Abstract
The purpose of this study was to investigate the feasibility and diagnostic efficacy of 68Ga-prostate-specific membrane antigen (PSMA) PET/CT combined with PET/ultrasound-guided biopsy in the diagnosis of prostate cancer (PCa). Methods: In total, 31 patients with a previously negative prostate biopsy but persistent elevated serum prostate-specific antigen (PSA) were imaged with a 68Ga-PSMA PET/CT ligand before undergoing repeat prostate biopsy. On the basis of the proposed Prostate Cancer Molecular Imaging Standardized Evaluation criteria, 68Ga-PSMA PET/CT results were interpreted as negative (molecular-imaging-for-PSMA expression score [miPSMA-ES] of 0–1) or positive (miPSMA-ES of 2–3). All patients underwent standard template systematic biopsy with up to 4 additional PET/ultrasound-guided biopsy cores. The sensitivity, specificity, positive and negative predictive values, and accuracy of 68Ga-PSMA PET/CT were determined. In addition, the correlation between the miPSMA-ES and the detection rate of PCa was also analyzed. Univariate logistic regression models were established using 68Ga-PSMA PET/CT semiquantitative analysis parameters to predict the outcome of repeat prostate biopsy. Results: The median age of patients was 65 y (range, 53–81 y), and the median PSA level was 18.0 ng/mL (range, 5.48–49.77 ng/mL). PCa was detected in 15 of 31 patients (48.4%), and 12 of 31 patients (38.7%) had clinically significant PCa (csPCa). The sensitivity, specificity, positive predictive value, negative predictive value, and accuracy of 68Ga-PSMA PET/CT in the diagnosis of csPCa were 100.0%, 68.4%, 66.7%, 100.0%, and 80.6%, respectively. The detection rate of PCa increased with the increase in miPSMA-ES. The detection rates of csPCa in the miPSMA-ES 0–1, 2, and 3 groups were 0%, 54.5%, and 85.7%, respectively. Semiquantitative analysis of 68Ga-PSMA PET/CT images showed that predictive models based on the SUVmax of prostate lesion, tumor–to–normal-prostate background SUVmax, and tumor–to–normal-liver background SUVmax could effectively predict csPCa; area under the curves were 0.930, 0.877, and 0.956, respectively. Conclusion: This study preliminarily confirmed that 68Ga-PSMA PET/CT imaging, combined with PET/ultrasound-guided prostate biopsy, can effectively detect csPCa. Prebiopsy 68Ga-PSMA PET/CT had predictive value for csPCa in the studied patient population.
Keywords: PSMA, PET/CT, biopsy, prostate cancer
For patients with suspected prostate cancer (PCa) because of an elevation in serum prostate-specific antigen (PSA) or an abnormality found on digital rectal examination, transrectal ultrasound–guided biopsy is currently a standard method for definitive diagnosis. However, traditional 10-core or 12-core systematic biopsy can lead to overdiagnosis in patients with PCa that is not clinically significant. On the other hand, it cannot avoid missed detection of some clinically significant PCa (csPCa) (1). These limitations persist in patients with suspected PCa who have had a negative prostate biopsy result but persistently elevated PSA. It is a challenging issue for clinicians to determine whether these patients need repeat biopsy and how to improve the detection of csPCa through biopsy.
A series of studies has suggested that multiparametric MRI (mpMRI) before biopsy, followed by targeted biopsy of suspected sites of PCa, can improve the detection of csPCa (2–5). In addition, some studies suggested that, given the high sensitivity and negative predictive value of mpMRI in the diagnosis of csPCa, patients with negative mpMRI results may avoid unnecessary biopsy (6,7). Indeed, both American Urological Association and European Association of Urology guidelines recommend mpMRI before repeat biopsy, and targeted biopsy of suspected lesions based on mpMRI findings can provide a basis for subsequent clinical decision making (8,9). However, the PROMIS trial (1) found that 24% of patients with negative mpMRI findings had csPCa, and it remains controversial whether mpMRI-negative patients need repeat biopsy (2). Furthermore, the interpretation of prostate mpMRI is based mainly on the Prostate Imaging Reporting and Data System, version 2.0. The image interpretation rules of this system are relatively complicated, and the experience of different readers and their understanding of the standards may lead to high interreader variability (10).
68Ga-labeled inhibitors of prostate-specific membrane antigen (PSMA) can be used for PET/CT and have demonstrated excellent sensitivity and specificity in the diagnosis of PCa (11). Multiple studies have shown that PSMA-targeted PET/CT and PET/MRI can accurately determine the location and extent of primary PCa, and some studies have demonstrated that this novel imaging technique is superior to traditional mpMRI in terms of diagnostic efficacy (12–14). Zamboglou et al. (14) compared the volume of primary PCa depicted by 68Ga-PSMA PET/CT and mpMRI and found that the 68Ga-PSMA PET/CT findings were highly consistent with postoperative pathologic results. In addition, unlike the complex image interpretation standards of mpMRI, the Prostate Cancer Molecular Imaging Standardized Evaluation (PROMISE) criteria (15) that were recently proposed for the interpretation of 68Ga-PSMA PET/CT and PET/MR images are relatively simple and may have less intrinsic interreader variability.
