Significance
Circadian clocks, biological oscillators with temperature-compensated periods close to 24 h, enhance fitness in Earth’s daily cycle. Although many circadian period mutants have helped to elucidate the circadian clock mechanism, the tuning mechanism of 24-h period remains unclear. In this study, we found that mutants at position 402 of cyanobacterial clock protein KaiC alter circadian periods dramatically, from 0.6 to 6.6 d. Despite the wide-range tunability, temperature compensation of period was kept nearly intact. As these astounding results can be observed in in vitro system, residue 402 is the important position for tuning mechanism of period. Furthermore, we found that the volume of residue 402 of KaiC is the key factor in determination of the length of circadian period.
Keywords: circadian clock, cyanobacteria, period, KaiC, ATPase
Abstract
The circadian clock of cyanobacteria consists of only three clock proteins, KaiA, KaiB, and KaiC, which generate a circadian rhythm of KaiC phosphorylation in vitro. The adenosine triphosphatase (ATPase) activity of KaiC is the source of the 24-h period and temperature compensation. Although numerous circadian mutants of KaiC have been identified, the tuning mechanism of the 24-h period remains unclear. Here, we show that the circadian period of in vitro phosphorylation rhythm of mutants at position 402 of KaiC changed dramatically, from 15 h (0.6 d) to 158 h (6.6 d). The ATPase activities of mutants at position 402 of KaiC, without KaiA and KaiB, correlated with the frequencies (1/period), indicating that KaiC structure was the source of extra period change. Despite the wide-range tunability, temperature compensation of both the circadian period and the KaiC ATPase activity of mutants at position 402 of KaiC were nearly intact. We also found that in vivo and in vitro circadian periods and the KaiC ATPase activity of mutants at position 402 of KaiC showed a correlation with the side-chain volume of the amino acid at position 402 of KaiC. Our results indicate that residue 402 is a key position of determining the circadian period of cyanobacteria, and it is possible to dramatically alter the period of KaiC while maintaining temperature compensation.
Circadian clocks, biological oscillators with temperature-compensated periods close to 24 h, serve as fundamental timing devices for living cells by encoding Earth’s physical rotation time as a pacemaker (1). To understand the fundamental principles underlying circadian clocks, the cyanobacterial circadian clock is a simple and useful model because it is the sole example thus far known to be reconstituted in vitro. When the three clock proteins KaiA, KaiB, and KaiC are mixed in a test tube, the phosphorylation state of KaiC exhibits a temperature-compensated circadian rhythm (2). KaiC, a duplicate P-loop ATPase belonging to the RecA superfamily, consists of N-terminal CI and C-terminal CII domains and forms an ATP-dependent hexametric ring (3–5). Although the ATPase activity of KaiC is extremely low (10–15 ATPs per day per KaiC at 30 °C), it exhibits temperature compensation and correlation with a circadian frequency even in the absence of KaiA and KaiB (6, 7). Therefore, KaiC functions as a circadian pacemaker that determines period and temperature compensation.
To enhance the fitness of living cells on Earth’s environment, one of the essential features of the circadian clock is the 24-h period. To reveal the tuning mechanism of the 24-h period, clock mutants showing unusually long and/or short periods would be useful (8). Generally, clock mutants that alter period length by more than 3–4 h are considered unusual period mutants, e.g., frq-1 (17 h) and frq-7 (29 h) of Neurospora, perT (16 h) and timUL (33 h) of Drosophila, hamster tau mutant (20 h), mouse Clock mutant (27 h), and toc1-1 (22 h) and ztl-1 (33 h) of Arabidopsis (9–15). In cyanobacteria, genetic analyses of circadian phenomena by using EMS-based mutagenesis have revealed numerous KaiC period mutants harboring point mutations, which have helped elucidate circadian systems of cyanobacteria (3). The periods of KaiC mutants range from 16 to 60 h, showing the extremely wide-range tunability of periods by KaiC point mutations. In this point, circadian clock system of cyanobacteria would be an ideal model for the study of tuning mechanism of the 24-h period. However, the tuning mechanism of the 24-h period installed in KaiC protein remains unclear, as the mutation sites are distributed in an apparently random fashion throughout the protein, and the effects of mutation on circadian period are not consistent.
In this study, we found that residue 402 of KaiC is an important position responsible for determining 24-h period. Both in vivo and in vitro circadian periods of mutants at position 402 of KaiC changed dramatically, from about half a day up to about 1 wk. The ATPase activity of mutants at position 402 of KaiC, in the absence of KaiA and KaiB, showed the correlation with the frequency (1/period), indicating that KaiC structure was the source of extreme period change. Despite the wide-range tunability, temperature compensation of both the circadian period and the KaiC ATPase activity of mutants at position 402 of KaiC were kept nearly intact. In this paper, we show that the volume of residue 402 of KaiC is involved in the tuning mechanism of 24-h period defined by KaiC ATPase activity.
