Abstract
Introduction
Clinical complications in sickle cell anemia (SCA) are heterogeneous and involve several molecules. It has been suggested that SCA individuals present a dyslipidemic phenotype and that lipid parameters are associated with severe clinical complications, such as pulmonary hypertension. We sought to investigate associations between lipid parameters and clinical manifestations, as well as other laboratory parameters in a population of pediatric SCA patients.
Methods
Our cross-sectional evaluation included 126 SCA patients in steady state and who were not undergoing lipid-lowering therapy. Hematological and biochemical parameters were characterized, and previous clinical manifestations were investigated.
Results
Total cholesterol and low-density lipoprotein cholesterol (LDL-C) levels were increased in patients with a previous history of pneumonia, which also positively correlated with HbS levels. Decreased LDL-C levels were also associated with leg ulcers and anemia. Elevated high-density lipoprotein cholesterol (HDL-C) levels were associated with pain crises, increased viscosity, and decreased hemolysis. Several studies have determined that lipids play a role in the vascular impairment seen in SCA, which was corroborated by our findings.
Conclusions
In sum, our results suggest that total cholesterol, HDL-C, and LDL-C levels are associated with hemolysis and anemia markers and, most importantly, with clinical complications related to vasculopathy in SCA.
1. Introduction
The single point mutation in sickle cell anemia (SCA) is responsible for the production of the variant hemoglobin S (HbS) which under low oxygen tension forms long polymers affecting the red cell morphology [1]. HbS polymerization is the first pathophysiological step leading to clinical manifestations in SCA; moreover, several different mechanisms are involved in the pathogenesis of the disease including ischemia reperfusion injury [2]; increased adhesiveness of leukocytes, reticulocytes, and endothelial cells culminating in vasoocclusion (VO) [3]; and the innate immune system activation with hemolysis products, known as erythrocyte damage-associated molecular pattern molecules (eDAMPs) [4]. Intravascular hemolysis and VO are hallmarks of SCA. Red blood cell lysis releases arginase, free heme, and hemoglobin which decreases the L-arginine pool, the main source for endothelial cells to produce nitric oxide (NO), and leads to endothelial dysfunction and VO [1]. In addition, many inflammatory molecules have been described in SCA such as cytokines, chemokines, adhesion molecules, NO, heme, reactive oxygen species, adenosine triphosphate (ATP), and lipid mediators [5–7].
Cholesterol is obtained from the diet or may be produced endogenously and is the precursor of steroid hormones, bile acids, and vitamin D and contributes to cell membrane fluidity [8]. It is transported to the tissues packaged with apolipoproteins, therefore generating the blood lipoproteins chylomicrons (CM), very low-density lipoprotein cholesterol (VLDL-C), low-density lipoprotein cholesterol (LDL-C), and high-density lipoprotein cholesterol (HDL-C) [8, 9]. Abnormal lipid homeostasis is related to different inflammatory diseases, including Alzheimer, where alterations in sphingolipid and cholesterol metabolism result in accumulation of long-chain ceramides and cholesterol [10], and psoriasis, since patients present significantly higher cholesterol levels in the VLDL-C and HDL-C fractions [11]. It is thought that LDL-C plays an important proinflammatory role in vascular diseases, while HDL-C is thought to be anti-inflammatory depending on the context [12].
Moreover, altered lipid parameters are directly and mostly associated to the development of cardiovascular diseases [13], due to the relevance of several cohort studies which have shown that elevations of plasma LDL-C levels in association with decreased HDL-C levels consist in an important risk factor for atherosclerosis and other vascular complications [14]. In atherosclerosis, several pathophysiological mechanisms are similar to SCA vasculopathy such as decreased NO bioavailability, oxidative stress, and endothelial dysfunction [15], although the formation of atheroma plaques is not observed in SCA. Vasculopathy in SCA is closely related to complications such as pulmonary hypertension, leg ulceration, priapism, and stroke [16].
It has been over 40 years since the first study suggesting a relationship of lipid determinations and laboratory parameters or clinical manifestations in SCA was published [17]. Since then, several studies attempted to investigate the role of lipids in the pathophysiological mechanism of SCA. It was shown that SCA individuals present decreased total cholesterol levels as well as HDL-C and LDL-C in addition to increased triglycerides and VLDL-C levels [18–20].
Clinical complications in SCA are heterogeneous and often associated to hemolysis such as pulmonary hypertension, leg ulcers, and priapism and to VO such as vasoocclusive pain crises, acute chest syndrome, and osteonecrosis, suggesting two subphenotypes [16]. However, there is evidence that pulmonary hypertension, for instance, seems to be associated to hemolysis, VO, and elevated triglyceride levels in a large cohort of individuals with sickle cell disease (SCD) with increased tricuspid regurgitant velocity [20]. In addition, during vasoocclusive crises, patients with SCA presented total cholesterol, triglyceride, and LDL-C levels significantly decreased whereas HDL-C levels were increased when compared to steady state [21]. Also, during steady state, HDL-C levels were found to be associated to nitric oxide metabolites (NOm) and fetal hemoglobin (HbF) levels [18], two of the most important prognostic biomarkers in SCA.
