Significance
Phosphatidylinositol 4,5-bisphosphate (PIP2), a plasma membrane lipid, is hydrolyzed by Gq-protein–coupled receptor (GqPCR) signaling into inositol 1,4,5-trisphosphate and diacylglycerol—extensively studied second messengers with profound regulatory effects in the vasculature. However, there is extensive evidence that PIP2 directly regulates ion channels, a finding with significant implications for vascular function. Beyond providing a previously unexplored perspective on how vascular GqPCR signaling influences vascular function, the concept of PIP2-mediated ion channel regulation helps to explain how vascular cell excitability is coordinated to support cerebral blood flow control mechanisms. Importantly, the link between the metabolic state of vascular cells and PIP2 content may provide insight into how metabolism affects vascular ion channel activity and, ultimately, vascular function in health and disease.
Keywords: PIP2, GPCR, ion channel, smooth muscle cell, endothelial cell
Abstract
The phosphoinositide, phosphatidylinositol 4,5-bisphosphate (PIP2), has long been established as a major contributor to intracellular signaling, primarily by virtue of its role as a substrate for phospholipase C (PLC). Signaling by Gq-protein–coupled receptors triggers PLC-mediated hydrolysis of PIP2 into inositol 1,4,5-trisphosphate and diacylglycerol, which are well known to modulate vascular ion channel activity. Often overlooked, however, is the role PIP2 itself plays in this regulation. Although numerous reports have demonstrated that PIP2 is critical for ion channel regulation, how it impacts vascular function has received scant attention. In this review, we focus on PIP2 as a regulator of ion channels in smooth muscle cells and endothelial cells—the two major classes of vascular cells. We further address the concerted effects of such regulation on vascular function and blood flow control. We close with a consideration of current knowledge regarding disruption of PIP2 regulation of vascular ion channels in disease.
The purpose of the vertebrate cardiovascular system is to deliver sufficient oxygen and nutrients to, and remove CO2 and waste products from, all cells of the body. The basic features of this system are familiar: The heart pumps blood into the vasculature, a delivery system of gradually narrowing arteries and arterioles that terminates in a vast arborizing network of capillaries—the sites of oxygen and nutrient exchange with tissue—before transitioning to a venous network of gradually increasing vessel diameter that collects the deoxygenated blood and sends it via the right ventricle to the lungs for reoxygenation and then back to the heart, where the cycle starts over again. Arteries and arterioles of the peripheral circulation are the main determinants of vascular resistance, which, together with cardiac output, determines blood pressure. These vessels have an outer layer of connective tissue, one (arterioles) or more (arteries) layers of smooth muscle cells (SMCs), and an interior lumen lined by endothelial cells (ECs). SMCs and ECs are equipped with a repertoire of voltage-gated ion channels, ligand-gated ion channels, and G-protein–coupled receptors (GPCRs). Collectively, these receptors and channels endow SMCs and ECs with the ability to sense, respond to, and balance multiple physiological inputs.
SMCs of arteries and arterioles possess the ability to contract in response to increases in intravascular pressure to produce a reduction in vessel diameter (1, 2). This feature, known as the myogenic response, is a homeostatic mechanism that establishes the tone (contractile activity) of the vessel and is an essential regulatory feature of small arteries and arterioles that contributes to the maintenance of relatively constant blood flow in the face of changes in blood pressure. As the name implies, the myogenic response is intrinsic to the smooth muscle myocytes, but SMC contractility is further controlled by ECs, changes in tissue metabolism, and importantly, humoral and neural stimuli.
Smooth muscle contractility is principally set by changes in SMC intracellular Ca2+ concentration ([Ca2+]i), which reflects Ca2+ release from intracellular stores and influx of Ca2+ from the extracellular space. The primary driver of Ca2+ influx into the cell is a change in SMC membrane potential (VM), and the central player governing Ca2+ entry in SMCs—and thus a major determinant of vascular tone—is the smooth muscle voltage-dependent Ca2+ channel, Cav1.2, which is activated by VM depolarization (2, 3). The membrane potential of SMCs and ECs in arteries constricted by physiological intravascular pressures is typically around −40 mV. Under these conditions, Cav1.2 channels mediate Ca2+ influx, which increases [Ca2+]i and leads to phosphorylation of myosin light chain, actin–myosin cross-bridge cycling, and ultimately, smooth muscle contraction and vasoconstriction. Hyperpolarization, on the other hand, deactivates Cav1.2 channels, decreasing Ca2+ entry into SMCs and leading to vasodilation. Therefore, signals that depolarize SMCs will tend to constrict arteries, and hyperpolarizing signals will counteract tone development and evoke vasodilation (Fig. 1). The relationship between membrane potential and arterial diameter is steep, with maximum dilation occurring at about −60 mV and maximum constriction at about −30 mV (2).
Fig. 1.
Principal vascular ion channels that regulate VM and smooth muscle contraction. A schematic depiction of prominent ion channels in SMCs and ECs. The arteriolar wall is composed of ECs facing the vessel lumen surrounded by an overlying layer of SMCs. Capillaries lack SMC coverage. Vascular cells are electrically coupled through gap junctions, which facilitate charge movement from one cell to the neighboring cell. Channels indicated in green are primary hyperpolarizing ion channels; those in red depolarize the VM of their corresponding cell type.
In addition to Cav1.2 channels, SMCs also express a variety of Na+-, Ca2+-, or Cl−-permeable ion channels that when activated, cause membrane depolarization, and thereby induce vasoconstriction (Fig. 1). Among these additional SMC depolarizing channels are transient receptor potential (TRP) cation channels of the canonical (TRPC3, TRPC6) and melastatin (TRPM4) subfamilies, and anion channels such as the Ca2+-activated Cl− channel, TMEM16A. Among ion channels in SMCs that contribute to VM by exerting a hyperpolarizing influence are voltage-dependent K+ (KV1.2, KV1.5, KV2.1) channels, strong inward-rectifier K+ (Kir2.1) channels, large-conductance, Ca2+- and voltage-sensitive K+ (BK) channels, and ATP-sensitive K+ (KATP) channels. Activation of any of these K+ channels mediates K+ efflux and causes SMC hyperpolarization and vasodilation (4).
SMC VM is also influenced by the endothelium. Heterocellular coupling between ECs and SMCs, enabled by gap junctions localized to specialized microdomains termed myoendothelial projections (Fig. 1), allows the transfer of electrical signals from ECs to SMCs. This electrical coupling between ECs and SMCs guarantees that a change in endothelial VM will alter the smooth muscle contractile state (tone). A prominent ion channel in the arterial/arteriolar endothelium that contributes to endothelial regulation of SMC VM is the Ca2+-permeable TRPV4 channel. When activated by epoxyeicosatrienoic acids (EETs) or acetylcholine-stimulated PKC, endothelial TRPV4 channels mediate an influx of Ca2+ that subsequently activates endothelial intermediate-conductance (IK) and small-conductance (SK) Ca2+-activated K+ (KCa) channels, triggering endothelial VM hyperpolarization via K+ efflux. This not only hyperpolarizes ECs, it also hyperpolarizes the membrane of adjacent SMCs (5). Importantly, ECs are electrically coupled to one another through EC–EC gap junctions, forming what can be viewed as an electrical syncytium that facilitates the transfer of electrical signals initiated in one cell to neighboring cells. ECs (and some types of SMCs) express Kir2.1 channels; notably, endothelial Kir2.1-mediated hyperpolarization can be transmitted directly to overlying SMCs in arteries, leading to vasodilation (6). Additionally, we have recently found that Kir2.1 channels are expressed in capillary ECs, which lack surrounding SMCs. Here, Kir2.1 activation hyperpolarizes capillary ECs, producing an electrical signal that is conducted to neighboring ECs by connexins (Fig. 1) until it reaches the upstream arteriole, whereupon it relaxes smooth muscle and evokes vasodilation (7). Another notable ion channel in ECs is the mechanosensitive Piezo1 channel, which senses mechanical forces in the vessel lumen, leading to influx of Ca2+ and Na+. It has therefore been suggested that the Piezo1 channel directly depolarizes ECs or, alternatively, is functionally associated with synthesis of the potent vasodilator, nitric oxide (for review, see ref. 8).
GPCRs, which signal through different heterotrimeric G-protein subtypes (Gq/11, Gs, Gi/o, and G12/13) to an array of downstream signaling cascades, are key elements in the repertoire of extracellular signal-regulated receptors in vascular ECs and SMCs with particular relevance to PIP2 regulatory dynamics. Among G-protein subtypes, Gq acts in pathways that serve to modulate arterial diameter; thus, Gq-protein–coupled receptor (GqPCR) signaling has been a central focus in vascular physiology. In the canonical signaling pathway, stimulation of GqPCRs activates phospholipase C (PLC), which hydrolyzes phosphatidylinositol 4,5-bisphosphate (hereafter, PIP2) into inositol 1,4,5-trisphosphate (IP3) and diacylglycerol (DAG). IP3 evokes Ca2+ release from the sarcoplasmic/endoplasmic reticulum (SR/ER) by sensitizing IP3 receptors (IP3Rs) to stimulatory Ca2+, whereas DAG and Ca2+ activate protein kinase C (PKC). These IP3/Ca2+ and DAG/Ca2+/PKC cascades are major signaling pathways that have significant effects in SMCs and ECs. For instance, smooth muscle IP3R-mediated Ca2+ release stimulates TRPM4 channels (9), and DAG directly activates different TRPC channel subtypes (10). Furthermore, DAG-activated PKC is an important regulator of voltage-dependent Ca2+ channels (11) and KATP channels (12) in SMCs. These PIP2 metabolites are also dynamically involved in modulating endothelial ion channels: IP3R-mediated Ca2+ release activates Ca2+-activated IK and SK channels (13), and PKC promotes endothelial TRPV4 channel activity (14).
Although the metabolites resulting from GqPCR signaling and PIP2 depletion—IP3 and DAG—are known to be important regulators of vascular ion channels, the fact that GqPCR signaling is associated with a concomitant dramatic decrease in PIP2 levels is often underappreciated in vascular studies. This less-studied aspect of vascular PIP2 metabolism has profound implications for the regulation of membrane proteins, including ion channels, many of which are positively or negatively regulated by association with PIP2 in the plasma membrane (15–18). In this review, we consider the important roles of PIP2 as a regulator of ion channels in vascular SMCs and ECs and address how this modulation affects (or could affect) vascular function. We additionally discuss how cellular PIP2 levels are determined, as well as the basis for PIP2–ion channel interactions. Finally, we review our current understanding of PIP2-mediated regulation of vascular ion channels in health and disease.
Determinants of PIP2 Levels in the Cell
Polyphosphoinositides—the phosphorylation products derived from phosphatidylinositol (PI)—exhibit different interconversions that reflect the number and sites of phosphorylated hydroxyl groups on the inositol ring (Fig. 2). The phosphoinositide PIP2 is a minor, negatively charged phospholipid that resides primarily in the inner leaflet of the plasma membrane. An important factor to appreciate in considering PIP2 involvement in signal transduction is that PIP2 levels are dynamic. The cellular levels of PIP2 reflect the net effect of lipid kinases and phosphatases, as well as GqPCR activity-induced PIP2 hydrolysis by phospholipases, the latter of which is the primary driver of dynamic changes in PIP2 levels.
Fig. 2.