This prospective study was designed to preliminarily investigate the following aspects in patients with prior negative prostate biopsy results but persistently elevated PSA: the diagnostic efficacy of 68Ga-PSMA PET/CT based on molecular-imaging-for-PSMA expression score (miPSMA-ES) criteria in the detection of csPCa and the feasibility and clinical value of PET/ultrasound-guided biopsy.
MATERIALS AND METHODS
Patient Population and Study Protocol
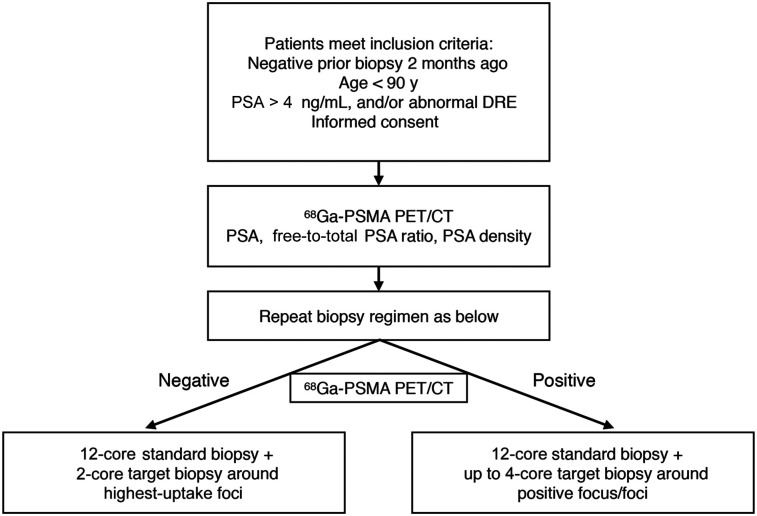
Patients with clinically suspected PCa but negative prostate biopsy results were scheduled to undergo repeat prostate biopsy. These patients were included between August 2016 and September 2018. The inclusion criteria were an age of less than 90 y, at least 1 negative prostate biopsy in the past, a PSA level of 4–50 ng/mL or an abnormality found on digital rectal examination, ability to understand the study procedures, and willingness to participate in the study. The exclusion criteria were acute prostatitis or the presence of any cancer other than PCa, with the exception of basal cell carcinoma or squamous cell skin cancer. All patients underwent 68Ga-PSMA PET/CT imaging and related laboratory examinations before biopsy, followed by PET/ultrasound-guided targeted biopsy and standard 12-core template biopsy of the prostate (Fig. 1).
FIGURE 1.
Prostate biopsy algorithm for study subjects. DRE = digital rectal examination.
68Ga-PSMA PET/CT
68Ga-PSMA-617 (68Ga-PSMA) was produced as described previously (16). Each patient received an intravenous injection of 68Ga-PSMA (median dose, 206.09 MBq; range, 121.73–361.12 MBq), followed by PET and CT scans at 60 ± 10 min using a Gemini TF scanner (Philips). The scanning range was from the skull base to the mid thigh. The CT acquisition and reconstruction parameters were a voltage of 120 keV, a current of 100 mAs, a pitch of 0.8 mm, and a tube-rotation time of 0.5 s. CT reconstruction used the standard method supplied by the vendor, with a matrix of 512 × 512 and a reconstructed slice thickness of 3–5 mm. The PET acquisition and reconstruction parameters included use of 3-dimensional emission mode, scanning at a total of 9–10 bed positions, a 90-s acquisition time for each bed position, and use of ordered-subsets expectation maximization. Attenuation correction was performed using CT. Fusion Viewer software on the Extended Brilliance Workstation (Philips) was used to fuse and analyze all 68Ga-PSMA PET/CT images.
PET/CT Image Analysis
68Ga-PSMA PET/CT images of all patients were jointly read by 2 nuclear medicine specialists with more than 10 y of experience. They conducted visual and semiquantitative analysis of PET/CT images while unaware of the patients’ clinical data. Whole-body CT images, PET images, and PET/CT images were viewed in the axial, coronal, and sagittal planes. The potential systematic and targeted biopsy areas were judged positive when lesion-related focal radiotracer uptake was higher than background uptake in the liver. 68Ga-PSMA uptake in biopsy areas was scored according to the PROMISE criteria (version 1.0) (15). The PROMISE scores of each biopsy were summarized, and the highest miPSMA-ES was determined. An miPSMA-ES of 0–1 was considered to be negative, and an miPSMA-ES of 2–3 was considered to be positive. The SUVmax of the prostate lesion, as well as of the normal prostate background and liver background, was determined. The tumor–to–normal-prostate background SUVmax was determined, as well as the tumor–to–normal-liver background SUVmax.
