Abstract
Background
The hemodialysis setting is suitable for trials that use cluster randomization, where intact groups of individuals are randomized. However, cluster randomized trials (CRTs) are complicated in their design, analysis, and reporting and can pose ethical challenges. We reviewed CRTs in the hemodialysis setting with respect to reporting of key methodological and ethical issues.
Methods
We conducted a systematic review of CRTs in the hemodialysis setting, published in English, between 2000 and 2019, and indexed in MEDLINE or Embase. Two reviewers extracted data, and study results were summarized using descriptive statistics.
Results
We identified 26 completed CRTs and five study protocols of CRTs. These studies randomized hemodialysis centers (n = 17, 55%), hemodialysis shifts (n = 12, 39%), healthcare providers (n = 1, 3%), and nephrology units (n = 1, 3%). Trials included a median of 28 clusters with a median cluster size of 20 patients. Justification for using a clustered design was provided by 15 trials (48%). Methods that accounted for clustering were used during sample size calculation in 14 (45%), during analyses in 22 (71%), and during both sample size calculation and analyses in 13 trials (42%). Among all CRTs, 26 (84%) reported receiving research ethics committee approval; patient consent was reported in 22 trials: 10 (32%) reported the method of consent for trial participation and 12 (39%) reported no details about how consent was obtained or its purpose. Four trials (13%) reported receiving waivers of consent, and the remaining 5 (16%) provided no or unclear information about the consent process.
Conclusion
There is an opportunity to improve the conduct and reporting of essential methodological and ethical issues in future CRTs in hemodialysis.
Review Registration
We conducted this systematic review using a pre-specified protocol that was not registered.
Keywords: Cluster randomized controlled trial, Systematic review, Ethics, Informed consent, Hemodialysis
Introduction
Patients on hemodialysis are often excluded from clinical trials, and many trials in the hemodialysis setting suffer from poor recruitment, inadequate sample sizes, and poor adherence to allocated treatment and treatment contamination [1–5]. Cluster randomized trials (CRTs) randomize intact groups of individuals (rather than independent individuals) to different arms. This design can offer a logistically convenient method to produce high-quality evidence, can be effective in avoiding treatment contamination, and may be better received by participants and healthcare staff when delivered to a group of individuals rather than select patients. The CRT is an attractive design in the hemodialysis setting, where interventions are often delivered at the center-level and where staff follow the same protocol for patients under their care.
Cluster randomization, however, introduces methodological issues that need to be addressed during the design and analysis stages [6, 7]. First, it may not be possible to identify and recruit participants until after the cluster has been randomized. This increases the risk of selection bias because knowledge of the allocated arm can influence both the identification of potential participants and their decisions to participate. Second, because outcomes are usually correlated within clusters, CRTs are statistically less efficient than individual-level randomized trials. As such, the CONSORT Statement for Cluster Randomized Trials requires that studies report how clustering was considered in both sample size calculation and analysis. Failing to account for clustering in the sample size calculation implies that the study may not have adequate power to detect meaningful differences between the groups, while failing to account for clustering in the analysis implies that standard errors of treatment effects will be under-estimated, increasing the risk of spurious statistical significance [6–9].
The CRT design also raises complex ethical issues. The Ottawa Statement on the Ethical Design and Conduct of Cluster Randomized Trials offers ethical guidance, providing 15 recommendations for those who design, conduct, and review CRTs [10–14]. For example, ethical issues that may challenge researchers including the following: When is a study considered research? Who is the research subject? And from whom, how, and when must informed consent be obtained? A summary of the Ottawa Statement recommendations and applicability of these recommendation for CRTs conducted in the hemodialysis setting is provided in Additional file 1: Appendix 1.
In the present study, we conducted a descriptive analysis of how CRTs in hemodialysis report key methodological (with regards to accounting for clustering effects and reporting of intra-class correlation coefficient) and ethical issues (with regards to the elements highlighted in the Ottawa Statement). This review will serve as a foundational step in a multi-year initiative that seeks to develop recommendations for the ethical design, conduct, and reporting of CRTs in the hemodialysis setting.
Materials and methods
Protocol and registration
We conducted this systematic review using a pre-specified protocol and reported our results according to published guidelines (PRISMA Checklist: Additional file 1: Appendix 2) [15].
Studies eligible for review
We did not set any limits on country of study and included published primary reports of CRTs or study protocols of CRTs with an unpublished primary report. We aimed to include English-language reports published between January 2000 and November 2019 that involved (1) patients on in-center hemodialysis or( 2) patients on in-center hemodialysis as a subgroup in a larger study of non-in-center hemodialysis patients. When we found a study protocol of a CRT with an identified completed trial, we used the protocol to supplement any missing information from the final published report. Other reports such as secondary analyses, conference abstracts, and pilot or feasibility CRTs were excluded. We excluded feasibility and pilot trials because they have different methodological [16] and ethical considerations than full scale CRTs.
Information sources
We implemented a search syntax on November 30, 2019, to identify published reports in MEDLINE and Embase.
Search
Our search strategy combined two published search filters designed to identify publications related to CRT [17] and dialysis [18] studies (Additional file 1: Appendix 3). Two reviewers (AAA and KC) screened titles and abstracts of articles. AAA manually searched for additional articles in bibliographies of all included articles, list of articles that cited the included studies in Google Scholar, and “Similar articles” feature in PubMed. The complete list of included studies was also reviewed by an expert in the field (AXG) to capture additional studies that may have been missed.
Study selection
We retrieved the full text of any article considered potentially relevant by any reviewer. Full-text articles were assessed for study eligibility by two reviewers (AAA and KC), with disagreements resolved through discussion. Agreement between the two reviewers was evaluated using the Kappa statistic [19].
Data collection process
We utilized a data abstraction form that was pilot tested on three studies by three reviewers (AAA, KC, and CEG). Thereafter, two reviewers (AAA and either KC or CEG) independently extracted data from each manuscript. Extracted details on trials considering the effect of clustering during sample size estimation and analysis were completed by AAA and either MT or SND. After each set of three studies, data extractions were compared within the pair and disagreements resolved by consensus. Details of extracted data are highlighted in Additional file 1: Appendix 4. We extracted data on study characteristics, methodological characteristics, data collection method, justification for using a CRT design, type of intervention, information regarding research ethics committee review, gatekeepers (i.e., an individual or body that represents the interests of cluster members, clusters, or organizations [20]), informed consent procedures, and any information about harm-benefit assessment or protection of vulnerable populations. Kidney disease disproportionally affects individuals traditionally considered vulnerable (e.g., patients with dementia). We defined vulnerable participants as any research participants who “may have increased likelihood of being wronged or of incurring additional harm,” as per the CIOMS international ethics guidelines [21]. This includes persons who have “impairments in decisional capacity, education, resources, strength, or other attributes needed to protect their own interests [21]”. We coded the presence of any vulnerable participants as clearly present, potentially present or unclear, and clearly absent or not relevant. If a vulnerability was clearly or potentially present, we looked for any reporting of additional protections provided by the authors.
Analysis
We summarized results using frequencies for categorical variables and medians with interquartile ranges for continuous variables. Given the small number of included studies, we did not test changes in reporting over time nor association between reporting of ethical elements and study characteristics. For all our analyses, we used R (Version 3.6.2) [22].
