Abstract
This study adapted the established glycemic index (GI) methodology used in human research to perform two studies in sled dogs in order to assess the blood glucose-raising potential of pulse-based dog foods. The first was a pilot study (n = 6 dogs) to determine the GI of single starch sources (white bread, cooked white rice, and cooked green lentils) using a glucose solution as control. Next, the effect on glycemic and insulinemic meal responses and GI of commercial extruded dog foods containing different categories of starch sources (traditional grain, whole grain, grain-free, and vegan) were investigated on 11 dogs using a glucose control. Results were compared using repeated measures analysis of variance (ANOVA). Consumption of 10 g of available carbohydrate (Av CHO) was insufficient to elicit a measurable response in blood glucose for GI determination, and as such, the amount was increased to 25 g for the second study. The GI (±SE) of the single starch sources and dog foods was: white bread: 47 ± 11, cooked white rice: 71 ± 14, cooked green lentils: 60 ± 20 (P = 0.569), traditional grain: 83 ± 17, whole grain: 56 ± 8, grain-free: 41 ± 6, and vegan: 65 ± 15 (P = 0.154). No statistical differences in glycemic response over time were observed between the single starch sources or the extruded diets tested (P = 0.1412; P = 0.2651). The insulinemic response elicited by the extruded diets was also not different (P = 0.079); however, the traditional grain diet did have the slowest time to peak for insulin (P = 0.0078). Among single starch sources and extruded dog foods, there were no differences in the glycemic indices measured in this study. The GI methodology has not been validated for use in canine species, and it is likely that our results were due to higher interindividual variation or inadequate study power. Regardless, this study will serve to better define future studies to investigate the potential physiological benefits of low GI foods for dogs.
Keywords: canine, glycemic index, grain-free, insulin, nutrition, pulses
Introduction
Starch is the primary digestible carbohydrate found in plants and is an economical source of dietary energy for both humans and pets (Bednar et al., 2001). Commercial pet foods typically contain a mixture of high-starch carbohydrate sources that fall into three broad categories: traditional grains (e.g., wheat, corn), novel whole grains (e.g., barley, oatmeal, rye), and nongrain carbohydrates (e.g., peas, lentils, tapioca, potato). Grain-free pet foods have been very popular with consumers for a number of years (Agriculture and Agri-Food Canada, 2016; Packaged Facts, 2017). However, in 2018, the U.S. Food and Drug Administration (FDA) issued a warning about a potential link between grain-free dog foods and the development of canine cardiomyopathy (Center for Veterinary Medicine FDA, 2018). Although the FDA report is inconclusive, it may have impacted the sales of grain-free pet food. Nonetheless, the grain-free trend had encouraged a switch from traditional starch ingredients, such as corn and rice, to novel, grain-free starch sources in pet food formulations, such as pulses (which include dry peas, lentils, beans, and chickpeas; Buff et al., 2014). As a result, the use of nongrain carbohydrate sources is of interest to pet food manufacturers.
Starch is made up of two glucose polymers: amylose and amylopectin (Nelson and Cox, 2008: 20). The linear structure of amylose makes it less susceptible to digestion, as compared with the branch-chained structure of amylopectin. The ratio of these polymers varies across starch sources; the amylose content of most starches ranges between 20% and 35% (Biliaderis, 1991; Joshi et al., 2013; Thakur et al., 2019), while legume starches contain between 29% and 65% amylose (Hoover and Sosulski, 1991; Joshi et al., 2013; Thakur et al., 2019). The proportion of amylose in a starch granule influences the rate of digestibility of starch sources and ultimately the quantity of resistant starch (RS; Biliaderis, 1991). RS refers to the portion of starch that cannot be enzymatically digested and goes on to be fermented in the large intestine (Englyst et al., 1992). As a result, RS becomes a source of volatile fatty acids as opposed to a source of glucose and ultimately does not contribute to the postprandial glycemic response (Bednar et al., 2001). As a result of the differences in amylose and RS content between starch sources, the postprandial glycemic response will vary. The glycemic index (GI) was created to rank foods based on their acute postprandial glycemic response in comparison to a control food, either a standard glucose solution or white bread (Jenkins et al., 1981). The regular consumption of low GI foods in at-risk human populations has been linked to numerous health benefits, such as decreased concentrations of cholesterol and triglycerides and reduced risk of obesity, diabetes, and cardiovascular disease (Wolever et al., 1991; Barclay et al., 2008; Ramdath, 2016). Common starch-containing pet food ingredients, such as corn and rice, have been shown in humans to have higher GI values compared with pulses, such as peas and lentils (Foster-Powell et al., 2002).
Given the limited research currently available on canine GI, it is unknown at this time what the physiological benefits to differences in GI may be for dogs. However, extruded foods that produce a delayed and lengthened postprandial glycemic response and improved insulin sensitivity may be beneficial in the dietary maintenance of dogs with diabetes mellitus or glucose intolerance resulting from obesity (Graham et al., 1994). Currently, pet food marketing claims concerning GI are ingredient based and communicate the inclusion of starch sources known to be low GI in humans; however, these claims are not based on standardized in vivo GI testing in dogs. Although research has demonstrated that the type of starch plays a role in determining postprandial glycemic responses in dogs (Carciofi et al., 2008), GI methodology has not been validated for use in companion animals, and limited work has been done investigating its use (Adolphe et al., 2012, 2015; Briens, 2018). Previous GI research in Beagles found that pulses may attenuate glycemic and insulinemic responses when fed alone and when included in an extruded kibble diet (Adolphe et al., 2012, 2015; Briens, 2018).
The aim of this two-part study was to further develop GI methodology in dogs. Since Beagles housed in controlled laboratory settings have been the only dogs previously used in published canine GI studies, the first study was performed to evaluate whether 10 g available carbohydrates (Av CHO) can also be considered an adequate quantity for canine GI testing with large breed client-owned dogs. Three high-starch foods (white bread, cooked white rice, and cooked green lentils) were tested. It was hypothesized that green lentils would have the lowest GI and white rice would have the highest GI. White bread was tested to assess its suitability as a control food similar to human GI studies. In the second study, the glycemic and insulinemic responses of four commercial extruded dog foods that contained different categories of starch sources (traditional grain, whole grain, grain-free, and vegan) were measured. It was hypothesized that the lowest and highest glycemic and insulinemic responses would result from the grain-free and traditional grain diets, respectively.
Materials and Methods
All experimental procedures for this study were approved by the University of Guelph Animal Care Committee (AUP#3650) and were in accordance with national and institutional guidelines for the care and use of animals.
Animals
Adult, client-owned Siberian Husky dogs were used for this research. Prior to the study, all dogs were deemed healthy based on a medical history, physical examination, complete blood count (CBC), and serum biochemistry profile. Dogs that had received medications 6 mo prior to enrollment, had abnormalities on their physical examination, CBC, or serum biochemistry, or were younger than 1 yr of age were not enrolled in the study. All dogs remained with their owner (Rajenn Kennel, Ayr, ON) throughout the course of the study and were housed together in an open-concept kennel facility at the owner’s home. The facility was visited by members of the Animal Care Committee at the University of Guelph and found to comply with the standards of care outlined by the Canadian Council on Animal Care and Ontario’s Animal Research Act. On the days of the study, the dogs were separated and individually handled. All dogs were transitioned onto the same background diet (GO! FIT + FREE Adult Dog Food, Petcurean Pet Nutrition, Chilliwack, BC, Canada) 2 wk prior to the start of the postprandial response tests. Initially, dogs were fed an amount determined to maintain optimal body condition score (BCS), based on their diet history. Body weight (BW) was recorded weekly and BCS was recorded monthly; food was adjusted to maintain stable BW. Dogs continued to eat the background diet throughout the entire study period.
Study 1
Six adult, client-owned Siberian Husky dogs (n = 3, male, neutered; n = 3, female, spayed) with a mean ± SD age of 5.63 ± 0.57 yr (range: 5.4 to 6.8 yr) were used to determine the GI of three starch-rich foods (white bread, cooked white rice, and cooked green lentils). Dogs had a BCS between 4 and 6 (mean ± SD: 4.80 ± 0.66; on a 9-point-scale; Laflamme, 1997), with a mean BW of 24.94 ± 0.99 kg (range: 21.51 to 28.72 kg).
