Abstract
Background:
Many older adults with limited life expectancy and/or advanced dementia (LLE/AD) are potentially overtreated for diabetes and may benefit from deintensification. We examined incidence and predictors of diabetes medication deintensification in older Veterans with LLE/AD who were potentially overtreated at admission to VA nursing home (Community Living Centers).
Design:
Retrospective cohort study using linked VA and Medicare clinical/administrative data and Minimum Data Set (MDS) assessments.
Setting:
VA Community Living Centers (CLCs).
Participants:
6,960 Veterans with diabetes and LLE/AD admitted to VA CLCs in fiscal years 2009–2015 with HbA1c measured within 90 days of admission.
Measurements:
We evaluated treatment deintensification (discontinuation or dose reduction for a consecutive 7-day period) among residents who were potentially overtreated (HbA1c≤7.5% and receiving hypoglycemic medications). Competing risk models assessed 90-day cumulative incidence of deintensification.
Results:
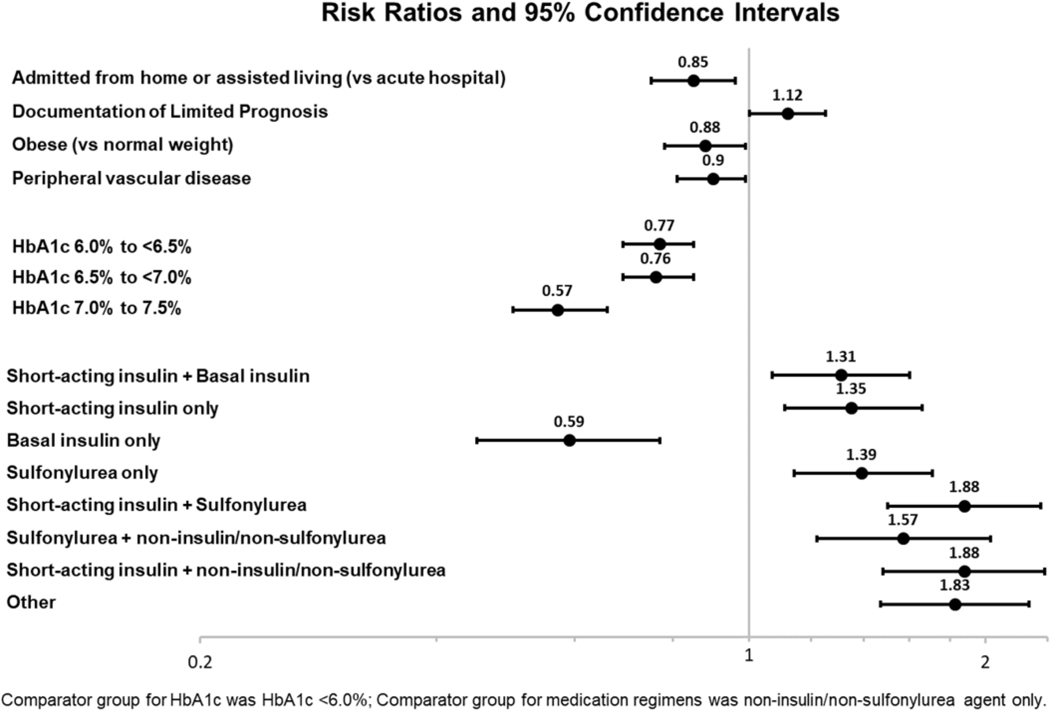
Over 40% (n=3,056) of Veteran CLC residents with diabetes were potentially overtreated. The cumulative incidence of deintensification at 90 days was 45.5%. Higher baseline HbA1c values were associated with lower likelihood of deintensification (e.g., HbA1c 7.0–7.5% vs. <6.0%, adjusted risk ratio (aRR): 0.57, 95% CI: 0.50–0.66). Compared to non-sulfonylurea oral agents (e.g. metformin), other treatment regimens were more likely to be deintensified ( aRR 1.31–1.88), except for basal insulin (aRR: 0.59, 95% CI: 0.52–0.66). The only resident factor associated with increased likelihood of deintensification was documented end-of-life status (aRR: 1.12, 95% CI:1.01–1.25). Admission from home/assisted living (aRR: 0.85, 95% CI: 0.75–0.96), obesity (aRR: 0.88, 95% CI: 0.78–0.99), and peripheral vascular disease (aRR: 0.90, 95% CI: 0.81–0.99) were associated with decreased likelihood of deintensification.
Conclusion:
Deintensification of treatment regimens occurred in under half of potentially overtreated Veterans, and was more strongly associated withlow HbA1c values and use of medications with high risk for hypoglycemia, rather than other resident characteristics.
Keywords: Nursing homes, deprescribing, diabetes, diabetes overtreatment, Veterans Affairs
INTRODUCTION
One in four older adults over age 65 has diabetes1, which is the seventh leading cause of death in the United States and a major contributor to cardiovascular disease.2 Guideline recommendations aimed at slowing the sequalae of diabetes progression have long recommended tight glycemic control, defined as hemoglobin A1C (HbA1c) < 6.5–7.0% for healthy, youngerindividuals.3–5 However, tight glycemic control may cause more harm than benefit in older adults with limited life expectancy and/or advanced dementia (LLE/AD).6 For example, these individuals may not live long enough to experience potential benefits.7,8 In addition, strict glycemic control increases the risk of adverse drug events such as hypoglycemia.9–11 Therefore, many guidelines now advocate for less stringent HbA1c targets (e.g., between 8.0–9.0%) in older adults who have multiple comorbidities, limited life expectancy, and/or reside in nursing homes.3,4,6,12,13
Many older adults with diabetes, including those with comorbid dementia, are potentially overtreated based on HbA1c measurements, according to updated recommendations14–16,17 Among potentially overtreated older outpatients with diabetes, few have their regimens deintensified.15 However, little is known about diabetes overtreatment and efforts to deintensify regimens among older adults with LLE/AD, who are least likely to benefit and most likely to experience harms of tight HbA1c control.18–21 Specifically, deintensifying diabetes treatment regimens in patients with LLE/AD has the potential to prevent unnecessary hospitalizations due to adverse drug events, reduce medication burden, and increase comfort.22,23 Similarly, potential overtreatment and deintensification has also not been well described in the nursing home setting. However, nursing home admission may present an opportunity to assess patients’ goals and preferences and review and adjust medications accordingly. The purpose of this study was to quantify: 1) the prevalence of potential diabetes overtreatment among older adults with LLE/AD residing in Veteran nursing homes, known as Community Living Centers (CLCs); 2) the extent to which potentially overtreated residents with LLE/AD had their regimens deintensified; and 3) association of resident-level characteristics with deintensification.