Results
Characteristics of included studies
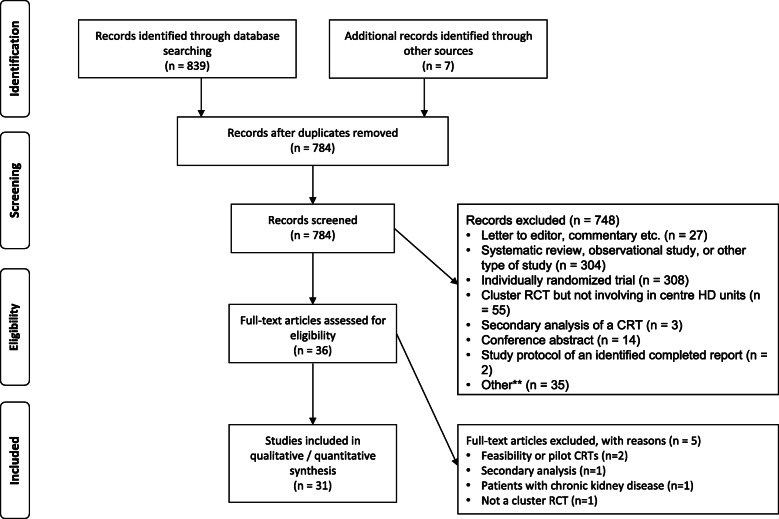
The study flow diagram is presented in Fig. 1. We screened 777 citations and retrieved 29 full-text articles to assess eligibility. We identified another seven articles by reviewing citation links (n total = 36). We had almost perfect between-reviewer agreement on which studies met the criteria for review (kappa statistic 0.96, 95% confidence interval: 0.91 to 1.00). Five articles were excluded after full-text review [23–27]. Thus, 31 articles were included in this review: 26 completed studies and 5 study protocols [28–58].
Fig. 1.
Flow diagram of study selection. **Other: One manuscript described the statistical plan for a main publication not related to cluster randomized trials, two described a program of research not related to the target population, and two were duplicate records not previously removed. Abbreviation: RCT, randomized controlled trial; CRT, cluster randomized trial
Study characteristics for the included trials are presented in Table 1. The 31 trials were published in 19 journals. Nineteen trials (61%) recruited patients from the USA, three (10%) were from the UK, three (10%) from Australia/New Zealand, and seven (23%) from other countries (some trials were multi-national and these categories are not mutually exclusive).
Table 1.
Included studies and their characteristics
| First author | Year | Country | Intervention arm (number) | Control arm (number) | Type of cluster | Type of patients₳ | Type of intervention₱ | Primary outcome | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Clusters | Patients | Clusters | Patients | |||||||
| Sehgal [28] | 2002 | USA | 21 | 85 | 23 | 84 | Individual providers | Prevalent only | 2 and 3 | Change in Kt/V and achievement of facility Kt/V goal |
| McClellan [29] | 2004 | USA | 21 | 2237 | 20 | 2044 | HD units | Prevalent and incident | 1, 2, and 3 | Proportion of patients whose urea reduction ratio was ≥65% |
| Leon [40] | 2006 | USA | 21 | 86 | 23 | 94 | HD units | Prevalent only | 3 | Serum albumin level |
| Pradel [51] | 2008 | USA | 14 | 107 | 14 | 107 | Shifts in HD unit | Prevalent and incident | 3 | See ¥ |
| Locatelli [53] | 2009 | EU** | NR* | 321 | NR | 278 | Nephrology Unit | Prevalent and incident | 2 | Proportions of patients with hemoglobin > 11 g/dL, serum ferritin > 100 μg/L, hypochromic red cell count < 10%, or transferrin saturation > 20% |
| Sullivan [48] | 2009 | USA | 14 | 145 | 14 | 134 | Shifts in HD unit | Prevalent only | 3 | Serum phosphorus level |
| Bond [49] | 2011 | USA | 38 | 3157 | 39 | 3135 | HD units | Prevalent and incident | 2 and 3 | Change in influenza vaccination rates |
| Kauric-Klein [54] | 2012 | USA | NR | 59 | NR | 59 | HD Units | Prevalent only | 3 | Changes in systolic blood pressure over time (primary outcome not explicitly stated) |
| Sullivan [50] | 2012 | USA | 11 | 92 | 12 | 75 | HD units | Prevalent and incident | 3 | Number of transplant process steps completed |
| Bennett [55] | 2013 | AUS/NZ | 2 | 38 | 2 | 41 | HD units | Prevalent and incident | 2 | Rate of referral to dietetic services for nutrition support |
| Karavetian [56] | 2013 | Lebanon | 1 | 37 | 1 | 24 | Shifts in HD unit | Prevalent and incident | 3 | Patient knowledge score£ |
| Weisbord [57] | 2013 | USA | 9 | 100 | 9 | 120 | Shifts in HD unit | Prevalent and incident | 2 | Changes in scores on pain, erectile dysfunction and depression surveys |
| Rosenblum [58] | 2014 | USA | 216 | 4609 | 216 | 4551 | HD units | Prevalent and incident | 2 and 4 | Positive blood culture rate |
| Wileman [30] | 2014 | UK | 6 | 45 | 6 | 45 | Shifts in HD unit | Prevalent and incident | 3 | Serum phosphate level |
| Karavetian [31] | 2015 | Lebanon | 6 | 88 | 6 | 96 | Shifts in HD unit | Prevalent and incident | 3 | Serum phosphorus level |
| Bennett [32] | 2016 | AUS/NZ | 15 | 171 | 15 | 171 | HD units | Prevalent and incident | 3 and 4 | 30-s sit-to-stand test |
| Graham-Brown [33] | 2016 | UK | 3 | NA*** | 3 | NA*** | Shifts in HD unit | Prevalent only | 4 | Left ventricular mass |
| Howren [34] | 2016 | USA | 11 | 61 | 11 | 58 | Shifts in HD unit | Prevalent and incident | 3 | Unclear: Mean interdialytic weight gain across for periods or Fluid nonadherent as defined by an interdialytic weight gain > 2.5 kg over a 4-week period |
| Wileman [35] | 2016 | UK | 6 | 49 | 6 | 40 | Shifts in HD unit | Prevalent and incident | 3 | Interdialytic weight gain |
| Hymes [36] | 2017 | USA | 20 | 1245 | 20 | 1225 | HD units | Prevalent and incident | 2 and 4 | Positive blood culture rate |
| Patzer [37] | 2017 | USA | 67 | 4203 | 20 | 1225 | HD units | Prevalent and incident | 1, 2, and 3 | Facility level transplant referral rate |
| Patzer [38] | 2017 | USA | NA*** | NA*** | NA*** | NA*** | HD units | Prevalent and incident | 1, 2, and 3 | Co-primary outcomes of (i) change in proportion of patients waitlisted and (ii) disparity reduction in proportion of patients waitlisted in a dialysis facility after 1 year |
| Brunelli [39] | 2018 | USA | 20 | 826 | 20 | 845 | HD units | Prevalent and incident | 4 | Positive blood culture rate |
| Delmas [41] | 2018 | Switzerland | NR | NR | NR | NR | HD Units | Prevalent only | 1 | Nurse quality of working life |
| Griva [42] | 2018 | Singapore | 14 | 101 | 14 | 134 | Shifts in HD unit | Prevalent only | 3 | Serum potassium/phosphate levels and interdialytic weight gains |
| Huang [43] | 2018 | China | 1 | 46 | 1 | 44 | Shifts in HD unit | Prevalent and incident | 3 | Blood pressure monitored before each hemodialysis |
| Milazi [44] | 2018 | AUS/NZ | 3 | 60 | 3 | 60 | Shifts in HD unit | Prevalent and incident | 3 | Serum phosphate level |
| Song [52] | 2018 | USA | NA*** | NA*** | NA*** | NA*** | HD units | Prevalent only | 3 and 5 | Patient and surrogate self-reported preparedness for end-of-life decision making |
| Sullivan [45] | 2018 | USA | 20 | 1041 | 20 | 836 | HD units | Prevalent and incident | 3 | Placement on kidney transplant waiting list |
| Waterman [46] | 2018 | USA | 10 | 133 | 10 | 120 | HD units | Prevalent and incident | 3 | Patients’ readiness to allow someone to be a living donor |
| Dember [47] | 2019 | USA | 133 | 1938 | 133 | 2532 | HD units | Incident only | 4 | Death |
NR not reported, USA United States of America, EU European Union, UK United Kingdom, AUS/NZ Australia/New Zealand, NA not applicable, g/dL grams per deciliter, μg/L micrograms per liter, Kt/V fractional urea clearance represented by K = dialyzer clearance of urea, t = dialysis time, V = distribution volume of urea
*Locatelli et al. did not report the number of clusters randomized to each arm; however, the authors reported a total of 53 nephrology units participated in the trial
**Included countries from Bulgaria, Croatia, Poland, Romania, and Serbia and Montenegro
***This was a study protocol of an ongoing trial and thus the final sample size used (or to be used) in the analysis was not available
₳We defined patients as “prevalent” if they were on hemodialysis for at least 6 months and “incident” if they are newly starting or started hemodialysis less than 6 months prior to baseline
