Abstract
Introduction:
The FIR phase II study (NCT01846416) evaluated the efficacy and safety of anti-programmed death-ligand 1 (PD-L1) atezolizumab in advanced non-small-cell lung cancer (NSCLC) selected by tumor cell (TC) or tumor-infiltrating immune cell (IC) PD-L1 expression.
Methods:
Patients with PD-L1 TC2/3 (PD-L1 staining on ≥5% of TC) or IC2/3 tumors (PD-L1 staining on ≥5% of IC; determined by SP142 PD-L1 immunohistochemistry assay) with paired fresh and archival histology samples were recruited into Cohort 1 (chemotherapy-naïve/>6 months between adjuvant chemotherapy and recurrence), Cohort 2 (≥ second-line without brain metastases), or Cohort 3 (≥ second-line with treated brain metastases). Patients received 1200 mg atezolizumab, Day 1 (21-day cycles). Primary endpoint: investigator-assessed modified Response Evaluation Criteria in Solid Tumors (mRECIST), objective response rate (ORR; RECIST v1.1). Secondary endpoints: overall survival, progression-free survival, duration of response, safety.
Results:
Patients (n=138) were enrolled (137 evaluable for response: Cohort 1, n=31; Cohort 2, n=93; Cohort 3, n=13). Investigator-assessed ORR was 32%, 21%, and 23% for Cohorts 1, 2, and 3, respectively. Treatment-related adverse events (TRAEs) were reported in 81%, 67%, and 69% of patients, respectively, including grade 3–4 TRAEs in 16%, 19%, and 15%. Moreover, 88.6% (n=86/97) paired baseline tumor samples had <5% change in TC/IC PD-L1 expression over time.
Conclusions:
Atezolizumab monotherapy showed clinical activity in patients with NSCLC, including those with brain metastases; safety was consistent with previous trials. Atezolizumab has completed phase III monotherapy studies in second-line; front-line trials are ongoing, confirming these favorable results.
Keywords: Non-small-cell lung cancer, Immunotherapy, Anti-PD-L1
Introduction
Non-small-cell lung cancer (NSCLC) is a leading cause of cancer death. Despite the range of first-line chemotherapy and targeted therapy options available in the metastatic setting, patients often progress, and second-line chemotherapy options have typically prolonged patient survival by less than a year.1,2 Immune checkpoint inhibitors targeting programmed death ligand-1 (PD-L1) or its receptor programmed death-1 (PD-1) have demonstrated clinical efficacy in patients with advanced or metastatic NSCLC,3–6 and this has led to approvals in this setting.7–9
It is hypothesized that in a healthy host, the immune system normally generates an anti-cancer immune response that recognizes and kills cancer cells.10 However, binding of PD-L1 to its receptors, PD-1 and B7.1 (CD80), on activated T cells can dampen the T-cell immune response and inhibit tumor cell death.10,11 Atezolizumab is a humanized anti-PD-L1 IgG1 monoclonal antibody that binds to PD-L1 and inhibits PD-L1 binding to PD-1 or B7.1.
Atezolizumab and nivolumab (an anti-PD-1 agent) are approved as monotherapy in patients with previously treated NSCLC,3,4,6 while pembrolizumab (anti-PD-1) is approved for use as monotherapy in patients with previously treated NSCLC showing PD-L1 expression (determined by a tumor proportion score [TPS] ≥1%).5 Pembrolizumab is the only immunotherapy currently approved for use in the first-line setting for metastatic NSCLC, as monotherapy in patients with NSCLC showing high PD-L1 expression (TPS ≥50%),12 and in combination with carboplatin and pemetrexed in patients with non-squamous, PD-L1-unselected NSCLC (as of the time of writing, accelerated approval in the U.S.A only, based on KEYNOTE-021 study results).13
PD-L1 is expressed on tumor cells (TCs) and immune cells (ICs),11 and in NSCLC high PD-L1 expression on TCs and ICs appears to represent a distinct subpopulation of tumors that are regulated by different molecular mechanisms.14 Several studies have suggested that increased PD-L1 expression on TCs and ICs may be independent predictors of enhanced clinical benefit with atezolizumab.6,15–17 Atezolizumab monotherapy has demonstrated a statistically significant and clinically meaningful improvement in overall survival (OS) over standard-of-care docetaxel chemotherapy in a PD-L1-unselected population of patients with previously treated NSCLC.6 While this benefit extended to patients with tumors showing low or no PD-L1 expression, PD-L1 expression was associated with a greater magnitude of benefit with atezolizumab.6 Atezolizumab monotherapy has also shown clinical benefit in terms of OS and objective response rate (ORR) in patients with PD-L1-selected, chemotherapy-naïve NSCLC, with data supporting an improved benefit in patients with higher PD-L1 expression.16
The FIR trial (NCT01846416; Genentech Inc. [a member of the Roche Group] study GO28625), a three-cohort, single-arm, phase II study, was initiated to evaluate the efficacy and safety of atezolizumab in both chemotherapy-naïve and previously treated patients with PD-L1-selected, advanced, incurable, or metastatic NSCLC. FIR included a cohort of patients with pretreated brain metastases, a population with a particularly high unmet need. Evaluating heterogeneity of tumor PD-L1 expression with respect to time of sample collection and sample location was an exploratory endpoint. FIR also assessed the utility of fluorodeoxyglucose positron emission tomography (FDG-PET) to distinguish pseudoprogression from true progression, and as a surrogate for response.
