Abstract
BACKGROUND/OBJECTIVE:
Uncertainty regarding benefits and risks associated with acetylcholinesterase inhibitors (AChEIs) in severe dementia means providers do not know if and when to deprescribe. We sought to identify which patient, provider, and system-level characteristics are associated with AChEI discontinuation.
DESIGN:
Analysis of 2015–16 data from Medicare claims, Part D prescriptions, Minimum Data Set v3.0, Area Health Resource File, and Nursing Home Compare. Cox-proportional hazards models with time-varying covariates were used to identify patient, provider, and system-level factors associated with AChEI discontinuation (≥30-day gap in supply).
SETTING:
U.S. Medicare certified nursing homes (NHs).
PARTICIPANTS:
Non-skilled NH residents aged 65+ with severe dementia receiving AChEIs within the first 14 days of an MDS assessment in 2016 (n=37,106).
RESULTS:
The sample was primarily white (78.7%), female (75.5%), and ≥80 years old (77.4%). The most commonly prescribed AChEIs were donepezil (77.8%), followed by transdermal rivastigmine (14.6%). The cumulative incidence of AChEI discontinuation was 29.7% at the end of follow-up (330 days), with mean follow-up times of 194 days for continuous users of AChEIs and 105 days for those who discontinued. Factors associated with increased likelihood of discontinuation were new admission, older age, difficulty being understood, aggressive behavior, poor appetite, weight loss, mechanically altered diet, limited prognosis designation, hospitalization in 90 days prior, and northeastern region. Factors associated with decreased likelihood of discontinuation included memantine use, use of strong anticholinergics, polypharmacy, rurality, and primary care prescriber vs. geriatric specialist.
CONCLUSION:
Among nursing home residents with severe dementia being treated with AChEIs, the cumulative incidence of AChEI discontinuation was just under 30% at 1-year of follow-up. Our findings provide insight into potential drivers of deprescribing AChEIs, identify system-level barriers to deprescribing, and help to inform covariates that are needed to address potential confounding in studies evaluating the potential risks and benefits associated with deprescribing.
Keywords: Dementia, Nursing Home, Cholinesterase Inhibitors, Deprescribing, Medicare
INTRODUCTION
The use of acetylcholinesterase inhibitors (AChEIs) in severe dementia is controversial. Although there are numerous efficacy studies of these agents, a limited number include patients with severe dementia, with variable findings.1–7 Most studies show minor improvements in cognition1–5,7, but few have demonstrated benefit for activities of daily living (ADLs) or behavioral and psychological symptoms of dementia (BPSD).8,9 There is also uncertainty regarding long-term efficacy, as few studies were conducted for longer than 6 months to one year.10
Deprescribing, the intentional and supervised reduction or discontinuation of medications in the setting of limited benefit11,12, may be warranted when reconsidering the appropriateness of AChEIs in severe dementia. There are a limited number of studies examining the effect of deprescribing AChEIs on functional, behavioral, or cognitive outcomes in severe dementia.13–15 However, these are limited by small samples, inadequate control groups, and questionable generalizability. In the absence of data on risks versus benefits of AChEIs in severe dementia, it is not surprising that a recent systematic review of clinical guidelines found inconsistency in recommendations for deprescribing AChEIs.16 Only three of 16 guidelines recommended deprescribing AChEIs (specifically complete discontinuation) in patients with severe dementia, with most others deferring to clinical judgement and family preferences.16 Qualitative interviews with prescribers echo this uncertainty.17–19
Quantitative studies of patients with varying dementia severity have found that AChEI discontinuation is driven by multiple factors, including demographics, health-related characteristics, medication use, setting, and provider specialty.20–34 Although several studies have found that patients with greater dementia severity are more likely to discontinue AChEIs21,26,31,33, none examined predictors of discontinuation within this subgroup. In addition, only three studies were conducted among nursing home (NH) residents.24,26,35 As a result, little is known about the frequency of AChEI discontinuation in NH residents with severe dementia, or associated factors.
This study sought to identify predictors of AChEIs discontinuation in a national sample of older NH residents with severe dementia and will inform covariates for future studies of the downstream effects of deprescribing.
METHODS
Design & Data Sources.
This was a retrospective analysis of linked data from Medicare enrollment, Part A and B claims, Master Beneficiary Summary File (MBSF), Part D prescriptions, the Minimum Data Set (MDS) version 3.0, the Area Health Resource File (AHRF), and Nursing Home Compare (NHC) for 2015–2016. The University of Pittsburgh Institutional Review Board (IRB) deemed this study exempt.
Data originated from a random sample of 1 million Medicare beneficiaries age 65 and older with continuous enrollment in Medicare Parts A, B, and D in 2015 and a dementia diagnosis prior to 2016 based on the Chronic Conditions Warehouse algorithm for identifying Alzheimer’s Disease or Related Disorders with International Classification of Diseases (ICD) codes.36 The MDS, a comprehensive health assessment tool administered to residents of CMS-certified NHs at admission and at least every 90 days thereafter, served as the primary source of variables. The Medicare MBSF and Part A/B claims were used to identify comorbidities, inpatient and outpatient healthcare utilization in the year prior, and death date. Medicare Part D data provided information on prescriptions dispensed in outpatient and long-term care settings, including drug name, National Drug Code (NDC), date filled, dose, strength, quantity, days’ supply, and prescriber characteristics. The NHC and AHRF provided facility characteristics.37,38
Sample.
The final cohort consisted of non-skilled nursing stays for patients with severe dementia receiving AChEIs at index (Figure 1). Skilled NH stays require more advanced care, including intravenous medications and physical therapy, compared to non-skilled stays. Skilled stays are also covered by Medicare Part A and thus medication data are not available. We used the MDS reason for assessment fields (A0310A, A0310B) to identify all MDS assessments for non-skilled NH stays,39,40 beginning in 2016. Nursing home episodes (n=335,487) were constructed by matching the first assessment in 2016 to the closest discharge form or assigning the end of the study period (12/31/2016) as the end date. We required that residents had continuous enrollment in Medicare Parts A, B, and D for the duration of all episodes and the year prior (n=14,388, 4.3% excluded). Episodes in which the resident had any Medicare skilled nursing facility claims overlapping the index date to episode end date were excluded (n=52,390, 16.3% excluded). Episodes in which residents had severe dementia were identified using cognitive assessments in the MDS (n=149,727, 55.6%). Specifically, the Brief Interview for Mental Status (BIMS)41 was used, or if unable to complete the BIMS, the Cognitive Performance Scale (CPS) was used.42 We used a BIMS ≤7 or CPS ≥4 to identify severe dementia, which have demonstrated acceptable sensitivity and specificity for severe cognitive impairment.41 We then limited to residents receiving AChEIs at index by searching Part D records for generic drug names (donepezil, rivastigmine, galantamine). Residents were considered treated if there was a prescription for an AChEI with an estimated days’ supply overlapping one of the initial 14 days of the episode (n=43,996, 29.4%). The first day in which AChEI supply was observed was assigned as the AChEI index date. We excluded episodes with ≤30 days of follow-up to allow time to observe potential discontinuation (n=4,158, 9.5% excluded). Finally, if residents had more than one episode meeting criteria, only the first was included (n=2,729, 6.9% excluded).
Figure 1.