Study 2
Eleven adult, client-owned Siberian Husky dogs (n = 4, male, neutered; n = 5, female, spayed; n = 2, female, intact) with a mean ± SD age of 5.63 ± 2.38 yr (range: 1.00 to 10.67 yr) were used to assess the glycemic and insulinemic responses of four commercial extruded dog foods containing different starch sources. The mean ± SEM BW of the dogs was 23.32 ± 1.15 kg (range: 19.00 to 30.68 kg). BCS for the dogs ranged between 3 and 6 on a 9-point scale (mean ± SD: 4.91 ± 0.63; Laflamme, 1997).
Diets
The total and RS content of each food was analyzed enzymatically using commercially available assay kits (Megazyme International, Wicklow, Ireland; AOAC methods 996.11 and 2002.02, respectively). Total and RS were calculated as a percentage of the total dry matter (DM). Free sugar content was determined by extracting and analyzing monosaccharides and disaccharides, as described by Brummer et al. (2015). Total Av CHO of each test food was calculated as the sum of the total starch and free sugar content (McCance and Lawrence, 1929).
Study 1
Three starch-rich foods were tested: white bread (Wonder Bread, Interstate Brands Companies, Kansas City, MO, USA), cooked white long-grain rice (Selection, St. Paul, MN, USA), and cooked Eston green lentils (AGT Food and Ingredients, Regina, SK, Canada). A 20% (wt/vol) glucose solution (d-Glucose, Thermo Fisher Scientific, Pittsburgh, PA, USA) was used as the standard. Using a 5 * 5 Latin Square design, each dog ingested each food once, except for the glucose control, which was tested twice, with a minimum washout period of 2 d between testing. Both the test foods and glucose standard were fed in amounts that provided 10 g of Av CHO (Adolphe et al., 2012, 2015).
Study 2
Four commercial dog foods were tested: Dog Chow (Nestlé Purina Petcare, St. Louis, MO, USA), SUMMIT Three Meat Adult Recipe (Petcurean Pet Nutrition, Chilliwack, BC, CA), GO! SENSITIVITY + SHINE Limited Ingredient Duck Recipe (Petcurean Pet Nutrition, Chilliwack, BC, CA), and Natural Balance Vegetarian Dry Dog Formula (Dick Van Patten’s Natural Balance Pet Foods, Burbank, CA, USA). The four test diets were classified based on the main starch sources that were listed on the ingredient panels: traditional grain (Dog Chow: corn, wheat), whole grain (SUMMIT: oats, brown rice, barley, rye), grain-free (GO!: peas, tapioca, lentils, chickpeas), or vegan (Natural Balance Vegetarian: rice, oats, barley, peas; no animal ingredients; Table 1). A 50% (wt/vol) glucose solution was again used as the standard. Similar to study 1, a 6 * 6 Latin Square design was used, with each dog testing each commercial diet once, and the glucose standard twice. The washout period between the testing periods was 7 d. The amount of Av CHO each dog received was increased from study 1; test diets and the control were fed in amounts that provided 25 g of Av CHO to provide approximately 1 g Av CHO per kg BW. Proximate analyses were performed by Central Testing Laboratories Ltd. (Winnipeg, MB, Canada) using the following methods: ash (AOAC 923.03), crude protein (AOAC 990.03), fat (AOCS Am 5-04), crude fiber (AOCS Ba6a-05), and moisture (AOAC 930.15).
Table 1.
In vitro starch and free sugar content of three single starch sources and their individual 10-g Av CHO portion sizes fed in a meal response test to six client-owned Siberian Huskies1
| Lentils | Rice | Bread | |
|---|---|---|---|
| Moisture, % | 9.25 | 10.45 | 37.65 |
| Total starch, % | 41.87 | 75.00 | 42.21 |
| RS, % | 2.72 | 0.11 | 0.82 |
| Galactose, % | 0.018 | nd | nd |
| Glucose, % | 0.017 | 0.020 | 0.87 |
| Sucrose, % | 1.25 | 0.18 | nd |
| Fructose, % | nd | nd | 1.74 |
| Av CHO, % | 39.79 | 75.00 | 42.27 |
| Portion Size for 10g Av CHO, g | 25.13 | 13.33 | 23.66 |
1Values equal to means (analyzed in duplicate); reported on a DM basis, except for moisture and portion size for 10 g Av CHO; nd, below level of detection.
Blood collection
The protocol for blood collection was the same for both studies. Dogs underwent an overnight (14 h) fast prior to each test. On test days, dogs were weighed; BCS was recorded; and Emla cream (2.5% Lidocaine) was applied to the dogs’ legs. A 20 Ga IV catheter (Insyte-W 20GA × 1.1, Becton Dickinson, Franklin Lakes, NJ, USA) was placed into a cephalic or saphenous vein. Once placed, catheters were immediately flushed with 2 mL of 0.9% sodium chloride solution (Baxter International, Deerfield, IL, USA), followed by 0.1 mL of 4.0% sodium citrate (Baxter International, Deerfield, IL, USA) to prevent clotting. Prior to blood collection, catheters were flushed with 0.5 mL sterile isotonic sodium chloride solution, and 0.5 mL of blood was withdrawn and discarded to avoid any dilution.
Dogs were allowed a minimum of 15 min to recover from catherization. Following this, two or three baseline blood samples (0.5 mL study 1 and 2.5 mL study 2) were taken before each test meal was fed. Baseline measurements were increased from two samples (study 1) to three samples in study 2 to account for variability seen in baseline blood glucose concentrations in the dogs. Postprandial blood samples were collected at 15, 30, 45, 60, 90, 120, and 150 min after the start of the meal (Wolever et al., 1991). For study 2, blood samples were placed into serum separation tubes (3.5 mL gold top, Vacutainer, Becton Dickinson, Franklin Lakes, NJ, USA). Time was started immediately when the dog started eating the meal or drinking the glucose standard. Each dog voluntarily consumed all dietary treatments in less than 5 min. Catheters were flushed after each time point with 2 mL saline and 0.1 mL sodium citrate to maintain patency.
Analytical methods
For studies 1 and 2, collected whole blood was immediately tested for glucose using a handheld blood glucose monitor (AlphaTRAK 2, Abbott Laboratories, North Chicago, IL, USA), validated for use in dogs (Kang et al., 2016). Each blood sample was tested twice for glucose. If results varied by more than 0.3 mmol/L, additional testing was done until two results that were 0.3 mmol/L apart or less were obtained.
In study 2, blood samples were immediately stored on ice following glucose analysis and moved into a fridge (4 °C) within 15 min for storage. Within 24 h after collection, the samples were centrifuged at 4 °C at 1,000 × g for 10 min (Legend RT, Kendro Laboratory Products, Asheville, NC, USA). Serum was aliquoted into two 1.5 mL microcentrifuge tubes and stored at −20 °C until analysis. Serum insulin analysis was performed in duplicate using a canine-specific Milliplex magnetic bead assay (Millipore Sigma, Burlington, MA, USA). Assays were performed according to manufacturer’s instructions and run on a Bio-Plex 2000 system (Biorad, Luminex 100/200, S/N: LX10010315403). The quality control samples and standard curves were evaluated based on manufacturer recommendations. Within-assay coefficients of variation (CV) for the results were assessed for each set of duplicates. If CV per duplicate was <20%, the results were deemed acceptable, and the mean of the duplicates was used for further analysis. However, if the CV was ≥20%, individual results were assessed. If the individual results of the duplicates were not in agreement, results from that sample were removed.
Calculations
The postprandial incremental area under the glycemic response curve (AUCgluc) of each participant and separate test diet was expressed as a percentage of the mean incremental AUCgluc of the two glucose controls taken by the same dog. The resulting values are averaged to obtain the GI value for the treatment (Wolever et al., 1991). The AUCgluc of each treatment was calculated as described by Brouns et al. (2005). In addition to AUCgluc from baseline to 150 min postprandial (i.e., overall AUCgluc), AUC from baseline to 30 min (i.e., immediate AUCgluc) and the AUC from 30 to 150 min (i.e., later AUCgluc) were assessed. Changes in serum insulin concentrations were used to calculate the postprandial incremental area under the insulinemic response curve (AUCins). Similar to glucose overall AUCins, immediate AUCins and later AUCins were calculated.