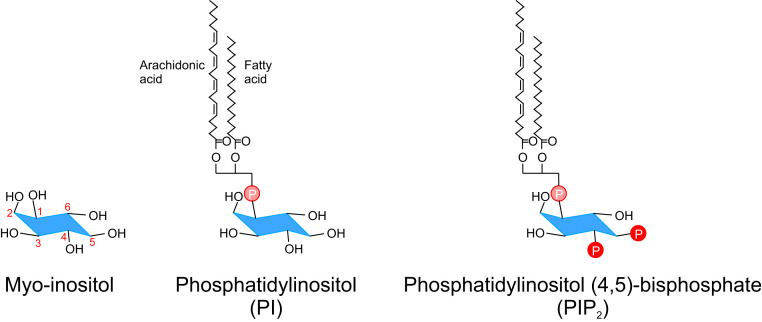
Structure of PIP2. Chemical structures of myo-inositol, phosphatidylinositol (PI), and phosphatidylinositol (4, 5)-bisphosphate (PIP2). The numbering in the myo-inositol structure (red) refers to the different positions on the inositol ring where specific hydroxyl groups can be altered. PI is biosynthesized from phosphatidic acid via the intermediate diacylglycerol (DAG) and inositol. One to three hydroxyl groups (positions 3, 4, and 5) on the inositol ring of PI can be phosphorylated by site-specific phosphoinositide kinases to give rise to different phosphoinositides.
PIP2 Synthesis.
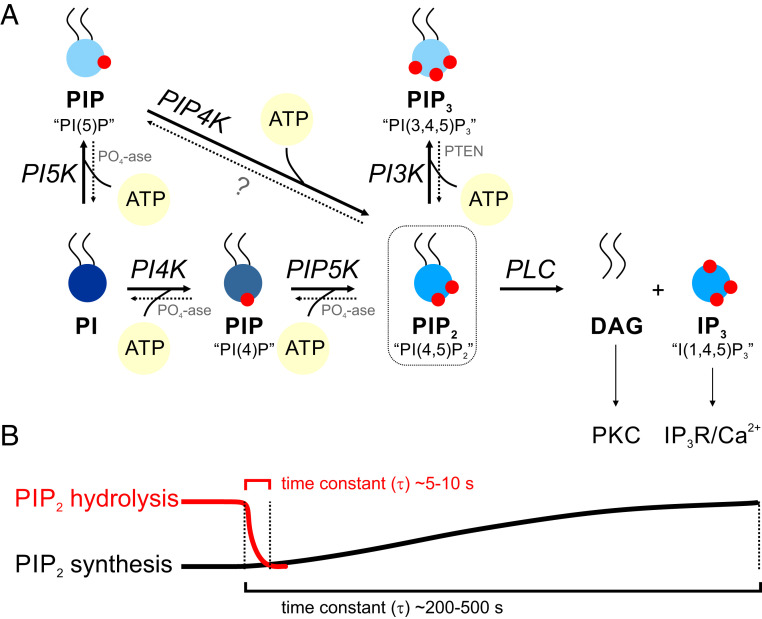
Distinct polyphosphoinositides can be generated from PI by phosphorylation of one to three hydroxyl groups at positions 3, 4, and 5 on the inositol ring (Fig. 2) by site-specific phosphoinositide kinases (Fig. 3). Phosphoryl transfer (to positions 4 and 5, in the case of PIP2) by kinases requires ATP and the cofactor Mg2+. Unlike protein kinases, most of which are maximally active at low-micromolar intracellular concentrations of ATP ([ATP]i), lipid kinases generally require much higher concentrations of ATP (hundreds-of-micromolar range) to support their activity (19). The formation of PIP2 reflects the sequential actions of phosphatidylinositol 4-kinase (PI4K) and phosphatidylinositol 4-phosphate 5-kinase (PIP5K). Some PIP2 is also generated through dephosphorylation of PI(3,4,5)P3 by phosphatases, such as PTEN (phosphatase and tensin homolog) (Fig. 3). Phosphorylation by PI4K is the rate-limiting step in PIP2 synthesis, with a Michaelis–Menten constant for ATP (KM, ATP) of ∼0.4 to 0.9 mM (19–22). One implication of this relatively high KM for ATP is that decreases in free cytoplasmic [ATP]i, and therefore the ATP:ADP (adenosine diphosphate) ratio, could significantly slow phosphoinositide synthesis by suppressing the phosphorylation potential of lipid kinases without substantially affecting cellular reactions with a low KM for ATP, such as those mediated by transporters or protein kinases. In other words, PIP2 synthesis is sensitive to the physiological energy state of the cell.
Fig. 3.
PIP2 synthesis and breakdown. (A) The precursor, PI, can be phosphorylated by the site-specific phosphoinositide kinase PI4K in the presence of ATP, resulting in phosphoryl transfer to position 4 on the inositol ring and formation of phosphatidylinositol-4-phosphate (PIP). The latter can be further phosphorylated at position 5 by the kinase PI(4)P5K to form PIP2. Maximum phosphorylation can occur through the action of PI3-kinase (PI3K), which acts specifically on position 3 to form phosphatidylinositol-(3,4,5)-trisphosphate (PIP3). PI can alternatively be phosphorylated to PI(5)P and then to PIP2 by the actions of PI5K and PI(5)P4K, respectively. The dotted lines represent phosphoinositide phosphatases that dephosphorylate different phosphoinositides at the 3, 4, and 5 positions of the inositol ring. PLC hydrolyzes PIP2 to DAG, which activates PKC, and IP3, which triggers Ca2+ release from intracellular stores. (B) An illustration highlighting the stark difference between the kinetics of PIP2 synthesis and hydrolysis (see Determinants of PIP2 Levels in the Cell).
PIP2 Breakdown.
The operation of kinases and phosphatases produces continuous fluctuations in polyphosphoinositides, including PIP2 (Fig. 3). Many of these reactions, however, are not capable of milliseconds-to-seconds regulation of phosphoinositide levels (18, 23–25). Because Gq activation can rapidly activate PLC, and the rate constant for PIP2 hydrolysis by activated PLC is high, Gq activation can decrease PIP2 concentration within seconds (time constant, ∼10 s) (18, 24) and is thus the predominant contributor to dynamic PIP2 depletion. In fact, GqPCR activation can rapidly deplete 90% of the cellular content of PIP2 (23–26). Notably, the continuously changing activity of GqPCRs, reflecting variations in the levels of receptor agonists released from perivascular cells (e.g., astrocytes, neurons) or circulating in the bloodstream, can lead to varying degrees of PIP2 depletion. However, restoration of PIP2 by ongoing lipid kinase-mediated synthesis is slower, spanning minutes (time constant, ∼200 to 500 s) (18), resulting in long-lasting effects of Gq activation on PIP2 cellular content and the activity of PIP2-regulated proteins (18, 27). Thus, GqPCR-mediated hydrolysis of PIP2 can outstrip synthesis and could therefore represent a major influence on proteins and ion channels that are targets of PIP2 regulation (Fig. 3).
Basis for PIP2–Ion Channel Interactions
It has been shown that many ion channels are regulatory targets of PIP2. As of this writing, the number of PIP2-modulated ion channels is approaching 100 (15, 28), a number that is likely to continue to grow. In pioneering work performed over two decades ago, Hilgemann and Ball (29) reported that the KATP channel is regulated by plasmalemmal PIP2. Many such studies have followed. In most instances, the realization that an ion channel required PIP2 stemmed from the electrophysiological observation that ionic currents in excised patches changed over time. This phenomenon was frequently abrogated by PIP2 supplementation or enhancement of PIP2 synthesis. Collectively, these observations led to the recognition of PIP2 as an important modulator of ion channel function (for review, see ref. 15).
The abundance of PIP2 targets raises the question of whether, and if so how, PIP2 can function to promote specific cellular functions that depend on coordinated enhancement of the activities of specific sets of channels and other proteins and suppression of the activities of others. The specificity of PIP2 signaling could reflect cell-specific differences in expression levels of potential targets, localization of signaling and targets in microdomains, and/or the dependence of activities on the coincident reinforcement of multiple signals (e.g., PIP2 and Ca2+ and depolarization) (24, 30–33).
PIP2 is negatively charged (Fig. 2) and thus contributes to the negative charge of the inner leaflet of the plasma membrane (34). This negative surface charge raises local concentrations of all cations, especially multivalent inorganic (e.g., Ca2+) and organic (e.g., spermine) cations, and freely diffusing, net positively charged proteins on the inner leaflet (35, 36). The high negative charge on PIP2 can also cause neighboring intrinsic membrane proteins to orient such that net positively charged regions are closer to the PIP2 headgroup and net negatively charged regions are farther from it (37, 38).
PIP2 binding to an ion channel depends on the phosphoinositide specificity of the channel. Some ion channels possess specific binding pockets for PIP2 and therefore show high PIP2 specificity. Other channels with lower PIP2 specificity display electrostatic binding to various negatively charged phospholipid molecules. Higher phosphoinositide specificity usually correlates with higher-affinity binding of PIP2 to the channel (39–42). Taken together with the relative scarcity of PIP2, the fact that PIP2 can reversibly bind to ion channels with high affinity has given rise to the “PIP2-gated ion channel theory,” which posits that PIP2 can be viewed as an agonist or activator of ion channels (41). Intriguingly, a lower PIP2–ion channel binding affinity is also physiologically important because it permits binding–unbinding to occur over physiological timescales (seconds) and concentrations of receptor agonists. This could translate into more rapid modulation, relative to high-affinity binding, of ion channel activity by factors that alter PIP2 levels.
Structural studies have demonstrated channel protein moieties complexed with PIP2 and confirmed that PIP2 positioning is essential for normal ion channel gating. A good example is the inward-rectifier K+ channel, Kir2.2 (KCNJ12). Elegant work by the MacKinnon laboratory (43) has revealed crystal structures of apo- and PIP2-bound Kir2.2 channels, clearly demonstrating that PIP2 binding induces profound structural changes to open the channel’s pore. Alternatively, PIP2 can alter ion channel function by interfering with channel binding to other regulators, such as ATP, calmodulin, βγ complexes of heterotrimeric G proteins, or even other lipids (44–48).
The fact that all ion channels possess basic motifs means that, in theory, all channels are capable of interacting with PIP2. An important question, however, is whether such interactions actually occur in vivo and, if so, whether they affect function in a physiologically meaningful manner. This highlights the necessity of performing experiments in native cells and tissues, where ion channels are expressed at physiological levels. It is also important to test whether physiological stimuli capable of changing PIP2 levels can alter the corresponding ion channel activity and thereby modulate tissue function.
Concerted PIP2 Regulation of Vascular Ion Channels
Our focus here will be on the physiological regulation of vascular ion channels in ECs and SMCs by PIP2. For information on the broader topic of phosphoinositides and ion channel function, we refer readers to excellent recent reviews (15, 49, 50). There are many reports describing the ability of PIP2 to regulate ion channel targets based on electrophysiological recordings of ionic currents in expression systems. In contrast to the wealth of literature on ion channel regulation by PIP2 in cell culture and cell-free systems, there are relatively few reports of such regulation in native vascular cells (27, 51–53).
Vascular K+ Channels.
As noted above, Hilgemann and Ball (29) were the first to describe K+ channel regulation by PIP2. Their work opened the door for subsequent studies that have implicated PIP2 as a modulator of most inwardly rectifying (Kir), voltage-gated (KV), and Ca2+-activated (KCa) K+ channels (for review, see ref. 15). Members of these K+ channel families are expressed in the vasculature and play key roles in vascular function.
Strong inward-rectifier K+ channels.
The Kir channel family is divided into seven subfamilies, Kir1–7, with variations within subfamilies giving rise to a total of 15 known isoforms (Kir1.1, Kir2.1–4, Kir3.1–4, Kir4.1–2, Kir5.1, Kir6.1–2, and Kir7.1). Among these, there is compelling evidence for the expression of strong inward-rectifier Kir2.1 (KCNJ2) and Kir2.2 (KCNJ12) channels, and weak inward-rectifier Kir6.1 (KCNJ8) channels, in the vasculature (Fig. 1).
In strong inward-rectifier Kir2 channels, outward current is blocked by voltage-dependent binding of intracellular polyamines (e.g., spermine) to the inner pore (54). Kir2.1 is highly expressed at both mRNA and protein levels in the vascular endothelium of arteries, arterioles, and capillaries (6, 7, 52, 55). It is also present and functional in most arterial SMCs, where it is necessary for K+-mediated vasodilation (56–59). Although Kir2.2 mRNA is present in ECs and SMCs, there is little evidence for Kir2.2 involvement in vascular function (7, 52, 58–60).
