Abstract
Background
The association between the lymphotoxin-α (LTA) A252G polymorphism and sepsis risk has been extensively studied, but the results have been controversial. This study is aimed at investigating the overall association between the LTA A252G polymorphism and the risk of sepsis/septic shock and sepsis-related mortality.
Methods
We searched the PubMed and EMBASE databases to identify studies that investigated the association between the LTA A252G polymorphism and risks of sepsis, septic shock, and mortality. The relevant data were extracted, and statistical analyses were performed using the Revman 5.0 and STATA 12 software.
Results
A total of 32 publications were included in the meta-analysis. The results demonstrated that the LTA A252G polymorphism showed no significant association with sepsis risk (GG+GA vs. AA: OR = 0.92, 95%CI = 0.79–1.07, p = 0.27) or with sepsis shock risk (GG+GA vs. AA: OR = 1.01, 95%CI = 0.84–1.22, p = 0.91). However, in the subgroup analyzed by ethnicity, the LTA A252G polymorphism significantly decreased sepsis risk in the Asian population for the recessive model [GG vs. GA+AA: OR = 0.82, 95%CI = 0.68–0.99, p = 0.04] but not in the Caucasian population. Moreover, comparisons between sepsis patients who survived and those who did not suggested that the LTA A252G polymorphism decreases the risk of mortality [GG+GA vs. AA: OR = 0.57, 95%CI = 0.41–0.80, p < 0.01].
Conclusion
Our results suggested that the A252G polymorphism in the LTA gene decreased the risk of sepsis in Asians and may reduce mortality in septic individuals.
1. Introduction
Sepsis is a severe condition in terms of mortality, morbidity, and the associated economic and social burden, worldwide. Despite advanced treatments, the sepsis mortality rate, which is around 20-30%, remains hard to ignore [1]. Although the pathogenesis of sepsis is complicated, several factors are known to contribute to sepsis susceptibility and these include aging, multidrug-resistant organisms, immune suppression, and invasive procedures [1]. Furthermore, an increasing number of studies suggest that host predisposition, mainly influenced by the individual's genetic variability, is closely linked with the incidence and outcome of sepsis [2]. As sepsis is potentially a damaging inflammatory response to infection, pro- and anti-inflammatory cytokines were recognized as candidate sepsis susceptibility genes. Several susceptibility genes have been identified in genome-wide association studies (GWAS) or genetic association case-control studies [3–6], and among these genes, the lymphotoxin-α gene (LTA, also termed as tumor necrosis factor-β) has been extensively studied.
Genotype frequency showed that the LTA+252 A allele frequency was the most predominant allele in most of the world populations; and the LTA+252 G allele was associated with the outcome of different diseases [7]. The higher level of TNFA and LTA production is associated with the mutant allele (G) [8]. LTA exerts anti-inflammatory effects and promotes normal lymphoid tissue development [9]. It has been found that LTA A252G polymorphism (NcoI, rs909253, the first intron) was associated with inflammatory response, including sepsis. The LTA A252G polymorphism has been reported as a sepsis susceptibility variant; however, the results have been inconclusive. In 2011, a meta-analysis was performed to assess the overall association between sepsis risk and the LTA A252G polymorphism [9]. In our study we aimed to perform an updated meta-analysis that also included subgroup analysis, as subgroup differences may affect the reliability of the conclusions. Furthermore, in the past five years, more studies have been conducted in different populations to evaluate the impact of the LTA A252G polymorphism on sepsis risk, and these studies should also be included. To obtain a more reliable and precise conclusion about the association between the LTA A252G polymorphism and sepsis/septic shock risk and sepsis-related mortality, we performed this updated meta-analysis with accurate data and current eligible studies.
2. Materials and Methods
2.1. Study Identification and Selection
We carried out a literature search in the PubMed and EMBASE databases to identify studies that investigated the association between the LTA A252G polymorphism and sepsis/septic shock risk and mortality, updated on July 14, 2020. The search terms used were as follows: “sepsis or severe sepsis or septic shock” in combination with “polymorphism or variant or mutation” and “lymphotoxin-α or LTA or tumor necrosis factor-β or TNF-β.” The inclusion criteria were as follows: (1) they were case-control genetic studies, (2) they evaluated the association between the LTA A252G polymorphism and sepsis/septic shock risk or mortality, and (3) the genotype distributions for cases and controls were sufficient to estimate the odd's ratio (OR) with a 95% confidence interval (95% CI). The exclusion criteria were as follows: (1) abstracts, letters, and review articles; (2) genotype frequency not shown, and (3) repeated or overlapping data.
2.2. Data Extraction
Two independent authors checked all the included studies and reached a consensus on every item. The following data were extracted from the included studies: author, year of publication, country of origin, ethnicity, sepsis source, sepsis definition, gene assay method, total number and distribution of genotypes, and genotyping methods.
2.3. Statistical Analysis
Hardy-Weinberg equilibrium (HWE) was tested using Pearson's χ2 test. A p value of <0.05 indicated deviation from HWE. The strength of the association between the LTA A252G polymorphism and sepsis risk was assessed by the odds ratio (OR) with its corresponding 95% confidence interval (95% CI). We applied a random effects (DerSimonian and Laird method) or fixed effects model (Mantel-Haenszel method) to pool the OR values according to the results of the heterogeneity examination. Heterogeneity was assessed by a χ2-based Q statistic and I2, and a p value of <0.10 was statistically significant. For p < 0.10, the pooled OR was calculated using a random effects model. Otherwise, a fixed effects model was used. The I2 statistic was used to estimate the degree of heterogeneity, and a value > 50% was considered an indication of a large degree of heterogeneity. The significance of the pooled OR was evaluated by a Z-test, and a p < 0.05 was statistically significant. The dominant genetic model (GG+GA vs. AA), recessive model (GG vs. GA+AA), codominant model (GG vs. AA), heterozygote model (GA vs. AA), and allele model (G vs. A) were used to pool ORs and assess the association of each genotype with the risk of sepsis. Subgroup analyses were performed for accordance with HWE, ethnic group, septic shock, and mortality of sepsis population.
