Significance
Hydrothermal fluid geochemistry exerts a key control on subseafloor microbial community structure and function. However, the effects of microbial metabolic activity, thermal decomposition of biomass, and abiotic reactions on geochemistry remain unconstrained. Depletions in molecular hydrogen and enrichments in methane in submarine hydrothermal mixing zones have been interpreted to reflect the influence of an active subseafloor biosphere. In contrast, our work reveals that these chemical shifts are driven by abiotic and thermogenic processes at temperatures beyond the limit for life. These findings have critical implications for constraining the extent to which global geochemical cycles can sustain a deep biosphere, and for the global molecular hydrogen budget.
Keywords: hydrothermal vent, subsurface biosphere, bioenergetics, biogeochemistry
Abstract
Subseafloor mixing of high-temperature hot-spring fluids with cold seawater creates intermediate-temperature diffuse fluids that are replete with potential chemical energy. This energy can be harnessed by a chemosynthetic biosphere that permeates hydrothermal regions on Earth. Shifts in the abundance of redox-reactive species in diffuse fluids are often interpreted to reflect the direct influence of subseafloor microbial activity on fluid geochemical budgets. Here, we examine hydrothermal fluids venting at 44 to 149 °C at the Piccard hydrothermal field that span the canonical 122 °C limit to life, and thus provide a rare opportunity to study the transition between habitable and uninhabitable environments. In contrast with previous studies, we show that hydrocarbons are contributed by biomass pyrolysis, while abiotic sulfate (SO42−) reduction produces large depletions in H2. The latter process consumes energy that could otherwise support key metabolic strategies employed by the subseafloor biosphere. Available Gibbs free energy is reduced by 71 to 86% across the habitable temperature range for both hydrogenotrophic SO42− reduction to hydrogen sulfide (H2S) and carbon dioxide (CO2) reduction to methane (CH4). The abiotic H2 sink we identify has implications for the productivity of subseafloor microbial ecosystems and is an important process to consider within models of H2 production and consumption in young oceanic crust.
At deep-sea hydrothermal vents, fluid–mineral reactions taking place at high temperatures and pressures substantially influence the chemical composition of the world’s oceans (1, 2) and sustain thriving microbiological communities below and at the seafloor (3–6). Mixing between hot, chemically reduced hydrothermal fluids and cold, oxidized seawater in the subsurface creates moderate-temperature diffuse vent fluids and large amounts of potential chemical energy. Chemosynthetic microbes harness this chemical energy by catalyzing thermodynamically favorable redox reactions that support the primary fixation of inorganic CO2 into biomass (5, 7, 8).
Numerous field investigations have demonstrated that vent fluid temperature and composition can control variability in modern-day microbial community structure and function (3–6) and provide insight into the environmental driving forces of evolution (9, 10). The close linkage between vent microbiology and fluid chemistry is further supported by thermodynamic models that estimate available energy for specific metabolic reactions (7, 11–13). Beyond serving as the primary producers at the base of the food web in hydrothermal environments, microbial activity has important biogeochemical implications, as metabolic processes can modulate the composition of diffuse fluids via the consumption and production of redox-reactive species within mixtures of endmember hydrothermal source fluids and seawater (14–23).
In addition to modification by active microbial processes, abiotic reactions and thermogenic reactions that involve thermal decomposition of living or once-living biomass can also exert important controls on diffuse fluid chemistry and microbial ecosystems. In particular, abiotic redox reactions that proceed rapidly and consume reactants utilized by microorganisms will reduce available metabolic energy, while thermogenic production of hydrocarbons represents a source of carbon and energy that can support a heterotrophic lifestyle.
Here, we report on the composition of low-temperature vent fluids (44 to 149 °C) sampled in 2012 and 2013 at the Piccard hydrothermal field, Mid-Cayman Rise (Tables 1 and 2, and SI Appendix, Tables S1 and S2). Hosted in unsedimented pillow basalts and sheet flows at a water depth of 4,970 m along the eastern flank of an ultraslow spreading (15 to 17 mm/y full rate), 12-km-long axial volcanic ridge, Piccard is Earth’s deepest known vent field (24). Low-temperature fluid compositions indicate that they are formed by subseafloor mixing of cold (5 °C) seawater and 398 °C vent fluids represented by the nearby Beebe Vents black smokers. These high-temperature vents share a common major ion and dissolved volatile chemistry that reflects a single vapor phase, low-Cl source fluid that originates at temperatures that potentially exceed 500 °C due to the great depth of the system (25). Many of the low-temperature Piccard fluids were turbid at the time of venting, suggesting that they contain fine-grained particulate material. The high-temperature Beebe Vents are characterized by dissolved H2 concentrations as high as 20.7 mmol/L due to high temperatures in subsurface reaction zones (25), making the lower temperature diffuse fluids at Piccard ideally suited to assess processes that may consume H2 and the abundance of other redox-reactive species in near seafloor mixing zones. The fate of H2 generated in young oceanic crust is of high interest, as a recent synthesis study cannot account for at least 30% of all H2 produced (26). Although uptake by subsurface microbial metabolic activity is inferred to be the primary driver for these H2 losses, it is critically important to consider the possibility of abiotic sinks for H2. An abiotic H2 sink could vastly affect global H2 budgets and estimates of subseafloor biosphere productivity.
Table 1.
Measured aqueous species abundances in mixing zones at the Piccard vent field
| Vent (year)/sample* | Tmax, °C | Mg, mm | pH, 25 °C | Ca, mm | SO4, mm | Fe, mm | ΣHCOOH, μm |
| Beebe endmember† | 0 | —‡ | 6.18 | 0.21 | 6.58 | − | |
| Hot Chimlet 1 (2012) | |||||||
| J2-619-IGT6 | 149 | 35.4 | 5.1 | 7.15 | 15.2 | 0.85 | 54 |
| J2-619-IGT3 | 147 | 33.4 | 5.0 | 6.93 | 13.8 | —§ | 58 |
| Hot Chimlet 1 (2013) | |||||||
| N59-IGT6 | 81 | 41.5 | 5.8 | 10.4 | 20.7 | 0.50 | 13 |
| N59-IGT4 | 85 | 50.4 | 6.3 | 10.3 | 26.4 | 0.17 | <1.0 |
| Hot Chimlet 3 (2013) | |||||||
| N62-IGT8 | 101 | 47.1 | 6.0 | 10.3 | 24.7 | —§ | 55 |
| N62-IGT7 | 97 | 35.1 | 5.4 | 10.2 | 16.6 | 0.35 | 167 |
| Hot Chimlet 2 (2012) | |||||||
| J2-620-IGT4 | 95 | 45.4 | 5.7 | 9.35 | 24.2 | 0.15 | 16 |
| J2-620-IGT8 | 87 | 47.8 | 5.9 | 9.75 | 23.1 | 0.16 | 12 |
| Shrimp Gulley 1 (2012) | |||||||
| J2-620-IGT1 | 111 | 42.4 | 5.5 | 9.67 | 22.6 | 1.89 | 24 |
| J2-620-IGT2 | 104 | 44.8 | 5.6 | 10.1 | 22.8 | 1.58 | 18 |
| Shrimp Gulley 3 (2013) | |||||||
| N63-IGT6 | 80 | 47.9 | 5.8 | 10.1 | 24.6 | 2.56 | <1.0 |
| Shrimp Gulley 2 (2012) | |||||||
| J2-618-IGT1 | 45 | 47.4 | 6.0 | 10.2 | 25.4 | 0.091 | 9.4 |
| J2-618-IGT3 | 44 | 48.9 | 5.9 | 10.1 | 25.2 | 0.11 | 8.8 |
| Bottom seawater | ∼5 | 52.5 | 8.0 | 10.3 | 28.0 | 0.0 | <1.0 |
Jason (J2) or Nereus (N##) dive number and isobaric gas-tight sampler number (-IGT).