METHODS
Data sources
We conducted a national retrospective cohort study by merging administrative and clinical data from fiscal years (FY) 2009–2015. This included the VA Residential History File (RHF)24, the VA Minimum Dataset (MDS) for CLCs25,26, the VA Corporate Data Warehouse (CDW)27–30, Medicare claims for Veterans dually enrolled in Medicare31,32, and the VA Vital Status File. The VA Pittsburgh Healthcare System Institutional Review Board approved this study.
The VA RHF (FY2009–2015) tracked the location of Veterans using linked VA, Medicare, Medicaid, and MDS records and was used to identify CLC episodes of care. The MDS is a standardized assessment of functional, psychosocial, and healthcare needs of nursing home residents that is mandated at CLC admission and at least quarterly thereafter. Because the MDS underwent a version change during our study period, we used MDS 2.0 data from Oct. 1, 2008 – June 30, 2012 and MDS 3.0 from July 1, 2013 – Sept. 30, 2015. The MDS was used to identify Veterans with LLE/AD at admission and to capture resident characteristics not available in utilization/claims data (e.g., physical functioning). The CDW provided VA inpatient and outpatient utilization data (FY2007–2015) needed to identify diabetes patients and develop covariates, laboratory records to obtain HbA1c values (FY2009–2015), and medications administered to CLC residents through the Bar Code Medication Administration system (BCMA; FY2009–2015). BCMA records captured drug names and doses each time a medication is administered to CLC resident. Medicare claims for Veterans dually enrolled in Medicare provided additional diagnosis and procedure information for non-VA healthcare settings to identify patients with diabetes and develop specific covariates. The VA Vital Status File provided date of death.
Study sample
We identified all CLC admissions between FY2009–2015 (n=200,333 episodes). We required all residents to meet at least one of three criteria for LLE/AD (n=81,271): 1) MDS Mortality Risk Index – Revised (MMRI-R) score ≥36, which has been validated in both MDS 2.0 and 3.0 and is associated with >50% likelihood of death within 6 months33,34; 2) endorsement of ≤6 months life expectancy on the MDS admission assessment (MDS 2.0: J5c; MDS 3.0: J1400); or 3) advanced dementia identified using either the Brief Interview for Mental Status for residents who were able to self-report with MDS 3.0 (scores ≤7 considered severely impaired)35 or the Cognitive Performance Scale for residents who were assessed with MDS 2.0 or unable to self-report with MDS 3.0 (scores ≥4 are considered severely impaired).36 Residents <65 years old at CLC admission (n=20,135); and those with lengths of stays <7 days (n=2,355) were excluded. We required patients to have diabetes, defined as having ≥1 inpatient or ≥2 outpatient encounters with an ICD-9 diagnosis code for diabetes in the 2 years prior to admission in VA and/or Medicare records37 or endorsement of diabetes as an active diagnosis on the MDS admission assessment (n=33,440 excluded). To assess potential overtreatment, we required residents to have at least one HbA1c measurement during the first 90 days of the CLC stay (n=18,381 excluded). We then identified episodes where residents were potentially overtreated for diabetes based on having HbA1c ≤ 7.5% and receiving ≥1 diabetes medication (i.e., insulin, metformin, sulfonylureas, biguanides, meglitinides, GLP-1 receptor antagnoists, alpha-glucosidase inhibitors, DPP-4 inhibitors, SGLT-2 inhibitors – see Table S3) on the day of or day after HbA1c measurement (n=3,539 excluded). In order to observe potential deintensification in this group, we required participants to have ≥7 days of follow-up after medication index date (i.e., HbA1c date + 1 day, n=267 excluded). Finally, for Veterans ≥2 admissions during the study time frame, we selected one episode at random (n=98 excluded). See Supplementary Figure 1 for further detail.
Overtreatment and baseline diabetes treatment regimens
Using laboratory data, we grouped the index HbA1c into the following categories: <6.0%, 6.0-<6.5%, 6.5%-<7.0%, and 7.0–7.5%. Consistent with treatment guidelines for this population which propose a HbA1c target of 8–9%,3,4,6 we defined potential overtreatment as having an index HbA1c ≤7.5% and receiving diabetes medications on the day of or day following HbA1c measurement. Among those potentially overtreated, we categorized baseline treatment regimens based on medications administered on these two days, specifically the maximum number of medications and the maximum dosages (for non-insulin medications) administered.
Treatment deintensification
The primary outcome for this analysis was treatment deintensification. Among residents identified as potentially overtreated, we examined the extent to which their baseline diabetes treatment regimens were deintensified within 90 days of follow-up after the medication index date. Similar to previous work15, deintensification was defined as decreasing the dose or completely discontinuing a non-insulin agent and/or stopping a type of insulin (e.g., switching from using both short- and long-acting insulin to just long-acting insulin; discontinuing insulin treatment altogether), with no addition of new agents or dose increase of a non-insulin agent. We did not consider insulin dose changes when defining deintensification, since insulin doses may be influenced by factors such as variable eating habits and thus cannot reliably be interpreted as deintensification. Changes had to be sustained over 7 days of follow-up to qualify as deintensification, with the first day of the 7-day period recorded as the deintensification event time. Although prior studies using prescription refill records have required longer gaps in medication supply (i.e. ≥30 days) for discontinuation38,39, we believed that the granularity contained in daily medication administration records would allow us to identify medication changes using a shorter time-frame with sufficient accuracy. In sensitivity analyses, we used a 14-day window to test the stability of our findings.
Follow-up
Residents were followed from the day after medication index date until the earliest of the following: deintensification, death, CLC discharge, administrative censoring (i.e. end of available data), or end of follow-up period (90 days). If a resident was censored or died, their follow-up time was truncated by seven days (e.g., censoring date – 7 days) because any deintensification occurring in this period would be unobservable.40
Resident characteristics associated with deintensification
We selected resident characteristics that may be associated with deintensification based on prior literature of diabetes overtreatment and deintensification, and behavioral theories applied to deprescribing.14–17,41,42 Resident characteristics fell into five categories: socio-demographics, environment of care, diabetes-related factors, cardiovascular risk factors, and markers of poor prognosis. Most characteristics were operationalized using the MDS admission assessment, with additional information on comorbidities captured using VA/Medicare records from the year preceding admission, as well as BCMA data for medication covariates.
