Abstract
The global panic of the novel coronavirus disease 2019 (COVID-19) triggered by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has led to an urgent requirement for effective therapy. COVID-19 infection, especially in severely ill patients, is likely to be associated with immune dysregulation, prompting the development of novel treatment approaches. Therefore, this systematic review was designed to assess the available data regarding the efficacy of the immunomodulatory drugs used to manage COVID-19. A systematic literature search was carried out up to May 27, 2020, in four databases (PubMed, Scopus, Web of Science, and Embase) and also Clinicaltrials.gov. Sixty-six publications and 111 clinical trials were recognized as eligible, reporting the efficacy of the immunomodulatory agents, including corticosteroids, hydroxychloroquine, passive and cytokine-targeted therapies, mesenchymal stem cells, and blood-purification therapy, in COVID-19 patients. The data were found to be heterogeneous, and the clinical trials were yet to post any findings. Medicines were found to regulate the immune system by boosting the innate responses or suppressing the inflammatory reactions. Passive and cytokine-targeted therapies and mesenchymal stem cells were mostly safe and could regulate the disease much better. These studies underscored the significance of severity profiling in COVID-19 patients, along with appropriate timing, duration, and dosage of the therapies. Therefore, this review indicates that immunomodulatory therapies are potentially effective for COVID-19 and provides comprehensive information for clinicians to fight this outbreak. However, there is no consensus on the optimal therapy for COVID-19, reflecting that the immunomodulatory therapies still warrant further investigations.
Keywords: COVID-19, SARS-CoV-2, Immunomodulatory-based therapy, Passive immunotherapy, Cytokine-targeted therapy, Mesenchymal stem cells
1. Introduction
The panic of novel coronavirus disease 2019 (COVID-19) triggered by SARS-CoV-2 has influenced people all over the world, which has been warned as a global health emergency [1]. SARS-CoV-2 belongs to the single-stranded positive-sense RNA β-coronavirus family, entering the cells through applying the angiotensin-converting enzyme 2 (ACE2) receptor distributed on the surface of the heart, kidney, intestine, and, particularly, lung’s alveolar type II (AT2) epithelial cells [2]. COVID-19 infection seems to be associated with more person-to-person transmissibility and less lethality than either severe acute respiratory syndrome (SARS) and the middle east respiratory syndrome (MERS), as the majority of the patients have experienced mild symptoms and good prognosis so far. However, it has been reported that approximately 14% of the patients with novel coronavirus pneumonia (NCP) have progressed severe or fatal conditions, along with a rising transition of the patients from the mild condition into severe pneumonia accompanied by a wide range of complications including the acute respiratory distress syndrome (ARDS), septic shock, and multiple organ failure, consequently leading to the death. Currently, management of these critically ill patients has turned into one of the principal challenges [3], [4], [5], [6], [7].
Clinically, the COVID-19 infection is likely to provoke the immune responses in two phases, and therefore, each phase requires tailored treatment approaches. So that, at the earlier stages, the virus replicates and activates both innate and adaptive immune responses during the non-severe disease period, in which boosting the immune system may be a suitable strategy for eliminating the virus. At the later stages associated with the expansion of severe conditions, COVID-19 is accompanied by the excessive inflammatory and dysregulated immune responses, leading to some life-threatening obstacles [8], [9], [10], [11]. Lymphopenia and higher levels of inflammatory indicators, e.g., D-dimer and C-reactive protein (CRP), are the main characteristics of the severe COVID-19 patients, with drastically reduced numbers of the helper T cells, cytotoxic suppressor T cells, regulatory T cells, and natural killer (NK) and B cells [5], [12], [13], [14]. Additionally, a large number of the leukocytes and circulating monocytes are irregularly activated, associated with the abnormal levels of cytokines/chemokines, especially, e.g., interleukin 6 (IL-6), IL-2, IL-7, IL-10, granulocyte-colony stimulating factor (GCSF), IFN-gamma-inducible protein 10 (IP-10), and Tumor necrosis factor-α (TNF-a) [15], [16], [17], [18], eventually leading to the macrophage activation syndrome (MAS) in some patients with severe respiratory failure (SRF) [15], [16], [17], [18], [19]. All these highlight that SARS-CoV-2 might mainly induce the cytokine release syndrome (CRS), in which the magnitude of the cytokine storm is correlated with the disease severity and fatal consequences. Therefore, we need to pay close attention to the immunological status of the patients and restrain the overt inflammatory responses timely through the immunomodulators and cytokine-storm-targeted therapies to control the progression of the disease cascade and decrease the mortality among the severe COVID-19 patients [18], [20], [21], [22], [23], [24], [25].
Seemingly, the medicines targeting the coronavirus alone might not be appropriately effective in controlling the extremely pathogenic infections and should probably be applied along with the immunomodulatory-based therapies [26], [27]. Various studies have focused on the efficacy of the immunomodulatory agents including corticosteroids, hydroxychloroquine or chloroquine, cytokine-targeted therapies (e.g., anakinra, siltuximab, or tocilizumab), passive immunotherapy (convalescent plasma and intravenous immunoglobulin), mesenchymal stem cells, and blood-purification therapy, mostly as adjuvant therapy for treatment of the patients with severe COVID-19 and partly have reported promising outcomes. To date, numerous immunomodulators have been investigated; however, data of the available literature do not provide a complete overview. Therefore, the present systematic review was conducted to review the available evidence on the therapeutic value of the immunomodulatory agents for the management of the COVID-19 patients.
2. Methods
2.1. Protocol and registration
This systematic review has been registered on the International Prospective Register of Systematic Reviews (http://www.crd.york.ac.uk/PROSPERO), with the registration number CRD42020179200. The protocol for this systematic review was conducted according to the Preferred Reporting Item for Systematic Review and Meta-analysis (PRISMA) guideline [28].