Results
Period of a KaiC Mutant, in Which Residue Tyr402 Is Replaced with Cys (Y402C), Is Longer than 3 d.
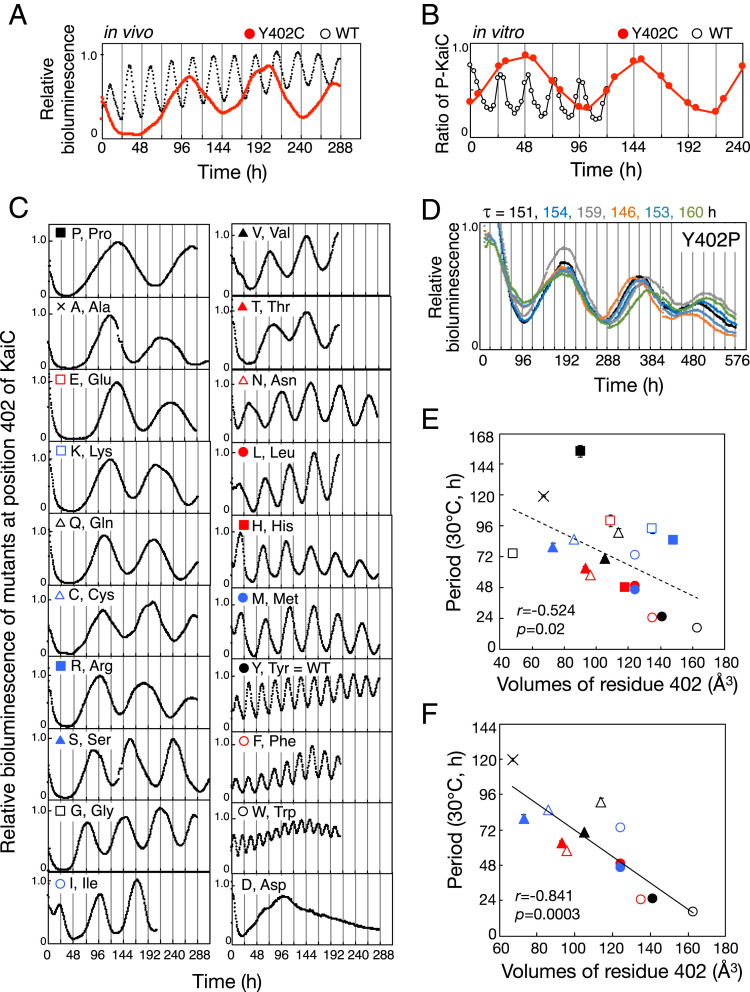
An effective strategy for identifying the critical determinant of the 24-h period is one focusing on mutation sites giving unusually long and/or short periods (8). In the collection of mutant cyanobacteria kept in our laboratory, we focused on a KaiC mutant strain, in which residue Tyr402 was replaced with Cys (Y402C), because the bioluminescence rhythm of Y402C showed only one peak under our standard bioluminescence measurement (∼5 d), suggesting that period of Y402C was very long. We performed the extended bioluminescence rhythm assay more than 10 d and confirmed that period of KaiC-Y402C was very long (∼85 h) (Fig. 1A). The in vitro phosphorylation rhythm of Y402C also exhibited a robust oscillation with a very long period (103 h) (Fig. 1B). The stable long-period phenotype of Y402C suggested that residue 402 of KaiC is one of the critical positions responsible for determining the 24-h period.
Fig. 1.
Aberrant circadian rhythms of mutants at position 402 of KaiC. (A) Representative bioluminescence rhythms of Y402C (red circles) and WT (black circles) at 30 °C. (B) Representative in vitro phosphorylation rhythm of Y402C (black circles) and WT (gray circles). KaiC protein was incubated at 30 °C in the presence of KaiA and KaiB, and aliquots of reaction mixtures were collected every 3 h (WT) or twice a day (Y402C). (C) Representative bioluminescence rhythms of the indicated KaiC mutants of Tyr402 at 30 °C. Data are shown in the order of period lengths. (D) Six independent measurements of bioluminescence rhythms and periods (τ) of Y402P at 30 °C. (E) Periods of bioluminescence rhythms of cyanobacteria with all possible amino acid substitutions at residue 402 of KaiC, plotted against van der Waals volumes of residue 402. Y402D is not shown because it was arrhythmic. (F) Periods of bioluminescence rhythms of cyanobacteria with uncharged amino acids other than proline and glycine at residue 402 of KaiC, plotted against van der Waals volumes of residue 402. Symbols are as in C. Values are represented as means ± SD. The sample sizes (n) are shown in SI Appendix, Table S1. r, correlation coefficient. p, P value. To determine period, peak times after 48 h (Y402P, Y402A, Y402E, Y402K, Y402Q, Y402C, Y402R, Y402S, Y402G, Y402I, Y402V, Y402T, Y402N, Y402L, Y402H, and Y402M) were used for the analysis to reject unstable peaks during the first 2 d.
