The placenta is an organ of fetal origin that develops at the interface with the maternal uterus (1). It performs numerous and diverse functions fundamental for the proper growth and development of the semiallogeneic fetus, including gas, nutrient, and waste exchange; production of hormones; and modulation of maternal immune response. Placental development begins, following implantation of the blastocyst-stage embryo, with the expansion of the trophectoderm (TE) and establishment of a villous trophoblast progenitor population, termed cytotrophoblast (CTB) (2). As this cell layer grows and invaginates to form chorionic villi (functional units of the human placenta), the CTB further expands and differentiates into two main mature trophoblast cell types: the syncytiotrophoblast (STB), involved in gas/nutrient exchange, and extravillous trophoblast (EVT), which invades the maternal decidua and remodels maternal spiral arterioles (Fig. 1). However, the mechanisms behind early events in human placentation, particularly CTB self-renewal and differentiation, are largely unknown. Using tools recently developed to study early human placental development, two studies in PNAS address the role of the Hippo pathway in trophoblast progenitor self-renewal. Both focus on the Hippo mediator complex containing TEAD4: Saha et al. (3) compare its function in early postimplantation mouse placenta with first-trimester human placenta and associate its deficiency with recurrent pregnancy loss (RPL), while Meinhardt et al. (4) focus on the mechanism by which its transcriptional cofactor yes-associated protein 1 (YAP1) regulates trophoblast stemness.
Fig. 1.
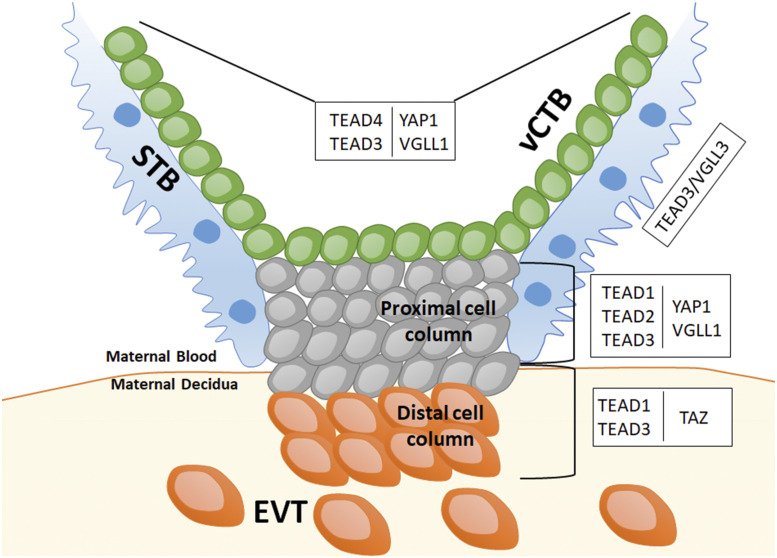
Human placental chorionic villus showing the different trophoblast subtypes: vCTB, STB, and EVTs in the maternal decidua, the latter arising from differentiation of vCTB through the cell column. Each placental trophoblast subcompartment expresses different combinations of the TEAD family members and their transcriptional cofactors, YAP1, TAZ, and VGLL family members. This particular spatial expression pattern suggests a dynamic exchange of binding partners that likely influences both cell fate and function.
The Hippo pathway is known to control organ size, tissue regeneration, cell fate decision, stemness, and differentiation (5). In the mouse preimplantation embryo, the differential activation of the Hippo pathway between the internal and external cells of the morula determines the first lineage specification, leading to distinction between the inner cell mass and outer TE layer of the blastocyst (6). The off state in the outer layer allows for nuclear translocation of the YAP1 cofactor protein, which in complex with TEAD4, drives the expression of TE-specific genes and specifies this lineage (7). There is some evidence that this mechanism is conserved in human trophoblast lineage specification (8); however, until now, the role of the TEAD4/YAP1 complex in human trophoblast self-renewal and differentiation had not been explored.