PET/Ultrasound-Guided Biopsy
PET/ultrasound-guided biopsy was performed in conjunction with the prebiopsy 68Ga-PSMA PET/CT images. The DICOM data of the 68Ga-PSMA PET/CT images were first imported into a navigated 3-dimensional ultrasound system (Logiq E9; GE Healthcare). According to previous studies (17), 68Ga-PSMA PET/CT and ultrasound image registration was performed using a 1-plane, 1-point method at first. Briefly, the plane was the puborectalis plane, and the point was the internal urethral orifice, a cyst, or a calcification, which could be corrected at any time. After the initial image registration was finished, real-time ultrasound was performed from the apex to the base of the prostate to evaluate the coincidence between ultrasonography and PET/CT. When the registration was not satisfied, an additional point was used to modify the registration. For patients with negative 68Ga-PSMA PET/CT results, only a 2-core targeted biopsy pass was conducted in the area of highest 68Ga-PSMA uptake in the prostate; for patients with positive 68Ga-PSMA PET/CT results, up to a 4-core targeted biopsy pass was conducted. All targeted biopsy procedures were conducted by an experienced urologist (>20 y), and systematic biopsy was conducted by another experienced sonologist (>8 y) without knowledge of the 68Ga-PSMA PET/CT results. The entire process for obtaining a PET/ultrasound-guided biopsy—from the image acquisition to the actual biopsy—is outlined in Supplemental Figure 1 (supplemental materials are available at http://jnm.snmjournals.org).
Pathology
A dedicated genitourinary pathologist was responsible for preparing sections of the biopsy samples and reporting the results according to International Society of Urological Pathology standards (2015 revision). In addition to the Gleason score (GS) for each biopsy specimen, the overall GS of the patient was provided on basis of systematic and targeted biopsy. In our study, csPCa on standard biopsy was defined by previously published studies (GS ≥ 3 + 4) (17,18). If pathologic examination showed a normal prostate or benign prostatic lesions, the patient was followed for at least 6 mo. Follow-up examination indices included PSA levels, imaging examinations, or transurethral resection of the prostate.
Statistics
Descriptive statistics were used, and data were expressed as either percentages or medians (with ranges). Depending on the specific case, the χ2 or Fisher exact test was used to compare categoric data, and the Wilcoxon–Mann–Whitney U test and the Kruskal–Wallis test were used to compare continuous data. The sensitivity, specificity, accuracy, and positive and negative predictive values for 68Ga-PSMA PET/CT were calculated for detection of csPCa. The detection rates for systematic biopsy versus targeted biopsy were compared using the McNemar mid-P test. A semiquantitative index for 68Ga-PSMA PET/CT was used to construct a univariate logistic prediction model for csPCa. The diagnostic efficacy of this model was analyzed using a receiver-operating-characteristic curve, and the optimal cutoff was obtained with the Youden index. All statistical analysis was performed using SPSS software (version 22.0; IBM). A P value of less than 0.05 was considered to have statistical significance.
RESULTS
Demographics and Clinical Characteristics
This study was approved by the Ethics Committee of Beijing Cancer Hospital. All patients were informed of the study procedures in detail, and all gave written informed consent. This study screened a total of 217 patients with suspected PCa treated in our hospital; among them, 34 patients who met the inclusion criteria without meeting any of the exclusion criteria were initially included. Among the enrolled patients, 2 were excluded because of failure to complete a 68Ga-PSMA PET/CT examination, and 1 was excluded because of failure to complete a biopsy. As such, 31 patients were included in the final analysis. Their median age was 65 y (range, 53–81 y). Twenty-four patients had 1 prior biopsy, 5 patients had 2, and 2 patients had 3. The median PSA of all patients was 18.0 ng/mL (range, 5.48–49.77 ng/mL).
In total, 31 subjects underwent PET/ultrasound-guided biopsy. Overall, PCa was detected in 15 of 31 patients (48.4%), including 12 patients with a GS of at least 3 + 4 and 3 patients with a GS of 3 + 3. The remaining 16 patients were negative for PCa. The patients (n = 12) with a GS of at least 3 + 4 were defined as csPCa. The patients (n = 19) with a GS of 3 + 3 (n = 3), a normal prostate, or benign prostatic disease (n = 16) were defined as non-csPCA.
When the 2 groups of patients with csPCA and non-csPCA were compared, there were significant differences in PSA level, prostate volume, and PSA density. There was no statistical difference in the number of previous biopsies or the free-to-total PSA ratio (Table 1).
TABLE 1.
Patients’ Clinical Characteristics
| Characteristic | Non-csPCa | csPCa | P |
| Patients (n) | 19 | 12 | |
| Age (y) | 63 (53–81) | 70.5 (57–81) | 0.152 |
| Prior biopsies (n) | 1 (1–3) | 1 (1–3) | 0.857 |
| Total PSA (ng/mL) | 10.63 (5.48–46.88) | 37.58 (8.96–49.77) | 0.002* |
| Free/total PSA (ng/mL) | 0.15 (0.07–0.47) | 0.13 (0.02–0.74) | 0.562 |
| Prostate volume (cm3) | 56.22 (22.39–108.63) | 33.69 (7.79–74.36) | 0.023* |
| PSA density (ng/mL/mL) | 0.16 (0.09–1.75) | 0.81 (0.29–2.31) | <0.001* |
Significant differences (P < 0.05).
csPCa has GS ≥ 7; non-csPCa has GS of 6 or is normal prostate or benign prostatic disease. Data are median followed by range in parentheses. P values are for Wilcoxon–Mann–Whitney U test.