The Circadian Period of Cyanobacteria Changes Dramatically, Correlating with the Volume of the Residue 402 of KaiC.
To investigate the effect of substitution of residue Tyr402 on period length, we prepared KaiC mutant strains in which this residue was replaced with 19 other natural amino acids and then measured the bioluminescence rhythms of each mutant (Fig. 1 C–E and SI Appendix, Table S1). The mutants exhibited rhythms with a very wide range of periods, from 16 h (Y402W) to 154 h (Y402P). Interestingly, mutants at position 402 of KaiC exhibited a correlation between their period lengths and the volume (16) of the residue at this position (Fig. 1E). The smaller the volume of the residue, the longer the period, suggesting that residue volume at this position is involved in the period determination. Although several studies have reported a correlation between residue volume at a specific position and a biochemical activity (17, 18), such correlation involving the circadian period would be interesting.
There appear to be two factors affecting this period–volume correlation. First, the Y402P mutant strain showed the longest period, and the Y402G mutant strain showed the shorter period than the approximate predictions (Fig. 1 D and E). We speculate that their poor correlations are due to a destabilization of the KaiC structure upon substituting Tyr for Pro/Gly, causing enhanced restriction/freedom for main-chain conformation (19), at position 402 constituting the α7 helix. Second, except for Y402H, the period lengths of mutant strains with charged amino acids (Y402E, Y402K, and Y402R) showed longer periods than the approximate predictions (SI Appendix, Fig. S1). The charged side chains may be solvent-exposed on seeking for countercharged ions or interact with countercharged side chains nearby Y402, such as Lys364, Gly398, and Glu406 (SI Appendix, Fig. S2A). The period lengths may be prolonged because of these structural perturbations associated with the charge neutralization by yet unknown mechanisms. As histidine is weakly basic, it is possible that histidine at position 402 of KaiC is neutral under our experimental condition. Accordingly, the 12 mutants with uncharged amino acids at position 402, other than proline and glycine, exhibited a high correlation between their period lengths and the volume of the residue at this position (Fig. 1F).
We also investigated the amplitudes of the bioluminescence rhythms of mutants at position 402 of KaiC (SI Appendix, Fig. S3). Unfortunately, we were unable to judge existence of relationships between period lengths and the amplitude of the mutants because the amplitude of the bioluminescence rhythms of some mutants included large deviations originated from instabilities of luciferase activity and/or metabolism of cyanobacterial cell.
Cyanobacteria Conserve the Identity of Amino Acids Involved in Period Determination.
Among natural amino acids, the volume of phenylalanine is closest to that of tyrosine. Consistent with this, the period of Y402F was close to 24 h (Fig. 1 C and E and SI Appendix, Table S1). Sequence alignment of KaiC proteins from cyanobacterial strains revealed that the amino acids corresponding to residue 402 of Synechococcus elongatus PCC (Pasteur culture collection) 7942-KaiC were always tyrosine or phenylalanine, indicating that side-chain volume at position 402 of KaiC plays a conserved role in the mechanism of period determination (SI Appendix, Fig. S2B).
We also investigated amino acid conservation of residues Lys364, Asn368, Gly398, and Glu406, which are located near residue Tyr402, and confirmed that residue Lys364, Gly398, and Glu406 are well conserved among cyanobacterial strains (SI Appendix, Fig. S2). Whereas the properties of the amino acids at residue Asn368 were less conserved among cyanobacterial strains, the side-chain volume of this residue was relatively conserved.
To confirm the significance of amino acid conservation in period determination, we focused on residues Ile65, Glu66, and Ala307 of KaiC, as these amino acids are less well conserved among cyanobacterial strains (SI Appendix, Fig. S4A). We investigated the bioluminescence rhythms of KaiC mutant strains in which residues Ile65, Glu66, and Ala307 were replaced with small, large, polar, positive-charged, and negative-charged amino acids (SI Appendix, Fig. S4 B and C and Table S1). Periods of the bioluminescence rhythm of 14 cyanobacteria strains with mutations in residue Ile65, Glu66, or Ala307 of KaiC were all close to 24 h, with only one exception of A307W (SI Appendix, Fig. S4 B and C and Table S1). These results indicated that cyanobacteria have actively conserved the identity of the amino acid involved in period determination.