Socio-demographics factors included age (65–74 years, 75–84 years, ≥85 years), gender, race/ethnicity (non-Hispanic White, non-Hispanic Black, Hispanic/Latino, other), and being married. Environment of care included admission source (hospital, home/assisted living, or other long-term care facility), hospice use in the year prior to CLC admission, and fiscal year of admission. Diabetes-related factors included baseline HbA1c (<6.0%, 6.0-<6.5%, 6.5%-<7.0%, and 7.0–7.5%), potential diabetes-related complications (e.g., diabetic eye disease, hospitalization for serious hypoglycemic events43) and baseline treatment regimens, with non-insulin medications aggregated into two categories of sulfonylureas and non-sulfonylurea/non-insulin agents, and insulin use classified as short-acting only, basal only, or both short-acting and basal. Cardiovascular factors included coronary artery disease, congestive heart failure, stroke, and hypertension. Markers of poor prognosis included advanced dementia, having explicit documentation at admission that the resident was nearing the end-of-life (admitted to a hospice treatment specialty, or MDS documentation of hospice care in the prior 14 days, or the item for <6 months life expectancy), physical functioning as measured by the MDS Activities of Daily Living Self-Performance Hierarchy44, the Elixhauser comorbidity index45, body mass index (underweight [<18.5], normal [18.5–24.9], overweight [25–29.9], obese [≥30]), recent weight loss, difficulty swallowing, pain and other comorbidities that may affect decision to deintensify from the MDS (e.g., history of falls/fractures). We also considered antidepressant and antipsychotic use, based on any use in the first 7 days of the episode, as these medications may induce metabolic syndromes that would subsequently affect diabetes management.46,47
Statistical Analysis
After estimating the prevalence of potential overtreatment in the entire sample, all subsequent analyses were restricted to residents who were potentially overtreated. In this restricted sample, we described resident characteristics, number of diabetes medications used, and treatment regimens overall and stratified by HbA1c categories (<6.0%, 6.0-<6.5%, 6.5%-<7.0%, and 7.0–7.5%).
We used competing risk survival methods for all time-to-event analyses to account for censoring (e.g., leaving the CLC) and competing risks (death). After describing the crude overall cumulative incidence of treatment deintensification, we then examined the crude cumulative incidence of specific hypoglycemic agents that were deintensified overall and stratified by the most common baseline treatment regimens. We then used Fine and Grey competing risk models to estimate marginal crude and adjusted risk, risk ratios, and risk differences for resident characteristics during 90 days of follow-up; 95% confidence intervals were estimated using 1,000 bootstrap samples. We concluded that all covariates sufficiently met the proportional sub-distribution hazards assumption after examining complimentary log-log transformation of the nonparametric cumulative incidence function and Schoenfeld residual plots.37 A more detailed discussion of our statistical approach is included in the Supplementary Methods Appendix.
In sensitivity analyses, we used a 14-day gap in medication administration (as opposed to a 7-day gap) to evaluate how these definitions affected our results. All analyses were conducted using Stata version 15.0.
RESULTS
We observed potential overtreatment of diabetes in 43.9% of CLC admissions (3,056 / 6,960) for Veterans with diabetes and LLE/AD who had HbA1c measured within the first 90 days. The CLC episodes contributed a total of 306 person-years of follow-up (median follow-up: 25 days; Interquartile range [IQR]: 8–67 days). Most episodes ended with discharge (36.8%) or deintensification (35.3%), followed by censoring at 90 days (18.1%), death (8.6%), and end of data (1.3%).
Potentially overtreated residents had a mean age of 77.6 years (standard deviation: 7.9) and were predominantly male (99.1%) and non-Hispanic white (75.8%; see Table 1). Two-thirds were admitted to CLCs from hospital settings. Twenty-nine percent had advanced dementia, 13.8% had explicit documentation of end-of-life status, and 79% had MMRI≥36. Many were physically dependent (37.1%) and had cardiovascular disease and/or potential diabetes-related complications, including 8.5% with serious hypoglycemic events in the previous year.
Table 1.
Characteristics of older Veterans with potentially overtreated diabetes living in Community Living Centers with limited life expectancy or advanced dementia, overall and stratified by baseline HbA1c (N=3,056).
| Stratified by baseline HbA1c, % |
|||||
|---|---|---|---|---|---|
| Characteristics, % | Overall (N=3,056) | <6.0 (n=811) | 6.0-<6.5 (n=769) | 6.5-<7.0 (n=816) | 7.0–7.5 (n=660) |
| Age in years | |||||
| 65–74 | 37.9 | 43.3 | 37.5 | 35.2 | 35.0 |
| 75–84 | 41.0 | 39.7 | 41.2 | 41.4 | 41.7 |
| ≥85 | 21.2 | 17.0 | 21.3 | 23.4 | 23.3 |
| Racial/ethnic minority | 24.2 | 26.1 | 23.1 | 24.1 | 23.0 |
| Married | 53.1 | 51.4 | 54.2 | 53.7 | 53.0 |
| Nursing home source of admission | |||||
| Acute hospital | 67.5 | 68.8 | 65.8 | 67.0 | 68.3 |
| Home / assisted living | 21.6 | 21.7 | 22.6 | 21.3 | 20.8 |
| Nursing home | 8.0 | 6.3 | 8.8 | 8.6 | 8.2 |
| Other | 2.9 | 3.2 | 2.7 | 3.1 | 2.7 |
| End of life conditions | |||||
| Advanced dementia1 | 29.0 | 25.2 | 31.9 | 29.3 | 29.8 |
| Documentation of end-of-life prognosis2 | 13.8 | 12.1 | 12.6 | 12.7 | 18.5 |
| Physical functioning3 | |||||
| Requiring extensive dependence | 38.9 | 37.7 | 39.8 | 35.2 | 43.8 |
| Physically dependent | 37.1 | 39.3 | 36.8 | 37.9 | 33.8 |
| Pain in the five days preceding MDS admission assessment | 67.7 | 67.9 | 66.8 | 66.2 | 70.5 |
| Body mass index | |||||
| Underweight | 3.8 | 4.3 | 3.4 | 3.1 | 4.5 |
| Normal or healthy weight | 33.8 | 33.3 | 35.2 | 35.4 | 30.9 |
| Overweight | 32.0 | 33.4 | 31.3 | 31.1 | 32.3 |
| Obese | 30.3 | 29.0 | 30.0 | 30.4 | 32.3 |
| Number of Elixhauser comorbidities | |||||
| 0–1 | 8.8 | 9.5 | 7.3 | 9.2 | 9.2 |
| 2–3 | 21.6 | 18.0 | 22.4 | 25.1 | 20.6 |
| 4–5 | 30.2 | 28.7 | 29.6 | 31.1 | 31.7 |
| ≥6 | 39.4 | 43.8 | 40.7 | 34.6 | 38.5 |
| Cardiovascular disease | |||||
| Coronary artery disease | 68.4 | 67.2 | 68.7 | 69.0 | 68.8 |
| Congestive heart failure | 44.9 | 46.7 | 45.3 | 44.2 | 43.2 |
| Stroke | 29.0 | 28.1 | 30.7 | 29.3 | 27.7 |
| Hypertension | 95.4 | 95.6 | 95.2 | 96.1 | 94.7 |
| Potential diabetes-related complications | |||||
| End-stage renal disease | 26.8 | 31.7 | 25.6 | 23.2 | 26.5 |
| Peripheral vascular disease | 31.9 | 34.3 | 31.3 | 30.6 | 31.2 |
| Diabetic eye disease | 16.7 | 16.3 | 14.8 | 17.3 | 18.8 |
| Lower extremity ulcers | 22.6 | 26.0 | 19.6 | 22.5 | 22.0 |
| Serious hypoglycemic event | 8.5 | 8.9 | 7.2 | 8.5 | 9.8 |
| Difficulty swallowing | 18.1 | 19.2 | 19.8 | 17.3 | 15.6 |
| Recent weight loss | 48.9 | 53.8 | 49.9 | 49.5 | 41.1 |
| History of falls / fractures | 47.1 | 44.0 | 47.5 | 48.7 | 48.5 |
| Specific medications | |||||
| Antidepressants | 39.3 | 39.8 | 43.6 | 37.6 | 35.9 |
| Antipsychotics | 15.6 | 14.9 | 17.4 | 14.7 | 15.3 |
Abbreviations: MDS: Minimum Data Set; MMRI-R: MDS Mortality Risk Index – Revised.