Publication bias was assessed by Begg's funnel plots and Egger's test. Sensitivity analyses indicating the reliability of a meta-analysis were conducted to identify the potential influence of individual data sets to the pooled OR. All statistical analyses were performed using the Revman 5.0 software (Review Manager, version 5.0, the Nordic Cochrane Centre, the Cochrane Collaboration, Copenhagen, 2008) and the STATA 12.0 software (Statistical Software, Release 12.0, College Station, TX: StataCorp LP, American, 2009).
3. Results
3.1. Characteristics of Included Studies
A total of 225 studies were identified after an initial search of the PubMed and EMBASE databases. After reading the full-text, one article [10] that was included in a previous meta-analysis [9] was excluded due to unavailable data for the genotype distribution in the sepsis group. A total of and 32 articles were included in this meta-analysis [11–42] (Supplementary Figure 1). HWE was performed in the control groups, and deviation from HWE was observed in eight studies. Seven studies were performed in Asians [22, 23, 28, 29, 36, 39, 41] and 19 in Caucasians [11–16, 18–20, 24–27, 30–33, 38, 42]. Three studies were performed in children [24, 30, 40], whereas 27 studies were performed in adults [11, 13–23, 25–29, 31–33, 35–39, 41, 42]. The relationship between the LTA A252G polymorphism and sepsis risk was reported in 25 studies [14, 17, 19–42], while nine studies were related to septic shock risk [20–23, 29, 31, 32, 38, 40], and 21 studies investigated the association with the mortality of sepsis [11–18, 21, 24, 26, 29, 31, 32, 34, 38–41]. The characteristics, genotype, and allele distributions of each case-control study are summarized in Table 1 and Supplement Table 1.
Table 1.
Characteristics of case-control studies.
| Author [Ref] | Country | Ethnicity | Age | Sepsis source | Sepsis type | SNP method | HWE | Primer | BM | Sepsis definition |
|---|---|---|---|---|---|---|---|---|---|---|
| Stuber et al. [11] | Germany | Caucasian | >18 | ICU | SS | PCR | Yes | Yes | No | Yes |
| Stuber et al. [12] | Germany | Caucasian | NA | ICU | SS | PCR | Yes | Yes | No | Yes |
| Fang et al. [13] | Germany | Caucasian | >18 | ICU | SS | PCR | No | Yes | No | Yes |
| Majetschak et al. [14] | Germany | Caucasian | ≥18 | Trauma | SS | PCR | Yes | Yes | No | Yes |
| Schroder et al. [15] | Germany | Caucasian | >18 | ICU | S | PCR | Yes | Yes | No | Yes |
| Schroeder et al. [16] | Germany | Caucasian | >18 | SICU | SS | PCR | Yes | Yes | No | Yes |
| Waterer et al. [17] | American | Mixed | ≥18 | CAP | SS | PCR | Yes | Yes | Yes | Yes |
| Rauchschwalbe et al. [18] | Germany | Caucasian | >18 | Surgery | S, SS | MS-PCR | Yes | Yes | No | Yes |
| Majetschak et al. [19] | Netherland | Caucasian | ≥18 | Trauma | SS | PCR-RFLP | Yes | Yes | No | Yes |
| Schaaf et al. [20] | Germany | Caucasian | ≥18 | CAP | S, SS, SSH | PCR | Yes | Yes | No | Yes |
| Calvano et al. [21] | American | Mixed | ≥18 | SICU | SH, S | PCR | Yes | Yes | Yes | Yes |
| Zhang 1 et al. [22] | China | Asian | ≥18 | ASP | SH | PCR | No | Yes | No | Yes |
| Zhang 2 et al. [23] | China | Asian | ≥18 | ASBP | SH | PCR | No | Yes | No | Yes |
| Balding et al. [24] | Ireland | Caucasian | Child | Sepsis | S | PCR | No | Yes | No | No |
| Riese et al. [25] | Germany | Caucasian | >18 | Surgery | S | PCR | No | Yes | No | Yes |
| Kahlke et al. [26] | Germany | Caucasian | ≥18 | Surgery | S | PCR-RFLP | Yes | Yes | No | Yes |
| Gordon et al. [27] | UK | Caucasian | ≥18 | ICU | SS, SSH | PCR-RFLP | Yes | Yes | Yes | Yes |
| Nakada et al. [28] | Japan | Asian | ≥18 | ICU | S | PCR-RFLP | Yes | Yes | No | Yes |
| Watanabe et al. [37] | Japan | Asian | >18 | ICU | S, SSH | PCR | NA | Yes | No | Yes |
| Schueller et al. [30] | Germany | Caucasian | Infant | Sepsis | S | PCR | Yes | Yes | Yes | Yes |
| Garnacho et al. [31] | Spain | Caucasian | >18 | ICU | S, SS, SSH | PCR | Yes | Yes | No | Yes |
| García-Segarra et al. [32] | Spain | Caucasian | >18 | ICU | S, SS, SSH | PCR | Yes | No | No | Yes |
| Menges et al. [33] | Germany | Caucasian | ≥18 | Trauma | S | PCR | Yes | Yes | Yes | Yes |
| Read et al. [34] | UK | Mixed | Mix | Sepsis | S | PCR | Yes | Yes | No | No |
| Carregaro et al. [35] | Brasil | Mixed | ≥18 | ICU | S, SS, SSH | Taqman | Yes | Yes | No | Yes |
| Gu et al. [36] | China | Asian | ≥18 | Trauma | S | PCR | Yes | No | No | Yes |
| Watanabe et al. [37] | American | Mixed | ≥18 | ICU | S, SS | PCR | No | No | Yes | Yes |
| Sole-Violan et al. [38] | Spain | Caucasian | ≥18 | CAP | S, SS, SSH | PCR | Yes | Yes | No | Yes |
| Song et al. [39] | China | Asian | ≥18 | Sepsis | S, SS | PCR | No | Yes | No | Yes |
| Azevedo et al. [40] | Brazil | Mixed | <18 | ICU | S, SS, SSH | PCR-RFLP | Yes | No | No | Yes |
| Baghel et al. [41] | Indian | Asian | >18 | Surgery | S | PCR | Yes | Yes | No | Yes |
| Montoya-Ruiz et al. [42] | American | Caucasian | >18 | Emergency | S | PCR | Yes | Yes | No | Yes |
S: sepsis; SS: severe sepsis; SSH: septic shock; NA: not available; HEW: Hardy-Weinberg equilibrium; PCR: Polymerase chain reaction; PCR-RFLP: Polymerase Chain Reaction-Restriction Fragment Length Polymorphism; BM: blind method.