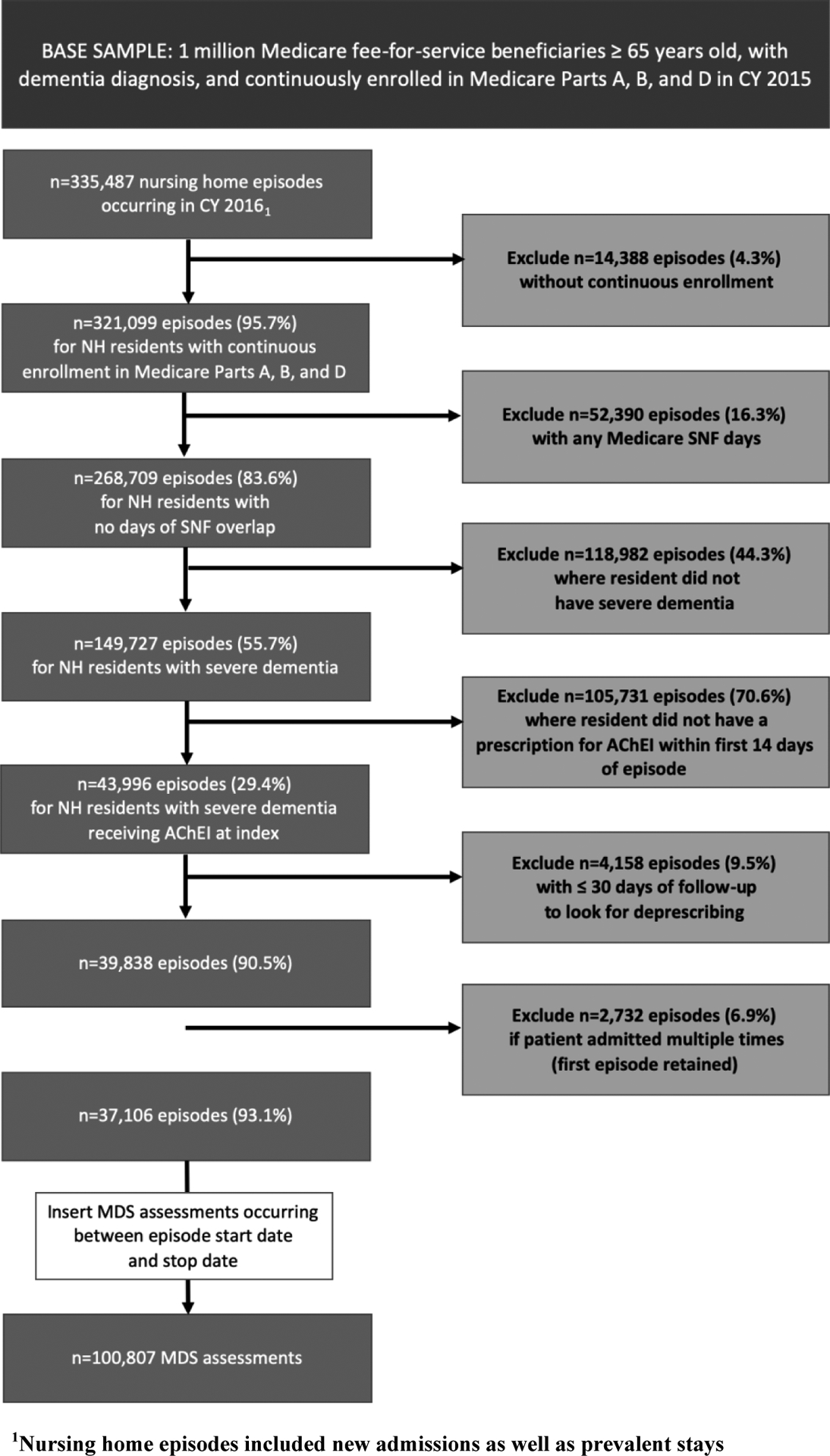
Cohort Construction
A longitudinal dataset was created in which each resident could have multiple MDS assessments from episode start until deprescribing or censoring. The final cohort included 37,106 residents contributing 100,807 MDS assessments.
Dependent Variable.
Discontinuation of AChEIs was defined as a gap in supply ≥30 days based on prescription fill dates and days’ supply, with the 1st gap day serving as the discontinuation date. For example, if a patient was issued a prescription on 01/01/2015 with a 28-day supply, this had an end date of 01/28/2015. If no subsequent fill was observed for any AChEI within the next 30 days, this was considered deprescribing, with a discontinuation date of 1/29/2015. A 30-day gap was chosen based on prior research in community-dwelling and NH residents.43,44
Independent Variables.
A conceptual model guided selection of covariates based on prior studies20–34 and behavioral theories applied to deprescribing.45–47 We identified four categories of covariates hypothesized to influence deprescribing: demographics, clinical assessment, environment of care, and provider specialty.
Demographics were captured as time-invariant covariates using the index MDS assessment. Clinical assessment factors were included as time-varying covariates measured at each MDS assessment. The type of MDS form (admission, quarterly, annual, significant change in status) was included as a measure of the trajectory of the resident’s stay. We included a scale rating ability to be understood to capture variation in cognitive ability.41 We created indicators for specific active conditions noted on the MDS that may impact deprescribing.48 These included indicators of poor prognosis (poor appetite, swallowing disorder, parenteral nutrition or tube feeds, mechanically altered diet, weight loss, shortness of breath, dehydration, cancer, end-stage renal disease, heart failure) or conditions that would be aggravated by AChEIs (urinary incontinence). Physical function was measured using a scale based on MDS items for ADLs.49 Aggression was measured using the Aggressive Behavior Scale (ABS)50, ranging from 0–12 and categorized as none (0), moderate (1–2), severe (3–5) or very severe (6+). Depression severity was assessed using the Patient Health Questionnaire (PHQ-9).51 Finally, we created an indicator for limited prognosis, based on the MDS item (J1400) for <6 months life expectancy, which has good accuracy for 6-month mortality48, and/or the MDS item (O0100K) for hospice use in the last 14 days.
Medications used to treat other conditions may influence deprescribing of AChEIs, because they may indicate the presence of psychiatric symptoms that could worsen after deprescribing. We used Part D records to create time-varying indicators for use of antidepressants, antipsychotics, and benzodiazepines on the first day of each assessment. We captured use of strongly anticholinergic medications from the Beers Criteria52, which may worsen cognition. We also counted the number of non-AChEI medications. Finally, we captured memantine use, and the index AChEI (donepezil, rivastigmine, or galantamine).
Healthcare utilization and comorbidities were measured as time-varying, using claims. We calculated the Charlson Comorbidity Index (CCI)53 using ICD-9 and ICD-10 codes from the prior year. We identified whether residents had experienced cause-specific hospitalizations for syncope and falls or fractures as well as other cause hospitalizations in the 90 days prior to each MDS assessment. We also identified location prior to NH residence.
Environment of care factors were captured as time-invariant and included geographic region, facility size, and rurality. NHC data37 provided facility size and ZIP codes to define rurality (urban, rural, highly rural) by linking to rural-urban continuum codes in the AHRF.38 Finally, we captured the Medicare provider specialty of the prescriber of the index AChEI prescription (primary care, geriatrics, or other) using taxonomy codes from the Part D Prescriber Characteristics file.54
Statistical Analyses.