Advanced dementia was defined as having a Brief Interview for Mental Status score ≤7 (range: 0–15) or a Cognitive Performance Score ≥4 (range: 0–6).
Documentation of end-of-life prognosis was defined as having hospice treatment specialty, receiving hospice care in the 14 days prior to the MDS admission assessment, or having ≤6 months life expectancy documented on the MDS assessment.
Physical functioning was defined using the MDS Activities of Daily Living Self-Performance Hierarchy (range: 0–6) to categorize residents as being independent to requiring mild assistance (0–2), requiring extensive assistance (3–4), or being physically dependent (5–6).
Table 2 summarizes overall and HbA1c-stratified diabetes treatment regimens administered to potentially overtreated Veterans. Nearly half of residents received ≥2 diabetes medications and those with higher HbA1c values (6.5-<7.0% or 7.0–7.5%) received more diabetes medications than those with lower HbA1c. Overall, the most common treatment regimens were combination short-acting and basal insulins (28.6%), short-acting insulin only (16.0%), basal insulin only (13.7%), and sulfonylureas only (13.8%), though these regimens varied across HbA1c levels. Three-quarters were administered at least one agent associated with a high risk of hypoglycemia48, defined as either short-acting insulin (56.7%) or sulfonylureas (26.4%). Use of short-acting insulin ranged from 50.7% (HbA1c 6.0-<6.5%) to 66.7% (HbA1c 7.0–7.5%).
Table 2.
Diabetes treatment regimens administered to older Veterans with potentially overtreated diabetes living in Community Living Centers with limited life expectancy or advanced dementia, overall and stratified by baseline HbA1c (N=3,056).1
| Stratified by baseline HbA1c, % |
|||||
|---|---|---|---|---|---|
| Treatment Administered, % | Overall (N=3,056) | <6.0 (n=811) | 6.0-<6.5 (n=769) | 6.5-<7.0 (n=816) | 7.0–7.5 (n=660) |
| Number of diabetes medications used | |||||
| 1 | 53.1 | 61.5 | 59.0 | 50.2 | 39.4 |
| 2 | 41.9 | 35.6 | 37.8 | 43.6 | 52.1 |
| ≥3 | 5.0 | 2.8 | 3.1 | 6.1 | 8.5 |
| Most common treatment regimens2 | |||||
| Short-acting insulin and basal insulin | 28.6 | 23.4 | 24.6 | 31.5 | 35.9 |
| Short-acting insulin only | 16.0 | 19.7 | 15.5 | 15.4 | 12.7 |
| Basal insulin only | 13.7 | 15.4 | 13.4 | 13.8 | 11.8 |
| Sulfonylureas only | 13.8 | 14.9 | 18.3 | 13.0 | 8.0 |
| Non-insulin / non-sulfonylurea agent only | 9.8 | 11.6 | 12.1 | 8.0 | 7.0 |
| Short-acting insulin and sulfonylureas | 5.0 | 4.3 | 5.5 | 3.8 | 6.8 |
| Sulfonylureas and non-insulin / non-sulfonylurea agent | 3.5 | 3.2 | 2.6 | 4.7 | 3.6 |
| Short-acting insulin and non-insulin / non-sulfonylurea agent | 2.6 | 2.8 | 2.5 | 2.0 | 3.2 |
| Other regimens3 | 7.1 | 4.6 | 5.6 | 7.8 | 10.9 |
| High-risk hypoglycemic agents | 75.3 | 71.9 | 73.2 | 77.3 | 79.4 |
| Short-acting insulin | 56.7 | 52.9 | 50.7 | 58.2 | 66.7 |
| Sulfonylurea | 26.4 | 25.0 | 29.8 | 27.1 | 23.3 |
Diabetes medications and treatment regimens were classified based on medications administered on the day of and day following the first HbA1C measurement following admission to the Community Living Center
Treatment regimens were classified after grouping medications into basal insulin, short-acting insulin, sulfonylureas, and non-insulin / non-sulfonylurea hypoglycemic agents. Most common treatment regimens are mutually exclusive and add to 100%.
3Includes all other regimens with a prevalence <2.0%. Note: all other regimens contained ≥3 of basal insulin, short-acting insulin, sulfonylureas, and non-insulin / non-sulfonylurea agents except for 1) basal insulin and other non-insulin / non-sulfonylurea use (1.2%) and 2) basal insulin and sulfonylurea use (1.1%).
