancer-specific distress, (c) general well-being, and (d) cancer-specific well-being. The effect size of the correlations between each of these aspects of psychological functioning and both social support and social constraint was evaluated, with attention given to bivariate analyses (Pearson’s r correlations unless otherwise noted) as opposed to multivariate analyses (e.g., beta weights from covariate-adjusted linear regressions) as more data were available for the former set of analyses and they allowed a more parsimonious and direct evaluation of the study question. To further aid interpretation of the results of this review, the Pearson’s r correlations extracted from each study were transformed into Z-scores, which were then used to calculate average effect sizes for the association between social support and social constraint on the one hand and distress and well-being on the other hand.
Results
Demographic and Clinical Characteristics of Study Samples
Table 1 contains details about some of the design features and sample characteristics of the 32 studies in this review, and a summary of that information follows. Most studies were based in the USA (71.88%; n = 23) [19, 23, 26, 46, 48–53, 56–58, 60, 63–69, 71, 72] with Australia being the second most common study location and most other studies originating from European countries. The number of cancer survivors in each study ranged from 25 [47] to 1,127 [23], with an M (±SD) of 227.94 ± 231.16 and median of 135. In every study, the data reported were comprised entirely of cancer survivors; no other patient populations are represented in this review. Across studies, the M age of participants was 56.62 ± 6.77 years, with a median of 56. Thirty-one percent (n = 10) of studies included only female cancer survivors [19, 23, 26, 46, 48, 59, 62, 68, 69, 72], while 21.88% (n = 7) included only males [51, 52, 55, 61, 65, 66, 71]. Of the remaining studies, the average sample was 48.89 ± 15.95% female, with a median of 51. Across all studies who reported on race and ethnicity, the majority of participants were White, non-Hispanic (M ± SD = 85.90 ± 19.13; median = 91), though one study had a singular focus on Hispanics/Latinxs [46]. Eight studies did not report race and ethnicity, all of which were conducted outside the USA [24, 47, 54, 55, 59, 61, 62, 70]. One third (n = 11) of studies only included married or partnered cancer survivors [26, 49–53, 57, 58, 66, 68, 70]. Of the remainder, an average of 74.87 ± 10.52% (median = 75) of participants were married or partnered.
Table 1.
Sample characteristics of the 32 studies in the qualitative synthesis
| Study | Country | n | Cancer typea |
Cancer stageb |
Cancer phasec | Age (M ± SD) | Female gender (%) | White NH (%) | Partneredd (%) |
|---|---|---|---|---|---|---|---|---|---|
| Badr et al. (2011) [68]; Badr et al. (2010) e [75] |
USA | 201 | Breast | Distant or metastatic | Mixed | 52.2 ± 10.5 | 100.0 | 92.0 | 100.0 |
| Bishop et al. (2007)f [63]; Wingard et al. (2010) [25]e |
USA | 662 | Mixed | Unknown | Mixed | 49.1 ± 10.3 | 62.0 | 92.0 | 73.0 |
| Boinon et al. (2012) [62] | France | 113 | Breast | Mixed, restricted to Stages 1–3 | Less than 1 year postdx | 52.8 ± 10.2 | 100.0 | Unknown | 73.4 |
| Champion et al. (2014)f [23] | USA | 1,127 | Breast | Unknown | Mixed | Unknown | 100.0 | 92.4 | 78.6 |
| Cordova et al. (2001) [69] | USA | 70 | Breast | Mixed, restricted to stages 0–3 | Mixed | 54.7 ± 12.1 | 100.0 | 90.0 | 50.0 |
| Dagan et al. (2011) [70] | Netherlands | 70 | Colorectal | Mixed | Less than 1 year postdx | 61.2 ± 10.0 | 25.7 | Unknown | 100.0 |
| Dunn et al. (2011) [24]; Green et al. (2013)e [73] |
Australia | 439 | Mixed | Unknown | Mixed | 59.3 ± 12.0 | 59.0 | Unknown | 70.0 |
| Eton et al. (2001) [71] | USA | 256 | Prostate | Mixed, restricted to Stages 1–3 | Less than 1 year postdx | 65.0 ± 0.5 | 0.0 | 89.0 | 86.0 |
| Figueiredo et al. (2004) [72] | USA | 66 | Breast | Mixed, restricted to Stages 1–2 | Less than 1 year postdx | 55.2 ± 10.5 | 100.0 | 72.7 | 65.2 |
| Gourvitz (2007) [64] | USA | 91 | Blood | Unknown | Mixed | 56.9 ± 13.2 | 52.8 | 94.5 | 75.8 |
| Graves et al. (2012) [46] | USA | 264 | Breast | Mixed | Mixed | 50.6 ± 9.9 | 100.0 | 0.0 | 57.2 |
| Kangas et al. (2012) [47] | Australia | 25g | Brain | Mixed | Less than 1 year postdx | 43.7 ± 14.0 | 56.3 | Unknown | 76.0 |
| Lepore et al. (1999) [48] | USA | 97 | Breasth | Mixed, restricted to Stages 1–3 | Less than 1 year postdx | 54.0 | 100.0 | 92.0 | 79.0 |
| Manne (1999) [49] | USA | 129 | Mixed | Mixed | Mixed | 54.0 | 67.4 | 95.0 | 100.0 |
| Manne et al. (1997) [50] Manne et al. (1999)e [76] |
USA | 158 | Mixed | Mixed, restricted to Stages 3 and 4 | Less than 1 year postdx | 56.0 | 41.3 | 94.0 | 100.0 |
| Manne et al. (2015) [51] | USA | 139 | Prostate | Mixed, restricted to Stages 1–3 | Mixed | 60.6 ± 7.5 | 0.0 | 76.3 | 100.0 |
| Manne et al. (2010) [52] | USA | 75 | Prostate | Mixed, restricted to Stages 1–2 | Less than 1 year postdx | 59.9 ± 10.9 | 0.0 | 88.0 | 100.0 |
| Manne and Badr (2010) [53] | USA | 109 | Mixed | Mixed | Mixed | Unknown | 37.6 | 92.7 | 100.0 |
| McDowell et al. (2011) [54]; Steginga et al. (2008)e [74] |
Australia | 439 | Mixed | Unknown | Mixed | 59.2 ± 12.0 | 59.0 | Unknown | 70.1 |
| Mehnert et al. (2010) [55] | Germany | 511 | Prostate | Mixed, restricted to Stages 2–4 | Mixed | 64.3 ± 6.0 | 0.0 | Unknown | 87.9 |
| Mosher et al. (2010)f [56] | USA | 253 | Blood | Unknown | 1–4 years postdx | 50.9 ± 12.5 | 51.4 | 87.4 | 73.9 |
| Mosher et al. (2011) [67] | USA | 195 | Blood | Unknown | 1–4 years postdx | 54.0 ± 12.0 | 50.0 | 87.0 | 96.0 |
| Oh (2009) [66] | USA | 50 | Prostate | Unknown | Less than 1 year postdx | 61.3 ± 7.2 | 0.0 | 86.0 | 100.0 |
| Pasipanodya et al. (2012) [26] | USA | 45 | Breast | Mixed, restricted to Stages 1–3 | Less than 1 year postdx | 52.4 ± 9.9 | 100.0 | 94.0 | 100.0 |
| Porter et al. (2005) [57] | USA | 47 | Gastrointestinal | Mixed | Mixed | 61.1 ± 12.2 | 23.4 | 91.5 | 100.0 |
| Porter et al. (2012)f [58] | USA | 130 | Gastrointestinal | Mixed (Stages 2–4) | Mixed | 59.4 ± 12.0 | 29.0 | 84.5 | 100.0 |
| Roberts (2004) [65] | USA | 89 | Prostate | Mixed, restricted to Stages 1–3 | Less than 1 year postdx | 65.7 ± 6.5 | 0.0 | 91.0 | 85.4 |
| Schmidt et al. (2004) [19] | USAi | 210 | Breast | Mixed | Mixed | 47.4 ± 8.4 | 100.0 | 91.0 | 75.2 |
| Shim et al. (2006) [59] | Multiple | 413 | Breast | Mixed | Mixed | 51.6 ± 10.4 | 100.0 | Unknown | 72.4 |
| Swartzman et al. (2017) [21] | Scotland | 205 | Colorectal | Mixed | Mixed | 71.0 ± 8.2 | 39.5 | 97.6 | Unknown |
| Widows et al. (2000) [60] | USA | 102 | Mixed | Mixed | Mixed | 45.0 ± 10.7 | 79.0 | 91.0 | 68.0 |
| Wilson et al. (2014) [61] | Australia | 514 | Prostate | Mixed | Mixed | 70.2 ± 8.36 | 0.0 | Unknown | 84.2 |
M ± SD = mean and standard deviation; Mixed = heterogeneous sample in relation to the variable of interest; NH = non-Hispanic ethnicity; Unknown = data missing or unclear in the paper.