Considering the vascular involvement of both pulmonary hypertension and vasoocclusive crises, the combined data indicate that lipid parameters may be associated to vasculopathy in SCA. Therefore, we attempted to investigate the association of lipid profile (total cholesterol, LDL-C, and HDL-C) with clinical complications and laboratory measurement of hemolysis, anemia, hemoglobin S (HbS), and systemic NO.
2. Methods
2.1. Study Design and Patients
A cross-sectional study was performed including 126 SCA individuals (homozygous HbSS genotype), all seen at the Bahia Hemotherapy and Hematology Foundation (HEMOBA), located in Salvador, Bahia, Brazil. The mean patient age was 14.5 ± 3.5 years; in addition, median patient age was 15 years (IQR 12-17 years) and 60 (47.6%) were female. Patients with SCA in steady state, defined as the absence of acute episodes in the past three months, were recruited to participate during routine clinical visits. All patients were taking folic acid supplementation, 60 were taking hydroxyurea, and none were undergoing therapy with lipid-lowering agents, such as statins. Data regarding the occurrence of previous clinical manifestations was collected using a standardized and confidential questionnaire (self-reported or reported by a legal guardian of the patient) at the time of enrollment and subsequently confirmed by medical records. The present study was approved by the Institutional Research Board of the São Rafael Hospital (protocol number 1400535) and was conducted in compliance with the ethical principles established by the Declaration of Helsinki and its later revisions. All patients were informed regarding the purpose and procedures of this study, and informed written consent was obtained from each SCA patient's legal guardian.
2.2. Hematological Parameters
Blood samples were collected at the time of enrollment after a 12-hour fast and analyzed immediately. Hematological parameters, including complete blood counts, were examined using a Beckman Coulter LH 780 Hematology Analyzer (Beckman Coulter, Brea, California, USA), and blood smears were stained with Wright's stain and examined by optical light microscopy. Reticulocytes were counted after staining supravitally with brilliant cresyl blue dye. Hemoglobin genotyping was performed by high-performance liquid chromatography on an HPLC/Variant II hemoglobin testing system (Bio-Rad, Hercules, California, USA) to confirm the presence of HbSS.
2.3. Biochemical Determinations
LDL-C and VLDL-C levels were determined by the Friedewald equation [22], while total cholesterol, HDL-C, and triglycerides as well as biochemical parameters, including total bilirubin and fractions, lactate dehydrogenase, iron, and hepatic (aspartate aminotransferase, alanine aminotransferase, and gamma-glutamyl transferase) and renal (urea, creatinine and uric acid) markers, were measured in serum samples using an automated A25 chemistry analyzer (Biosystems S.A., Barcelona, Catalunya, Spain).
Ferritin levels were determined using an Access 2 Immunochemistry System (Beckman Coulter Inc., Pasadena, California, USA). NO metabolites (NOm) were quantified in serum samples with Griess reagent employing SoftMax Pro software, as previously described [23]. Laboratory analyses were performed at the Clinical and Toxicological Analysis Laboratory of the College of Pharmaceutical Sciences, Federal University of Bahia (LACTFAR-UFBA).
2.4. Statistical Analyses
Statistical analyses were performed using the Statistical Package for the Social Sciences (SPSS) version 20.0 software (IBM, Armonk, New York, USA) and GraphPad Prism version 6.0 (GraphPad Software, San Diego, California, USA), which was also used to assemble graphs. The clinical characteristics of the study participants are expressed as means and respective standard variations. The distribution of each variable was tested by employing the Shapiro-Wilk test. The Mann-Whitney U test was used when the variables presented a nonparametric distribution, and independent t-test was used when the variables presented a parametric distribution. Fisher's exact test was used to compare categorical variables. Spearman correlation rank analysis was performed to test correlations between lipid parameters and hematological parameters. p values < 0.05 were considered statistically significant.
3. Results
3.1. Investigation of Clinical Manifestations and Lipid Parameters in SCA
We first decided to investigate associations between lipid parameters and previous clinical events using the Mann-Whitney U test. We found that patients with a previous history of pneumonia (postpneumonic) presented increased total cholesterol levels (Figure 1(a)), a previous history of leg ulcers was associated with decreased LDL-C levels (Figure 1(b)), and patients with a previous history of pain crises had increased HDL-C levels (Figure 1(c)).
Figure 1.
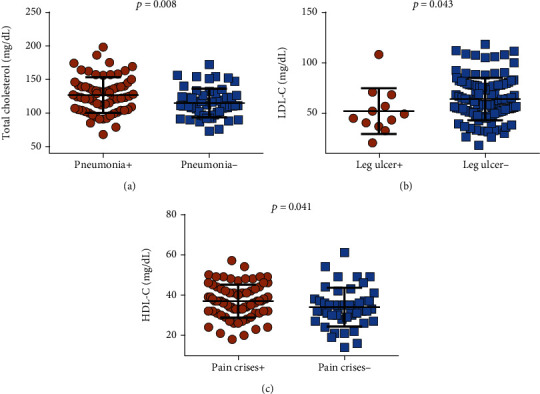
Associations between total cholesterol, LDL-C, and HDL-C levels and clinical manifestations in SCA. (a) Patients with a previous history of pneumonia (N = 69) exhibited increased total cholesterol levels. (b) Patients with a previous history of leg ulcers (N = 12) presented decreased LDL-C levels. (c) Patients with a previous history of pain crises (N = 78) had increased HDL-C levels. p values obtained using the Mann-Whitney U test.