Materials and Methods
Study Design and Participants
FIR comprised three cohorts: Cohort 1 enrolled patients who had not received platinum-based chemotherapy for metastatic disease or adjuvant therapy within 6 months of recurrence; patients in Cohorts 2 and 3 had received prior platinum-based chemotherapy for metastatic disease, with Cohort 3 enrolling patients with brain metastases (required to be treated and asymptomatic at screening). Patients with PD-L1-selected tumors (i.e. >5% PD-L1 staining in TC or IC) were identified by the VENTANA SP142 PD-L1 immunohistochemistry (IHC) assay. TC scores (TC0, TC1, TC2, and TC3) were assigned based on the percentage of tumor cells with PD-L1 staining (Supplementary Table 1). IC scores (IC0, IC1, IC2, and IC3) were based on the percentage of tumor area occupied by PD-L1-expressing ICs.6 Patients were selected for enrollment based on PD-L1 IHC status in archival or freshly collected samples. Initially, only patients with IC2 or IC3 tumors were enrolled (74 patients), but study eligibility was later expanded to include patients with TC2 or TC3 tumors (a further 64 patients). This change in PD-L1 selection criteria was made in response to emerging data external to this study that highlighted the importance of PD-L1 expression on both TC and IC as a predictive biomarker of atezolizumab efficacy in NSCLC. Patients provided an archival tumor specimen in addition to a sample obtained after the most recent systemic therapy, if clinically feasible. Resections or biopsy samples with ≥50 viable tumor cells with associated stroma were acceptable (~15 slides); fine needle aspiration/cytology specimens were not acceptable. Eastern Cooperative Oncology Group performance status (ECOG PS) of 0–1 was required. Patients with activating epidermal growth factor receptor or anaplastic lymphoma kinase gene mutations were eligible for enrollment, but were required to have received prior tyrosine kinase inhibitor therapy. Cohorts 1 and 2 excluded patients with any history of central nervous system (CNS) disease. Cohort 3 specifically enrolled patients with treated brain metastases. Other exclusion criteria included uncontrolled hypercalcemia or pleural effusion, receipt of a live attenuated vaccine within 4 weeks of cycle 1, and prior or current malignancies other than NSCLC within 5 years. Patients with a history of autoimmune disease or idiopathic pulmonary fibrosis, active tuberculosis, severe infection, hepatitis B or C infection, or significant cardiovascular disease were also excluded.
All cohorts received 1200 mg of intravenous atezolizumab on Day 1 of 21-day cycles until disease progression, unacceptable toxicity, or death. Cohort 1 patients were required to discontinue treatment if they experienced progression as per Response Evaluation Criteria in Solid Tumors (RECIST) v1.1; patients in Cohorts 2 and 3 could continue treatment upon progression if they were experiencing clinical benefit in the opinion of the investigator.
FIR was approved by the relevant institutional review or ethics committees and was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines. All patients provided written informed consent.
Procedures
Tumor assessments were undertaken at baseline, every 6 weeks for 12 months, then every 9 weeks, and at disease progression. ORR comprised confirmed complete and partial responses. Progression-free survival (PFS) and OS were estimated using Kaplan–Meier methodology. Mandatory FDG-PET scans were acquired at baseline, Week 6, and at radiographic progression, if clinically feasible. FDG-PET data were assessed using European Organization for Research and Treatment of Cancer (EORTC) criteria. At progression, tumor biopsies were taken, where clinically feasible. Adverse events (AEs) were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0. AEs of special interest (AESIs) were predefined based on the known mechanism of action for atezolizumab and precautions reported for other checkpoint inhibitors (Supplementary Appendix).
Outcomes
The primary endpoint was investigator-assessed ORR by modified RECIST (mRECIST; Supplementary Table 2) rather than ORR by RECIST v1.1, to provide context for the possibility of delayed anti-tumor activity. ORR by RECIST v1.1 was a secondary endpoint, along with PFS and duration of response (DOR) by RECIST v1.1 and mRECIST, OS, pharmacokinetics, and safety. Exploratory endpoints included changes in PD-L1 status between paired archival and fresh tumor specimens, and the use of FDG-PET imaging to distinguish between pseudoprogression and true progression.
Statistical Analysis
The planned study enrollment was approximately 130 patients (Cohort 1, n=45; Cohort 2, n=75; Cohort 3, n=10). ORR (across the three cohorts in a combined analysis) was estimated using 95% confidence intervals (CIs). Efficacy and safety analyses included all patients who received at least one dose of study treatment. There was no plan to perform a formal statistical comparison of the response rates between cohorts.
Results
Patients
Between May 2013 and June 2014, tumor specimens from 1009 patients were assessed for PD-L1 status; 918 had valid PD-L1 results with 41% being deemed as PD-L1 positive (i.e. >5% PD-L1 staining in TC or IC). Subsequently, 201 patients with a positive PD-L1 result were screened for clinical eligibility, with 138 patients enrolled; 137 patients were evaluable for efficacy and safety (Cohort 1, n=31; Cohort 2, n=93; Cohort 3, n=13; one patient withdrew from Cohort 2 before receiving study treatment; Fig. 1). Baseline characteristics are shown in Table 1. Data cut-off was March 31, 2017, with median survival follow-up of 33.5 months, 36.5 months, and 31.1 months for Cohorts 1, 2, and 3, respectively.
Figure 1.