We described demographics, environment of care factors and provider specialty at the index MDS assessment, and time-varying clinical factors at index and across all assessments. Missing observations (<5% total) were addressed with single imputation using chained equations.55
Associations of time to AChEI discontinuation with all covariates were evaluated using Cox proportional hazards models. Residents were followed from index date to discontinuation or censoring (death, NH discharge, end of study period). Bi-variable models were estimated to determine unadjusted associations, followed by multivariable models. The proportional hazards assumption was evaluated using Schoenfeld residuals. Variance inflation factors were used to evaluate potential collinearity. We used robust standard errors to address facility-level clustering.
Two sensitivity analyses were conducted: 1) analyses stratified by documentation of limited prognosis to determine whether associated factors were robust in residents recognized as end-of-life; and 2) using an alternative measure of discontinuation that required a 60-day gap in medication supply, to account for potential measurement error. Analyses were performed using SAS version 9.4 (SAS Institute Inc, Cary, NC) and STATA 15 (College Station, TX).
RESULTS
Sample Characteristics.
Table 1 presents time invariant characteristics measured at index (n=37,106) and Table 2 presents assessment-level clinical characteristics for all MDS assessments (n=100,807). At index, over 75% were ≥80 years old, primarily female (75.5%), and white (78.7%). Most residents were not married (77.9%), entered the NH following hospitalization (68.1%), and were not newly admitted (i.e., prevalent stays; 87.7%).
Table 1.
Time-invariant Baseline Characteristics of Elderly Nursing Home Residents with Severe Dementia Receiving AChEIs
| Variable | Baseline Characteristics n (%) N=37,106 episodes |
|---|---|
| DEMOGRAPHICS | |
| Age in years | |
| 65–69 | 901 (2.4) |
| 70–79 | 7,496 (20.2) |
| 80–89 | 17,922 (48.3) |
| 90+ | 10,787 (29.1) |
| Sex | |
| Male | 9,092 (24.5) |
| Female | 28,014 (75.5) |
| Race/ethnicity | |
| White | 29,210 (78.7) |
| Black | 4,405 (11.9) |
| Hispanic | 2,049 (5.5) |
| Other | 1,442 (3.9) |
| Current marital status | |
| Married | 8,176 (22.0) |
| Not Married | 28,930 (78.0) |
| Entered from | |
| Community | 7,575 (20.4) |
| Hospital | 25,275 (68.1) |
| NH or other LTC facility | 4,256 (11.5) |
| ENVIRONMENT OF CARE | |
| Geographic region | |
| Midwest | 10,108 (27.2) |
| Northeast | 6,737 (17.9) |
| South | 17,286 (46.6) |
| West | 3,075 (8.3) |
| Certified beds | |
| <50 | 1,899 (5.1) |
| 50–99 | 10,617 (28.6) |
| 100–199 | 20,339 (54.8) |
| 200+ | 4,252 (11.5) |
| Rural/urban continuum | |
| Urban | 25,719 (69.3) |
| Rural | 9,910 (26.7) |
| Highly rural | 1,477 (4.0) |
| PROVIDER SPECIALTY | |
| Prescriber specialty | |
| Geriatrics | 3,168 (8.5) |
| Primary care | 29,972 (80.8) |
| Other | 3,966 (10.7) |
Note: Missing data was imputed using chained equations. All item-level missingness was <5%.
Table 2.
Time-varying Clinical Characteristics of Elderly Nursing Home Residents with Severe Dementia Receiving AChEIs
| Variable | Baseline Characteristics n (%) N=37,106 episodes | Time-varying Characteristics n (%) N=100,807 assessments |
|---|---|---|
| MDS Assessment Type | ||
| Admission | 4,576 (12.3) | 4,576 (4.5) |
| Quarterly | 24,434 (65.9) | 71,976 (71.4) |
| Annual | 6,001 (16.2) | 18,387 (18.2) |
| Significant Change in Status | 2,095 (5.7) | 5,868 (5.8) |
| Charlson Comorbidity Index | ||
| 0–1 | 6,001 (16.3) | 15,514 (15.4) |
| 2–3 | 9,101 (24.7) | 25,761 (25.6) |
| 4–5 | 8,659 (23.5) | 24,614 (24.4) |
| ≥6 | 13,127 (35.6) | 34,918 (34.6) |
| Makes self understood | ||
| Understood | 14,719 (39.7) | 38,964 (38.7) |
| Usually understood | 9,961 (26.8) | 27,333 (27.1) |
| Sometimes understood | 8,086 (21.8) | 21,830 (21.7) |
| Rarely/never understood | 4,340 (11.7) | 12,680 (12.6) |
| PHQ-9 score | ||
| Minimal | 30,155 (81.3) | 82,783 (82.1) |
| Mild | 4,808 (13.0) | 12,364 (12.3) |
| Moderate | 1,623 (4.4) | 4,338 (4.3) |
| Moderate-severe or severe | 520 (1.4) | 1,322 (1.3) |
| Aggressive behavior scale | ||
| None | 28,391 (76.5) | 78,048 (77.4) |
| Moderate | 5,741 (15.5) | 15,178 (15.1) |
| Severe | 2,335 (6.3) | 6,024 (6.0) |
| Very severe | 639 (1.7) | 1,557 (1.5) |
| Activities of Daily Living Score | ||
| 1 to 7 | 2,849 (7.7) | 7709 (7.7) |
| 8 to 14 | 6,033 (16.3) | 15,917 (15.8) |
| 15 to 21 | 19,426 (52.4) | 52,903 (52.5) |
| 22 to 28 | 8,798 (23.7) | 24,278 (24.1) |
| Urinary incontinence | ||
| Continent | 3,954 (10.7) | 9,893 (9.8) |
| Occasionally incontinent | 5,439 (14.7) | 13,988 (13.9) |
| Frequently incontinent | 12,281 (33.1) | 33,382 (33.1) |
| Always incontinent | 14,644 (39.5) | 41,899 (41.6) |
| Indwelling catheter | 788 (2.1) | 1,645 (1.6) |
| Cancer | 1,557 (4.2) | 4,081 (4.1) |
| Heart failure | 5,808 (15.7) | 15,490 (15.4) |
| End Stage Renal Disease | 3,433 (9.3) | 9,140 (9.1) |
| Short of breath | 2,469 (6.7) | 6,412 (6.4) |
| Poor appetite | 4,946 (13.3) | 12,811 (12.7) |
| Weight loss | 2,392 (6.5) | 5,891 (5.8) |
| Swallowing difficulty | 1,247 (3.4) | 3,266 (3.2) |
| Mechanically altered diet | 16,780 (45.2) | 47,426 (47.1) |
| IV/parenteral nutrition or feeding tube | 1,262 (3.4) | 2,892 (2.9) |
| Limited Prognosis | 1,875 (5.1) | 4,623 (4.6) |
| Hospitalizations/ED Visits (90 days prior) | ||
| None | 27,937 (75.3) | 86,397 (85.7) |
| Cause-specific (fall, fracture, syncope) | 3,193 (8.6) | 5,560 (5.5) |
| Other cause | 5,976 (16.1) | 8,850 (8.8) |
| AChEI at index date | ||
| Donepezil | 28,877 (77.8) | 77,772 (77.2) |
| Donepezil/memantine | 483 (1.3) | 1,926 (1.9) |
| Galantamine | 832 (2.2) | 2,280 (2.3) |
| Rivastigmine (oral) | 1,511 (4.1) | 4,230 (4.2) |
| Rivastigmine (transdermal) | 5,403 (14.6) | 14,599 (14.5) |
| Memantine use | 15,199 (41.0) | 42,692 (42.3) |
| Benzodiazepine and/or Z drug | 5,505 (14.8) | 14,282 (14.2) |
| Antipsychotic use | 9,128 (24.6) | 23,039 (22.9) |
| Antidepressant use | 21,310 (57.4) | 57,578 (57.1) |
| Highly Anticholinergic Drugs | 5,519 (14.9) | 14,179 (14.1) |
| Total number of medications | ||
| 0 to 5 | 17,798 (48.0) | 49,798 (49.4) |
| 6 to 10 | 16,183 (43.6) | 43,261 (42.9) |
| >10 | 3,125 (8.4) | 7,868 (7.7) |
In total, 20.4% of the sample had their AChEI deprescribed, whereas the remainder were censored due to death (8.8%), discharge (23.7%), or end of follow-up (47.2%). The median follow-up time for continuous users of AChEIs was 242 days (IQR=83–290) and 82 days (IQR=30–165) for those who were deprescribed. Figure 2 shows the cumulative incidence of discontinuation, which was 29.7% at day 330 (end of follow-up).