aCancer type refers to the primary cancer site included in the sample.
b Cancer stage was coded as: in situ (Stage 0), local (Stage 1), regional (stages 2–3), distant/metastatic (Stage 4), or mixed if it included more than one of the aforesaid categories.
cCancer phase reflects the sample’s average time since cancer diagnosis and was coded as follows: less than 1 year, 1–4 years, 5 years, more than 5 years, or mixed.
dIndicates the proportion of the sample that was in a romantic relationship.
eData was extracted from this article as it provides relevant information that was not included in the selected paper.
fInformation missing from the selected paper was provided by one of its authors in separate correspondence.
gThe original sample size for this study was (n = 70); however, the results were divided between benign (n = 45) and malignant (n = 25) brain tumors.
hEighty-five percent of the sample was breast cancer survivors.
iEighty percent of the sample was recruited from the USA.
Many studies were exclusive to female breast cancer survivors (31.25%; n = 10) [19, 23, 26, 46, 48, 59, 62, 68, 69, 72]. Other common single cancer type studies were prostate (21.88%; n = 7) [51, 52, 55, 61, 65, 66, 71], gastrointestinal (12.50%; n = 4) [57, 58, 61, 70], hematological/blood (9.38%; n = 3) [56, 64, 67], and brain (3.13%; n = 1) [47] cancers. For studies with mixed cancer types (21.88%; n = 7) [24, 49, 50, 53, 54, 60, 63], a wide range of cancer sites were included. Twenty-five percent (n = 8) of studies did not report participants’ cancer stage [23, 24, 54, 56, 63, 64, 66, 67]. Of those that did, all except one [68] included participants with different cancer stages (95.65%; n = 23). Notably, 25.00% (n = 8) of studies expressly excluded cancer survivors with metastatic cancer [26, 48, 50, 51, 59, 62, 65, 69, 71, 72], while three (9.38%) studies focused exclusively on those with late stage disease [50, 58, 68]. Similar to cancer stage, most studies included participants at different phases of cancer survivorship (59.38%; n = 19) [19, 21, 23, 24, 46, 49, 51, 53–55, 57–61, 63, 64, 68, 69]. However, 34.38% (n = 11) of studies were with recently diagnosed cancer survivors, often diagnosed within the last year [26, 47, 48, 50, 52, 62, 65, 66, 70–72].
Study Design and Construct Measurement
The Supplementary Material 1 describes the basic study design of the 32 studies reviewed and the measures used to assess psychological and social functioning. Only 21.88% (n = 7) of studies were longitudinal [26, 47–49, 65, 66, 70]. Of those seven studies, six included two time points (spanning 3–8 months) and 1 included seven daily assessments [26]. The methodological quality of studies, as judged by STROBE checklists [45], was generally high. Total scores for each study ranged from 70.00 [21, 24, 69, 72] to 87.10 [66, 68], with the best possible score being 100.00. The average methodological quality score was 76.80 ± 4.63 (median = 76.67); the information for each study is available in Supplementary Material.
Psychological functioning
Ninety percent (n = 29) of studies measured distress (exceptions [46, 59, 71]), while only 46.88% measured well-being (n = 15) [23, 24, 26, 46, 48, 50, 51, 57, 59, 61, 63, 65, 66, 69, 71]. Of those that measure distress, 72.41% (n = 21) measure general distress [19, 23, 24, 26, 47–53, 56, 58, 61–63, 66–70] and 44.83% (n = 13) measure cancer-specific distress [19, 21, 23, 24, 49, 54, 56, 57, 60, 61, 64, 65, 69]. Of the 15 that measure well-being, 93.33% (n = 14) measure general well-being [23, 24, 26, 46, 48, 50, 51, 57, 59, 63, 65, 66, 69, 71] and 26.67% (n = 4) evaluate cancer-specific well-being [23, 24, 61, 63].
The most common measures of general distress were the 20-item Center for Epidemiological Studies Depression Scale (23.81%; n = 5), the 7- or 10-item version of the Positive and Negative Affect Schedule—Negative Affect Scale (19.05%; n = 4), the 14-item Hospital Anxiety and Depression Scale (14.29%; n = 3), and the 23- or 24-item version of the Mental Health Inventory—Psychological Distress Scale (14.29%; n = 3); see Table 2. For cancer-specific distress, the most common measures were different versions of the Impact Event Scale (61.53%; n = 8) and 17-item Post-Traumatic Stress Disorder Checklist (46.15%; n = 6); see Table 2. All distress measures were multi-item; however, 20.69% (n = 6) of studies did not provide a coefficient α [21, 23, 47, 55, 63, 64]. Of those that did, the average coefficient α fell well within the acceptable range (M ± SD = .88 ± .06; median = .89).
Table 2.