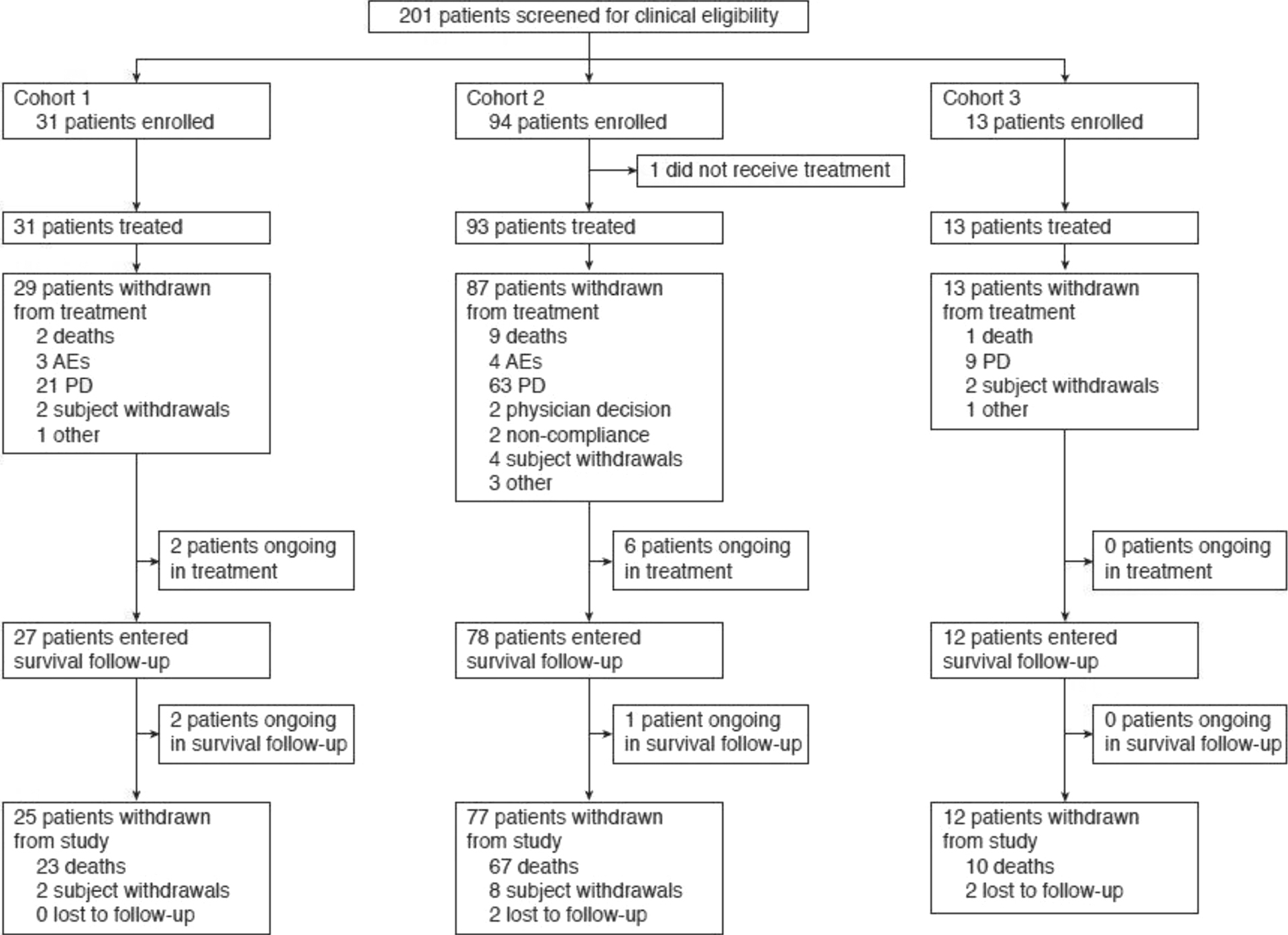
Study design.
AE, adverse event; PD, progressive disease
Table 1.
Baseline Characteristics
| Characteristics | Cohort 1 n = 31 |
Cohort 2 n = 93 |
Cohort 3 n = 13 |
Total N = 137 |
|---|---|---|---|---|
| Median age (range), years | 68 (42–85) | 65 (44–85) | 65 (52–74) | 66 (42–85) |
| Sex, n (%) | ||||
| Male | 14 (45) | 59 (63) | 6 (46) | 79 (58) |
| Female | 17 (55) | 34 (37) | 7 (54) | 58 (42) |
| Histology, n (%) | 11 (36) | 26 (28) | 1 (8) | 38 (28) |
| Non-squamous | ||||
| Squamous | 20 (65) | 67 (72) | 12 (92) | 99 (72) |
| Tobacco use, n (%) | ||||
| Never | 3 (10) | 16 (17) | 1 (8) | 20 (15) |
| Current | 3 (10) | 13 (14) | 2 (15) | 18 (13) |
| Previous | 25 (81) | 64 (69) | 10 (77) | 99 (72) |
| ECOG PS, n (%) | ||||
| 0 | 11 (36) | 24 (26) | 6 (46) | 41 (30) |
| 1 | 20 (65) | 68 (74) | 7 (54) | 95 (70) |
| Missing | 0 (0) | 1 (1) | 0 (0) | 1 (1) |
| EGFR mutation status, n (%) | 13 | 51 | 7 | 71 |
| Positive | 0 (0) | 5 (10) | 3 (43) | 8 (11) |
| Negative | 13 (100) | 44 (86) | 4 (57) | 61 (86) |
| T790M | 0 (0) | 2 (4) | 0 (0) | 2 (3) |
| ALK mutation positive, n (%) | 20 | 65 | 10 | 95 |
| Positive | 0 (0) | 1 (2) | 0 (0) | 1 (1) |
| Negative | 20 (100) | 64 (99) | 10 (100) | 94 (99) |
| PD-L1 TC/IC status, n (%) | ||||
| TC2 or IC2/3 | 28 (90) | 78 (84) | 12 (92) | 118 (86) |
| TC3 or IC3 | 7 (23) | 38 (41) | 8 (62) | 53 (39) |
ALK, anaplastic lymphoma kinase; ECOG PS, Eastern Cooperative Oncology Group performance status; EGFR, epidermal growth factor receptor; IC, tumor-infiltrating immune cell (IC2/3; 5% to <10%/≥10% PD-L1 staining); PD-L1, programmed death ligand-1; TC, tumor cell (TC2/3; 5% to <50%/≥50% PD-L1 staining).
Efficacy
Overall, there was agreement in ORR between mRECIST and RECIST v1.1 (Table 2). The primary endpoint of investigator-assessed ORR per mRECIST was 32% (95% CI: 17–51; n=10/31), 21% (95% CI: 13–30; n=19/92 [one patient in Cohort 2 did not have measurable disease]), and 23% (95% CI: 5–54; n=3/13) for Cohorts 1, 2, and 3, respectively. In the subset of patients with the highest level of PD-L1 expression (IC3 or TC3), ORR by mRECIST was 43% (95% CI: 10–82; n=3/7), 32% (95% CI: 18–49; n=12/38), and 25% (95% CI: 3–65; n=2/8), in Cohorts 1, 2 and 3, respectively. Pseudoprogression was rare, occurring in two patients who experienced a partial response after initially progressing per RECIST v1.1. The ORR by RECIST v1.1 in patients with TC3 or IC3 tumors was 43%, 26%, and 25%, for Cohorts 1, 2, and 3, respectively (Table 2).
Table 2.