3.2. Associations between Laboratory Parameters and Lipid Markers
In addition to clinical manifestations, we also investigated associations between laboratory and lipid parameters. The patients presented overall median total cholesterol levels of 118 mg/dL (IQR: 103.5–135.5 mg/dL) and median LDL-C levels of 58.8 mg/dL (IQR: 48.6–77.5 mg/dL). None of the patients presented total cholesterol levels higher than 200 mg/dL or LDL-C levels higher than 110 mg/dL, because of that we decided to use the median value.
The patients were then stratified considering each median lipid parameter value; moreover, regarding HDL-C, patients were stratified according to pathologically decreased (below 40 mg/dL) and normal HDL-C levels (over or equal to 40 mg/dL). Thus, association analyses were performed.
Patients with total cholesterol levels higher than the median value (≥118 mg/dL) were also found to present increased LDL-C, VLDL-C, triglyceride, and direct bilirubin levels, as well as decreased indirect bilirubin and HbF levels, in addition to basophil counts (Table 1).
Table 1.
Associations between lipid profile and laboratory parameters in SCA patients.
| Laboratory parameters | Total cholesterol < 118 mg/dL (N = 64) | Total cholesterol ≥ 118 mg/dL (N = 62) | p value |
|---|---|---|---|
| Basophil (mL) | 111.02 ± 107.40 | 76.69 ± 106.97 | 0.035 |
| LDL-C (mg/dL) | 47.98 ± 11.40 | 77.44 ± 18.63 | 0.000 |
| VLDL-C (mg/dL) | 19.41 ± 9.04 | 25.67 ± 12.42 | 0.000 |
| Triglycerides (mg/dL) | 94.17 ± 39.24 | 124.97 ± 55.95 | 0.000 |
| Direct bilirubin (mg/dL) | 0.38 ± 0.14 | 0.44 ± 0.16 | 0.021 |
| Indirect bilirubin (mg/dL) | 2.91 ± 1.74 | 2.32 ± 1.47 | 0.046 |
| HbF (%) | 10.37 ± 5.97 | 7.87 ± 5.14 | 0.015 |
| HDL‐C < 40 mg/dL (N = 89) | HDL‐C ≥ 40 mg/dL (N = 37) | ||
| Hemoglobin (g/dL) | 8.26 ± 0.99 | 8.99 ± 1.00 | 0.001 |
| Hematocrit (%) | 24.50 ± 3.25 | 26.85 ± 3.24 | 0.001∗ |
| RDW (%) | 23.14 ± 3.83 | 21.54 ± 3.52 | 0.027∗ |
| Eosinophil (mL) | 561 ± 548 | 326 ± 249 | 0.011 |
| Total cholesterol (mg/dL) | 118.27 ± 25.18 | 128.17 ± 22.96 | 0.025 |
| VLDL-C (mg/dL) | 24.32 ± 11.53 | 18.22 ± 9.65 | 0.001 |
| Triglycerides (mg/dL) | 119.43 ± 55.14 | 85.37 ± 25.01 | 0.001 |
| GGT (U/L) | 26.16 ± 23.31 | 30.7 ± 20.4 | 0.035 |
| LDH (U/L) | 1358.3 ± 1513.8 | 984.6 ± 355.8 | 0.006 |
| Ferritin (ηg/mL) | 362.85 ± 506.69 | 482.64 ± 426.72 | 0.035 |
| NOm (μM) | 26.38 ± 15.91 | 17.45 ± 5.52 | 0.001 |
| HbF (%) | 8.43 ± 5.60 | 10.73 ± 5.80 | 0.044 |
| LDL‐C < 58.8 mg/dL (N = 65) | LDL‐C ≥ 58.8 mg/dL (N = 61) | ||
| MCV (fL) | 94.63 ± 11.45 | 90.07 ± 11.44 | 0.028∗ |
| MCH (ρg/mL) | 32.10 ± 3.91 | 30.52 ± 3.91 | 0.025∗ |
MCV: mean corpuscular volume; MCH: mean corpuscular hemoglobin; GGT: gamma-glutamyl transferase; RDW: red cell distribution width; LDH: lactate dehydrogenase; HbS: hemoglobin S; HbF: fetal hemoglobin; HDL-C: high-density lipoprotein cholesterol; LDL-C: low-density lipoprotein cholesterol; VLDL-C: very low-density lipoprotein cholesterol; NOm: nitric oxide metabolites. p value obtained using Mann-Whitney U test. ∗p value obtained using independent t-test.
Patients with higher HDL-C levels (≥40 mg/dL) also presented decreased red cell distribution width (RDW) and eosinophil counts as well as decreased VLDL-C, triglyceride, LDH, and NOm levels. In addition, they presented increased hemoglobin, hematocrit, total cholesterol, gamma-glutamyl transferase (GGT), ferritin, and HbF levels (Table 1).
Moreover, patients with higher LDL-C levels (≥58.8 mg/dL) also exhibited decreased mean corpuscular volume (MCV) and mean corpuscular hemoglobin (MCH) values (Table 1).
3.3. Associations between Clinical Manifestations and Lipid Parameters
As some clinical events were previously found to be associated with lipid parameters, we further investigated these associations in patients stratified according to each median lipid variable value. Patients with a previous history of pneumonia were found to exhibit total cholesterol (≥118 mg/dL) and LDL-C (≥58.8 mg/dL) levels higher than both median values (Table 2). No statistical significance was found regarding pain crises and leg ulcers.