Statistical analyses
Statistical analyses were performed using the GLIMMIX procedure of SAS (SAS Studio, Version 9.4, SAS Institute Inc., Cary, NC, USA). Prior to analysis, Q–Q plots, box plots, and the Kolmogorov–Smirnov test were used to assess the normality of the residuals. Data were log-transformed when necessary. The effect of removing outliers more than 2SD from the mean was explored. Outliers were not removed for the final analysis, as this did not affect the results. An analysis of variance (ANOVA) model was used to compare AUCgluc, AUCins, peak glucose and insulin concentrations, time to peak, and GI among dietary treatments. Dog was defined as the random effect and treatment as the fixed effect. Period was also included as a fixed effect; however, as it had no significant effects on any of the assessed outcomes, it was removed from the final MODEL statement. Additionally, the effect of sex along with baseline blood glucose and serum insulin values, for both studies, was investigated and initially added to the model as possible covariates. These were removed from the final model due to lack of significance. A first-order autoregressive covariance structure for the period was used to account for repeated measures, with dog as the experimental unit. This model was also used to assess the dogs’ BWs over the course of the study. A repeated measures ANOVA model was also used to compare glycemic and insulinemic response over time, using dog as the random effect, and treatment, time, and the interaction as the fixed effects. Similarly, period was included as a fixed effect but was later removed from the final MODEL statement. Time was defined as the repeated term, with dog as the experimental unit. Tukey’s post hoc test was performed for all multiple comparisons where a significant treatment effect was present. Results are expressed as mean ± standard error of mean (SEM). A P-value < 0.05 was considered significant, and a P-value of <.10 was considered a trend.
Results
All dogs tolerated the background diet, test diets, and glucose control well; no dog refused to eat the diets or drink the glucose solution. BW and BCS were constant for all dogs throughout the entire study period (P > 0.05).
Study 1
Dietary treatments
White bread, cooked white rice, and cooked green lentils had 42.27, 75.00, and 39.79 g of Av CHO per 100 g of food, respectively (Table 1). As a result, in order to provide 10 g of Av CHO, 24 g of bread, 13 g of rice, and 25 g of lentils were fed. The quantity of rice and lentils was weighed out dry, prior to cooking.
GI and glycemic response
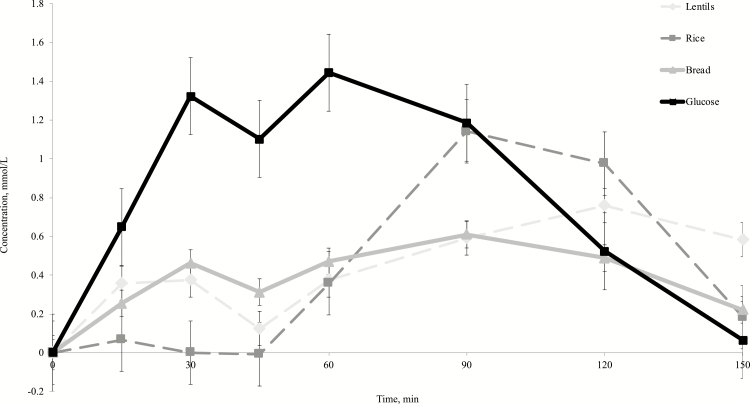
The postprandial glycemic responses to the starch sources and glucose standard are presented in Figure 1 and Table 2. As expected, there was a significant effect of time on postprandial glycemic response (P = 0.0029). However, the effects of treatment and treatment × time were not significant (P = 0.371; P = 0.1412). The numerical order of GI for the starch sources, from highest to lowest, was: cooked white rice > cooked green lentils > white bread, with no significant differences found among treatments (P = 0.569). Additionally, there were no significant differences found among any of the treatments, including the glucose control, for the peak glucose concentration or time to peak (P = 0.175; P = 0.149). There was a treatment effect for overall AUCgluc (P = 0.0496) and immediate AUCgluc (P = 0.0024). For overall AUCgluc, when a conservative multiple post hoc test such as Tukey’s was performed, there were no individual differences between treatments. There was a tendency for glucose to produce a larger overall AUCgluc as compared with bread (P = 0.0967). However, when a less conservative post hoc test was performed, such as PDIFF, glucose produced a significantly larger overall AUCgluc as compared with all other treatments (P < 0.05). Immediate AUCgluc was significantly greater for the glucose control compared with all other dietary treatments, following a Tukey’s post hoc (P < 0.05). Significant differences in the later AUCgluc were not observed among treatments (P = 0.110).
Figure 1.
Mean increases in measured concentration of whole blood glucose from baseline (mmol/L) following acute feedings of single starch sources and a glucose control (20% wt/vol). Treatments were given as 10 g of Av CHOs to fasted client-owned Siberian Huskies (n = 6). Values expressed as mean ± SEM.
Table 2.
Postprandial glycemic responses to acute feedings of 10 g of Av CHO of a glucose solution (20% wt/vol) and single starch sources in fasted client-owned Siberian Huskies1
| Glucose | Lentils | Rice | Bread |
P-value Treatment |
|
|---|---|---|---|---|---|
| Peak Glucose Concentration, mmol/L | 7.24 ± 0.22 | 6.58 ± 0.29 | 7.23 ± 0.57 | 6.73 ± 0.20 | 0.175 |
| Time to peak, min | 56.3 ± 8.3 | 87.5 ± 20.7 | 92.5 ± 16.6 | 60.0 ± 15.0 | 0.149 |
| Overall AUC: AUCgluc ≤ 150 min, mmol/L min | 144.2 ± 20.7 | 75.8 ± 23.7 | 92.9 ± 24.4 | 74.0 ± 25.2 | 0.0496 |
| Immediate AUC: AUCgluc ≤ 30 min, mmol/L min | 20.5 ± 2.7a | 8.4 ± 2.3b | 6.0 ± 2.7b | 9.3 ± 3.4b | 0.0024 |
| Later AUC: AUCgluc ≥ 30 min, mmol/L min | 123.7± 20.8 | 67.3 ± 22.5 | 86.9 ± 22.8 | 64.6 ± 22.2 | 0.110 |
| GI | — | 60 ± 20 | 71 ± 14 | 47 ± 11 | 0.569 |
1Values expressed as mean ± SEM; n = 6; values in a row with superscripts without a common letter differ; P < 0.05, ANOVA with Tukey–Kramer post hoc test.
Study 2
Dietary treatments
The percentages of total starch, RS, free sugars, and Av CHO for each extruded diet are listed in Table 3. In order to provide 25 g of Av CHO, 62 g of the traditional grain diet, 77 g of the whole-grain diet, 65 g of the grain-free diet, and 66 g of the vegan diet were fed. The grain-free diet had the lowest percentage of total starch, and the highest percentage of RS of the commercial diets analyzed. Proximate analyses for the diets are shown in Table 3.
Table 3.
In vitro starch and free sugar content and proximate analysis1 of four commercial extruded dog foods fed in a meal response test to 11 client-owned Siberian Huskies
| Traditional grain diet2 | Whole-grain diet3 | Grain-free diet4 | Vegan diet5 | |
|---|---|---|---|---|
| Moisture, % | 5.85 | 6.32 | 6.56 | 7.15 |
| Total starch, % | 41.82 | 40.93 | 34.03 | 47.59 |
| RS, % | 0.41 | 0.16 | 0.56 | 0.26 |
| Glucose, % | 0.10 | 0.023 | 0.032 | 0.029 |
| Sucrose, % | 1.40 | 0.56 | 1.38 | 0.86 |
| Av CHO, % | 42.62 | 41.23 | 34.75 | 48.05 |
| Portion size for 25 g Av CHO, g | 62 | 77 | 65 | 55 |
| Ash, % | 7.24 | 10.10 | 7.66 | 5.00 |
| Crude fiber, % | 1.05 | 1.24 | 3.14 | 2.10 |
| Crude protein, % | 25.55 | 26.10 | 28.59 | 23.46 |
| Fat, % | 10.25 | 10.21 | 11.58 | 8.20 |
| Gross energy (GE), kcal/kg6 | 4,712 | 4,593 | 4,728 | 4,619 |
| Metabolizable energy (ME), kcal/kg7 | 3,959 | 3,841 | 3,816 | 3,846 |
1Values reported on a DM basis, except for moisture and portion size for 25 g Av CHO.