ORR, DOR in Confirmed Responders, Median PFS, and 6-Month PFS by mRECIST and RECIST v1.1 for All Patients and Those with TC3 or IC3 Expression
| Cohort 1 | Cohort 2 | Cohort 3 | ||||
|---|---|---|---|---|---|---|
| mRECIST | RECIST v1.1 | mRECIST | RECIST v1.1 | mRECIST | RECIST v1.1 | |
| Response | ||||||
| ORR, % (95% CI), All | n = 31 32 (17–51) | n = 31 29 (14–48) | n = 92 21 (13–30) | n = 92 19 (11–28) | n = 13 23 (5–54) | n = 13 23 (5–54) |
| ORR, % (95% CI), TC3 or IC3 | n = 7 43 (10–82) | n = 7 43 (10–82) | n = 38 32 (18–49) | n = 38 26 (13–43) | n = 8 25 (3–65) | n = 8 25 (3–65) |
| Median DOR, months (range), All | n = 10 11.5 (2.3–30.4+) | n = 9 9.2 (2.3–30.4+) | n = 19 17.0 (5.6+–44.2+) | n = 17 17.0 (2.8–44.2+) | n = 3 NE (5.6+–9.9+) | n = 3 NE (2.8–9.9+) |
| Median DOR, months (range), TC3 or IC3 | n = 3 19.8 (2.9–30.4+) | n = 3 8.7 (2.9–30.4+) | n = 12 29.0 (5.6+–44.2+) | n = 10 29.0 (2.8–44.2+) | n = 2 NE (5.6+–9.9+) | n = 2 NE (5.6+–9.9+) |
| PFS | ||||||
| Median PFS, months (range), All | n = 31 5.5 (0.9–37.9+) | n = 31 4.5 (0.9–37.9+) | n = 93 3.7 (0.0+–45.5+) | n = 93 2.7 (0.0+–45.5+) | n = 13 4.3 (1.1–16.2) | n = 13 2.5 (1.0–11.3+) |
| Median PFS, months (range), TC3 or IC3 | n = 7 5.4 (3.3–34.3+) | n = 7 5.4 (3.3–34.3+) | n = 38 7.7 (0.0+–45.5+) | n = 38 4.1 (0.0+–45.5+) | n = 8 5.6 (1.4–16.2) | n = 8 2.3 (1.1–11.3+) |
| PFS rates | ||||||
| 12-month PFS, % (95% CI), All | n = 31 31 (14–48) | n = 31 20 (6–34) | n = 93 29 (19–39) | n = 93 23 (14–32) | n = 13 24 (0–50) | n = 13 NE |
| 12-month PFS, % (95% CI), TC3 or IC3 | n = 7 29 (0–62) | n = 7 14 (0–40) | n = 38 41 (24–57) | n = 38 33 (18–49) | n = 8 38 (4–71) | n = 8 NE |
| 30-month PFS, % (95% CI), All | n = 31 12 (0–25) | n = 31 13 (0–25) | n = 93 10 (3–17) | n = 93 10 (4–17) | n = 13 NE | n = 13 NE |
| 30-month PFS, % (95% CI), TC3 or IC3 | n = 7 14 (0–40) | n = 7 14 (0–40) | n = 38 20 (6–35) | n = 38 20 (6–34) | n = 8 NE | n = 8 NE |
CI, confidence interval; IC, immune cell; DOR, duration of response; ORR, objective response rate; PFS, progression-free survival; mRECIST, modified Response Evaluation Criteria in Solid Tumors; TC, tumor cell; NE, not estimable; + denotes a censored value.
Median DOR by mRECIST was 11.5 months and 17.0 months for Cohorts 1 and 2, respectively, and not estimable for Cohort 3, where the median DOR was not reached. In patients with PD-L1 TC3 or IC3 tumors, the median DOR was 19.8 months and 29.0 months in Cohorts 1 and 2, respectively, and not estimable in Cohort 3. Sample size was too small to infer differences in DOR by TC/IC status. Median time to response was 2.0 months, 2.6 months, and 1.4 months for Cohorts 1, 2, and 3, respectively.
Median PFS assessed by mRECIST was 5.5 months (95% CI: 0.9–37.9+) for Cohort 1, 3.7 months (95% CI: 0.0–45.5+) for Cohort 2, and 4.3 months (95% CI: 1.1–16.2) for Cohort 3. Median PFS by mRECIST in patients with PD-L1 TC3 or IC3 tumors was 5.4 months, 7.7 months, and 5.6 months for Cohorts 1, 2, and 3, respectively. Median PFS by RECIST v1.1 was 4.5 months (95% CI: 3.3–8.3) for Cohort 1, 2.7 months (95% CI: 1.5–3.5) for Cohort 2, and 2.5 months (95% CI: 1.2–4.2) for Cohort 3 (Fig. 2A). Many patients were discontinued from treatment after progressing per RECIST v1.1 but before recording PD per mRECIST, resulting in a high rate of censoring and wide confidence intervals around median PFS estimates by mRECIST.
Figure 2.