Table 2.
Frequency of clinical manifestations associated with lipid parameters in SCA patients.
| Clinical data | Lipid parameters | p value | |
|---|---|---|---|
| Pneumonia + (N = 69) |
Total cholesterol < 118 mg/dL | Total cholesterol ≥ 118 mg/dL | 0.007 |
| 27 (39%) | 42 (61%) | ||
| Pneumonia + (N = 69) |
LDL‐C < 58.8 mg/dL | LDL‐C ≥ 58.8 mg/dL | 0.048 |
| 29 (42%) | 40 (58%) | ||
Data comparisons performed using Fisher's exact test.
3.4. Correlations between Lipid Markers and Laboratory Parameters
Correlation analysis was performed to investigate associations between laboratory and lipid parameters in patients with a previous history of pneumonia or pain crises. In patients with a previous history of pneumonia, total cholesterol levels were found to be negatively correlated with mean platelet volume (Figure 2(a)) and positively correlated with HbS (Figure 2(b)) levels. LDL-C levels were found to be positively correlated with HbS levels (Figure 2(c)), negatively correlated with mean corpuscular volume (Figure 2(d)), and positively correlated with reticulocyte counts (Figure 2(e)). HDL-C levels were positively correlated with ferritin levels (Figure 2(f)).
Figure 2.
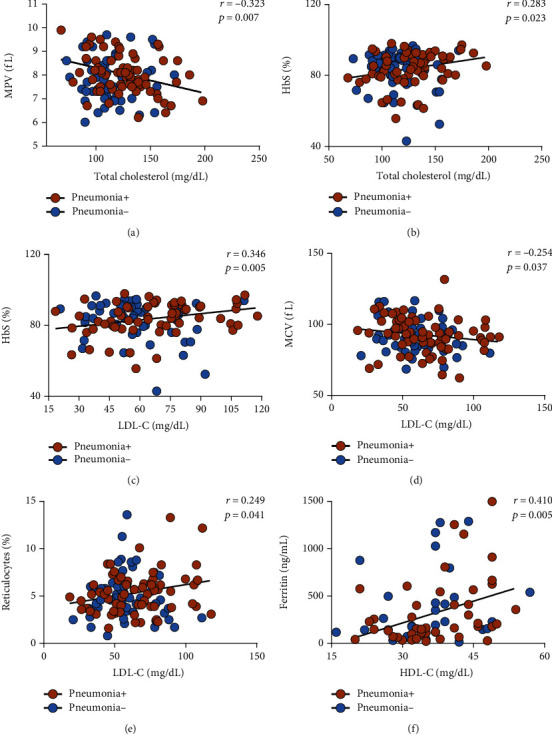
Correlations between lipid and hematological parameters in SCA patients with a previous history of pneumonia (N = 69) indicated by the brown circles. Patients without a previous history of pneumonia (N = 57) are indicated by the blue circles. (a) Total cholesterol levels were negatively correlated with mean platelet volume (MPV). (b) Total cholesterol levels were positively correlated with HbS levels. (c) LDL-C levels were positively correlated with HbS levels. (d) LDL-C levels were negatively correlated with MCV. (e) LDL-C levels were positively correlated with reticulocyte counts. (f) HDL-C levels were positively correlated with ferritin levels. Data comparisons made using Spearman's correlation rank test. None of the correlations described herein were statistically significant in patients without a previous history of pneumonia.
In patients with a previous history of pain crises, HDL-C levels were found to be negatively correlated with RDW (Figure 3(a)), positively correlated with total cholesterol levels (Figure 3(b)), and negatively correlated with HbF levels (Figure 3(c)) and MCHC (Figure 3(d)). Total cholesterol levels were found to be positively correlated with albumin levels (Figure 3(e)); in addition, LDL-C levels were also positively correlated with albumin levels (Figure 3(f)). It is relevant to highlight that these correlations were not statistically significant in patients without a previous history of pneumonia or pain crises.
Figure 3.
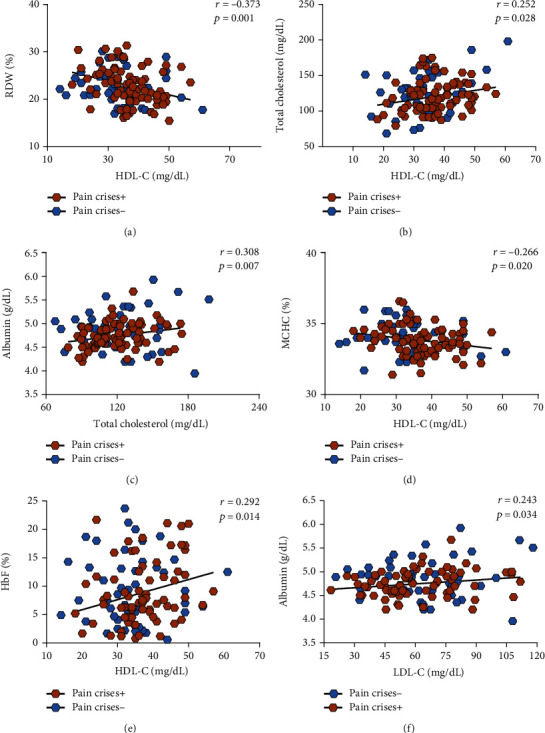
Correlations between HDL-C and triglyceride levels and hematological parameters in SCA patients with a previous history of pain crises (N = 78) indicated by the brown hexagons. Patients without a previous history of pain crises (N = 48) are indicated by the blue hexagons. (a) HDL-C levels were negatively correlated with RDW levels. (b) HDL-C levels were positively correlated with total cholesterol levels. (c) HDL-C levels were negatively correlated with HbF levels. (d) HDL-C levels were negatively correlated with MCHC. (e) Total cholesterol levels were positively correlated with albumin levels. (f) LDL-C levels were positively correlated with albumin levels. Data were compared using Spearman's correlation rank test. None of the correlations described herein were statistically significant in patients without a previous history of pain crises.