2.2. Search strategy
A comprehensive search was carried out on April 21, 2020, updated on May 27, 2020, from the following main electronic databases: PubMed, Scopus, Web of Science, and Embase, for studies that focused on the efficacy of immunomodulatory medicines without any language restriction. We also checked the reference lists of all principal publications for further eligible publications. We then systematically searched for the published articles in Google, and for ongoing trials in clinical trial registries (clinicaltrials.gov). The following search strategy has been used regarding specific search tips of each database: “COVID-19” and “Immunomodulation”, “Anti Inflammatory”, “Mesenchymal Stem Cell”, “anti-interleukin-6”, “tocilizumab”, “siltuximab”, “Janus Kinase Inhibitor”, “Anakinra”, “glucocorticoids”, “convalescent plasma”, “intravenous immunoglobulin”, “hydroxychloroquine or chloroquine” or “blood purification therapy”. The details of the search strategy have been provided in Supporting Information 1.
2.3. Eligibility criteria
Publications eligible for inclusion were case reports, case series, case-control, and cohort studies characterizing the efficacy of immunomodulatory therapies in patients with COVID-19.
The following exclusion criteria were considered: 1) reviews, meta-analysis, and abstracts; 2) non-human studies; 3) The topics were not related to the review question (e.g., when the articles addressed other virus-related diseases); 4) Studies that patients received any type of anti-virals not related to immunomodulatory property; 5) studies with incomplete, non-detailed or non-useful data; 6) studies with patients coinfected with influenza virus and SARS-CoV-2; 7) COVID-19 disease in transplant recipients with long-term immunosuppression.
2.4. Study selection
The selection of studies was conducted in two steps. Step 1: two reviewers (MR and FH), independently, screened titles and abstracts of publications retrieved through the search strategies based on the inclusion criteria. Any publications that did not fulfill the eligibility criteria were excluded. Step 2: where a publication was potentially eligible, the full text was reviewed by the same reviewers. Any disagreements were resolved by a third author.
2.5. Data extraction
For each eligible publication, the following descriptive information was extracted: study characteristics (first author and study country), clinical characteristics (age, sex and population), characteristics of intervention (types, dosage, frequency, etc.), and main findings (all outcomes and mortality). The primary outcome was mortality risk. Other outcomes of interest were adverse reactions, clinical laboratory benefits, and computed tomographic (CT) findings. All the retrieved information was cross-checked by MR, FH, EG, and MD.
2.6. Statistical analysis
It was not feasible to perform a meta-analysis because there were not adequate, appropriate research studies on this issue.
3. Results
3.1. Literature search
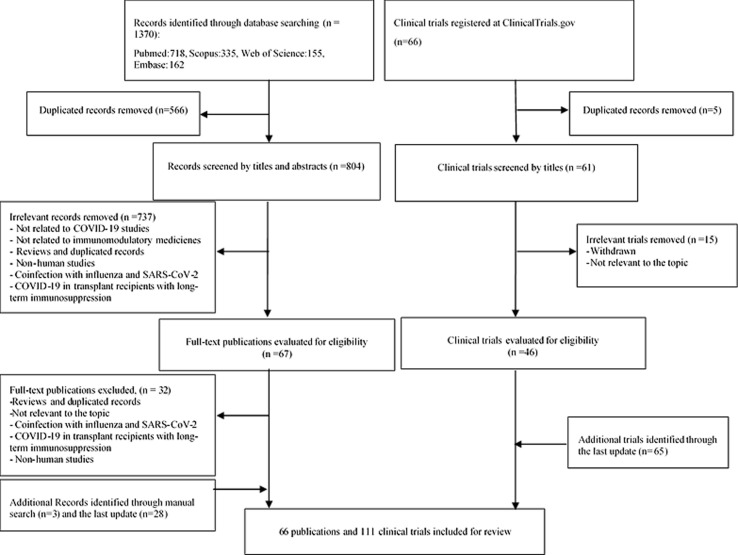
In the first step, a total of 1370 publications were identified from the PubMed (7 1 8), Scopus (3 3 5), Web of Science (1 5 5), and Embase (1 6 2) databases. Subsequently, the obtaining records were transferred to the EndNote reference management software to remove the duplicate publications (n = 566), after which 804 studies remained. Following the subsequent screening of the titles and abstracts, 737 publications were excluded as they were review studies or not relevant to the topic, and 67 records met the inclusion criteria. In the second step, a full-text review was performed for the remaining 67 papers, from which 32 publications were removed according to the exclusion criteria, leading to the inclusion of 35 publications in the qualitative analysis. Additionally, 3 relevant studies were found via manual search. Twenty-eight new publications were also identified through updating the search. Ultimately, a total of 66 publications were included in this systematic review. Fig. 1 shows a flowchart of the search and selection procedure of the studies.
Fig. 1.
Flow chart of the search strategy according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.
Herein, the details of the ongoing clinical trials registered at the ClinicalTrials.gov by April 21, 2020, updated on May, 27 were also provided. The resulting trials were collected, were evaluated for eligibility, and were checked to remove any duplicates. After the screening procedure, 111 ongoing clinical trials were included related to the topic of the present review (Fig. 1.).
The main objective of these included publications and clinical trials was determining the safety and efficacy of the various immunomodulatory medicines in patients with mild/moderate-to-severe COVID-19. Table 1, Table 2, Table 3 present the characteristics of the publications, patients demographics, therapeutic interventions, and their main findings. The enrolled trials consisted of the registry number of trials, study design, patient population, intervention, and outcomes (Supporting Information 2).
Table 1.