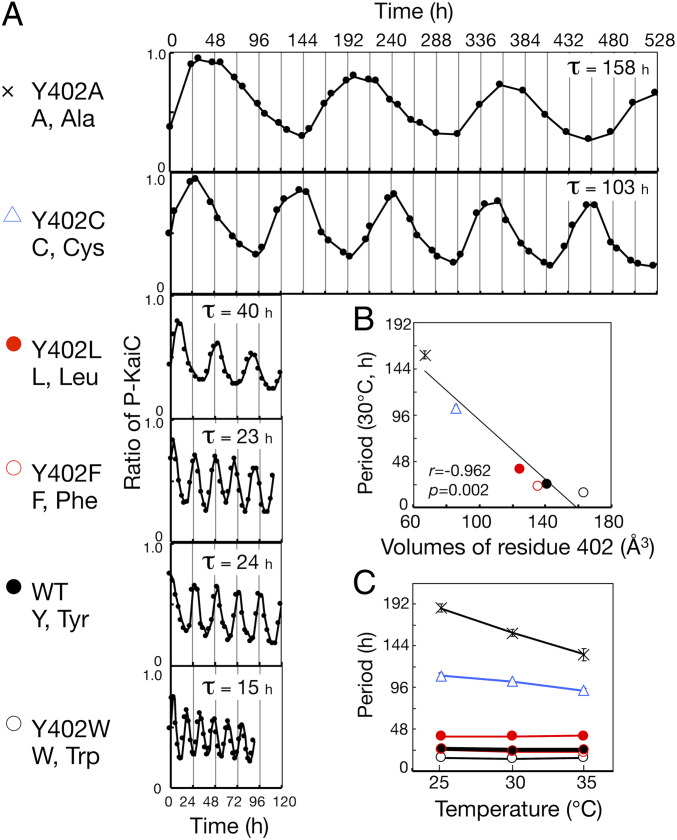
The Circadian Period of In Vitro KaiC Phosphorylation Rhythm of Mutants at Position 402 of KaiC Also Changes Dramatically, from 15 to 158 h.
Next, we compared the in vitro KaiC phosphorylation rhythms of Y402A, Y402C, Y402L, Y402F, and Y402W. These five mutant KaiC proteins exhibited robust phosphorylation rhythms, with periods correlated with their in vivo bioluminescence rhythms (Fig. 2A and SI Appendix, Table S1). Especially, the in vitro KaiC phosphorylation rhythm of Y402A was extremely stable, persisting for ∼20 d with an ultralong period of 158 h (6.6 d). The period length of Y402A was 10 times longer than that of Y402W (15 h), indicating that residue 402 confers a wide-range tuning of the period length. As with their in vivo bioluminescence rhythms, the period lengths of the in vitro KaiC phosphorylation rhythms of the five mutants were correlated with the volume of the residue at position 402 (Fig. 2B). Unfortunately, we could not purify recombinant Y402P protein because it became insoluble during preparation.
Fig. 2.
In vitro phosphorylation rhythms of mutants at position 402 of KaiC. (A) Representative in vitro phosphorylation rhythms and periods (τ) of Y402A, Y402C, Y402L, Y402F, WT, and Y402W at 30 °C. (B) Periods of in vitro phosphorylation rhythms of Y402A, Y402C, Y402L, Y402F, WT, and Y402W, plotted against van der Waals volumes of residue 402. r, correlation coefficient. p, P value. (C) Effect of temperature on period lengths of the in vitro phosphorylation rhythms of Y402A, Y402C, Y402L, Y402F, WT, and Y402W. Symbols are as in A. Values are represented as means ± SD. The sample sizes (n) are shown in SI Appendix, Table S1.
We also investigated the amplitude of in vitro KaiC phosphorylation rhythms of five mutants (SI Appendix, Fig. S5). When we compared the amplitudes of wild-type (WT), Y402F, and Y402W (short period), the shorter the period, the lower the amplitude. The amplitudes of Y402L, Y402C, and Y402A (long period) included large deviations, compared to the accuracy of period lengths of these mutant proteins (SI Appendix, Fig. S5 and Fig. 2B). Therefore, we were unable to evaluate the correlation between the amplitudes and the periods of Y402L, Y402C, and Y402A.
Period Lengths of Mutants at Position 402 of KaiC Are Approximately Temperature-Compensated.
Temperature compensation of period lengths is the most fundamental and unique feature of the circadian clock, as ambient temperature generally influences biochemical activity with a temperature coefficient (Q10) of ∼2–3. Under standard conditions, within temperature ranges from 25 to 35 °C, the periods of the in vitro phosphorylation rhythms of WT, Y402W, Y402F, and Y402L were temperature-compensated, with Q10 values of 1.05, 0.99, 1.13, and 0.98, respectively (Fig. 2C). To exhibit an in vitro phosphorylation rhythm, Y402C and Y402A required 3-fold (3.6 μM) and 3.5-fold (4.2 μM) higher KaiA concentrations, respectively, relative to the standard condition. Excess KaiA affected temperature compensation of period even in WT: the Q10 values of WT were 1.30 at 3.6 μM KaiA and 1.27 at 4.2 μM KaiA (SI Appendix, Fig. S6). Q10 of Y402C was 1.19 (3.6 μM KaiA), indicating that it was better temperature-compensated than the WT (SI Appendix, Fig. S7 and Table S1). The period of the in vitro phosphorylation rhythm of Y402A was slightly more sensitive to temperature than WT, with a Q10 of 1.40 (4.2 μM KaiA), indicating a small effect on temperature compensation relative to the drastic period change (SI Appendix, Fig. S7 and Table S1).