Average high-temperature vent endmember [for Beebe 1, 3, 5, and Beebe Woods; McDermott et al. (25)]. See McDermott et al. (25) for IGT data from high-temperature fluid samples.
Lowest measured pH for high-temperature source fluids: 3.1 (Beebe Vent 1, 2012); 3.2 (Beebe Vent 3, 2012); 3.0 (Beebe Vent 5, 2012); 3.2 (Beebe Vent 5, 2013); 3.2 (Beebe Woods, 2012); from McDermott et al. (25).
A dregs aliquot was not collected for sample J2-619-IGT3 or N62-IGT8. The dregs aliquot is composed of particles remaining in the IGT sampler following extraction of the aqueous sample; see Methods and Materials.
Table 2.
Measured volatile species abundances and isotope values in mixing zones at the Piccard vent field
| Vent (year)/sample* | ΣH2S, mm | δ34SH2S, ‰ | H2, mm | ΣCO2, mm | CH4, μm | C2H6, nm | C3H8, nm | n-C4H10, nm | i-C4H10, nm | δ13CCH4, ‰ | δ13CC2H6, ‰ | δ13CC3H8, ‰ |
| Beebe endmember† | 11.7 | 6.1 | 19.4 | 25.7 | 121 | 13 | 7.6 | 1.9 | 1.3 | −23.9 | −24.9 | − |
| Hot Chimlet 1 (2012) | ||||||||||||
| J2-619-IGT6 | 2.47 | 6.6 | 1.94 | 9.09 | 51.2 | 43 | 28 | 9.0 | 9.7 | −28.4 | −33.3 | −29.7 |
| J2-619-IGT3 | 2.60 | — | 2.06 | 10.1 | 56.5 | 92 | 66 | 27 | 11 | −29.9 | −33.5 | −28.2 |
| Hot Chimlet 1 (2013) | ||||||||||||
| N59-IGT6 | 1.47 | — | 0.027 | — | 39.6 | 27 | 22 | 5.4 | 5.2 | — | — | — |
| N59-IGT4 | 0.086 | — | 0.010 | — | 9.78 | — | — | — | — | — | — | — |
| Hot Chimlet 3 (2013) | ||||||||||||
| N62-IGT8 | 0.45 | — | 0.13 | — | 23.0 | — | — | — | — | — | — | — |
| N62-IGT7 | 2.03 | — | 0.45 | — | 64.3 | 36 | 28 | 7.3 | 7.7 | — | −36.9 | −29.5 |
| Hot Chimlet 2 (2012) | ||||||||||||
| J2-620-IGT4 | 1.04 | — | 0.68 | 4.89 | 21.8 | 17 | 11 | 4.3 | 3.4 | −29.2 | — | — |
| J2-620-IGT8 | 0.96 | — | 0.52 | 4.08 | 15.6 | 11 | 8.3 | 3.1 | <1.0 | — | — | — |
| Shrimp Gulley 1 (2012) | ||||||||||||
| J2-620-IGT1 | 1.95 | — | 0.40 | 5.95 | 24.0 | 14 | 9.7 | 3.8 | 1.6 | — | — | — |
| J2-620-IGT2 | 1.12 | — | 0.28 | 5.56 | 20.5 | 12 | 7.3 | 2.7 | <1.0 | — | — | — |
| Shrimp Gulley 3 (2013) | ||||||||||||
| N63-IGT6 | 0.49 | — | <0.002 | — | 19.4 | — | — | — | — | — | — | — |
| Shrimp Gulley 2 (2012) | ||||||||||||
| J2-618-IGT1 | 0.83 | — | 0.11 | 3.39 | 10.8 | 5.1 | — | 1.6 | <1.0 | — | — | — |
| J2-618-IGT3 | 0.84 | — | 0.13 | 3.64 | 10.9 | 5.1 | 4.0 | 1.8 | <1.0 | — | — | — |
| Bottom seawater | 0 | — | 0 | 2.21 | 0 | 0 | 0 | 0.0 | 0 | — | — | — |
Abiotic Processes in Subseafloor Mixing Environments
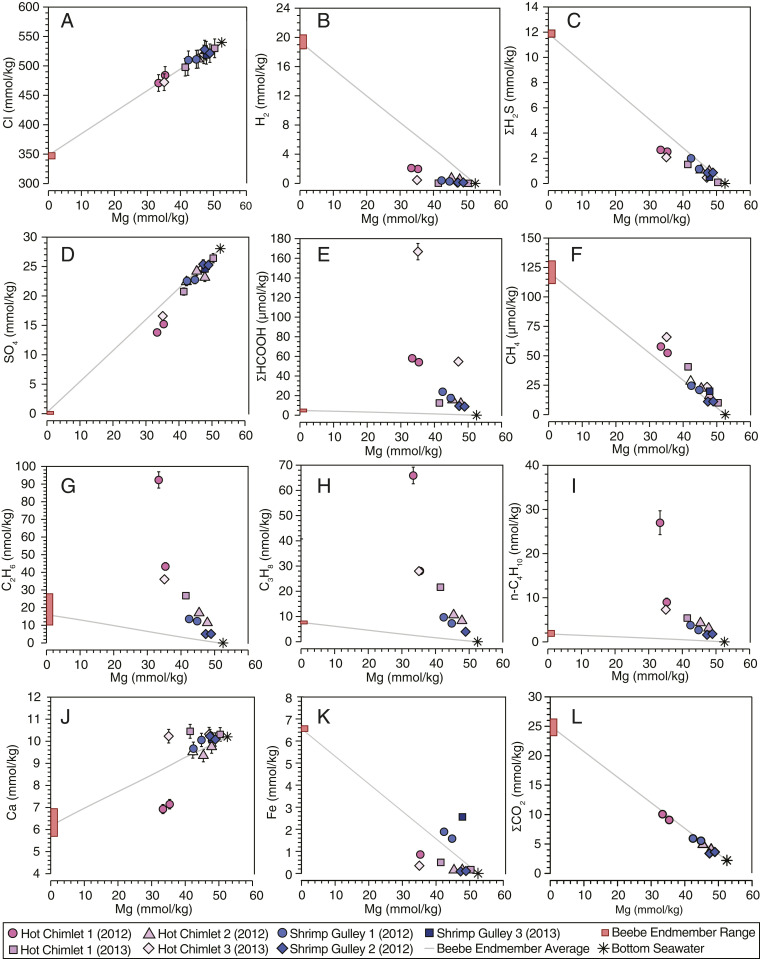
Because dissolved Mg2+ behaves conservatively during mixing of near-zero–Mg endmember high-temperature vent fluids and Mg-rich seawater, the effects of subseafloor mixing on a given aqueous species in a mixed vent fluid can be examined by plotting its concentration against Mg2+ (Fig. 1). Many species such as Cl− show conservative behavior during mixing in the Piccard fluids (Fig. 1A), demonstrating their origin from simple two-component subsurface mixing between seawater and Beebe Vents fluids. However, several redox-reactive species behave nonconservatively and show significant depletions or enrichments (Fig. 1 and SI Appendix, Table S3). For example, concentrations of dissolved H2, ΣH2S (ΣH2S = H2S + HS− + S2−), and SO42− show substantial depletions (Fig. 1 B–D), while dissolved ΣHCOOH (ΣHCOOH = HCOO− + HCOOH) and hydrocarbons show varying levels of enrichment (Fig. 1 E–I). Dissolved Ca2+ and Fe2+ concentrations show both enrichments and depletions relative to conservative mixing (Fig. 1 J and K). In the case of Fe2+, enriched concentrations may reflect entrainment of Fe-rich particles during sample collection (27). Some of the most pronounced depletions and enrichments are observed in the Hot Chimlet 1 fluids sampled in 2012. This fluid is characterized by a maximum measured temperature of 149 °C that exceeds the known temperature limit for life at 122 °C (28). Accordingly, we infer that consumption of H2, the production of CH4, and the nonconservative behavior of many other species at Hot Chimlet 1 (2012) must proceed via one or more abiotic processes.