2Traditional grain diet was Purina Dog Chow (Nestlé Purina Petcare, St. Louis, MO, USA). Ingredients: whole-grain corn, meat and bone meal, corn gluten meal, animal fat preserved with mixed-tocopherols, soybean meal, poultry byproduct meal, egg and chicken flavor, whole-grain wheat, animal digest, salt, calcium carbonate, potassium chloride, l-lysine monohydrochloride, mono and dicalcium phosphate, choline chloride, zinc sulfate, yellow 6, vitamin E supplement, ferrous sulfate, yellow 5, red 40, manganese sulfate, niacin, blue 2, vitamin A supplement, copper sulfate, calcium pantothenate, garlic oil, pyridoxine hydrochloride, vitamin B-12 supplement, thiamine mononitrate, vitamin D-3 supplement, riboflavin supplement, calcium iodate, menadione sodium bisulfite complex (source of vitamin K activity), folic acid, biotin, and sodium selenite.
3Whole-grain diet was SUMMIT Three Meat Adult Recipe (Petcurean Pet Nutrition, Chilliwack, BC, Canada). Ingredients: chicken meal, oatmeal, whole brown rice, rye, barley, chicken fat (preserved with mixed tocopherols), salmon meal, lamb meal, natural chicken flavor, whole dried egg, rice bran, dried kelp, flaxseed, dicalcium phosphate, calcium carbonate, potassium chloride, choline chloride, l-lysine, sodium chloride, vitamins (vitamin A supplement, vitamin D-3 supplement, vitamin E supplement, inositol, niacin, l-ascorbyl-2-polyphosphate [a source of vitamin C], d-calcium pantothenate, thiamine mononitrate, beta-carotene, riboflavin, pyridoxine hydrochloride, folic acid, biotin, vitamin B-12 supplement), minerals (zinc proteinate, iron proteinate, copper proteinate, zinc oxide, manganese proteinate, copper sulfate, ferrous sulfate, calcium iodate, manganous oxide, selenium yeast), dl-methionine, and dried rosemary.
4Grain-free diet was GO! SENSITIVITY + SHINE Limited Ingredient Duck Recipe (Petcurean Pet Nutrition, Chilliwack, BC, Canada). Ingredients: de-boned duck, duck meal, peas, tapioca, lentils, chickpeas, pea flour, canola oil (preserved with mixed tocopherols), coconut oil (preserved with mixed tocopherols), natural flavor, salmon oil, calcium carbonate, dicalcium phosphate, sodium chloride, potassium chloride, dried chicory root, choline chloride, vitamins (vitamin A supplement, vitamin D3 supplement, vitamin E supplement, inositol, niacin, l-ascorbyl-2-polyphosphate [a source of vitamin C], d-calcium pantothenate, thiamine mononitrate, beta-carotene, riboflavin, pyridoxine hydrochloride, folic acid, biotin, vitamin B-12 supplement), minerals (zinc proteinate, iron proteinate, copper proteinate, zinc oxide, manganese proteinate, copper sulfate, ferrous sulfate, calcium iodate, manganous oxide, selenium yeast), taurine, and dried rosemary.
5Vegan diet was Vegetarian Dry Dog Formula (Dick Van Patten’s Natural Balance Pet Foods, Burbank, CA, USA). Ingredients: brown rice, oatmeal, cracked pearled barley, peas, potato protein, canola oil, potatoes, tomato pomace, vegetable flavoring, calcium carbonate, dicalcium phosphate, flaxseed, potassium chloride, choline chloride, taurine, natural mixed tocopherols, spinach, parsley flakes, cranberries, l-lysine, l-carnitine, Yucca schidigera extract, kelp, vitamin E supplement, iron proteinate, zinc proteinate, copper proteinate, ferrous sulfate, zinc sulfate, copper sulfate, potassium iodide, thiamine mononitrate (vitamin B-1), manganese proteinate, manganous oxide, ascorbic acid, vitamin A supplement, biotin, niacin, d-calcium pantothenate, manganese sulfate, sodium selenite, pyridoxine hydrochloride (vitamin B-6), vitamin B-12 supplement, riboflavin (vitamin B-2), vitamin D-2 supplement, and folic acid.
6GE (kcal/kg) = (5.7 × g protein) + (9.4 × g fat) + [4.1 × (g NFE + g crude fiber)] (National Research Council, 2006).
7ME (kcal/kg) = 575 + [0.816 × GE (kcal/kg)] + (12.08 × percentage fat) –(52.76 × percentage crude fiber) – (20.61 × percentage protein) – (6.07 × percentage moisture) (Hall et al., 2013).
GI and glycemic response
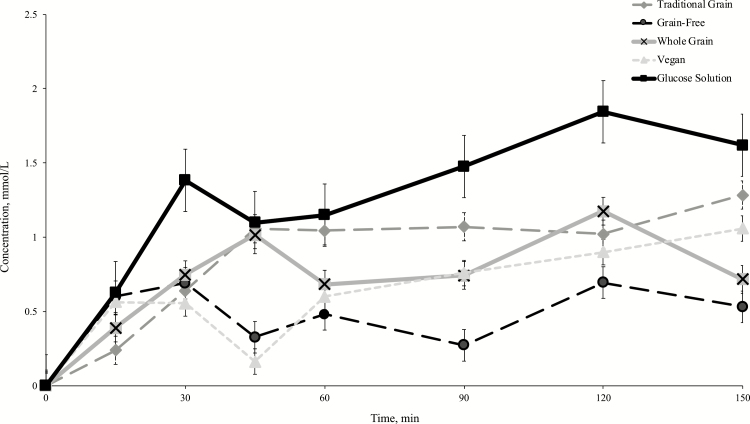
The postprandial glycemic responses to the extruded diets and glucose control are presented in Figure 2 and Table 4. Similar to study 1, there was a significant effect of time on postprandial glycemic response (P < 0.0001). There was also a significant effect of treatment (P = 0.0010), as the glucose solution produced a greater overall postprandial response than the grain-free and vegan diets, following a Tukey’s post hoc (P = 0.0007; P = 0.0228). There was no significant treatment × time effect (P = 0.2651). The order of GI, from highest to lowest, was observed as: traditional grain diet > vegan diet > whole-grain diet > grain-free diet. Although numerical differences were observed, there was no overall significant difference in GI (P = 0.1537). A treatment effect was observed for overall AUCgluc and later AUCgluc (P = 0.0004; P = 0.0494), but not for the immediate AUCgluc (P = 0.1132). Tukey’s post hoc test revealed that dogs receiving the glucose standard had a greater overall AUCgluc when compared with dogs fed the whole-grain diet and grain-free diet (P = 0.0476; P = 0.0027). The glucose standard also elicited significantly higher later AUCgluc as compared with the vegan diet (P = 0.0323), following a Tukey’s post hoc. Additionally, the glucose standard resulted in higher peak blood glucose concentrations as compared with dogs fed all of the extruded commercial diets (P < 0.0001). Significant differences in time to peak for glucose was not observed among treatments (P = 0.6981).
Figure 2.
Mean increases in measured concentration of whole blood glucose from baseline (mmol/L) following acute feedings of 25 g Av CHOs of a glucose control (50% wt/vol) and four commercial extruded dog foods in fasted client-owned Siberian Huskies (n = 11). Values expressed as mean ± SEM.
Table 4.