Fan and Makielski (61) provided evidence that a mixture of anionic phospholipids that included PIP2 activated strong inward-rectifier Kir2.1 channels overexpressed in cultured mammalian cells. Huang et al. (16) subsequently discovered that, when expressed in Xenopus oocytes, Kir2 channels were directly activated by PIP2. Using electrophysiological approaches, they showed that Kir2.1 currents gradually declined and that providing PIP2 to the cytoplasmic side reversed this effect. They further showed that channel activity was enhanced by ATP, which promotes PIP2 generation by supporting lipid kinase activity, and was inhibited by antibodies against PIP2. Inhibition of Kir2.1 current, whether due to the lack of PIP2 or induced by an anti-PIP2 antibody, was found to be slow compared with that of other inwardly rectifying K+ channels. On the other hand, they found that addition of PIP2 (or ATP) led to a recovery of Kir2.1 channel activity (16). About a decade later, MacKinnon’s laboratory (43) solved the crystal structure of Kir2.2 channel subunits (encoded by KCNJ12), revealing that PIP2 molecules are bound to a highly structured site in the channel transmembrane domain comprising positively charged residues. This Kir2.2–PIP2 relationship was shown to be essential for normal channel activity. Like Kir2.2, the Kir2.1 channel (KCNJ2) requires PIP2 to function (62–64).
Only recently has the PIP2–Kir2 relationship in the vasculature been explored. Both arterial SMCs and ECs express Kir2 channels (Fig. 1) (6, 56, 59). Recent studies have additionally shown that brain capillaries, composed only of ECs without surrounding SMCs, express Kir2.1 channels that act as sensors of neural activity (7). Conventional whole-cell recordings from freshly isolated and dialyzed mouse capillary ECs revealed a decline in Kir2.1 currents (27) reminiscent of Kir2.2 current rundown reported in excised patches from Xenopus oocytes (43). When recordings were made using the perforated-patch configuration, which maintains an intact cytoplasm, capillary Kir2.1 currents did not decline, suggesting the presence of an intracellular factor that sustains Kir2.1 activity. Dialyzing capillary ECs with Mg-ATP, but not with nonhydrolyzable ATP-γ-S, prevented the decline of Kir2.1 channel currents, suggesting that dialysis of the cell caused the loss of ATP, resulting in deactivation of the lipid kinases that synthesize PIP2. Consistent with this, dialysis with water-soluble PIP2 in the absence of Mg-ATP was sufficient to sustain Kir2.1 activity (27). Collectively, these observations are consistent with the idea that intracellular ATP helps sustain PIP2 synthesis, and that PIP2, in turn, is necessary for capillary EC Kir2.1 activity. The arterial EC Kir2.1 channel similarly requires PIP2 for activity (52).
Kir2 channels are activated by external K+ through relief of block by intracellular cationic polyamines (54), a biophysical property that confers on SMCs and ECs the ability to sense local increases in [K+]o and convert them into membrane hyperpolarization and vasodilation (7, 65, 66). Membrane potential hyperpolarization also activates Kir2 channels by driving polyamines out of the pore. This property enables electrically coupled ECs to transmit a regenerating hyperpolarizing signal through the endothelium to the smooth muscle layer (7, 67) (Fig. 1).
In the cerebral circulation, activation of the Kir2.1 channel by neuronal activity-driven elevation of [K+]o provides a mechanism for translating neural activity into vascular responses, a process termed neurovascular coupling that links neural demand to increases in blood flow and thus O2 and glucose delivery (7, 65). Modest increases in [K+]o during neural activity—a local consequence of neuronal action potentials—leads to sustained hyperpolarization only when Kir2.1 outward current exceeds overall cellular inward current (68). Thus, this mechanism, in which retrograde electrical signaling to upstream arterioles increases cerebral blood flow, is critically dependent on the number of functional Kir2.1 channels, which, in turn, is dependent on PIP2 occupancy (27). Although dialysis of the cytoplasmic compartment is capable of causing ATP depletion and inhibition of PIP2 synthesis in whole-cell patch-clamp electrophysiology experiments, under physiological conditions, it is unlikely that ATP would fall to levels low enough to deactivate lipid kinases and suppress PIP2 synthesis. Instead, hydrolysis of PIP2 by GqPCR activation is likely the major mechanism for producing rapid changes in PIP2 in vivo. In fact, acute depletion of endothelial PIP2 due to GqPCR activation and PLC-mediated hydrolysis inhibits capillary Kir2.1 channel activity and abolishes the ability of these channels to communicate to upstream arterioles to enhance cerebral blood flow (Fig. 4) (27). Whether this regulatory dynamic operates in ECs in all vascular beds, however, is not clear. Distinct behaviors at different points in the vascular tree could, in theory, reflect differences in the availability of PIP2 or cellular localization of receptors and proteins that determine PIP2 levels (30, 31).
Fig. 4.
GqPCR signaling alters PIP2 levels and ion channel activity in a coordinated manner. In the absence of GqPCR agonists (or when GqPCR activity is minimal), PIP2 levels at the inner leaflet of the plasma membrane are maintained. PIP2 can have dual effects on different ion channels, supporting (activating) one ion channel type while simultaneously inhibiting another (tonic inhibition). As GqPCR activity increases, PIP2 is hydrolyzed to the metabolites DAG and IP3. The attendant dramatic depletion of PIP2 can exert an inhibitory effect on ion channels that require PIP2 for activation and a disinhibitory effect on ion channels that are tonically inhibited by PIP2.
In summary, the activity of strong inwardly rectifying K+ channels in the cerebral vasculature depends on PIP2 (Fig. 5). Therefore, the ability of arteries/arterioles to hyperpolarize and dilate in response to neuronal activity and consequent increases in extracellular K+ is weakened by the loss of PIP2.
Fig. 5.
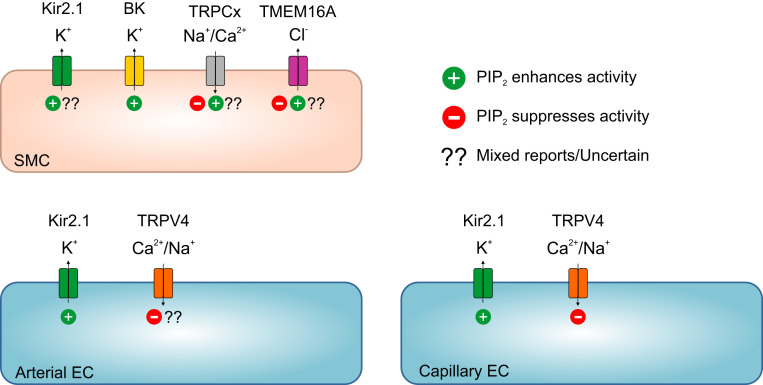
PIP2-mediated regulation of vascular ion channels. Different vascular cells express ion channels that are regulatory targets of PIP2. Capillary ECs: PIP2 enhances Kir2.1 activity and suppresses TRPV4 activity (27, 51). Arterial ECs: PIP2 is essential for Kir2 channel activity (52) and presumably suppresses TRPV4 channels, similar to capillary TRPV4. Smooth muscle cells: Extensive evidence supports the conclusion that PIP2 is required for Kir2 channel activity, although Welsh and colleagues (52) have suggested that PIP2 is a minor physiological regulator of Kir2 channels in SMCs. PIP2 directly activates BK channels (53). Studies have variably reported that PIP2 facilitates or inhibits TRPC channels (see TRPC Channels). Greenwood and colleagues (136) have suggested that PIP2 inhibits TMEM16A in pulmonary artery smooth muscle, but several groups studying nonvascular TMEM16A suggest otherwise (see Vascular Chloride Channels).
Weak inward-rectifier K+ channels.
In contrast to the strong inward rectification of Kir2.1 and Kir2.2, the Kir6.1 isoform, which is expressed primarily in SMCs and pericytes, is a weak inward-rectifier channel that associates with sulfonylurea receptor subunits (SUR2B) to form ATP-sensitive K+ (KATP) channels (69, 70). Notably, the first demonstration that PIP2 acts as an ion channel regulator was provided by studies on the cardiac KATP channel, Kir6.2 (encoded by KCNJ11), in guinea pig cardiac myocytes (29). The closely related channel, Kir6.1 (encoded by KCNJ8), is the pore-forming subunit of KATP channels in SMCs. Several vasoconstrictor GqPCR agonists clearly inhibit KATP channel currents in arterial smooth muscle. However, this inhibition has been attributed to downstream activation of PKC rather than PIP2 depletion via PLC (12, 70). Several studies support this conclusion. Although PIP2 can bind to the C terminus of the Kir6.1 channel (71), the effects of PIP2 depletion on SUR2B/Kir6.1, the complex found in SMCs, appear to be minimal (72). In contrast, the SUR2A/Kir6.2 channel, the predominant complex in ventricular cardiac myocytes, is inhibited by GqPCR activation through PIP2 depletion independent of PKC (72). In summary, the lack of PIP2 regulation of smooth muscle KATP channels—likely reflecting structural differences in channel complex composition between cardiac myocytes (SUR2A/Kir6.2) and vascular SMCs (SUR2B/Kir6.1)—suggests that PIP2-mediated regulation of vascular KATP channels is not of major physiological significance.
Ca2+-activated K+ channels.
Ca2+-activated K+ (KCa) channels are important players in vascular physiology and are classified based on their conductances into small (SK)-, intermediate (IK)-, and large-conductance (BK) channels. Activation of KCa channels leads to membrane hyperpolarization (Fig. 1). SK3 (KCa2.3/KCNN3) and IK (KCa3.1/KCNN4) channels, which are expressed in most ECs (but see below), are voltage insensitive and are thus activated solely by elevations in cytosolic [Ca2+]i. BK channels, in contrast, are voltage and Ca2+ sensitive. Although it has been shown that other SK channels in nonvascular cells are modulated by PIP2 levels (73, 74), this has not been investigated in ECs.
BK channels are activated by depolarization, with the voltage for half-maximal activation (V50) depending on intracellular [Ca2+]i. They are expressed in virtually all SMCs, where they are arrayed in the plasma membrane in close apposition to ryanodine receptors (RyRs) in the SR. RyR-mediated Ca2+-release events from the SR, termed “Ca2+ sparks” (75), provide the Ca2+ that supports activation of these channels. During a Ca2+ spark, [Ca2+]i transiently rises well into the micromolar range. One Ca2+ spark has been estimated to increase nearby BK channel activity in cerebral arterial SMCs by ∼105- to 106-fold (76). This high [Ca2+]i causes channel opening, and rapidly hyperpolarizes the SMC membrane, thereby closing voltage-dependent Ca2+ channels and limiting Ca2+-dependent contraction.
BK channels in vascular SMCs are activated by endogenous PIP2 (53). In cerebral arterial SMCs, polyphosphoinositides, including PIP2, activate BK channels independently of the PIP2 metabolites, IP3 and DAG. The PIP2 headgroup has been proposed to bind to the BK channel, increasing its Ca2+ sensitivity and thereby increasing BK open probability (53). Ca2+ binding to the channel also enhances the positive effects of PIP2 on BK channels (77). In the absence of accessory β-subunits, the open probability of the pore-forming α-subunit of the BK channel (encoded by KCNMA1) is enhanced approximately fivefold by PIP2 in vascular SMCs, an effect that is attributable to a leftward shift in V50 and amplification of Ca2+-driven gating. However, the accessory β-subunit strongly enhances PIP2-mediated regulation of BK channels. Association of the vascular SMC BK α-subunit with the β1-subunit (KCNMB1), the predominant β-subtype in vascular SMCs (78), potentiates PIP2 effects on BK channel open probability, increasing it by ∼25-fold (vs. 5-fold in the absence of the β1-subunit) (53). In contrast to the β1-subunit, the β4-subunit (KCNMB4), which predominates in skeletal muscle, does not sensitize BKα to PIP2 (53).