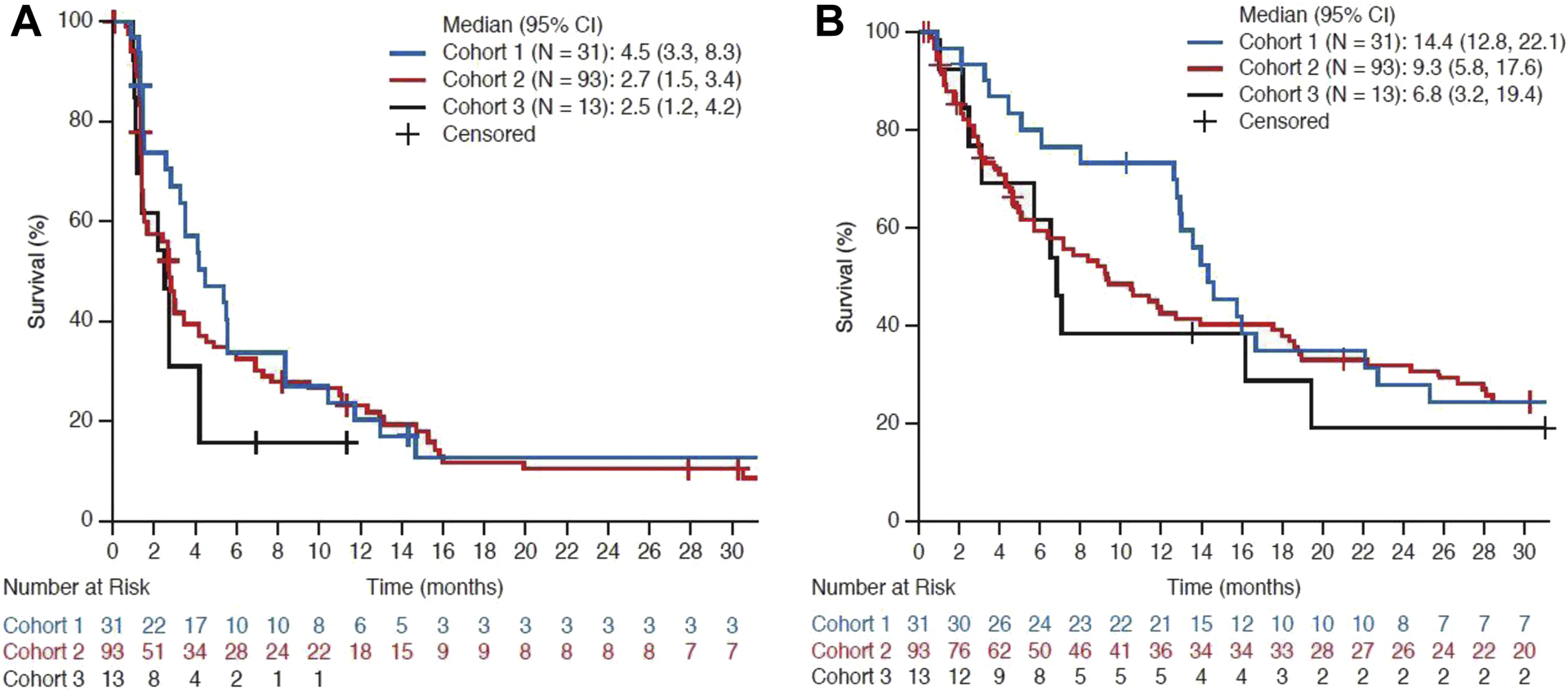
Kaplan–Meier curves of progression-free survival (A) and overall survival (B) by RECIST v1.1.
Median OS in patients with TC2/3 or IC2/3 tumors was 14.4 months in Cohort 1, 9.3 months in Cohort 2, and 6.8 months in Cohort 3, with 30-month OS rates of 25% in Cohort 1, 25% in Cohort 2, and 19% in Cohort 3 (Table 3; Fig. 2B). In patients with TC3 or IC3 tumors, median OS was 15.8 months in Cohort 1, 22.2 months in Cohort 2, and 7.0 months in Cohort 3, with 30-month OS rates of 29%, 43%, and 19% respectively.
Table 3.
Summary of Overall Survival Endpoints
| Cohort 1 | Cohort 2 | Cohort 3 | ||||
|---|---|---|---|---|---|---|
| All n = 31 |
TC3 or IC3 n = 7 |
All n = 93 |
TC3 or IC3 n = 38 |
All n = 13 |
TC3 or IC3 n = 8 |
|
| Median OS | ||||||
| Months (95% CI) | 14.4 (12.8–22.1) | 15.8 (13.0–32.3) | 9.3 (5.8–17.6) | 22.2 (5.8–NE) | 6.8 (3.2–19.5) | 7.0 (2.5–16.2) |
| 12-month OS rate | ||||||
| % (95% CI) | 74 (58–89) | 86 (60–100) | 43 (32–53) | 55 (39–71) | 38 (12–65) | 38 (4–71) |
| 30-month OS rate | ||||||
| % (95% CI) | 25 (9–40) | 29 (0–62) | 25 (15–34) | 43 (26–59) | 19 (0–42) | 19 (0–50) |
CI, confidence interval; IC, immune cell; NE, not estimable; OS, overall survival; TC, tumor cell.
Safety
All grade treatment-related AEs (TRAES) occurred in 70% (n = 96/137) of patients overall: 81% (n = 25/31) of Cohort 1, 67% (n = 62/93) of Cohort 2, and 69% (n = 9/13) of Cohort 3 (Table 4). Grade 3–4 TRAEs were seen in 18% (n = 25/137) of patients: 16% (n = 5/31) of Cohort 1, 19% (n = 18/93) of Cohort 2, and 15% (n = 2/13) of Cohort 3. The most common TRAEs (all grades) were fatigue (27%, n=37/137), decreased appetite (15%, n = 21/137), nausea (15%, n = 20/137), diarrhea (9%, n = 13/137), pyrexia (8%, n = 11/137), and pruritus (7%, n = 10/137; Supplementary Table 3). Five deaths resulting from grade 5 AEs occurring occurred ≤30 days after the last dose of atezolizumab (cardiac arrest; hemoptysis; disseminated intravascular coagulation; constrictive pericarditis; respiratory disorder). Only the event of constrictive pericarditis was considered related to atezolizumab by the investigator.
Table 4.
Overall Safety Summary
| Cohort 1 n = 31 |
Cohort 2 n = 93 |
Cohort 3 n = 13 |
All N = 137 |
|
|---|---|---|---|---|
| Median treatment duration, months | 4.3 | 2.5 | 3.5 | 2.9 |
| All grade AEs (any cause), n (%) | 31 (100) | 92 (99) | 13 (100) | 136 (99) |
| Treatment-related AEs, n (%) | 25 (81) | 62 (67) | 9 (69) | 96 (70) |
| Grade 3–4 AEs (any cause), n (%) | 16 (52) | 53 (57) | 7 (54) | 76 (56) |
| Treatment-related grade 3–4 AEs, n (%) | 5 (16) | 18 (19) | 2 (15) | 25 (18) |
| Grade 5 AEs (any cause), n (%)a | 1 (3) | 3 (3) | 0 (0) | 4 (3) |
| Treatment-related grade 5 AEs, n (%) | 0 (0) | 1 (1) | 0 (0) | 1 (1) |
| Patients withdrawing from treatment due to AEs, n (%) | 3 (10) | 5 (5) | 0 (0) | 8 (6) |
Grade 5 AEs occurring ≤30 days after last treatment dose included cardiac arrest, cardiac tamponade, and hemoptysis in Cohort 1 and disseminated intravascular coagulation, constrictive pericarditis, and respiratory disorder in Cohort 2; of these, only constrictive pericarditis was considered related to atezolizumab. Grade 5 AEs occurring >30 days after last treatment dose included euthanasia and pneumonia.