Fig. 1.
Measured concentrations of aqueous Mg versus aqueous Cl (A), H2 (B), ΣH2S (C), SO4 (D), ΣHCOOH (E), CH4 (F), C2H6 (G), C3H8 (H), n-C4H10 (I), Ca (J), Fe (K), and ΣCO2 (L) for mixed fluids sampled at the Piccard vent field in 2012 and 2013. For each species, the range in extrapolated zero-Mg high-temperature endmember composition is shown with the red bar along the y axis, and the average is shown with a gray line projected from seawater composition. Species that exhibit conservative behavior during mixing plot on the line, while species that behave nonconservatively plot above or below the line. The 2s errors are shown (or are smaller than the symbols).
Removal of dissolved ΣH2S and Fe2+ from mixed fluids can be attributed to the precipitation of iron sulfide minerals due to decreases in their solubility with decreasing temperature and increasing pH. Near equimolar depletions of ΣH2S and Fe2+ that range from 0.88 to 1.85 mmol/kg in the Hot Chimlet 1 fluids in both 2012 and 2013 and the Hot Chimlet 3 fluids (SI Appendix, Table S3) suggest pyrrhotite (FeS) formation during mixing according to the following reaction:
| [1] |
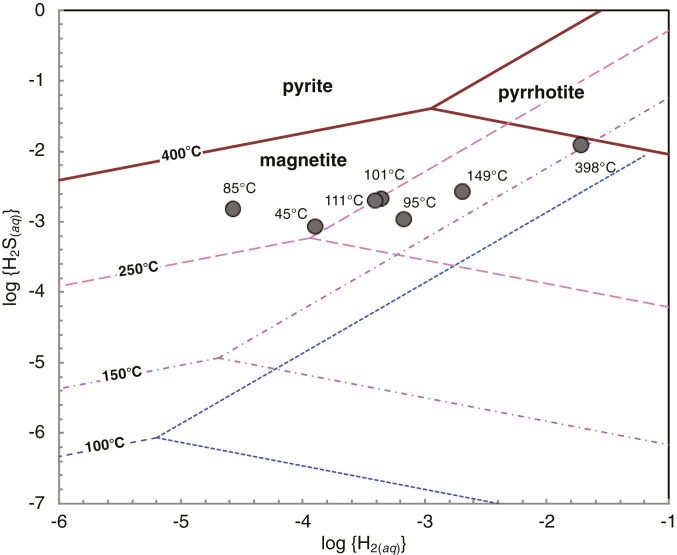
Phase relations in the chemical system Fe–S–O–H indicate that pyrrhotite is the stable iron phase for the measured concentrations of ΣH2S and H2 in the endmember fluids to temperatures down to 150 °C (Fig. 2), consistent with pyrrhotite formation during the early stages of vent fluid mixing with cold seawater. Continued seawater mixing produces temperatures below 150 °C and reduces dissolved H2 concentrations, shifting fluid compositions into the pyrite (FeS2) stability field (Fig. 2). Accordingly, pyrite formation may be responsible for additional depletion in ΣH2S and Fe2+ during the later stages of mixing under temperature conditions compatible with life.
Fig. 2.
Activity diagram showing phase relations in the chemical system Fe–S–O–H at 400, 250, 150, and 100 °C and 500 bar. The symbols indicate measured H2(aq) and H2S(aq) concentrations in Piccard vent fluids. Requisite thermodynamic data for the construction of the diagram were calculated using SUPCRT92 computer code (56) and corrected for the nonstoichiometric composition of pyrrhotite in equilibrium with pyrite (57).
The formation of iron sulfides such as pyrrhotite according to reaction 1 results in the generation of 2 equivalents of acidity for each mole of ΣH2S and Fe2+ that precipitates. Thus, the Hot Chimlet 3 fluids that display the largest ΣH2S and Fe2+ depletion of 1.85 mmol/kg will generate acidity at a level sufficient to titrate all of the alkalinity contributed by admixed seawater and create fluids characterized by pH (25 °C) <3 in the absence of other reactions that consume H+. Measured pH (25 °C) values for all of the mixed fluids are >5, providing evidence for titration of acidity by additional geochemical processes.
Enrichment of the mixed Piccard fluids in ΣHCOOH suggests that CO2 reduction during mixing may account for a portion of the observed H2 depletions. Reducing conditions, coupled with the lower temperatures and higher pH conditions of a mixed fluid, provide a thermodynamic drive for the abiotic reduction of CO2 by H2 to ΣHCOOH according to the following reaction:
| [2] |
Calculated chemical affinities for reaction 2 in >100 °C mixed fluids are less than ±5 kJ/mol, suggesting that they have attained metastable thermodynamic equilibrium between CO2, H2, and ΣHCOOH. Calculated affinities in the <100 °C fluids are slightly higher than those at >100 °C with positive values of +8.6 kJ/mol, suggesting a thermodynamic drive for additional ΣHCOOH production that may reflect slower reaction kinetics at lower temperatures of these fluids. A similar approach to equilibrium involving CO2, H2, and ΣHCOOH has been documented at the nearby Von Damm vent field (29) and at the Lost City vent field, Mid-Atlantic Ridge (30). Attainment of metastable equilibrium according to reaction 2 in subseafloor mixing zones represents an abiotic pathway for the production of ΣHCOOH that may fuel microbial metabolism (6).