Bivariate correlations among psychological distress and social functioning for the 32 studies in the qualitative synthesis
| Study | Distress measures | Social constraint measures | Effect size (Pearson’s r)a | Social support measures | Effect size (Pearson’s r)a | Overall finding (Z-score)a |
|---|---|---|---|---|---|---|
| Badr et al. (2011) [68]; Badr et al. (2010)b [75] |
CES-D | MPI-SPc | .20 | MPI-HDRc MPI-SSc |
.01 −.05 |
SC > SS (.20 vs. −.02) |
| Bishop et al. (2007)d [63]; Wingard et al. (2010)b [25] |
CES-D | SCSc | .49 | FSSQ | −.49 | SC > SS (.52 vs. −.35) |
| IES | SCSc | .46 | FSSQ | −.17 | ||
| Boinon et al. (2012) [62] | BDI | PSS-N SS-A |
.22 .10 |
PSS-E PSS-Inf PSS-Inst |
−.01 −.03 −.08 |
SC > SS (.16 vs. .02) |
| PANAS-NA | PSS-N SS-A |
.24 .09 |
PSS-E PSS-Inf PSS-Inst |
.09 .04 .08 |
||
| Champion et al. (2014)d [23] | CES-D | SCSc | .40 | NSSSc | −.36 | SC > SS (.41 vs. −.32) |
| CARS* | SCSc | .32 | NSSSc | −.22 | ||
| IES | SCSc | .42 | NSSSc | −.27 | ||
| TAS | SCSc | .40 | NSSSc | −.40 | ||
| Cordova et al. (2001) [69] | CES-D | SCSe | .72 | TAC | −.31 | SC > SS (.69 vs. −.32) |
| IES-A* | SCSe | .42 | TAC | −.29 | ||
| IES-I* | SCSe | .62 | TAC | −.14 | ||
| Dagan et al. (2011) [70] | CES-D | ISSL-UBc | .17 | ISSL-SBc | −.11 | SC > SS (.17 vs. −.11) |
| Dunn et al. (2011) [24]; Green et al. (2013)b [73] |
HADS-A | SCS | .49 | ESSI | −.36 | SC > SS (.47 vs. −.32) |
| HADS-D | SCS | .35 | ESSI | −.40 | ||
| IES-A* | SCS | .46 | ESSI | −.26 | ||
| IES-I* | SCS | .45 | ESSI | −.22 | ||
| Figueiredo et al. (2004) [72] | MOS-RL | USII | .33f | SSQ | −.21f | SC > SS (.33 vs. −.21) |
| Gourvitz (2007) [64] | PCL-C* | SCS SIP-Nc,e |
.55 .41, .50 |
SIP-P c, e | .05, −.02 | SC > SS (.53 vs. .02) |
| Kangas et al. (2012) [46] | PCL-Sg | SCSc,e | .33, .48 | PRCIe | .23 | SC > SS (.43 vs. .23) |
| Lepore et al. (1999) [48] | PANAS-NA | SCSc,e | .20, .32 | UCLA-SSIc,e | .16, .10 | SC > SS (.27 vs. .13) |
| Manne (1999) [49] | IES* | PNSB-ARc PNSB-CRc |
.23 .17 |
ASc | .06 | SC > SS (.27 vs. −.02) |
| MHI-PDS | PNSB-ARc PNSB-CRc |
.35 .29 |
ASc | −.10 | ||
| Manne et al. (1997) [50]; Manne et al. (1999)b [76] |
MHI-PDS | PNSB-ARc PNSB-CRc |
.36h, .38i .38h, .44i |
PSSBc | −.21h, .18i | SC > SS (.41 vs. −.02) |
| Manne et al. (2015) [51] | MHI-PDS | HBc | .49 | PAIRc | −.33 | SC > SS (.53 vs. −.34) |
| Manne et al. (2010) [52] | BSI-GSI | HBc MAc PD-PWc |
.12 .25 .13 |
MCCc PAIRc LDc |
−.36 −.40 .07 |
SC < SS (.17 vs. −.24) |
| Manne and Badr (2010) [53] | BSI-GSI | PBc | .39 | CSRIc PAIRc SDc |
−.14 −.14 −.22 |
SC > SS (.41 vs. −.14) |
| McDowell et al. (2011) [54]; Steginga et al. (2008)b [74] |
IES* | NASH SCS |
.26 .48 |
ESSI PASH |
−.26 .39 |
SC > SS (.39 vs. .07) |
| Mehnert et al. (2010) [55] | Psych- composite | ISSS-NS | 1.38j | ISSS-PS | −.69 j | SC > SS (1.38 vs. −.69) |
| Mosher et al. (2010)d [56] | BSI-GSI | SCS | .44 | PRCIe | −.24 | SC > SS (.45 vs. −.23) |
| PCL-C* | SCS | .41 | PRCIe | −.21 | ||
| Mosher et al. (2011) [57] | BSI-GSI | SCSc,e UCLA-LS |
.42, .52 .61 |
PANSES-ESc,e | −.10, −.19 | SC > SS (.58 vs. −.15) |
| Oh (2009) [66] | PANAS-NA | SCSc PB-Ptc PB-Spc |
.03 .10 .18 |
BSS-Rc DAS-Rc |
−.24 −.39 |
SC < SS (.10 vs. −.33) |
| Pasipanodya et al. (2012) [26] | PANAS-NA | SCS | .51 | DAS-RHc LIc QMIc |
−.12 −.40 −.34 |
SC > SS (.56 vs. −.30) |
| Porter et al. (2005) [57] | IES-A* | HBc | .33 | LDc | .20 | SC > SS (.36 vs. .17) |
| IES-I* | HBc | .36 | LDc | .14 | ||
| Porter et al. (2012)d [58] | POMS | HBc PNSBc |
.34 .29 |
LDc MSISc |
−.19 .11 |
SC > SS (.33 vs. −.04) |
| Roberts (2004) [65] | IES* | SCSe | .43 | SPS | −.36 | SC > SS (.46 vs. −.38) |
| Schmidt et al. (2004) [19] | HADS-A | SCS | .40 | FSSQ | −.28 | SC > SS (.42 vs. −.27) |
| HADS-D | SCS | .44 | FSSQ | −.42 | ||
| IES-A* | SCS | .42 | FSSQ | −.18 | ||
| IES-I* | SCS | .32 | FSSQ | −.17 | ||
| Swartzman et al. (2017) [21] | PCL* | SCS | .62 | MOS-SSS GISe |
−.24 −.37 |
SC > SS (.73 vs. −.32) |
| Widows et al. (2000) [60] | PCL* | SCS | .44 | ISEL | −.43 | SC > SS (.47 vs. −.46) |
| Wilson et al. (2014) [61] | ERRI-IR | SCS | .37 | MEIM-CP MEIM-UP |
.29 .33 |
SC > SS (.50 vs. .22) |
| IES-A* | SCS | .51 | MEIM-CP MEIM-UP |
.18 .19 |
||
| IES-I* | SCS | .51 | MEIM-CP MEIM-UP |
.15 .15 |
Acronym explanation for each measure is available in Supplementary Material 1.
Unknown = information not provided. SS = social support. SC = social constraint.
*Cancer-specific measure.
aData in the first and second effect size columns correspond to the Pearson’s r correlation between the measure(s) of distress and social constraint and social support, respectively. Data in the overall finding column summarize the head-to-head comparison of the Z-score transformed average effect size for social constraint and social support based on the aforementioned effect size data. Here, the information inside the parentheses compares the average effect size for social constraint versus the average effect size for social support across all measures of all variables considered.
bData was extracted from this article as it provides relevant information that was not included in the selected paper.
cData correspond to a measure specific to spouses or partners.
dInformation missing from the selected paper was provided by one of its authors in separate correspondence.
eThe data correspond to a measure specific to family or friends.
fData shown corresponds to a covariate-adjusted, standardized beta weight from a hierarchical linear regression.
gCorrelations of social functioning at baseline with Time 2 psychological distress variables.
hCorrelations reported for female participants in the study sample.
iCorrelations reported for male participants in the study sample.
jData shown corresponds to a covariate-adjusted, standardized beta weight from a logistic regression.
The most common measures of general well-being were the Medical Outcomes Study—Mental Health Index (21.43%; n = 3), the 5- and 10-item version of the Positive and Negative Affect Schedule—Positive Affect Scale (21.43%; n = 3), the 6-item Functional Assessment of Cancer Therapy—Emotional Well-Being Scale (14.29%; n = 2), and the 14-item Mental Health Inventory—Psychological Well-Being Scale (14.29%; n = 2); see Table 3. Few measures of well-being were identified as cancer specific, and they included the 21-item Posttraumatic Growth Inventory (75.00%; n = 3) and 17-item Benefit Finding Questionnaire (25.00%; n = 1); see Table 3. Only one study had a single-item measure for well-being [26] and 50.00% (n = 7) of studies with multi-item measures did not provide a coefficient α [23, 24, 46, 59, 63, 65, 71]. The average coefficient α reported for the multi-item measures was high (M ± SD = .91 ± .06; median = .94).
Table 3.