In summary, PIP2 increases BK channel activity in vascular SMCs (Fig. 5) by as much as 25-fold. However, this pales in comparison with the ∼105- to 106-fold increase in open probability induced by a single Ca2+ spark. Because BK channel activity in vascular smooth muscle is largely controlled by the frequency of Ca2+ sparks (79), it is unlikely that the functional output of these channels is significantly impacted by dynamic modulation of PIP2.
Voltage-gated K+ channels.
Vascular SMCs express functional KV1.2, KV1.5, and KV2.1 channels (Fig. 1). PIP2 has been shown to modulate KV1.2 (80, 81), KV1.5 (82), and KV2.1 (83) channels in heterologous expression systems, and may also promote changes in their biophysical properties. However, direct evidence for a regulatory role of PIP2 on SMC KV channels in native preparations is lacking. It has also been reported that KV7.x channels, which appear to be expressed by SMCs in some vascular beds (for review, see ref. 84), but not others (85), are activated by PIP2, although different KV7.x subtypes display widely varying affinities for PIP2 (86–88). The activity of several KV7 channel subtypes is suppressed by depletion of PIP2 (89, 90), consistent with an enhancing role for PIP2. These studies on all KV7 subtypes, performed in cultured cells, cell-free systems, or nonvascular systems (88, 91, 92), show that PIP2 acts by stabilizing an open state of the channel. Whether PIP2 plays a physiological role in regulating SMC KV7 activity awaits experiments in native cells and vascular tissues.
Vascular TRP Channels.
TRP channels are a family of cation channels whose members are ubiquitously expressed across diverse cell types. There are 28 TRP channel members in mammals that vary with respect to their expression pattern, cation permeability, activation mechanism, and cellular function. A diverse array of TRP channels has been reported in the vasculature (for review, see ref. 10). For instance, TRPC channels and TRPM4 channels are expressed in vascular SMCs and play key roles in membrane potential depolarization (Fig. 1). The vanilloid subfamily channel, TRPV4, is widely expressed in the vascular endothelium and represents a crucial Ca2+-influx route in ECs. Studies performed to date suggest that PIP2 modulates almost all TRP channels, sensitizing some (93–95) and desensitizing others (96–99).
TRPC channels.
It has been shown that norepinephrine, angiotensin II, endothelin-1, uridine triphosphate, and vasopressin, acting through GqPCRs or receptor tyrosine kinases, activate canonical TRP (TRPC) channels in vascular SMCs (10, 100, 101). Determining the contribution of each TRPC channel subtype to SMC function has been difficult and has led to disparate conclusions. Studies of this type face several obstacles. First, available pharmacological agents exhibit limited subtype selectivity; second, genetic manipulations often lead to compensatory changes in other TRPC subtypes; and third, TRPC channels heteromultimerize. Collectively, these factors complicate interpretation of experimental results, making it difficult to discern the roles of specific isoforms (for review, see refs. 10 and 101).
There is considerable evidence supporting the expression of five TRPC channel subtypes in SMCs: TRPC1, TRPC3, TRPC4, TRPC5, and TRPC6 (101). The TRPC1 subtype mediates depolarization in SMCs in response to endothelin-1 or norepinephrine exposure (102, 103). The TRPC3 channel has been implicated in vasoconstriction induced by different GqPCR agonists via a mechanism involving DAG and possibly IP3R, but it does not appear to contribute to constriction in response to increased intravascular pressure (myogenic response) (104–106). TRPC3 subunits can multimerize with TRPC1 and TRPC6, and also directly associate with IP3Rs (102–104, 107). TRPC4 and TRPC5 subtypes, which are also thought to heteromultimerize with other subunits (108–111), have poorly defined roles in vascular SMCs. The TRPC6 channel likely plays a key role in receptor-mediated vasoconstriction, reflecting production of DAG, an activator of TRPC6, by PLC-mediated hydrolysis of PIP2 downstream of activated GqPCRs. The other product of this hydrolysis, IP3, might also enhance TRPC6 activity (112–115). Ca2+ influx through TRPC6 can facilitate IP3R-mediated Ca2+ release from the SR, which subsequently activates depolarizing TRPM4 channels. This latter outcome could explain the contribution of TRPC6 channels to the development of myogenic tone (116, 117).
Earlier studies explored the potential regulation of TRPC channels in vascular SMCs by PIP2 (95, 118, 119). Such regulation is complicated by the fact that the PIP2 metabolites, DAG and IP3, are themselves standard activators and/or modulators of TRPC channels. In vascular SMCs, it has been shown that PIP2 is obligatory for TRPC1 activation (95), a regulatory role that requires PKC activation and subsequent channel phosphorylation and ultimately leads to PIP2 binding to the channel (118). These observations suggest that both PIP2 and PKC are required for TRPC1 activation. Studies of PIP2 regulation of TRPC6 channels have produced contradictory results, with one group reporting that PIP2 enhances TRPC3 and TRPC6 activity in heterologous expressions systems (120), and another proposing that PIP2 in mesenteric artery SMCs associates with TRPC6 and inhibits its angiotensin II-induced activity (119). Because TRPC6 opening requires PLC-mediated hydrolysis of PIP2 to DAG, as noted above, it is conceivable that both PIP2 depletion and DAG generation lead to channel activation. However, prolonged depletion of PIP2 could ultimately reduce DAG production and decrease channel activation. Thus, the role of PIP2 in the regulation of SMC TRPC6 activity remains unclear (Fig. 5).
TRPV4 channel.
The TRPV4 channel is permeable to Ca2+ and Na+ (121). It is activated by EETs (122), heat (123), and possibly mechanical stimuli (124). TRPV4 channels are also activated by GqPCR signaling (for review, see ref. 125). Several studies have established TRPV4 expression and function in vascular SMCs and ECs and shown that activation of the channel in either cell type leads to arteriolar dilation, albeit through different mechanisms in each cell type (5, 126).
Earley et al. (126) reported that, in vascular SMCs, TRPV4 channel activation by EETs induces Ca2+ influx, which stimulates RyR activity through a Ca2+-induced Ca2+-release mechanism to generate Ca2+ sparks, which, in turn, activate BK channels. The resulting BK channel-mediated K+ efflux causes SMC hyperpolarization and feedback deactivation of voltage-dependent Ca2+ channels, leading to arterial dilation. Santana and colleagues (127) also reported that activation of Gq-coupled angiotensin II receptors stimulates SMC TRPV4 channels in a PKC-dependent manner.
Strong evidence for TRPV4 channel expression and function in the endothelium has accumulated over the past decade. Activation of TRPV4 channels in mesenteric artery ECs, either by a potent synthetic agonist (GSK1016790A) or in response to muscarinic GqPCR activation, results in an influx of Ca2+ that can be detected optically. These events, termed “TRPV4 sparklets,” are elementary Ca2+ signals that reflect Ca2+ influx through individual TRPV4 channels, which appear to exist predominantly in the form of four-channel clusters in mesenteric ECs (5). This Ca2+ influx subsequently activates KCa channels (IK and SK), increasing K+ efflux. This not only hyperpolarizes EC VM, it also hyperpolarizes adjacent SMCs (5) (Fig. 1). Notably, muscarinic receptor activation of mesenteric endothelial TRPV4 channels stimulates TRPV4 sparklets only at myoendothelial projections linking ECs to SMCs (Fig. 1) (5, 14). This microdomain signaling enables activation of as few as three TRPV4 channels per EC to cause hyperpolarization and near-maximal vasodilation in response to acetylcholine (5). In addition to regulation of membrane potential and diameter, vascular TRPV4 channels have been implicated in angiogenesis and control of vascular permeability (125).
Recent work has shown that, similar to arterial ECs, brain capillary ECs express functional TRPV4 channels (51). Because capillary ECs are apparently unique compared with arterial/arteriolar ECs in that they lack functional Ca2+-activated IK/SK channels (7), activation of TRPV4 channels should depolarize capillary ECs (Fig. 1), as is the case in the lymphatic system (128). One intriguing observation is that the open probability of TRPV4 channels in brain ECs is exceedingly low under basal conditions, an observation that might indicate tonic TRPV4 inhibition in the cerebral vascular bed. Electrophysiological and pharmacological experiments have revealed that PIP2 suppresses TRPV4 channel activity in capillary ECs (51) (Fig. 5). These findings in native vascular ECs are in line with previous results in heterologous expression systems showing that PIP2 interacts directly with N-terminal residues of the TRPV4 channel (99, 129) and suppresses TRPV4 channel activity (99). Consistent with an inhibitory role for PIP2, we found that suppressing PIP2 synthesis by decreasing intracellular ATP or inhibiting lipid kinases significantly enhanced TRPV4 activity. Enhancing PIP2 hydrolysis through prostanoid or muscarinic GqPCR signaling has also been shown to activate TRPV4 channels in brain capillary ECs (51). Therefore, receptor agonists that are postulated to mediate neurovascular coupling in the brain, such as prostaglandin E2 (PGE2) (130, 131), could be physiological activators of brain capillary EC TRPV4 channels. Because GqPCR signaling causes PIP2 depletion, it not only activates a depolarizing channel (TRPV4), but simultaneously deactivates a hyperpolarizing channel (Kir2.1) (27, 51). This dual mechanism ensures more efficient control of the membrane potential of brain capillaries, such that PIP2 facilitates K+/Kir2.1-mediated hyperpolarization and GqPCR-mediated PIP2 depletion depolarizes VM.
In contrast to cerebral ECs, where GqPCR agonists increase TRPV4 activity through depletion of PIP2 (51), muscarinic GqPCR activation in mesenteric arteries promotes TRPV4-mediated hyperpolarization in a manner that depends on A-kinase anchoring protein (AKAP150)-bound PKC (14). These apparent vascular bed-specific modes of physiological TRPV4 activation are reminiscent of the different mechanisms of KATP channel inhibition by GqPCRs in vascular SMCs (PKC-mediated) (12) and cardiac myocytes (PIP2-dependent) (29). Another important consideration is the possibility that normal PIP2 levels are different at different points in vascular networks. A higher PIP2 set point could dramatically alter vascular ion channel regulation and could reflect diminished PIP2 breakdown owing to reduced levels of GqPCR agonists, lower constitutive GqPCR/PLC activity, and/or decreased GqPCR expression (24). The proximity of GqPCRs to ion channels under PIP2 control—and thus the ability of an agonist-activated GqPCR to deplete PIP2 in the vicinity of the channel—could also dictate ion channel regulation (24, 30, 31). As an alternative (or in addition) to reduced PIP2 hydrolysis, more robust PIP2 synthesis could translate into higher levels of PIP2 and stronger tonic inhibition of TRPV4 channels in brain capillary ECs. Physiologically, a higher PIP2 set point in the brain endothelium could have favorable implications for sustaining electrical signaling in the brain circulation. Specifically, the PIP2 set point in brain capillaries is apparently sufficient to maximally activate Kir2.1 channels; this facilitates VM hyperpolarization and suppresses TRPV4 channels, thereby limiting VM depolarization (Fig. 5) (27, 51, 67). Conversely, one could envision a scenario in which a lower PIP2 set point, and therefore higher activity of cerebral TRPV4 channels, would limit hyperpolarization in response to Kir2.1 activation (67). The resulting failure to evoke VM hyperpolarization would be detrimental to Kir2.1-mediated capillary electrical signaling, with deleterious consequences for regulation of cerebral blood flow.
Vascular Chloride Channels.