Figure 2.
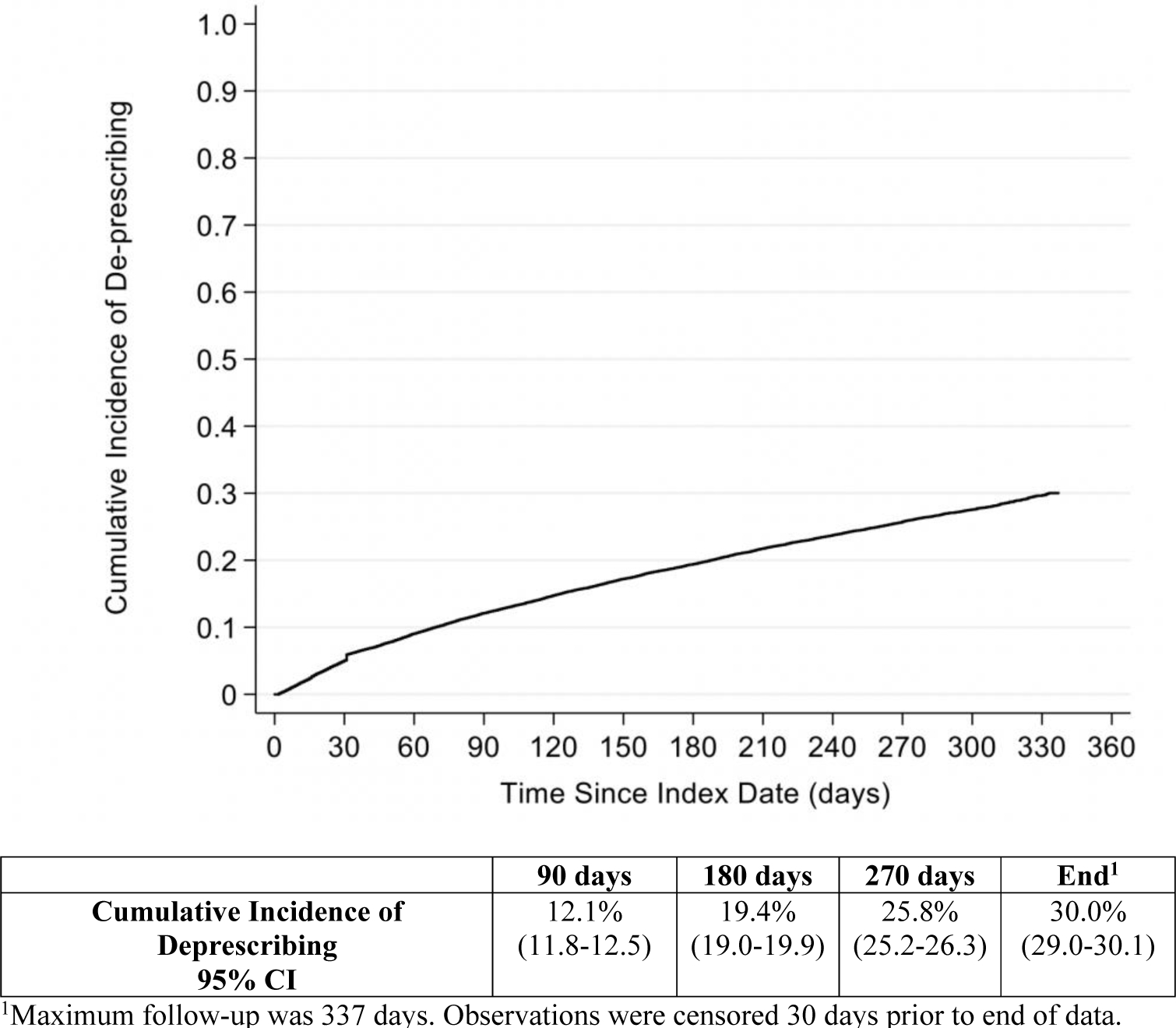
Cumulative Incidence of Deprescribing During Observation Period
Table 3 presents statistically significant results from Cox models (full results presented in Supplementary Table S1). In unadjusted and adjusted analyses, age ≥70 versus 65–69 was associated with increased likelihood of discontinuation (70–79yo aHR=1.21, 95% CI [1.02–1.43]; 80–89yo aHR=1.20, 95% CI [1.02–1.41]; 90+yo aHR=1.26, 95% CI [1.06–1.48]). Clinical factors associated with increased likelihood for discontinuation in unadjusted and adjusted models were: being understood only sometimes (aHR=1.13, 95% CI [1.05–1.21]) or rarely (aHR=1.22, 95% CI [1.11–1.34]); moderate (aHR=1.11, 95% CI [1.04–1.18]) or severe (aHR=1.24, 95% CI [1.15–1.37]) aggressive behavior; ADL impairment (ADL scale 15–21 aHR=1.26, 95% CI [1.10–1.44]; ADL scale 22–28 aHR=1.41, 95% CI [1.22–1.63]); poor appetite (aHR=1.20, 95% CI [1.11–1.28]); recent weight loss (aHR=1.31, 95% CI [1.21–1.41]); mechanically altered diet (aHR=1.07, 95% CI [1.02–1.13]); limited prognosis (aHR=3.92, 95% CI [3.65–4.20]); and cause-specific (aHR=1.28, 95% CI [1.17–1.40]) or other cause hospitalization (aHR=1.25, 95% CI [1.16–1.35]) in the prior 90 days. Clinical factors associated with decreased likelihood for discontinuation were use of combination donepezil/memantine as AChEI therapy versus donepezil alone (aHR=0.65, 95% CI [0.52–0.82]); memantine use (aHR=0.87, 95% CI [0.82–0.91]); strong anticholinergic use (aHR=0.92, 95% CI [0.86–0.99]); use of >5 concurrent medications (6 to 10 medications aHR=0.89, 95% CI [0.84–0.93]; >10 medications aHR=0.79, 95% CI [0.71–0.87]). Discontinuation was also less likely following quarterly or annual assessments versus admission (aHR=0.39, 95% CI [0.36–0.43]; aHR=0.36, 95% CI [0.33–0.40], respectively).