Bivariate correlations among psychological well-being and social functioning for the 32 studies in the qualitative synthesis
| Study | Well-being measures | Social constraint measures | Effect size (Pearson’s r)a | Social support measures | Effect size (Pearson’s r)a | Overall finding (Z-score)a |
|---|---|---|---|---|---|---|
| Bishop et al. (2007)b [63]; Wingard et al. (2010)c [25] |
MOS-MHI | SCSd | −.41 | FSSQ | .48 | SC < SS (−.19 vs. .34) |
| PTGI* | SCSd | .05 | FSSQ | .15 | ||
| Champion et al. (2014)b [23] | IWB | SCSd | .04 | NSSSd | .13 | SC < SS (−.17 vs. .31) |
| PTGI* | SCSd | −.37 | NSSSd | .45 | ||
| Cordova et al. (2001) [69] | RWBS | SCSe | −.57 | TAC | .36 | SC > SS (−.65 vs. .38) |
| Dunn et al. (2011) [24]; Green et al. (2013)c [73] |
BFQ* | SCS | .10 | ESSI | .18 | SC < SS (−.09 vs. −.33) |
| MOS-MHI | SCS | −.28 | ESSI | −.69 | ||
| Eton et al. (2001) [71] | PCI-MF | SCSd,e | −.26, −.26 | SPSd,e | .16, .16 | SC > SS (−.27 vs. .16) |
| Graves et al. (2012) [46] | FACT-EWB | SCS | −.23 | FSSQ | .19 | SC > SS (−.23 vs. .19) |
| Lepore et al. (1999) [48] | PANAS-PA | SCSd,e | .01, −.03 | UCLA-SSId,e | .07, 07 | SC < SS (−.01 vs. .07) |
| Manne et al. (1997) [50] Manne et al. (1999)c [76] |
MHI-PWB | PNSB-CRd PNSB-ARd |
−.30f, −.39g −.30f, −.39g |
PSSBd | .24f, −.14g | SC > SS (−.36 vs. .05) |
| Manne et al. (2015) [51] | MHI-PWB | HBd | −.54 | PAIRd | .49 | SC > SS (−.60 vs. .54) |
| Oh (2009) [66] | PANAS-PA | SCSc PB-Ptc PB-Spc |
.18 −.10 −.02 |
BSS-Rc DAS-Rc |
−.16 −.04 |
SC < SS (.02 vs. −.10) |
| Pasipanodya et al. (2012) [26] | PANAS-PA | SCS | −.05 | DAS-RHd LId QMId |
.24 .27 .35 |
SC < SS (−.23 vs. .46) |
| SE | SCS | −.39 | DAS-RHd LId QMId |
.58 .59 .50 |
||
| Porter et al. (2005) [57] | FACT-EWB | HBd | −.24 | LDd | −.07 | SC > SS (−.24 vs. −.07) |
| Roberts (2004) [65] | MOS-MHI | SCSe | −.16 | SPS | .21 | SC < SS (−.16 vs. .21) |
| Shim et al. (2006) [59] | SF12-MCS | ISSS-NS | −.33, −.19h | ISSS-PS | .29, .12h | SC > SS (−.27 vs. .21) |
| Wilson et al. (2014) [61] | PTGI-AL* | SCS | .14 | MEIM-CP MEIM-UP |
.34 .32 |
SC < SS (.10 vs. .36) |
| PTGI-NP* | SCS | .11 | MEIM-CP MEIM-UP |
.37 .43 |
||
| PTGI-PS* | SCS | .05 | MEIM-CP MEIM-UP |
.29 .32 |
Acronym explanation for each measure is available in Supplementary Material 1.
Unknown = information not provided; SS = social support. SC = social constraint.
*Cancer-specific measure.
aData in the first and second effect size columns correspond to the Pearson’s r correlation between the measure(s) of well-being and social constraint and social support, respectively. Data in the overall finding column summarize the head-to-head comparison of the Z-score transformed average effect size for social constraint and social support based on the aforementioned effect size data. Here, the information inside the parentheses compares the average effect size for social constraint versus the average effect size for social support across all measures of all variables considered.
bThe author provided additional information for inclusion.
cThe article provides additional relevant information (demographics information and/or results) not included in the selected article.
dData correspond to a measure specific to the spouse or partner.
eThe data correspond to a measure specific to family or friends.
fThe correlations reported for the female sample.
gCorrelations reported for the male sample.
hData shown corresponds to a covariate-adjusted, standardized beta weight from a linear regression. The first value corresponds to data from Germany and the second to Japan.
Social functioning
The most common social constraint measures were at least four different versions of the Social Constraint Scale (59.38%; n = 19) followed by a 10-item measure of Holding Back (12.50%; n = 4), 13-item measure of Perceived Negative Spouse Behaviors (9.38%; n = 3), and 9-item Illness-Specific Social Support Scale—Negative Support Subscale (6.25%; n = 2). All measures included were multi-item. However, 31.25% (n = 10) of studies did not provide a coefficient α [21, 23, 47, 55, 58, 59, 62–64, 71]. The reported coefficient α for the social constraint measures ranged from poor to excellent (M ± SD = .83 ± .10; median = .88), with 21.88% (n = 7) of studies having a social constraint measure with a coefficient α < .80 [46, 50, 53, 62, 66, 68, 70].
In contrast to social constraint, less consistency across studies was found for the social support measures. Social support was more commonly measured through the 8-item Duke-UNC Functional Social Support Questionnaire (9.38%; n = 3), a 10-item Level of Self-Disclosure measure (9.38%; n = 3), and the 7-item Personal Assessment of Intimacy in Relationships (9.38%; n = 3). Nine percent (n = 3) of studies had a single-item measure for social support [26, 49, 69], and 28.13% (n = 9) of studies did not provide a coefficient α for the multi-item measures [21, 23, 47, 55, 58, 59, 63, 64, 71]. The reported coefficient α for the multi-item social support measures ranged from poor to excellent (M ± SD = .83 ±.10; median = .85), with 25.00% (n = 8) of studies having a social support measure with a coefficient α < .80 [48, 50, 54, 61, 65, 67, 68, 70].
Seventy-two percent (n = 23) of studies reported a correlation between social constraint and social support. These variables were negatively correlated to each other 79.17% (n = 38) of the time, and the mean effect size was Z = −.26 (SD = .27). No clear pattern emerged in terms of the relationship between measures used and direction or strength of the association.
Association between Psychological and Social Functioning
General and cancer-specific distress
Table 2 shows the relationship between general distress and both social constraint and social support. Social constraint was positively correlated with general distress in all cases (n = 42 correlations) and social support was negatively correlated with general distress 72.72% (n = 32 correlations) of the time. With the exception of two studies [52, 66], social constraint had a stronger overall association with general distress than social support. This can be observed in the last column of Table 2, which compares the mean effect size for social constraint to social support across the various measures of distress in any given study. The mean effect size across all studies was Z = .39 (SD = .18) for social constraint and Z = −.15 (SD = .21) for social support. As an example, in the Manne et al. study [51], the Holding Back measure of social constraint was correlated with the Mental Health Inventory—Psychological Distress Scale at r = .49, while the Personal Assessment of Intimacy in Relationships measure of social support was correlated with this same distress measure at r = −.33. Similarly, Dunn et al. [24] found that the Social Constraint Scale was correlated with Hospital Anxiety and Depression Scale anxiety and depression subscales at r = .49 and .35, respectively (Z-score M ± SD = .45 ± .12), while the ENRICHD Social Support Instrument measure of social support was correlated with the same distress measures at r = −.36 and −.40, respectively (Z-score M ± SD = −.40 ± .03).
Table 2 also includes the bivariate correlations for the outcome of cancer-specific distress. Similar to general distress, social constraint was positively correlated with cancer-specific distress in all cases (n = 23 correlations), while social support was negatively correlated with this outcome only 62.50% (n = 15 correlations) of the time. Across the 13 relevant studies, social constraint had a stronger overall association with distress in all studies. The mean effect size was Z = .42 (SD = .15) for social constraint versus Z = −.14 (SD = .21) for social support. As an example, in the Champion et al. study [23], the Social Constraint Scale was correlated with the Concerns About Recurrence Scale at r = .32, while the Northouse Social Support Scale was correlated with this same distress measure at r = −.22. Similarly, in the Schmidt et al. study [19], another Social Constraint Scale was correlated with the Impact Event Scale Avoidance and Intrusion subscales at r = .42 and .32, respectively (Z-score M ± SD = .39 ± .08), while the Duke-UNC Functional Social Support Questionnaire measure was correlated with these same distress measures at r = −.18 and −.17, respectively (Z-score M ± SD = −.18 ± .01). For both general and cancer-specific distress, the effect size varied across the measures used in any given study, but the overall trend of social constraint demonstrating a more consistent and stronger association with distress than social support is clear.