AE, adverse event.
AESIs were reported in 27.7% (n=38/137) of patients. Most patients with an AESI had a grade 1–2 event (73.7%, n = 28/38). The most commonly reported events were dermatologic reactions, such as rash. Ten patients (7.3%; n = 10/137) experienced a grade 3–4 AESI (dermatitis, aspartate aminotransferase [AST] increased, alanine aminotransferase [ALT] increased, Guillain–Barré syndrome, autoimmune colitis, pneumonitis, and systemic inflammatory response syndrome).
PD-L1 Prevalence
Tumor specimens, including biopsies and resections/excisions, from 1009 patients were assessed for PD-L1 status. Of these, 91 specimens were not evaluable for PD-L1 expression. The overall prevalence of PD-L1 expression was 16% in TC3 or IC3, 41% in TC2/3 or IC2/3, and 77% in TC1/2/3 or IC1/2/3. The prevalence of PD-L1 expression subgroups in biopsy samples compared with resections/excisions is shown in Supplementary Figure 1. Among 700 biopsy samples, PD-L1 expression was TC3 or IC3 in 15%, TC2 or IC2 in 22%, and TC1 or IC1 in 35% within mutually exclusive PD-L1 subgroups. Overall, 37% of biopsies were TC2/3 or IC2/3 and 72% were TC1/2/3 or IC1/2/3. No expression of PD-L1 was detected in 28% of biopsy samples (TC0 and IC0). Among resections/excisions (n=211), PD-L1 expression was TC3 or IC3 in 19% of samples, TC2 or IC2 in 35%, TC1 or IC1 in 39%, with no expression in 7% (TC0 and IC0). Overall, 54% of resections/excisions were TC2/3 or IC2/3 and 93% were TC1/2/3 or IC1/2/3 (Supplementary Figure 1).
PD-L1 Expression in Metachronous Tumor Pairs
From the 1009 prescreened patients, 97 had paired archival and freshly collected tumor samples. The median time between obtaining archival and fresh samples was 11.2 months (range, 0.6–87.9 months). Altogether, 37 patients who submitted paired samples received non-trial therapy between their archival sample and the fresh sample (n=20 received chemotherapy, n=17 received chemotherapy plus targeted therapy; line of therapy dependent on assigned cohort), and 60 patients did not receive any treatment between the collection of paired samples. Changes in PD-L1 expression are shown in Supplementary Figure 2. The median change in PD-L1 expression over time between archival and freshly collected samples on TC was 0% (95% distribution-free CI: 0–0), and on IC was -1% (95% distribution-free CI: -2–0%). Overall, 88.6% of paired samples showed a change of <5% in either TC or IC. When assessed at individual TC or IC cut-offs, the overall agreement between paired samples regardless of procurement method (resection or biopsy) was 75% (n = 73/97) for the TC1/2/3 or IC1/2/3 cut-off, 67% (n = 65/97) for the TC2/3 or IC2/3 cut-off, and 79% (n = 77/97) for the TC3 or IC3 cut-off. When paired samples were obtained using the same tissue procurement method, agreement was 91% (n = 49/54) at the TC3 or IC3 cut-off, 70% (n = 38/54) at the TC2/3 or IC2/3 cut-off, and 74% (n = 40/54) at the TC1/2/3 or IC1/2/3 cut-off (Supplementary Table 4; Supplementary Figure 3).
FDG-PET Imaging
Evaluable baseline and Week 6 FDG-PET scans were available from 103 patients. Patients with metabolic response by EORTC criteria on 6-week scans had a higher ORR than metabolic non-responders (73.9% [n = 17/23] versus 6.3% [n = 5/80]). In patients with FDG-PET data, response by EORTC criteria at Week 6 was not significantly different than response based on diagnostic computed tomography (CT) at Week 6. However, in patients with stable disease at Week 6 by CT, an increase in metabolic tumor burden was associated with a worse survival outcome (Supplementary Figure 4). As there were few instances of pseudoprogression (two patients; pseudoprogression observed at ~6 months), it was not possible to test the utility of FDG-PET as a non-invasive method to distinguish between pseudoprogression and true progression.
Discussion
An association between tumor PD-L1 expression and response to checkpoint inhibitors was proposed relatively early in their clinical development.18–20 The FIR study was designed to investigate the efficacy of atezolizumab in previously treated and chemotherapy-naïve PD-L1-selected patients.
FIR demonstrated that atezolizumab provides clinically meaningful activity in terms of ORR (by RECIST v1.1 or mRECIST) and OS, with durable responses. For patients with previously treated NSCLC, the clinical activity of atezolizumab in terms of ORR and OS was similar between patients with previously treated brain metastases and those without, consistent with subgroup analyses from the OAK phase III trial.6
Patients with the highest levels of PD-L1 expression (TC3 or IC3) achieved higher response rates than the overall study population (TC2/3 or IC2/3). For patients with previously treated NSCLC, the ORR in both the overall study population (TC2/3 or IC2/3: 21% Cohort 2, 23% Cohort 3) and patients with the highest levels of PD-L1 expression (TC3 or IC3: 32% Cohort 2, 25% Cohort 3) were comparable with those seen in the BIRCH phase II monotherapy trial (TC2 or IC2: 18% Cohort 2 [patients who had 1 prior platinum chemotherapy], 19% Cohort 3 [patients who had ≥2 prior chemotherapies] and TC3 or IC3: 26% Cohort 2, 27% Cohort 3, respectively) and OAK (TC2/3 or IC2/3: 23% and TC3 or IC3: 31%, respectively).6,16 The ORR in patients receiving first-line atezolizumab in FIR (32% in TC2/3 or IC2/3; 43% in TC3 or IC3) also compares favorably with the promising results reported from BIRCH for atezolizumab monotherapy as first-line treatment for PD-L1-selected NSCLC (22% in TC2/3 or IC2/3; 31% in TC3 or IC3).16 Data from FIR are consistent with those published for other immunotherapy agents, where higher response rates have also been reported in populations enriched for tumor PD-L1 expression versus an unselected population.3–5,21 However, comparisons should be treated with caution due to the use of different assays that cannot be directly compared and different cut-off criteria to define PD-L1.