Diagnostic Performance of 68Ga-PSMA PET/CT for PCa Detection
On a patient-by-patient basis, 68Ga-PSMA PET/CT imaging was positive (miPSMA-ES, 2–3) in 18 patients (58%) and negative in the remaining 13 patients (42%) (typical cases are illustrated in Supplemental Figs. 2 and 3). The detection rate of csPCa was 0% (0/13), 54.5% (6/11), and 85.7% (6/7), respectively, in the miPSMA-ES 0–1, 2, and 3 groups; that difference was statistically significant (P < 0.001). The sensitivity, specificity, and accuracy of 68Ga-PSMA PET/CT in the diagnosis of PCa were 93.3%, 75.0%, and 83.9%, respectively. The sensitivity, specificity, and accuracy in the diagnosis of csPCa were 100.0%, 68.4%, and 80.6%, respectively. The positive predictive value of 68Ga-PSMA PET/CT (miPSMA-ES, 2–3) was 77.8% in determining the presence of PCa and 66.7% in identifying csPCa. The main causes of false-positives were believed to be prostate hyperplasia (2 cases) and chronic prostatitis (2 cases); miPSMA-ES was 2 in all cases. All patients with a miPSMA-ES of 3 had PCa, and most of them (85.7%) had csPCa. The negative predictive value of an miPSMA-ES of 0–1 was 92.3% in ruling out PCa and 100.0% in ruling out csPCa. Among patients with negative 68Ga-PSMA PET/CT results, there was 1 case of PCa (GS 6), 6 cases of benign prostatic hyperplasia, 2 cases of chronic inflammation, 2 cases of prostatic intraepithelial neoplasia (9), and 1 case of atypical hyperplasia.
From the 31 patients enrolled in this study, 440 prostate biopsy cores were obtained in total, among which 105 cores (23.8%) detected PCa and 75 cores (17.0%) detected csPCa. The detection rate of csPCa gradually increased as miPSMA-ES increased from 0 to 3 in each group; the detection rate was 0.0%, 6.7%, 44.9%, and 78.6%, respectively. The intergroup differences had statistical significance (P < 0.001). A total of 88 cores detected PCa (69.8%) in 126 68Ga-PSMA PET/CT–positive lesions, among which 66 cores (52.4%) detected csPCa. In total, 314 biopsy cores were obtained from 68Ga-PSMA PET/CT–negative areas (miPSMA-ES, 0–1), among which 297 cores (94.6%) were normal prostate tissues or benign prostatic lesions and 17 cores were false-negative lesions (GS of 6 in 8 cores, 7 in 7 cores, and 8 in 2 cores); however, none of the false-negative results affected the patient’s final GS.
Semiquantitative Analysis of 68Ga-PSMA PET/CT and Its Predictive Value for Prostate Biopsy Outcomes
The median highest SUVmax in prostate-lesion areas of interest was 5.61 (range, 2.90–30.95) in all patients. The median background SUVmax in normal prostate tissues was 3.40 (range, 2.00–4.40). The median SUVmax in the liver was 5.47 (range, 3.05–9.74). The median SUVmax for the prostate lesion versus the prostate background was 1.62 (range, 1.12–10.32). The median SUVmax for the prostate lesion versus the liver background was 1.02 (range, 0.64–6.03). When the pathologic results were used for grouping, the SUVmax of the prostate lesion, the tumor–to–normal-prostate background SUVmax, and the tumor–to–normal-liver background SUVmax were significantly higher in csPCa (GS ≥ 7) than in non-csPCa (P < 0.001). There was no statistical difference in SUVmax or liver background between the 2 groups (P = 0.13 and 0.484, respectively; Table 2).
TABLE 2.
Comparison of Semiquantitative Analysis Parameters of 68Ga-PSMA PET/CT Between csPCa and Non-csPCa
| Parameter | Non-csPCa | csPCa | P |
| SUVmax of prostate lesion | 4.63 (2.90–9.87) | 10.15 (5.45–30.95) | <0.001* |
| SUVmax for… | |||
| Liver background | 5.47 (3.05–9.74) | 5.42 (4.62–8.60) | 0.484 |
| Prostate background | 3.3 (2.00–4.40) | 3.5 (2.70–4.20) | 0.130 |
| Prostate lesions vs. prostate background | 1.43 (1.12–4.94) | 3.36 (1.44–10.32) | <0.001* |
| Prostate lesions vs. liver background | 0.91 (0.64–2.12) | 1.48 (1.03–6.03) | <0.001* |
Significant differences (P < 0.05).
csPCa has GS ≥ 7; non-csPCa has GS of 6 or is normal prostate or benign prostatic disease. Data are median followed by range in parentheses. P values are for Wilcoxon–Mann–Whitney U test.