Although formation of ΣHCOOH in subseafloor mixing zones consumes H2 according to reaction 2, the relatively low concentrations observed indicate that it can account for only a small fraction of the H2 depletions in the mixed fluids. For example, close examination of the 149 °C Hot Chimlet 1 fluid in 2012 (J2-619-IGT6, Tables 1 and 2) reveals a H2 depletion of 4.3 mmol/kg (Fig. 1B) that was accompanied by production of only 0.052 mmol/kg ΣHCOOH (Fig. 1E). Relatively minor depletions in ΣCO2 (Fig. 1L; ΣCO2 = H2CO3* + HCO3− + CO32−) indicate that reduction of ΣCO2 to other reduced carbon species is not responsible for the large H2 depletions in the mixed fluids. Rather, the linear relationship between ΣCO2 and Mg abundances (Fig. 1L) largely reflects mixing of a high ΣCO2 endmember with lower ΣCO2 seawater. Minor amounts of H2 may also be consumed by reaction with dissolved O2 and NO3− present in admixed seawater at concentrations of ∼0.2 and ∼0.02 mmol/kg, respectively (31). Collectively, ΣHCOOH production and O2 and NO3− reduction can consume ∼0.4 mmol/kg of H2 in the 2012 Hot Chimlet 1 fluid, requiring an additional sink for the remaining 3.9 mmol/kg of H2 depletion.
High concentrations of dissolved SO42− in conjunction with depletions relative to conservative values in mixed fluids suggest that reactions involving the reduction of SO42− are responsible for the observed H2 depletions. Complete SO42− reduction results in the production of ΣH2S, while partial reduction may form sulfur species in intermediate states such as sulfur (S), thiosulfate (S2O32–), or polysulfide (Sx2–). Equilibrium fractionation factors for isotopic exchange between SO42– and ΣH2S (32) indicate that reduction of SO42− at temperatures below 400 °C should produce ΣH2S that is highly depleted in 34S relative to initial seawater SO42− (δ34Sseawater SO4 = 21.0‰). For example, ΣH2S produced from seawater SO42− at 350 °C would have a δ34S value of 4.1‰ at 350 °C and −2.5‰ at 250 °C (32), resulting in decreasing δ34S values for dissolved ΣH2S in low-temperature mixed fluids. Comparison of the 34S content of ΣH2S in the high-temperature endmember fluid with the 34S content of ΣH2S in the 2012 Hot Chimlet 1 fluid reveals near-identical values of 6.1 and 6.6‰, respectively, indicating that significant amounts of ΣH2S produced by SO42− reduction during mixing have not been added to the low-temperature fluids. The absence of evidence for reduction of SO42− to ΣH2S suggests that the observed H2 depletions relect formation of intermediate oxidation state sulfur species.
The formation of intermediate oxidation state sulfur species has been reported for several low-temperature vent environments (18, 33, 34) but was not determined in this study. We postulate that the H2 depletions observed in the low-temperature Piccard fluids result from the formation of elemental S, S2O32–, and Sx2– (Fig. 3). Indeed, the distinct turbidity that characterized many of the low-temperature Piccard fluids may reflect condensation of liquid or particulate sulfur. Reduction of seawater SO42− to an intermediate oxidation state such as elemental S can be represented by the following reaction:
| [3] |
Reaction 3 predicts that each mole of SO42− reduced will consume 3 mol of H2 and 2 mol of H+, indicating that formation of elemental S may represent a sink for acidity produced by precipitation of iron sulfides according to reaction 1 in addition to dissolved H2. The 3.9 mmol/kg H2 depletion in the 2012 Hot Chimlet 1 fluid that cannot be attributed to reduction of ΣCO2, O2, and NO3−would be consistent with the reduction of 1.3 mmol/kg SO42−. The observed SO42− depletion of 3.7 mmol/kg in this fluid (Fig. 1D and SI Appendix, Table S3) is substantially greater than this value, but includes removal of SO42− due to anhydrite (CaSO4) precipitation. Due to significant variability in the endmember fluid Ca concentration (Fig. 1K), estimates for the amount of anhydrite precipitation based on Ca depletions vary from 1.6 to 2.3 mmol/kg, suggesting that 1.4 to 2.1 mmol/kg of the SO42− depletion can be attributed to formation of intermediate oxidation state sulfur species. Although the low end of this range is consistent with the observed H2 depletion via elemental S formation (reaction 3), the higher values may suggest production of more oxidized intermediate oxidation state sulfur species, such as S2O32–, that would consume less H2 for a given amount of SO42− reduced.
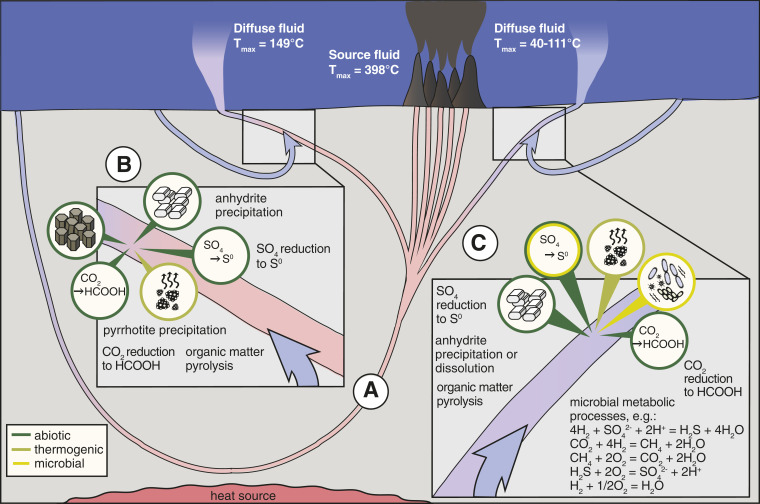
Fig. 3.
Schematic representation of key processes that occur when ascending high-temperature source fluids mix with seawater (A) under relatively higher temperature (∼149 °C) (B) and lower temperature (<122 °C) conditions (C). At temperatures of ∼149 °C and higher, mixed fluid chemistry is impacted by abiotic reactions involving the aqueous reduction of dissolved species and mineral precipitation, along with thermogenic reactions involving the pyrolysis of living or previously living organic matter. At temperatures at and below 122 °C, microbial metabolic processes may occur in addition to abiotic and thermogenic processes. The metabolic processes could include hydrogenotrophic SO42− reduction, anaerobic methanogenesis from CO2 and H2, methanotrophy by O2, sulfide oxidation by O2, and hydrogen oxidation by O2.
In contrast to the Hot Chimlet 1 (2012) fluid, measured temperatures of all other mixing zone fluids collected in 2012 and 2013 are below the canonical 122 °C limit for life. Thus, the chemistry of these fluids could be impacted by active microbial processes in addition to abiotic processes (Fig. 3). It is important to note, however, that this 122 °C record is held by a methanogen grown in the laboratory under optimal conditions (28). In the natural environment, the limit for life may be closer to 80 °C, as has been shown in petroleum basin systems (35). Eleven of 13 fluids in this study match or exceed 80 °C, and thus the chemistry of the majority of our samples may reflect only abiotic or thermogenic inputs. All fluids have lost significant quantities of H2 relative to conservative mixing calculations (0.77 to 5.98 mmol/kg H2 lost; Fig. 1B and SI Appendix, Table S3). Common metabolic reactions that directly impact H2, such as aerobic H2 oxidation, can be eliminated as significant, due to insufficient O2 availability to account for the magnitude of H2 lost.