3.2. Quantitative Synthesis
For an overall analysis of sepsis risk, we analyzed the heterogeneity of GG+GA vs. AA for all 24 studies and the χ2 value was 59.5 with 23 degrees of freedom (p = 0.27). In addition, the I-square value, another index of heterogeneity, was 61%. A fixed effects model was used to pool the data. The overall OR was 0.92 (95%CI = 0.79–1.07), and the overall effect Z value was 1.10 (p = 0.27) for the GG+GA vs. AA model (Figure 1). The results showed that GG homozygote and GA heterozygote carriers did not increase the sepsis risk when compared with AA homozygote individuals. The results for the recessive model (GG vs. GA+AA), codominant model (GG vs. AA), and allele model (G vs. A), which did not indicate any associations with the risk of sepsis, are listed in Table 2.
Figure 1.
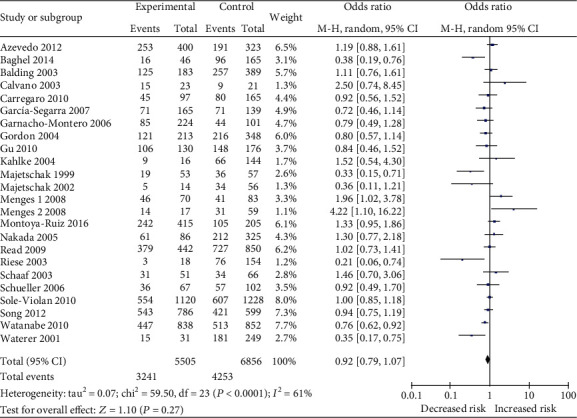
Forest plot of the association between lymphotoxin-α A252G (GG+GA vs. AA) polymorphism and sepsis risk.
Table 2.
Summary of results from different comparative genetic models.
| Comparison | Stratification | No | OR (95% CI) | p | I 2 (%) (p∗) | Model |
|---|---|---|---|---|---|---|
| GG + GA vs. AA | Overall | 24 | 0.92 [0.79, 1.07] | 0.27 | 61 (<0.01) | Random |
| HWE | 20 | 0.94 [0.79, 1.13] | 0.51 | 60 (<0.01) | Random | |
| Caucasian | 14 | 0.95 [0.76, 1.19] | 0.65 | 62 (<0.01) | Random | |
| Asian | 4 | 0.84 [1.57, 1.25] | 0.39 | 63 (0.04) | Random | |
| Shock | 9 | 1.01 [0.84, 1.22] | 0.91 | 50 (0.05) | Fixed | |
| Mortality | 19 | 0.57 [0.41, 0.80] | <0.01 | 63 (<0.01) | Random | |
| GG vs. GA + AA | Overall | 25 | 0.92 [0.84, 1.02] | 0.12 | 26 (0.12) | Fixed |
| HWE | 18 | 1.01 [0.88, 1.15] | 0.93 | 14 (0.29) | Fixed | |
| Caucasian | 12 | 1.08 [0.90, 1.30] | 0.39 | 19 (0.26) | Fixed | |
| Asian | 7 | 0.82 [0.68, 0.99] | 0.04 | 0 (0.72) | Fixed | |
| Shock | 8 | 0.92 [0.70, 1.22] | 0.58 | 38 (0.13) | Fixed | |
| Mortality | 19 | 0.73 [0.57, 0.93] | 0.01 | 28 (0.13) | Fixed | |
| GG vs. AA | Overall | 22 | 0.94 [0.79, 1.12] | 0.48 | 39 (0.03) | Random |
| HWE | 18 | 0.99 [0.85, 1.15] | 0.92 | 29 (0.12) | Fixed | |
| Caucasian | 12 | 1.04 [0.86, 1.26] | 0.70 | 29 (0.17) | Fixed | |
| Asian | 4 | 0.84 [0.65, 1.07] | 0.15 | 0 (0.91) | Fixed | |
| Shock | 5 | 1.02 [0.71, 1.46] | 0.92 | 39 (0.16) | Fixed | |
| Mortality | 17 | 0.52 [0.31, 0.85] | 0.009 | 56 (<0.01) | Random | |
| G vs. A | Overall | 22 | 0.94 [0.85, 1.03] | 0.19 | 56 (<0.01) | Random |
| HWE | 18 | 0.95 [0.85, 1.07] | 0.41 | 50 (<0.01) | Random | |
| Caucasian | 12 | 0.96 [0.83, 1.12] | 0.63 | 53 (0.01) | Random | |
| Asian | 4 | 0.91 [0.80, 1.03] | 0.13 | 27 (0.25) | Fixed | |
| Shock | 5 | 0.97 [0.82, 1.13] | 0.67 | 49 (0.10) | Fixed | |
| Mortality | 17 | 0.70 [0.54, 0.90] | 0.005 | 67 (<0.01) | Random | |
| GA vs. AA | Overall | 22 | 0.89 [0.77, 1.03] | 0.13 | 53 (0.002) | Random |
| HWE | 18 | 0.89 [0.74, 1.06] | 0.19 | 54 (0.003) | Random | |
