Abstract
Peptide receptor radiotherapy using 177Lu-labeled somatostatin ligand analogs is a well-established treatment for neuroendocrine tumors, with 177Lu-DOTATATE having acquired marketing authorization in Europe and the United States. The investigation of the pharmacokinetics of these radiopharmaceuticals in vivo in humans is crucial for personalized treatment management and understanding of treatment effects. Such an investigation requires input data on the in vivo stability of the radiopharmaceuticals in blood and plasma. The work presented here is devoted to the investigation of the in vivo stability of 177Lu-DOTATATE in humans affected by neuroendocrine tumors. Methods: Blood samples of 6 patients undergoing 177Lu-DOTATATE were taken at 0.5, 4, 24, and 96 h after injection. Analysis of metabolic stability was performed using high-performance liquid chromatography. Results: A fast metabolism of the radiopharmaceutical was observed, with the fraction of intact 177Lu-DOTATATE in plasma decreasing rapidly to 23% ± 5% (mean ± SD) at 24 h and 1.7% ±0. 9% at 96 h after injection. Conclusion: The in vivo stability of 177Lu-DOTATATE is much lower than previously assumed, with the major part of radioactivity in plasma consisting of 177Lu-labeled metabolites already at 24 h after injection.
Keywords: neuroendocrine, radionuclide therapy, radiopharmaceuticals, 177Lu-DOTATATE, Lutathera, dosimetry
In the treatment of patients with neuroendocrine tumors, 177Lu-DOTATATE has shown promising results. It has recently been approved as Lutathera (Novartis) by the Food and Drug Administration and the European Medicines Agency for peptide receptor radionuclide therapy (PRRT) on the basis of the results of the NETTER-1 trial, showing significantly better survival than high-dose treatment with an unlabeled somatostatin analog (1). 177Lu-DOTATATE is assumed to have a high level of in vivo stability based on a previously published analysis of urine samples from patients during the NETTER-1 trial, showing a radiochemical purity close to 100% up to 48 h after administration. In addition, in vitro studies using human hepatocytes similarly showed no hepatic metabolic degradation, whereas metabolism was seen in human kidney homogenates (2,3).
Thus far, we have treated more than 800 patients with 177Lu-DOTATATE using a patient-specific protocol, where the number of treatment cycles is determined by a combination of image-based dosimetry to assess absorbed doses to the kidneys and combined image- and blood-based dosimetry to estimate absorbed doses to the bone marrow (4,5). For a limit of 23 Gy to the kidneys, only mild (grade 1–2) renal toxicity has been observed (4). However, even though bone marrow absorbed doses were significantly below 2 Gy (<0.2 Gy per cycle (5)), hemotoxicity has repeatedly led to a delay of subsequent therapy cycles or a decrease in the amount of administered activity. With similar methods, others have reported higher absorbed doses to the bone marrow (6), and image-based bone marrow dosimetry generally results in higher absorbed doses, depending on the specific methodology (7,8). Blood-based bone marrow dosimetry assumes similar concentrations of 177Lu in blood and bone marrow, based on the work by Forrer et al., who found radioactivity concentrations in blood similar to those in bone marrow aspirates at 4–8 d after administration of 177Lu-DOTATATE (9). However, if in vivo stability of 177Lu-DOTATATE is not as high as assumed, differential kinetics of 177Lu-DOTATATE and its radioactive metabolites in bone marrow during the first days after administration might explain differences between blood- and image-based dosimetry.
In recent work, we have attempted to apply the kinetic rate constants estimated on the basis of dynamic 68Ga-DOTATATE PET to the measured total plasma data during PRRT to predict 177Lu-DOTATATE tumor uptake during therapy, assuming 177Lu-DOTATATE to be stable in blood (10,11). However, this assumption resulted in large overestimations compared with the measured tumor uptake using SPECT. Although this may in part be explained by clearance of radioactivity from tumors, another possible explanation is that the assumption of only intact 177Lu-DOTATATE in plasma for several days after administration is not valid.
To improve our understanding of the kinetics of 177Lu-DOTATATE during PRRT and the observations described above, information on the plasma metabolism of 177Lu-DOTATATE is essential. Hence, the aim of the present study was to assess the in vivo stability of 177Lu-DOTATATE using blood sampling and high-performance liquid chromatography (HPLC) in patients undergoing PRRT.