Mixed fluids sampled in 2013 at Hot Chimlet 1, Hot Chimlet 3, and Shrimp Gulley 3 have temperatures of 80, 101, and 85 °C, respectively, and are characterized by significant SO42− depletions from 0.5 to 2.2 mmol/kg (Fig. 1D and SI Appendix, Table S3). These SO42− depletions are accompanied by Ca2+ enrichments relative to conservative mixing (Fig. 1J and SI Appendix, Table S3) indicating net dissolution of anhydrite, which would have contributed additional SO42− to the fluid in 1:1 proportion to Ca2+. The observation that SO42− has been consumed in these fluids despite an additional source from dissolving anhydrite, highlights the importance of abiotic or microbially mediated SO42− reduction pathways as a sink for H2.
Thermogenic Processes
In contrast to the depletions observed for inorganic redox reactive species, measured concentrations of CH4, C2H6, C3H8, n-C4H10, and i-C4H10 in the low-temperature mixed fluids at the Piccard vent field are significantly greater than abundances expected for conservative mixing of endmember fluids and seawater, especially within the 149 °C Hot Chimlet fluid in 2012 (Table 2 and Fig. 1 F–I). Several lines of evidence suggest that these hydrocarbon enrichments are the product of thermal degradation of immature organic material. Relative to biogenic and abiotic mantle-derived hydrocarbons that are dominated by CH4, thermogenic hydrocarbons generally contain substantially greater proportions of C2+ alkanes (36). Thus, substantially lower CH4/C2+ values (mol/mol) of 290 to 1,000 in the low-temperature mixed fluids relative to the high-temperature endmember fluids characterized by CH4/C2+ values of 2,300 to 4,500, likely reflect a large increase in the relative abundance of thermogenic C2+ hydrocarbons. Similar processes have been noted in the high-temperature fluids at Piccard. For example, a higher C2H6 concentration at Beebe Woods (354 °C, 28 nmol/kg) relative to the higher temperature Beebe Vents (398 °C, 11 to 15 nmol/kg) has been attributed to pyrolysis of dissolved organic carbon (DOC) in admixed seawater (25). Thermogenic hydrocarbon inputs are far more apparent in the mixed fluids presented here and represent a substantially greater fraction of the total hydrocarbon budgets. The linear inverse correlation of individual hydrocarbons with Mg in the diffuse fluids (Fig. 1) suggests that thermal alteration of organic matter occurs above the maximum temperature of 149 °C for the mixed fluids at Piccard, with subsequent seawater mixing producing decreasing hydrocarbon concentrations due to dilution.
The carbon isotopic composition of Hot Chimlet 1 hydrocarbons also supports a thermogenic origin for the hydrocarbon enrichment in the mixed fluids. The isotopic compositions of Hot Chimlet 1 hydrocarbons vary from −28.4 to −29.9‰ for CH4 and −33.3 to −36.9‰ for C2H6 (Table 2). These values are significantly depleted in 13C relative to the 13C content of hydrocarbons in the endmember fluids that are characterized by uniform δ13C values of −23.9‰ and −24.9‰ for CH4 and C2H6, respectively. An isotope mass balance assuming two-component conservative mixing of hydrocarbons in endmember fluid (Table 2) with a thermogenic component added during mixing yields calculated δ13C values of −39.7, −33.8, and −28.9‰ for thermogenic CH4, C2H6, and C3H8, respectively. The calculated values fall within the range of −25‰ to −50‰ and show progressive 13C enrichment with increasing carbon chain length, consistent with trends typically observed for thermogenic hydrocarbons (37–39). The presence of hydrocarbons with similar isotopic compositions in mixed fluids with temperatures above and below 122 °C suggests that they are derived from a common process occurring above and below the temperature limit for life (Fig. 3). The Piccard mixed fluids are also characterized by substantial enrichments in dissolved ΣNH4 (ΣNH4 = NH3 + NH4+) and methanethiol (CH3SH) relative to the high-temperature endmembers (40) (SI Appendix, Table S1). Both species are products of thermal degradation of fresh organic matter and provide additional evidence that thermogenic processes are contributing to the chemical inventory of mixed fluids at Piccard.
Implications for Subsurface Life and Chemical Fluxes to the Ocean
Sulfate reduction rates and mechanisms have largely been studied within the context of petroleum reservoirs and sedimented basins, where SO42− is reduced by organic matter via microbially or abiotically mediated processes (41). Sulfur cycling is also important within ancient hydrothermal systems, deep terrestrial aquifers, and in applied fields such as nuclear waste disposal. The reaction kinetics of SO42− reduction by H2 have been investigated in a two-phase (water plus gas) experiment conducted under hydrothermal conditions (42). Several conditions that enhance reduction kinetics were identified, including temperature, the pH-dependent speciation of SO42−, and the initial presence of H2S to contribute to the formation of S2O32– and Sx2– intermediates. Our results suggest that conditions in the Piccard mixing zone, where fluid residence times are estimated to be on the order of hours to days, support the reduction of SO42− to intermediate oxidation state sulfur species such as elemental S, S2O32–, and Sx2–.
The existence of a chemosynthetic subsurface biosphere inhabiting fractures in the shallow oceanic crust has been widely discussed (43). Evidence for subsurface life at oceanic spreading centers ranges from direct observations of microbial blooms following eruptive events (44), to microbial communities inhabiting the surface and interior of young basaltic crust (45), to indirect measurements of chemical signatures in venting fluids that are presumed to be generated by thermal degradation of subseafloor microbial biomass (46). For example, elevations in long-chained and cyclic hydrocarbons and microbial lipid residues have been identified in high- and low-temperature vents, relative to their abundances in seawater (46). Some endmember fluids, including those at Piccard and Von Damm vent fields along the Mid-Cayman Rise, contain lower bulk DOC concentrations than ambient seawater, implying that thermal degradation at high temperatures is an important DOC removal mechanism in deep-sea vents (47, 48). In contrast, lower temperature vent fluids contain elevated DOC and microbial cell counts that are higher than ambient seawater (6, 47, 49).
Past studies have documented substantial depletions in H2 and enrichments in CH4 in low-temperature mixed hydrothermal vent fluids relative to values expected for conservative mixing (14, 15, 21, 22). Due to the presence of active microbial ecosystems in contact with the fluids (16, 17) and measured temperatures below 122 °C, the H2 depletions and CH4 enrichments in those studies were attributed to active microbial methanogenesis. Here, we demonstrate that H2 depletions and CH4 enrichments may, instead, reflect abiotic and thermogenic reaction pathways such as those occurring within hydrothermal mixing zones at the Piccard vent field at temperatures in excess of the known limits to life.