¥Study assessed three distinct behaviors to explore patients’ readiness to pursue living donor kidney transplant: (1) considering living donor kidney transplant, (2) talking with family or friends about living donor kidney transplant, and (3) asking someone to be a kidney transplant donor
£Patient knowledge questionnaire was utilized to assess patients’ knowledge of kidney disease, renal diet, phosphate binders, and vitamin D therapy
₱1 = educational/ quality improvement interventions targeted at health professionals (e.g., transplant education and engagement activities targeting health professionals, etc.); 2 = quality improvement interventions targeted at organization of health care or health services delivery (e.g., nutrition screening, change in catheter exit-site care, etc.); 3 = patient health promotion or educational intervention (e.g., education about benefits of resistance exercise program, dietary counseling, education on avoiding foods with phosphorus additives, etc.); 4 = direct patient therapeutic intervention (e.g., intradialytic resistance training, antimicrobial barrier caps for catheters, etc.); and 5 = other
Reporting of methodological characteristics
Table 2 provides a description of the reporting of study characteristics. Thirty trials (97%) utilized a parallel arm design and one trial (3%) used a stepped-wedge design. All trials were designed as superiority trials. The types of randomized clusters were hemodialysis centers (n = 17; 55%), hemodialysis shifts or sessions (n = 12; 39%), providers or professionals (n = 1; 3%), and nephrology units (n = 1; 3%; it was not clear how a “nephrology unit” was defined). Clusters were randomly allocated to the treatment arm using unrestricted (n = 8; 26%), pair-matched (n = 4; 13%), stratified (n = 4; 13%), split-cluster (n = 11, 35% [i.e., day shifts within centers]), covariate-constrained randomization (n = 1, 3%), or an unreported method of allocation (n = 3, 10%).
Table 2.
Reporting of study characteristics
| Component | Number of studies (%) (N total = 31) |
|---|---|
| Trial design | |
| Parallel arm | 30 (97%) |
| Stepped-wedge design | 1 (3%) |
| Types of randomized clusters | |
| Hemodialysis centers | 17 (55%) |
| Hemodialysis shifts or sessions | 12 (39%) |
| Providers or professionals | 1 (3%) |
| Nephrology units₱ | 1 (3%) |
| Method of random allocation | |
| Completely randomized design (unrestricted randomization) | 8 (26%) |
| Stratified design | 4 (13%) |
| Pair-matched design | 4 (13%) |
| Split-cluster (i.e., shifts within a hemodialysis center) | 11 (35%) |
| Covariate-constrained | 1 (3%) |
| Not reported | 3 (10%) |
| Number of clusters per trial [median (25th, 75th percentile)]¥ | 28 (12, 43) |
| Number of patients per trial [median (25th, 75th percentile)]₳ | 228 (120, 1723) |
| Number of patients per cluster [median (25th, 75th percentile)]€,Ϫ | 20 (8, 32) |
₱It is not clear how a “nephrology unit” was defined
Estimate is based on ¥32, ₳29, and €28 trials. Missing data may have been a result of not reporting or the study being a protocol with no final information on the number of clusters/patients being available
ϪFor each study, we estimated the average cluster size by dividing the total number of patients recruited by the number of clusters (e.g., 200 patients recruited in a trial/10 clusters = 20 patients per cluster). We then took the median of the calculated average of patients per clusters from each trial
The median (25th, 75th percentile) number of clusters included per trial was 28 (12, 43), and all trials used 1:1 randomization. One trial (3%) had one cluster per arm, and six trials (19%) had fewer than the minimum recommendation of four clusters per arm [7, 8]. The median number of participants per trial was 228 (120, 1723). All trials included patients (as opposed to providers alone) as the research participants with a median number of 20 (8, 32) patients per cluster.
One study (3%) reported the intra-class correlation coefficient (ICC) for their primary outcome. Table 3 describes whether and how clustering was accounted for during sample size estimation and analysis. Fourteen trials (45%) accounted for clustering during sample size estimation for the primary outcome, three (10%) did not account for clustering, two (6%) accounted for clustering but using a different outcome measure than the primary outcome, one (3%) was unclear, and 11 (35%) did not report a sample size or power estimate. At the analysis stage, 22 trials (71%) accounted for clustering using either an individual-level analysis adjusting for clustering or using a cluster-level summary method. The remaining nine trials (29%) either did not account for clustering in their primary analysis or it was unclear if clustering was accounted for in the analysis. A total of 13 trials (42%) accounted for clustering in both the sample size calculation and analysis.
Table 3.
Reporting of (a) how clustering was considered during sample size estimation and analysis and (b) justification for using a cluster randomized design
| N = 31 trials (%) | |
|---|---|
| Did sample size/power calculations account for the cluster design? | |
| Not presented₳ | 11 (35%) |
| Yes, used patient-level data and accounted for clustering (e.g., random effects model) | 11 (35%) |
| Yes, used cluster-level summaries | 3 (10%) |
| No, used patient-level data without accounting for clustering | 3 (10%) |
| Unclear | 1 (3%) |
| Other¥ | 2 (6%) |
| Did the analysis for primary outcome account for clustering? | |
| Yes, used patient-level data and accounted for clustering | 17 (55%) |
| Yes, used cluster-level summaries | 5 (16%) |
| No, used patient-level data without accounting for clustering ₱ | 7 (23%) |
| Unclear/other¥ | 2 (6%) |
| Justification for utilizing a cluster randomized design (categories were not mutually exclusive) | |
| None provided | 16 (52%) |
| Avoid contamination | 15 (48%) |
| Logistical or administrative convenience | 2 (6%) |
₳One study presented power calculation, but it was a post hoc power analysis
¥This may have included using an inappropriate method for the proposed primary outcome, or the study accounted for clustering but not based on the primary outcome measure (e.g., they assumed a continuous outcome, but the primary endpoint was a proportion)
₱One study accounted for repeated events within patients but did not report accounting for within-cluster correlation; another study reported using a generalized linear mixed model but did not specify whether they accounted for the effect of the cluster as random effect
Reporting of justification for cluster randomization
Of all 31 trials, 15 trials (48%) reported a justification for using a cluster randomized design (Table 3). Thirteen trials (42%) reported using a CRT design to avoid contamination and two trials (6%) reported using a CRT design to avoid contamination and for logistical/administrative convenience.