MATERIALS AND METHODS
Patients
Six patients enrolled in a prospective study (EudraCT 2009-012660-14) approved by the Regional Ethical Review Board in Uppsala were included in this work (4). All patients provided written informed consent. 177Lu-DOTATATE (7.4 GBq [n = 5] or 6.0 GBq [n = 1] in 100 mL of saline) was administered as a 30-min infusion. For kidney protection, 2 L of amino acid solution infusion were started 30 min before administration of radioactivity. The peptide was a kind gift from Prof. Eric Krenning, 177Lu was purchased from IDB Holland BV (Petten), and labeling was performed in-house. Arterialized venous blood samples were taken immediately after the end of 177Lu-DOTATATE infusion and at 4, 24, and 96 h after the end of infusion.
Analysis of Metabolic Stability
The blood samples were collected in heparin sample-collection tubes that were kept on ice until preanalytic processing (5–10 min). They were centrifuged at 3,082g for 2 min at 4°C to separate red blood cells and plasma. From the plasma, 0.5 mL (in triplicates) was taken and an equal volume of acetonitrile was added to precipitate the proteins. The mixture was then centrifuged at 16,100g at 4°C for 1 min. The radioactivity in supernatant and pellets was measured to assess recovery, representing the fraction of plasma radioactivity not bound to plasma proteins. The supernatant was filtered through a 0.2-μm nylon membrane by centrifugation at 16,100g at 4°C for 1 min. Then, 500 μL of the filtered supernatant were diluted with 1,500 μL of deionized water. Radioactivity in the filter and diluted supernatant was measured to assess recovery during the filtration step.
HPLC analysis was performed using a binary pump system (Gilson) with ultraviolet (Gilson) and radioactive (Radiomatic 610TR; Packard) detectors coupled in series. The sample of 1.8 mL was injected using an automated solid-phase extraction controller (ASPEC Gilson) connected to a dilutor (Gilson). Separation was performed on an Xbridge Prep BEH130 C18 column (250 × 10 mm, 5 μm, peptide separation technology; Waters) with a 10 × 10 mm C18 security guard from the same supplier. The HPLC system was operated at a flow rate of 6 mL/min. Mobile phase A consisted of 0.1% trifluoroacetic acid in deionized water, and mobile phase B consisted of 0.1% trifluoroacetic acid in acetonitrile. The gradient elution mode was used for the separation (gradient B: 0–7 min, 5%–35%; 7–9 min, 35%, 9–10 min; 35%–70%, 12–13 min, 70%–5%; 13–15 min, 5%). Unlabeled DOTATATE, used as an internal reference to spike the radioactive samples, was detected at 220 nm. Radioactivity recovery from the HPLC column was assessed as the total eluate radioactivity as a fraction of the total amount of radioactivity loaded onto the column.
Since the sensitivity of the online radioactivity detector was insufficient for online radioactivity measurements, fractions were collected from the HPLC system outlet. The automated sampling system was programmed to collect 30 uniform fractions during 15 min of HPLC run. The relative amount of radioactivity in the fractions was measured off-line by a high-sensitivity automatic well-type γ-counter (Wizard 3″; Perkin Elmer Life Science). Compensation for the spillover from one well to the others, background correction, and decay correction were done automatically.