Characteristics of included publications related with conventional therapy.
|
Author Country |
Aim of study |
N. cases |
Male/Female Age |
Treatment plan | Main Findings | Mortality |
|---|---|---|---|---|---|---|
| Zheng China [29] |
The risk-adapted therapy of COVID-19 cases using corticosteroid based on the illness severity | 55 mild-to-severe patients | 24/31 62(29-91) |
1- Non-severe group (n = 34): SOC 2- Severe group (n = 21): SOC, corticosteroid (MP, 0.5mg∼1mg/kg.d× 5 days) within the first three days |
- Benefits: 1- After two weeks, clinical manifestations had completely disappeared in most of patients in both groups (p = 0.85) 2- This approach was beneficial in manifestations relief and chest CT resolution 3- No considerable differences in SARS-CoV-2 RNA elimination (p = 0.92) and the amounts of IgM and IgG antibody production between two groups (p = 0.13, 0.62) - Outcome: 45 patients were discharged, and all patients were alive |
0/55 (0%) |
| Wu China [30] |
Clinical characteristics associated with the development of ARDS and progression from ARDS to death in COVID-19 cases |
201 | 128/73 51 (21-83) |
SOC, MP (62/201), immunomodulators (70/201) (IVIG, thymosin, and recombinant human granulocyte colony-stimulating factor) |
- Outcome: 144 of the total 201 cases were discharged and all of the patients who died (44/201) had developed ARDS - Benefits: 1- The use of MP reduced the rate of death in cases with ARDS (HR, 0.38; 95% CI, 0.20-0.72; P = .003). 2- Among the cases with ARDS (84/201, 41.8%), of those who received MP (n = 50), 23 (46.0%) patients died, while of those who did not receive MP (n = 34), 21 (61.8%) died. 3- - Patients with ARDS compared with patients without ARDS were less likely to be treated with anti-virals (P = .005) and more likely to be treated with MP (P < .001). |
44/201 (21.9%) |
| Zhou China [31] |
Possible profits of corticosteroids for severe NCP patients | 15 critical patients | 10/5 61.7 |
SOC, corticosteroid (MP, median dose of 400.0 mg/day) for an average of 9.5 days |
- Benefits (Early administration of corticosteroid (in the first 3−5 days after ICU admission)): 1- Increasing of oxygen saturation (SaO2) (p = 0.012) and reducing of CRP (p = 0.035) and D-dimer (P = 0.047) levels. 2- Augment arterial oxygen tension (PaO2)/inspiratory oxygen fraction (FiO2) (p = 0.034) 3- Acquire precious time for management of inflammatory storm - Adverse events (Delayed administration of corticosteroids): When COVID-19 cases were complicated by both ARDS and shock or multiorgan failure, corticosteroid therapy did not exert survival advantage (7/15 Died) |
7/15 (46.7%) |
| Wang China [32] |
Assess the effect and safety of corticosteroid therapy in the severe NCP patients | 46 severe patients | 26/20 54 |
1- Non-corticosteroid group (n = 20): SOC, thymosin 2- Corticosteroid-treated group (n = 26): SOC, thymosin, i.v. corticosteroid (MP, a dose of 1-2mg/kg/d during 5-7 days) |
- Benefits: 1- The periods for body temperature recovery were shorter in MP-treated group compared to control group (2.06±0.28 vs. 5.29±0.70, P = 0.010). 2- The MP-treated patients had a quicker recovery of SpO2 compared to control group (8.2 days (7.0-10.3) vs. 13.5days (10.3-16); P < 0.001). 3- The CT results were significantly better in cases with MP therapy - Outcome: Among the three deaths, two happened in the patients with MP administration |
1- 1/20 (5%) 2- 2/26 (7.6%) |
| Lu China [33] |
The effect of corticosteroids as adjuvant therapy in critically NCP patients | 244 critically ill patients | 128/116 62 (50-71) |
1- Non-Corticosteroid group (n = 93): SOC 2- Corticosteroid-treated group (n = 151): SOC, corticosteroid (dosage range: 100-800mg/d, 8 (4-12) days) |
- Outcome: 1- Corticosteroid therapy was independent of overall mortality (adjusted OR: 1.05; 95% CI: -1.92-2.01). 2- Subgroup analyses of 147 cases who developed dyspnea and 87 cases with ARDS displayed corticosteroid was not involved in enhanced 28-day mortality (P>0.3) | NA |
| case-control: 62 critically ill patients in 31 pairs | 32/32 62 (50-71) |
1- Control (non-corticosteroid) group (n = 31): SOC 2- Case (corticosteroid-treated) group (n = 31) : SOC, corticosteroid |
- Adverse events: Raised corticosteroid dose was associated with enhanced mortality rate (P = 0.003) in matched cases after adjustment for administration duration; every ten-milligram increase was associated with additional 4% mortality risk (adjusted HR: 1.04, 95% CI: 1.01-1.07). - Outcome: The 28-day mortality risk was 39% (12 out of 31) in case group and 16% (5 out of 31) in control group (P = 0.09). |
1- 5/31 (16%) 2- 12/31 (39%) |
||
| Xu China [34] |
Identify risk factors associated with persistent virus RNA shedding in NCP cases | 113 mild-to-severe patients |
70/43 52 years |
SOC, Corticosteroid (56.6%, 0.5-1 mg MP/kg body weight) |
- Adverse events: 1- Cases with longer SARS-CoV-2 RNA shedding (≥15 d after disease onset) was associated with several risk factors, like sex (p = 0.009), old age (p = 0.033), delayed admission to hospital (p = 0.001), invasive mechanical ventilation (p = 0.006) and corticosteroid use (p = 0.025) compared to cases with early (<15 d) RNA clearance. 2- Cases with persistent viral RNA shedding duration had slower retrieval of temperature (p < 0.001) and radiological images (p < 0.001) than cases with early viral RNA shedding - Corticosteroid was not an independent risk factor of longer viral RNA shedding - SARS-CoV-2 RNA was detected with the median periods of 17 d from disease onset. |