Postprandial glycemic and insulinemic responses to acute feedings of 25 g of Av CHOs of a glucose solution (50% wt/vol) and four commercial extruded dog foods in fasted client-owned Siberian Huskies1
| Glucose | Traditional grain diet | Whole-grain diet | Grain-free diet | Vegan diet |
P-value Treatment |
|
|---|---|---|---|---|---|---|
| Glucose | ||||||
| Peak concentration, mmol/L | 8.27± 0.24a | 7.59 ± 0.29a | 7.48 ± 0.30b | 6.81 ± 0.21b | 7.41 ± 0.28b | <0.0001 |
| Time to peak, min | 96.8 ± 7.8 | 100.9 ± 15.0 | 76.4 ± 13.6 | 94.1 ± 14.0 | 84.6 ± 17.9 | 0.6981 |
| Overall AUC: AUCgluc ≤ 150 min, mmol/L min |
233.9 ± 21.2a | 177.2 ± 28.6ab | 142.5 ± 20.1b | 104.7 ± 14.4b | 164.6 ± 38.9ab | 0.0004 |
| Immediate AUC: AUCgluc ≤ 30 min, mmol/L min |
19.8 ± 2.3 | 10.0 ± 2.9 | 11.9 ± 2.3 | 14.5 ± 3.9 | 13.8 ± 3.9 | 0.1132 |
| Later AUC: AUCgluc ≥ 30 min, mmol/L min |
196.3 ± 50.23a | 162.8 ± 61.3ab | 110.8 ± 41.7ab | 92.5 ± 34.9ab | 51.4 ± 19.4b | 0.0494 |
| GI | — | 83 ± 17 | 56 ± 8 | 41 ± 6 | 65 ± 15 | 0.1537 |
| Insulin | ||||||
| Peak concentration, pg/mL | 675.26 ± 63.9 | 537.54 ± 66.53 | 574.51 ± 71.07 | 500.16 ± 61.95 | 684.33 ± 84.89 | 0.1398 |
| Time to peak, min | 40.4± 6.7a | 109.1 ± 24.9b | 41.9 ± 9.6a | 47.7 ± 10.9ab | 67.6 ± 15.4ab | 0.0078 |
| Overall AUC: AUCins ≤ 150 min, pg/mL min |
28,095.3 ± 5,723.9 | 12,787.6 ± 3,505.1 | 14,437.8 ± 3,954.5 | 16,694.1 ± 4,580.4 | 20,537.9 ± 5,627.1 | 0.0785 |
| Immediate: AUCins ≤ 30 min, pg/mL min |
5,250.0 ± 1,129.0a | 1,251.2 ± 394.7b | 2,842.3 ± 850.8ab | 1,764.6 ± 505.8b | 2,141.7 ± 641.8ab | 0.0009 |
| Later AUC: AUCins ≥ 30 min, pg/mL min |
22,309.7 ± 4,972.0 | 12,114.6 ± 3,709.6 | 12,785.6 ± 3,912.7 | 13,332.2 ± 4,085.7 | 13,724.1 ± 4,200.7 | 0.2948 |
1Values expressed as mean ± SEM; n = 11; values in a row with superscripts without a common letter differ; P < 0.05, ANOVA with Tukey–Kramer post hoc test.
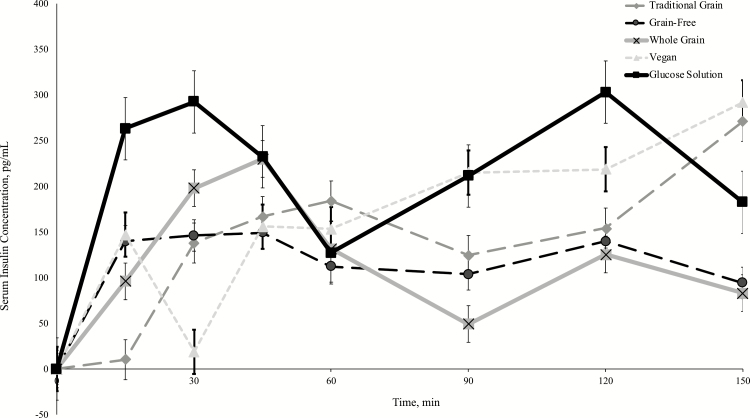
Insulinemic response
The postprandial insulinemic responses to all dietary treatments, including the glucose standard, are presented in Figure 3 and Table 4. The overall AUCins tended to be different among treatments (P = 0.0785), where the glucose standard resulted in the highest AUC and the traditional grain diet presented the lowest. A treatment effect was found for immediate AUCins (P = 0.0009). A Tukey’s post hoc test showed that dogs fed the glucose solution had significantly greater immediate AUCins as compared with the grain-free diet and the traditional grain diet (P = 0.0132; P = 0.0014). No differences were observed for later AUCins and peak postprandial serum insulin concentration among any of the treatments (P = 0.2948; P = 0.1398). Time to peak for insulin was different among treatments (P = 0.0078), with a faster time to peak for the glucose solution compared with the traditional grain diet (P = 0.0065), which had the slowest time to peak. The traditional grain diet also had a significantly slower time to peak compared with the whole-grain diet (P = 0.0276), following Tukey’s post hoc.
Figure 3.
Mean increases in measured concentration of serum insulin from baseline (pg/mL) following acute feedings of 25 g Av CHOs of a glucose control (50% wt/vol) and four commercial extruded dog foods in fasted client-owned Siberian Huskies (n = 11). Values expressed as mean ± SEM.
Discussion
The current study is the first to report on the derivation of GI values in dogs for cooked white rice (GI 71 ± 14), cooked green lentils (GI 60 ± 20), and white bread (GI 47 ± 11); however, no differences were observed among treatments. To date, only two studies investigated the GI of various individual uncooked starch sources in dogs (Adolphe et al., 2012; Briens, 2018). These studies tested canine GI by feeding 10 g of Av CHO to Beagles in a laboratory setting. Both Adolphe et al. (2012) and Briens (2018) reported the GI of rice in dogs as 55; however, in both studies, rice was ground and mixed with water, as opposed to cooked. It is, therefore, possible that in the current study the cooking temperature may have contributed to starch gelatinization, resulting in a higher content of digestible starch and, therefore, the higher GI compared with previous research (Fernandes et al., 2005; Nayak et al., 2014). Briens (2018) reported a GI of 47 ± 10 after feeding dogs a slurry composed of ground and hydrated lentils. Similar to white rice, the present study obtained a higher GI for cooked green lentils, which may have again been attributed to the cooking process. In humans, the GI of cooked white rice and cooked green lentils has been reported to be 56 ± 2 and 30 ± 4, respectively, with glucose as the control (Foster-Powell et al., 2002). The GI values observed in the present canine study were notably higher than that reported for humans. Similarly, the low GI of 47 ± 11 calculated for white bread does not agree with human research. White bread is considered a high GI food in humans, with a reported GI of 73 ± 2 using glucose as the control (Foster-Powell et al., 2002). Although GI is influenced by a number of factors, including the extent it is chewed, moisture content, processing and cooking methods, and temperature storage (Ramdath, 2016), it is unclear what may have caused this unexpectedly low GI value for white bread in dogs. Additionally, as a result of the small meal size offered to these dogs (23.7 g of white bread) and the previous work done on canine gastric emptying rates (Gooding et al., 2013; McKnight et al., 2015), it can be speculated that the peak postprandial glycemic response elicited by this treatment may have occurred prior to the first sample taken at 15 min postprandial. Regardless, due to the low overall AUCgluc, using white bread to calculate the GI values of the other treatments would result in GI values over 100 in this study. Therefore, based on these results, the glucose standard may be a better and more reliable control than white bread for future GI testing in dogs. However, future studies aimed at testing human GI methodologies in dogs should investigate the use of white bread as a control food in doses higher than 10 g of Av CHO.
Following the completion of the first study, the authors believed that increasing the quantity of Av CHO fed to the dogs was warranted for the second study. Feeding 10 g of Av CHO was based on previous GI canine studies using Beagles of normal body condition (Adolphe et al., 2012, 2015). In the study by Adolphe et al. (2015), the dogs had a reported mean weight of 9.8 kg. Although the mean weight of the dogs was not reported by Briens (2018), the author reported feeding 1 g of Av CHO per kilogram BW. In contrast, human GI research is based on portions of 50 g of Av CHO, independent of BW (Wolever et al., 1991). This dose has been proposed as the standard due to a curvilinear dose–response curve and the tendency for blood glucose concentrations to plateau as intake becomes greater than 50 g Av CHOs in humans (Wolever and Bolognesi, 1996; Lee and Wolever, 1998; Brouns et al., 2005). In comparison, intakes of 10 to 25 g in humans have demonstrated insignificant or only slight increases in blood glucose concentrations (Wolever and Bolognesi, 1996; Lee and Wolever, 1998; Brouns et al., 2005). To better compare with the amount of Av CHO offered in human GI testing methodology, a suggested equation for the quantity of Av CHO fed in canine GI testing was proposed by Nguyen et al. (1998) as 2 g per metabolic kg (2 g/kg BW0.75). As a result, the quantity of Av CHO was increased to 25 g for the second study in order to see more pronounced postprandial glycemic responses in Siberian Huskies, which is a considerably larger breed than Beagles.