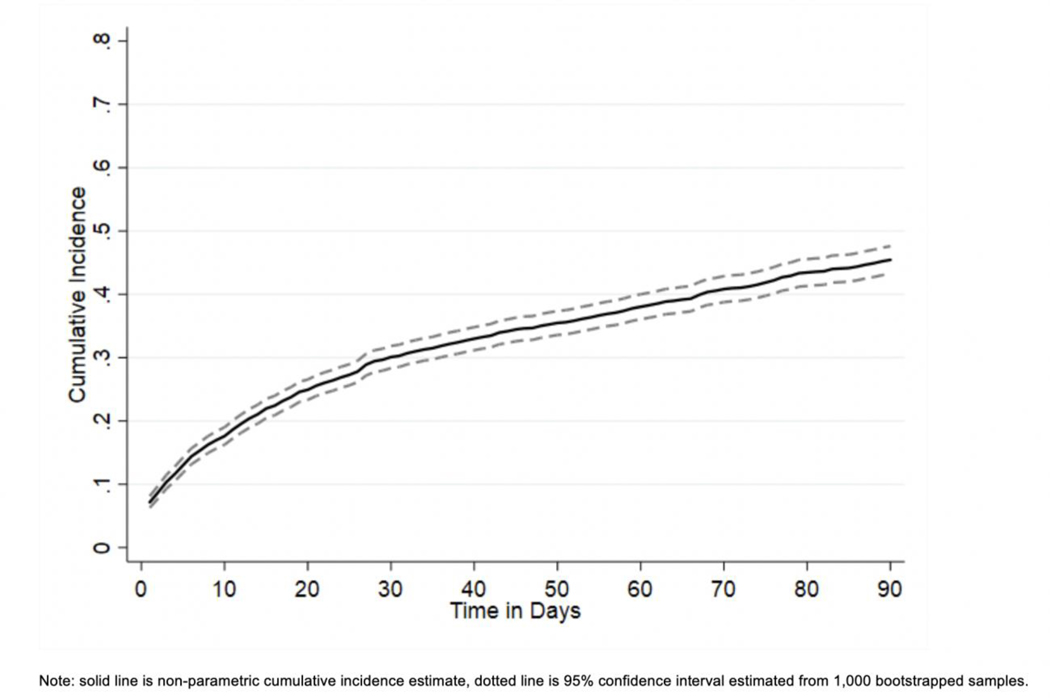
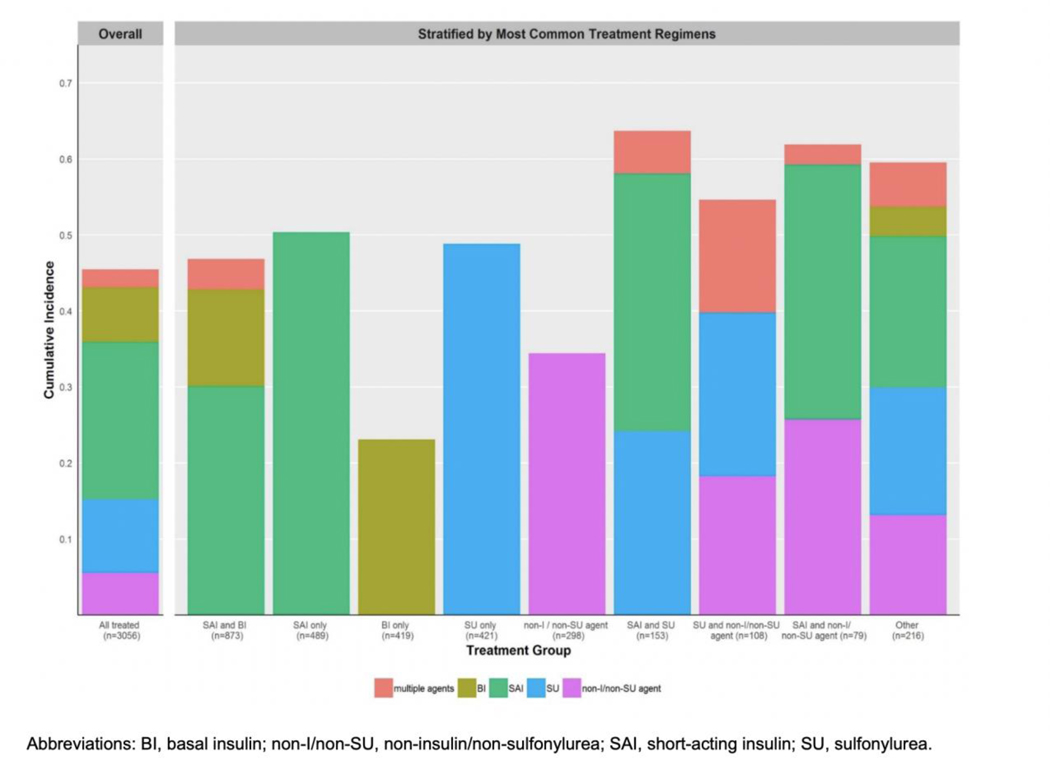
The respective cumulative incidence of deintensification at 15, 30, 60, and 90 days of follow-up was 22.0%, 30.1%, 38.0%, and 45.5% (see Figure 1). Figure 2 provides detail on specific medications deintensified overall and after stratifying by most common baseline treatment regimens. The most common hypoglycemic medications to be deintensified overall were short-acting insulin (20.7%), followed by sulfonylureas (9.7%), basal insulin (7.2%), and non-insulin / non-sulfonylurea agents (5.6%). Deintensification of multiple medications simultaneously only occurred in 2.3% of Veterans.
Figure 1.
Cumulative incidence of diabetes treatment deintensification during 90 days of follow-up.
Figure 2.
Crude 90-day cumulative incidence of deintensifying specific hypoglycemic medications overall and stratified by most common treatment regimens.
Figure 3 shows selected adjusted risk ratios (aRR) for resident characteristics and deintensification at 90 days of follow-up. Higher baseline HbA1c values were associated with lower likelihood of deintensification (e.g., HbA1c 7.0–7.5% vs. <6.0%, aRR: 0.57, 95% CI: 0.50–0.66). Treatment regimens other than non-sulfonylurea oral agents (e.g., short-acting insulin only) were associated with higher likelihood of deintensification (aRR ranging from 1.31–1.88). The only exception was basal insulin which was associated with decreased likelihood of deintensification (aRR: 0.59, 95% CI: 0.52–0.66). The associations of other non-diabetes resident factors with deintensification at 90 days were not as profound: source of admission (e.g., home / assisted living versus hospital admission (aRR: 0.85, 95% CI: 0.75–0.96), explicit documentation of end-of-life prognosis (versus none; aRR: 1.12, 95% CI:1.01–1.25 ), obese versus normal/healthy weight (aRR: 0.88, 95% CI: 0.78–0.99), and peripheral vascular disease (vs. none; aRR: 0.90, 95% CI: 0.81–0.99)). Other characteristics including demographics, pain, functional status, other cardiovascular and diabetes-related complications, antipsychotic use, and antidepressant use had risk ratios that were not statistically significant, with ≤10% change in risk compared to the referent group, albeit with varying degrees of precision. We did not observe consistent time trends in deintensification. See Appendix Table S1 for complete model-based results including crude/adjusted risk, risk ratios, and risk differences.