To confirm the temperature dependency of the periods of Y402C and Y402A, we also investigated the temperature dependency of periods of the bioluminescence rhythms of Y402A and Y402C cyanobacterial mutant strains (SI Appendix, Fig. S8 and Table. S1). Within temperature ranges from 25 to 30 °C, the periods of the bioluminescence rhythms of WT and Y402C were temperature-compensated, with Q10 values of 1.04 and 1.02, respectively. The period of the bioluminescence rhythm of Y402A mutant strain was slightly more sensitive to temperature than WT, with a Q10 of 1.20, as with in vitro phosphorylation rhythm of Y402A. In this experiment, the period of Y402C mutant strain at 30 °C was about 15 h longer than that shown in Fig. 1E (SI Appendix, Table. S1). We extended the dark period from 12 h (Fig. 1C) to 24 h (SI Appendix, Fig. S8), to optimize the clock synchronization of long-period mutants. It is possible that the waveform of the bioluminescence rhythm of long-period mutants changed and affected the calculation of the period (Fig. 1C and SI Appendix, Fig. S8; see Materials and Methods).
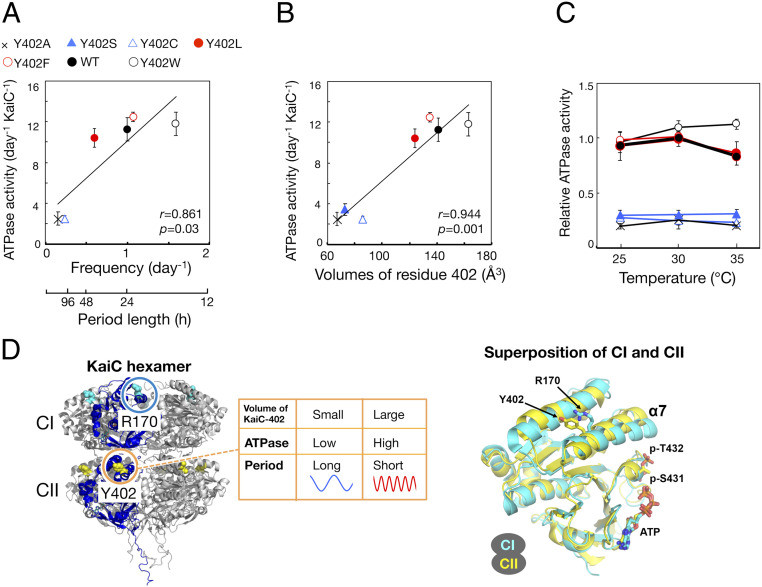
The ATPase Activities of Mutants at Position 402 of KaiC Correlate with the Frequencies and the Volumes of Residue 402.
Next, we investigated whether these prominent features of mutants at position 402 of KaiC (period-changed significantly but temperature-compensated) were derived from the ATPase activity of KaiC as a circadian pacemaker. Consistent with our previous study, in the absence of KaiA and KaiB, the ATPase activities of five mutants at position 402 of KaiC (Y402A, Y402C, Y402L, Y402F, and Y402W) were correlated with the frequencies of their in vitro phosphorylation rhythms: the higher the KaiC ATPase activity, the shorter the period (Fig. 3A). As with the periods of the circadian rhythms, the ATPase activities of six mutants at position 402 of KaiC (Y402A, Y402C, Y402S, Y402L, Y402F, and Y402W) were correlated with the volume of residue 402: the larger the volume, the higher the KaiC ATPase activity (Fig. 3B). On the other hand, the ATPase activities of Y402W and Y402L were less correlated with the frequencies of their in vitro phosphorylation rhythms (Fig. 3A) (see Discussion).
Fig. 3.
ATPase activities of mutants at position 402 of KaiC. (A and B) ATPase activity of Y402A, Y402S, Y402C, Y402L, Y402F, WT, and Y402W plotted against frequencies of in vitro phosphorylation rhythms (A) and van der Waals volumes of residue 402 of KaiC (B). In the absence of KaiA and KaiB, KaiC was incubated at 30 °C for 3 d (n = 11 for WT; n = 7 for Y402A, Y402S, Y402C, Y402W and Y402L; n = 9 for Y402F). r, correlation coefficient. p, P value. (C) Effects of temperature on ATPase activity of Y402A, Y402C, Y402L, Y402F, WT, and Y402W (n = 4 for WT, Y402A, Y402S, Y402C, Y402W and Y402L; n = 3 for Y402F). Values are presented as means ± SD. (D) (Left) Overall structure of KaiC hexamer and positions Y402 and R170, colored yellow and cyan (PDB ID: 3DVL). (Right) Superposition of CI (residues 19–250; cyan) and CII domains (residues 261–482; yellow) (accession code: 3DVL) (20). Y402, R170, phosphorylated T432, phosphorylated S432, and ATP are shown in the stick model. The rmsd of the Cα pairs is 1.52 Å.