RESULTS
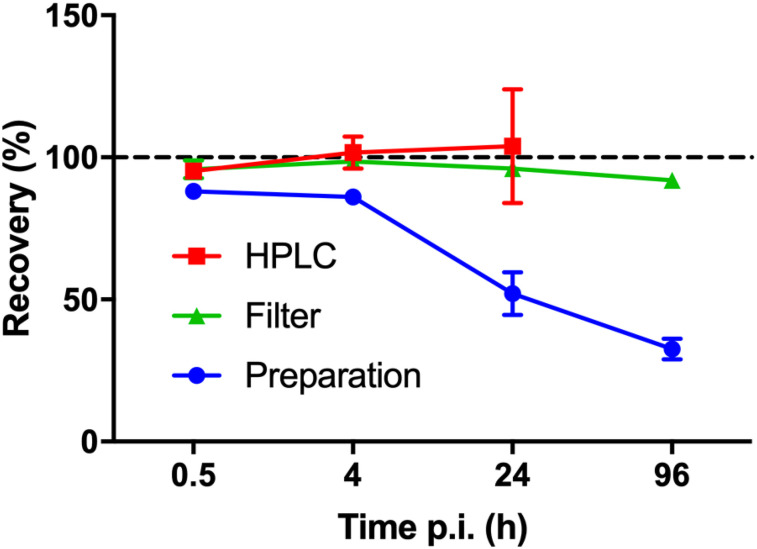
Specific activity at the time of administration was 26.0 MBq/μg (range, 25.1–26.4 MBq/μg), and radiochemical purity was more than 99%. Blood data at 30 min, 4 h, and 24 h after injection were collected for all 6 patients, whereas blood at 96 h after injection was available from 4 patients. Radioactivity concentrations in blood were 259 ± 102, 49 ± 16, 3.9 ± 0.6, and 1.1 ± 0.2 kBq/g (mean ± SD) at 30 min, 4 h, 24 h, and 96 h, respectively, corresponding to 10, 2, 0.15, and 0.04 ng of peptide per gram of blood. Recovery could not be assessed for all samples and stages, and some samples had to be rejected because of poor sample preparation. HPLC recovery could not be reliably measured at 96 h after injection because of the low amounts of radioactivity in the samples, with total counts detected in the HPLC fractions reducing by a factor of 600 between 0.5 and 96 h. Figure 1 shows the average recovery of the remaining samples during the sample workup and HPLC separation. The radioactivity recovery from the plasma protein precipitation step declined for the later time points (24 and 96 h).
FIGURE 1.
Recovery (mean ± SD) of radioactivity from HPLC column (n = 5, 4, and 4 at 0.5, 4, and 24 h after injection), after supernatant filtration (n = 5, 2, 4, and 3 at 0.5, 4, 24, and 96 h after injection), and plasma protein precipitation (n = 5, 2, 4, and 4 at 0.5, 4, 24, and 96 h after injection) as function of blood sampling time point. p.i. = after injection.
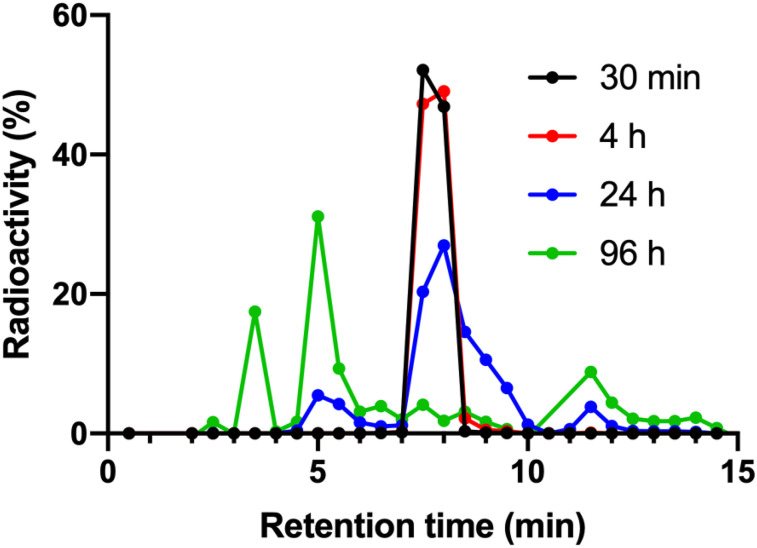
Plasma–to–whole-blood ratio was constant and similar across patients and time points at around 1.65, indicating that all blood radioactivity was contained in plasma rather than blood cells. Figure 2 shows the average chromatograms (mean of all subjects) at each time point. Three radioactive metabolite fractions could be consistently identified at the 3-, 9-, and 12-min retention time, with the parent fraction at 8 min. A fourth metabolite fraction could be identified in only 1 patient at 96 h after injection.
FIGURE 2.
Average (n = 5, 6, 6, and 3 at 0.5, 4, 24, and 96 h) chromatograms at 30 min (n = 5), 4 h (n = 6), 24 h (n = 6), and 96 h (n = 3) after injection. Symbols represent fractions collected from HPLC outlet. Figure 3 shows SD of intact 177Lu-DOTATATE fractions.