Figure 3.
Select Risk Ratios for Factors Associated with Diabetes Regimen Deintensification
In sensitivity analyses using a 14-day gap in medication administration as our definition for deintensification, associations were substantively unchanged (Table S2), although the cumulative incidences of deintensification were lower at all time points (Figure S2 - 15-day: 16.1%; 30-day: 22.9%; 60-day: 29.6%; 90-day: 36.1%).
DISCUSSION
A substantial portion of Veteran CLC residents with LLE/AD may be overtreated for management of their diabetes at the time of admission. Among those who were potentially overtreated, the cumulative incidence of deintensification was 45% within the 90 days after having an HbA1c level measured that fell below minimum levels recommended for older patients with greater comorbidity burden and reduced life expectancy. Residents with higher HbA1c values, but who were still defined as overtreated, tended to have more complex diabetes treatment regimens at baseline and were less likely to be deintensified during follow-up. The most common agents to be deintensified were those with a high risk for hypoglycemia (i.e., short-acting insulin and sulfonylureas). Overall, the medications received for management of diabetes appeared to be stronger predictors of deintensification than other resident characteristics.
This is one of the first national studies to evaluate potential overtreatment and deintensification of diabetes management in a sub-group of nursing home residents with LLE/AD. Although patterns of overtreatment and deintensification in diabetes have been described previously in the literature14,16,17,41,42,49–51, only one investigation has examined the influence of life expectancy on diabetes management.15 The proportion of residents who were overtreated in our sample (nearly half) aligns with previously reported estimates from large observational studies conducted in non-nursing home populations.16,17 The fact that the frequency of potential overtreatment remains just as high in this more vulnerable population is concerning and signals a need for interventions to increase the uptake and implementation of treatment deintensification or deprescribing in other care settings (e.g. office visits and hospital stays), prior to nursing home admission. Although almost half of residents in this study eventually had their regimens deintensified after admission, it begs the question of whether this should have occurred at an earlier point in time, especially given that a striking number of potentially overtreated Veterans (8.5%) had evidence of a serious hypoglycemic event in the prior year. Although this proportion is conservative compared to prior estimates52, it nevertheless emphasizes the importance of reducing potential overtreatment in frail older individuals and the need to screen patients at all points on the care continuum for medications with high risk for hypoglycemia.
Treatment deintensification was more strongly associated with the characteristics of each resident’s treatment regimen rather than other resident-level factors. We observed that residents with higher baseline HbA1c values were less likely to have medications deintensified and those receiving medications known to have a high risk of hypoglycemia (e.g. short-acting insulin and sulfonylureas) were more likely to have their regimens deintensified. One study of deintensification conducted in the outpatient setting also found that lower HbA1c was associated with greater likelihood for deintensification, in agreement with our findings.15 This makes sense given the potential increased risk of hypoglycemia in these patients. However, other studies of community-dwelling older adults with diabetes identified several other factors that were associated with increased likelihood for deintensification including more chronic conditions, greater frailty and more outpatient visits.41,42 One could argue that patients with these characteristics are common among older adults in the nursing home setting and that some may actually serve as drivers of institutionalization. Taken together, our findings indicate that in older CLC residents with LLE/AD, a population in which complex comorbidity and frailty are likely common, deintensification is not so much driven by individual clinical characteristics, but rather by a general concern for hypoglycemic adverse events that apply to all older adults.
We identified no strong time trends in terms of deintensification rates during FYs 2009–2015. This was surprising, given the increasing awareness of hypoglycemia risk associated with certain classes of medications as well as updated recommendations that have advocated for less conservative management of diabetes in older nursing home residents.12,4–6,14 There has also been a major system-wide initiative rolled out within the VA, the VHA Choosing Wisely Hypoglycemia Safety Initiative, which aims to improve diabetes management and reduce the risk for hypoglycemic events. However, this initiative was only implemented in 2014, so it is possible that its impact is not reflected given the limited period of overlap with our data.
There are several strengths to this analysis compared to previous studies. Our sample included a large number of residents and evaluated patterns of medication use over a period of several years. We used detailed daily medication administration data (BCMA data) to characterize medication exposures. The level of granularity contained within these data allowed us to identify deintensification with greater certainty by evaluating dosages administered each day as opposed to using medication dispensing data, where the dosages and administration are assumed, based on days-supply. We also implemented statistical models that accounted for death as a competing event, which provided more accurate estimation of cumulative incidence over prior studies, given the increased likelihood for mortality in this population. Finally, our investigation focused on a clinically relevant population of nursing home residents, those with LLE/AD, who likely have the least to gain from overly intense diabetes management considering long-term benefits relative to risk for adverse events.
There are several limitations to this study that should be acknowledged. First, in using administrative data to capture medication use, we were not able to identify intentional versus unintentional discontinuation of diabetes medications. Although we can be reasonably confident that the data from BCMA records captured periods during which patients did not actually receive medication, without information on actual medication orders, we were not able to definitively discern whether gaps may have been due to temporary discontinuations. Our ability to identify Veterans as potentially overtreated was dependent on availability of HbA1c data from the electronic health record. There were a large portion of residents with diabetes who did not have HbA1c measured within the first 90 days of their stay. Although the lack of monitoring may serve as an indicator of less aggressive disease management, by excluding these individuals it is possible that we have over- or underestimated the proportion of Veterans who were potentially overtreated. We did not have complete laboratory or medication data prior to each CLC episode and we therefore have not captured deintensification that occurred prior to admission. We also used a crude definition for deintensification with regards to insulin use that did not take into account the number of units of insulin administered. This study was focused on resident-level factors and did not examine provider or facility characteristics, which may provide additional insight into diabetes management in this setting and should be examined in future research. Finally, we acknowledge the potentially limited generalizability of our findings to non-veterans, women, or patients in other care settings (e.g. community and hospital).
CONCLUSION
This study found that a substantial portion of CLC residents with diabetes may be overtreated for management of diabetes at the time of admission, despite having LLE/AD. Deintensification of treatment regimens among occurred in just under half of potentially overtreated residents, and was more strongly associated with low HbA1c values and use of medications with high risk for hypoglycemia, rather than other resident characteristics. Future studies should examine the impact of deintensification on health outcomes and adverse events to better understand the risks and benefits of diabetes management strategies in this population.