The chloride equilibrium potential in SMCs is between −30 and −20 mV (132, 133), and the cell’s membrane potential is about −50 to −40 mV (2). Therefore, activation of chloride channels in SMCs leads to membrane depolarization. TMEM16A (transmembrane member 16A, encoded by ANO1) is a Ca2+-activated Cl− channel expressed in vascular SMCs that is a determinant of reactivity in some vascular beds (134, 135). Pritchard et al. (136), working on pulmonary arterial SMCs, were the first to report the regulation of TMEM16A by PIP2. On the basis of biochemical and electrophysiological analyses, these authors suggested that PIP2 physically associates with TMEM16A in SMCs and that this association suppresses TMEM16A currents. However, the effects of PIP2 on TMEM16A appear more complex (Fig. 5), given that several studies from different groups have demonstrated a requirement for PIP2 to sustain TMEM16A activity, albeit in nonvascular cells (137, 138). Along these lines, Carlson and colleagues (139) recently reported that, in addition to Ca2+, PIP2 is required for TMEM16A activity. GqPCR signaling-induced PLC activation and subsequent Ca2+ signals have also been suggested to activate TMEM16A (140), despite the simultaneous PIP2 hydrolysis that this signaling produces, highlighting the complexity of PIP2 modulation. Recent structural findings provide some insights into the molecular basis of PIP2-dependent regulation of the TMEM16A channel (141). The ion-conducting pore of TMEM16A has two modules: a Ca2+-binding module that mediates channel activation, and a PIP2-binding module that mediates desensitization. GqPCR activation triggers Ca2+ signals that activate the TMEM16A channel. PIP2 dissociation from the channel during prolonged stimulation and Ca2+ saturation, as occurs during extended GqPCR stimulation, leads to channel desensitization due to a conformational change that causes the ionic pore to collapse (141). Clearly, further investigations are warranted to understand the consequences of PIP2–TMEM16A interactions in the vasculature and how such a regulatory axis could alter vascular function.
PIP2–Ion Channel Vascular Pathologies
Pathological changes in PIP2 interactions with an ion channel can increase or decrease channel function. These pathologies are categorized under one of two mechanisms, depending on the nature of the defect. In the first, an ion channel mutation involving residues critical for PIP2 binding can cripple channel regulation, even if cellular PIP2 levels are unchanged. In the second, compromised PIP2 levels could lead to altered ion channel regulation in the absence of mutations in the target channel (Fig. 6). Despite the critical roles of ion channels in vascular physiology, only a few vascular pathologies are currently recognized to involve PIP2. Given the emerging interest in PIP2 and its crucial role in vascular ion channel regulation, it is likely that additional PIP2-dependent channelopathies will be identified in the future. In this section, we discuss the limited available information on pathological conditions in which altered PIP2–ion channel interactions have been implicated and speculate on others.
Fig. 6.
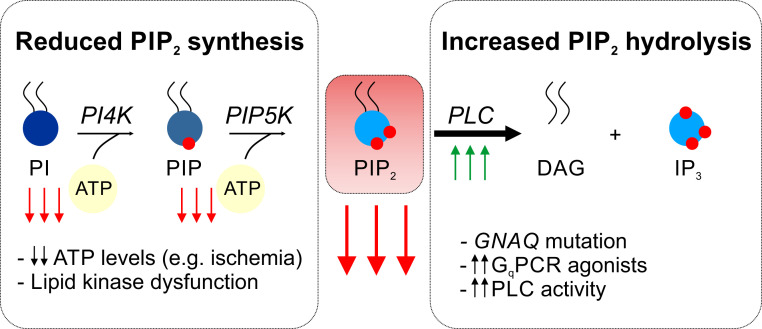
Altered PIP2 levels in pathology. A profound decline in cellular levels of PIP2 is likely associated with different vascular pathologies. Impaired biosynthesis of PIP2 occurs when ATP:ADP ratio necessary for phosphoryl transfer is not maintained or when phosphoinositide kinases are dysfunctional. A reduction in ATP levels is linked to certain pathological conditions (e.g., ischemia) or mitochondrial dysfunction, the latter of which is a hallmark of many vascular diseases. On the other hand, enhanced breakdown of PIP2 by GqPCR/PLC signaling can reduce PIP2 levels at rates exceeding those of PIP2 repletion. Pathological increases in GqPCR activity (e.g., due to a gain-of-function mutation in GNAQ or increased receptor agonist levels) or PLC activity can result in reduced availability of PIP2, thereby affecting vascular ion channel activity.
Ion Channel Mutation.
Andersen–Tawil syndrome (ATS) is characterized by cardiac arrhythmias, periodic paralysis, and developmental abnormalities. About 60% of ATS patients harbor loss-of-function mutations in the KCNJ2 gene encoding the Kir2.1 channel. Many of these mutations map to Kir2.1 residues involved in PIP2 binding; these mutations lead to defective PIP2 binding to the channel and thus decrease channel activity (142, 143). In addition to the hallmark triad of ATS symptoms—arrhythmias, periodic paralysis, and dysmorphic features—impaired flow-mediated vasodilation, indicative of impaired endothelial function and presumably reflecting dysfunctional Kir2.1 channels (144), has been reported in ATS patients (145). ATS patients also often display multiple white matter lesions characteristic of small-vessel diseases of the brain (146). Vascular abnormalities attributable to Kir2.1 mutations in ATS have yet to be systematically investigated.
Mutations in the TRPV4 gene cause a wide range of human disorders (for review, see ref. 147). Although a direct link between TRPV4 channelopathies and PIP2 regulation has not been clearly established, many human TRPV4 mutations are located in the ankyrin repeat domain, which regulates binding of PIP2, ATP, and calmodulin to the channel (99, 129, 147, 148). Despite the remarkably high number of disease-causing TRPV4 mutations (compared with other TRP channels), it is unclear why vascular manifestations of these mutations are mild in affected patients, who are more frequently affected by skeletal disease and neuropathies.
PIP2 Availability.
A change in PIP2 levels in the inner leaflet of the plasma membrane, in most pathophysiological settings, a decrease, could disrupt the function of PIP2-regulated proteins. As noted above, cellular PIP2 levels are dynamic, such that PIP2 availability is determined largely by PIP2 synthesis and depletion. Other cellular mechanisms, such as PIP2 sequestration, might also play a role in making the phosphoinositide less available to PIP2-interacting proteins (149, 150).
Altered PIP2 synthesis.
PIP2 synthesis requires an appropriate ATP:ADP ratio and functional lipid kinases. Interruption of either or both could decrease PIP2 and compromise ion channel regulation (Fig. 6) (151, 152). There are pathological situations in which ATP levels decline dramatically, such as during ischemic events (e.g., cerebral or coronary ischemia) (153, 154). These events could therefore be associated with compromised PIP2 synthesis. Mitochondrial dysfunction in the vasculature, a key feature associated with aging (155) and different diseases such as diabetes (156, 157) and CADASIL (cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy) (158), can lead to compromised oxidative phosphorylation and ATP synthesis. Several studies have additionally reported disrupted phosphoinositide levels in the brains of Alzheimer’s disease patients (159–161), changes that, in theory, could contribute to altered blood flow control. In addition to ATP, phosphoinositide kinases are required for PIP2 synthesis, and their dysregulation causes human diseases, such as cancer and developmental disorders (162).
Altered PIP2 hydrolysis.
GqPCR/PLC activation affects Kir2 and TRPV4 channels in a PIP2-dependent manner (27, 51). There are several instances in which GqPCR activity would be abnormally high, which could disrupt maintenance of PIP2 levels (Fig. 6). For example, most capillary malformation patients (nonsyndromic and Sturge–Weber syndrome) harbor an R183Q gain-of-function or activating mutation in the Gαq protein, encoded by GNAQ, which is expressed at high levels in ECs (163–167). These patients exhibit altered cerebral blood flow [e.g., generalized hypoperfusion, severe ischemia, and impaired cerebral hemodynamic responses to seizure activity (168–170)], but how mutant GNAQ affects endothelial signaling and blood flow is not fully understood. It is conceivable that the constitutively active Gαq R183Q mutant could deplete PIP2 in ECs, leading to Kir2 channel deactivation and TRPV4 channel activation (27, 51). If so, it could explain the reported cerebral ischemia and poor cerebral blood flow in infants with capillary malformation (163). Another example in the brain is cortical spreading depression, during which a slow depolarizing wave propagates across the cerebral cortex. This depolarization is associated with global release of neurotransmitters, many of which are receptor agonists and could therefore enhance GqPCR activation and PIP2 breakdown (171). Whether this affects vascular signaling remains to be tested. Downstream PLC activity can also be altered in disease. For example, PLC is increased and accumulates in the brains of Alzheimer’s disease patients (172, 173), a finding that aligns with a reported reduction in phosphoinositides in these patients (160).
Concluding Remarks
We have summarized our current perspective on known and potential physiological regulation of ion channels in the vasculature by the phosphoinositide PIP2. There are significant implications of such regulation for vascular function in health and disease. First, the ability of PIP2 to tune ion channel function facilitates the coordination of vascular cell excitability through dual actions of PIP2 on hyperpolarizing and depolarizing channels so as to favor a membrane potential shift in one direction or the other. Second, the concept of PIP2-mediated ion channel regulation provides an important and underexamined mechanism by which vascular GqPCR signaling is coupled to vascular function. Third, the strong link between the metabolic state of SMCs or ECs and PIP2 content suggests a potential mechanism to explain how metabolism affects vascular ion channel activity and, ultimately, vascular function. These insights establish a strong foundation for further investigations that will advance our understanding of how disruption of PIP2-mediated ion channel regulation—whether due to channel mutation or altered PIP2 availability—can be detrimental to vascular function and blood flow control.
Acknowledgments
This work was supported by a Postdoctoral Fellowship (17POST33650030 to O.F.H.), and a Career Development Award (20CDA35310097 to O.F.H.) from the American Heart Association (AHA), the Totman Medical Research Trust (to M.T.N.), the Fondation Leducq Transatlantic Network of Excellence on the Pathogenesis of Small Vessel Disease of the Brain (to M.T.N.), European Union’s Horizon 2020 Research and Innovation Programme (Grant Agreement 666881, SVDs@target, to M.T.N.), a grant from the Henry M. Jackson Foundation for the Advancement of Military Medicine (HU0001-18-2-0016), and grants from the NIH (P01-HL-095488, R01-HL-121706, R37-DK-053832, 7UM-HL-1207704, and R01-HL-131181 to M.T.N.). Research was also supported by grants from the National Institute of Neurological Disorders and Stroke and National Institute of Aging (R01-NS-110656) and by the National Heart, Lung, and Blood Institute of the NIH under Award R35-HL-140027.
Footnotes
The authors declare no competing interest.
See QnAs on page 20346.
Data Availability.
This article includes no unpublished data.