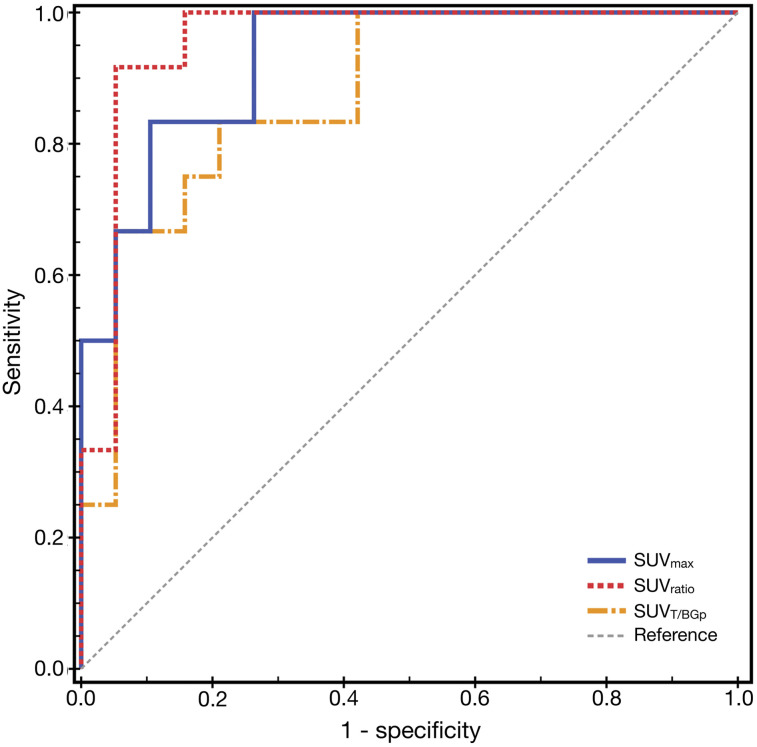
Univariate logistic regression analysis suggested that the SUVmax of the prostate lesion, the tumor–to–normal-prostate background SUVmax, and the tumor–to–normal-liver background SUVmax could all be used as predictors of csPCa in patients undergoing repeat biopsy. The predictive model based on the SUVmax of the prostate lesion, the tumor–to–normal-prostate background SUVmax, and the tumor–to–normal-liver background SUVmax demonstrated an excellent diagnostic efficacy. The results of receiver-operating-characteristic curve analysis showed that the area under the curve for 68Ga-PSMA PET/CT semiquantitative parameters in the prediction of csPCa was 0.93 (SUVmax of prostate lesion), 0.877 (tumor–to–normal-prostate background SUVmax), and 0.956 (tumor–to–normal-liver background SUVmax), respectively (Fig. 2). When calculated by the Youden index, the optimal cutoff for the SUVmax of the prostate lesion was 5.27, the diagnostic sensitivity was 100%, and the specificity was 73.7% (0.93). The optimal cutoff for the tumor–to–normal-liver background SUVmax was 1.19, with sensitivity and specificity of 91.7% and 94.7%, respectively (0.956). The optimal cutoff for the tumor–to–normal-prostate background SUVmax was 1.81, and the sensitivity and specificity were 83.3% and 78.9%, respectively (0.956).
FIGURE 2.
Receiver-operating-characteristic curve analysis of 68Ga-PSMA PET/CT semiquantitative analysis index for prediction of csPCa. SUVT/BGp = tumor–to–normal-prostate background SUVmax.
Comparison Between Systematic Biopsy and PET/Ultrasound-Guided Targeted Biopsy
PET/ultrasound-guided targeted biopsy detected 12 cases of csPCa, and systematic biopsy detected 10 cases. The detection rate of targeted biopsy was slightly higher, 38.7% versus 32.3%, but the difference had no statistical significance (P = 0.25). The positive and negative predictive values were 50.0% and 96.9%, respectively, for systematic biopsy and 57.1% and 100%, respectively, for targeted biopsy. Two patients with a systematic biopsy GS of 6 were confirmed to have csPCa (GS, 3 + 4) by targeted biopsy, and 1 patient with negative results on targeted biopsy was confirmed to have GS 6 PCa by systematic biopsy. The detection rate of csPCa using PET/ultrasound-guided biopsy increased to 66.7% in patients with positive imaging. Neither targeted biopsy nor systematic biopsy detected csPCa in patients with negative 68Ga-PET/CT results, but targeted biopsy detected more prostatic intraepithelial neoplasia (2 vs. 0) and dysplasia (1 vs. 0) than systematic biopsy. In addition, in the case of similar cancer detection rates, the number of cores was significantly lower with targeted biopsy than with systematic biopsy. For each patient, the median cores of the 2 biopsy methods were 2 and 12, respectively (P < 0.001). Overall, the percentages of total positive cores for targeted biopsy and systematic biopsy were 47.0% (31/68) and 19.4% (72/372), respectively. Extrapolating from these findings, the diagnosis of a single case would require 36 cores by systematic biopsy and only 5.5 cores by targeted biopsy.