2/113 (1.7%) |
| Fang China [35] |
Investigate the efficiency of low-dose corticosteroid use on SARS-CoV-2 clearance time | 78 general-to-severe patients | 44/34 General group: 40 Severe group: 57.4 |
1- Non-corticosteroid group (n = 53): SOC 2- Corticosteroid-treated group (n = 25): SOC, Corticosteroids (9 cases in the general group received oral MP with a median dose of 237.5 mg/day for 7 d while 16 cases in the severe group, i.v. MP, median dose of 250 mg/day for 4.5 d) |
- There was no significant difference in virus clearance time between the corticosteroid-treated group and the non-corticosteroid in the general group (17.6±4.9 vs. 18.7±7.7 days) and cases in the severe group (18.8±5.3 vs. 18.3±4.2 days). - Low-dose corticosteroid does not prolong viral clearance in NCP cases |
NA |
| Wang China [9] |
Evaluate the clinical features of hospitalized NCP patients |
138 patients | 75/63 56 (22-92) |
SOC, glucocorticoid (62 /138) |
- Outcome: Most patients were still hospitalized, 47 cases (34.1%) discharged, and six died. - No effective outcomes were observed. |
6/138 (4.3%) |
| Wan China [20] |
Describe the clinical characteristics, therapies and outcomes of NCP patients | 135 mild-to-severe patients | 72/63 47 (36–55) |
SOC, traditional Chinese medicine, corticosteroids (36/135) |
- Outcome: Most patients were still hospitalized at the end of study, a total of 15 patients had been discharged, and one patient had died. The 28-day mortality rate was 2.5%. - Kaletra was shown to be effective in the early treatment of COVID‐19 patients - Recommendations for the usage of glucocorticoids for NCP patients |
2.5% |
| Mo China [36] |
Clinical characteristics of refractory COVID-19 pneumonia | 155 general and refractory patients | 86/69 54 (42∼66) |
SOC, i.v. corticosteroid (79/155), immune-enhancing treatment (thymalfasin, 7.1%; IVIG, 5.8%). | - Benefits: Most patients recovered | NA |
| Zheng China [37] |
Clinical Characteristics of Children with COVID-19 pneumonia | 25 pediatric cases | 14/11 3 |
SOC, Two critical cases received corticosteroids and IVIG | - Children were prone to NCP like adults, while the clinical symptoms were more desirable in children - Outcome: The abnormalities in 24 of 25 cases were mitigated and one patient discharged |
0/25 (0%) |
| Sun China [38] |
Assess clinical features, therapies and outcomes of pediatric NCP cases | 8 severe or critical pediatric patients | 6/2 2 months to 15 years |
SOC, i.v. glucocorticoids (5/8) and IVIG (4/8) | - Severely ill cases had a disease duration of over 10 d, but critical patients had over 20 d - Outcome: Three patients remained in ICU, the other five recovered and discharged |
0/8 (0%) |
| Xu China [39] |
Assess the clinical features of NCP patients | 62 mild or moderate patients | 35/27 41 |
SOC, corticosteroid (16/62, 40-80 mg/day), IVIG (16/62, 15-20 g/day) for 3-5 d | - Outcome: 61 cases still remain in hospital, one case had been discharged, and no patients had died. | 0/62 (0%) |
| Wang China [40] |
Describe clinical features in COVID-19 patients | 125 mild-to-critical patients | 71/54 38.76 |
SOC, glucocorticoids (35/125), IVIG (24/125) | - Outcome: 47 patients were discharged with 14.8 d hospital length of stay, none of the cases died and 78 (62.4%) patients remained in hospital. | 0/125 (0%) |
| Liu China [41] |
Analyze the clinical symptoms, treatments, and prognosis of NCP cases | 137 critically ill patients | 61/ 76 57 (20–83) |
SOC, corticosteroid (40/137, 30– 80 mg/day, 3–5 days), IVIG (44/137) |
- Adverse events: Intravenous MP neither reduced the illness course nor ameliorated the prognosis. - Outcome: 32.1% patients discharged (n = 44/137) and 11% dead (n = 16/137) |
16/137 (11%) |
| Zhou China [42] |
Treatment of a COVID-19 patient with spontaneous pneumomediastinum | One patient | A 38-year-old man | First 10 days: SOC, corticosteroid After 10 days: corticosteroid was stopped, SOC, and bronchodilators for 14 days |
- Adverse events: After ten days of therapy, the patient had pneumomediastinum, and corticosteroid was stopped. Then, therapeutic regimen changed, and bronchodilators were added. - Outcome: By day 25, the patient’s temperature had recovered and then discharged |
0/1 (0%) |
| Zha China [43] |
Evaluate the effect of corticosteroid in cases with COVID-19 | 31 mild COVID-19 without ARDS | 20/11 39 (32–54) |
1- Non-corticosteroid group (n = 20): SOC 2- Corticosteroid-treated group (n = 11): SOC, MP (40 mg once or twice daily) within 24 h of admission for a median 5 d |
- No correlation between corticosteroid use and viral clearance time (HR, 1.26; 95% CI, 0.58–2.74), hospital length of stay (HR, 0.77; 95% CI, 0.33–1.78), discharge (HR, 0.77; 95% CI, 0.33–1.78), and clinical alleviation (HR, 0.86; CI, 0.40–1.83) - Outcome: 26 of the 31 cases were discharged, and five were yet hospitalized. |
0/31 (0%) |
| Gautret France [44] |
Assess the impact of HCQ on respiratory viral loads |