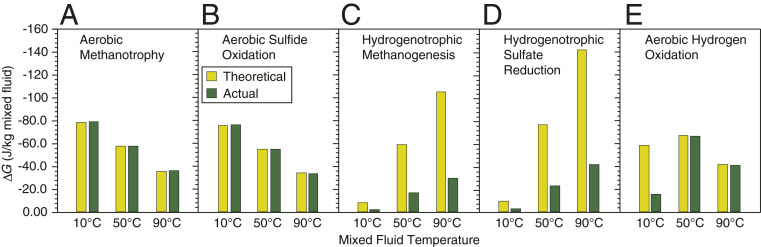
In general, previous studies that estimate Gibbs free energy available to support the subseafloor biosphere in hydrothermal settings do not consider abiotic and thermogenic reactions that consume H2 and generate CH4 and C2–4 alkanes. To further quantify the implications of abiotic reactions on the energy landscape for the subsurface biosphere at Piccard, two geochemical mixing models were constructed that allow calculation of the maximum amount of potential chemical energy available to support five chemolithoautotrophic metabolic pathways using an approach that is similar to previous investigations (7, 50) (see Fig. 3 for metabolic reactions; see SI Appendix for calculation details). Both models involved incremental mixing of a hydrothermal fluid with 2 °C seawater over a temperature range of 160 to 5 °C at 500-bar pressure. For the first model, the starting composition of the hydrothermal fluid represents a conservative mixture of the endmember Beebe Vent fluid with anaerobic seawater to achieve a Mg concentration identical to the Hot Chimlet 1 fluid (SI Appendix, Table S7). For the second model, the composition of the fluid in 2012 (J2-619-IGT6, Table 1) was used for the starting composition of the hydrothermal fluid. At each step in the models, the potential Gibbs free energy available to support sulfate reduction, methanogenesis, methanotrophy, sulfide oxidation, and hydrogen oxidation was calculated using the calculated equilibrium distribution of aqueous species at temperatures less than or equal to 122 °C. Results demonstrate that the amount of Gibbs free energy available for aerobic methanotrophy by O2 and sulfide oxidation by O2 increase only marginally, up to 0.1% and 0.2% per kg of mixed fluid, respectively, at a given temperature (Fig. 4 A and B). On a percent basis, a negligible amount of additional energy is made available to chemo-heterotrophic organisms that oxidize CH4. In contrast, the potential chemical energy available for both methanogenesis involving reduction of CO2 by H2 and for hydrogenotrophic SO42− reduction is reduced by 71 to 86% across the habitable temperature range (Fig. 4 C and D). In addition, the potential energy available for H2 oxidation by O2 is reduced by 1 to 84%, with the greatest impact at 10 °C (Fig. 4E). Within 50 to 90 °C mixing regimes where H2-utilizing metabolic reactions may otherwise be expected to dominate, the energy landscape shifts to favor O2-utilizing reactions. While thermogenic degradation of biomass can release energy to support chemo-heterotrophic organisms in submarine hydrothermal systems, our study demonstrates that abiotic processes may substantially reduce the amounts of H2-based energy available to the subsurface biosphere on Earth. This abiotic H2 sink could vastly affect global H2 budgets and should be considered in tandem with biologically mediated sinks in global H2 mass balance models.
Fig. 4.
Catabolic energy available (joules per kilogram mixed fluid) to support five microbial metabolic pathways (A–E) in 10, 50, and 90 °C Piccard mixing zone fluids. Greater energy availability is indicated by more negative ΔG values. Energy was determined for a theoretical mixed fluid reflecting conservative endmember–seawater mixing (yellow bars) and for the actual measured mixed fluid composition that is affected by abiotic and thermogenic processes (green bars).
Materials and Methods
Hydrothermal Vent Fluid Sampling.
Hydrothermal fluid samples were collected by ROV Jason II during cruise AT18-16 aboard the R/V Atlantis in January 2012 and by HROV Nereus during cruise FK008 aboard the R/V Falkor in June 2013, following the approach reported in McDermott et al. (25). All fluids were collected using titanium isobaric gas-tight (IGT) samplers (51). Replicate samples were collected at each orifice when feasible. Fluid temperatures were monitored continuously with thermocouples mounted flush to the inlet of each IGT during the ∼2 min required to fill each sampler. A National Institute of Standards and Technology temperature calibrator was used to confirm that all thermocouples were accurate to ±2 °C. Reported temperatures for each sample (Table 1) reflect the maximum measured temperature recorded during its collection. The elapsed time interval between sampling on the seafloor and sample recovery and processing ranged from 5 to 20 h due to differences in dive duration. Onboard extraction and processing of vent fluid samples took place within 24 h of sampler recovery.
Analytical Methods.
Shipboard sampling handling and analysis, as well as shore-based analysis, were performed for pH, volatile species, nonvolatile species, and C, S, and Sr isotopes as in McDermott et al. (25). Measured metal concentrations in the dissolved, filter, and dregs fractions are reported in SI Appendix, Tables S4–S6. Concentration units for species measured at sea, including H2, CH4, and ΣH2S, were converted from moles per liter solution to moles per kilogram solution using a uniform fluid density of 1.025 kg/L. The analytical uncertainty (2s) was ±0.05 units for pH (25 °C, 1 atm), ±5% for dissolved ΣCO2, CH4, C2H6, C3H8, ΣNH4, and H2 and ±10% for dissolved n-C4H10, i-C4H10, and ΣH2S. Analytical uncertainties (2s) were ±3% for dissolved Na, Cl, Ca, K, SO4, Mg, SiO2, and Sr; ±5% for dissolved Li, Rb, Fe, Mn, Zn, Cu, Al, Pb, Co, and Cd; and ±10% for dissolved Br and Ag. The pooled SD (2s) for replicate samples was ±0.3‰ for δ13CCO2 and ±0.8‰ for δ13CCH4, and the instrumental analytical uncertainty (2s) was ±0.4‰ for δ13CC2H6, ±0.7‰ for δ13CC3H8, and ±0.3‰ for δ34SΣH2S.
Mixed and Endmember Fluid Compositions.
At high temperatures (>300 °C) and low water/rock ratio, hydrothermal fluids undergo near quantitative removal of Mg2+ and SO42− during fluid–rock reaction with basalt and gabbro (52–55). The measurable dissolved Mg2+ in high-temperature ridge-crest hydrothermal fluids can be attributed to the dead volume of the IGT samplers (∼4 mL), which are prefilled with bottom seawater prior to deployment, and to inadvertent entrainment of ambient seawater during sample collection. The composition of the zero-Mg2+ endmembers prior to mixing were calculated via least-squares regression of an individual conservative chemical species as a function of Mg2+ for all samples from one vent orifice, weighted to pass through the background seawater composition and extrapolated to 0 mmol/kg Mg2+.
Diffuse hydrothermal fluids (45 to 149 °C) are produced when a zero-Mg2+ “endmember” fluid mixes with ambient seawater below the seafloor and/or within chimney structures prior to sampling. Diffuse fluid compositions therefore reflect two-component mixing of these two compositionally distinct fluids. Nonconservative behavior occurs during subsurface mixing for some species reported here (e.g., Ca2+, Sr2+, SO42−, ΣNH4, ΣHCOOH, H2, ΣH2S, CH4, C2+ alkanes, and transition metals). Only measured abundances are reported for these nonconservative species in lower temperature mixed fluids (Tables 1 and 2 and SI Appendix, Tables S1 and S2), because extrapolation to an endmember composition has no physical meaning in these instances.
Thermodynamic Affinity Calculations.
The affinity for the reduction of CO2 with H2 to produce HCOO− and H+ via reaction 1 was calculated as in McDermott et al. (29).
Numerical Geochemical Mixing Model.
See SI Appendix.
Supplementary Material
Acknowledgments
Financial support was provided by the National Aeronautics and Space Administration (NASA) Astrobiology program (Awards NNX09AB75G and 80NSSC19K1427 to C.R.G. and J.S.S.) and the NSF (Award OCE-1061863 to C.R.G. and J.S.S.). Ship and vehicle time for cruise FK008 was provided by the Schmidt Ocean Institute. We thank the ROV Jason II and HROV Nereus groups, and the captain, officers, and crew of R/V Atlantis (AT18-16) and R/V Falkor (FK008) for their dedication to skillful operations at sea. We thank our scientific colleagues from both cruises, as well as Meg Tivey, Frieder Klein, and Scott Wankel for insightful discussions. We are grateful to the editor and two anonymous reviewers for providing helpful comments and suggestions.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2003108117/-/DCSupplemental.