General and cancer-specific well-being
Table 3 contains the bivariate correlations between general well-being and both social constraint and social support. Of all the associations between social constraint and general well-being, 86.36% (n = 19 correlations) were negative, and the associations between social support and general well-being were positive 82.61% (n = 19 correlations) of the time. Of the 14 relevant studies, 50.00% (n = 7) reported a stronger association with social support than social constraint. The mean effect size across studies was Z = −.26 (SD = .21) for social constraint and Z = .14 (SD = .35) for social support. For example, in the Graves et al. study [46], the Social Constraint Scale was correlated with the Functional Assessment of Cancer Therapy—Emotional Well-Being Scale measure of general well-being at r = −.23, while the Duke-UNC Functional Social Support Questionnaire was correlated with this same well-being measure at r = .19. However, in the Bishop et al. study [63], the Social Constraint Scale was correlated with Medical Outcomes Study—Mental Health Index measure of general well-being at r = −.41 while the Duke-UNC Functional Social Support Questionnaire was correlated with this same outcome at r = .48.
Information pertinent to cancer-specific well-being is also found in Table 3. Only 16.67% (n = 1 correlation) of the correlations between social constraint and cancer-specific well-being were negative, while all the correlations between social support and cancer-specific well-being were positive (n = 9 correlations). Overall, in each of the four relevant studies, social support exhibited a stronger relationship with cancer-specific well-being than social constraint. The mean effect size across studies was Z = −.03 (SD = .24) for social constraint and Z = .29 (SD = .15) for social support. For example, Bishop et al.’s study [63] found that the Social Constraint Scale was correlated with a Posttraumatic Growth Inventory measure of cancer-specific well-being at r = .05, while the Duke-UNC Functional Social Support Questionnaire was correlated with the same well-being measure at r = .15. Another example can be seen in Champion et al.’s study [23]; the Social Constraint Scale was correlated with the Posttraumatic Growth Inventory at r = −.37, while the Northouse Social Support Scale measure of social support was correlated with it at r = .45. In summary, there appear to be differences in the nature and magnitude of the influence of social constraint and social support, with the effects at least partly dependent upon the outcome of interest (i.e., general versus cancer-specific well-being).
Discussion
Social functioning is theorized to help explain the psychological functioning of cancer survivors and other patient populations [1, 2] yet, as a multidimensional construct, it is likely that the nature and magnitude of social functioning’s association with psychological functioning depend on the specific constructs under consideration. This is the first systematic review to evaluate if social constraint or social support has a stronger association with two specific dimensions of psychological functioning, namely distress and well-being. The findings of this review unequivocally reaffirm the relevance of social constraint for understanding the distress of cancer survivors as was recently shown in another review [35]. A particularly noteworthy contribution of this review is the collection of evidence that supports the hypothesis that social constraint is more consistently and strongly associated with distress—both general distress and cancer-specific distress—than social support [35, 39, 77]. Another key finding is that, while social constraint and social support demonstrated the expected pattern of associations with general well-being (i.e., mainly negative associations for social constraint, while mainly positive ones for social support), social constraint was more strongly associated with general well-being than social support, which actually runs counter to a priori hypotheses. Remarkably, the same finding was not observed for cancer-specific well-being, albeit only four studies evaluated these relationships. As it pertains to cancer-specific well-being, social support, as opposed to social constraint, demonstrated a much more consistent and stronger association as predicted. In sum, for three of the four psychological outcomes evaluated (specifically, general distress, cancer-specific distress, and general well-being), social constraint exhibited a stronger connection than social support.
As noted in the Introduction, the social-cognitive processing model could explain the influence of social functioning on psychological functioning. Based on this model, one would expect cancer survivors to cope with the stress and aftermath of their cancer diagnosis via complex emotional and cognitive processes [15]. From this vantage point, social constraint would hinder cancer survivors’ adjustment because it limits their opportunities to process their cancer experience [15], while social support would hasten cancer survivors’ adjustment because it affords the opportunity for sincere condolences, communal experiences, and solace in the face of stress. Studies with breast cancer survivors have demonstrated that intrusive thoughts do mediate the relationship between social constraint and depressive symptoms [69, 78], which indicates that social constraint is in fact linked with adaptive emotional/cognitive processing or the lack thereof. The importance of social support is not to be discounted though as it is consistently (even if only modestly) correlated with cancer survivors’ distress and well-being as demonstrated here. That said, it may be that negative interactions have a stronger effect on psychological functioning than positive interactions [40, 77], which would mean that a copious amount of social support is needed to counter the negative psychological effects of social constraint.
Although psychological functioning was treated as the outcome in this review, the findings are based on the results of bivariate correlational analyses. Because of this, the possibility of a reciprocal relationship between social functioning and psychological functioning cannot be ignored, and it should actually be expected based on the foremost quality-of-life models [79]. That said, four studies in this review evaluated the longitudinal relationship between the quality-of-life domains of interest here and, in each study, social functioning was the predictor and psychological functioning the outcome. While these studies are not in the position to rule in or out the possibility that psychological functioning influences social functioning, they do underscore the potential for social functioning to impact cancer survivors’ future psychological functioning. Furthermore, with only one exception [66], social constraint was indeed a stronger predictor of distress than social support [49, 70, 73]. Only two studies evaluated well-being as an outcome: in one case, social support had a stronger association with well-being than social constraint [73] and, in the other case, social constraint exhibited the stronger association [66]. Although small in number, these longitudinal studies are consistent with the results of the review as a whole.
Perhaps the best test of the relative importance of social constraint versus social support for cancer survivors’ psychological functioning is one in which any overlap between the constructs is controlled and only their unique variance evaluated. Unfortunately, few studies in this review reported the results of analyses in which social constraint and social support were examined in the same regression or structural equation model. For those that did, it was generally found that social constraint had a stronger association with psychological functioning [19, 21, 25, 55, 59, 62, 67, 72], but social support was the winner in some cases [24, 60, 61, 73, 80]. This inconsistency in findings across studies could be explained by multiple factors, including but not limited to the combination of covariates included in the models, diversity in the measures used, and sample characteristics. Given this, no definitive conclusions can be reached about the relative importance of one variable versus the other, but the evidence is certainly mounting that negative exchanges (including the experience of social constraint) may be more impactful than positive exchanges when it comes to psychological functioning [40, 77].
In addition to the key findings and implications just discussed, this review documents a strikingly large number of measures of each variable under investigation. In the records reviewed, the psychometric properties of these measures were missing in some cases and inconsistently reported in others but, based on available information, most measures are valid and reliable. All of the studies reviewed herein have sound methodology and adhere to reporting standards but, to facilitate replicability and comparability of study findings, future quality-of-life researchers should consider adopting a standard, agreed-upon set of psychometrically sound measures. In this review, the Social Constraint Scale was utilized in most studies and it is the “gold standard” measure of social constraint [35]. For the other variables of interest, where many sound measures are available, the universality of the measure should be considered. The Patient-Reported Outcomes Measurement Information System (PROMIS) provides valid, reliable measures of social support, distress, and well-being, all of which are publicly available and many of which are available in languages other than English [81]. PROMIS is intended to increase consistency in measurement of health and related outcomes across research and clinical settings, and it is acceptable for use with cancer survivors [82, 83]. Thus, one clear recommendation that arises from this review is that researchers adopt greater uniformity in measures to facilitate comparability across studies and ease conduct of meta-analyses, with one option being use of the Social Constraint Scale and PROMIS.