| Caucasian | 12 | 0.86 [0.69, 1.07] | 0.18 | 53 (0.02) | Random | |
| Asian | 4 | 0.86 [0.54, 1.36] | 0.51 | 69 (0.02) | Random | |
| Shock | 5 | 0.54 [0.18, 1,61] | 0.27 | 94 (<0.01) | Random | |
| Mortality | 17 | 0.61 [0.44, 0.86] | 0.004 | 57 (0.002) | Random |
For studies in accordance with HWE, no significant association was found between the LTA A252G polymorphism and sepsis risk (OR = 0.94, 95%CI = 0.79–1.13, p = 0.51 for GG+GA vs. AA). In the subgroup analysis by ethnicity (Caucasian and Asian), no association was identified between the LTA A252G polymorphism and sepsis risk in Caucasians (OR = 0.95, 95%CI = 0.76–1.19, p = 0.65 for GG+GA vs. AA) and Asians (OR = 0.84, 95%CI = 0.57–1.25, p = 0.39 for GG+GA vs. AA). However, the recessive model for the Asian populations showed decreased risk of sepsis (OR = 0.82, 95%CI = 0.68–0.99, p = 0.04 for GG vs. GA+AA) (Figure 2).
Figure 2.
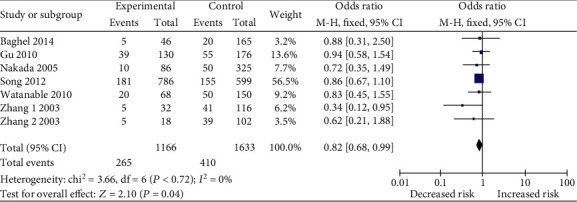
Forest plot of the association between lymphotoxin-α A252G (GG vs. GA+AA) polymorphism and sepsis risk in Asians.
Nine studies had reported a potential effect of the LTA A252G polymorphism on septic shock risk [18–21, 27, 29, 30, 36, 38], while no significant association between this polymorphism and septic shock susceptibility was identified (OR = 1.01, 95%CI = 0.84–1.22, p = 0.91 for GG+GA vs. AA). Furthermore, a total of 21 studies had determined the association between the LTA A252G polymorphism and the mortality of sepsis [9–16, 19, 22, 24, 27, 29, 30, 32, 36–39], and the results of all the four models showed that the LTA A252G polymorphism significantly decreased the mortality risk of sepsis patients (Figure 3). A summary of all the results of statistical analysis is shown in Table 2.
Figure 3.
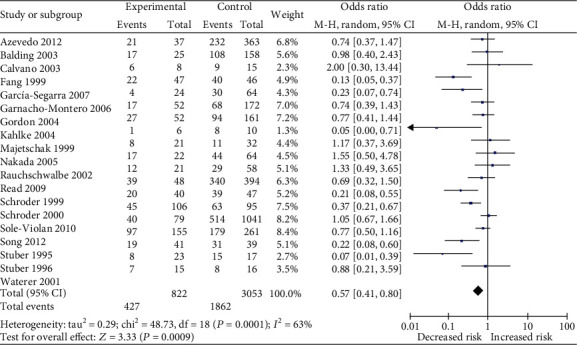
Forest plot of the association between lymphotoxin-α A252G (GG+GA vs. AA) polymorphism and sepsis mortality.
3.3. Sensitivity Analysis and Publication Bias
A sensitivity analysis was performed to evaluate the stability of the individual data to the pooled OR (GG+GA vs. AA). After sequentially excluding each one of the 25 studies that assessed the overall relationship between the LTA A252G polymorphism and sepsis risk, statistically similar results were obtained, suggesting the results of this meta-analysis were stable (Figure 4). Furthermore, similar findings were identified in other statistical models (data not shown). Moreover, publication bias was assessed by Begg's funnel plots and Egger's test. The shape of the funnel plots appeared symmetrical in the GG+GA vs. the AA comparison model, suggesting the absence of publication bias (Figure 5). Egger's test was performed to provide statistical evidence of funnel plot asymmetry. The p value was 0.42, indicating an absence of publication bias. In addition, no publication bias was identified in other statistical models (data not shown).
Figure 4.