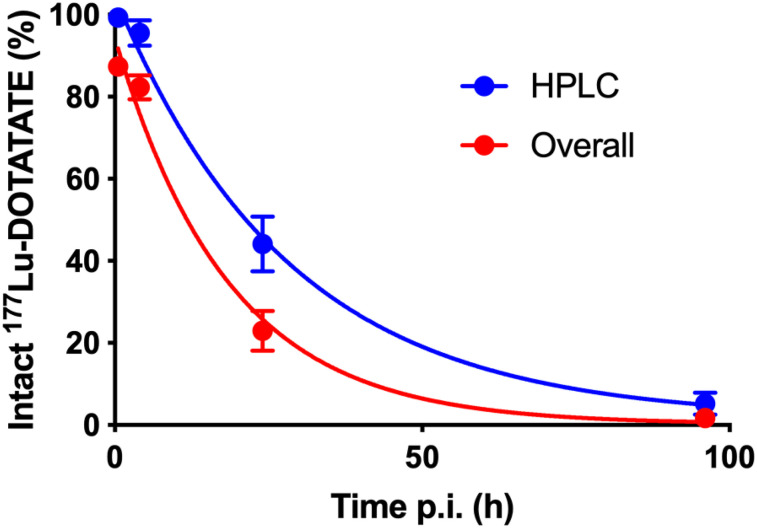
Figure 3 shows the intact 177Lu-DOTATATE fraction in the HPLC analysis, as well as the overall intact 177Lu-DOTATATE fraction in plasma accounting for protein-bound metabolites in the pellet. This last curve was calculated by multiplication of the mean intact 177Lu-DOTATATE fraction in the HPLC analysis by the mean recovery in the pellet at each time point. The mean fraction of free 177Lu-DOTATATE in plasma was 87% ± 2% immediately after infusion, decreasing to 82% ± 3% at 4 h after injection, 23% ± 5% at 24 h after injection, and 1.7% ± 0.9% at 96 h after injection. Interpatient variability in metabolism was small, as indicated by the error bars in Figure 3.
FIGURE 3.
Intact 177Lu-DOTATATE fraction in HPLC analysis (n = 5, 6, 6, and 3 at 0.5, 4, 24, and 96 h after injection) and overall intact 177Lu-DOTATATE fraction in plasma accounting for protein-bound fraction in pellet as function of time after injection. Error bars show SD.
DISCUSSION
To the best of our knowledge, this is the first report on the in vivo metabolic stability of 177Lu-DOTATATE in blood or plasma. The chemical formulation of our in-house–labeled 177Lu-DOTATATE is identical to that of Lutathera. The only difference lies in the administration, with Lutathera being coinfused with saline whereas we dissolve the product in a similar amount of saline before infusion. The in vivo stability of 177Lu-DOTATATE was much lower than previously hypothesized, with the major part of radioactivity in plasma consisting of 177Lu-labeled metabolites already at 24 h after injection. This finding appears to contradict the conclusions drawn from previous data showing stability of the radiopharmaceutical in urine up to 48 h after injection and no hepatic metabolism based on in vitro studies. However, our result is somewhat in line with earlier studies on normal and tumor-bearing rats regarding the metabolism of the somatostatin analog 111In-diethylenetriaminepentaacetic acid-octreotide, which, although not being structurally identical, showed rapid metabolism, with 15% of the activity in urine accounted for by metabolites and less than 30% of the activity due to intact 111In-diethylenetriaminepentaacetic acid-octreotide in tumors, liver, kidney, and pancreas at 4 h after injection (12).
The present metabolite analysis was challenging because of the low amounts of radioactivity in the blood samples at later time points. For the first 3 time points, radioactivity recovery from the HPLC column was close to 100%, but because the concentration of 177Lu-DOTATATE in plasma decreased considerably, recovery could not be reliably measured for the 96-h-postinjection samples. As shown in Figure 1, radioactivity recovery in plasma during the plasma protein precipitation was decreasing for the 24- and 96-h samples, with consistently more than 60% of the radioactivity in the pellets at 96 h after injection, likely indicating plasma protein binding of radioactive metabolites rather than 177Lu-DOTATATE itself. The result is that the overall fraction of intact 177Lu-DOTATATE is even lower than that based on HPLC alone, as shown in Figure 3. Thus, image-derived blood values would have to be multiplied by the plasma-to-blood ratio of 1.65 and the red curve in Figure 3 to result in a time–activity curve describing the available amount of 177Lu-DOTATATE in plasma. The Lutathera multidiscipline review (3) states that the nonradioactive analog 175Lu-DOTATATE is 43% bound to plasma proteins. This may indicate that (part of) the radioactivity in the pellets is instead due to 177Lu-DOTATATE itself, which would suggest that the blue line in Figure 3 is more representative of the fraction of intact 177Lu-DOTATATE in plasma. This possibility would, however, not alter the overall conclusion of the present work.