4. Discussion
As has been previously demonstrated by several studies, the significant alterations in lipid parameters presented by SCA patients have been associated with hemolysis, anemia, vasoocclusive crises, activation of the TGF-β pathway, pulmonary hypertension, acute chest syndrome, and other complications [18–21, 24, 25]. Thus, we decided to investigate associations between the lipid profile in SCA individuals and previous history of clinical manifestations.
We found that SCA patients with increased total cholesterol levels (yet within physiological range) also had increased levels of LDL-C, VLDL-C, and triglycerides, as well as decreased levels of indirect bilirubin and HbF and a previous history of pneumonia. In postpneumonic patients, total cholesterol levels were also correlated with HbS and MPV, while LDL-C levels were correlated with HbS, MCV, and xferritin levels as well as reticulocyte counts. Pulmonary complications in SCA are mostly associated with vascular impairment and vasoconstriction, leading to VO. The occurrence of pneumonia in SCA patients has been closely linked to an increased frequency of acute chest syndrome, since the presentation of these conditions may overlap, and both are usually associated with pulmonary fat embolism and infectious pathogens [26]. In SCA patients, increasing levels of triglycerides were associated with more frequent episodes of acute chest syndrome [27]. Moreover, in a large cohort of adults, several associations were found between serum increased cholesterol levels and an increased risk of hospitalization leading to death due to respiratory disease [28]. The present study found a positive correlation between HbS levels and levels of total cholesterol and LDL-C in SCA patients with a previous history of pneumonia, which suggests that altered lipid parameters may indicate a more severe disease phenotype. Additionally, another study investigating lipoproteins in patients with SCD found a positive correlation between total cholesterol levels and hemopexin and hemoglobin, which were also negatively correlated with reticulocyte counts and LDH and bilirubin levels [29]. Together, these findings suggest that total cholesterol levels are related to pulmonary complications and hematological alterations in SCA.
We further observed that SCA individuals with increased HDL-C levels also had increased hemoglobin, hematocrit, total cholesterol, GGT, ferritin, and HbF levels, in addition to decreased RDW, VLDL-C, triglyceride, LDH, and NOm levels. Moreover, increased HDL-C levels were also associated with previous pain crises, and in the patients who reported painful episodes, HDL-C levels were also found to be correlated with hematological markers and HbF levels as well as total cholesterol levels. Additionally, total cholesterol and LDL-C levels were correlated with albumin levels. These results are consistent with a previous study that associated HDL-C levels with hemoglobin and hematocrit levels [30]. Likewise, in a study carried out by Zorca and colleagues that evaluated associations between lipid parameters and pulmonary hypertension, HDL-C levels were negatively correlated with LDH levels [20]. In the same study, triglyceride levels were correlated with markers of hemolysis, endothelial activation, and leukocyte counts [20]. These results reinforce the potential participation of HDL-C in modulating hemolysis and vascular dysfunction [31]. Albumin levels are thought to be increased in SCA individuals when compared to individuals with HbAS or HbAA genotype [32] and may be higher during hospitalization [33]. A previous study suggests that patients with chronic obstructive pulmonary disease present increased levels of ischemia-modified albumin as well as oxLDL, due to hypoxia, inflammation, and oxidative stress that these patients experience [34]. These mechanisms are also present in SCA pathogenesis; while the clinical presentation of SCA is highly variable, the most widely recognized clinical event is acute pain crisis driven by VO [35]. VO initiates a cascade of events, leading to tissue ischemia, which is responsible for the acute systemic vasoocclusive crises that frequently necessitate medical care for SCA patients [1]. Increased hematocrit levels are associated with blood rheology and red blood cell deformability, which can also contribute to VO and pain crises [1, 36]. Correspondingly, although the frequency of acute pain varies among SCA patients, it tends to be more frequent in patients presenting increased hematocrit and reduced HbF levels [37]. Moreover, besides the known anti-inflammatory and vasoprotective properties of HDL-C [14], recent data suggests the participation of HDL-C in the vascular environment with regard to hemolysis and anemia [31], which are important mechanisms underlying pain crises in SCA.