Table 3.
Unadjusted and Adjusted Hazards Ratios for Significant AssociationsTable 3. Unadjusted and Adjusted Hazards Ratios for Significant Associations
| Variable | Unadjusted Hazard Ratio [CI] | Adjusted Hazard Ratio [CI] |
|---|---|---|
| Age in years | ||
| 65–69 | ref | ref |
| 70–79 | 1.20 (1.01–1.41)* | 1.21 (1.02–1.42)* |
| 80–89 | 1.19 (1.02–1.41)* | 1.20 (1.02–1.41)* |
| 90+ | 1.32 (1.11–1.56)^ | 1.25 (1.06–1.48)+ |
| Assessment Form Type | ||
| Admission | ref | ref |
| Quarterly | 0.38 (0.35–0.42)^ | 0.39 (0.35–0.43)^ |
| Annual | 0.35 (0.31–0.38)^ | 0.36 (0.33–0.41)^ |
| Significant Change in Status | 1.77 (1.61–1.95)^ | 0.93 (0.84–1.04) |
| Makes self understood | ||
| Understood | ref | ref |
| Usually understood | 1.15 (1.08–1.22)^ | 1.06 (0.99–1.13) |
| Sometimes understood | 1.42 (1.33–1.50)^ | 1.14 (1.07–1.22)^ |
| Rarely/never understood | 1.69 (1.58–1.81)^ | 1.24 (1.14–1.34)^ |
| PHQ-9 score | ||
| Minimal | ref | ref |
| Mild | 1.24 (1.16–1.32)^ | 0.98 (0.92–1.05) |
| Moderate | 1.50 (1.35–1.66)^ | 1.11 (0.99–1.23) |
| Mod. severe or severe | 1.78 (1.50–2.11)^ | 1.06 (0.88–1.27) |
| Aggressive behavior scale | ||
| None | ref | ref |
| Moderate | 1.14 (1.07–1.21)^ | 1.12 (1.05–1.19)^ |
| Severe | 1.37 (1.23–1.49)^ | 1.26 (1.15–1.38)^ |
| Very severe | 1.35 (1.14–1.59)^ | 1.15 (0.97–1.36) |
| Activities of Daily Living Scale | ||
| 0 to 7 | ref | ref |
| 8 to 14 | 1.27 (1.13–1.45)^ | 1.13 (0.99–1.28) |
| 15 to 21 | 1.75 (1.57–1.96)^ | 1.26 (1.10–1.44)^ |
| 22 to 28 | 2.45 (2.19–2.75)^ | 1.41 (1.22–1.63)^ |
| Urinary incontinence | ||
| Continent | ref | ref |
| Occasionally incontinent | 1.14 (1.02–1.27)+ | 0.98 (0.88–1.10) |
| Frequently incontinent | 1.38 (1.25–1.52)^ | 1.03 (0.92–1.15) |
| Always incontinent | 1.75 (1.60–1.92)^ | 1.09 (0.97–1.22) |
| Indwelling catheter | 2.09 (1.74–2.51)^ | 1.02 (0.84–1.24) |
| Poor appetite | 1.65 (1.56–1.75)^ | 1.20 (1.12–1.29)^ |
| Weight loss | 2.29 (2.13–2.46)^ | 1.31 (1.21–1.42)^ |
| Swallowing difficulty | 1.56 (1.39–1.74)^ | 1.09 (0.98–1.22) |
| Mechanically altered diet | 1.32 (1.26–1.38)^ | 1.08 (1.02–1.13)+ |
| IV/parenteral nutrition or feeding tube | 1.29 (1.13–1.45)^ | 1.02 (0.89–1.18) |
| Limited Prognosis | 6.88 (6.43–7.36)^ | 3.91 (3.64–4.19)^ |
| Hospitalizations/ED Visits (90 days prior) | ||
| Cause-specific (fall, fracture, syncope) | 1.61 (1.48–1.75)^ | 1.28 (1.17–1.39)^ |
| Other cause | 1.55 (1.45–1.67)^ | 1.25 (1.16–1.35)^ |
| AChEI Use at Index | ||
| Donepezil | ref | ref |
| Donepezil/memantine | 0.61 (0.49–0.77)^ | 0.65 (0.52–0.83)^ |
| Galantamine | 0.90 (0.77–1.06) | 0.85 (0.72–1.00) |
| Rivastigmine (oral) | 1.00 (0.89–1.12) | 1.00 (0.89–1.13) |
| Rivastigmine (transdermal) | 1.05 (0.99–.12) | 0.97 (0.90–1.03) |
| Memantine | 0.78 (0.74–0.81)^ | 0.87 (0.83–0.91)^ |
| Antidepressant | 0.94 (0.90–0.99)* | 1.01 (0.97–1.07) |
| Highly Anticholinergic Drugs | 0.88 (0.83–0.95)^ | 0.93 (0.87–0.99)* |
| Total number of medications | ||
| 0 to 5 | ref | ref |
| 6 to 10 | 0.83 (0.79–0.87)^ | 0.89 (0.85–0.94)^ |
| >10 | 0.75 (0.68–0.82)^ | 0.79 (0.72–0.88)^ |
| Geographic region | ||
| Midwest | ref | ref |
| Northeast | 1.20 (1.11–1.29)^ | 1.12 (1.03–1.22)+ |
| South | 1.15 (1.08–1.22)^ | 1.07 (0.99–1.14) |
| West | 0.91 (0.81–1.01) | 0.85 (0.76–0.96)+ |
| Rural/urban continuum | ||
| Urban | ref | ref |
| Rural | 0.79 (0.74–0.83)^ | 0.82 (0.77–0.87)^ |
| Highly rural | 0.69 (0.60–0.80)^ | 0.76 (0.65–0.89)^ |
| Prescriber specialty | ||
| Geriatrics | ref | ref |
| Primary care | 0.85 (0.78–.92)^ | 0.90 (0.83–0.98)* |
| Other | 0.91 (0.82–1.01) | 0.94 (0.84–1.04) |
p<0.05;
p<0.01;
p<0.001
Regional characteristics exhibited significant relationships with discontinuation. Residing in a facility in the West versus the Midwest (aHR=0.86, 95% CI [0.76–0.96]) and in a rural (aHR=0.82, 95% CI [0.76–0.87]) or highly rural facility (aHR=0.77, 95% CI [0.66–0.89]) versus an urban facility, were associated with decreased likelihood of discontinuation. In addition, having an AChEI prescriber with a primary care specialty (vs. geriatrics) was associated with decreased likelihood of discontinuation (aHR=0.91, 95% CI [0.83–0.99]).
Supplementary Tables S2 and S3 show results of the sensitivity analysis stratifying by documentation of limited prognosis. Among patients without documentation of limited prognosis, findings were similar to the primary analysis. However, in patients with documented limited prognosis, many factors originally associated with discontinuation of AChEIs were no longer significant. The direction of association changed for several variables, but none were statistically significant. Supplementary Figure S1 shows the cumulative incidence curves for time to discontinuation, stratified by limited prognosis.
In the sensitivity analysis using a 60-day gap in AChEI supply to define discontinuation, adjusted associations remained substantively unchanged (Supplementary Table S4). Several additional factors became statistically significantly associated with discontinuation, including female sex, black race, significant change in status assessment, and facility size 50–99 beds and 100–199 beds. However, their point estimates changed less than 12%.