The second study investigated postprandial glycemic and insulinemic response to extruded dry foods featuring various categories of starches. Commercial diets were selected based on the broad category of starch sources listed on the ingredient panel and were categorized into the following: traditional grains (e.g., wheat, corn), novel whole grains (e.g., brown rice, barley, oatmeal, rye), nongrain carbohydrates (e.g., peas, lentils, tapioca, chickpeas), and vegan (e.g., no animal-sourced ingredients). Despite the varying starch sources listed on the ingredient panel of each diet, the mean percentage of RS remained very low across all four diets, ranging from 0.16% to 0.56% on a DM basis. This low amount of RS is likely due to the high temperature and pressure used for extrusion processing, which led to increased starch gelatinization. However, the grain-free diet had the lowest proportion of total starch and the highest proportion of RS. Research on pulses has regularly reported high RS levels (Murphy et al., 2008). Therefore, the higher RS content of the grain-free diet is reflective of the inclusion of pulses within this diet. In comparison, the mean percentage of total starch between the extruded diets ranged from 34.03% to 47.59% on a DM basis. The vegan diet contained the largest proportion of total starch. This may have been due to the increased proportion of overall plant matter used in this diet, as compared with the others that also contained animal-derived ingredients.
The present study found the GI of the test diets as follows: traditional grain 83 ± 17, vegan 65 ± 15, whole grain 56 ± 8, and grain-free 41± 6, but these were not found to be statistically different. Similarly, both Adolphe et al. (2015) and Briens (2018) found that extruded pulse-based diets had numerically lower GI values as compared with extruded diets containing corn or rice; however, these results were not statistically significant. In the study by Adolphe et al. (2015), the extruded diet containing peas had a reported GI of 56 ± 12, while Briens (2018) reported that extruded diets containing either peas, lentils, or fava beans had GI values of 55 ± 20, 37 ± 11, and 48 ± 11, respectively. The GI of the grain-free diet tested in our research, therefore, falls within this range. It is also worth mentioning that the GI of the grain-free diet did show a large numerical difference compared with the traditional grain diet, although this difference was not statistically significant. Based on human GI methodology and categorization, the grain-free diet would be categorized as a low GI food (GI of ≤55), while the traditional grain diet would be classified as a high GI food (GI of ≥70) (Atkinson et al., 2008). In human GI testing, pulses have been observed to be low GI foods with values of 10 to 54, based on the type of pulse as well as storage and processing conditions. In comparison, corn typically has a higher GI value of 37 to 69, again based on the type and the conditions it has been exposed to (Foster-Powell et al., 2002). However, it should be noted that postprandial blood glucose values did not return to baseline in study 2, as would have been considered ideal for the calculation of GI. That being said, published GI research in humans shows that blood glucose values do not always return to baseline within 120 or 150 min (Chlup et al., 2010). In human GI research, postprandial blood samples are taken at 15, 30, 45, 60, 90, and 120 min from healthy participants (Brouns et al., 2005). We chose to extend our postprandial sampling to 150 min, as previous published GI research in Beagle dogs found them to return to baseline within this time period. It is unknown why the sled dogs did not similarly return to baseline within this time, and whether this was a result of differences in breed and/or environment.
In agreement with previous research, the present study noted delayed and lengthened responses in postprandial glucose and insulin following the consumption of the grain-free diet containing pulses. Adolphe et al. (2015) and Briens (2018) found that feeding 10 g Av CHOs from extruded diets containing pulses produced delayed and lengthened glycemic responses compared with the diets containing corn or rice. Carciofi et al. (2008) also demonstrated that sorghum-, lentil-, and pea-based diets produced delayed and lengthened glycemic and insulinemic responses compared with those made of rice, corn, and cassava flour, when fed as a meal in amounts to sustain maintenance energy requirements. The overall AUC was not lower for the sorghum, lentil, and pea diet as compared with the other diets. Similarly, the present study did not reveal a lower overall AUC for the grain-free diet as compared with the other commercial diets. Moreover, the grain-free diet had lower peak concentrations in postprandial glucose and insulin, as well as a longer time to peak for both of these responses, than the other commercial diets. This is again in agreement with Adolphe et al. (2015) and Briens (2018) who found that a diet containing pulses, as compared with corn- or rice-based diets, also resulted in lower peak concentrations and a longer time to peak for both postprandial glucose and insulin.
As this research aimed to investigate commercially available extruded diets, the amount of fat and protein provided by the test meals could not be kept consistent. This was also the case for Carciofi et al. (2008), while the macronutrient profiles of the extruded diets were formulated to be identical for Adolphe et al. (2015) and Briens (2018). The dietary starch content has been proposed as the main determinant for postprandial glycemic response in dogs, while in contrast, the insulinemic response was also influenced by dietary fat and protein content, and not only by starch content (Nguyen et al., 1998). Similarly, GI testing done in humans has reported that the protein and fat contents within the dietary treatments provided may influence the postprandial glycemic response and the overall calculated GI for the food (Jenkins et al., 1981; Wolever et al., 1991). Therefore, it is possible that postprandial glycemic and insulinemic responses observed in the present study, and the final mean calculated GI of each commercial pet food, were influenced by the fat and protein contents of the meal provided. However, this study aimed to utilize in vivo GI methodology in dogs that mimicked human GI methodology, providing a constant amount of Av CHOs between treatments, and not solely focus on postprandial responses to the starch sources in extruded pet foods.
Although test conditions were kept the same between dogs and dietary treatments, the variability in glycemic and insulinemic responses noted between the dogs was quite large. As chewing contributes to the glycemic response, it can be speculated that any variation in how much the dogs chewed the dietary treatments could have been a source of variability in the results (Ranawana et al., 2010). Future studies investigating the GI and postprandial glycemic and/or insulinemic responses in dogs should consider removing the effect of chewing by grinding the dietary treatments provided. Furthermore, investigations into blood glucose monitors have revealed variability within and between different monitors in their ability to accurately measure blood glucose concentrations (Johnson and Baker, 2001). It can, therefore, be speculated that this may have been another potential source of variation within the present study. Previous studies investigating both GI and glycemic response in dogs have also demonstrated notable variability among dogs (Nguyen et al., 1994; Carciofi et al., 2008; Adolphe et al., 2015; Briens, 2018). As a result, the variability in glycemic responses observed in this study, as well as previous research, suggest that GI testing using the same methodology as in human research may not be a reliable tool for dogs.
It has been proposed that breeds similar to the Siberian Husky, such as the Samoyed and the Greenland Sled dog, may have reduced copy numbers of the gene coding for pancreatic Alpha-amylase 2B (AMY2B), shown to correlate with the activity of serum amylase in dogs (Arendt et al., 2014). However, as noted by the authors, only a small percentage of the differences in serum amylase can be explained by this gene coding. Serum amylase levels are also influenced by diet, circadian rhythm, and age (Piccione et al., 2008), which had not been considered. Moreover, the Beagle, a common breed used for research, and the only breed used for published GI testing, demonstrated the most variation in its AMY2B copy numbers. As a result, it is difficult to interpret these results, and how they may affect the ability of these dogs to digest dietary starch and absorb its products of digestion. Although Siberian Huskies competing in high-intensity Iditarod races may have differences in their overall glucose transport activity (Davis et al., 2014) and oxidation of various macronutrients (Miller et al., 2015, 2017), the Siberian Huskies used in this research were not trained or competing to such a caliber. Additionally, these dogs had not been actively racing or training during this research, or for at least 2 mo prior; however, they may have been more insulin sensitive due to the effect of previous training (Dela et al., 1992). Any potential differences in the postprandial responses observed as well as calculated GI caused by breed cannot be speculated at this time.