References
- 1.Bayliss W. M., On the local reactions of the arterial wall to changes of internal pressure. J. Physiol. 28, 220–231 (1902). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Knot H. J., Nelson M. T., Regulation of arterial diameter and wall [Ca2+] in cerebral arteries of rat by membrane potential and intravascular pressure. J. Physiol. 508, 199–209 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nelson M. T., Patlak J. B., Worley J. F., Standen N. B., Calcium channels, potassium channels, and voltage dependence of arterial smooth muscle tone. Am. J. Physiol. 259, C3–C18 (1990). [DOI] [PubMed] [Google Scholar]
- 4.Nelson M. T., Quayle J. M., Physiological roles and properties of potassium channels in arterial smooth muscle. Am. J. Physiol. 268, C799–C822 (1995). [DOI] [PubMed] [Google Scholar]
- 5.Sonkusare S. K., et al. , Elementary Ca2+ signals through endothelial TRPV4 channels regulate vascular function. Science 336, 597–601 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sonkusare S. K., Dalsgaard T., Bonev A. D., Nelson M. T., Inward rectifier potassium (Kir2.1) channels as end-stage boosters of endothelium-dependent vasodilators. J. Physiol. 594, 3271–3285 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Longden T. A., et al. , Capillary K+-sensing initiates retrograde hyperpolarization to increase local cerebral blood flow. Nat. Neurosci. 20, 717–726 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Murthy S. E., Dubin A. E., Patapoutian A., Piezos thrive under pressure: Mechanically activated ion channels in health and disease. Nat. Rev. Mol. Cell Biol. 18, 771–783 (2017). [DOI] [PubMed] [Google Scholar]
- 9.Gonzales A. L., Amberg G. C., Earley S., Ca2+ release from the sarcoplasmic reticulum is required for sustained TRPM4 activity in cerebral artery smooth muscle cells. Am. J. Physiol. Cell Physiol. 299, C279–C288 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Earley S., Brayden J. E., Transient receptor potential channels in the vasculature. Physiol. Rev. 95, 645–690 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Navedo M. F., Amberg G. C., Votaw V. S., Santana L. F., Constitutively active L-type Ca2+ channels. Proc. Natl. Acad. Sci. U.S.A. 102, 11112–11117 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bonev A. D., Nelson M. T., Vasoconstrictors inhibit ATP-sensitive K+ channels in arterial smooth muscle through protein kinase C. J. Gen. Physiol. 108, 315–323 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ledoux J., et al. , Functional architecture of inositol 1,4,5-trisphosphate signaling in restricted spaces of myoendothelial projections. Proc. Natl. Acad. Sci. U.S.A. 105, 9627–9632 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sonkusare S. K., et al. , AKAP150-dependent cooperative TRPV4 channel gating is central to endothelium-dependent vasodilation and is disrupted in hypertension. Sci. Signal. 7, ra66 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hille B., Dickson E. J., Kruse M., Vivas O., Suh B.-C., Phosphoinositides regulate ion channels. Biochim. Biophys. Acta 1851, 844–856 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang C.-L., Feng S., Hilgemann D. W., Direct activation of inward rectifier potassium channels by PIP2 and its stabilization by Gbetagamma. Nature 391, 803–806 (1998). [DOI] [PubMed] [Google Scholar]
- 17.Shyng S. L., Nichols C. G., Membrane phospholipid control of nucleotide sensitivity of KATP channels. Science 282, 1138–1141 (1998). [DOI] [PubMed] [Google Scholar]
- 18.Suh B.-C., Hille B., Recovery from muscarinic modulation of M current channels requires phosphatidylinositol 4,5-bisphosphate synthesis. Neuron 35, 507–520 (2002). [DOI] [PubMed] [Google Scholar]
- 19.Knight Z. A., Shokat K. M., Features of selective kinase inhibitors. Chem. Biol. 12, 621–637 (2005). [DOI] [PubMed] [Google Scholar]
- 20.Balla A., Balla T., Phosphatidylinositol 4-kinases: Old enzymes with emerging functions. Trends Cell Biol. 16, 351–361 (2006). [DOI] [PubMed] [Google Scholar]
- 21.Gehrmann T., et al. , Functional expression and characterisation of a new human phosphatidylinositol 4-kinase PI4K230. Biochim. Biophys. Acta 1437, 341–356 (1999). [DOI] [PubMed] [Google Scholar]
- 22.Suer S., Sickmann A., Meyer H. E., Herberg F. W., Heilmeyer L. M. Jr, Human phosphatidylinositol 4-kinase isoform PI4K92. Expression of the recombinant enzyme and determination of multiple phosphorylation sites. Eur. J. Biochem. 268, 2099–2106 (2001). [DOI] [PubMed] [Google Scholar]
- 23.Falkenburger B. H., Dickson E. J., Hille B., Quantitative properties and receptor reserve of the DAG and PKC branch of Gq-coupled receptor signaling. J. Gen. Physiol. 141, 537–555 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dickson E. J., Falkenburger B. H., Hille B., Quantitative properties and receptor reserve of the IP3 and calcium branch of Gq-coupled receptor signaling. J. Gen. Physiol. 141, 521–535 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Falkenburger B. H., Jensen J. B., Hille B., Kinetics of PIP2 metabolism and KCNQ2/3 channel regulation studied with a voltage-sensitive phosphatase in living cells. J. Gen. Physiol. 135, 99–114 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Horowitz L. F., et al. , Phospholipase C in living cells: Activation, inhibition, Ca2+ requirement, and regulation of M current. J. Gen. Physiol. 126, 243–262 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harraz O. F., Longden T. A., Dabertrand F., Hill-Eubanks D., Nelson M. T., Endothelial GqPCR activity controls capillary electrical signaling and brain blood flow through PIP2 depletion. Proc. Natl. Acad. Sci. U.S.A. 115, E3569–E3577 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hilgemann D. W., et al. , Lipid signaling to membrane proteins: From second messengers to membrane domains and adapter-free endocytosis. J. Gen. Physiol. 150, 211–224 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hilgemann D. W., Ball R., Regulation of cardiac Na+,Ca2+ exchange and KATP potassium channels by PIP2. Science 273, 956–959 (1996). [DOI] [PubMed] [Google Scholar]
- 30.Cho H., et al. , Low mobility of phosphatidylinositol 4,5-bisphosphate underlies receptor specificity of Gq-mediated ion channel regulation in atrial myocytes. Proc. Natl. Acad. Sci. U.S.A. 102, 15241–15246 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cho H., Lee D., Lee S. H., Ho W.-K., Receptor-induced depletion of phosphatidylinositol 4,5-bisphosphate inhibits inwardly rectifying K+ channels in a receptor-specific manner. Proc. Natl. Acad. Sci. U.S.A. 102, 4643–4648 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hilgemann D. W., Feng S., Nasuhoglu C., The complex and intriguing lives of PIP2 with ion channels and transporters. Sci. STKE 2001, re19 (2001). [DOI] [PubMed] [Google Scholar]
- 33.Wang J., Richards D. A., Segregation of PIP2 and PIP3 into distinct nanoscale regions within the plasma membrane. Biol. Open 1, 857–862 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McLaughlin S., Wang J., Gambhir A., Murray D., PIP2 and proteins: Interactions, organization, and information flow. Annu. Rev. Biophys. Biomol. Struct. 31, 151–175 (2002). [DOI] [PubMed] [Google Scholar]
- 35.Won D. H., et al. , PI(3,4,5)P3 and PI(4,5)P2 lipids target proteins with polybasic clusters to the plasma membrane. Science 314, 1458–1461 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Suh B. C., Hille B., Electrostatic interaction of internal Mg2+ with membrane PIP2 Seen with KCNQ K+ channels. J. Gen. Physiol. 130, 241–256 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McLaughlin S., Murray D., Plasma membrane phosphoinositide organization by protein electrostatics. Nature 438, 605–611 (2005). [DOI] [PubMed] [Google Scholar]
- 38.van den Bogaart G., et al. , Membrane protein sequestering by ionic protein-lipid interactions. Nature 479, 552–555 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dickson E. J., Hille B., Understanding phosphoinositides: Rare, dynamic, and essential membrane phospholipids. Biochem. J. 476, 1–23 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hammond G. R. V., Balla T., Polyphosphoinositide binding domains: Key to inositol lipid biology. Biochim. Biophys. Acta 1851, 746–758 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hansen S. B., Lipid agonism: The PIP2 paradigm of ligand-gated ion channels. Biochim. Biophys. Acta 1851, 620–628 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Suh B.-C., Hille B., PIP2 is a necessary cofactor for ion channel function: How and why? Annu. Rev. Biophys. 37, 175–195 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hansen S. B., Tao X., MacKinnon R., Structural basis of PIP2 activation of the classical inward rectifier K+ channel Kir2.2. Nature 477, 495–498 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Baukrowitz T., et al. , PIP2 and PIP as determinants for ATP inhibition of KATP channels. Science 282, 1141–1144 (1998). [DOI] [PubMed] [Google Scholar]
- 45.Sui J. L., Petit-Jacques J., Logothetis D. E., Activation of the atrial KACh channel by the βγ subunits of G proteins or intracellular Na+ ions depends on the presence of phosphatidylinositol phosphates. Proc. Natl. Acad. Sci. U.S.A. 95, 1307–1312 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tobelaim W. S., et al. , Competition of calcified calmodulin N lobe and PIP2 to an LQT mutation site in Kv7.1 channel. Proc. Natl. Acad. Sci. U.S.A. 114, E869–E878 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee S. J., et al. , Secondary anionic phospholipid binding site and gating mechanism in Kir2.1 inward rectifier channels. Nat. Commun. 4, 2786 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee S. J., et al. , Structural basis of control of inward rectifier Kir2 channel gating by bulk anionic phospholipids. J. Gen. Physiol. 148, 227–237 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Logothetis D. E., et al. , Phosphoinositide control of membrane protein function: A frontier led by studies on ion channels. Annu. Rev. Physiol. 77, 81–104 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Robinson C. V., Rohacs T., Hansen S. B., Tools for understanding nanoscale lipid regulation of ion channels. Trends Biochem. Sci. 44, 795–806 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Harraz O. F., Longden T. A., Hill-Eubanks D., Nelson M. T., PIP2 depletion promotes TRPV4 channel activity in mouse brain capillary endothelial cells. eLife 7, e38689 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sancho M., et al. , Membrane lipid-KIR2.x channel interactions enable hemodynamic sensing in cerebral arteries. Arterioscler. Thromb. Vasc. Biol. 39, 1072–1087 (2019). [DOI] [PubMed] [Google Scholar]
- 53.Vaithianathan T., et al. , Direct regulation of BK channels by phosphatidylinositol 4,5-bisphosphate as a novel signaling pathway. J. Gen. Physiol. 132, 13–28 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lopatin A. N., Makhina E. N., Nichols C. G., Potassium channel block by cytoplasmic polyamines as the mechanism of intrinsic rectification. Nature 372, 366–369 (1994). [DOI] [PubMed] [Google Scholar]
- 55.Zhao G., Joca H. C., Nelson M. T., Lederer W. J., ATP- and voltage-dependent electro-metabolic signaling regulates blood flow in heart. Proc. Natl. Acad. Sci. U.S.A. 117, 7461–7470 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bradley K. K., et al. , Kir2.1 encodes the inward rectifier potassium channel in rat arterial smooth muscle cells. J. Physiol. 515, 639–651 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Smith P. D., et al. , KIR channels function as electrical amplifiers in rat vascular smooth muscle. J. Physiol. 586, 1147–1160 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tykocki N. R., Bonev A. D., Longden T. A., Heppner T. J., Nelson M. T., Inhibition of vascular smooth muscle inward-rectifier K+ channels restores myogenic tone in mouse urinary bladder arterioles. Am. J. Physiol. Renal Physiol. 312, F836–F847 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zaritsky J. J., Eckman D. M., Wellman G. C., Nelson M. T., Schwarz T. L., Targeted disruption of Kir2.1 and Kir2.2 genes reveals the essential role of the inwardly rectifying K+ current in K+-mediated vasodilation. Circ. Res. 87, 160–166 (2000). [DOI] [PubMed] [Google Scholar]