OS was a secondary endpoint in FIR, and across all cohorts appeared longer in the subgroup of patients with the highest levels of PD-L1 expression (TC3 or IC3) compared with all treated patients. However, the OS difference between the TC3 or IC3 subgroup and all treated patients in those with chemotherapy-naïve NSCLC was marginal, consistent with the findings from the BIRCH study showing that OS benefit appeared independent of PD-L1 status.16 In patients with previously treated NSCLC without brain metastases, median OS was considerably longer in patients with the highest levels of PD-L1 expression (TC3 or IC3) compared with all treated patients. This is consistent with findings from the OAK study where OS was longer in patients with higher PD-L1 expression, although in OAK atezolizumab improved OS over standard-of-care docetaxel across all PD-L1 expression subgroups including those patients with low or no PD-L1 expression (TC0 and IC0).6 Comparisons with other studies should be made with caution due to differences in study size, patient characteristics, and PD-L1 selection criteria.
It must also be noted that, as the PD-L1 selection criteria were changed during the FIR study, it is likely that the study population contains a higher proportion of IC2/3 patients than a natural TC2/3 or IC2/3 population, which is a limitation of this study. In FIR, atezolizumab demonstrated an acceptable safety profile that is consistent with findings from previous trials of atezolizumab monotherapy, including rate of patient withdrawal due to AEs and incidence of grade 3–4 TRAEs.6,16
The utility of FDG-PET as a biomarker of response was similar to previous reports with chemotherapy in NSCLC.22,23 Metabolic response at 6 weeks was predictive for clinical benefit, but due to the inherent increased sensitivity of FDG-PET, earlier evaluation would likely be more valuable in identifying non-responders, particularly in the first-line setting. The incidence of pseudoprogression (defined as a radiographic increase in tumor volume due to the influx of immune cells) in NSCLC was unknown at the start of the study, but ultimately turned out to be much lower than anticipated in FIR (n = 2). The incidence has also been reported to be low in the majority of solid tumors treated with checkpoint inhibitors,24 including melanoma. Therefore, the use of FDG-PET to distinguish pseudoprogression could not be assessed.
PD-L1 prevalence in FIR was in line with other studies of metastatic NSCLC.6,16 The TC3 or IC3 prevalence in resection/excision specimens was comparable to prevalence in biopsies (19% versus 15%, respectively). TC1/2/3 or IC1/2/3 prevalence was numerically higher in resections/excision compared with biopsies (97% versus 72%), mostly driven by the TC2 or IC2 subgroup. These data are in contrast to findings by Ilie et al.,25 who reported a much larger difference in PD-L1 prevalence between resections and biopsies (74% versus 25%, respectively), and may reflect differences in the assay type (investigational use only used in our study versus research use only used by Ilie et al.), potential differences in the staining protocol, as well as the scoring algorithm. Analysis of PD-L1 expression on TC and IC revealed a minimal overall change in PD-L1 expression in both cell types over time and/or due to intervening therapies, as comparison of fresh and archival tissue showed that 88.6% of paired samples showed a change of <5% in TC or IC. Similarly, a high level of agreement in PD-L1 expression was observed across all PD-L1 expression thresholds. The agreement was not dependent on the procurement method, and was similar for paired resections and biopsies (apart from the TC3 or IC3 cut-off). In the highly selected TC3 or IC3 group, the agreement in PD-L1 expression between metachronous samples was particularly high. These data suggest that intrapatient heterogeneity of PD-L1 expression is relatively low in NSCLC tumor specimens, implying that fresh or archival samples can be reliably used to assess PD-L1 status via IHC.
Conclusions
FIR demonstrated that atezolizumab monotherapy has clinical activity in patients with PD-L1-selected NSCLC, both in the first and later lines, with favorable ORR, PFS, and OS. Atezolizumab monotherapy showed clinical activity in patients with and without previously treated brain metastases. The overall PD-L1 prevalence in resections/excisions appeared to be higher compared to small biopsy (e.g. 93% vs 72% for TC1/2/3 or IC1/2/3), except for PD-L1 high (TC3 or IC3) population that had similar prevalence in both types of specimens (19% and 15%, respectively). PD-L1 expression appears to be relatively stable over time. The role of FDG-PET assessment in characterizing tumor response to immunotherapy remains unchanged based on the FIR data, but may identify non-responders earlier than conventional imaging, which may be beneficial particularly in patients with significant toxicity.
In addition to FIR, the BIRCH study has also shown favorable ORR and OS outcomes for atezolizumab in PD-L1 selected patients, in both first and later lines.16 Furthermore, the POPLAR and OAK studies demonstrated an OS benefit with atezolizumab over standard-of-care docetaxel chemotherapy in PD-L1-unselected patients with previously treated NSCLC,6,15 supporting the approval of atezolizumab in this setting. Based on data from our single arm phase II trials, FIR and BIRCH, a confirmatory phase III study IMpower110 has been initiated in chemotherapy-naïve NSCLC comparing atezolizumab monotherapy versus platinum-based chemotherapy in PD-L1-selected patients. This will provide further evidence of the efficacy of atezolizumab in NSCLC.
Supplementary Material
Acknowledgments
Third-party medical writing assistance, under the direction of the authors, was provided by Sophie Powell, PhD, of Gardiner-Caldwell Communications, and was funded by F. Hoffmann-La Roche Ltd.