DISCUSSION
This is the first national study of deprescribing AChEIs in U.S. Medicare NH residents with severe dementia. We found a cumulative incidence of discontinuation of 30% over approximately one year of follow-up. In a time-to-event analysis, discontinuation was driven by several important clinical characteristics, as well as a few facility- and prescriber-level factors.
There are a number of strengths to our analysis that set it apart from existing literature. We studied a large, national sample of U.S. NH residents with severe dementia using a data source with hundreds of clinical variables. Inclusion of prevalent stays and newly admitted NH residents yielded a larger and more generalizable sample, thereby increasing statistical power. By limiting to residents with severe dementia, our study is the first to identify predictors of discontinuation in patients for whom treatment recommendations are most controversial. In addition, utilization of a time-to-event analysis with time-varying covariates also strengthened our ability to identify associations between fluctuating clinical factors and discontinuation. A recent by Maclagan et al. also examined predictors of AChEI discontinuation in NH residents in Canada.34 In addition to country, this study differed from ours in their inclusion of all dementia severities, restriction to newly admitted residents, and earlier years of data (2011–2015). Overall, however, the magnitude and direction of the associations in our study align with the Maclagan study, with a few exceptions. Maclagan et al. found that older residents were less likely to be deprescribed, contradicting the findings of our analysis and others.20,22,25,29,32 We also identified several factors associated with discontinuation that were not associated with discontinuation in the Maclagan study, including markers of poor prognosis, polypharmacy, strong anticholinergics, and rurality.
The strongest predictor of AChEI discontinuation in this study was explicit MDS documentation of limited prognosis. Residents with severe dementia who had less than 6-months life expectancy or hospice use had almost 4 times the likelihood of discontinuation compared to other residents. This is expected, as de-escalation of care, including medications, is an integral part of hospice. Sensitivity analyses demonstrated that although the effect of many of the patient-, system-, and provider-level factors on discontinuation was eclipsed by limited prognosis status, they remained significantly associated in other residents.
Many clinical factors associated with increased likelihood of discontinuation have previously been identified as surrogate markers for decline in clinical status (e.g. poor appetite, recent weight loss).48 This aligns with current, albeit limited, recommendations to reconsider these agents in this population.16 We also found that discontinuation was more likely to occur during observation periods identified by MDS admission assessments, rather than routine care (i.e. quarterly or annual assessments). Evaluations at the time of transfer to a new care setting are likely more comprehensive, and thus medications may be scrutinized more closely for appropriateness. This points out a potential area for intervention, as medications should be regularly re-evaluated for appropriateness and not just at the time of care transition.
Interestingly, the presence of polypharmacy and use of strongly anticholinergic medications were associated with decreased likelihood of discontinuation. Our findings suggest that these may act as surrogates of poor prescribing that carry over into reduced deprescribing of medications with questionable benefit. It is also possible that medication use may represent greater comorbidity burden or more conservative deprescribing driven by family preferences.17–19
Important facility- and prescriber-level factors were associated with a decreased likelihood of discontinuation; for example, residence in a NH in a rural or highly rural region was associated with decreased likelihood for discontinuation as compared to urban regions. This may represent proximity to an academic medical center where initiatives that promote deprescribing may be more common. Residents prescribed AChEIs by a primary care physician versus geriatrician were less likely to be deprescribed. Geriatricians are likely more attuned to medication-related issues, especially deprescribing, given the increased risk for adverse effects in the setting of advanced age.56 These system-level factors may be seen as targets for educational interventions to improve uptake and implementation of deprescribing.
Our study has several limitations. One of the main limitations of this analysis is that we were unable to identify the intent behind gaps in medication supply. Thus, while a number of these gaps may have been the result of intentional deprescribing, it is also possible that human error, systems error, or withheld doses contributed to gaps in medication. Most prior studies examining patterns of AChEI use refer to gaps in therapy as either discontinuation or non-persistence, avoiding the term deprescribing because of the inability to establish intent.20–23,25–30,32,34 However, in a nursing home setting, it may be more likely that sustained therapy gaps greater than 30 or 60 days are intentional. The optimal gap in days’ supply for identifying intentional AChEI discontinuation using Part D data is unknown. Although sensitivity analyses using a 60-day gap in medication supply revealed no significant differences, the incidence of deprescribing dropped considerably (30.0% vs. 18.3%). Given the potential for medication withdrawal syndromes and worsening behavioral symptoms, prescribers may opt for deprescribing by dosage reduction, which we may not have captured. Future studies should examine different definitions for deprescribing using data sources that contain greater detail (e.g. medication administration data).
CONCLUSIONS:
This study found a cumulative incidence of deprescribing of AChEIs just under 30% over a one-year period among older NH residents with severe dementia. A number of clinical factors corresponding to limited prognosis or deteriorating clinical status were associated with increased likelihood for deprescribing. However, several system-level factors that may act as barriers to implementation were also associated with deprescribing. Future studies examining downstream effects of deprescribing should account for these potential confounders.
Supplementary Material
Figure S1. Cumulative Incidence of Deprescribing AChEIs Stratified by Limited Prognosis Designation
Table S1. Complete Results for Unadjusted and Adjusted Hazard Ratios for Primary Analysis
Table S2. Sample Characteristics for Sub-group Analysis at Index Assessment
Table S3. Adjusted Hazards Ratios for Sub-group Analysis of Residents with and without Limited Prognosis
Table S4. Unadjusted and Adjusted Hazards Ratios for Sensitivity Analysis using 60-day gap
IMPACT STATEMENT:
We certify that the research presented in this manuscript is novel and has high potential to impact clinical care for older adults from both a clinical perspective and a research perspective. We found the incidence of discontinuing acetylcholinesterase inhibitors (AChEIs) in a large national sample of nursing home residents with severe dementia was approximately 30% over a one-year period. In addition, we identified a number of patient-level clinical prognostic factors that were associated with increased likelihood of discontinuation, many of which may signify a decline clinical status. We also identified that several system-level factors were associated with decreased likelihood of discontinuation and thus may serve as barriers to implementation of deprescribing in the nursing home setting.
There have been no studies to date that have examined patterns of AChEI discontinuation specifically in nursing home residents with severe dementia, a sub-group that may be most likely to have these agents deprescribed based on limited clinical benefit. We implemented a robust methodological approach using a time-to-event analysis that incorporated time-varying covariates to evaluate the dynamic relationship between fluctuating clinical prognostic factors and discontinuation of AChEIs over time. Our study is also innovative because our sample included both newly admitted nursing home residents as well as those who had been residing the nursing home, which allowed us to identify that while a significant portion of discontinuation occurs around the time of admission, it can also occur later during a resident’s stay.
The findings presented in this manuscript address an important gap in the literature, providing insight into what factors clinicians may view as important in decision-making regarding deprescribing AChEIs in severe dementia. Our study also provides a springboard for future research by identifying potential confounders that should be addressed in any studies of the downstream effects of deprescribing AChEIs on outcomes.
FUNDING:
Funding was provided by: The Patrick and Catherine Weldon Donaghue Medical Research Foundation, the University of Pittsburgh Older American’s Independence Center (P30 AG024827). Dr. Niznik was funded by a T32 Award from the National Institutes on Aging (T32AG021885)
SPONSOR’S ROLE:
Funding sources had no role in the study design, data collection and analysis, manuscript preparation or the decision to submit the manuscript for publication.