Temperature compensation of ATPase activity was preserved in six mutant KaiC proteins, with Q10 values of 0.9 (WT), 1.1 (Y402A), 1.0 (Y402S), 0.8 (Y402C), 0.9 (Y402L), 0.9 (Y402F), and 1.2 (Y402W) (Fig. 3C). These results indicated that the volume of residue 402 of KaiC affects pacemaker activity defined by KaiC ATPase activity and changed the circadian period without altering temperature compensation (Fig. 3D).
The Mutation of Residue 402 in CII Domain of KaiC Affects the ATPase Activity of CI through the CI/CII Contact Region.
Residue 402 of KaiC is located at the α7 helix in C-terminal CII domain, near the contact region between the N-terminal CI and C-terminal CII domains (7, 20). Multiple sequence alignment and superposition of structures of CI and CII domain confirmed that the residue corresponding to Tyr402 in CI is Arg170 (Fig. 3D and SI Appendix, Fig. S9A). The periods of 12 mutants at position 170 were correlated with residue volume (SI Appendix, Fig. S9 B and C and Table S1). The periods of in vitro phosphorylation rhythm and the ATPase activity of R170T and R170H were also correlated with residue volume (SI Appendix, Fig. S10 and Table S1). However, the effects of substitution of Arg170 on period and KaiC ATPase activity were much milder than those of substitution of Tyr402, indicating that residue 402 has an important function due to its localization at the contact region between CI and CII.
It is reported that CI domain mainly assumes the ATPase activity of KaiC because the ATPase activity of full-length KaiC (WT) and a truncated version of KaiC consisting only of the CI domain (KaiC-CI) is 10–15/KaiC per day and ∼10/KaiC per day, respectively (6, 7) (Fig. 3A). The ATPase activities of full-length Y402A and Y402C (∼3/KaiC per day) were significantly lower than that of KaiC-CI (∼10/KaiC per day) (Fig. 3A) (6, 7). Therefore, it is thought that volume of residue 402 affects the ATPase activity of CI through the CI/CII contact region (Fig. 3D).
Discussion
In this study, we found that circadian period of mutants at position 402 of KaiC changes severely (Figs. 1 and 2). Period of mutants at residue 402 of KaiC changes with 10-fold dynamic range (Figs. 1 and 2). Especially, the period of in vitro phosphorylation rhythm of KaiC-Y402A (6.6 d = 158 h) is much longer than clock mutants so far reported in cyanobacteria (from 16 to 60 h) (3) (Fig. 2). These extreme period changes of mutants are caused by the volumes of residue 402. As the ATPase activity of KaiC, in the absence of KaiA and KaiB, correlates with the volume of residue 402, the volume of this residue could change the KaiC ATPase activity and thereby defines the circadian period (Fig. 3). The substantial effect of mutation in residue 402 on period and the simple correlation between period and residue volume at this position indicate that residue 402 of KaiC is one of the critical determinants of the period of the cyanobacterial circadian clock. In fact, cyanobacterial strains conserve the volume of this residue (SI Appendix, Fig. S2B). Structural analysis on the function of residue 402 of KaiC would provide important information about the mechanism of period determination based on the KaiC ATPase activity.
In this experiment, the ATPase activities of Y402W and Y402L were less correlated with the frequencies of their in vitro phosphorylation rhythms (Fig. 3A). This result might suggest that the mutation at position 402 of KaiC also affects the CII ATPase activity, in addition to the period-determining step by the CI ATPase activity. On the other hand, it is also possible that the mutation at position 402 of KaiC affects the quality or stability of purified protein.
Whereas temperature compensation of both KaiC ATPase activity and period are kept intact in almost all mutants at residue 402 examined, the period of Y402A is slightly more sensitive to temperature (Q10 = 1.40 for in vitro phosphorylation rhythm, and Q10 = 1.20 for in vivo bioluminescence rhythm) than that of WT (Q10 = 1.27 for in vitro phosphorylation rhythm, and Q10 = 1.04 for in vivo bioluminescence rhythm) (Fig. 2C and SI Appendix, Fig. S8). It is possible that the extreme long period of Y402A partly affects the temperature compensation of period through the unknown mechanism because the ATPase activity of KaiC-Y402A is temperature-compensated (Q10 = 1.1) (Fig. 3C). On the other hand, the effect of temperature on the period of in vivo bioluminescence rhythm of Y402A (Q10 = 1.20) is much smaller, compared to the general biochemical activity (Q10 = 2–3).
Therefore, our results indicate that the circadian pacemaker of cyanobacteria, which functions without a transcription–translation feedback loop, can define the period of extra wide dynamic range, while keeping temperature compensation intact. These features are installed in the structure of KaiC protein, as ATPase activity of KaiC can determine both circadian period and temperature compensation. To establish both flexibility of period determination and persistence of temperature compensation within KaiC protein, it is possible that novel physical mechanism inside the KaiC structure links energy derived from the ATP hydrolysis with circadian pacemaker function. By maintaining such mechanism, cyanobacteria may have been able to adapt to the period of Earth’s rotation, which lengthened due to the tidal friction, and thus succeed among the organisms to inhabit this planet (21, 22).