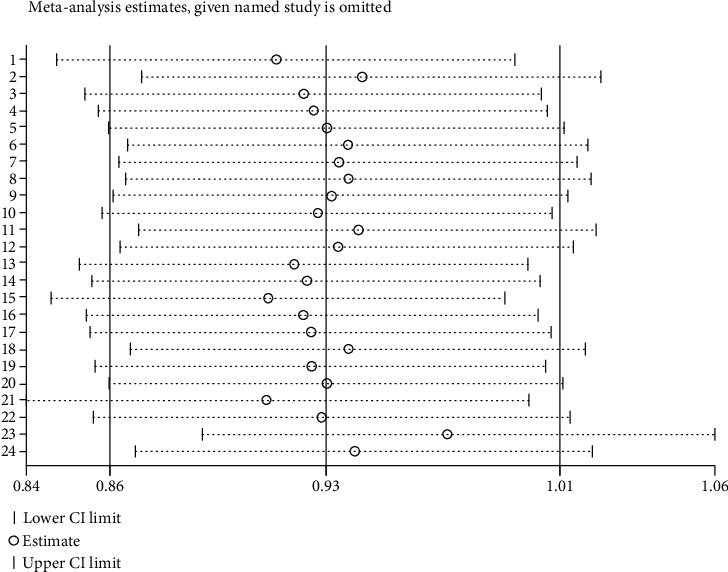
Sensitivity analysis of included studies investigated the association between lymphotoxin-α A252G (GG+GA vs. AA) polymorphism and sepsis risk.
Figure 5.
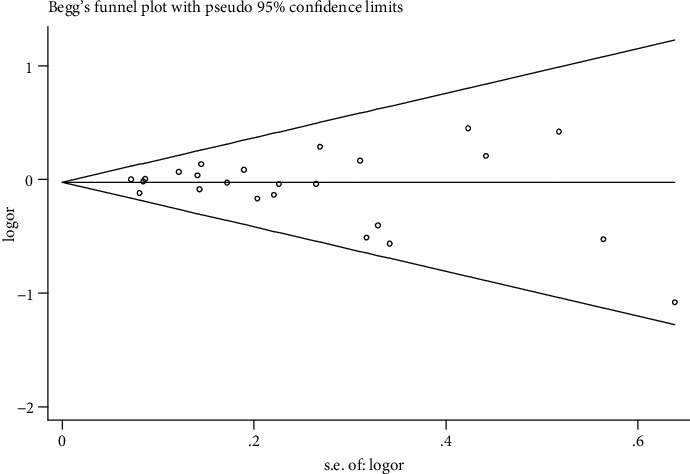
Begg's funnel plot for publication bias in selection of studies on lymphotoxin-α A252G (GG+GA vs. AA) polymorphism.
4. Discussion
Sepsis is a severe complication of infectious diseases which may develop to severe sepsis, septic shock or even death. Even with advanced life support and antibiotics, the mortality of sepsis is still remarkable [43]. Host genetic and immune factors play an important role in the prognosis of sepsis patients. Genetic variants can predict an individual's susceptibility to sepsis and may be helpful in determining the risk for serious complications and death in sepsis patients [43]. As inflammatory cells and cytokines are essential for the pathogenesis of sepsis, many researchers have studied polymorphisms of inflammatory cytokines. The A252G polymorphism of the LTA gene is one of the most studied gene polymorphisms, but the results have been conflicting. To reach a more accurate and objective conclusion, we performed this updated meta-analysis to assess the overall association between the LTA A252G polymorphism and sepsis risk based on current available publications. Compared with the previous meta-analysis, there are some advantages in the article. First, the article is an updated meta-analysis and included subgroup analysis. Second, the previous meta-analysis analyzed the correlation between A allele and sepsis risk. However, according to the allele frequency studies, the A allele is the predominant allele. So, we mainly analyzed the G allele in our meta-analysis. Third, we analyzed publication bias and sensitivity in our meta-analysis which was deficient in the previous article.
The meta-analysis involved 32 articles; considering the genetic background, subgroup analyses were performed for accordance with HWE, ethnic group, septic shock, and mortality of the sepsis population. The results of both overall studies and studies in accordance with HWE showed no association between the LTA A252G polymorphism and risk of sepsis. However, ethnicity is an important factor for the pathogenesis of sepsis, and single nucleotide polymorphisms can be used to distinguish among different ethnic populations. In terms of allele frequencies, a significant difference of G allele was found between Moroccan, African, and Asian populations; however, no difference was found in Mediterranean, European, and Japanese populations [44, 45]. In this meta-analysis, 19 of the included studies were conducted in Caucasian and seven in Asian populations. While no association was found in the Caucasian populations, in the Asian populations, the G allele was found to decrease sepsis risk, indicating the importance of ethnic differences. Only two studies included African-Americans; therefore, due to the small sample size, additional studies are needed to assess this association in the future.
Since sepsis, in severe cases, can progress to septic shock and death, we also analyzed the association between the LTA A252G polymorphism and the risk of septic shock and mortality. In this meta-analysis, nine studies had reported the effect of the LTA A252G polymorphism on septic shock susceptibility, and 21 studies had analyzed the LTA A252G gene variants in septic patients who survived and those who did not. The results suggested no significant effect of the LTA A252G polymorphism on septic shock susceptibility. The genetic distribution of GG, GA, and AA could not be extracted independently in four of the nine articles, and therefore, the negative associations for septic shock could be attributed to the small sample size. Further studies are needed for future evaluation. In the mortality analysis, all the results indicated that the LTA A252G polymorphism decreased the risk of sepsis-related mortality, suggesting that the presence of the G allele (GG and G) could decrease the mortality rate in septic patients.
GWAS is the most appropriate method to identify susceptible genes for sepsis [43], and many sepsis-susceptibility genes have been so far identified by GWAS [3, 4]. However, no GWAS has reported a significant association between the LTA A252G polymorphism and sepsis risk, indicating that this polymorphism might not have been included in those GWAS arrays. Thus, future studies are needed to further assess and validate our results.
Heterogeneity and publication bias play a determining role in the reliability of the results in a meta-analysis. Significant heterogeneity was detected in some comparisons; however, this may be due to study design differences among the included studies. When significant heterogeneity was found, a random effects model was applied for analysis. In addition, the genetic distribution of GG, GA, and AA could not be extracted independently in some cases, probably partly contributing to the existence of heterogeneity.