DISCUSSION
The study described in this article preliminarily confirmed the following findings: for patients with a previous negative prostate biopsy but elevated PSA, miPSMA-ES helped predict csPCa. The SUVmax of the prostate lesion, the tumor–to–normal-prostate background SUVmax, and the tumor–to–normal-liver background SUVmax of 68Ga-PSMA PET/CT were all useful predictors of csPCa. PET/ultrasound-guided prostate biopsy was clinically feasible and improved the detection of csPCa. To our knowledge, this was the first prospective study of 68Ga-PSMA PET/CT combined with PET/ultrasound-guided biopsy based on miPSMA-ES for the diagnosis of patients with negative biopsy findings but suspected of harboring PCa.
Prior studies have suggested that PSMA PET may be a valuable alternative or adjunct in patients with suspected primary PCa. In recent years, some case reports and small-scale studies have successively confirmed that 68Ga-PSMA PET combined with either PET/CT-plus-ultrasound–guided prostate biopsy or PET/MRI-plus-ultrasound–guided prostate biopsy can effectively improve the detection of csPCa. Simopoulos et al. (19) first reported a successful case of 68Ga-PSMA PET/CT and MRI/ultrasound-guided prostate biopsy. Subsequently, Westenfelder et al. (20) successfully detected csPCa (GS, 4 + 3) using 68Ga-PSMA PET/MR-plus-ultrasound–guided biopsy in a patient with a previous negative prostate biopsy and MRI. Lopci et al. (18) included 45 patients with a negative prostate biopsy and negative or equivocal MRI findings or MRI contraindications. All patients underwent 68Ga-PSMA PET/CT. Among them, 25 patients with confirmed positive lesions immediately received PET/ultrasound-guided targeted prostate biopsy, and the detection rate of PCa was 44%.
In our study, PSMA PET demonstrated high sensitivity (85.7%–100%) and high specificity (75%–100%) in the detection of PCa, with similar performance characteristics for the detection of csPCa. That imaging performance is based on miPSMA-ES from the PROMISE criteria (15), a different approach from what has generally been used in other studies, which have focused on using the uptake value of the normal prostate for reference (12,13,18,21). Using the liver uptake as a reference may be a superior methodology, as prostate tissues often may have associated hyperplasia, inflammation, and even glandular fibrosis caused by repeated biopsy, which could potentially cause false-positive PSMA PET uptake. When the above lesions are present with PCa, in addition to the impact of volume effect, it is possible that interpretation of PET images may be more difficult and that observer error may increase between different readers. On the basis of our results, during the diagnosis of patients with complicated suspected PCa, miPSMA-ES criteria are more appropriate, stable, and objective for the determination of positive lesions.
As shown by the results of this study, when an miPSMA-ES of 0–1 is used as a negative 68Ga-PSMA-PET/CT imaging criterion, a higher negative predictive value is provided for the diagnosis, and a higher probability to obtain a negative result after a repeat biopsy is suggested. This finding concords with a recent report by Zhang et al. (22); among patients requiring prostate biopsy according to the European Randomized Study of Screening for Prostate Cancer risk-calculator level 3 criteria, 19% of these patients could potentially avoid unnecessary biopsy if negative 68Ga-PSMA PET/CT imaging results were followed. On the other hand, positive 68Ga-PSMA PET results with an miPSMA-ES of 2–3 provided high accuracy in the diagnosis of csPCa. In a recent retrospective study, Chen et al. (23) proposed that PCa should be considered when the mpMRI had an expression score of 3 in the Prostate Imaging Reporting and Data System but the miPSMA-ES was higher than 2. If an miPSMA-ES of at least 2 alone was used as the criterion for the diagnosis of csPCa in our study, the sensitivity and specificity were 89% and 71%, respectively, which were similar to the results we obtained. In summary, 68Ga-PSMA PET/CT examination based on miPSMA-ES criteria has a high diagnostic value for patients with suspected PCa who previously had a negative biopsy but have a persistently elevated PSA.
Previous studies on 68Ga-PSMA PET/CT have been based mostly on 68Ga-PSMA-11, whereas studies focusing on the newly developed radiotracer 68Ga-PSMA-617 have been less numerous. In preclinical studies, 68Ga-PSMA-617 showed improved affinity that could further increase tumor uptake, as well as improved internalization that may keep tumor uptake stable until late time points (24). Furthermore, 68Ga-PSMA-617 has become a valuable diagnostic agent in detecting primary PCa and in predicting risk stratification for PCa (16,25).
Moreover, several 18F-labeled PSMA ligands have recently been introduced clinically. 18F-DCFPyL and 18F-PSMA-1007 are among the most promising candidates. On the basis of the physical properties of 18F, radiotracers labeled with that nuclide should show a spatial resolution equal to or better than that of 68Ga (26). In addition, delayed imaging is more feasible because of the longer half-life of 18F, which could reduce urinary excreted 18F-PSMA in the bladder. Therefore, 18F-PSMA–targeting radiotracers may have advantages in detecting small lesions in the prostate.