Our results and the earlier findings of no hepatic metabolism and no urinary excretion of radioactive metabolites during the first 48 h after injection do not necessarily contradict each other, but our findings add to the previously published data. If indeed no hepatic metabolism occurs, the radioactive metabolites could consist of 177Lu-labeled fragments being cleared from the tumors or other somatostatin receptor–expressing cells. These metabolites subsequently appear to be removed from the body by hepatic clearance and not by renal excretion, explaining the lack of radioactive metabolites found in urine. Previous studies on tumor-bearing Lewis rats have shown 8% fecal excretion at 24 h, compared with a 73% total excretion rate (3). To our knowledge, this work has not been replicated in humans, and no information is available on whether the radioactivity excreted in the feces was due to 177Lu-DOTATATE itself or to radioactive metabolites. However, a urinary excretion rate of 65% in 24 h is within the range of the 58%–71% found in humans (3,5). If indeed metabolites are not cleared renally, fecal excretion of metabolites may explain the lack of signs of long-term buildup of radioactive metabolites, such as liver and bone marrow uptake, in images taken more than 5 wk after administration (13). On the other hand, blood radioactivity due to metabolites amounts to less than 1 kBq/mL at 96 h after injection, compared with typical concentrations in tumors on the order of MBq/mL. Since there is very little 177Lu-DOTATATE available in blood at that time (<0.02 kBq/mL), blood concentrations due to metabolites will continue to decline after 96 h. Even if there is preferential uptake of radioactive metabolites in liver or bone, these would be much lower than those in tumors and might be hard to detect on scintigraphy after more than 5 wk because of the very low amounts of radioactivity. In the present work, no attempt was made to identify the produced metabolites, and hepatic clearance was not verified. These issues consequently need to be confirmed in future studies.
As stated in the introduction, we have previously found a much larger fraction of patients experiencing hematologic toxicity during treatment with 177Lu-DOTATATE than would be expected from the combined image- and blood-based estimates of absorbed doses to red marrow. Notably, these estimates implicitly assume that concentrations in bone marrow are similar to those in blood (9). On the basis of our results, it is likely that nearly all of the radioactivity measured in blood samples in the work by Forrer et al. was due to radioactive metabolites rather than 177Lu-DOTATATE itself. If bone marrow kinetics of 177Lu-DOTATATE and its metabolites are different, extrapolation of the observed similar radioactivity concentrations in blood and bone marrow to the first 4 d after administration will not be straightforward. For example, if the activity concentrations in marrow aspirates as measured by Forrer et al. at 4–8 d after injection are due to intact 177Lu-DOTATATE, with only about 2% of plasma activity at this time point representing 177Lu-DOTATATE itself, this finding could implicate much higher concentrations in marrow than in blood also at earlier time points and could explain the much higher marrow-absorbed doses found for image-based than for blood-based dosimetry (5,7) and the underestimation of marrow toxicity in previous work using blood-based dosimetry. Similarly, a rapidly decreasing fraction of intact 177Lu-DOTATATE in plasma may help in explaining the discrepancy between estimated tumor uptake based on dynamic 68Ga-DOTATATE PET and tumor uptake observed during 177Lu-DOTATATE therapy. A more detailed investigation into this this topic will be subject of further studies.
CONCLUSION
The present work showed that the in vivo stability of 177Lu-DOTATATE is much lower than previously assumed, with the major part of radioactivity in plasma already consisting of 177Lu-labeled metabolites at 24 h after injection. The possible impact of this finding on the interpretation of the effects of 177Lu-DOTATATE therapy needs to be further assessed.
DISCLOSURE
This work was financed by the regional agreement on medical training and clinical research (ALF) between Uppsala University Hospital and Uppsala County. No other potential conflict of interest relevant to this article was reported.