Publication bias and sensitivity analysis constitute an essential index for the quality and reliability of the study. Publication bias was analyzed using Begg's funnel plots and Egger's test in our study. The results indicated the reliability of our meta-analysis.
Hitherto, this is the most specific and comprehensive meta-analysis to investigate the association of the LTA A252G polymorphism with sepsis risk. However, this study had some limitations. First, since our literature search was conducted only in the selected databases, we might have missed relevant studies deposited in other databases. Second, since we only included published studies written in English, studies in other languages were excluded. Third, most of the included studies were conducted in Caucasian and Asian populations; therefore, the results may only be applicable to these populations. Hence, future studies are warranted to explore these associations further, particularly in African-American, African, and Latin populations. Nevertheless, this meta-analysis has made an important contribution to this field. A comprehensive evaluation of the association between the LTA A252G polymorphism and sepsis risk is more powerful than a single study. Furthermore, the reliability of this meta-analysis was confirmed by heterogeneity, publication bias, and sensitivity analyses.
To our knowledge, this is the most comprehensive meta-analysis to assess the relationship between the A252G polymorphism in the LTA gene and sepsis risk. Our results suggested that the LTA A252G polymorphism was significantly associated with a decreased risk of sepsis in Asian populations and with a decreased risk for mortality among septic individuals.
Acknowledgments
This work was funded by Grant 81700044 from the National Natural Science Foundation of China and Grant 16PJ416 from the Health and Family Planning Commission of Sichuan Province to Dr. Shujin Guo.
Data Availability
The raw data supporting the conclusions of this manuscript will be made available by the authors, without undue reservation, to any qualified researcher.
Additional Points
Highlights. Hitherto, this is the most specific and comprehensive meta-analysis to investigate the association of the LTA A252G polymorphism with sepsis risk, septic shock, and mortality.
Disclosure
The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflicts of Interest
The authors declare that they have no conflict of interest.
Authors' Contributions
Shujin Guo, Qiunan Zuo, and Xiaohui Li searched the studies and extracted and analyzed the data. Ye He contributed to the statistical analyses. Shujin Guo edited the manuscript. Yutian Zhou reviewed and edited the manuscript. Shujin Guo, Qiunan Zuo, and Xiaohui Li contributed equally to this work and are joint first authors.
Supplementary Materials
Supplementary Table 1: characteristics of case-control studies and distributions of LTA genotype and allele among sepsis patients and controls [11–42]. Supplementary Figure 1: flow chart of study inclusion.
References
- 1.Dellinger R. P., Levy M. M., Rhodes A., et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Medicine. 2013;39(2):165–228. doi: 10.1007/s00134-012-2769-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Namath A., Patterson A. J. Genetic polymorphisms in sepsis. Critical Care Nursing Clinics of North America. 2011;23(1):181–202. doi: 10.1016/j.ccell.2010.12.011. [DOI] [PubMed] [Google Scholar]
- 3.Rautanen A., Mills T. C., Gordon A. C., et al. Genome-wide association study of survival from sepsis due to pneumonia: an observational cohort study. The Lancet Respiratory Medicine. 2015;3(1):53–60. doi: 10.1016/S2213-2600(14)70290-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scherag A., Schöneweck F., Kesselmeier M., et al. Genetic factors of the disease course after sepsis: a genome-wide study for 28 day mortality. eBioMedicine. 2016;12:239–246. doi: 10.1016/j.ebiom.2016.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cernada M., Serna E., Bauerl C., Collado M. C., Perez-Martinez G., Vento M. Genome-wide expression profiles in very low birth weight infants with neonatal sepsis. Pediatrics. 2014;133(5):e1203–e1211. doi: 10.1542/peds.2013-2552. [DOI] [PubMed] [Google Scholar]
- 6.Savva A., Plantinga T. S., Kotanidou A., et al. Association of autophagy-related 16-like 1 (ATG16L1) gene polymorphism with sepsis severity in patients with sepsis and ventilator-associated pneumonia. European Journal of Clinical Microbiology & Infectious Diseases. 2014;33(9):1609–1614. doi: 10.1007/s10096-014-2118-7. [DOI] [PubMed] [Google Scholar]
- 7.Qidwai T., Khan F. Tumour necrosis factor gene polymorphism and disease prevalence. Scandinavian Journal of Immunology. 2011;74(6):522–547. doi: 10.1111/j.1365-3083.2011.02602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Warzocha K., Ribeiro P., Bienvenu J., et al. Genetic polymorphisms in the tumor necrosis factor locus influence non-Hodgkin’s lymphoma outcome. Blood. 1998;91(10):3574–3581. doi: 10.1182/blood.V91.10.3574. [DOI] [PubMed] [Google Scholar]
- 9.Tiancha H., Huiqin W., Jiyong J., Jingfen J., Wei C. Association between lymphotoxin-α intron +252 polymorphism and sepsis: a meta-analysis. Scandinavian Journal of Infectious Diseases. 2011;43(6-7):436–447. doi: 10.3109/00365548.2011.562528. [DOI] [PubMed] [Google Scholar]