The results and implications of this review must be considered in light of its shortcomings. First, because of substantial heterogeneity across studies, a meta-analysis was not done. However, standardized effect sizes for the correlations between social support and social constraint with psychological functioning were reported and the evaluation of bivariate analysis allowed a more direct comparison across studies by reducing the effects of analysis heterogeneity. Second, because this review relies heavily on cross-sectional studies, the findings cannot be used to indicate causality and should not be interpreted as such. Third, although social functioning is a multidimensional concept, this review only evaluated two of its many dimensions, and this evaluation was further limited by the nature of the measures used in the studies reviewed (primarily, perceived social support alongside perceived social constraint). Fourth, this review only included published studies and dissertations, ignoring the “grey literature” altogether. This is problematic because it reduces the amount of relevant data available for review and raises the possibility that publication bias influenced the results. Fifth, these findings are not generalizable across cancer survivors from any and all countries and cultures. Most studies were done in the USA, which is likely a result of the review’s English language restriction. Most studies also included samples that were mostly White non-Hispanic, which may further limit generalizability. Evaluating the study location and cancer survivors’ race and ethnicity is recommended, given that culturally specific social norms could influence individuals’ perception of their social functioning and its relationship with psychological functioning. Sixth, one third of studies reviewed herein focused on married couples. Being in a romantic relationship, whether married or partnered, is associated with better quality of life in breast cancer survivors [84]; thus, greater representation of partnered participants in this review could bias the results toward better overall quality of life. Additionally, a focus on married couples at the exclusion of others in a committed, romantic relationship is not an inclusive approach to research on social functioning and unfairly limits representation of the cancer survivors in any review. Seventh, this review excludes studies that focus on children and adolescents. This is because cancer is primarily a disease of old age [85] and child/adolescent cancer survivors are expected to have social networks, relationship dynamics, and cognitive and psychological capacities that differ from adult cancer survivors [86], giving rise to the possibility of unclear or inconsistent findings simply due to too much variation in participants’ age. Finally, most studies reviewed focus on breast and prostate cancer survivors, perhaps because they are the most common gender-specific types in females and males, respectively [85]. However, lung cancer was not the focus of any study and very few focused on colorectal cancer despite the predominance of these cancer types in the USA [85] and other countries [87].
Conclusions
This review highlights the relevance of social constraint for studies of cancer survivors’ psychological functioning. The stronger overall association between social constraint and psychological functioning relative to social support supports the claim that these constructs are distinct, albeit related dimensions of social functioning [15, 35], with potentially distinct impacts on other quality-of-life outcomes. It should, therefore, be appreciated that social constraint occurs in individuals with other chronic diseases (e.g., HIV and rheumatoid arthritis) [88, 89], and its observed relationship with cancer survivors’ psychological functioning may well replicate in other patient populations. The results of this review suggest that social constraint should be a central target when designing interventions to reduce general and cancer-specific distress or promote overall well-being in cancer survivors; however, in interventions that attempt to increase cancer-specific well-being, social support might instead be the principal target. In conclusion, the interconnectedness of social functioning and psychological functioning is at least partly dependent upon the underlying dimensions at play, with social constraint and social support having unique associations with the distress and well-being of cancer survivors.
Supplementary Material
Acknowledgments
The authors acknowledge the work of Spencer Acadia, PhD, who assisted with the search of electronic databases, Gabriella Puleo, MS, and Shannon Kelly who assisted with the coding and data extraction process, and Will Bowling who assisted in the evaluation of quality of the records included in this review. In addition, the authors would like to thank Catherine Mosher, PhD, Dennis Turk, PhD, Kathleen M. Ingram, PhD, Mariko Shiozaki, PhD, Michelle Bishop, PhD, Patrick Monahan, MD, PhD, Timothy Stump, MS, Victoria Champion, PhD, and Laura Porter, PhD, for responding to email requests for additional information about their studies.
Funding
This research was supported by grant K07 CA181351 from the National Cancer Institute (J.L.B.) and grant UL1TR001998 from the National Center for Advancing Translational Sciences(REDCap). Its content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Compliance with Ethical Standards
Authors’ Statement of Conflict of Interest and Adherence to Ethical Standards The authors have no conflict of interest to disclose.
Authors’ Contributions Jessica N. Rivera Rivera and Jessica L. Burris jointly conceived and designed the systematic review; Jessica N. Rivera Rivera served as the primary reviewer, coder, and analyst for the systematic review; Jessica N. Rivera Rivera and Jessica L. Burris collaborated on data syntehsis and manuscript preparation. Both authors read and approved the final manuscript.
Ethical Approval Approval by an Institutional Review Board was not required given that this study did not involve human subjects or primary data collection.
Informed Consent Informed consent did not apply to this study as no human subjects were involved.
References
- 1. Ferrell BR, Hassey Dow K. Quality of life among long-term cancer survivors. Oncology (Williston Park). 1997;11:565–568, 571; discussion 572, 575. [PubMed] [Google Scholar]
- 2. Bloom JR, Petersen DM, Kang SH. Multi-dimensional quality of life among long-term (5+ years) adult cancer survivors. Psychooncology. 2007;16:691–706. [DOI] [PubMed] [Google Scholar]
- 3. Burris JL, Andrykowski MA. Physical and mental health status and health behaviors of survivors of multiple cancers: A national, population-based study. Ann Behav Med. 2011;42:304–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lee LJ, Chung CW, Chang YY, et al. . Comparison of the quality of life between patients with non-small-cell lung cancer and healthy controls. Qual Life Res. 2011;20:415–423. [DOI] [PubMed] [Google Scholar]
- 5. LeMasters T, Madhavan S, Sambamoorthi U, Kurian S. A population-based study comparing HRQoL among breast, prostate, and colorectal cancer survivors to propensity score matched controls, by cancer type, and gender. Psychooncology. 2013;22:2270–2282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Richardson LC, Wingo PA, Zack MM, Zahran HS, King JB. Health-related quality of life in cancer survivors between ages 20 and 64 years: Population-based estimates from the Behavioral Risk Factor Surveillance System. Cancer. 2008;112:1380–1389. [DOI] [PubMed] [Google Scholar]
- 7. Carlson LE, Waller A, Mitchell AJ. Screening for distress and unmet needs in patients with cancer: Review and recommendations. J Clin Oncol. 2012;30:1160–1177. [DOI] [PubMed] [Google Scholar]
- 8. Andrykowski MA, Lykins E, Floyd A. Psychological health in cancer survivors. Semin Oncol Nurs. 2008;24:193–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jim HS, Jacobsen PB. Posttraumatic stress and posttraumatic growth in cancer survivorship: A review. Cancer J. 2008;14:414–419. [DOI] [PubMed] [Google Scholar]
- 10. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology—Distress management—Version 3.2019.2019. DIS-2. Available at: https://www.nccn.org/professionals/physician_gls/pdf/distress.pdf. Accessibility verified September 4, 2019.