This study showed that the liver could be used as an appropriate reference tissue for thresholding images when assessing the 68Ga-PSMA PET/CT scans. However, liver is not always a suitable reference, as the biodistribution of 18F-PSMA-1007 is different from that of other PSMA-related radiotracers (specifically, 18F-PSMA-1007 has significant hepatobiliary clearance, whereas other radiotracers [e.g., 18F-DCFPyL, 68Ga-PSMA-11, and 68Ga-PSMA-617] have predominantly renal clearance). For agents such as 18F-PSMA-1007, the tumor–to–normal-liver background SUVmax may not be suitable as a semiquantitative analysis parameter.
This study does have limitations. Although it is currently the largest prospective PSMA PET diagnostic study on patients with negative biopsy results previously but persistently elevated PSA, the overall sample size is still small. Considering the total number of patients in this study and the limited number of cases for grouping analysis, we were unable to analyze the potential correlation between miPSMA-ES and GS. As limited by the sample size, we were unable to conduct multiparametric regression analysis of semiquantitative indices. In addition, this study did not compare radical prostatectomy with the pathologic results of biopsy, as a large proportion of patients included in this study did not undergo surgical operation because of advanced age, high-risk grading, and other factors. It should also be pointed out that this study included only those patients visiting the Peking University cancer hospital—a restriction that may cause a bias in patient composition. As a result, our patient population may not represent a more general patient composition.
CONCLUSION
This study confirmed the feasibility of 68Ga-PSMA PET imaging combined with PET/ultrasound-guided prostate biopsy in patients with prior negative results from prostate biopsy. This technique can effectively detect PCa. Prebiopsy 68Ga-PSMA PET/CT imaging based on miPSMA-ES criteria can aid in the diagnosis of csPCa.
DISCLOSURE
This work was financially supported by the Interdisciplinary Medicine Seed Fund of Peking University (BMU2017MX007), National Natural Science Foundation of China projects (81501519, 81571705, and 81671733), and the Beijing Natural Science Foundation (7202028 and 7171002). No other potential conflict of interest relevant to this article was reported.
KEY POINTS
QUESTION: Can 68Ga-PSMA PET/CT and PET/ultrasound-targeted prostate biopsy diagnose csPCa in men with previous negative biopsy results?
PERTINENT FINDINGS: In this prospective study, 31 patients with a previously negative prostate biopsy but persistent elevated PSA underwent 68Ga-PSMA PET/CT and PET/ultrasound-targeted prostate biopsy. 68Ga-PSMA PET/CT helped predict csPCa, and PET/ultrasound-guided prostate biopsy was clinically feasible and improved the detection of csPCa.
IMPLICATIONS FOR PATIENT CARE: 68Ga-PSMA PET imaging combined with PET/ultrasound-guided prostate biopsy has a high diagnostic value for patients with suspected PCa who previously had negative biopsy results but have a persistently elevated PSA.
Supplementary Material
Acknowledgments
We thank all the physicians, chemists, nurses, and technicians from the Department of Nuclear Medicine, Peking University Cancer Hospital, for their contributions to this project. We thank Yuehan Zheng, Puyun Chen, Weiai Peng, and Yeqing Liu for their technical assistance.
REFERENCES
- 1.Ahmed HU, El-Shater Bosaily A, Brown LC, et al. Diagnostic accuracy of multi-parametric MRI and TRUS biopsy in prostate cancer (PROMIS): a paired validating confirmatory study. Lancet. 2017;389:815–822. [DOI] [PubMed] [Google Scholar]
- 2.Schoots IG. Omission of systematic transrectal ultrasound guided biopsy from the MRI targeted approach in men with previous negative prostate biopsy might still be premature. Ann Transl Med. 2016;4:205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mendhiratta N, Meng X, Rosenkrantz AB, et al. Prebiopsy MRI and MRI-ultrasound fusion-targeted prostate biopsy in men with previous negative biopsies: impact on repeat biopsy strategies. Urology. 2015;86:1192–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sonn GA, Chang E, Natarajan S, et al. Value of targeted prostate biopsy using magnetic resonance-ultrasound fusion in men with prior negative biopsy and elevated prostate-specific antigen. Eur Urol. 2014;65:809–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wegelin O, van Melick HHE, Hooft L, et al. Comparing three different techniques for magnetic resonance imaging-targeted prostate biopsies: a systematic review of in-bore versus magnetic resonance imaging-transrectal ultrasound fusion versus cognitive registration—is there a preferred technique? Eur Urol. 2017;71:517–531. [DOI] [PubMed] [Google Scholar]
- 6.Barry MJ, Rosenkrantz AB. MRI-targeted versus ultrasonography-guided biopsy for suspected prostate cancer. N Engl J Med. 2018;378:1835–1836. [DOI] [PubMed] [Google Scholar]
- 7.Kasivisvanathan V, Emberton M, Moore CM. “Don’t let the perfect be the enemy of the good”: time to embrace magnetic resonance imaging before first prostate biopsy. Eur Urol. 2018;74:411–412. [DOI] [PubMed] [Google Scholar]
- 8.Prostate cancer. European Association of Urology website. http://uroweb.org/guideline/prostate-cancer. Accessed April 28, 2020.