Funding: Genentech Inc. (a member of the Roche Group)
Disclosures:
RF, MK, LL, AS, and JF are employees of Genentech and own stocks in Roche/Genentech. DS has advised for Bristol-Myers Squibb, Roche/Genentech, Novartis, and Pfizer and serves on the Data Safety Monitoring Board for Merck. LC has received research grants from Novartis, Bristol-Myers Squibb, Merck, Eli Lilly/Imclone, OSI Pharma, Genentech, VentiRx, Pfizer, NCCN/GlaxoSmithKline, AstraZeneca/MedImmune, and Incyte, has advised for Emergent BioSolutions, Merck, Bristol-Myers Squibb, and Novartis, and has received honoraria from Astellas Pharma. SG has received research grants from Bristol-Myers Squibb, AstraZeneca, ARIAD, and Genentech, and has advised and received honoraria from Bristol-Myers Squibb and ARIAD. JC has advised for Genentech, Clovis, and AstraZeneca. BC is an employee of Eli Lilly. NR has advised for Merck, Roche, AstraZeneca, and Novartis. PS, RH, and LD have nothing to disclose.
Abbreviations:
- AE
adverse event
- AESI
adverse event of special interest
- ALK
anaplastic lymphoma kinase
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- CI
confidence interval
- CNS
central nervous system
- CR
complete response
- CT
computed tomography
- DOR
duration of response
- ECOG PS
Eastern Cooperative Oncology Group performance status
- EGFR
epidermal growth factor receptor
- EORTC
European Organisation for Research and Treatment of Cancer
- FDG-PET
fluorodeoxyglucose positron emission tomography
- IC
immune cell
- IHC
immunohistochemistry
- mRECIST
modified Response Evaluation Criteria in Solid Tumors
- NE
not estimable
- NSCLC
non-small-cell lung cancer
- ORR
objective response rate
- OS
overall survival
- PD
progressive disease
- PD-1
programmed death-1
- PD-L1
programmed death-ligand 1
- PFS
progression-free survival
- PR
partial response
- RECIST
Response Evaluation Criteria in Solid Tumors
- SD
stable disease
- TC
tumor cell
- TPS
tumor proportion score
- TRAE
treatment-related adverse event
References
- 1.Fossella FV, DeVore R, Kerr RN, et al. Randomized phase III trial of docetaxel versus vinorelbine or ifosfamide in patients with advanced non-small-cell lung cancer previously treated with platinum-containing chemotherapy regimens. The TAX 320 Non-Small Cell Lung Cancer Study Group. J Clin Oncol. 2000;18:2354–2362. [DOI] [PubMed] [Google Scholar]
- 2.Shepherd FA, Dancey J, Ramlau R, et al. Prospective randomized trial of docetaxel versus best supportive care in patients with non-small-cell lung cancer previously treated with platinum-based chemotherapy. J Clin Oncol. 2000;18:2095–2103. [DOI] [PubMed] [Google Scholar]
- 3.Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373:123–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373:1627–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387:1540–1550. [DOI] [PubMed] [Google Scholar]
- 6.Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017;389:255–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Merck Sharp & Dohme. KEYTRUDA (pembrolizumab) prescribing information, December 2015. https://www.merck.com/product/usa/pi_circulars/k/keytruda/keytruda_pi.pdf.
- 8.Genentech. TECENTRIQ (atezolizumab) prescribing information, April 2017. https://www.gene.com/download/pdf/tecentriq_prescribing.pdf.
- 9.Squibb Bristol-Myers. OPDIVO (nivolumab) prescribing information, May 2016. https://packageinserts.bms.com/pi/pi_opdivo.pdf.
- 10.Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity. 2013;39:1–10. [DOI] [PubMed] [Google Scholar]
- 11.Chen DS, Irving BA, Hodi FS. Molecular pathways: next-generation immunotherapy--inhibiting programmed death-ligand 1 and programmed death-1. Clin Cancer Res. 2012;18:6580–6587. [DOI] [PubMed] [Google Scholar]
- 12.Reck M, Rodriguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016;375:1823–1833. [DOI] [PubMed] [Google Scholar]
- 13.Langer CJ, Gadgeel SM, Borghaei H, et al. Carboplatin and pemetrexed with or without pembrolizumab for advanced, non-squamous non-small-cell lung cancer: a randomised, phase 2 cohort of the open-label KEYNOTE-021 study. Lancet Oncol. 2016;17:1497–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gettinger SN, Kowanetz M, Koeppen H, et al. Molecular, immune and histopathologic characterization of NSCLC based on PD-L1 expression on tumor and immune cells and association with response to the anti-PDL1 antibody atezolizumab. J Clin Oncol. 2015;33(suppl):3015 [abstract]. [Google Scholar]
- 15.Fehrenbacher L, Spira A, Ballinger M, et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet. 2016;387:1837–1846. [DOI] [PubMed] [Google Scholar]
- 16.Peters S, Gettinger S, Johnson ML, et al. Phase II trial of atezolizumab as first-line or subsequent therapy for patients with programmed death-ligand 1-selected advanced non-small-cell lung cancer (BIRCH). J Clin Oncol. 2017:35:2781–2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Horn L, Spigel DR, Gettinger SN, et al. Clinical activity, safety and predictive biomarkers of the engineered antibody MPDL3280A (anti-PDL1) in non-small cell lung cancer (NSCLC): update from a phase Ia study. J Clin Oncol. 2015;33(suppl):8029 [abstract]. [Google Scholar]
- 18.Herbst RS, Soria JC, Kowanetz M, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515:563–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brahmer JR, Drake CG, Wollner I, et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol. 2010;28:3167–3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372:2018–2028. [DOI] [PubMed] [Google Scholar]
- 22.Hicks RJ. Role of 18F-FDG PET in assessment of response in non-small cell lung cancer. J Nucl Med. 2009;50(suppl 1):31S–42S. [DOI] [PubMed] [Google Scholar]
- 23.Skoura E, Datseris IE, Platis I, Oikonomopoulos G, Syrigos KN. Role of positron emission tomography in the early prediction of response to chemotherapy in patients with non-small-cell lung cancer. Clin Lung Cancer. 2012;13:181–187. [DOI] [PubMed] [Google Scholar]
- 24.Chiou VL, Burotto M. Pseudoprogression and immune-related response in solid tumors. J Clin Oncol. 2015;33:3541–3543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ilie M, Long-Mira E, Bence C, et al. Comparative study of the PD-L1 status between surgically resected specimens and matched biopsies of NSCLC patients reveal major discordances: a potential issue for anti-PD-L1 therapeutic strategies. Ann Oncol. 2016;27:147–153. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