- 10.Nuntayanuwat S., Dharakul T., Chaowagul W., Songsivilai S. Polymorphism in the promoter region of tumor necrosis factor-alpha gene is associated with severe melioidosis. Human Immunology. 1999;60(10):979–983. doi: 10.1016/s0198-8859(99)00073-7. [DOI] [PubMed] [Google Scholar]
- 11.Stüber F., Petersen M., Bokelmann F., Schade U. A genomic polymorphism within the tumor necrosis factor locus influences plasma tumor necrosis factor-alpha concentrations and outcome of patients with severe sepsis. Critical Care Medicine. 1996;24(3):381–384. doi: 10.1097/00003246-199603000-00004. [DOI] [PubMed] [Google Scholar]
- 12.Stuber F., Udalova I. A., Book M., et al. -308 tumor necrosis factor (TNF) polymorphism is not associated with survival in severe sepsis and is unrelated to lipopolysaccharide inducibility of the human TNF promoter. Journal of Inflammation. 1996;46(1):42–50. [PubMed] [Google Scholar]
- 13.Fang X. M., Schröder S., Hoeft A., Stüber F. Comparison of two polymorphisms of the interleukin-1 gene family: interleukin-1 receptor antagonist polymorphism contributes to susceptibility to severe sepsis. Critical Care Medicine. 1999;27(7):1330–1334. doi: 10.1097/00003246-199907000-00024. [DOI] [PubMed] [Google Scholar]
- 14.Majetschak M., Flohé S., Obertacke U., et al. Relation of a TNF gene polymorphism to severe sepsis in trauma patients. Annals of Surgery. 1999;230(2):207–214. doi: 10.1097/00000658-199908000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schroeder S., Reck M., Hoeft A., Stüber F. Analysis of two human leukocyte antigen-linked polymorphic heat shock protein 70 genes in patients with severe sepsis. Critical Care Medicine. 1999;27(7):1265–1270. doi: 10.1097/00003246-199907000-00006. [DOI] [PubMed] [Google Scholar]
- 16.Schröder J., Kahlke V., Book M., Stüber F. Gender differences in sepsis: genetically determined? Shock. 2000;14:310–313. [PubMed] [Google Scholar]
- 17.Waterer G. W., Quasney M. W., Cantor R. M., Wunderink R. G. Septic shock and respiratory failure in community-acquired pneumonia have different TNF polymorphism associations. American Journal of Respiratory and Critical Care Medicine. 2001;163(7):1599–1604. doi: 10.1164/ajrccm.163.7.2011088. [DOI] [PubMed] [Google Scholar]
- 18.Rauchschwalbe S. K., Maseizik T., Mittelkötter U., et al. Effect of the LT-α (+250 G/A) polymorphism on markers of inflammation and clinical outcome in critically ill patients. The Journal of Trauma. 2004;56(4):815–822. doi: 10.1097/01.TA.0000085852.55853.3A. [DOI] [PubMed] [Google Scholar]
- 19.Majetschak M., Obertacke U., Schade F. U., et al. Tumor necrosis factor gene polymorphisms, leukocyte function, and sepsis susceptibility in blunt trauma patients. Clinical and Diagnostic Laboratory Immunology. 2002;9(6):1205–1211. doi: 10.1128/cdli.9.6.1205-1211.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schaaf B. M., Boehmke F., Esnaashari H., et al. Pneumococcal septic shock is associated with the interleukin-10-1082 gene promoter polymorphism. American Journal of Respiratory and Critical Care Medicine. 2003;168(4):476–480. doi: 10.1164/rccm.200210-1164OC. [DOI] [PubMed] [Google Scholar]
- 21.Calvano J. E., Um J. Y., Agnese D. M., et al. Influence of the TNF-alpha and TNF-beta polymorphisms upon infectious risk and outcome in surgical intensive care patients. Surgical Infections. 2003;4(2):163–169. doi: 10.1089/109629603766956951. [DOI] [PubMed] [Google Scholar]
- 22.Zhang D. L., Li J. S., Jiang Z. W., Yu B. J., Tang X. M., Zheng H. M. Association of two polymorphisms of tumor necrosis factor gene with acute biliary pancreatitis. World Journal of Gastroenterology. 2003;9(4):824–828. doi: 10.3748/wjg.v9.i4.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang D., Li J., Jiang Z. W., Yu B., Tang X. Association of two polymorphisms of tumor necrosis factor gene with acute severe pancreatitis. The Journal of Surgical Research. 2003;112(2):138–143. doi: 10.1016/S0022-4804(03)00085-4. [DOI] [PubMed] [Google Scholar]
- 24.Balding J., Healy C. M., Livingstone W. J., et al. Genomic polymorphic profiles in an Irish population with meningococcaemia: is it possible to predict severity and outcome of disease? Genes & Immunity. 2003;4(8):533–540. doi: 10.1038/sj.gene.6364020. [DOI] [PubMed] [Google Scholar]
- 25.Riese J., Woerner K., Zimmermann P., Denzel C., Hohenberger W., Haupt W. Association of a TNFbeta gene polymorphism with complications after major abdominal operations. Shock. 2003;19(1):1–4. doi: 10.1097/00024382-200301000-00001. [DOI] [PubMed] [Google Scholar]
- 26.Kahlke V., Schafmayer C., Schniewind B., Seegert D., Schreiber S., Schröder J. Are postoperative complications genetically determined by TNF-β NcoI gene polymorphism? Surgery. 2004;135(4):365–373. doi: 10.1016/j.surg.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 27.Gordon A. C., Lagan A. L., Aganna E., et al. TNF and TNFR polymorphisms in severe sepsis and septic shock: a prospective multicentre study. Genes and Immunity. 2004;5(8):631–640. doi: 10.1038/sj.gene.6364136. [DOI] [PubMed] [Google Scholar]
- 28.Nakada T. A., Hirasawa H., Oda S., et al. Influence of toll-like receptor 4, CD14, tumor necrosis factor, and interleukine-10 gene polymorphisms on clinical outcome in Japanese critically ill patients. Journal of Surgical Research. 2005;129(2):322–328. doi: 10.1016/j.jss.2005.05.020. [DOI] [PubMed] [Google Scholar]