Footnotes
Conflict of Interest: The authors have no conflicts of interest to disclose.
This paper will be presented as a poster at the 2019 American Geriatrics Society Annual Meeting in Portland, OR.
Supplementary Materials – Sample characteristics, adjusted hazard ratios, and cumulative incidence of deprescribing for sub-group analysis stratified by limited prognosis designation; Unadjusted and adjusted hazard ratios for sensitivity analysis using deprescribing definition of 60-day gap in supply.
REFERENCES
- 1.Winblad B, Kilander L, Eriksson S, et al. Donepezil in patients with severe Alzheimer’s disease: double-blind, parallel-group, placebo-controlled study. Lancet. 2006;367(9516):1057–1065. [DOI] [PubMed] [Google Scholar]
- 2.Black SE, Doody R, Li H, et al. Donepezil preserves cognition and global function in patients with severe Alzheimer disease. Neurology. 2007;69(5):459–469. [DOI] [PubMed] [Google Scholar]
- 3.Homma A, Imai Y, Tago H, et al. Donepezil treatment of patients with severe Alzheimer’s disease in a Japanese population: results from a 24-week, double-blind, placebo-controlled, randomized trial. Dement Geriatr Cogn Disord. 2008;25(5):399–407. [DOI] [PubMed] [Google Scholar]
- 4.Burns A, Bernabei R, Bullock R, et al. Safety and efficacy of galantamine (Reminyl) in severe Alzheimer’s disease (the SERAD study): a randomised, placebo-controlled, double-blind trial. Lancet Neurol. 2009;8(1):39–47. [DOI] [PubMed] [Google Scholar]
- 5.Feldman H, Gauthier S, Hecker J, et al. Efficacy and safety of donepezil in patients with more severe Alzheimer’s disease: a subgroup analysis from a randomized, placebo-controlled trial. Int J Geriatr Psychiatry. 2005;20(6):559–569. [DOI] [PubMed] [Google Scholar]
- 6.Ballard C, Margallo-Lana M, Juszczak E, et al. Quetiapine and rivastigmine and cognitive decline in Alzheimer’s disease: randomised double blind placebo controlled trial. BMJ. 2005;330(7496):874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cummings J, Jones R, Wilkinson D, et al. Effect of donepezil on cognition in severe Alzheimer’s disease: a pooled data analysis. J Alzheimers Dis. 2010;21(3):843–851. [DOI] [PubMed] [Google Scholar]
- 8.Rodda J, Morgan S, Walker Z. Are cholinesterase inhibitors effective in the management of the behavioral and psychological symptoms of dementia in Alzheimer’s disease? A systematic review of randomized, placebo-controlled trials of donepezil, rivastigmine and galantamine. Int Psychogeriatr. 2009;21(5):813–824. [DOI] [PubMed] [Google Scholar]
- 9.Cooper C, Mukadam N, Katona C, et al. Systematic review of the effectiveness of pharmacologic interventions to improve quality of life and well-being in people with dementia. Am J Geriatr Psychiatry. 2013;21(2):173–183. [DOI] [PubMed] [Google Scholar]
- 10.Birks J Cholinesterase inhibitors for Alzheimer’s disease. Cochrane Database Syst Rev. 2006(1):CD005593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scott IA, Hilmer SN, Reeve E, et al. Reducing inappropriate polypharmacy: the process of deprescribing. JAMA Intern Med. 2015;175(5):827–834. [DOI] [PubMed] [Google Scholar]
- 12.Bruyère Research Institute. What is Deprescribing? [Web Page]. 2019; https://deprescribing.org/what-is-deprescribing/. Accessed April 11, 2019, 2019.
- 13.Suzuki H, Inoue Y, Mikami K, et al. The influence and changes in the dosages of concomitantly used psychotropic drugs associated with the discontinuation of donepezil in severe Alzheimer’s disease with behavioral and psychological symptoms on dementia: a preliminary open-label trial. Ther Adv Psychopharmacol. 2014;4(1):37–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burns E, Kane R, Akbar ST, et al. Stopping pharmacological treatment for advanced dementia: No change in status. J Am Geriatr Soc. 2010;58(S104). [Google Scholar]
- 15.Simpson S, Beavis D, Leddy A, et al. Naturalistic audit of NICE criteria for the use of cholinesterase inhibitors. Psychiatr Bull. 2005;29(11):410–412. [Google Scholar]
- 16.Renn BN, Asghar-Ali AA, Thielke S, et al. A Systematic Review of Practice Guidelines and Recommendations for Discontinuation of Cholinesterase Inhibitors in Dementia. Am J Geriatr Psychiatry. 2018;26(2):134–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Herrmann N, Black SE, Li A, et al. Discontinuing cholinesterase inhibitors: results of a survey of Canadian dementia experts. Int Psychogeriatr. 2011;23(4):539–545. [DOI] [PubMed] [Google Scholar]
- 18.Shega JW, Ellner L, Lau DT, et al. Cholinesterase inhibitor and N-methyl-D-aspartic acid receptor antagonist use in older adults with end-stage dementia: a survey of hospice medical directors. J Palliat Med. 2009;12(9):779–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ray R, Prettyman R. When do we discontinue anti-dementia drugs? Views expressed by clinicians in a national survey within the United Kingdom. Int Psychogeriatr. 2013;25(9):1559–1560. [DOI] [PubMed] [Google Scholar]
- 20.Ahn SH, Choi NK, Kim YJ, et al. Drug persistency of cholinesterase inhibitors for patients with dementia of Alzheimer type in Korea. Arch Pharm Res. 2015;38(6):1255–1262. [DOI] [PubMed] [Google Scholar]
- 21.Amuah JE, Hogan DB, Eliasziw M, et al. Persistence with cholinesterase inhibitor therapy in a population-based cohort of patients with Alzheimer’s disease. Pharmacoepidemiol Drug Saf. 2010;19(7):670–679. [DOI] [PubMed] [Google Scholar]
- 22.Bohlken J, Weber S, Rapp MA, et al. Continuous treatment with antidementia drugs in Germany 2003–2013: a retrospective database analysis. Int Psychogeriatr. 2015;27(8):1335–1342. [DOI] [PubMed] [Google Scholar]
- 23.Kogut SJ, El-Maouche D, Abughosh SM. Decreased persistence to cholinesterase inhibitor therapy with concomitant use of drugs that can impair cognition. Pharmacotherapy. 2005;25(12):1729–1735. [DOI] [PubMed] [Google Scholar]
- 24.Mansour D, Wong R, Kuskowski M, et al. Discontinuation of acetylcholinesterase inhibitor treatment in the nursing home. Am J Geriatr Pharmacother. 2011;9(5):345–350. [DOI] [PubMed] [Google Scholar]
- 25.Pariente A, Pinet M, Moride Y, et al. Factors associated with persistence of cholinesterase inhibitor treatments in the elderly. Pharmacoepidemiol Drug Saf. 2010;19(7):680–686. [DOI] [PubMed] [Google Scholar]
- 26.Parsons C, Briesacher BA, Givens JL, et al. Cholinesterase inhibitor and memantine use in newly admitted nursing home residents with dementia. J Am Geriatr Soc. 2011;59(7):1253–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Suh DC, Thomas SK, Valiyeva E, et al. Drug persistency of two cholinesterase inhibitors: rivastigmine versus donepezil in elderly patients with Alzheimer’s disease. Drugs & aging. 2005;22(8):695–707. [DOI] [PubMed] [Google Scholar]