Data Availability.
All study data are included in the article and SI Appendix.
References
- 1.Staudigel H., Hart S. R., Alteration of basaltic glass: Mechanisms and significance for the oceanic crust-seawater budget. Geochim. Cosmochim. Acta 47, 337–350 (1983). [Google Scholar]
- 2.German C. R., Seyfried W. E. Jr., “Hydrothermal processes” in Treatise on Geochemistry: Second Edition, Holland H. D., Turekian K. K., Eds. (Elsevier, 2014), Vol. 8, pp. 191–233. [Google Scholar]
- 3.Opatkiewicz A. D., Butterfield D. A., Baross J. A., Individual hydrothermal vents at Axial Seamount harbor distinct subseafloor microbial communities. FEMS Microbiol. Ecol. 70, 413–424 (2009). [DOI] [PubMed] [Google Scholar]
- 4.Perner M. et al., Driving forces behind the biotope structures in two low-temperature hydrothermal venting sites on the southern Mid-Atlantic Ridge. Environ. Microbiol. Rep. 3, 727–737 (2011). [DOI] [PubMed] [Google Scholar]
- 5.Nakamura K., Takai K., Theoretical constraints of physical and chemical properties of hydrothermal fluids on variations in chemolithotrophic microbial communities in seafloor hydrothermal systems. Prog. Earth Planet. Sci. 1, 1–24 (2014). [Google Scholar]
- 6.Reveillaud J. et al., Subseafloor microbial communities in hydrogen-rich vent fluids from hydrothermal systems along the Mid-Cayman Rise. Environ. Microbiol. 18, 1970–1987 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McCollom T. M., Shock E. L., Geochemical constraints on chemolithoautotrophic metabolism by microorganisms in seafloor hydrothermal systems. Geochim. Cosmochim. Acta 61, 4375–4391 (1997). [DOI] [PubMed] [Google Scholar]
- 8.Sievert S., Vetriani C., Chemoautotrophy at deep-sea vents: Past, present, and future. Oceanography 25, 218–233 (2012). [Google Scholar]
- 9.Reysenbach A.-L., Shock E., Merging genomes with geochemistry in hydrothermal ecosystems. Science 296, 1077–1082 (2002). [DOI] [PubMed] [Google Scholar]
- 10.Anderson R. E. et al., Genomic variation in microbial populations inhabiting the marine subseafloor at deep-sea hydrothermal vents. Nat. Commun. 8, 1114 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Amend J. P., McCollom T. M., Hentscher M., Bach W., Catabolic and anabolic energy for chemolithoautotrophs in deep-sea hydrothermal systems hosted in different rock types. Geochim. Cosmochim. Acta 75, 5736–5748 (2011). [Google Scholar]
- 12.Hentscher M., Bach W., Geochemically induced shifts in catabolic energy yields explain past ecological changes of diffuse vents in the East Pacific Rise 9°50’N area. Geochem. Trans. 13, 2 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dahle H., Økland I., Thorseth I. H., Pederesen R. B., Steen I. H., Energy landscapes shape microbial communities in hydrothermal systems on the Arctic Mid-Ocean Ridge. ISME J. 9, 1593–1606 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Von Damm K. L., Lilley M. D., “Diffuse flow hydrothermal fluids from 9° 50′ N East Pacific Rise: Origin, evolution and biogeochemical controls” in The Subseafloor Biosphere at Mid-Ocean Ridges, Wilcock W. S. D., Delong E. F., Kelley D. S., Baross J. A., Cary S. C., Eds. (American Geophysical Union, 2004), Vol. 144, pp. 245–268. [Google Scholar]
- 15.Wankel S. D. et al., Influence of subsurface biosphere on geochemical fluxes from diffuse hydrothermal fluids. Nat. Geosci. 4, 461–468 (2011). [Google Scholar]
- 16.McNichol J. et al., Primary productivity below the seafloor at deep-sea hot springs. Proc. Natl. Acad. Sci. U.S.A. 115, 6756–6761 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McNichol J. et al., Assessing microbial processes in deep-sea hydrothermal systems by incubation at in situ temperature and pressure. Deep. Res. Part I Oceanogr. Res. Pap. 115, 221–232 (2016). [Google Scholar]
- 18.Gartman A. et al., Sulfide oxidation across diffuse flow zones of hydrothermal vents. Aquat. Geochem. 17, 583–601 (2011). [Google Scholar]
- 19.Fortunato C. S., Larson B., Butterfield D. A., Huber J. A., Spatially distinct, temporally stable microbial populations mediate biogeochemical cycling at and below the seafloor in hydrothermal vent fluids. Environ. Microbiol. 20, 769–784 (2018). [DOI] [PubMed] [Google Scholar]
- 20.Sylvan J. B. et al., Evidence for microbial mediation of subseafloor nitrogen redox processes at Loihi Seamount, Hawaii. Geochim. Cosmochim. Acta 198, 131–150 (2017). [Google Scholar]
- 21.Butterfield D. A., et al. , “Mixing, reaction and microbial activity in the sub-seafloor revealed by temporal and spatial variation in diffuse flow vents at Axial volcano” in The Subseafloor Biosphere at Mid-Ocean Ridges, Wilcock W. S. D., Delong E. F., Kelley D. S., Baross J. A., Cary S. C., Eds. (American Geophysical Union, 2004), Vol. 144, pp. 269–289. [Google Scholar]
- 22.Proskurowski G., Lilley M. D., Olson E. J., Stable isotopic evidence in support of active microbial methane cycling in low-temperature diffuse flow vents at 9°50′N East Pacific Rise. Geochim. Cosmochim. Acta 72, 2005–2023 (2008). [Google Scholar]
- 23.Beinart R. A., Gartman A., Sanders J. G., Luther G. W., Girguis P. R., The uptake and excretion of partially oxidized sulfur expands the repertoire of energy resources metabolized by hydrothermal vent symbioses. Proc. Biol. Sci. 282, 20142811 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kinsey J. C., German C. R., Sustained volcanically-hosted venting at ultraslow ridges: Piccard hydrothermal field, Mid-Cayman Rise. Earth Planet. Sci. Lett. 380, 162–168 (2013). [Google Scholar]
- 25.McDermott J. M., Sylva S. P., Ono S., German C. R., Seewald S., Geochemistry of fluids from Earth’s deepest ridge-crest hot-springs: Piccard hydrothermal field, Mid-Cayman Rise. Geochim. Cosmochim. Acta 228, 95–118 (2018). [Google Scholar]
- 26.Worman S. L., Pratson L. F., Karson J. A., Schlesinger W. H., Abiotic hydrogen (H2) sources and sinks near the Mid-Ocean Ridge (MOR) with implications for the subseafloor biosphere. Proc. Natl. Acad. Sci. U.S.A. 117, 13283–13293 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Trefry J. H. et al., Trace metals in hydrothermal solution from Cleft segment on the southern Juan de Fuca Ridge. J. Geophys. Res. 99, 4925–4935 (1994). [Google Scholar]