Saha et al. (3) investigated TEAD4 function using human trophoblast stem cells (hTSCs). Using both two-dimensional and three-dimensional (organoid) culture conditions, they demonstrated that knockdown of TEAD4 led to loss of self-renewal of these cells, associated with the inability to maintain colonies or self-renewing organoids. Interestingly, hTSCs isolated from patients experiencing idiopathic RPL showed low TEAD4 expression; they were also associated with lower proliferation and higher differentiation, a phenotype that was mostly rescued by reintroduction of TEAD4. Saha et al. (3) also evaluated Tead4 in the early postimplantation mouse placenta, a period equivalent to first-trimester human placenta. They found that Tead4 knockdown in mouse trophoblast stem cell (mTSC) compromised self-renewal in vitro, and Tead4 knockout impaired placental development in vivo, demonstrating a conserved function for this transcription factor between the two species. Further analysis of genome occupancy and gene expression pointed to a direct role for TEAD4 in regulation of cell cycle in both mTSC and hTSC.
Using tools recently developed to study early human placental development, two studies in PNAS address the role of the Hippo pathway in trophoblast progenitor self-renewal.
Meinhardt et al. (4) focused on the role of YAP1, the best-known partner for TEAD4, and evaluated its role using a combination of trophoblast organoids and genetically manipulated JEG3 choriocarcinoma cells. In the human placenta, YAP1 showed overlapping expression with TEAD4 in the CTB progenitor compartment of early first-trimester placentae. Manipulation of YAP1 expression and activity showed alterations in both cell cycle and STB-associated genes: In fact, the authors showed that the TEAD4/YAP1 complex can be either activating (with respect to proliferation-associated genes) or repressive (with respect to STB-associated genes), with the latter complexes containing the histone methyltransferase EZH2. Interestingly, down-regulation of YAP1 was also associated with up-regulation of TAZ, another TEAD4 partner, suggesting partial functional overlap.
While these two studies establish a role for TEAD4/YAP1 in maintenance of stemness of human villous cytotrophoblast (vCTB; by both promoting proliferation and inhibiting differentiation), they also point to involvement of other family members in regulation of other trophoblast compartments. In fact, there are four members of the TEAD family (TEAD1 to -4), each of which binds different cofactors, including members of the YAP/TAZ and VGLL families, all of which also show specific spatiotemporal expression patterns within chorionic villi (Fig. 1). Specifically, while TEAD4 is primarily expressed in vCTB, TEAD1 to -3 are enriched in differentiating/differentiated trophoblast, with TEAD2 in proximal cell column (9) and TEAD3 in STB progenitors. Similarly, of the cofactors, YAP1 is highly expressed in vCTB, while TAZ and VGLL3 are expressed in the cell column and STB, respectively. As our own previous study has also shown (10), VGLL1 is expressed in vCTB and proximal cell column. While there is likely some redundancy of function, this pattern of spatial expression suggests a dynamic exchange of binding partners that can influence cell fate and function within each trophoblast subcompartment. How the different cofactors modify DNA binding properties of each transcription factor, and thus, orchestrate changes in gene expression, remains to be elucidated. Meanwhile, it should be noted that, while the TEAD4/YAP1 complex appears to have a conserved role in mTSC and hTSC, VGLL members appear to be uniquely expressed in human trophoblast, thus introducing an additional species-specific layer of complexity in regulation of human placental development.
It is important to recognize that the study of human placental development, particularly of processes early in gestation, has long been hampered by lack of proper models that recapitulate human trophoblast cell types in vivo. These two studies have been possible because of the recent development of novel tools for such studies, namely the identification of culture conditions for the isolation and maintenance of self-renewing hTSCs (11) and trophoblast organoids (12, 13). These tools will continue to help elucidate mechanisms of human trophoblast self-renewal and differentiation and thus, improve our understanding of early human placental development. This is particularly crucial because abnormal placental development and function are associated with numerous pregnancy complications, ranging from idiopathic RPL to preeclampsia, intrauterine growth restriction, preterm birth, and stillbirth (14–16). Moreover, abnormal placental function in utero has been associated with fetal developmental reprogramming, with consequences on postnatal health, including higher incidence of metabolic dysregulation and cardiovascular disease (17, 18). Finally, understanding mechanisms of trophoblast progenitor self-renewal and differentiation may also help, not just to improve pregnancy management and outcome but also, to improve implantation and in vitro fertilization success. Many more studies such as the ones by Saha et al. (3) and Meinhardt et al. (4) are needed to address the dearth of knowledge on human placental development.