- 28.Sverdrup Efjestad A, Ihle-Hansen H, Hjellvik V, et al. Comedication and Treatment Length in Users of Acetylcholinesterase Inhibitors. Dement Geriatr Cogn Dis Extra. 2017;7(1):30–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Taipale H, Tanskanen A, Koponen M, et al. Antidementia drug use among community-dwelling individuals with Alzheimer’s disease in Finland: a nationwide register-based study. Int Clin Psychopharmacol. 2014;29(4):216–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thorpe CT, Fowler NR, Harrigan K, et al. Racial and Ethnic Differences in Initiation and Discontinuation of Antidementia Drugs by Medicare Beneficiaries. J Am Geriatr Soc. 2016;64(9):1806–1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Umegaki H, Itoh A, Suzuki Y, et al. Discontinuation of donepezil for the treatment of Alzheimer’s disease in geriatric practice. Int Psychogeriatr. 2008;20(4):800–806. [DOI] [PubMed] [Google Scholar]
- 32.Sun Y, Lai MS, Lu CJ, et al. How long can patients with mild or moderate Alzheimer’s dementia maintain both the cognition and the therapy of cholinesterase inhibitors: a national population-based study. Eur J Neurol. 2008;15(3):278–283. [DOI] [PubMed] [Google Scholar]
- 33.Gardette V, Lapeyre-Mestre M, Piau A, et al. A 2-year prospective cohort study of antidementia drug non-persistency in mild-to-moderate Alzheimer’s disease in Europe : predictors of discontinuation and switch in the ICTUS study. CNS Drugs. 2014;28(2):157–170. [DOI] [PubMed] [Google Scholar]
- 34.Maclagan LC, Bronskill SE, Guan J, et al. Predictors of Cholinesterase Discontinuation during the First Year after Nursing Home Admission. J Am Med Dir Assoc. 2018. [DOI] [PubMed] [Google Scholar]
- 35.Dybicz SB, Keohane DJ, Erwin WG, et al. Patterns of cholinesterase-inhibitor use in the nursing home setting: a retrospective analysis. Am J Geriatr Pharmacother. 2006;4(2):154–160. [DOI] [PubMed] [Google Scholar]
- 36.Buccaneer Computer Systems & Service I Chronic Condition Data Warehouse Medicare Administrative Data User Guide. In:2013.
- 37.Center for Medicare and Medicaid Services. Design for Nursing Home Compare Five-Star Quality Rating System: Technical User’s Guide. In. Vol January 2017.
- 38.US Department of Health and Human Services HRaSA BoHP. Area Health Resource File (AHRF). In. Rockville, MD. [Google Scholar]
- 39.Intrator O, Hiris J, Berg K, et al. The residential history file: studying nursing home residents’ long-term care histories(*). Health Serv Res. 2011;46(1 Pt 1):120–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wei YJ, Simoni-Wastila L, Zuckerman IH, et al. Algorithm for Identifying Nursing Home Days Using Medicare Claims and Minimum Data Set Assessment Data. Med Care. 2016;54(11):e73–e77. [DOI] [PubMed] [Google Scholar]
- 41.Saliba D, Buchanan J, Edelen MO, et al. MDS 3.0: brief interview for mental status. J Am Med Dir Assoc. 2012;13(7):611–617. [DOI] [PubMed] [Google Scholar]
- 42.Morris JN, Fries BE, Mehr DR, et al. MDS Cognitive Performance Scale. J Gerontol. 1994;49(4):M174–182. [DOI] [PubMed] [Google Scholar]
- 43.Daiello LA, Ott BR, Lapane KL, et al. Effect of discontinuing cholinesterase inhibitor therapy on behavioral and mood symptoms in nursing home patients with dementia. Am J Geriatr Pharmacother. 2009;7(2):74–83. [DOI] [PubMed] [Google Scholar]
- 44.Tjia J, Cutrona SL, Peterson D, et al. Statin discontinuation in nursing home residents with advanced dementia. J Am Geriatr Soc. 2014;62(11):2095–2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Holmes HM, Hayley DC, Alexander GC, et al. Reconsidering medication appropriateness for patients late in life. Arch Intern Med. 2006;166(6):605–609. [DOI] [PubMed] [Google Scholar]
- 46.Ailabouni NJ, Nishtala PS, Mangin D, et al. Challenges and Enablers of Deprescribing: A General Practitioner Perspective. PLoS One. 2016;11(4):e0151066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Palagyi A, Keay L, Harper J, et al. Barricades and brickwalls--a qualitative study exploring perceptions of medication use and deprescribing in long-term care. BMC Geriatr. 2016;16:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Niznik JD, Zhang S, Mor MK, et al. Adaptation and Initial Validation of Minimum Data Set (MDS) Mortality Risk Index to MDS Version 3.0. J Am Geriatr Soc. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Morris JN, Fries BE, Morris SA. Scaling ADLs within the MDS. J Gerontol A Biol Sci Med Sci. 1999;54(11):M546–553. [DOI] [PubMed] [Google Scholar]
- 50.Perlman CM, Hirdes JP. The aggressive behavior scale: a new scale to measure aggression based on the minimum data set. J Am Geriatr Soc. 2008;56(12):2298–2303. [DOI] [PubMed] [Google Scholar]
- 51.Saliba D, DiFilippo S, Edelen MO, et al. Testing the PHQ-9 interview and observational versions (PHQ-9 OV) for MDS 3.0. J Am Med Dir Assoc. 2012;13(7):618–625. [DOI] [PubMed] [Google Scholar]
- 52.By the American Geriatrics Society Beers Criteria Update Expert P. American Geriatrics Society 2015 Updated Beers Criteria for Potentially Inappropriate Medication Use in Older Adults. J Am Geriatr Soc. 2015;63(11):2227–2246. [DOI] [PubMed] [Google Scholar]
- 53.Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130–1139. [DOI] [PubMed] [Google Scholar]
- 54.Centers for Medicare and Medicaid Services/ Center for Program Integrity/Provider Enrollment Oversight Group/Division Enrollment Operations. Crosswalk Medicare Provider/Supplier to Healthcare Provider Taxonomy. 2017; https://www.cms.gov/medicare/provider-enrollment-and-certification/medicareprovidersupenroll/downloads/taxonomycrosswalk.pdf. Accessed July 11, 2018.
- 55.StataCorp. Multiple-Imputation Reference Manual. In: Stata Press; 2017: https://www.stata.com/bookstore/multiple-imputation-reference-manual/. Accessed April 18, 2017. [Google Scholar]
- 56.Hilmer SN, Gnjidic D. Deprescribing: the emerging evidence for and the practice of the ‘geriatrician’s salute’. Age Ageing. 2018;47(5):638–640. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Cumulative Incidence of Deprescribing AChEIs Stratified by Limited Prognosis Designation
Table S1. Complete Results for Unadjusted and Adjusted Hazard Ratios for Primary Analysis
Table S2. Sample Characteristics for Sub-group Analysis at Index Assessment
Table S3. Adjusted Hazards Ratios for Sub-group Analysis of Residents with and without Limited Prognosis
Table S4. Unadjusted and Adjusted Hazards Ratios for Sensitivity Analysis using 60-day gap