- 9.Fulgham PF, Rukstalis DB, Turkbey IB, et al. AUA policy statement on the use of multiparametric magnetic resonance imaging in the diagnosis, staging and management of prostate cancer. J Urol. 2017;198:832–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sonn GA, Fan RE, Ghanouni P, et al. Prostate magnetic resonance imaging interpretation varies substantially across radiologists. Eur Urol Focus. 2019;5:592–599. [DOI] [PubMed] [Google Scholar]
- 11.Perera M, Papa N, Christidis D, et al. Sensitivity, specificity, and predictors of positive 68Ga-prostate-specific membrane antigen positron emission tomography in advanced prostate cancer: a systematic review and meta-analysis. Eur Urol. 2016;70:926–937. [DOI] [PubMed] [Google Scholar]
- 12.Fendler WP, Schmidt DF, Wenter V, et al. 68Ga-PSMA PET/CT detects the location and extent of primary prostate cancer. J Nucl Med. 2016;57:1720–1725. [DOI] [PubMed] [Google Scholar]
- 13.Eiber M, Weirich G, Holzapfel K, et al. Simultaneous 68Ga-PSMA HBED-CC PET/MRI improves the localization of primary prostate cancer. Eur Urol. 2016;70:829–836. [DOI] [PubMed] [Google Scholar]
- 14.Zamboglou C, Drendel V, Jilg CA, et al. Comparison of 68Ga-HBED-CC PSMA-PET/CT and multiparametric MRI for gross tumour volume detection in patients with primary prostate cancer based on slice by slice comparison with histopathology. Theranostics. 2017;7:228–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eiber M, Herrmann K, Calais J, et al. Prostate Cancer Molecular Imaging Standardized Evaluation (PROMISE): proposed miTNM classification for the interpretation of PSMA-ligand PET/CT. J Nucl Med. 2018;59:469–478. [DOI] [PubMed] [Google Scholar]
- 16.Liu C, Liu T, Zhang N, et al. 68Ga-PSMA-617 PET/CT: a promising new technique for predicting risk stratification and metastatic risk of prostate cancer patients. Eur J Nucl Med Mol Imaging. 2018;45:1852–1861. [DOI] [PubMed] [Google Scholar]
- 17.Davenport MS, Montgomery JS, Kunju LP, et al. 18F-choline PET/mpMRI for detection of clinically significant prostate cancer: part 1. Improved risk stratification for MRI-guided transrectal prostate biopsies. J Nucl Med. 2020;61:337–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lopci E, Saita A, Lazzeri M, et al. 68Ga-PSMA positron emission tomography/computerized tomography for primary diagnosis of prostate cancer in men with contraindications to or negative multiparametric magnetic resonance imaging: a prospective observational study. J Urol. 2018;200:95–103. [DOI] [PubMed] [Google Scholar]
- 19.Simopoulos DN, Natarajan S, Jones TA, Fendler WP, Sisk AE, Jr, Marks LS. Targeted prostate biopsy using 68gallium PSMA-PET/CT for image guidance. Urol Case Rep. 2017;14:11–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Westenfelder KM, Lentes B, Rackerseder J, et al. Gallium-68 HBED-CC-PSMA positron emission tomography/magnetic resonance imaging for prostate fusion biopsy. Clin Genitourin Cancer. 2018;16:245–247. [DOI] [PubMed] [Google Scholar]
- 21.Koerber SA, Utzinger MT, Kratochwil C, et al. 68Ga-PSMA-11 PET/CT in newly diagnosed carcinoma of the prostate: correlation of intraprostatic PSMA uptake with several clinical parameters. J Nucl Med. 2017;58:1943–1948. [DOI] [PubMed] [Google Scholar]
- 22.Zhang J, Shao S, Wu P, et al. Diagnostic performance of 68Ga-PSMA PET/CT in the detection of prostate cancer prior to initial biopsy: comparison with cancer-predicting nomograms. Eur J Nucl Med Mol Imaging. 2019;46:908–920. [DOI] [PubMed] [Google Scholar]
- 23.Chen M, Zhang Q, Zhang C, et al. Combination of 68Ga-PSMA PET/CT and multiparameter MRI improves the detection of clinically significant prostate cancer: a lesion by lesion analysis. J Nucl Med. 2019;60:944–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Benešova M, Schafer M, Bauder-Wust U, et al. Preclinical evaluation of a tailor-made DOTA-conjugated PSMA inhibitor with optimized linker moiety for imaging and endoradiotherapy of prostate cancer. J Nucl Med. 2015;56:914–920. [DOI] [PubMed] [Google Scholar]
- 25.Afshar-Oromieh A, Hetzheim H, Kratochwil C, et al. The theranostic PSMA ligand PSMA-617 in the diagnosis of prostate cancer by PET/CT: biodistribution in humans, radiation dosimetry, and first evaluation of tumor lesions. J Nucl Med. 2015;56:1697–1705. [DOI] [PubMed] [Google Scholar]
- 26.Sanchez-Crespo A. Comparison of gallium-68 and fluorine-18 imaging characteristics in positron emission tomography. Appl Radiat Isot. 2013;76:55–62. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.