Supplementary Material
ACKNOWLEDGMENTS
Funding: This study was funded by a grant from the U.S. Department of Veterans Affairs (IIR 14–306, PI: Carolyn T. Thorpe). Dr. Niznik was funded by a T32 award from the National Institutes on Aging (T32AG021885) and Drs. Hunnicutt and Springer were funded by postdoctoral fellowships through the Veterans Affairs Office of Academic Affiliations. Support for VA/CMS data is provided by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Health Services Research and Development, VA Information Resource Center (Project Numbers SDR 02–237 and 98–004). The views expressed are those of the authors and do not represent the views of the Department of Veterans Affairs.
Prior presentations: Parts of this manuscript were presented at the 2019 American Geriatrics Society Annual Meeting.
Sponsor’s Role: The VA had no role in the study design, data collection or analysis, manuscript preparation, or the decision to submit the manuscript for publication. The views expressed in this paper are those of the authors, and no official endorsement by the Department of Veterans Affairs or the U.S government is intended or should be inferred.
Footnotes
Conflict of Interest: JNH is now a fulltime employee at GlaxoSmithKline; however, he was a VA postdoctoral fellow during the conduct of this study. All other authors have no conflicts of interest to disclose.
References:
- 1.Kirkman MS, Briscoe VJ, Clark N, et al. Diabetes in older adults. Diabetes Care. 2012;35(12):2650–2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murphy SL, Xu J, Kochanek KD, et al. Deaths: Final Data for 2015. Natl Vital Stat Rep. 2017;66(6):1–75. [PubMed] [Google Scholar]
- 3.American Diabetes Association. 6. Glycemic Targets: Standards of Medical Care in Diabetes-2018. Diabetes Care. 2018;41(Suppl 1):S55–S64. [DOI] [PubMed] [Google Scholar]
- 4.Conlin PR, Colburn J, Aron D, et al. Synopsis of the 2017 U.S. Department of Veterans Affairs/U.S. Department of Defense Clinical Practice Guideline: Management of Type 2 Diabetes Mellitus. Ann Intern Med. 2017;167(9):655–663. [DOI] [PubMed] [Google Scholar]
- 5.Garber AJ, Abrahamson MJ, Barzilay JI, et al. Consensus Statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the Comprehensive Type 2 Diabetes Management Algorithm - 2018 Executive Summary. Endocr Pract. 2018;24(1):91–120. [DOI] [PubMed] [Google Scholar]
- 6.American Geriatrics Society Expert Panel on Care of Older Adults with Diabetes. Guidelines abstracted from the American Geriatrics Society Guidelines for Improving the Care of Older Adults with Diabetes Mellitus: 2013 update. J Am Geriatr Soc. 2013;61(11):2020–2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holmes HM, Hayley DC, Alexander GC, et al. Reconsidering medication appropriateness for patients late in life. Arch Intern Med. 2006;166(6):605–609. [DOI] [PubMed] [Google Scholar]
- 8.Kirsh SR, Aron DC. Choosing targets for glycaemia, blood pressure and low-density lipoprotein cholesterol in elderly individuals with diabetes mellitus. Drugs Aging. 2011;28(12):945–960. [DOI] [PubMed] [Google Scholar]
- 9.Duckworth W, Abraira C, Moritz T, et al. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360(2):129–139. [DOI] [PubMed] [Google Scholar]
- 10.ADVANCE Collaborative Group, Patel A, MacMahon S, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358(24):2560–2572. [DOI] [PubMed] [Google Scholar]
- 11.Group AS, Gerstein HC, Miller ME, et al. Long-term effects of intensive glucose lowering on cardiovascular outcomes. N Engl J Med. 2011;364(9):818–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Munshi MN, Florez H, Huang ES, et al. Management of Diabetes in Long-term Care and Skilled Nursing Facilities: A Position Statement of the American Diabetes Association. Diabetes Care. 2016;39(2):308–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.LeRoith D, Biessels GJ, Braithwaite SS, et al. Treatment of Diabetes in Older Adults: An Endocrine Society* Clinical Practice Guideline. J Clin Endocrinol Metab. 2019;104(5):1520–1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lipska KJ, Ross JS, Miao Y, et al. Potential overtreatment of diabetes mellitus in older adults with tight glycemic control. JAMA Intern Med. 2015;175(3):356–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sussman JB, Kerr EA, Saini SD, et al. Rates of Deintensification of Blood Pressure and Glycemic Medication Treatment Based on Levels of Control and Life Expectancy in Older Patients With Diabetes Mellitus. JAMA Intern Med. 2015;175(12):1942–1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tseng CL, Soroka O, Maney M, et al. Assessing Potential Glycemic Overtreatment in Persons at Hypoglycemic Risk. Jama Internal Medicine. 2014;174(2):259–268. [DOI] [PubMed] [Google Scholar]
- 17.Thorpe CT, Gellad WF, Good CB, et al. Tight glycemic control and use of hypoglycemic medications in older veterans with type 2 diabetes and comorbid dementia. Diabetes Care. 2015;38(4):588–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cigolle CT, Langa KM, Kabeto MU, et al. Geriatric conditions and disability: the Health and Retirement Study. Ann Intern Med. 2007;147(3):156–164. [DOI] [PubMed] [Google Scholar]
- 19.Freedman VA, Spillman BC. The residential continuum from home to nursing home: size, characteristics and unmet needs of older adults. J Gerontol B Psychol Sci Soc Sci. 2014;69 Suppl 1:S42–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jokanovic N, Tan EC, Dooley MJ, et al. Prevalence and factors associated with polypharmacy in long-term care facilities: a systematic review. J Am Med Dir Assoc. 2015;16(6):535 e531–512. [DOI] [PubMed] [Google Scholar]
- 21.Kojima G Prevalence of Frailty in Nursing Homes: A Systematic Review and Meta-Analysis. J Am Med Dir Assoc. 2015;16(11):940–945. [DOI] [PubMed] [Google Scholar]
- 22.Budnitz DS, Lovegrove MC, Shehab N, et al. Emergency hospitalizations for adverse drug events in older Americans. N Engl J Med. 2011;365(21):2002–2012. [DOI] [PubMed] [Google Scholar]