- 60.Yang Y., et al. , Diverse Kir expression contributes to distinct bimodal distribution of resting potentials and vasotone responses of arterioles. PLoS One 10, e0125266 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fan Z., Makielski J. C., Anionic phospholipids activate ATP-sensitive potassium channels. J. Biol. Chem. 272, 5388–5395 (1997). [DOI] [PubMed] [Google Scholar]
- 62.D’Avanzo N., Cheng W. W. L., Doyle D. A., Nichols C. G., Direct and specific activation of human inward rectifier K+ channels by membrane phosphatidylinositol 4,5-bisphosphate. J. Biol. Chem. 285, 37129–37132 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.D’Avanzo N., Lee S.-J., Cheng W. W. L., Nichols C. G., Energetics and location of phosphoinositide binding in human Kir2.1 channels. J. Biol. Chem. 288, 16726–16737 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Du X., et al. , Characteristic interactions with phosphatidylinositol 4,5-bisphosphate determine regulation of kir channels by diverse modulators. J. Biol. Chem. 279, 37271–37281 (2004). [DOI] [PubMed] [Google Scholar]
- 65.Filosa J. A., et al. , Local potassium signaling couples neuronal activity to vasodilation in the brain. Nat. Neurosci. 9, 1397–1403 (2006). [DOI] [PubMed] [Google Scholar]
- 66.Knot H. J., Zimmermann P. A., Nelson M. T., Extracellular K., Extracellular K+-induced hyperpolarizations and dilatations of rat coronary and cerebral arteries involve inward rectifier K+ channels. J. Physiol. 492, 419–430 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Moshkforoush A., et al. , The capillary Kir channel as sensor and amplifier of neuronal signals: Modeling insights on K+-mediated neurovascular communication. Proc. Natl. Acad. Sci. U.S.A. 117, 16626–16637 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Longden T. A., Nelson M. T., Vascular inward rectifier K+ channels as external K+ sensors in the control of cerebral blood flow. Microcirculation 22, 183–196 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hibino H., et al. , Inwardly rectifying potassium channels: Their structure, function, and physiological roles. Physiol. Rev. 90, 291–366 (2010). [DOI] [PubMed] [Google Scholar]
- 70.Quayle J. M., Nelson M. T., Standen N. B., ATP-sensitive and inwardly rectifying potassium channels in smooth muscle. Physiol. Rev. 77, 1165–1232 (1997). [DOI] [PubMed] [Google Scholar]
- 71.MacGregor G. G., et al. , Nucleotides and phospholipids compete for binding to the C terminus of KATP channels. Proc. Natl. Acad. Sci. U.S.A. 99, 2726–2731 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Quinn K. V., Cui Y., Giblin J. P., Clapp L. H., Tinker A., Do anionic phospholipids serve as cofactors or second messengers for the regulation of activity of cloned ATP-sensitive K+ channels? Circ. Res. 93, 646–655 (2003). [DOI] [PubMed] [Google Scholar]
- 73.Lu M., Hebert S. C., Giebisch G., Hydrolyzable A. T. P., Hydrolyzable ATP and PIP2 modulate the small-conductance K+ channel in apical membranes of rat cortical-collecting duct (CCD). J. Gen. Physiol. 120, 603–615 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhang M., et al. , Selective phosphorylation modulates the PIP2 sensitivity of the CaM-SK channel complex. Nat. Chem. Biol. 10, 753–759 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nelson M. T., et al. , Relaxation of arterial smooth muscle by calcium sparks. Science 270, 633–637 (1995). [DOI] [PubMed] [Google Scholar]
- 76.Pérez G. J., Bonev A. D., Nelson M. T., Micromolar Ca2+ from sparks activates Ca2+-sensitive K+ channels in rat cerebral artery smooth muscle. Am. J. Physiol. Cell Physiol. 281, C1769–C1775 (2001). [DOI] [PubMed] [Google Scholar]
- 77.Tang Q.-Y., Zhang Z., Meng X.-Y., Cui M., Logothetis D. E., Structural determinants of phosphatidylinositol 4,5-bisphosphate (PIP2) regulation of BK channel activity through the RCK1 Ca2+ coordination site. J. Biol. Chem. 289, 18860–18872 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Brenner R., et al. , Vasoregulation by the β1 subunit of the calcium-activated potassium channel. Nature 407, 870–876 (2000). [DOI] [PubMed] [Google Scholar]
- 79.Jaggar J. H., Porter V. A., Lederer W. J., Nelson M. T., Calcium sparks in smooth muscle. Am. J. Physiol. Cell Physiol. 278, C235–C256 (2000). [DOI] [PubMed] [Google Scholar]
- 80.Abderemane-Ali F., et al. , Dual effect of phosphatidyl (4,5)-bisphosphate PIP2 on shaker K+ channels. J. Biol. Chem. 287, 36158–36167 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rodriguez-Menchaca A. A., et al. , PIP2 controls voltage-sensor movement and pore opening of Kv channels through the S4-S5 linker. Proc. Natl. Acad. Sci. U.S.A. 109, E2399–E2408 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Decher N., et al. , Structural determinants of Kvβ1.3-induced channel inactivation: A hairpin modulated by PIP2. EMBO J. 27, 3164–3174 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Delgado-Ramírez M., et al. , Regulation of Kv2.1 channel inactivation by phosphatidylinositol 4,5-bisphosphate. Sci. Rep. 8, 1769 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Byron K. L., Brueggemann L. I., Kv7 potassium channels as signal transduction intermediates in the control of microvascular tone. Microcirculation 25, e12419 (2018). [DOI] [PubMed] [Google Scholar]
- 85.Lee S., Yang Y., Tanner M. A., Li M., Hill M. A., Heterogeneity in Kv7 channel function in the cerebral and coronary circulation. Microcirculation 22, 109–121 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hernandez C. C., Falkenburger B., Shapiro M. S., Affinity for phosphatidylinositol 4,5-bisphosphate determines muscarinic agonist sensitivity of Kv7 K+ channels. J. Gen. Physiol. 134, 437–448 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Loussouarn G., et al. , Phosphatidylinositol-4,5-bisphosphate, PIP2, controls KCNQ1/KCNE1 voltage-gated potassium channels: A functional homology between voltage-gated and inward rectifier K+ channels. EMBO J. 22, 5412–5421 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhang H., et al. , PIP2 activates KCNQ channels, and its hydrolysis underlies receptor-mediated inhibition of M currents. Neuron 37, 963–975 (2003). [DOI] [PubMed] [Google Scholar]
- 89.Selyanko A. A., et al. , Inhibition of KCNQ1-4 potassium channels expressed in mammalian cells via M1 muscarinic acetylcholine receptors. J. Physiol. 522, 349–355 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Suh B.-C., Inoue T., Meyer T., Hille B., Rapid chemically induced changes of PtdIns(4,5)P2 gate KCNQ ion channels. Science 314, 1454–1457 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kim K. S., Duignan K. M., Hawryluk J. M., Soh H., Tzingounis A. V., The voltage activation of cortical KCNQ channels depends on global PIP2 levels. Biophys. J. 110, 1089–1098 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zaydman M. A., Cui J., PIP2 regulation of KCNQ channels: Biophysical and molecular mechanisms for lipid modulation of voltage-dependent gating. Front. Physiol. 5, 195 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Nilius B., et al. , The Ca2+-activated cation channel TRPM4 is regulated by phosphatidylinositol 4,5-biphosphate. EMBO J. 25, 467–478 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rohács T., Lopes C. M. B., Michailidis I., Logothetis D. E., PI(4,5)P2 regulates the activation and desensitization of TRPM8 channels through the TRP domain. Nat. Neurosci. 8, 626–634 (2005). [DOI] [PubMed] [Google Scholar]
- 95.Saleh S. N., Albert A. P., Large W. A., Obligatory role for phosphatidylinositol 4,5-bisphosphate in activation of native TRPC1 store-operated channels in vascular myocytes. J. Physiol. 587, 531–540 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Doerner J. F., Hatt H., Ramsey I. S., Voltage- and temperature-dependent activation of TRPV3 channels is potentiated by receptor-mediated PI(4,5)P2 hydrolysis. J. Gen. Physiol. 137, 271–288 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ma R., et al. , PKD2 functions as an epidermal growth factor-activated plasma membrane channel. Mol. Cell. Biol. 25, 8285–8298 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Prescott E. D., Julius D., A modular PIP2 binding site as a determinant of capsaicin receptor sensitivity. Science 300, 1284–1288 (2003). [DOI] [PubMed] [Google Scholar]
- 99.Takahashi N., et al. , TRPV4 channel activity is modulated by direct interaction of the ankyrin domain to PI(4,5)P2. Nat. Commun. 5, 4994 (2014). [DOI] [PubMed] [Google Scholar]
- 100.Abramowitz J., Birnbaumer L., Physiology and pathophysiology of canonical transient receptor potential channels. FASEB J. 23, 297–328 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tykocki N. R., Boerman E. M., Jackson W. F., Smooth muscle ion channels and regulation of vascular tone in resistance arteries and arterioles. Compr. Physiol. 7, 485–581 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Salomonsson M., Braunstein T. H., Holstein-Rathlou N.-H., Jensen L. J., Na+-independent, nifedipine-resistant rat afferent arteriolar Ca2+ responses to noradrenaline: Possible role of TRPC channels. Acta Physiol. (Oxf.) 200, 265–278 (2010). [DOI] [PubMed] [Google Scholar]
- 103.Tai K., et al. , Agonist-evoked calcium entry in vascular smooth muscle cells requires IP3 receptor-mediated activation of TRPC1. Eur. J. Pharmacol. 583, 135–147 (2008). [DOI] [PubMed] [Google Scholar]
- 104.Liu D., et al. , Increased transient receptor potential canonical type 3 channels in vasculature from hypertensive rats. Hypertens. 53, 70–76 (2009). [DOI] [PubMed] [Google Scholar]
- 105.Peppiatt-Wildman C. M., Albert A. P., Saleh S. N., Large W. A., Endothelin-1 activates a Ca2+-permeable cation channel with TRPC3 and TRPC7 properties in rabbit coronary artery myocytes. J. Physiol. 580, 755–764 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Reading S. A., Earley S., Waldron B. J., Welsh D. G., Brayden J. E., TRPC3 mediates pyrimidine receptor-induced depolarization of cerebral arteries. Am. J. Physiol. Heart Circ. Physiol. 288, H2055–H2061 (2005). [DOI] [PubMed] [Google Scholar]
- 107.Adebiyi A., et al. , Isoform-selective physical coupling of TRPC3 channels to IP3 receptors in smooth muscle cells regulates arterial contractility. Circ. Res. 106, 1603–1612 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Shi J., et al. , TRPC1 proteins confer PKC and phosphoinositol activation on native heteromeric TRPC1/C5 channels in vascular smooth muscle: Comparative study of wild-type and TRPC1−/− mice. FASEB J. 26, 409–419 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Shi J., Ju M., Saleh S. N., Albert A. P., Large W. A., TRPC6 channels stimulated by angiotensin II are inhibited by TRPC1/C5 channel activity through a Ca2+- and PKC-dependent mechanism in native vascular myocytes. J. Physiol. 588, 3671–3682 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Strübing C., Krapivinsky G., Krapivinsky L., Clapham D. E., TRPC1 and TRPC5 form a novel cation channel in mammalian brain. Neuron 29, 645–655 (2001). [DOI] [PubMed] [Google Scholar]
- 111.Kiselyov K., Shin D. M., Kim J. Y., Yuan J. P., Muallem S., TRPC channels: Interacting proteins. Handb. Exp. Pharmacol. 179, 559–574 (2007). [DOI] [PubMed] [Google Scholar]
- 112.Albert A. P., Large W. A., Synergism between inositol phosphates and diacylglycerol on native TRPC6-like channels in rabbit portal vein myocytes. J. Physiol. 552, 789–795 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Inoue R., et al. , The transient receptor potential protein homologue TRP6 is the essential component of vascular α1-adrenoceptor-activated Ca2+-permeable cation channel. Circ. Res. 88, 325–332 (2001). [DOI] [PubMed] [Google Scholar]
- 114.Ju M., Shi J., Saleh S. N., Albert A. P., Large W. A., Ins(1,4,5)P3 interacts with PIP2 to regulate activation of TRPC6/C7 channels by diacylglycerol in native vascular myocytes. J. Physiol. 588, 1419–1433 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Large W. A., Receptor-operated Ca2+-permeable nonselective cation channels in vascular smooth muscle: A physiologic perspective. J. Cardiovasc. Electrophysiol. 13, 493–501 (2002). [DOI] [PubMed] [Google Scholar]