36 | 15/21 45.1 ± 22.0 |
1- Control group (n = 16): SOC 2- HCQ-treated group (n = 20): SOC, oral HCQ (200 mg, three times per day for ten days), AZ (6/20, 500mg on d 1 then 250mg per day, for another 4 d) |
- Benefits: 1- Ay day6 post-therapy, 70% of HCQ-treated group was virologically treated compared to 12.5% in the control group (p = 0.001), showing the efficacy of HCQ in clearing SARS-CoV-2. 2- The addition of AZ to HCQ therapy was more effective for viral clearance. |
1/36 (2.7%) |
| Gautreta France [45] |
Assess the Clinical impact of a combination of HCQ and AZ on NCP cases | 80 | 43/37 52 (18 to 88) |
HCQ orally 200 mg three times daily for ten days, AZ (500 mg on d 1 followed by 250 mg daily for another four days) |
- Benefits: 1- A fast clearance of nasopharyngeal viral load, with 93% negative at day8. - Outcome: All cases recovered and discharged with a length of stay of 5 days except one case who died and the other one still in ICU. |
1/80 (1.2%) |
| Milliona France [46] |
Assess the early therapy of NCP cases with HCQ and AZ | 1061 | 492/569 43.6 (14–95) |
HCQ (200 mg three times daily for ten days) + AZ (500 mg on day 1 followed by 250 mg daily for another four days) |
- Benefits: 1- Well clinical recovery and viral cure were gained in 973 cases within 10 days (91.7%). 2- Use of the HCQ+AZ combination before COVID-19 complications onset was safe and associated with a very low fatality rate in cases. - Outcome: Five patients were still remained in hospital (98.7% of patients cured) - Adverse events: 1- Prolonged viral carriage was identified in 47 patients (4.4%). 2- A total of 2.3% of cases reported mild side effects (gastrointestinal or skin symptoms, headache, insomnia and transient blurred vision) |
10/1061 (0.9%) |
| Chen China [47] |
Evaluate the effect of HCQ in the curing of COVID-19 patients |
62 | 29/33 44.7+- 15.3 |
1- Control group (n = 31): SOC 2- HCQ-treated group (n = 31): SOC, oral HCQ (400 mg/d (200 mg/bid) during days 1 and 5) |
- Benefits: 1- TTCR was greatly shortened in the HCQ-treated group. 2- HCQ could improve pneumonia (80.6%) compared with the control group (54.8%). - Adverse events: There were two cases with mild adverse effects in the HCQ-treated group (rash and headache) without appearance of severe adverse reactions. |
NA |
| Huang China [48] |
Assess the effect and safety of CQ in NCP cases compared to the cases treated with Lopinavir/Ritonavir | 22 (8 sever and 14 moderate patients) | 13/9 44.0 (36.5–57.5) |
1- Control group (n = 12): 5 severe and 7 moderate cases received 400/100 mg of oral Lopinavir/Ritonavir twice daily for 10 d 2- CQ group (n = 10): 3 severe and 7 moderate cases given oral CQ 500 mg twice daily for 10 d |
- Benefits: 1- At day 13, all of the CQ-treated cases turned SARS-CoV-2 negative, while all Lopinavir/ Ritonavir-treated patients became at day 14. 2- At day 14, the rate of lung recovery was more than doubled in the CQ group compared to the control group. 3- Patients treated with CQ recovered better without serious adverse events. - Outcome: By day 14, all 10 patients (100%) from the CQ group were discharged compared to 6 patients (50%) from the Lopinavir/ Ritonavir group. |
0/22 (0%) |
| Wan China [49] |
Investigate the relationship between cytokines and PII in COVID-19 progression and response to treatment | 123 mild-to-severe patients | 66/57 15–82 |
SOC, CQ, TCM |
- Benefits: 1- The CD4+ T, CD8+ T, and IL-6 recovered before discharge in severe cases, suggesting the restoration of the cellular immune function. - Outcome: All patients were discharged except the four severe cases who died with the longest follow-up of 20 days. |
4/123 (3.2%) |
| Mahévas France [50] |
Assess the effectiveness of oral HCQ in preventing admission to ICU and/or death | 181 patients requiring oxygenation | 128/53 60 |
1- No-HCQ group (n = 97): SOC, the absence of HCQ use during 48 h of admission 2- HCQ-treated group (n = 84): SOC, HCQ within 48 h of admission, 600 mg/day |
- Outcome: 1- 20.2% cases in the HCQ group were transferred to the ICU or died during 7 days vs 22.1% in the no-HCQ group. 2- In the HCQ group, the lethality was 2.8% within 7 days vs 4.6% in the no-HCQ group (3 vs 4 events, RR 0.61, 95% CI 0.13–2.89) - Adverse events: 1- Eight cases given HCQ (9.5%) experienced electrocardiogram modifications needing HCQ termination. 2- These results did not recommend the use of HCQ in cases hospitalized for COVID-19. |
1- 4.6% 2- 2.8% |
| Geleris USA [51] |
Examine the association between HCQ use and respiratory failure | 1376 mild-to-severe patients | 781/595 | 1- Control group (n = 562): SOC, some cases given ACE inhibitor, glucocorticoid, AZ, TCZ, remdesivir 2- HCQ-treated group (n = 811): SOC, oral HCQ (600 mg twice on day 1, then 400 mg daily for a median of 5 days), some cases given ACE inhibitor, glucocorticoid, TCZ, remdesivir, AZ |
- Outcome: Over a median follow-up of 22.5 days, 346 cases (25.1%) had a primary end-point event (166 cases died without being intubated, and 180 were intubated). At the time of data cutoff, a total of 232 patients had died, 1025 had survived to hospital discharge, and 119 were still hospitalized. - There was no considerable association between HCQ use and a lowered or an enhanced rate of intubation or death (HR, 1.04, 95% CI, 0.82 to 1.32) |