Overall, despite the notable popularity that grain-free dog food has had with consumers, the results of this study suggest that different starch sources in commercial extruded diets may not have significant effects on GI or postprandial glycemic and insulinemic response in dogs. Given the high interindividual variation seen, more research is recommended to further improve and streamline canine GI testing before it can become a validated method for use in dogs. Future studies should investigate by increasing the number of dogs used in this study, grinding the dietary treatments to remove the effect of chewing, as well as adjusting the amount of Av CHO offered to dogs of different breeds and sizes. Additionally, future canine GI studies should consider further modifying the standard timeline that postprandial blood glucose is tested in human GI research and consider taking samples earlier than 15 min postprandial. Based on previous canine gastric emptying research (Gooding et al., 2013; McKnight et al., 2015), control foods, such as glucose or white bread, may have peaked prior to the initial samples, taken at 15 min postprandial, in the present study. Gastric emptying is also predicated on meal size, and in contrast to the daily requirements of these dogs, the amount of food offered in the present studies may have resulted in faster gastric emptying times. At this time, the physiological relevance of low GI foods for dogs, and the health benefits they may produce, is unknown. However, based on these results, it does not appear that the dog foods containing different types of carbohydrate sources provide improved glucose and/or insulin control. Claims regarding the GI of pet foods cannot be substantiated due to the postprandial variability in glucose and insulin responses in dogs.
Acknowledgments
This study was funded by a Natural Science & Engineering Research Council Collaborative Research Development grant (#CRDPJ488705–15) in partnership with Petcurean Pet Nutrition. Support for A.R. was provided by a Mitacs Accelerate grant in partnership with Petcurean Pet Nutrition. We would like to acknowledge our volunteers for their assistance with data collection. We also thank Aileen Hawke, Mary Ellen Clarke, and Michelle Edwards for their help with laboratory and statistical analyses. Additionally, we would like to extend a special thank you to the owners of the sled dogs.
Glossary
Abbreviations
- AUCgluc
area under the glycemic response curve
- AUCins
area under the insulinemic response curve
- Av CHO
available carbohydrate
- BCS
body condition score
- BW
body weight
- CBC
complete blood count
- DM
dry matter
- FDA
Food and Drug Administration
- GI
glycemic index
- RS
resistant starch
Conflict of interest statement
J.L.A. is a paid employee of PPN Limited Partnership (Petcurean). A.V. is the Royal Canin Veterinary Diets Endowed Chair in Canine and Feline Clinical Nutrition at the Ontario Veterinary College. The remaining authors declare no real or perceived conflicts of interest.
Literature Cited
- Adolphe J L, Drew M D, Huang Q, Silver T I, and Weber L P. . 2012. Postprandial impairment of flow-mediated dilation and elevated methylglyoxal after simple but not complex carbohydrate consumption in dogs. Nutr. Res. 32:278–284. doi: 10.1016/j.nutres.2012.03.002 [DOI] [PubMed] [Google Scholar]
- Adolphe J L, Drew M D, Silver T I, Fouhse J, Childs H, and Weber L P. . 2015. Effect of an extruded pea or rice diet on postprandial insulin and cardiovascular responses in dogs. J. Anim. Physiol. Anim. Nutr. (Berl). 99:767–776. doi: 10.1111/jpn.12275 [DOI] [PubMed] [Google Scholar]
- Agriculture and Agri-Food Canada 2016. Pet food sales in Canada [accessed July 12, 2019]. Available from https://www.agr.gc.ca/resources/prod/Internet-Internet/MISB-DGSIM/ATS-SEA/PDF/6747-eng.pdf [Google Scholar]
- Arendt M, Fall T, Lindblad-Toh K, and Axelsson E. . 2014. Amylase activity is associated with AMY2B copy numbers in dog: implications for dog domestication, diet and diabetes. Anim. Genet. 45:716–722. doi: 10.1111/age.12179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson F S, Foster-Powell K, and Brand-Miller J C. . 2008. International tables of glycemic index and glycemic load values: 2008. Diabetes Care 31:2281–2283. doi: 10.2337/dc08-1239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barclay A W, Petocz P, McMillan-Price J, Flood V M, Prvan T, Mitchell P, and Brand-Miller J C. . 2008. Glycemic index, glycemic load, and chronic disease risk–a meta-analysis of observational studies. Am. J. Clin. Nutr. 87:627–637. doi: 10.1093/ajcn/87.3.627 [DOI] [PubMed] [Google Scholar]
- Bednar G E, Patil A R, Murray S M, Grieshop C M, Merchen N R, and Fahey G C Jr. 2001. Starch and fiber fractions in selected food and feed ingredients affect their small intestinal digestibility and fermentability and their large bowel fermentability in vitro in a canine model. J. Nutr. 131:276–286. doi: 10.1093/jn/131.2.276 [DOI] [PubMed] [Google Scholar]
- Biliaderis C G. 1991. The structure and interactions of starch with food constituents. Can. J. Physiol. Pharmacol. 69:60–78. doi: 10.1139/y91-011 [DOI] [PubMed] [Google Scholar]
- Briens J. 2018. Linking a toxic glucose metabolite to glycemic and cardiovascular responses in an omnivore compared to a carnivore [master’s thesis]. University of Saskatchewan; Available from http://hdl.handle.net/10388/8471. [Google Scholar]
- Brouns F, Bjorck I, Frayn K N, Gibbs A L, Lang V, Slama G, and Wolever T M. . 2005. Glycaemic index methodology. Nutr. Res. Rev. 18:145–171. doi: 10.1079/NRR2005100 [DOI] [PubMed] [Google Scholar]
- Brummer Y, Kaviani M, and Tosh S. . 2015. Structural and functional characteristics of dietary fibre in beans, lentils, peas and chickpeas. Food Res. Int. 67:117–125. doi: 10.1016/j.foodres.2014.11.009 [DOI] [Google Scholar]
- Buff P R, Carter R A, Bauer J E, and Kersey J H. . 2014. Natural pet food: a review of natural diets and their impact on canine and feline physiology. J. Anim. Sci. 92:3781–3791. doi: 10.2527/jas.2014-7789 [DOI] [PubMed] [Google Scholar]
- Carciofi A C, Takakura F S, de-Oliveira L D, Teshima E, Jeremias J T, Brunetto M A, and Prada F. . 2008. Effects of six carbohydrate sources on dog diet digestibility and post-prandial glucose and insulin response. J. Anim. Physiol. Anim. Nutr. (Berl). 92:326–336. doi: 10.1111/j.1439-0396.2007.00794.x [DOI] [PubMed] [Google Scholar]
- Center for Veterinary Medicine FDA 2018. FDA investigating potential connection between diet and cases of canine heart disease [accessed September 16, 2019]. Available from: https://www.fda.gov/animal-veterinary/cvm-updates/fda-investigating-potential-connection-between-diet-and-cases-canine-heart-disease.