Reporting of intervention type and target population
Table 4 lists the types of intervention used in each arm of included trials. The most common type of study intervention was health promotion or an educational intervention (n = 22 trials; 71%) for which patients were the intended recipients. Six trials (19%) examined a direct patient therapeutic intervention—for example, intradialytic resistance training or antimicrobial barrier caps for central venous catheters. Among all trials, the intervention was necessarily administered at the cluster-level (e.g., education of providers) for 18 trials (58%). In the control arm, 23 trials (74%) utilized “usual care,” four (13%) used some form of augmented care (usual care plus some minimal elements of active intervention), three (10%) used an active control, and one (3%) used an attention-placebo. Four trials (13%) utilized interventions that included an educational or quality improvement component targeting health professionals (e.g., transplant education and engagement activities). Both prevalent and incident patients on hemodialysis were included in 22 trials (71%), eight trials (26%) included only prevalent patients, and one trial (3%) included only incident patients on hemodialysis.
Table 4.
Summary of results for type(s) of interventions, data collection procedures, reporting of participant consent procedures for study interventions and data collection, timing of any participant consent, and whether participants can opt out of the intervention or data collection
| Component | Intervention arm n (%) |
Control arm n (%) |
|---|---|---|
| Type(s) of interventions (i.e., all components of intervention)¥ | N total = 31 | N total = 8** |
| Educational/ quality improvement interventions targeted at health professionals (e.g., transplant education and engagement activities targeting health professionals, etc.) | 4 (13%) | 0 (0%) |
| Quality improvement interventions targeted at organization of health care or health services delivery (e.g., nutrition screening, change in catheter exit-site care, etc.) | 10 (32%) | 2 (25%) |
| Patient health promotion or educational intervention (e.g., education about benefits of resistance exercise program, dietary counseling, education on avoiding foods with phosphorus additives, etc.) | 22 (71%) | 4 (50%) |
| Direct patient therapeutic intervention (e.g., intradialytic resistance training, antimicrobial barrier caps for catheters, etc.) | 6 (19%) | 1 (12%) |
| Other € | 1 (3%) | 1 (12%) |
| Types of Data collection procedures ¥ | N total = 31 | N total = 31 |
| Routinely collected outcomes extracted locally from existing patient medical records (physical charts or electronic records) | 30 (97%) | 30 (97%) |
| Data query from clinical data registry or other central source of routinely collected data (e.g., administrative data) | 11 (35%) | 11 (35%) |
| Specimen collection or physical examination that were not required for usual patient care | 4 (13%) | 4 (13%) |
| Interviewer-administered patient questionnaires done face-to-face or by telephone that were not required for usual patient care | 9 (29%) | 9 (29%) |
| Self-administered patient questionnaires (done by mail, e-mail or Internet) that were not required for usual patient care | 18 (58%) | 16 (52%) |
| Other ₳ | 5 (16%) | 2 (6%) |
| Reporting of participant consent procedures for study interventions | N total = 31 | N total = 31 |
| Reported written informed consent | 9 (29%) | 10 (32%) |
| Reported verbal informed consent | 1 (3%) | 0 (0%) |
| Reported informed consent but no details about method or what consent was for | 12 (39%) | 11 (35%) |
| Reported the study was exempt from research ethics committee review, received waiver of consent, or explicitly stated no consent | 4 (13%) | 4 (13%) |
| Unclear if participants consented | 1 (3%) | 2 (6%) |
| Not mentioned | 4 (13%) | 4 (13%) |
| Reporting of participant consent procedures for data collection | N total = 31 | N total = 31 |
| Reported written informed consent | 7 (22%) | 6 (19%) |
| Reported verbal informed consent | 1 (3%) | 1 (3%) |
| Reported informed consent but no details about method or what consent was for | 14 (45%) | 14 (45%) |
| Reported the study was exempt from research ethics committee review, received waiver of consent, or explicitly stated no consent | 4 (13%) | 4 (13%) |
| Unclear if participants consented | 1 (3%) | 2 (6%) |
| Not mentioned | 4 (13%) | 4 (13%) |
¥The responses to these questions were not mutually exclusive
₳Active data collection, including using case report form
€Surrogate decision-maker educational intervention in the intervention arm; audit feedback from previous year in the control arm
**These questions were not applicable when the comparator arm was usual care
Data collection procedures
Data collection procedures in the intervention and control arm were similar for most trials (Table 4). In the intervention arm, 30 (97%) trials used local routinely collected data (e.g., medical charts or electronic medical records) as the primary source for data collection. Eleven trials (35%) used clinical registry data and 24 (77%) supplemented routinely collected data with additional sources: self-administered questionnaires (n = 18; 58%), interviewer administered questionnaires (n = 9; 29%), specimen collection or physical examination not required for usual patient care (n = 4; 13%), as well as active data collection (n = 5; 16%), for example, using case report forms.
Gatekeepers
Five trials (16%) reported that a gatekeeper provided permission for clusters to participate in the study (Table 5). For the remaining trials (84%), no information about gatekeepers was provided.
Table 5.
Summary of results for reported information about gatekeepers, research ethic committee review, timing of any participant consent, and whether participants can opt out of the intervention or data collection
| Component | Number of trials N total = 31 (%) |
|---|---|
| Whether a gatekeeper was identified that allowed access to each cluster | |
| Yes—a clearly identified individual or body | 3 (10%) |
| Yes—but the gatekeeper not clearly identified | 2 (6%) |
| No gatekeeper information provided | 26 (84%) |
| Reporting of research ethics review | |
| Stated research ethics committee approval | 26 (84%) |
| Stated research ethics committee exempt (specify reason) | 1 (3%) |
| Not reported | 4 (13%) |
| Timing of any participant consent | |
| Not applicable | 4 (13%) |
| Any consent was before randomization of clusters | 7 (23%) |
| Any consent was after randomization of clusters | 10 (32%) |
| Timing of consent was unclear and could not be deduced from the report | 10 (32%) |
| Whether participants can opt out of the data collection | |
| Yes—it is clearly reported that participants could opt out of data collection | 7 (23%) |
| No—participants could not opt out of data collection | 3 (10%) |
| Not reported or Unclear if participants could opt out | 21 (68%) |
| Presence of any vulnerable participants* in the trial | |
| Clearly present | 6 (20%) |
| Potentially present or unclear | 24 (77%) |
| Clearly absent or not relevant | 1 (3%) |
Reporting of research ethics review
We found that 26 trials (84%) reported research ethics committee approval, one (3%) reported that the study was exempt from review, and four (13%) did not report whether the study was reviewed by a research ethics committee (Table 5).
Reporting of consent procedures
One trial (3%) reported they received an exemption from ethics review, three (10%) received a waiver of consent from the research ethics committee (see Additional file 1: Appendix 5), 22 trials (71%) reported obtaining consent from patients, and five (16%) trials either did not discuss the consent process or it was unclear if patients provided informed consent. For the 22 trials (71%) that reported obtaining consent from patients, written or verbal informed consent was reported in 10 trials (45%) for the study intervention and eight (36%) for data collection; the remaining trials provided no details about the method of consent for study intervention and/or for data collection (Table 4).
Among the remaining 27 trials that did not receive an exemption from ethics review or had a waiver of consent, the timing of consent took place before randomization for seven trials (26%), after randomization for 10 (37%), and was unclear for the remaining 10 trials (37%).
The ability for participants to opt out of the data collection was reported in seven trials (23%); three trials (10%) reported patients could not opt out of data collection, and ability to opt out was unclear for the remaining 21 trials (68%) (Table 5).