232/1376 (16%) |
| Molina France [52] |
Explore virologic and clinical outcomes of HCQ and AZ on NCP cases | 11 | 7/4 58.7 (20-77) |
HCQ (600 mg/d for 10 days), AZ (500 mg day 1 and 250 mg the next 4 days) |
- Adverse events: 1- During 5 days, one case died, two were transferred to the ICU and therapy of one case was discontinued after 4 days because of a prolongation of the QT interval. 2- At days 5 to 6 post-treatment, viral RNA was positive in 8/10 patients (80%). 3- No data of a strong anti-viral potency or clinical recovery of the combination of HCQ and AZ. |
1/11 (9.09%) |
| Tang China [53] |
An open–label, randomized, controlled trial assessing the efficacy and safety of HCQ plus SOC compared with SOC alone in NCP patients | 150 patients (148 mild/ moderate and 2 severe cases) |
82/68 46 |
1- Control group (n = 75): SOC 2- HCQ-treated group (n = 75): SOC, HCQ 1, 200 mg daily for 3 d followed by a dose of 800 mg daily (2 or 3 weeks) |
- Adverse events: This trial did not support the use of HCQ in cases with mild to moderate NCP due to slight impacts on viral clearing and significantly enhanced adverse effects (specially diarrhea). | NA |
| Chorin USA [54] |
Evaluate the changes in QTc interval and arrhythmic events in cases with COVID-19 received HCQ/AZ | 251 hospitalized patients | 188/63 64±13 |
HCQ orally at 400 mg BID for one day followed by 200 mg BID for 4 days, AZ orally at a dose of 500 mg daily for 5 days | - Adverse events: 1- Increasing HCQ/AZ exposure was associated with QTc prolongation, especially in 23% of COVID-19 patients, which occurred on day 4.1 ± 2 of therapy and seven cases required premature discontinuation of therapy. 2- This prolongation may be responsible for life-threatening arrhythmia. | 44/251 (17.5%) |
| Magagnoli USA [55] |
Assess the effect of HCQ alone or in combination with AZ, in the therapy of NCP patients |
368 | 368 male 65 |
1- No-HCQ (n = 158) 2- HCQ (n = 97) 3- HCQ+AZ (n = 113) |
- Outcome: Rates of lethality in the HCQ, HCQ +AZ, and no-HCQ groups were 27.8%, 22.1%, 11.4%, respectively. - Adverse events: The rate of lethality from any cause was more in the HCQ group (adjusted HR, 2.61; 95% CI, 1.10 to 6.17) but not in the HCQ+AZ group (adjusted HR, 1.14; 95% CI, 0.56 to 2.32) compared to the no-HCQ group. - The risk of ventilation was similar in all groups. |
1- 18/158 (11.4%) 2- 27/97 (27.8%) 3- 25/113 (22.1%) |
| Borba Brazil [56] |
Evaluate the safety and efficacy of two different CQ doses in severe NCP cases | 81 severe patients | 60/21 51.1+- 13.9 |
1- High-dosage group (n = 41): SOC, AZ, CQ (600 mg CQ; 4 × 150 mg tablets twice daily for 10 days; total dose 12 g) 2- low-dosage group (n = 40): SOC, AZ, CQ (ie, 450 mg twice daily on day 1 and once daily for 4 days; total dose 2.7 g) |
- Outcome: 1- Death by day 13 was 39.0% in the high-dosage group and 15.0% in the low-dosage group (OR, 3.6; 95% CI, 1.2-10.6). 2- The high-dosage CQ was no longer associated with lethality when controlled by age (OR, 2.8; 95% CI, 0.9-8.5) - Adverse events: Higher CQ dosage should not be used for critical cases due to its potential safety risks (more instance of QTc interval), notably when given with AZ and oseltamivir. |
1- 16/41 (39%) 2- 6/40 (15%) |
| Perinel France [57] |
Explore the PK characteristic of HCQ in ICU NCP cases to define the optimal dosing regimen | 13 | 11/2 68 (38 – 82) |
SOC, oral HCQ (200 mg, three times per day, four cases underwent dose limitation and subsequently given 200 mg of HCQ twice daily), CRRT (one case) |
- HCQ levels 1–2 mg/L were regarded to be curative. Only 8/13 cases (61%) reached the minimum curative level of 1 mg/L and 2/13 cases exceeded over 2 mg/L. - The mean time to reach the minimum therapeutic level was 2.7 days - Adverse events: HCQ was withdrawn in two patients due to QT interval prolongation - Based on simulation results, 800 mg once on day 1, followed by 200 mg twice daily for seven days, can more rapidly reach therapeutic levels in ICU patients |
NA |
COVID-19: coronavirus disease 2019, SARS-CoV-2: severe acute respiratory syndrome coronavirus 2, SOC: standard of care (antibiotics, anti-virals, oxygen therapy, non-invasive or invasive ventilation, ECMO and supportive care according to severity of disease), NCP: novel coronavirus pneumonia, MP: methylprednisolone, IL-6: interleukin-6, PII: pulmonary inflammation index, SOFA: sequential organ failure assessment score (range 0–24, with higher scores indicating more severe illness), CT: computed tomography, IVIG: intravenous immunoglobulin, SpO2: percutaneous oxygen saturation, TTCR: time to clinical recovery, FiO2: fraction of inspired oxygen. PaO2: partial pressure of oxygen, i.v.: intravenous, ICU: intensive care unit, CRP: C-reactive protein, ARDS: acute respiratory distress syndrome, HR: hazard ratio, HCQ: hydroxychloroquine, AZ: azithromycin, CQ: chloroquine, d: day(s), TCM: traditional Chinese medicine, CRRT: continuous renal replacement therapy, PK: pharmacokinetic, ACE2: angiotensin-converting enzyme 2, OR: odds ratio.
Table 2.
Characteristics of included publications related with modern therapy.