Decreased LDL-C levels were found in SCA individuals with a previous history of leg ulcers and increased in patients with a previous history of pneumonia, in addition to being associated with decreased MCV and MCH levels. The anemia presented by SCA patients is related to decreased red blood cell survival [38] and intravascular hemolysis [7], which creates a prooxidant and proinflammatory vascular milieu that contributes to endothelial dysfunction [39]. In this same vascular environment, LDL-C exerts a strong proinflammatory role [8], which could also contribute to SCA vasculopathy. This is further supported by evidence that LDL-C is also susceptible to oxidative modifications in SCA, based on the observation of increased binding of free heme to LDL-C fractions, which could favor the production of oxLDL [29]. Moreover, multiple vascular mechanisms have been attributed to the pathogenesis of leg ulcers in SCA, such as the physical obstruction caused by irreversibly sickled red blood cells, poor venous recirculation, bacterial infection, anemia, in situ thrombosis, and reduced NO bioavailability [40]. Patients with a previous history of leg ulcers exhibit elevated hemolytic laboratory parameters, increased uric acid, and decreased albumin levels [41]. Since the frequency of leg ulcers was associated with priapism and pulmonary hypertension, venous stasis could justify the causal relationship between pulmonary hypertension and leg ulcers, due to the overlapping of pathophysiological mechanisms [41]. Accordingly, the association between anemia and LDL-C levels suggests the vascular involvement of this molecule, which could also contribute to clinical manifestations.
Our findings stand in agreement with other studies that also reported decreased total cholesterol, HDL-C, and LDL-C levels among SCA individuals in comparison to HbAA individuals [19, 20, 25, 27]. It is thought that the hypocholesterolemia seen in SCA results from the augmented cholesterol utilization in erythropoiesis consequent to anemia and hemolysis. In addition, the occurrence of hypocholesterolemia in patients with nonhemolytic anemia suggests increased erythropoietic activity [17]. Moreover, it is also relevant to point that our investigation was carried out in a pediatric population of SCA patients, which could explain some discrepancies with data in the literatures, such as lack of association with different clinical manifestations; however, many of our results are in accordance with previous publications involving patients of a similar age, as well as adults [19, 20, 25]. The cross-sectional design of our study made it difficult to establish any causative roles for lipid parameters with regard to clinical manifestations in SCA, yet the relevant associations found herein will be useful in guiding further evaluations.
5. Conclusions
In summary, the present findings serve to affirm and extend the knowledge surrounding the abnormal lipid profile presented by SCA individuals in association with pain crises, leg ulcers, and pneumonia, in addition to upholding established correlations with laboratory markers of hemolysis and anemia.
Acknowledgments
We would like to thank all of the sickle cell anemia patients who agreed to participate in the present study. We also would like to thank all the staff at the Fundação de Hematologia e Hemoterapia do Estado da Bahia (HEMOBA) for their kind support in assisting with sample collection. We would like to thank Andris K. Walter for English language revision and manuscript copyediting assistance. The work from MSG was supported by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) (470959/2014-2 and 405595/2016-6). SCMAY, RPS, and SPC received scholarship from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, Brasil (CAPES), Finance Code 001.
Abbreviations
- GGT:
Gamma-glutamyl transferase
- HbF:
Fetal hemoglobin
- HbS:
Hemoglobin S
- HDL-C:
High-density lipoprotein cholesterol
- LDH:
Lactate dehydrogenase
- LDL-C:
Low-density lipoprotein cholesterol
- MCH:
Mean corpuscular hemoglobin
- MCHC:
Mean corpuscular hemoglobin concentration
- MCV:
Mean corpuscular volume
- NO:
Nitric oxide
- RDW:
Red cell distribution width
- SCA:
Sickle cell anemia
- VLDL-C:
Very low-density lipoprotein cholesterol
- VO:
Vasoocclusion.
Data Availability
All relevant data are within the paper.
Ethical Approval
The present study was approved by the Institutional Research Board of the São Rafael Hospital (protocol number 1400535) and was conducted in compliance with the ethical principles established by the Declaration of Helsinki and its later revisions.
Consent
All patients were informed regarding the purpose and procedures of this study, and informed written consent was obtained from each SCA patient's legal guardian.
Disclosure
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflicts of Interest
The authors have not received any funding or benefits from industry companies or otherwise to conduct this study.
Authors' Contributions
CCG designed the project, performed all experiments and statistical analyses, and wrote the manuscript. RPS organized the data and helped with statistical analyses. CFLF, JSS, and AMJO performed the hematological measurements and helped with sample collection. SCMAY, MMA, CVBF, and LMF performed biochemical characterizations and helped with sample collection. SPC and RMO supervised the study and helped with sample collection, laboratory characterization, and the discussion of results. VMLN and LCR assisted the patients and helped with sample collection. MSG conceived and supervised the study and critically revised the manuscript.