Assessment of benefit-harm and protections for vulnerable groups
Vulnerable participants were clearly present in 6 trials (20%); in another 24 (77%), vulnerable participants were considered to be potentially present or their presence could not be clearly ruled out (Table 5). None of the trials reported additional protections for vulnerable patients.
Discussion
The hemodialysis population is suitable for the CRT design, especially for interventions that are implemented at the center-level; in our review, approximately 60% of trials utilized an intervention that was necessarily administered at the cluster-level. This review presents a descriptive analysis of the reporting of key methodological and ethical characteristics of CRTs involving hemodialysis patients. Guidance on the reporting of CRTs is provided in the CONSORT extension for CRTs, while the Ottawa Statement is currently the only guidance document specific to the ethical design and conduct of CRTs in health research [8, 14]. While several studies were published prior to the dissemination of the CONSORT, the Ottawa Statement or both, the interpretation of our results would not change had we presented our results based on the period pre- and post-publication of these statements.
We found that cluster randomized trials in hemodialysis have poor methodological quality and sub-optimally report ethical considerations around this design. While many of the identified issues are not unique to the hemodialysis setting, we consider three issues that require special attention: (1) taking clustering into account at the sample size estimation and analysis stages, (2) methodological and contamination issues around designs that randomize shifts within hemodialysis centers, and (3) reporting on how the rights of vulnerable participants are protected.
First, patients on hemodialysis within the same center have similar characteristics compared to patients from other centers. For example, small satellite hemodialysis centers might have patients that are medically stable compared to large academic centers that might treat sicker patients requiring close medical monitoring. It is concerning that more than half of included trials did not report a method that appropriately accounts for within-cluster correlation when estimating sample size and more than a quarter of trials did not account for clustering in the analysis, putting the study results at an increased risk of spurious statistical significance [7–9, 14]. Adjusting for clustering is especially important in this setting because there is generally high practice variation between hemodialysis centers and low variation with-in centers [59–61], factors that increase the ICC [62].
Currently, there is limited information in the literature to inform estimates of the ICC for outcomes of patients on hemodialysis; thus, researchers in hemodialysis must rely on estimates from other disciplines or historic data. As such, it is important for completed trials to report the observed ICC or design effect estimates for their outcomes so that the community can begin to build a repository that might help in the design of future trials. In our review, only one trial reported an ICC [47].
Second, a common experimental design was to randomize shifts within hemodialysis centers (e.g., Mon, Wed, Fri versus Tues, Thu, Sat). This type of randomization requires additional considerations in design and conduct. For example, the same healthcare staff will care for patients dialyzing in a single center in both arms of the trial. Contamination of the two arms of the trial can still occur if staff observe better patient outcomes in one arm and then begin to implement the treatment in clusters (i.e., shifts) in the other arm. This type of design also requires additional considerations in the analysis because clustering can occur at two levels, i.e., center and shift. It was beyond this review to assess whether authors reported the appropriate analyses accounting for this type of experimental design.
Third, authors should report how the rights of vulnerable participants are protected, especially those that have limited health literacy or may not be capable to provide informed consent. When including these subgroups, it raises ethical concerns about the extent to which these participants are truly informed. There are no clear standards for “how much” understanding is adequate [63]. Additionally, lower education levels, lower health literacy, and a participant’s primary language are all associated with poor comprehension of the informed consent process [64]. These characteristics are particularly important in the hemodialysis setting, where vulnerable participants are overrepresented [65–68].
In general, trials in this setting were small with both a limited number of clusters and patients within clusters. One trial randomized only one cluster to each arm of the trial and a fifth of reviewed trials had four or fewer clusters [43, 56]. Randomizing two clusters effectively precludes any inferences about the intervention because it is impossible to disentangle natural variation between clusters from the effect of the intervention [69]. While some have suggested that parallel arm CRTs should have at least four clusters per study arm [7, 8], with such a small number of clusters, the study may be severely under-powered, parametric statistical tests (e.g., t tests) may not meet the assumption of normality, and there is a high risk of baseline imbalances between trial arms that might complicate the interpretation of the trial results [70].
There is room for improved reporting of consent procedures. When consent is required, study authors ought to report adequate details to assess what consent was for (e.g., enrollment, receiving the interventions, data collection), as well as from whom (e.g., patients, providers, etc.), when (before or after randomization), and how (e.g., written, verbal) consent was obtained [8, 14]. The timing of informed consent was either not reported or took place after randomization for 20 trials. Post-randomization consent, especially when the study is unblinded, is a key risk of bias that can introduce selection bias through differential recruitment [8]. When applicable, researchers must justify how their study meets the criteria for a waiver or alteration of informed consent as outlined by national regulations or international guidelines [21, 71, 72].
Our study has several limitations. We were unable to examine changes in quality of reporting over time, or factors associated with better reporting due to the small number of trials. When a study protocol was published for one of the completed trials, we used both references to complete study extraction; however, we did not have access to the original research ethics submissions or non-peer-reviewed study protocols, did not follow-up with study authors, and did not conduct a search of any trial registries or Green Open Access options (e.g., ResearchGate). Thus, our results are based exclusively on what was reported in peer-reviewed published articles; for example, we are aware of other ongoing CRTs not included here because no study protocol or a primary report was available at the time of our search [73, 74].
Our study also has several strengths. We utilized an abstraction tool that has been developed and refined over several studies [75–78]. It is unlikely that a substantial number of relevant primary trials were missed, as we combined two validated search strategies supplemented with an extensive manual search of reference resources [17, 18]. To reduce the risk of misclassification of trial characteristics and reporting practices, we used consensus between two reviewers who independently extracted information from published articles.
Conclusion
There is suboptimal conduct and reporting of methodological issues of CRTs in the hemodialysis setting and incomplete reporting of key ethical issues. The Ottawa Statement on the Ethical Design and Conduct of Cluster Randomized Trials provides specific recommendations for CRTs, but did not consider unique characteristics of the hemodialysis setting [14]. This systematic review was conducted as a first step to describe key study design characteristics and document reporting of ethical practices in CRTs in the hemodialysis setting. Our future work builds on the information from this review to explore the views/perceptions of researchers and patients with regard to the ethical issues for CRTs in the hemodialysis setting.
Supplementary information
Additional file 1: Appendix 1. Recommendations from the Ottawa Statement. Appendix 2. PRISMA Checklist. Appendix 3. Search syntax to identify relevant articles in Medline between January 1st, 2000 and July 20th, 2018 in Embase Classic+Embase, Ovid MEDLINE(R) Epub Ahead of Print, In-Process & Other Non-Indexed Citations, Ovid MEDLINE(R) Daily and Ovid MEDLINE(R). Appendix 4. Extracted data. Appendix 5. Reported information about waiver of consent for the four studies that reported a waiver of informed patient consent or research ethic committee exemption.
Acknowledgements
We are grateful for Risa Shorr for creating and executing the search strategy for this review.
Ahmed Al-Jaishi was supported by the Allied Health Doctoral Fellowship from the Kidney Foundation of Canada, CIHR Doctoral Award, and McMaster University Michael DeGroote Scholarship. Cory E. Goldstein, Kelly Carroll, Stuart G. Nicholls, and Stephanie Dixon’s research is supported through a SPOR Innovative Clinical Trial Multi-Year Grant (Grant number: MYG-151209) from the Canadian Institutes of Health Research (CIHR). Amit Garg was supported by the Dr. Adam Linton Chair in Kidney Health Analytics and a Clinician Investigator Award from the CIHR. Jeremy Grimshaw holds a Canada Research Chair in Health Knowledge Transfer and Uptake.