KEY POINTS
QUESTION: How stable is 177Lu-DOTATATE in vivo during PRRT?
PERTINENT FINDINGS: Contrary to conclusions based on earlier work using urine sampling and in vitro data, the fraction of intact 177Lu-DOTATATE decreased rapidly after administration, and the major part of radioactivity in plasma consisted of 177Lu-labeled metabolites already at 24 h after injection.
IMPLICATIONS FOR PATIENT CARE: The assumptions underlying blood-based bone marrow dosimetry during PRRT may be wrong. The impact of this finding on the interpretation of the effects of 177Lu-DOTATATE therapy needs to be further assessed.
Acknowledgments
We thank Prof. Eric Krenning for generously supplying the peptide. We thank Mevan Abdulkarim, Uppsala University Hospital, for discussions on the formulation of 177Lu-DOTATATE. We thank the staff at the PET Centre and the Department of Nuclear Medicine at Uppsala University Hospital for their contribution to this work.
REFERENCES
- 1.Strosberg J, El-Haddad G, Wolin E, et al. Phase 3 trial of 177Lu-dotatate for midgut neuroendocrine tumors. N Engl J Med. 2017;376:125–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lutathera European Public Assessment Report. London, U.K.: European Medicines Agency; 2017. EMA/506460/2017.
- 3. Lutathera Multi-Discipline Review. Silver Spring, MD: United States Food and Drug Administration; 2016. NDA 208700.
- 4.Garske-Román U, Sandstrom M, Fross Baron K, et al. Prospective observational study of 177Lu-DOTA-octreotate therapy in 200 patients with advanced metastasized neuroendocrine tumours (NETs): feasibility and impact of a dosimetry-guided study protocol on outcome and toxicity. Eur J Nucl Med Mol Imaging. 2018;45:970–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sandström M, Garske-Roman U, Granberg D, et al. Individualized dosimetry of kidney and bone marrow in patients undergoing 177Lu-DOTA-octreotate treatment. J Nucl Med. 2013;54:33–41. [DOI] [PubMed] [Google Scholar]
- 6.Bergsma H, Konijnenberg MW, Kam BL, et al. Subacute haematotoxicity after PRRT with 177Lu-DOTA-octreotate: prognostic factors, incidence and course. Eur J Nucl Med Mol Imaging. 2016;43:453–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hagmarker L, Svensson J, Ryden T, et al. Bone marrow absorbed doses and correlations with hematologic response during 177Lu-DOTATATE treatments are influenced by image-based dosimetry method and presence of skeletal metastases. J Nucl Med. 2019;60:1406–1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Svensson J, Ryden T, Hagmarker L, Hemmingsson J, Wangberg B, Bernhardt P. A novel planar image-based method for bone marrow dosimetry in 177Lu-DOTATATE treatment correlates with haematological toxicity. EJNMMI Phys. 2016;3:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Forrer F, Krenning EP, Kooij PP, et al. Bone marrow dosimetry in peptide receptor radionuclide therapy with [177Lu-DOTA0,Tyr3]octreotate. Eur J Nucl Med Mol Imaging. 2009;36:1138–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ilan E, Sandstrom M, Velikyan I, Sundin A, Eriksson B, Lubberink M. Parametric net influx rate images of 68Ga-DOTATOC and 68Ga-DOTATATE: quantitative accuracy and improved image contrast. J Nucl Med. 2017;58:744–749. [DOI] [PubMed] [Google Scholar]
- 11.Ilan E, Lubberink M, Sandström M, et al. Comparison of Ga-68-DOTATATE and Lu-177-DOTATATE kinetics in neuroendocrine tumors [abstract]. Eur J Nucl Med Mol Imaging. 2018;45(suppl):S276–S277. [Google Scholar]
- 12.Bass LA, Lanahan MV, Duncan JR, et al. Identification of the soluble in vivo metabolites of indium-111-diethylenetriaminepentaacetic acid-D-Phe1-octreotide. Bioconjug Chem. 1998;9:192–200. [DOI] [PubMed] [Google Scholar]
- 13.Gleisner KS, Brolin G, Sundlov A, et al. Long-term retention of 177Lu/177mLu-DOTATATE in patients investigated by gamma-spectrometry and gamma-camera imaging. J Nucl Med. 2015;56:976–984. [DOI] [PubMed] [Google Scholar]