Materials and Methods
Cyanobacterial Strains and Culture Conditions.
S. elongatus PCC 7942 was used as the background strain for this study. NUC43 is a kaiABC-deleted strain carrying the PkaiBC::luxAB reporter construct at neutral site I, and pCkaiABC is a targeting plasmid carrying kaiABC and the omega fragment (23). NUC43 harboring pCkaiABC was used as the WT strain. All Synechococcus cells were cultured at 30 °C in either modified BG-11 liquid medium or solid BG-11 medium containing 1.5% Bacto Agar (Difco) under continuous light illumination (LL) of 100 µmol m−2 s−1. Light was provided by an light emitting diode (LED) daylight lamp.
Bioluminescence Rhythm Assay.
Bioluminescence assay and analysis were performed as previously described (3, 23). Cyanobacterial cells were cultured on BG-11 solid medium under LL at 100 µmol m−2 s−1 at 30 °C for 3 d. After two 12-h light/12-h dark treatments, the cells were transferred to LL at 30 °C (27 µmol m−2 s−1) from an LED daylight lamp. Bioluminescence profiles were monitored using a photomultiplier tube detector or a cooled-CCD (charge-coupled device) camera system. Bioluminescence traces are representative of three or more experiments. For the analysis of the effect of temperature on the period lengths (SI Appendix, Fig. S8), the cells were entrained by one 24-h dark treatment. For the bioluminescence monitoring at 25 and 30 °C, the dishes were reversed to avoid dew condensation on the lid under low temperature.
Preparation of Kai Proteins.
Recombinant KaiC protein was expressed and purified as described previously (24). Recombinant KaiA and KaiB proteins were expressed and purified as described previously (24), with slight modifications. After Resource Q anion exchange chromatography (GE Healthcare), the proteins were purified using a Bio-Scale CHT2-I Column (BIO-RAD). KaiA and KaiB were then further purified using a Superdex 200 column (GE Healthcare) and a Superdex 75 column (GE Healthcare), respectively.
Reconstitution of In Vitro KaiC Phosphorylation Rhythm.
Reconstitution of the in vitro KaiC phosphorylation rhythm was performed as described previously (2), except that reaction buffer (20 mM Tris [pH 7.8], 150 mM NaCl, 5 mM MgCl2, and 1 mM ATP) was used for the assay. Concentrations of KaiA, KaiB, and KaiC were 1.2, 3.5, and 3.5 μM, respectively (standard conditions). For reconstruction of the in vitro phosphorylation rhythm of Y402C and Y402A, the concentrations of KaiA were 3.6 and 4.2 μM, respectively. In addition, for reconstruction of the in vitro phosphorylation rhythm of Y402C and Y402A, the ATP concentration was 3 mM, and 3 μg/mL vancomycin (final concentration) was added to repress bacterial contamination. After estimation of protein concentration of KaiC using the Quick Start Bradford Protein Assay (Bio-Rad), the reaction mixture was prepared. Periods were estimated as described previously (25), except that KaiC phosphorylation ratio after second peak was used for the analysis.
ATPase Assay Using UPLC System.
The ATPase activity of KaiC was measured using an ACQUITY UPLC (ultra-performance liquid chromatography) system (Waters) as described previously (26), except that the buffer composition was modified to improve peak separation. ADP was separated from ATP on a BEH C18 column (2.1 ID × 50 mm, 1.7 μm) (Waters) at a flow rate of 0.8 mL min−1 with a mobile phase of 14 mM ammonium phosphate, 7 mM tetrabutylammonium hydrogen sulfate (pH 8.5), and 15% (vol/vol) acetonitrile. ADP concentrations were calculated from the corresponding peak areas.
Supplementary Material
Acknowledgments
We are grateful for the technical assistance of K. Izuka, T. Nishikawa, and S. Yi. This work was supported by Grants-in-Aid for Scientific Research (24000016 and 17H01427 to T.K. and 17H06165 to S.A.).
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2005496117/-/DCSupplemental.
Data Availability.
All data, associated protocols, methods, and sources of materials are available in the main text or SI Appendix, and the source data are available at DOI: 10.6084/m9.figshare.12694043.