Competing interests
CW receives consulting income from Cardialen, Eli Lilly & Company, and Research Triangle Institute (RTI) International. Other authors have nothing to disclose.
We declare that the results presented in this paper have not been published previously in whole or part.
Authors’ contributions
AXG and MT conceived the overall project idea and co-led the funding application with substantial contributions from all authors. MT developed the data extraction tool, and AAA, KC, and CEG extracted the study data. AAA, MT, and SND extracted details on trials considering the effect of clustering during sample size estimation and analysis. AAA, KC, CEG, and MT wrote the initial draft of the manuscript with substantial input from all authors. All authors contributed to the interpretation of results, made critical revisions, and approved the final manuscript.
Funding
We received funding for this review from partnering organization including Lawson Health Research Institute, Ontario Renal Network, Dialysis Clinic Inc., Heart and Stroke Foundation of Canada, and Canadian Institutes of Health Research (CIHR) Innovative Clinical Trials (iCT) Initiative. Funding is also provided by the Ontario Strategy for Patient-Oriented Research SUPPORT Unit, which is supported by the Canadian Institutes of Health Research and the Province of Ontario. Funding bodies had no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Availability of data and materials
Study data can be made available upon request.
Ethics approval and consent to participate
We did not apply for ethics approval as we conducted a systematic review of published literature.
Consent for publication
Consent for publication was obtained from all authors.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s13063-020-04657-9.
References
- 1.Strippoli GFM, Craig JC, Schena FP. The number, quality, and coverage of randomized controlled trials in nephrology. J Am Soc Nephrol. 2004;15:411–419. doi: 10.1097/01.asn.0000100125.21491.46. [DOI] [PubMed] [Google Scholar]
- 2.Palmer SC, Sciancalepore M, Strippoli GFM. Trial quality in nephrology: how are we measuring up? Am J Kidney Dis. 2011;58:335–337. doi: 10.1053/j.ajkd.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 3.Deo A, Schmid CH, Earley A, Lau J, Uhlig K. Loss to analysis in randomized controlled trials in CKD. Am J Kidney Dis. 2011;58:349–355. doi: 10.1053/j.ajkd.2011.04.023. [DOI] [PubMed] [Google Scholar]
- 4.Inrig JK, Califf RM, Tasneem A, et al. The landscape of clinical trials in nephrology: a systematic review of Clinicaltrials.gov. Am J Kidney Dis. 2014;63:771–780. doi: 10.1053/j.ajkd.2013.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaushalm P, March D, Eborall H, Gray L, Burton J. Poor reporting and conduct of cluster and crossover randomised trials in the haemodialysis population. Nephrol Dialysis Transplant. 2020;35:SO063. [Google Scholar]
- 6.Donner A, Klar N. Design and analysis of cluster randomization trials in Health Research. London: Arnold; 2000. [Google Scholar]
- 7.Hayes RJ, Moulton LH. Cluster randomised trials. Boca Raton: CRC Press; 2009. .
- 8.Campbell MK, Piaggio G, Elbourne DR, Altman DG, CONSORT Group Consort 2010 statement: extension to cluster randomised trials. BMJ (Clinical research ed.) 2012;345:e5661. doi: 10.1136/bmj.e5661. [DOI] [PubMed] [Google Scholar]
- 9.Campbell MJ, Walters SJ. How to design, analyse and report cluster randomised trials in medicine and health related research. Chichester: Wiley; 2014.
- 10.Klar N, Donner A. Wiley Encyclopedia of Clinical Trials. Hoboken: Wiley; 2007. Ethical challenges posed by cluster randomization. [Google Scholar]
- 11.Goldstein CE, Weijer C, Taljaard M, et al. Ethical issues in pragmatic cluster-randomized trials in dialysis facilities. Am J Kidney Dis. 2019;74:659–666. doi: 10.1053/j.ajkd.2019.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eldridge SM, Ashby D, Feder GS. Informed patient consent to participation in cluster randomized trials: an empirical exploration of trials in primary care. CliniTrials. 2005;2:91–98. doi: 10.1191/1740774505cn070oa. [DOI] [PubMed] [Google Scholar]
- 13.Edwards SJL, Braunholtz DA, Lilford RJ, Stevens AJ. Ethical issues in the design and conduct of cluster randomised controlled trials. BMJ. 1999;318:1407–1409. doi: 10.1136/bmj.318.7195.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weijer C, Grimshaw JM, Eccles MP, et al. The Ottawa statement on the ethical design and conduct of cluster randomized trials. PLoS Med. 2012;9:e1001346. doi: 10.1371/journal.pmed.1001346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62:1006–1012. doi: 10.1016/j.jclinepi.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 16.Eldridge SM, Chan CL, Campbell MJ, et al. CONSORT 2010 statement: extension to randomised pilot and feasibility trials. Pilot Feasibility Stud. 2016;2:64. doi: 10.1186/s40814-016-0105-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Taljaard M, McGowan J, Grimshaw JM, et al. Electronic search strategies to identify reports of cluster randomized trials in MEDLINE: low precision will improve with adherence to reporting standards. BMC Med Res Methodol. 2010;10:15. doi: 10.1186/1471-2288-10-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iansavichus AV, Haynes RB, Lee CWC, et al. Dialysis search filters for PubMed, Ovid MEDLINE, and Embase databases. Clin J Am Soc Nephrol. 2012;7:1624–3161. doi: 10.2215/CJN.02360312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cohen J. A coefficient of agreement for nominal scales. Educ Psychol Meas. 1960;20:37–46. [Google Scholar]
- 20.Gallo A, Weijer C, White A, et al. What is the role and authority of gatekeepers in cluster randomized trials in health research? Trials. 2012;13:116. doi: 10.1186/1745-6215-13-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Council for International Organizations of Medical Sciences (CIOMS). International ethical guidelines for health-related research involving humans. 2016. https://cioms.ch/shop/product/international-ethical-guidelines-for-health-related-research-involving-humans/. Accessed 9 July 2020.
- 22.R Core Team. R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2019. .