Footnotes
The authors declare no competing interest.
See companion article, “TEAD4 ensures postimplantation development by promoting trophoblast self-renewal: An implication in early human pregnancy loss,” 10.1073/pnas.2002449117.
References
- 1.Burton G. J., Fowden A. L., The placenta: A multifaceted, transient organ. Philos. Trans. R. Soc. Lond. B Biol. Sci. 370, 20140066 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Knöfler M., et al. , Human placenta and trophoblast development: Key molecular mechanisms and model systems. Cell. Mol. Life Sci. 76, 3479–3496 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saha B., et al. , TEAD4 ensures postimplantation development by promoting trophoblast self-renewal: An implication in early human pregnancy loss. Proc. Natl. Acad. Sci. U.S.A. 117, 17864–17875 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meinhardt G., et al. , Pivotal role of the transcriptional co-activator YAP in trophoblast stemness of the developing human placenta. Proc. Natl. Acad. Sci. U.S.A. 117, 13562–13570 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Varelas X., The Hippo pathway effectors TAZ and YAP in development, homeostasis and disease. Development 141, 1614–1626 (2014). [DOI] [PubMed] [Google Scholar]
- 6.Nishioka N., et al. , Tead4 is required for specification of trophectoderm in pre-implantation mouse embryos. Mech. Dev. 125, 270–283 (2008). [DOI] [PubMed] [Google Scholar]
- 7.Nishioka N., et al. , The Hippo signaling pathway components Lats and Yap pattern Tead4 activity to distinguish mouse trophectoderm from inner cell mass. Dev. Cell 16, 398–410 (2009). [DOI] [PubMed] [Google Scholar]
- 8.Home P., et al. , Altered subcellular localization of transcription factor TEAD4 regulates first mammalian cell lineage commitment. Proc. Natl. Acad. Sci. U.S.A. 109, 7362–7367 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wakeland A. K., et al. , Hypoxia directs human extravillous trophoblast differentiation in a hypoxia-inducible factor-dependent manner. Am. J. Pathol. 187, 767–780 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Soncin F., et al. , Comparative analysis of mouse and human placentae across gestation reveals species-specific regulators of placental development. Development 145, dev156273 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Okae H., et al. , Derivation of human trophoblast stem cells. Cell Stem Cell 22, 50–63.e6 (2018). [DOI] [PubMed] [Google Scholar]
- 12.Turco M. Y., et al. , Trophoblast organoids as a model for maternal-fetal interactions during human placentation. Nature 564, 263–267 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haider S., et al. , Self-renewing trophoblast organoids recapitulate the developmental program of the early human placenta. Stem Cell Reports 11, 537–551 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hustin J., Jauniaux E., Schaaps J. P., Histological study of the materno-embryonic interface in spontaneous abortion. Placenta 11, 477–486 (1990). [DOI] [PubMed] [Google Scholar]
- 15.Lim K. H., et al. , Human cytotrophoblast differentiation/invasion is abnormal in pre-eclampsia. Am. J. Pathol. 151, 1809–1818 (1997). [PMC free article] [PubMed] [Google Scholar]
- 16.Romero R., Kusanovic J. P., Chaiworapongsa T., Hassan S. S., Placental bed disorders in preterm labor, preterm PROM, spontaneous abortion and abruptio placentae. Best Pract. Res. Clin. Obstet. Gynaecol. 25, 313–327 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Godfrey K. M., Barker D. J. P., Fetal nutrition and adult disease. Am. J. Clin. Nutr. 71(5 suppl.), 1344S–1352S (2000). [DOI] [PubMed] [Google Scholar]
- 18.Gluckman P. D., Hanson M. A., Beedle A. S., Early life events and their consequences for later disease: A life history and evolutionary perspective. Am. J. Hum. Biol. 19, 1–19 (2007). [DOI] [PubMed] [Google Scholar]