- 116.Gonzales A. L., et al. , A PLCγ1-dependent, force-sensitive signaling network in the myogenic constriction of cerebral arteries. Sci. Signal. 7, ra49 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Welsh D. G., Morielli A. D., Nelson M. T., Brayden J. E., Transient receptor potential channels regulate myogenic tone of resistance arteries. Circ. Res. 90, 248–250 (2002). [DOI] [PubMed] [Google Scholar]
- 118.Shi J., Birnbaumer L., Large W. A., Albert A. P., Myristoylated alanine-rich C kinase substrate coordinates native TRPC1 channel activation by phosphatidylinositol 4,5-bisphosphate and protein kinase C in vascular smooth muscle. FASEB J. 28, 244–255 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Albert A. P., Saleh S. N., Large W. A., Inhibition of native TRPC6 channel activity by phosphatidylinositol 4,5-bisphosphate in mesenteric artery myocytes. J. Physiol. 586, 3087–3095 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lemonnier L., Trebak M., Putney J. W. Jr, Complex regulation of the TRPC3, 6 and 7 channel subfamily by diacylglycerol and phosphatidylinositol-4,5-bisphosphate. Cell Calcium 43, 506–514 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Strotmann R., Harteneck C., Nunnenmacher K., Schultz G., Plant T. D., OTRPC4, a nonselective cation channel that confers sensitivity to extracellular osmolarity. Nat. Cell Biol. 2, 695–702 (2000). [DOI] [PubMed] [Google Scholar]
- 122.Watanabe H., et al. , Anandamide and arachidonic acid use epoxyeicosatrienoic acids to activate TRPV4 channels. Nature 424, 434–438 (2003). [DOI] [PubMed] [Google Scholar]
- 123.Güler A. D., et al. , Heat-evoked activation of the ion channel, TRPV4. J. Neurosci. 22, 6408–6414 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Vriens J., et al. , Cell swelling, heat, and chemical agonists use distinct pathways for the activation of the cation channel TRPV4. Proc. Natl. Acad. Sci. U.S.A. 101, 396–401 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.White J. P. M., et al. , TRPV4: Molecular conductor of a diverse orchestra. Physiol. Rev. 96, 911–973 (2016). [DOI] [PubMed] [Google Scholar]
- 126.Earley S., Heppner T. J., Nelson M. T., Brayden J. E., TRPV4 forms a novel Ca2+ signaling complex with ryanodine receptors and BKCa channels. Circ. Res. 97, 1270–1279 (2005). [DOI] [PubMed] [Google Scholar]
- 127.Mercado J., et al. , Local control of TRPV4 channels by AKAP150-targeted PKC in arterial smooth muscle. J. Gen. Physiol. 143, 559–575 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Behringer E. J., et al. , Calcium and electrical dynamics in lymphatic endothelium. J. Physiol. 595, 7347–7368 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Garcia-Elias A., et al. , Phosphatidylinositol-4,5-biphosphate-dependent rearrangement of TRPV4 cytosolic tails enables channel activation by physiological stimuli. Proc. Natl. Acad. Sci. U.S.A. 110, 9553–9558 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lacroix A., et al. , COX-2-derived prostaglandin E2 produced by pyramidal neurons contributes to neurovascular coupling in the rodent cerebral cortex. J. Neurosci. 35, 11791–11810 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zonta M., et al. , Neuron-to-astrocyte signaling is central to the dynamic control of brain microcirculation. Nat. Neurosci. 6, 43–50 (2003). [DOI] [PubMed] [Google Scholar]
- 132.Bulley S., Jaggar J. H., Cl− channels in smooth muscle cells. Pflugers Arch. 466, 861–872 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kitamura K., Yamazaki J., Chloride channels and their functional roles in smooth muscle tone in the vasculature. Jpn. J. Pharmacol. 85, 351–357 (2001). [DOI] [PubMed] [Google Scholar]
- 134.Davis A. J., et al. , Expression profile and protein translation of TMEM16A in murine smooth muscle. Am. J. Physiol. Cell Physiol. 299, C948–C959 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Thomas-Gatewood C., et al. , TMEM16A channels generate Ca2+-activated Cl− currents in cerebral artery smooth muscle cells. Am. J. Physiol. Heart Circ. Physiol. 301, H1819–H1827 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Pritchard H. A. T., Leblanc N., Albert A. P., Greenwood I. A., Inhibitory role of phosphatidylinositol 4,5-bisphosphate on TMEM16A-encoded calcium-activated chloride channels in rat pulmonary artery. Br. J. Pharmacol. 171, 4311–4321 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.De Jesús-Pérez J. J., et al. , Phosphatidylinositol 4,5-bisphosphate, cholesterol, and fatty acids modulate the calcium-activated chloride channel TMEM16A (ANO1). Biochim. Biophys. Acta Mol. Cell Biol. Lipids 1863, 299–312 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ta C. M., Acheson K. E., Rorsman N. J. G., Jongkind R. C., Tammaro P., Contrasting effects of phosphatidylinositol 4,5-bisphosphate on cloned TMEM16A and TMEM16B channels. Br. J. Pharmacol. 174, 2984–2999 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Tembo M., Wozniak K. L., Bainbridge R. E., Carlson A. E., Phosphatidylinositol 4,5-bisphosphate (PIP2) and Ca2+ are both required to open the Cl− channel TMEM16A. J. Biol. Chem. 294, 12556–12564 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Schroeder B. C., Cheng T., Jan Y. N., Jan L. Y., Expression cloning of TMEM16A as a calcium-activated chloride channel subunit. Cell 134, 1019–1029 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Le S. C., Jia Z., Chen J., Yang H., Molecular basis of PIP2-dependent regulation of the Ca2+-activated chloride channel TMEM16A. Nat. Commun. 10, 3769 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Donaldson M. R., Yoon G., Fu Y.-H., Ptacek L. J., Andersen-Tawil syndrome: A model of clinical variability, pleiotropy, and genetic heterogeneity. Ann. Med. 36 (suppl. 1), 92–97 (2004). [DOI] [PubMed] [Google Scholar]
- 143.Tristani-Firouzi M., Etheridge S. P., Kir 2.1 channelopathies: The Andersen-Tawil syndrome. Pflugers Arch. 460, 289–294 (2010). [DOI] [PubMed] [Google Scholar]
- 144.Ahn S. J., et al. , Inwardly rectifying K+ channels are major contributors to flow-induced vasodilatation in resistance arteries. J. Physiol. 595, 2339–2364 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Takeda I., et al. , Autosomal recessive Andersen-Tawil syndrome with a novel mutation L94P in Kir2.1. Neurol. Clin. Neurosci. 1, 131–137 (2013). [Google Scholar]
- 146.Chan H.-F., et al. , A novel neuropsychiatric phenotype of KCNJ2 mutation in one Taiwanese family with Andersen-Tawil syndrome. J. Hum. Genet. 55, 186–188 (2010). [DOI] [PubMed] [Google Scholar]
- 147.Nilius B., Voets T., The puzzle of TRPV4 channelopathies. EMBO Rep. 14, 152–163 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Inada H., Procko E., Sotomayor M., Gaudet R., Structural and biochemical consequences of disease-causing mutations in the ankyrin repeat domain of the human TRPV4 channel. Biochemistry 51, 6195–6206 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Abd Halim K. B., Koldsø H., Sansom M. S. P., Interactions of the EGFR juxtamembrane domain with PIP2-containing lipid bilayers: Insights from multiscale molecular dynamics simulations. Biochim. Biophys. Acta 1850, 1017–1025 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Golebiewska U., et al. , Membrane-bound basic peptides sequester multivalent (PIP2), but not monovalent (PS), acidic lipids. Biophys. J. 91, 588–599 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Xie L. H., Takano M., Kakei M., Okamura M., Noma A., Wortmannin, an inhibitor of phosphatidylinositol kinases, blocks the MgATP-dependent recovery of Kir6.2/SUR2A channels. J. Physiol. 514, 655–665 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Shyng S. L., et al. , Modulation of nucleotide sensitivity of ATP-sensitive potassium channels by phosphatidylinositol-4-phosphate 5-kinase. Proc. Natl. Acad. Sci. U.S.A. 97, 937–941 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Kawauchi S., et al. , Light scattering change precedes loss of cerebral adenosine triphosphate in a rat global ischemic brain model. Neurosci. Lett. 459, 152–156 (2009). [DOI] [PubMed] [Google Scholar]
- 154.Matsunaga A., et al. , Energy-dependent redox state of heme a + a3 and copper of cytochrome oxidase in perfused rat brain in situ. Am. J. Physiol. 275, C1022–C1030 (1998). [DOI] [PubMed] [Google Scholar]
- 155.Dai D.-F., Rabinovitch P. S., Ungvari Z., Mitochondria and cardiovascular aging. Circ. Res. 110, 1109–1124 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Blake R., Trounce I. A., Mitochondrial dysfunction and complications associated with diabetes. Biochim. Biophys. Acta 1840, 1404–1412 (2014). [DOI] [PubMed] [Google Scholar]
- 157.Kowluru R. A., Diabetic retinopathy: Mitochondrial dysfunction and retinal capillary cell death. Antioxid. Redox Signal. 7, 1581–1587 (2005). [DOI] [PubMed] [Google Scholar]
- 158.Zellner A., et al. , CADASIL brain vessels show a HTRA1 loss-of-function profile. Acta Neuropathol. 136, 111–125 (2018). [DOI] [PubMed] [Google Scholar]
- 159.Jope R. S., Song L., Li X., Powers R., Impaired phosphoinositide hydrolysis in Alzheimer’s disease brain. Neurobiol. Aging 15, 221–226 (1994). [DOI] [PubMed] [Google Scholar]
- 160.Stokes C. E., Hawthorne J. N., Reduced phosphoinositide concentrations in anterior temporal cortex of Alzheimer-diseased brains. J. Neurochem. 48, 1018–1021 (1987). [DOI] [PubMed] [Google Scholar]
- 161.Wallace M. A., Effects of Alzheimer’s disease-related beta amyloid protein fragments on enzymes metabolizing phosphoinositides in brain. Biochim. Biophys. Acta 1227, 183–187 (1994). [DOI] [PubMed] [Google Scholar]
- 162.McCrea H. J., De Camilli P., Mutations in phosphoinositide metabolizing enzymes and human disease. Physiology (Bethesda) 24, 8–16 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Bichsel C., Bischoff J., A somatic missense mutation in GNAQ causes capillary malformation. Curr. Opin. Hematol. 26, 179–184 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Couto J. A., et al. , Endothelial cells from capillary malformations are enriched for somatic GNAQ mutations. Plast. Reconstr. Surg. 137, 77e–82e (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Nakashima M., et al. , The somatic GNAQ mutation c.548G>A (p.R183Q) is consistently found in Sturge-Weber syndrome. J. Hum. Genet. 59, 691–693 (2014). [DOI] [PubMed] [Google Scholar]
- 166.Shirley M. D., et al. , Sturge-Weber syndrome and port-wine stains caused by somatic mutation in GNAQ. N. Engl. J. Med. 368, 1971–1979 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Tan W., et al. , The somatic GNAQ mutation (R183Q) is primarily located within the blood vessels of port wine stains. J. Am. Acad. Dermatol. 74, 380–383 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Aylett S. E., et al. , Sturge-Weber syndrome: Cerebral haemodynamics during seizure activity. Dev. Med. Child Neurol. 41, 480–485 (1999). [PubMed] [Google Scholar]
- 169.Chiron C., et al. , Regional cerebral blood flow by SPECT imaging in Sturge-Weber disease: An aid for diagnosis. J. Neurol. Neurosurg. Psychiatry 52, 1402–1409 (1989). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Griffiths P. D., et al. , 99mTechnetium HMPAO imaging in children with the Sturge-Weber syndrome: A study of nine cases with CT and MRI correlation. Neuroradiology 39, 219–224 (1997). [DOI] [PubMed] [Google Scholar]
- 171.Ayata C., Lauritzen M., Spreading depression, spreading depolarizations, and the cerebral vasculature. Physiol. Rev. 95, 953–993 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Shimohama S., et al. , Aberrant accumulation of phospholipase C-delta in Alzheimer brains. Am. J. Pathol. 139, 737–742 (1991). [PMC free article] [PubMed] [Google Scholar]
- 173.Shimohama S., et al. , Phospholipase C isozymes in the human brain and their changes in Alzheimer’s disease. Neuroscience 82, 999–1007 (1998). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article includes no unpublished data.