|
Author Country |
Aim of study |
N. cases |
Male/Female Age |
Treatment plan | Main Findings | Mortality |
|---|---|---|---|---|---|---|
| Xu China [17] |
The efficacy of TCZ in curing severe or critical COVID-19 cases | 21 severe or critical patients | 18/3 56.8 25-88 |
SOC, one (n = 18) or two (n = 3) doses of TCZ, i.v. 12 hours apart (4–8 mg/kg body weight) |
- Benefits: 1- The fever returned to near baseline the first day after giving TCZ, 75.0% had recovered oxygenation, and 90.5% patients had resolved CT scans. 2- On the fifth day after therapy, the value of peripheral lymphocytes and CRP returned to normal in 52.6% and 84.2% of patients, respectively, without reduction of IL-6 in the short time after infusion. - Outcome: All cases have been discharged on average 15.1 d after receiving TCZ. |
0/21 (0%) |
| Giamarellos-BourboulisGreece [19] |
Describe immune responses of 54 NCP patients, 28 of whom had severe respiratory failure (SRF) | 54 patients Non-SRF: 26 SRF: 28 |
40/14 Non-SRF: 59.2 ± 10.3 SRF: 67.8 ± 10.8 |
TCZ | - All patients with SRF revealed either MAS or immune dysregulation characterized by low expression of HLA-DR on CD14 monocytes, along with intense exhaustion of CD4 lymphocytes, CD19 lymphocytes, and NK cells. - IL-6 over-production by circulating monocytes led to the low HLA-DR expression on CD14 monocytes of COVID-19 patients. - Benefits: The use of the particular suppressor of the IL-6 cascade TCZ partly improved the expression of HLA-DR on monocytes of all cases with immune dysregulation, accompanied by elevation in circulating lymphocytes. |
NA |
| Luo China [58] |
Assess the therapeutic effects of TCZ in the COVID‐19 cases |
15 moderate-to- critical patients |
12/3 73 (62-80) |
TCZ at dose range from 80 mg to 600 mg per time (5 cases received TCZ twice or more), MP (8/15) |
- Benefits: In 11 moderately and seriously ill patients, CRP levels were reduced rapidly (P < .01) and returned near the baseline level during 1 week. - After therapy, IL‐6 level in 10 cases raised shortly in first and then reduced slightly. - Outcome: for the 4 critical patients who used only one dose of TCZ, 3 of them were dead and one patient experienced a worsening condition. |
3/15 (20%) |
| Guo China [59] |
The immune network with TCZ therapy at single cell resolution | 2 severe patients | - | TCZ | - Based on the single-cell transcriptomes profile of peripheral blood mononuclear cells isolated from two severe COVID-19 cases pre-treated with TCZ, a monocyte subpopulation was identified only in patients at severe stage connected by the inflammatory cytokines and their receptors. - Benefits: The hyper-inflammatory responses were rescued after TCZ therapy, yet immune cells, including plasma B cells and CD8+ T cells, still exhibited an intense humoral and cell-mediated anti-viral immune response in recovered COVID-19 cases. |
NA |
| Cellina Italy [60] |
Assessing of CT results in a COVID-19 patient, who received TCZ | one patient | 64-year-old man | SOC, 2 doses of TCZ (8 mg/kg), 12 hours apart, on day 7 and 8. | - Benefits: 1- One day after fusion, CRP, and white blood cell count dropped significantly. 2- One week later, patient clinical condition and chest CT progressively improved. | 0/1 (0%) |
| Giambenedetto Italy [61] |
Off-label administration of TCZ in NCP patients | 3 | 3 male 45-71 |
Anti-virals (lopinavir/ritonavir plus HCQ), two or three doses of TCZ, 12 hours apart | - Benefits: 1- Fever resolution and improvement in PaO2-to-Fio2 ratio within 3 days of therapy. 2- Clinical condition improved and CRP returned to near normal within 6 days’ post-therapy. | 0/3 (0%) |
| Sciascia Italy [62] |
Assess on off-label usage of TCZ in severe NCP patients |
63 hospitalized severe COVID-19 patients |
56/7 62.6±12.5 |
SOC, either TCZ i.v. (8 mg/ kg) or s.c. (324 mg); a second dose within 24 h in 52 out of 63 cases |
- Benefits: 1- Great recovery in the levels of ferritin, CRP and D-dimer post-treatment. 2- The ratio of PaO2 to FiO2 improved 7 days after therapy (p < 0.05). 2- TCZ use within 6 days from hospital admission was associated with an enhanced likelihood of survival (HR 2.2 95%CI 1.3-6.7, p < 0.05). 3- No cases reported severe to moderate side events directly associated with TCZ. - Outcome: Mortality at 14 days was 11%. There were no differences between the route of use in lethality, as rates were 12.9% and 10.3% in the TCZ i.v. and s.c. use, respectively |
7/63 (11%) |
| Klopfenstein France [63] |
A retrospective case-control study compared the outcome of cases cured with TCZ and patients without TCZ | 45 severely or critically ill patients | NA 18- >80 |
1- Patients without TCZ (n = 25): SOC 2- TCZ group (n = 20): SOC, TCZ (1 or 2 doses) |
- Benefits: 1- TCZ therapy, used on average 13 days after NCP symptom onset, decreased ICU admissions and/or mortality in patients than cases without TCZ (25% vs 72%, P = 0.002). 2- Cases without TCZ more often required invasive mechanical ventilation than the TCZ group (32% vs 0%, P = 0.006) with more lethality of patients without TCZ than in the TCZ group (48% vs 25%, P = 0.066) | 1- 48% 2- 25% |
| Roumier France [64] |
IL-6 blockade for severe COVID-19 | 30 severe patients | 24/6 50 |
SOC, TCZ (8 mg/kg, second dose in case of insufficient response to treatment) |
- Benefits: After a median follow-up of 8 days, IL-6 blockade could mitigate the cytokine storm, reduce ICU admission and ventilation compared with a control group of patients - Adverse events: TCZ was well-tolerated. Mild hepatic cytolysis (n = 2) and ventilator-acquired pneumonia (n = 1). |
3/30 (10%) |
| Alattar Qatar [65] |
Explore the clinical features and outcomes of severe NCP patients in ICU treated with the TCZ | 25 severe patients |
23/2 58 (50‐63) |
SOC, TCZ (a median of one TCZ dose (IQR, 1‐3) and a median total dose of 5.7 mg/kg (IQR, 4.8‐9.5), i.v. infusion over 60 minutes |
- Benefits: In patients with severe NCP, TCZ led to marked diminish in inflammation markers, radiological recovery and decreased ventilation requirements. - Adverse events: The majority (92%) of patients experienced at least one adverse event like anemia, ALT rise, and QT interval prolongation. - Outcome: By day 14, nine patients (36%) were discharged alive from ICU. Of the remaining 16, three (12%) patients died, and 13 (52%) were still in ICU. |