References
- 1.Johnson C. H. et al., “Fundamental properties of circadian rhythms” in Chronobiology: Biological Timekeeping, Dunlap J. C., Loros J. J., Decoursey P. J., Eds. (Sinauer, 2004), pp. 67–105. [Google Scholar]
- 2.Nakajima M. et al., Reconstitution of circadian oscillation of cyanobacterial KaiC phosphorylation in vitro. Science 308, 414–415 (2005). [DOI] [PubMed] [Google Scholar]
- 3.Ishiura M. et al., Expression of a gene cluster kaiABC as a circadian feedback process in cyanobacteria. Science 281, 1519–1523 (1998). [DOI] [PubMed] [Google Scholar]
- 4.Iwasaki H., Taniguchi Y., Ishiura M., Kondo T., Physical interactions among circadian clock proteins KaiA, KaiB and KaiC in cyanobacteria. EMBO J. 18, 1137–1145 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mori T. et al., Circadian clock protein KaiC forms ATP-dependent hexameric rings and binds DNA. Proc. Natl. Acad. Sci. U.S.A. 99, 17203–17208 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Terauchi K. et al., ATPase activity of KaiC determines the basic timing for circadian clock of cyanobacteria. Proc. Natl. Acad. Sci. U.S.A. 104, 16377–16381 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abe J. et al., Circadian rhythms. Atomic-scale origins of slowness in the cyanobacterial circadian clock. Science 349, 312–316 (2015). [DOI] [PubMed] [Google Scholar]
- 8.Takahashi J. S., Finding new clock components: Past and future. J. Biol. Rhythms 19, 339–347 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gardner G. F., Feldman J. F., The frq locus in Neurospora crassa: A key element in circadian clock organization. Genetics 96, 877–886 (1980). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Konopka R. J., Hamblen-Coyle M. J., Jamison C. F., Hall J. C., An ultrashort clock mutation at the period locus of Drosophila melanogaster that reveals some new features of the fly’s circadian system. J. Biol. Rhythms 9, 189–216 (1994). [DOI] [PubMed] [Google Scholar]
- 11.Rothenfluh A., Abodeely M., Price J. L., Young M. W., Isolation and analysis of six timeless alleles that cause short- or long-period circadian rhythms in Drosophila. Genetics 156, 665–675 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lowrey P. L. et al., Positional syntenic cloning and functional characterization of the mammalian circadian mutation tau. Science 288, 483–492 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vitaterna M. H. et al., Mutagenesis and mapping of a mouse gene, clock, essential for circadian behavior. Science 288, 483–491 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Millar A. J., Carré I. A., Strayer C. A., Chua N. H., Kay S. A., Circadian clock mutants in Arabidopsis identified by luciferase imaging. Science 267, 1161–1163 (1995). [DOI] [PubMed] [Google Scholar]
- 15.Somers D. E., Schultz T. F., Milnamow M., Kay S. A., ZEITLUPE encodes a novel clock-associated PAS protein from Arabidopsis. Cell 101, 319–329 (2000). [DOI] [PubMed] [Google Scholar]
- 16.Darby N. J., Creighton T. E., “Basic aspects of polypeptide structure” in Protein Structure, Creighton T. E., Ed. (Oxford University Press, 1993), pp. 1–22. [Google Scholar]
- 17.el-Zaatari F. A., Sams K. C., Taurog J. D., In vitro mutagenesis of HLA-B27. Amino acid substitutions at position 67 disrupt anti-B27 monoclonal antibody binding in direct relation to the size of the substituted side chain. J. Immunol. 144, 1512–1517 (1990). [PubMed] [Google Scholar]
- 18.Atta-Asafo-Adjei E., Daldal F., Size of the amino acid side chain at position 158 of cytochrome b is critical for an active cytochrome bc1 complex and for photosynthetic growth of Rhodobacter capsulatus. Proc. Natl. Acad. Sci. U.S.A. 88, 492–496 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O’Neil K. T., DeGrado W. F., A thermodynamic scale for the helix-forming tendencies of the commonly occurring amino acids. Science 250, 646–651 (1990). [DOI] [PubMed] [Google Scholar]
- 20.Pattanayek R. et al., Visualizing a circadian clock protein: Crystal structure of KaiC and functional insights. Mol. Cell 15, 375–388 (2004). [DOI] [PubMed] [Google Scholar]
- 21.Deines S. D., Williams C. A., Earth’s rotational deceleration : Determination of tidal friction independent of timescales. Astron. J. 151, 103 (2016). [Google Scholar]
- 22.Dvornyk V., Vinogradova O., Nevo E., Origin and evolution of circadian clock genes in prokaryotes. Proc. Natl. Acad. Sci. U.S.A. 100, 2495–2500 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nishimura H. et al., Mutations in KaiA, a clock protein, extend the period of circadian rhythm in the cyanobacterium Synechococcus elongatus PCC 7942. Microbiology 148, 2903–2909 (2002). [DOI] [PubMed] [Google Scholar]
- 24.Nishiwaki T. et al., A sequential program of dual phosphorylation of KaiC as a basis for circadian rhythm in cyanobacteria. EMBO J. 26, 4029–4037 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nakajima M., Ito H., Kondo T., In vitro regulation of circadian phosphorylation rhythm of cyanobacterial clock protein KaiC by KaiA and KaiB. FEBS Lett. 584, 898–902 (2010). [DOI] [PubMed] [Google Scholar]
- 26.Murayama Y. et al., Tracking and visualizing the circadian ticking of the cyanobacterial clock protein KaiC in solution. EMBO J. 30, 68–78 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data, associated protocols, methods, and sources of materials are available in the main text or SI Appendix, and the source data are available at DOI: 10.6084/m9.figshare.12694043.