- 11. DiMatteo MR, Lepper HS, Croghan TW. Depression is a risk factor for noncompliance with medical treatment: Meta-analysis of the effects of anxiety and depression on patient adherence. Arch Intern Med. 2000;160:2101–2107. [DOI] [PubMed] [Google Scholar]
- 12. Prieto JM, Blanch J, Atala J, et al. . Psychiatric morbidity and impact on hospital length of stay among hematologic cancer patients receiving stem-cell transplantation. J Clin Oncol. 2002;20:1907–1917. [DOI] [PubMed] [Google Scholar]
- 13. Carlson LE, Bultz BD. Efficacy and medical cost offset of psychosocial interventions in cancer care: Making the case for economic analyses. Psychooncology. 2004;13:837–849; discussion 850. [DOI] [PubMed] [Google Scholar]
- 14. Koutrouli N, Anagnostopoulos F, Potamianos G. Posttraumatic stress disorder and posttraumatic growth in breast cancer patients: A systematic review. Women Health. 2012;52:503–516. [DOI] [PubMed] [Google Scholar]
- 15. Lepore SJ, Reverson TA. Social constraints on disclosure and adjustment to cancer. Soc Personal Psychol Compass. 2007;1:313–33. [Google Scholar]
- 16. Carpenter KM, Fowler JM, Maxwell GL, Andersen BL. Direct and buffering effects of social support among gynecologic cancer survivors. Ann Behav Med. 2010;39:79–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. De Leeuw JR, De Graeff A, Ros WJ, Hordijk GJ, Blijham GH, Winnubst JA. Negative and positive influences of social support on depression in patients with head and neck cancer: A prospective study. Psychooncology. 2000;9:20–28. [DOI] [PubMed] [Google Scholar]
- 18. Naughton MJ, Herndon JE II, Shumaker SA, et al. . The health-related quality of life and survival of small-cell lung cancer patients: Results of a companion study to CALGB 9033. Qual Life Res. 2002;11:235–248. [DOI] [PubMed] [Google Scholar]
- 19. Schmidt JE, Andrykowski MA. The role of social and dispositional variables associated with emotional processing in adjustment to breast cancer: An internet-based study. Health Psychol. 2004;23:259–266. [DOI] [PubMed] [Google Scholar]
- 20. Smith SG, Turner B, Pati J, Petrides KV, Sevdalis N, Green JS. Psychological impairment in patients urgently referred for prostate and bladder cancer investigations: The role of trait emotional intelligence and perceived social support. Support Care Cancer. 2012;20:699–704. [DOI] [PubMed] [Google Scholar]
- 21. Swartzman S, Sani F, Munro AJ. The role of social support, family identification, and family constraints in predicting posttraumatic stress after cancer. Psychooncology. 2017;26:1330–1335. [DOI] [PubMed] [Google Scholar]
- 22. Dakof GA, Taylor SE. Victims’ perceptions of social support: What is helpful from whom? J Pers Soc Psychol. 1990;58:80–89. [DOI] [PubMed] [Google Scholar]
- 23. Champion VL, Wagner LI, Monahan PO, et al. . Comparison of younger and older breast cancer survivors and age-matched controls on specific and overall quality of life domains. Cancer. 2014;120:2237–2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dunn J, Occhipinti S, Campbell A, Ferguson M, Chambers SK. Benefit finding after cancer: The role of optimism, intrusive thinking and social environment. J Health Psychol. 2011;16:169–177. [DOI] [PubMed] [Google Scholar]
- 25. Wingard JR, Huang IC, Sobocinski KA, et al. . Factors associated with self-reported physical and mental health after hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2010;16:1682–1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pasipanodya EC, Parrish BP, Laurenceau JP, et al. . Social constraints on disclosure predict daily well-being in couples coping with early-stage breast cancer. J Fam Psychol. 2012;26:661–667. [DOI] [PubMed] [Google Scholar]
- 27. Arora NK, Finney Rutten LJ, Gustafson DH, Moser R, Hawkins RP. Perceived helpfulness and impact of social support provided by family, friends, and health care providers to women newly diagnosed with breast cancer. Psychooncology. 2007;16:474–486. [DOI] [PubMed] [Google Scholar]
- 28. Badr H, Pasipanodya EC, Laurenceau JP. An electronic diary study of the effects of patient avoidance and partner social constraints on patient momentary affect in metastatic breast cancer. Ann Behav Med. 2013;45:192–202. [DOI] [PubMed] [Google Scholar]
- 29. Luszczynska A, Pawlowska I, Cieslak R, Knoll N, Scholz U. Social support and quality of life among lung cancer patients: A systematic review. Psychooncology. 2013;22:2160–2168. [DOI] [PubMed] [Google Scholar]
- 30. Nausheen B, Gidron Y, Peveler R, Moss-Morris R. Social support and cancer progression: A systematic review. J Psychosom Res. 2009;67:403–415. [DOI] [PubMed] [Google Scholar]
- 31. Salonen P, Tarkka MT, Kellokumpu-Lehtinen PL, Koivisto AM, Aalto P, Kaunonen M. Effect of social support on changes in quality of life in early breast cancer patients: A longitudinal study. Scand J Caring Sci. 2013;27:396–405. [DOI] [PubMed] [Google Scholar]
- 32. Turner-Cobb JM, Sephton SE, Koopman C, Blake-Mortimer J, Spiegel D. Social support and salivary cortisol in women with metastatic breast cancer. Psychosom Med. 2000;62:337–345. [DOI] [PubMed] [Google Scholar]
- 33. Koopman C, Hermanson K, Diamond S, Angell K, Spiegel D. Social support, life stress, pain and emotional adjustment to advanced breast cancer. Psychooncology. 1998;7:101–111. [DOI] [PubMed] [Google Scholar]
- 34. Dukes Holland K, Holahan CK. The relation of social support and coping to positive adaptation to breast cancer. Psychol Health. 2003;18:15–29. [Google Scholar]
- 35. Adams RN, Winger JG, Mosher CE. A meta-analysis of the relationship between social constraints and distress in cancer patients. J Behav Med. 2015;38:294–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Thoits PA. Mechanisms linking social ties and support to physical and mental health. J Health Soc Behav. 2011;52:145–161. [DOI] [PubMed] [Google Scholar]
- 37. Taylor SE. Social support: A review. In: Friedman M. S., ed. The Oxford Handbook of Health Psychology. New York: Oxford University Press; 2011:189–214. [Google Scholar]
- 38. Ingersoll-Dayton B, Morgan D, Antonucci T. The effects of positive and negative social exchanges on aging adults. J Gerontol B Psychol Sci Soc Sci. 1997;52:S190–S199. [DOI] [PubMed] [Google Scholar]
- 39. Newsom JT, Rook KS, Sorkin DH. Understanding the relative importance of positive and negative social exchanges: Examining specific domains and appraisals. J Gerontol. 2005;6:304–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Newsom JT, Nishishiba M, Morgan DL, Rook KS. The relative importance of three domains of positive and negative social exchanges: A longitudinal model with comparable measures. Psychol Aging. 2003;18:746–754. [DOI] [PubMed] [Google Scholar]
- 41. Mallinckrodt B, Armer JM, Heppner PP. A threshold model of social support, adjustment, and distress after breast cancer treatment. J Couns Psychol. 2012;59:150–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mendeley. Mendeley Ltd. London, UK: Elsevier; 2018. [Google Scholar]
- 44. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative The strengthening the reporting of observational studies in epidemiology (STROBE) statement: Guidelines for reporting observational studies. J Clin Epidemiol. 2008;61:344–349. [DOI] [PubMed] [Google Scholar]
- 46. Graves KD, Jensen RE, Cañar J, et al. . Through the lens of culture: Quality of life among Latina breast cancer survivors. Breast Cancer Res Treat. 2012;136:603–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kangas M, Tate RL, Williams JR, Smee RI. The effects of radiotherapy on psychosocial and cognitive functioning in adults with a primary brain tumor: A prospective evaluation. Neuro Oncol. 2012;14:1485–1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lepore SJ, Ituarte PHG. Optimism about cancer enhances mood by reducing negative social relations. Cancer Res Ther Control. 1999;8:165–74. [Google Scholar]
- 49. Manne SL. Intrusive thoughts and psychological distress among cancer patients: The role of spouse avoidance and criticism. J Consult Clin Psychol. 1999;67:539–546. [DOI] [PubMed] [Google Scholar]
- 50. Manne SL, Taylor KL, Dougherty J, Kemeny N. Supportive and negative responses in the partner relationship: Their association with psychological adjustment among individuals with cancer. J Behav Med. 1997;20:101–125. [DOI] [PubMed] [Google Scholar]