- 23.Huml AM, Sullivan CM, Pencak JA, Sehgal AR. Accuracy of dialysis medical records in determining patients’ interest in and suitability for transplantation. Clin Transpl. 2013;27:541–545. doi: 10.1111/ctr.12147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leonardis D, Mallamaci F, Enia G, et al. The MAURO study: baseline characteristics and compliance with guidelines targets. J Nephrol. 2012;25:1081–1090. doi: 10.5301/jn.5000239. [DOI] [PubMed] [Google Scholar]
- 25.Dai C-Y, Lin S-C, Peng H-L, et al. Effectiveness of vestibular rehabilitation in hemodialysis patients with dizziness. Rehabil Nurs. 2017;42:125–130. doi: 10.1002/rnj.198. [DOI] [PubMed] [Google Scholar]
- 26.Carswell C, Reid J, Walsh I, McAneney H, Noble H. Implementing an arts-based intervention for patients with end-stage kidney disease whilst receiving haemodialysis: a feasibility study protocol. Pilot Feasibility Stud. 2019;5:1. doi: 10.1186/s40814-018-0389-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Griva K, Rajeswari M, Nandakumar M, et al. The combined diabetes and renal control trial (C-DIRECT) - a feasibility randomised controlled trial to evaluate outcomes in multi-morbid patients with diabetes and on dialysis using a mixed methods approach. BMC Nephrol. 2019;20:2. doi: 10.1186/s12882-018-1183-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sehgal AR, Leon JB, Siminoff LA, Singer ME, Bunosky LM, Cebul RD. Improving the quality of hemodialysis treatment: a community-based randomized controlled trial to overcome patient-specific barriers. JAMA. 2002;287:1961–1967. doi: 10.1001/jama.287.15.1961. [DOI] [PubMed] [Google Scholar]
- 29.McClellan WM, Hodgin E, Pastan S, McAdams L, Soucie M. A randomized evaluation of two health care quality improvement program (HCQIP) interventions to improve the adequacy of hemodialysis care of ESRD patients: feedback alone versus intensive intervention. J Am Soc Nephrol. 2004;15:754–760. doi: 10.1097/01.asn.0000115701.51613.d7. [DOI] [PubMed] [Google Scholar]
- 30.Wileman V, Farrington K, Chilcot J, et al. Evidence that self-affirmation improves phosphate control in hemodialysis patients: a pilot cluster randomized controlled trial. Ann Behav Med. 2014;48:275–281. doi: 10.1007/s12160-014-9597-8. [DOI] [PubMed] [Google Scholar]
- 31.Karavetian M, de Vries N, Elzein H, Rizk R, Bechwaty F. Effect of behavioral stage-based nutrition education on management of osteodystrophy among hemodialysis patients, Lebanon. Patient Educ Couns. 2015;98:1116–1122. doi: 10.1016/j.pec.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 32.Bennett PN, Fraser S, Barnard R, et al. Effects of an intradialytic resistance training programme on physical function: a prospective stepped-wedge randomized controlled trial. Nephrol Dialysis Transplant. 2016;31:1302–1309. doi: 10.1093/ndt/gfv416. [DOI] [PubMed] [Google Scholar]
- 33.Graham-Brown MPM, March DS, Churchward DR, et al. Design and methods of CYCLE-HD: improving cardiovascular health in patients with end stage renal disease using a structured programme of exercise: a randomised control trial. BMC Nephrol. 2016;17:69. doi: 10.1186/s12882-016-0294-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Howren MB, Kellerman QD, Hillis SL, Cvengros J, Lawton W, Christensen AJ. Effect of a behavioral self-regulation intervention on patient adherence to fluid-intake restrictions in hemodialysis: a randomized controlled trial. Ann Behav Med. 2016;50:167–176. doi: 10.1007/s12160-015-9741-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wileman V, Chilcot J, Armitage CJ, et al. Evidence of improved fluid management in patients receiving haemodialysis following a self-affirmation theory-based intervention: a randomised controlled trial. Psychol Health. 2016;31:100–114. doi: 10.1080/08870446.2015.1073729. [DOI] [PubMed] [Google Scholar]
- 36.Hymes JL, Mooney A, Van Zandt C, Lynch L, Ziebol R, Killion D. Dialysis catheter–related bloodstream infections: a cluster-randomized trial of the ClearGuard HD antimicrobial barrier cap. Am J Kidney Dis. 2017;69:220–227. doi: 10.1053/j.ajkd.2016.09.014. [DOI] [PubMed] [Google Scholar]
- 37.Patzer RE, Paul S, Plantinga L, et al. A randomized trial to reduce disparities in referral for transplant evaluation. J Am Soc Nephrol. 2017;28:935–942. doi: 10.1681/ASN.2016030320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Patzer RE, Smith K, Basu M, et al. The ASCENT (Allocation System Changes for Equity in Kidney Transplantation) study: a randomized effectiveness-implementation study to improve kidney transplant waitlisting and reduce racial disparity. Kidney Int Rep. 2017;2:433–441. doi: 10.1016/j.ekir.2017.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brunelli SM, Van Wyck DB, Njord L, Ziebol RJ, Lynch LE, Killion DP. Cluster-randomized trial of devices to prevent catheter-related bloodstream infection. J Am Soc Nephrol. 2018;29:1336–1343. doi: 10.1681/ASN.2017080870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Leon JB, Albert JM, Gilchrist G, et al. Improving albumin levels among hemodialysis patients: a community-based randomized controlled trial. Am J Kidney Dis. 2006;48:28–36. doi: 10.1053/j.ajkd.2006.03.046. [DOI] [PubMed] [Google Scholar]
- 41.Delmas P, O’Reilly L, Cara C, et al. Effects on nurses’ quality of working life and on patients’ quality of life of an educational intervention to strengthen humanistic practice among hemodialysis nurses in Switzerland: a protocol for a mixed-methods cluster randomized controlled trial. BMC Nurs. 2018;17:47. doi: 10.1186/s12912-018-0320-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Griva K, Nandakumar M, Ng JH, Lam KFY, McBain H, Newman SP. Hemodialysis Self-management Intervention Randomized Trial (HED-SMART): a practical low-intensity intervention to improve adherence and clinical markers in patients receiving hemodialysis. Am J Kidney Dis. 2018;71:371–381. doi: 10.1053/j.ajkd.2017.09.014. [DOI] [PubMed] [Google Scholar]
- 43.Huang B, Li Z, Wang Y, et al. Effectiveness of self-management support in maintenance haemodialysis patients with hypertension: a pilot cluster randomized controlled trial. Nephrology. 2018;23:755–763. doi: 10.1111/nep.13098. [DOI] [PubMed] [Google Scholar]
- 44.Milazi M, Douglas C, Bonner A. A bundled phosphate control intervention (4Ds) for adults with end-stage kidney disease receiving haemodialysis: a cluster randomized controlled trial protocol. J Adv Nurs. 2018;74(10):2431–2441. doi: 10.1111/jan.13774. [DOI] [PubMed] [Google Scholar]
- 45.Sullivan CM, Barnswell KV, Greenway K, et al. Impact of navigators on first visit to a transplant center, waitlisting, and kidney transplantation: a randomized, controlled trial. Clin J Am Soc Nephrol. 2018;13:1550–1555. doi: 10.2215/CJN.03100318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Waterman AD, Peipert JD. An explore transplant group randomized controlled education trial to increase dialysis patients’ decision-making and pursuit of transplantation. Prog Transplant (Aliso Viejo, Calif.) 2018;28:174–183. doi: 10.1177/1526924818765815. [DOI] [PubMed] [Google Scholar]
- 47.Dember LM, Lacson E, Brunelli SM, et al. The TiME trial: a fully embedded, cluster-randomized, pragmatic trial of hemodialysis session duration. J Am Soc Nephrol. 2019;30:890–903. doi: 10.1681/ASN.2018090945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sullivan C, Sayre SS, Leon JB, et al. Effect of food additives on hyperphosphatemia among patients with end-stage renal disease. JAMA. 2009;301:629. doi: 10.1001/jama.2009.96. [DOI] [PubMed] [Google Scholar]
- 49.Bond TC, Patel PR, Krisher J, et al. A group-randomized evaluation of a quality improvement intervention to improve influenza vaccination rates in dialysis centers. Am J Kidney Dis. 2011;57:283–290. doi: 10.1053/j.ajkd.2010.09.019. [DOI] [PubMed] [Google Scholar]