References
- 1.Sundd P., Gladwin M. T., Novelli E. M. Pathophysiology of sickle cell disease. Annual Review of Pathology: Mechanisms of Disease. 2019;14(1):263–292. doi: 10.1146/annurev-pathmechdis-012418-012838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akinola N. O., Stevens S. M., Franklin I. M., Nash G. B., Stuart J. Subclinical ischaemic episodes during the steady state of sickle cell anaemia. Journal of Clinical Pathology. 1992;45(10):902–906. doi: 10.1136/jcp.45.10.902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang D., Xu C., Manwani D., Frenette P. S. Neutrophils, platelets, and inflammatory pathways at the nexus of sickle cell disease pathophysiology. Blood. 2016;127(7):801–809. doi: 10.1182/blood-2015-09-618538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pitanga T. N., Oliveira R. R., Zanette D. L., et al. Sickle red cells as danger signals on proinflammatory gene expression, leukotriene B4 and interleukin-1 beta production in peripheral blood mononuclear cell. Cytokine. 2016;83:75–84. doi: 10.1016/j.cyto.2016.03.016. [DOI] [PubMed] [Google Scholar]
- 5.Carvalho M. O. S., Araujo-Santos T., Reis J. H. O., et al. Inflammatory mediators in sickle cell anaemia highlight the difference between steady state and crisis in paediatric patients. British Journal of Haematology. 2018;182(6):933–936. doi: 10.1111/bjh.14896. [DOI] [PubMed] [Google Scholar]
- 6.Conran N., Belcher J. D. Inflammation in sickle cell disease. Clinical Hemorheology and Microcirculation. 2018;68(2-3):263–299. doi: 10.3233/CH-189012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.da Guarda C. C., Santiago R. P., Fiuza L. M., et al. Heme-mediated cell activation: the inflammatory puzzle of sickle cell anemia. Expert Review of Hematology. 2017;10(6):533–541. doi: 10.1080/17474086.2017.1327809. [DOI] [PubMed] [Google Scholar]
- 8.Goldstein J. L., Brown M. S. A century of cholesterol and coronaries: from plaques to genes to statins. Cell. 2015;161(1):161–172. doi: 10.1016/j.cell.2015.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang Y., Zanotti I., Reilly M. P., Glick J. M., Rothblat G. H., Rader D. J. Overexpression of apolipoprotein A-I promotes reverse transport of cholesterol from macrophages to feces in vivo. Circulation. 2003;108(6):661–663. doi: 10.1161/01.CIR.0000086981.09834.E0. [DOI] [PubMed] [Google Scholar]
- 10.Cutler R. G., Kelly J., Storie K., et al. Involvement of oxidative stress-induced abnormalities in ceramide and cholesterol metabolism in brain aging and Alzheimer’s disease. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(7):2070–2075. doi: 10.1073/pnas.0305799101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mallbris L., Granath F., Hamsten A., Stahle M. Psoriasis is associated with lipid abnormalities at the onset of skin disease. Journal of the American Academy of Dermatology. 2006;54(4):614–621. doi: 10.1016/j.jaad.2005.11.1079. [DOI] [PubMed] [Google Scholar]
- 12.Navab M., Berliner J. A., Subbanagounder G., et al. HDL and the inflammatory response induced by LDL-derived oxidized phospholipids. Arteriosclerosis, Thrombosis, and Vascular Biology. 2001;21(4):481–488. doi: 10.1161/01.ATV.21.4.481. [DOI] [PubMed] [Google Scholar]
- 13.Mozaffarian D., Benjamin E. J., Go A. S., et al. Executive summary: heart disease and stroke Statistics—2016 update. Circulation. 2016;133(4):447–454. doi: 10.1161/CIR.0000000000000366. [DOI] [PubMed] [Google Scholar]
- 14.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. executive summary of the third report of the national cholesterol education program (ncep) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel iii) JAMA. 2001;285(19):2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 15.Libby P., Ridker P. M., Hansson G. K. Progress and challenges in translating the biology of atherosclerosis. Nature. 2011;473(7347):317–325. doi: 10.1038/nature10146. [DOI] [PubMed] [Google Scholar]
- 16.Kato G. J., Gladwin M. T., Steinberg M. H. Deconstructing sickle cell disease: reappraisal of the role of hemolysis in the development of clinical subphenotypes. Blood Reviews. 2007;21(1):37–47. doi: 10.1016/j.blre.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Westerman M. P. Hypocholesterolaemia and anaemia. British Journal of Haematology. 1975;31(1):87–94. doi: 10.1111/j.1365-2141.1975.tb00835.x. [DOI] [PubMed] [Google Scholar]
- 18.Aleluia M. M., da Guarda C. C., Santiago R. P., et al. Association of classical markers and establishment of the dyslipidemic sub-phenotype of sickle cell anemia. Lipids in Health and Disease. 2017;16(1):p. 74. doi: 10.1186/s12944-017-0454-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seixas M. O., Rocha L. C., Carvalho M. B., et al. Levels of high-density lipoprotein cholesterol (HDL-C) among children with steady-state sickle cell disease. Lipids in Health and Disease. 2010;9(1):p. 91. doi: 10.1186/1476-511X-9-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zorca S., Freeman L., Hildesheim M., et al. Lipid levels in sickle-cell disease associated with haemolytic severity, vascular dysfunction and pulmonary hypertension. British Journal of Haematology. 2010;149(3):436–445. doi: 10.1111/j.1365-2141.2010.08109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Akinlade K. S., Adewale C. O., Rahamon S. K., Fasola F. A., Olaniyi J. A., Atere A. D. Defective lipid metabolism in sickle cell anaemia subjects in vaso-occlusive crisis. Nigerian Medical Journal. 2014;55(5):428–431. doi: 10.4103/0300-1652.140388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Friedewald W. T., Levy R. I., Fredrickson D. S. Estimation of the concentration of lowdensity lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clinical Chemistry. 1972;18(6):499–502. doi: 10.1093/clinchem/18.6.499. [DOI] [PubMed] [Google Scholar]