- 51. Manne SL, Kissane D, Zaider T, et al. . Holding back, intimacy, and psychological and relationship outcomes among couples coping with prostate cancer. J Fam Psychol. 2015;29:708–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Manne S, Badr H, Zaider T, Nelson C, Kissane D. Cancer-related communication, relationship intimacy, and psychological distress among couples coping with localized prostate cancer. J Cancer Surviv. 2010;4:74–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Manne S, Badr H. Intimacy processes and psychological distress among couples coping with head and neck or lung cancers. Psychooncology. 2010;19:941–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. McDowell ME, Occhipinti S, Ferguson M, Chambers SK. Prospective predictors of psychosocial support service use after cancer. Psychooncology. 2011;20:788–791. [DOI] [PubMed] [Google Scholar]
- 55. Mehnert A, Lehmann C, Graefen M, Huland H, Koch U. Depression, anxiety, post-traumatic stress disorder and health-related quality of life and its association with social support in ambulatory prostate cancer patients. Eur J Cancer Care (Engl). 2010;19:736–745. [DOI] [PubMed] [Google Scholar]
- 56. Mosher CE, DuHamel KN, Rini CM, et al. . Barriers to mental health service use among hematopoietic SCT survivors. Bone Marrow Transplant. 2010;45:570–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Porter LS, Keefe FJ, Hurwitz H, Faber M. Disclosure between patients with gastrointestinal cancer and their spouses. Psychooncology. 2005;14:1030–1042. [DOI] [PubMed] [Google Scholar]
- 58. Porter LS, Keefe FJ, Baucom DH, et al. . Partner-assisted emotional disclosure for patients with GI cancer: 8-week follow-up and processes associated with change. Support Care Cancer. 2012;20:1755–1762. [DOI] [PubMed] [Google Scholar]
- 59. Shim EJ, Mehnert A, Koyama A, et al. . Health-related quality of life in breast cancer: A cross-cultural survey of German, Japanese, and South Korean patients. Breast Cancer Res Treat. 2006;99:341–350. [DOI] [PubMed] [Google Scholar]
- 60. Widows MR, Jacobsen PB, Fields KK. Relation of psychological vulnerability factors to posttraumatic stress disorder symptomatology in bone marrow transplant recipients. Psychosom Med. 2000;62:873–882. [DOI] [PubMed] [Google Scholar]
- 61. Wilson B, Morris BA, Chambers S. A structural equation model of posttraumatic growth after prostate cancer. Psychooncology. 2014;23:1212–1219. [DOI] [PubMed] [Google Scholar]
- 62. Boinon D, Sultan S, Charles C, Rosberger Z, Delaloge S, Dauchy S. How social sharing and social support explain distress in breast cancer after surgery: The role of alexithymia. J Psychosoc Oncol. 2012;30:573–592. [DOI] [PubMed] [Google Scholar]
- 63. Bishop MM, Beaumont JL, Hahn EA, et al. . Late effects of cancer and hematopoietic stem-cell transplantation on spouses or partners compared with survivors and survivor-matched controls. J Clin Oncol. 2007;25:1403–1411. [DOI] [PubMed] [Google Scholar]
- 64. Gourvitz RL. Posttraumatic Stress Disorder and Sub-Syndromal Trauma Symptoms in Cancer Patients Treated with Autologous Hematopoietic Stem Cell Transplant and Standard Dose Chemotherapy. Doctoral dissertation. Ann Arbor, MI: Fielding Graduate University; 2008. [Google Scholar]
- 65. Roberts KJ. Applying the Social Cognitive Processing Model to Predict Adjustment in Men Treated for Prostate Cancer. Doctoral dissertation. Ann Arbor, MI: Columbia University; 2004. [Google Scholar]
- 66. Oh S. Social Support, Constraints, and Protective Buffering in Prostate Cancer Patients and their Partners. Doctoral dissertation. Ann Arbor, MI: University of Southern California; 2009. [Google Scholar]
- 67. Mosher CE, Lepore SJ, Wu L, et al. . Social correlates of distress following hematopoietic stem cell transplantation: Exploring the role of loneliness and cognitive processing. J Health Psychol. 2012;17:1022–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Badr H, Milbury K. Associations between depression, pain behaviors, and partner responses to pain in metastatic breast cancer. Pain. 2011;152:2596–2604. [DOI] [PubMed] [Google Scholar]
- 69. Cordova MJ, Cunningham LL, Carlson CR, Andrykowski MA. Social constraints, cognitive processing, and adjustment to breast cancer. J Consult Clin Psychol. 2001;69:706–711. [PubMed] [Google Scholar]
- 70. Dagan M, Sanderman R, Schokker MC, et al. . Spousal support and changes in distress over time in couples coping with cancer: The role of personal control. J Fam Psychol. 2011;25:310–318. [DOI] [PubMed] [Google Scholar]
- 71. Eton DT, Lepore SJ, Helgeson VS. Early quality of life in patients with localized prostate carcinoma. Cancer. 2001;92:1451–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Figueiredo MI, Fries E, Ingram KM. The role of disclosure patterns and unsupportive social interactions in the well-being of breast cancer patients. Psychooncology. 2004;13:96–105. [DOI] [PubMed] [Google Scholar]
- 73. Green HJ, Ferguson M, Shum DH, Chambers SK. Prospective individual and social predictors of changes in adjustment for patients attending a regional cancer service. Qual Life Res. 2013;22:759–770. [DOI] [PubMed] [Google Scholar]
- 74. Steginga SK, Campbell A, Ferguson M, et al. . Socio-demographic, psychosocial and attitudinal predictors of help seeking after cancer diagnosis. Psychooncology. 2008;17:997–1005. [DOI] [PubMed] [Google Scholar]
- 75. Badr H, Carmack CL, Kashy DA, Cristofanilli M, Revenson TA. Dyadic coping in metastatic breast cancer. Health Psychol. 2010;29:169–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Manne SL, Pape SJ, Taylor KL, Dougherty J. Spouse support, coping, and mood among individuals with cancer. Ann Behav Med. 1999;21:111–121. [DOI] [PubMed] [Google Scholar]
- 77. Baumeister RF, Bratslavsky E, Finkenauer C, Vohs KD. Bad is stronger than good. Rev Gen Psychol. 2001;5:323–70. [Google Scholar]
- 78. Cohee AA, Adams RN, Fife BL, et al. . Relationship between depressive symptoms and social cognitive processing in partners of long-term breast cancer survivors. Oncol Nurs Forum. 2017;44:44–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Bakas T, McLennon SM, Carpenter JS, et al. . Systematic review of health-related quality of life models. Health Qual Life Outcomes. 2012;10:134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Nenova M, DuHamel K, Zemon V, Rini C, Redd WH. Posttraumatic growth, social support, and social constraint in hematopoietic stem cell transplant survivors. Psychooncology. 2013;22:195–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. PROMIS [Internet]. Health Measures.2019. Available at: http://www.healthmeasures.net/explore-measurement-systems/promis. Accessibility verified August 8, 2019.
- 82. Yost KJ, Eton DT, Garcia SF, Cella D. Minimally important differences were estimated for six PROMIS—Cancer scales in advanced-stage cancer patients. J Clin Epidemiol. 2011;64:507–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Jensen RE, Potosky AL, Moinpour CM, et al. . United States population-based estimates of patient—reported outcomes measurement information system symptom and functional status reference values for individuals with cancer. J Clin Oncol. 2017;35:1913–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Leung J, Smith MD, McLaughlin D. Inequalities in long term health-related quality of life between partnered and not partnered breast cancer survivors through the mediation effect of social support. Psychooncology. 2016;25:1222–1228. [DOI] [PubMed] [Google Scholar]
- 85. American Cancer Society. Cancer Facts & Figures 2018. Atlanta, GA: American Cancer Society; 2018. [Google Scholar]
- 86. Brand S, Wolfe J, Samsel C. The impact of cancer and its treatment on the growth and development of the pediatric patient. Curr Pediatr Rev. 2017;13:24–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- 88. Danoff-Burg S, Revenson TA, Trudeau KJ, Paget SA. Unmitigated communion, social constraints, and psychological distress among women with rheumatoid arthritis. J Pers. 2004;72:29–46. [DOI] [PubMed] [Google Scholar]
- 89. Buscher A, Latini DM, Hartman C, Kallen M, Sansgiry S, Giordano TP. Utility of a partner communication scale and a personal meaning scale in newly diagnosed HIV-infected persons. J Assoc Nurses AIDS Care. 2013;24:126–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.