- 29.Galan P., Viteri F. E., Bertrais S., et al. Cytokine-related genotypic differences in peak interleukin-6 blood levels of patients with SIRS and septic complications. The Journal of Trauma. 2005;59:1181–1190. doi: 10.1097/00005373-200511000-00025. [DOI] [PubMed] [Google Scholar]
- 30.Schueller A. C., Heep A., Kattner E., et al. Prevalence of two tumor necrosis factor gene polymorphisms in premature infants with early onset sepsis. Biology of the Neonate. 2006;90(4):229–232. doi: 10.1159/000093605. [DOI] [PubMed] [Google Scholar]
- 31.Garnacho-Montero J., Aldabo-Pallas T., Garnacho-Montero C., et al. Timing of adequate antibiotic therapy is a greater determinant of outcome than are TNF and IL-10 polymorphisms in patients with sepsis. Critical Care. 2006;10(4):p. R111. doi: 10.1186/cc4995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.García-Segarra G., Espinosa G., Tassies D., et al. Increased mortality in septic shock with the 4G/4G genotype of plasminogen activator inhibitor 1 in patients of white descent. Intensive Care Medicine. 2007;33(8):1354–1362. doi: 10.1007/s00134-007-0695-y. [DOI] [PubMed] [Google Scholar]
- 33.Menges T., König I. R., Hossain H., et al. Sepsis syndrome and death in trauma patients are associated with variation in the gene encoding tumor necrosis factor. Critical Care Medicine. 2008;36(5, article 1456-e6) doi: 10.1097/CCM.0B013E318170ABB6. [DOI] [PubMed] [Google Scholar]
- 34.Read R. C., Teare D. M., Pridmore A. C., et al. The tumor necrosis factor polymorphism TNF (-308) is associated with susceptibility to meningococcal sepsis, but not with lethality. Critical Care Medicine. 2009;37(4):1237–1243. doi: 10.1097/CCM.0b013e31819c39bc. [DOI] [PubMed] [Google Scholar]
- 35.Carregaro F., Carta A., Cordeiro J. A., Lobo S. M., Silva E. H. T., Leopoldino A. M. Polymorphisms IL10-819 and TLR-2 are potentially associated with sepsis in Brazilian patients. Memórias do Instituto Oswaldo Cruz. 2010;105(5):649–656. doi: 10.1590/S0074-02762010000500008. [DOI] [PubMed] [Google Scholar]
- 36.Gu W., Zeng L., Zhou J., et al. Clinical relevance of 13 cytokine gene polymorphisms in Chinese major trauma patients. Intensive Care Medicine. 2010;36(7):1261–1265. doi: 10.1007/s00134-010-1797-5. [DOI] [PubMed] [Google Scholar]
- 37.Watanabe E., Buchman T. G., Hirasawa H., Zehnbauer B. A. Association between lymphotoxin-alpha (tumor necrosis factor-beta) intron polymorphism and predisposition to severe sepsis is modified by gender and age. Critical Care Medicine. 2010;38(1):181–193. doi: 10.1097/CCM.0b013e3181bc805d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Solé-Violán J., Rodríguez de Castro F., García-Laorden M. I., et al. Genetic variability in the severity and outcome of community-acquired pneumonia. Respiratory Medicine. 2010;104(3):440–447. doi: 10.1016/j.rmed.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 39.Song Z., Song Y., Yin J., et al. Genetic variation in the TNF gene is associated with susceptibility to severe sepsis, but not with mortality. PLoS One. 2012;7(9, article e46113) doi: 10.1371/journal.pone.0046113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Azevedo Z. M., Moore D. B., Lima F. C., et al. Tumor necrosis factor (TNF) and lymphotoxin-alpha (LTA) single nucleotide polymorphisms: Importance in ARDS in septic pediatric critically ill patients. Human Immunology. 2012;73(6):661–667. doi: 10.1016/j.humimm.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 41.Baghel K., Srivastava R. N., Chandra A., et al. Tumor necrosis factor-β Nco 1 polymorphism and susceptibility to sepsis following major elective surgery. Surgical Infections. 2014;15(3):213–220. doi: 10.1089/sur.2012.211. [DOI] [PubMed] [Google Scholar]
- 42.Montoya-Ruiz C., Jaimes F. A., Rugeles M. T., López J. Á., Bedoya G., Velilla P. A. Variants in LTA, TNF, IL1B, and IL10, genes associated with the clinical course of sepsis. Immunologic Research. 2016;64(5-6):1168–1178. doi: 10.1007/s12026-016-8860-4. [DOI] [PubMed] [Google Scholar]
- 43.Esposito S., Zampiero A., Pugni L., et al. Genetic polymorphisms and sepsis in premature neonates. PLoS One. 2014;9(7, article e101248) doi: 10.1371/journal.pone.0101248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brick C., Atouf O., Bouayad A., Essakalli M. Moroccan study of HLA (-A, -B, -C, -DR, -DQ) polymorphism in 647 unrelated controls: updating data. Molecular and Cellular Probes. 2015;29(4):197–207. doi: 10.1016/j.mcp.2015.04.002. [DOI] [PubMed] [Google Scholar]
- 45.Aznag F. Z., Moutaoufik M. T., Korrida A., Izaabel E. H. Genetic distribution of the LTA+252 A>G and TNFA−308 G > A polymorphisms in the Moroccan population. Genetic Testing and Molecular Biomarkers. 2019;23(12):871–876. doi: 10.1089/gtmb.2019.0116. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1: characteristics of case-control studies and distributions of LTA genotype and allele among sepsis patients and controls [11–42]. Supplementary Figure 1: flow chart of study inclusion.
Data Availability Statement
The raw data supporting the conclusions of this manuscript will be made available by the authors, without undue reservation, to any qualified researcher.