- 28.Takai K. et al., Geochemical and microbiological evidence for a hydrogen-based, hyperthermophilic subsurface lithoautotrophic microbial ecosystem (HyperSLiME) beneath an active deep-sea hydrothermal field. Extremophiles 8, 269–282 (2004). [DOI] [PubMed] [Google Scholar]
- 29.McDermott J. M., Seewald J. S., German C. R., Sylva S. P., Pathways for abiotic organic synthesis at submarine hydrothermal fields. Proc. Natl. Acad. Sci. U.S.A. 112, 7668–7672 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lang S. Q. et al., Deeply-sourced formate fuels sulfate reducers but not methanogens at Lost City hydrothermal field. Sci. Rep. 8, 755 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Joyce T. M., Hernandez-Guerra A., Smethie M. Jr., Zonal circulation in the NW Atlantic and Caribbean from a meridional World Ocean Circulation Experiment hydrographic section at 66°W. J. Geophys. Res. 106, 22095–22113 (2001). [Google Scholar]
- 32.Eldridge D. L., Guo W., Farquhar J., Theoretical estimates of equilibrium sulfur isotope effects in aqueous sulfur systems: Highlighting the role of isomers in the sulfite and sulfoxylate systems. Geochim. Cosmochim. Acta 195, 171–200 (2016). [Google Scholar]
- 33.Luther G. W. III et al., Chemical speciation drives hydrothermal vent ecology. Nature 410, 813–817 (2001). [DOI] [PubMed] [Google Scholar]
- 34.Mullaugh K. M. et al., Voltammetric (micro)electrodes for the in situ study of Fe2+ oxidation kinetics in hot springs and S2O32− production at hydrothermal vents. Electroanalysis 20, 280–290 (2008). [Google Scholar]
- 35.Head I. M., Jones D. M., Larter S. R., Biological activity in the deep subsurface and the origin of heavy oil. Nature 426, 344–352 (2003). [DOI] [PubMed] [Google Scholar]
- 36.Schoell M., Multiple origins of methane in the Earth. Chem. Geol. 71, 1–10 (1988). [Google Scholar]
- 37.Schoell M., The hydrogen and carbon isotopic composition of methane from natural gases of various origins. Geochim. Cosmochim. Acta 44, 649–661 (1980). [Google Scholar]
- 38.Lorant F., Prinzhofer A., Behar F., Huc A. Y., Carbon isotopic and molecular constraints on the formation and the expulsion of thermogenic hydrocarbon gases. Chem. Geol. 147, 249–264 (1998). [Google Scholar]
- 39.Rooney M. A., Claypool G. E., Chung H. M., Modeling thermogenic gas generation using carbon isotope ratios of natural gas hydrocarbons. Chem. Geol. 126, 219–232 (1995). [Google Scholar]
- 40.Reeves E. P., McDermott J. M., Seewald J. S., The origin of methanethiol in midocean ridge hydrothermal fluids. Proc. Natl. Acad. Sci. U.S.A. 111, 5474–5479 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Machel H. G., Bacterial and thermochemical sulfate reduction in diagenetic settings—old and new insights. Sediment. Geol. 140, 143–175 (2001). [Google Scholar]
- 42.Truche L. et al., Experimental reduction of aqueous sulphate by hydrogen under hydrothermal conditions: Implication for the nuclear waste storage. Geochim. Cosmochim. Acta 73, 4824–4835 (2009). [Google Scholar]
- 43.Orcutt B. N., Sylvan J. B., Knab N. J., Edwards K. J., Microbial ecology of the dark ocean above, at, and below the seafloor. Microbiol. Mol. Biol. Rev. 75, 361–422 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Meyer J. L., Akerman N. H., Proskurowski G., Huber J. A., Microbiological characterization of post-eruption “snowblower” vents at Axial Seamount, Juan de Fuca Ridge. Front. Microbiol. 4, 153 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Santelli C. M. et al., Abundance and diversity of microbial life in ocean crust. Nature 453, 653–656 (2008). [DOI] [PubMed] [Google Scholar]
- 46.Brault M., Simoneit B. R. T., Marty J. C., Saliot A., Hydrocarbons in waters and particulate material from hydrothermal environments at the East Pacific Rise, 13°N. Org. Geochem. 12, 209–219 (1988). [Google Scholar]
- 47.Lang S. Q., Butterfield D. A., Lilley M. D., Johnson H. P., Hedges J. I., Dissolved organic carbon in ridge-axis and ridge-flank hydrothermal systems. Geochim. Cosmochim. Acta 70, 3830–3842 (2006). [Google Scholar]
- 48.Hawkes J. A. et al., Efficient removal of recalcitrant deep-ocean dissolved organic matter during hydrothermal circulation. Nat. Geosci. 8, 856–860 (2015). [Google Scholar]
- 49.Lang S. Q., Butterfield D. A., Schulte M., Kelley D. S., Lilley M. D., Elevated concentrations of formate, acetate and dissolved organic carbon found at the Lost City hydrothermal field. Geochim. Cosmochim. Acta 74, 941–952 (2010). [Google Scholar]
- 50.McCollom T. M., Geochemical constraints on sources of metabolic energy for chemolithoautotrophy in ultramafic-hosted deep-sea hydrothermal systems. Astrobiology 7, 933–950 (2007). [DOI] [PubMed] [Google Scholar]
- 51.Seewald J. S., Doherty K. W., Hammar T. R., Liberatore S. P., A new gas-tight isobaric sampler for hydrothermal fluids. Deep. Res. Part I Oceanogr. Res. Pap. 49, 189–196 (2002). [Google Scholar]
- 52.Bischoff J. L., Dickson F. W., Seawater-basalt interaction at 200°C and 500 bars: Implications for origin of seafloor heavy-metal deposits and regulation of seawater chemistry. Earth Planet. Sci. Lett. 25, 385–397 (1975). [Google Scholar]
- 53.Mottl M. J., Holland H. D., Chemical exchange during hydrothermal alteration of basalt by seawater—I. Experimental results for major and minor components of seawater. Geochim. Cosmochim. Acta 42, 1103–1115 (1978). [Google Scholar]
- 54.Seyfried W. E., Bischoff J. L., Experimental seawater-basalt interaction at 300°C, 500 bars, chemical exchange, secondary mineral formation and implications for the transport of heavy metals. Geochim. Cosmochim. Acta 45, 135–147 (1981). [Google Scholar]
- 55.Von Damm K. L. et al., Chemistry of submarine hydrothermal solutions at 21°N, East Pacific Rise. Geochim. Cosmochim. Acta 49, 2197–2220 (1985). [Google Scholar]
- 56.Johnson J. W., Oelkers E. H., Helgeson H. C., SUPCRT92: A software package for calculating the standard molal thermodynamic properties of minerals, gases, aqueous species, and reactions from 1 to 5000 bar and 0 to 1000°C. Comput. Geosci. 18, 899–947 (1992). [Google Scholar]
- 57.Toulmin P., Barton P. B., A thermodynamic study of pyrite and pyrrhotite. Geochim. Cosmochim. Acta 28, 641–671 (1964). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the article and SI Appendix.