- 50.Sullivan C, Leon JB, Sayre SS, et al. Impact of navigators on completion of steps in the kidney transplant process: a randomized, controlled trial. Clin J Am Soc Nephrol. 2012;7:1639–1645. doi: 10.2215/CJN.11731111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pradel FG, Suwannaprom P, Mullins CD, Sadler J, Bartlett ST. Short-term impact of an educational program promoting live donor kidney transplantation in dialysis centers. Prog Transpl (Aliso Viejo, Calif.) 2008;18:263–272. doi: 10.1177/152692480801800409. [DOI] [PubMed] [Google Scholar]
- 52.Song M-K, Unruh ML, Manatunga A, et al. SPIRIT trial: a phase III pragmatic trial of an advance care planning intervention in ESRD. Contemp Clin Trials. 2018;64:188–194. doi: 10.1016/j.cct.2017.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Locatelli F, Covic A, MacDougall IC, Scherhag A, Wiecek A. Effect of computer-assisted European best practice guideline implementation on adherence and target attainment: ORAMA results. J Nephrol. 2009;22:662–674. [PubMed] [Google Scholar]
- 54.Kauric-Klein Z. Improving blood pressure control in end stage renal disease through a supportive educative nursing intervention. Nephrol Nurs J. 2012;39:217–228. [PubMed] [Google Scholar]
- 55.Bennett PN, Miller MD, Woodman RJ, Hill K, Murray S, Gleadle JM. Nutrition screening by nurses in dialysis. J Clin Nurs. 2013;22:723–732. doi: 10.1111/j.1365-2702.2012.04286.x. [DOI] [PubMed] [Google Scholar]
- 56.Karavetian M, Ghaddar S. Nutritional education for the management of osteodystrophy (NEMO) in patients on haemodialysis: a randomised controlled trial. J Renal Care. 2013;39:19–30. doi: 10.1111/j.1755-6686.2012.00327.x. [DOI] [PubMed] [Google Scholar]
- 57.Weisbord SD, Mor MK, Green JA, et al. Comparison of symptom management strategies for pain, erectile dysfunction, and depression in patients receiving chronic hemodialysis: a cluster randomized effectiveness trial. Clin J Am Soc Nephrol. 2013;8:90–99. doi: 10.2215/CJN.04450512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rosenblum A, Wang W, Ball LK, Latham C, Maddux FW, Lacson E. Hemodialysis catheter care strategies: a cluster-randomized quality improvement initiative. Am J Kidney Dis. 2014;63:259–267. doi: 10.1053/j.ajkd.2013.08.019. [DOI] [PubMed] [Google Scholar]
- 59.Fuller DS, Robinson BM. Facility practice variation to help understand the effects of public policy: insights from the Dialysis Outcomes and Practice Patterns Study (DOPPS) Clin J Am Soc Nephrol. 2017;12:190–199. doi: 10.2215/CJN.03930416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fuller DS, Zepel L, Bieber BA, Robinson BM, Pisoni RL. Hemodialysis facility variation in hospitalization and transfusions using Medicare claims: the DOPPS Practice Monitor for US dialysis care. Am J Kidney Dis. 2016;67:337–340. doi: 10.1053/j.ajkd.2015.10.002. [DOI] [PubMed] [Google Scholar]
- 61.Al Salmi I, Larkina M, Wang M, et al. Missed hemodialysis treatments: international variation, predictors, and outcomes in the Dialysis Outcomes and Practice Patterns Study (DOPPS) Am J Kidney Dis. 2018;72:634–643. doi: 10.1053/j.ajkd.2018.04.019. [DOI] [PubMed] [Google Scholar]
- 62.Campbell MK, Grimshaw JM, Elbourne DR. Intracluster correlation coefficients in cluster randomized trials: empirical insights into how should they be reported. BMC Med Res Methodol. 2004;4:9. doi: 10.1186/1471-2288-4-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Falagas ME, Korbila IP, Giannopoulou KP, Kondilis BK, Peppas G. Informed consent: how much and what do patients understand? Am J Surg. 2009;198:420–435. doi: 10.1016/j.amjsurg.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 64.Sherlock A, Brownie S. Patients’ recollection and understanding of informed consent: a literature review. ANZ J Surg. 2014;84:207–210. doi: 10.1111/ans.12555. [DOI] [PubMed] [Google Scholar]
- 65.United States Renal Data System. 2018 USRDS annual data report. Epidemiology of kidney disease in the United States. Bethesda: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2018. .
- 66.Moist LM, Fenton S, Kim JS, et al. Canadian Organ Replacement Register (CORR): reflecting the past and embracing the future. Can J Kidney Health Dis. 2014;1:26. doi: 10.1186/s40697-014-0026-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liyanage T, Ninomiya T, Jha V, et al. Worldwide access to treatment for end-stage kidney disease: a systematic review. Lancet. 2015;385:1975–1982. doi: 10.1016/S0140-6736(14)61601-9. [DOI] [PubMed] [Google Scholar]
- 68.Green JA, Mor MK, Shields AM, et al. Associations of health literacy with dialysis adherence and health resource utilization in patients receiving maintenance hemodialysis. Am J Kidney Dis. 2013;62:73–80. doi: 10.1053/j.ajkd.2012.12.014. [DOI] [PubMed] [Google Scholar]
- 69.Varnell SP, Murray DM, Baker WL. An evaluation of analysis options for the one-group-per-condition design. Can any of the alternatives overcome the problems inherent in this design? Eval Rev. 2001;25:440–453. doi: 10.1177/0193841X0102500402. [DOI] [PubMed] [Google Scholar]
- 70.Taljaard M, Teerenstra S, Ivers NM, Fergusson DA. Substantial risks associated with few clusters in cluster randomized and stepped wedge designs. Clin Trials (London, England) 2016;13:459–463. doi: 10.1177/1740774516634316. [DOI] [PubMed] [Google Scholar]
- 71.Canadian Institutes for Health Research, Natural Sciences and Engineering Research Council of Canada, Social Sciences and Humanities Research Council of Canada. Tri-Council Policy Statement. Ottawa: Ethical Conduct for Research Involving Humans; 2014.
- 72.International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH). Geneva: Guideline For Good Clinical Practice E6(R2); 2014.
- 73.HiLo | A Pragmatic Clinical Trial. Available from: https://hilostudy.org/. Cited 2020 Jan 20.
- 74.Outcomes of a higher versus lower hemodialysate magnesium concentration. Available from: https://clinicaltrials.gov/ct2/show/NCT04079582. Cited 2020 Jan 20.
- 75.Taljaard M, Goldstein CE, Giraudeau B, et al. Cluster over individual randomization: are study design choices appropriately justified? Review of a random sample of trials. Clin Trials. 2020;17(3):253–63. [DOI] [PubMed]
- 76.Ivers NM, Taljaard M, Dixon S, et al. Impact of CONSORT extension for cluster randomised trials on quality of reporting and study methodology: review of random sample of 300 trials, 2000-8. BMJ. 2011;343:d5886. doi: 10.1136/bmj.d5886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Taljaard M, Hemming K, Shah L, Giraudeau B, Grimshaw JM, Weijer C. Inadequacy of ethical conduct and reporting of stepped wedge cluster randomized trials: Results from a systematic review. Clin Trials (London, England) 2017;14:333–341. doi: 10.1177/1740774517703057. [DOI] [PubMed] [Google Scholar]
- 78.Taljaard M, McRae AD, Weijer C, et al. Inadequate reporting of research ethics review and informed consent in cluster randomised trials: review of random sample of published trials. BMJ (Clinical research ed.) 2011;342:d2496. doi: 10.1136/bmj.d2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Appendix 1. Recommendations from the Ottawa Statement. Appendix 2. PRISMA Checklist. Appendix 3. Search syntax to identify relevant articles in Medline between January 1st, 2000 and July 20th, 2018 in Embase Classic+Embase, Ovid MEDLINE(R) Epub Ahead of Print, In-Process & Other Non-Indexed Citations, Ovid MEDLINE(R) Daily and Ovid MEDLINE(R). Appendix 4. Extracted data. Appendix 5. Reported information about waiver of consent for the four studies that reported a waiver of informed patient consent or research ethic committee exemption.
Data Availability Statement
Study data can be made available upon request.