- Chlup R, Peterson K, Zapletalová J, Kudlová P, and Seckar P. . 2010. Extended prandial glycemic profiles of foods as assessed using continuous glucose monitoring enhance the power of the 120-minute glycemic index. J. Diabetes Sci. Technol. 4:615–624. doi: 10.1177/193229681000400316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M S, Bonen A, Snook L A, Jain S S, Bartels K, Geor R, and Hueffer K. . 2014. Conditioning increases the gain of contraction-induced sarcolemmal substrate transport in ultra-endurance racing sled dogs. PLoS One. 9:e103087. doi: 10.1371/journal.pone.0103087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dela F, Mikines K J, von Linstow M, Secher N H, and Galbo H. . 1992. Effect of training on insulin-mediated glucose uptake in human muscle. Am. J. Physiol. 263:E1134–E1143. doi: 10.1152/ajpendo.2006.263.6.E1134 [DOI] [PubMed] [Google Scholar]
- Englyst H N, Kingman S M, and Cummings J H. . 1992. Classification and measurement of nutritionally important starch fractions. Eur. J. Clin. Nutr. 46(Suppl 2):S33–S50. doi: 10.1111/j.1750-3841.2010.01627.x [DOI] [PubMed] [Google Scholar]
- Fernandes G, Velangi A, and Wolever T M. . 2005. Glycemic index of potatoes commonly consumed in North America. J. Am. Diet. Assoc. 105:557–562. doi: 10.1016/j.jada.2005.01.003 [DOI] [PubMed] [Google Scholar]
- Foster-Powell K, Holt S, and Brand-Miller J. . 2002. International table of glycemic index and glycemic load. Am. Soc. Clin. Nutr. 76:5–56. doi: 10.1093/ajcn/76.1.5 [DOI] [PubMed] [Google Scholar]
- Gooding M A, Cant J P, Pencharz P B, Davenport G M, Atkinson J L, and Shoveller A K. . 2013. Oral and intravenous l-[1-13C]phenylalanine delivery measure similar rates of elimination when gastric emptying and splanchnic extraction are accounted for in adult mixed hounds1–4. J. Anim. Physiol. Anim. Nutr. 97:181–189. doi: 10.1111/j.1439-0396.2011.01256.x [DOI] [PubMed] [Google Scholar]
- Graham P A, Maskell I E, and Nash A S. . 1994. Canned high fiber diet and postprandial glycemia in dogs with naturally occurring diabetes mellitus. J. Nutr. 124(12 Suppl):2712S–2715S. doi: 10.1093/jn/124.suppl_12.2712S [DOI] [PubMed] [Google Scholar]
- Hall J. A., Melendez L. D., and Jewell D. E.. 2013. Using gross energy improves metabolizable energy predictive equations for pet foods whereas undigested protein and fiber content predict stool quality. PLoS ONE 8(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover R, and Sosulski F W. . 1991. Composition, structure, functionality, and chemical modification of legume starches: a review. Can. J. Physiol. Pharmacol. 69:79–92. doi: 10.1139/y91-012 [DOI] [PubMed] [Google Scholar]
- Jenkins D J, Wolever T M, Taylor R H, Barker H, Fielden H, Baldwin J M, Bowling A C, Newman H C, Jenkins A L, and Goff D V. . 1981. Glycemic index of foods: a physiological basis for carbohydrate exchange. Am. J. Clin. Nutr. 34:362–366. doi: 10.1093/ajcn/34.3.362 [DOI] [PubMed] [Google Scholar]
- Johnson R N, and Baker J R. . 2001. Error detection and measurement in glucose monitors. Clin. Chim. Acta. 307:61–67. doi: 10.1016/s0009-8981(01)00433-8 [DOI] [PubMed] [Google Scholar]
- Joshi M, Aldred P, McKnight S, Panozzo J F, Kasapis S, Adhikari R, and Adhikari B. . 2013. Physicochemical and functional characteristics of lentil starch. Carbohydr. Polym. 92:1484–1496. doi: 10.1016/j.carbpol.2012.10.035 [DOI] [PubMed] [Google Scholar]
- Kang M H, Kim D H, Jeong I S, Choi G C, and Park H M. . 2016. Evaluation of four portable blood glucose meters in diabetic and non-diabetic dogs and cats. Vet. Q. 36:2–9. doi: 10.1080/01652176.2015.1092617 [DOI] [PubMed] [Google Scholar]
- Laflamme D. 1997. Development and validation of a body condition score system for dogs. Canine Pract. 22:10–15. [Google Scholar]
- Lee B M, and Wolever T M. . 1998. Effect of glucose, sucrose and fructose on plasma glucose and insulin responses in normal humans: comparison with white bread. Eur. J. Clin. Nutr. 52:924–928. doi: 10.1038/sj.ejcn.1600666 [DOI] [PubMed] [Google Scholar]
- McCance R A, and Lawrence R D. . 1929. The carbohydrate content of foods. Lancet. 213:1264–1265. doi: 10.1016/s0140-6736(00)49171-3 [DOI] [Google Scholar]
- McKnight L L, Shoveller A K, Lopez S, and France J. . 2015. A kinetic model of whole-body glucose metabolism with reference to the domestic dog (Canis lupus familiaris). Int. Sch. Res. Notices 2015:286076. doi: 10.1155/2015/286076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller B F, Drake J C, Peelor F F, Biela L M, Geor R, Hinchcliff K, Davis M, and Hamilton K L. . 2015. Participation in a 1,000-mile race increases the oxidation of carbohydrate in Alaskan sled dogs. J. Appl. Physiol. 118:1502–1509. doi: 10.1152/japplphysiol.00588.2014 [DOI] [PubMed] [Google Scholar]
- Miller B, Hamilton K, Boushel R, Williamson K, Laner V, Gnaiger E, and Davis M. . 2017. Mitochondrial respiration in highly aerobic canines in the non-raced state and after a 1600-km sled dog race. PLoS One. 12:e0174874. doi: 10.1371/journal.pone.0174874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy M M, Douglass J S, and Birkett A. . 2008. Resistant starch intakes in the United States. J. Am. Diet. Assoc. 108:67–78. doi: 10.1016/j.jada.2007.10.012 [DOI] [PubMed] [Google Scholar]
- National Research Council 2006. Nutrient requirements of dogs and cats. Washington (DC):The National Academies Press. [Google Scholar]
- Nayak B, Berrios J De J, and Tang J. . 2014. Impact of food processing on the glycemic index (GI) of potato products. Food Res. Int. 56:35–46. doi: 10.1016/j.foodres.2013.12.020 [DOI] [Google Scholar]
- Nelson D, and Cox M. . 2008. Carbohydrates and glycobiology. In: K. Ahr and R. Rossignol, editors. Lehinger principles of biochemistry. 5th ed. New York (NY):W.H. Freeman; p. 235–270. [Google Scholar]
- Nguyen P, Dumon H, Biourge V, and Pouteau E. . 1998. Glycemic and insulinemic responses after ingestion of commercial foods in healthy dogs: influence of food composition. J. Nutr. 128:2654S. doi: 10.1093/jn/128.12.2654s [DOI] [PubMed] [Google Scholar]
- Nguyen P, Dumon H, Buttin P, Martin L, and Gouro A S. . 1994. Composition of meal influences changes in postprandial incremental glucose and insulin in healthy dogs. J. Nutr. 124(12 Suppl):2707S–2711S. doi: 10.1093/jn/124.suppl_12.2707S [DOI] [PubMed] [Google Scholar]
- Packaged Facts 2017. Pet food in the U.S. 13th edition [accessed July 7, 2018]. Available from https://www.packagedfacts.com/Pet-Food-13th-Edition-11295405/. [Google Scholar]
- Piccione G, Giannetto C, Fazio F, and Giudice E. . 2008. Daily rhythm of serum lipase and alpha-amylase activity in fed and fasted dogs. J. Vet. Diagn. Invest. 20:795–799. doi: 10.1177/104063870802000614 [DOI] [PubMed] [Google Scholar]
- Ramdath D. 2016. Glycemic index, glycemic load, and their health benefits. In: Wrigley, C. W., H. Corke, K. Seetharaman, and J. Faubion, editors. Encyclopedia of food grains. 2nd ed. Waltham (MA): Elsevier Ltd; p. 241–247. [Google Scholar]
- Ranawana V, Monro J A, Mishra S, and Henry C J. . 2010. Degree of particle size breakdown during mastication may be a possible cause of interindividual glycemic variability. Nutr. Res. 30:246–254. doi: 10.1016/j.nutres.2010.02.004 [DOI] [PubMed] [Google Scholar]
- Thakur R, Pristijono P, Scarlett C J, Bowyer M, Singh S P, and Vuong Q V. . 2019. Starch-based films: major factors affecting their properties. Int. J. Biol. Macromol. 132:1079–1089. doi: 10.1016/j.ijbiomac.2019.03.190 [DOI] [PubMed] [Google Scholar]
- Wolever T M S, and Bolognesi C. . 1996. Source and amount of carbohydrate affect postprandial glucose and insulin in normal subjects. J. Nutr. 126:2798–2806. doi: 10.1093/jn/126.11.2798 [DOI] [PubMed] [Google Scholar]
- Wolever T M, Jenkins D J, Jenkins A L, and Josse R G. . 1991. The glycemic index: methodology and clinical implications. Am. J. Clin. Nutr. 54:846–854. doi: 10.1093/ajcn/54.5.846 [DOI] [PubMed] [Google Scholar]