- 23.Einterz SF, Gilliam R, Lin FC, et al. Development and testing of a decision aid on goals of care for advanced dementia. J Am Med Dir Assoc. 2014;15(4):251–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Intrator O, Hiris J, Berg K, et al. The residential history file: studying nursing home residents’ long-term care histories(*). Health Serv Res. 2011;46(1 Pt 1):120–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mor V A comprehensive clinical assessment tool to inform policy and practice: applications of the minimum data set. Med Care. 2004;42(4 Suppl):III50–59. [DOI] [PubMed] [Google Scholar]
- 26.Saliba D, Buchanan J. Making the investment count: revision of the Minimum Data Set for nursing homes, MDS 3.0. J Am Med Dir Assoc. 2012;13(7):602–610. [DOI] [PubMed] [Google Scholar]
- 27.Johnson CL, Carlson RA, Tucker CL, et al. Using BCMA software to improve patient safety in Veterans Administration Medical Centers. J Healthc Inf Manag. 2002;16(1):46–51. [PubMed] [Google Scholar]
- 28.Gonsoulin M VIReC Factbook: Corporate Data Warehouse (CDW) Outpatient 2.1 Domain. Hines, IL. 2016. [Google Scholar]
- 29.Gonsoulin M VIReC Factbook: Corporate Data Warehouse (CDW) Inpatient 3.0 Domain (Part 1 - Inpatient). Hines, IL. 2017. [Google Scholar]
- 30.Gonsoulin M ViReC Factbook: Corporate Data Warehouse (CDW) Patient 3.0 Domain. Hines, IL. 2018. [Google Scholar]
- 31.Hynes DM, Koelling K, Stroupe K, et al. Veterans’ access to and use of Medicare and Veterans Affairs health care. Med Care. 2007;45(3):214–223. [DOI] [PubMed] [Google Scholar]
- 32.US Department of Veterans Affairs. VA/CMS Data. VHA Directive 1153: Access to Centers for Medicare and Medicaid Services (CMS) and the United States Renal Data System (USRD) Data for Veterans Health Administration (VHA) Users within the Department of Veterans Affairs (VA) Information Technology (IT) Systems. April 15, 2016. [Google Scholar]
- 33.Niznik JD, Zhang S, Mor MK, et al. Adaptation and Initial Validation of Minimum Data Set (MDS) Mortality Risk Index to MDS Version 3.0. J Am Geriatr Soc. 2018;66(12):2353–2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Porock D, Parker-Oliver D, Petroski GF, et al. The MDS Mortality Risk Index: The evolution of a method for predicting 6-month mortality in nursing home residents. BMC Res Notes. 2010;3:200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saliba D, Buchanan J, Edelen MO, et al. MDS 3.0: brief interview for mental status. J Am Med Dir Assoc. 2012;13(7):611–617. [DOI] [PubMed] [Google Scholar]
- 36.Morris JN, Fries BE, Mehr DR, et al. MDS Cognitive Performance Scale. J Gerontol. 1994;49(4):M174–182. [DOI] [PubMed] [Google Scholar]
- 37.Buccaneer Computer Systems & Service Inc. Chronic Condition Data Warehouse Medicare Administrative Data User Guide. Buccaneer Computer Systems & Service Inc; Minneapolis, MN: https://www.ccwdata.org/documents/10280/19002246/ccw-medicare-data-user-guide.pdf. Accessed June 18,2018 [Google Scholar]
- 38.Tjia J, Cutrona SL, Peterson D, et al. Statin discontinuation in nursing home residents with advanced dementia. J Am Geriatr Soc. 2014;62(11):2095–2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Niznik JD, Zhao X, He M, et al. Factors Associated With Deprescribing Acetylcholinesterase Inhibitors in Older Nursing Home Residents With Severe Dementia. J Am Geriatr Soc. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Suissa S Immortal time bias in pharmaco-epidemiology. Am J Epidemiol. 2008;167(4):492–499. [DOI] [PubMed] [Google Scholar]
- 41.Maciejewski ML, Mi X, Sussman J, et al. Overtreatment and Deintensification of Diabetic Therapy among Medicare Beneficiaries. J Gen Intern Med. 2018;33(1):34–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McAlister FA, Youngson E, Eurich DT. Treatment Deintensification Is Uncommon in Adults With Type 2 Diabetes Mellitus: A Retrospective Cohort Study. Circ Cardiovasc Qual Outcomes. 2017;10(4). [DOI] [PubMed] [Google Scholar]
- 43.Ginde AA, Blanc PG, Lieberman RM, et al. Validation of ICD-9-CM coding algorithm for improved identification of hypoglycemia visits. BMC Endocr Disord. 2008;8:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Morris JN, Fries BE, Morris SA. Scaling ADLs within the MDS. J Gerontol A Biol Sci Med Sci. 1999;54(11):M546–553. [DOI] [PubMed] [Google Scholar]
- 45.Elixhauser A, Steiner C, Harris DR, et al. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8–27. [DOI] [PubMed] [Google Scholar]
- 46.Roerig JL, Steffen KJ, Mitchell JE. Atypical antipsychotic-induced weight gain: insights into mechanisms of action. CNS Drugs. 2011;25(12):1035–1059. [DOI] [PubMed] [Google Scholar]
- 47.Andersohn F, Schade R, Suissa S, et al. Long-term use of antidepressants for depressive disorders and the risk of diabetes mellitus. Am J Psychiatry. 2009;166(5):591–598. [DOI] [PubMed] [Google Scholar]
- 48.American Geriatrics Society Beers Criteria Update Expert Panel. American Geriatrics Society 2019 Updated AGS Beers Criteria(R) for Potentially Inappropriate Medication Use in Older Adults. J Am Geriatr Soc. 2019;67(4):674–694. [DOI] [PubMed] [Google Scholar]
- 49.Newton CA, Adeel S, Sadeghi-Yarandi S, et al. Prevalence, quality of care, and complications in long term care residents with diabetes: a multicenter observational study. J Am Med Dir Assoc. 2013;14(11):842–846. [DOI] [PubMed] [Google Scholar]
- 50.Lee SJ, Stijacic-Cenzer I, Barnhart C, et al. Changing Patterns of Glucose-Lowering Medication Use in VA Nursing Home Residents With Diabetes, 2005 to 2011. J Am Med Dir Assoc. 2015;16(10):898 e899–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lederle L, Jing B, Rodriguez A, et al. Glycemic Over- and Undertreatment in VA Nursing Home Residents with Type 2 Diabetes: a Retrospective Cohort Study. J Gen Intern Med. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Feil DG, Rajan M, Soroka O, et al. Risk of hypoglycemia in older veterans with dementia and cognitive impairment: implications for practice and policy. J Am Geriatr Soc. 2011;59(12):2263–2272. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.