- 23.Bryan N. S., Grisham M. B. Methods to detect nitric oxide and its metabolites in biological samples. Free Radical Biology & Medicine. 2007;43(5):645–657. doi: 10.1016/j.freeradbiomed.2007.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Figueiredo C. V. B., Carvalho M. O. S., Santiago R. P., et al. _Leptin_ − 2548 G > A gene polymorphism is associated with lipids metabolism and TGF-β alteration in sickle cell disease. Meta Gene. 2016;10:27–31. doi: 10.1016/j.mgene.2016.10.001. [DOI] [Google Scholar]
- 25.Teixeira R. S., Arriaga M. B., Terse-Ramos R., et al. Higher values of triglycerides: HDL-cholesterol ratio hallmark disease severity in children and adolescents with sickle cell anemia. Brazillian Journal of Medical and Biological Research. 2019;52(10, article e8833) doi: 10.1590/1414-431x20198833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vichinsky E. P., Neumayr L. D., Earles A. N., et al. Causes and outcomes of the acute chest syndrome in sickle cell disease. National Acute Chest Syndrome Study Group. The New England Journal of Medicine. 2000;342(25):1855–1865. doi: 10.1056/NEJM200006223422502. [DOI] [PubMed] [Google Scholar]
- 27.Lalanne-Mistrih M. L., Connes P., Lamarre Y., et al. Lipid profiles in French West Indies sickle cell disease cohorts, and their general population. Lipids in Health and Disease. 2018;17(1):p. 38. doi: 10.1186/s12944-018-0689-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iribarren C. Serum total cholesterol and risk of hospitalization, and death from respiratory disease. International Journal of Epidemiology. 1997;26(6):1191–1202. doi: 10.1093/ije/26.6.1191. [DOI] [PubMed] [Google Scholar]
- 29.Vendrame F., Olops L., Saad S. T. O., Costa F. F., Fertrin K. Y. Differences in heme and hemopexin content in lipoproteins from patients with sickle cell disease. Journal of Clinical Lipidology. 2018;12(6):1532–1538. doi: 10.1016/j.jacl.2018.08.002. [DOI] [PubMed] [Google Scholar]
- 30.Emokpae A., Kuliya-Gwarzo A. The influence of decreased levels of high density lipoprotein cholesterol on hematological indices in sickle cell disease patients. Annals of Medical and Health Sciences Research. 2014;4(2):157–161. doi: 10.4103/2141-9248.129020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ji X., Feng Y., Tian H., et al. The mechanism of proinflammatory HDL generation in sickle cell disease is linked to cell-free hemoglobin via haptoglobin. PLoS One. 2016;11(10, article e0164264) doi: 10.1371/journal.pone.0164264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matthew A., Adu E. M., Okosun R. E., Ophori E. A. Effects of the sickle cell (S) gene on serum protein profile. Continental Journal of Biomedical Sciences. 2012;6:1–5. [Google Scholar]
- 33.Curtis S. A., Danda N., Etzion Z., Cohen H. W., Billett H. H. Elevated steady state WBC and platelet counts are associated with frequent emergency room use in adults with sickle cell anemia. PLoS One. 2015;10(8, article e0133116) doi: 10.1371/journal.pone.0133116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Can U., Yerlikaya F. H., Yosunkaya S. Role of oxidative stress and serum lipid levels in stable chronic obstructive pulmonary disease. Journal of the Chinese Medical Association. 2015;78(12):702–708. doi: 10.1016/j.jcma.2015.08.004. [DOI] [PubMed] [Google Scholar]
- 35.Williams T. N., Thein S. L. Sickle cell anemia and its phenotypes. Annual Review of Genomics and Human Genetics. 2018;19(1):113–147. doi: 10.1146/annurev-genom-083117-021320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barabino G. A., Platt M. O., Kaul D. K. Sickle cell biomechanics. Annual Review of Biomedical Engineering. 2010;12(1):345–367. doi: 10.1146/annurev-bioeng-070909-105339. [DOI] [PubMed] [Google Scholar]
- 37.Ballas S. K., Gupta K., Adams-Graves P. Sickle cell pain: a critical reappraisal. Blood. 2012;120(18):3647–3656. doi: 10.1182/blood-2012-04-383430. [DOI] [PubMed] [Google Scholar]
- 38.Quinn C. T., Smith E. P., Arbabi S., et al. Biochemical surrogate markers of hemolysis do not correlate with directly measured erythrocyte survival in sickle cell anemia. American Journal of Hematology. 2016;91(12):1195–1201. doi: 10.1002/ajh.24562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reiter C. D., Wang X., Tanus-Santos J. E., et al. Cell-free hemoglobin limits nitric oxide bioavailability in sickle-cell disease. Nature Medicine. 2002;8(12):1383–1389. doi: 10.1038/nm1202-799. [DOI] [PubMed] [Google Scholar]
- 40.Mohan J. S., Marshall J. M., Reid H. L., Thomas P. W., Serjeant G. R. Postural vasoconstriction and leg ulceration in homozygous sickle cell disease. Clinical Science (London, England) 1997;92(2):153–158. doi: 10.1042/cs0920153. [DOI] [PubMed] [Google Scholar]
- 41.Minniti C. P., Eckman J., Sebastiani P., Steinberg M. H., Ballas S. K. Leg ulcers in sickle cell disease. American Journal of Hematology. 2010;85(10):831–833. doi: 10.1002/ajh.21838. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.