3/25 (12%) |
| Radbel New Jersey [66] |
Assess the efficacy of TCZ therapy for COVID-19-induced CRS | 2 patients complicated by CRS |
1/1 40 & 69 |
SOC, one or two dosing of TCZ (400 mg-700 mg i.v.) |
- Adverse events: Both patients deteriorated to sHLH despite TCZ therapy and one developed viral myocarditis. - Outcome: Both patients passed away. |
2/2 (100%) |
| Morrison USA [67] |
Evaluate the short-term side effects of TCZ including hypertriglyceridemia in COVID-19 cases | 2 severe patients with ARDS | 2 male 65 & 43 |
lopinavir/ritonavir, ribavirin, HCQ, propofol (discontinued before TCZ), TCZ (800 mg (8mg/kg), one or two doses) | - Adverse events: Both COVID-19 cases, treated with TCZ, progressed acute hypertriglyceridemia: one with enhanced biomarkers related to acute pancreatitis the other without. These cases highlighted several main monitoring parameters and pharmacotherapy. | NA |
| Antinori Italy [68] |
Assess the safety and efficacy of TCZ in severe COVID-19 patients | 43 severe patients |
NA | SOC, TCZ (maximum dose 800 mg/d) repeated within 12 h from the first use. |
- Adverse events: 3 patients (16,3%) showed candidaemia after the median time of 13 days from the last use of TCZ, indicating the possibility of the blood infection with the suppression of IL-6. | NA |
| Gritti Italy [69] |
Assess the effect of siltuximab in COVID-19 pneumonia requiring ventilation | 21 patients with ARDS | 18/3 64 (48-75) |
SOC, intravenous siltuximab at a median dose of 900 mg (700 to 1,200 mg) (a dose of 11 mg/kg/day) (five patients received a second dose) |
- Benefits: By day 5 following therapy, serum CRP levels returned to the normal range and remained stable in 16 patients with available data. - Outcome: 33% (7/21) of patients experienced an amelioration in the clinical condition, 43% (9/21) of patients stabilized, and 24% (5/21) deteriorated with one death. |
1/21 (5%) |
| Filocamo Italy [70] |
Evaluate the efficacy of anakinra in a critical COVID-19 case | A critical COVID-19 | 50 year-old man | SOC, anakinra (200mg, i.v., followed by 100 mg every 6 hours, s.c.) |
- Benefits: Within 72 h of therapy, a sharp decrease of inflammatory markers, liver enzymes and ferritin and an enhance in lymphocyte count were observed. Respiratory parameters recovered by day 13, followed by a desirable radiographic resolution. - Outcome: Case was discharged from the hospital by day 29 |
0/1 (0%) |
| Aouba France [71] |
Targeting the inflammatory pathways with anakinra in NCP patients | 9 severe patients | 8/1 46-84 |
SOC, anakinra s.c. at 100mg/12h from day (d) 1 to d3, then at 100mg/24h from d4 to d10. |
- Benefits: 1- By the third day, fever stopped in the remaining eight cases. 2- Desirable outcomes, including oxygen flow and inflammatory blood biomarkers, were recovered. CRP reduced slightly at d6 in all and normalized in 5/8 patients at d11. 3- In all cases, an early chest CT scan was controlled between d5 and d8. - Adverse events: 1- Among the nine cases, a patient revealed an acute respiratory failure 6 h after the first dose of anakinra, leading to withdrawal. 2- Anakinra was safe. However, mild elevation of transaminase and triglyceride was seen. |
0/9 (0%) |
| Cavalli Italy [72] |
Assess the clinical outcomes of NCP patients with ARDS, who administrated anakinra compared with a historical cohort without anakinra. | 52 moderate-to-severe patients with ARDS | 43/9 51-78 |
1- Historical control group (n = 16): SOC 2- High-dose anakinra-treated group (n = 29): SOC, anakinra (5 mg/kg twice daily, i.v., a median duration of 9 days) 3- Low-dose anakinra-treated group (n = 7): SOC, anakinra (100 mg twice a daily, s.c., infused over 1 h) |
- Low-dose anakinra was discontinued after 7 days because neither was associated with decreases in CRP nor with recovery in clinical conditions. - Benefits: Within 21 days, therapy with high-dose anakinra led to decreases in serum CRP and great amelioration in respiratory function of 72% patients (21/29); five (17%) patients required ventilation, and three (10%) died. In the control group, eight (50%) of 16 patients showed respiratory recovery at 21 days; one (6%) patient was on ventilation, and seven (44%) died. - Outcome: Within 21 days, survival was 90% in the high-dose anakinra group compared to 56% in the control group (p = 0·009). - Adverse events: Bacteraemia appeared in four (14%) of 29 cases given high-dose anakinra and two (13%) of 16 cases in the control group. |
1- 7/16 (44%) 2- 3/29 (10%) |
| Leng China [21] |
Explore the therapeutic potential of MSC transplantation for COVID-19 cases | 10 patients: including 1 critical type, 7 severe types and 2 common types |
4/6 18–95 |
1- placebo control group (n = 3): SOC, 2- MSCs-treated Group (n = 7): SOC, i.v. MSCs (in 100 ml of normal saline, and the total number of 1×106 cells/kg of weight infused in forty minutes) |
- Benefits: The pneumonic signs of seven cases were relieved in 2 days after therapy without obvious adverse events. 2- The peripheral lymphocytes, CRP, and the immune cells with secreting of hyperinflammatory cytokines, T cells and NK cells restored to normal range in 3-6 days, specifying that the inflammatory condition was tempered immediately.3- In severe cases, both the regulatory T cells and DC enhanced post-transplantation. 4- The amount of TNF-α was notably reduced, while IL-10 enhanced in MSCs-treated group in comparison with the control group (p < 0.05). 5- SPA and SPC were greatly expressed in MSCs, showing the possibility of MSCs differentiation to AT2 cells. - Outcome: Three cases were discharged and 4 recovered within 10 days after transplantation, whilst in placebo group, one died, one progressed ARDS and one recovered. |
1- 1/3 (33%) 2- 0/10 (0%) |
| Liang China [73] |
The clinical outcome of hUCMSCs therapy in a critical NCP patient | One critical patient | 65-year-old female | SOC, glucocorticoid, thymosin α1, three i.v. infusions of 5 × 107 (each time) hUCMSC |
- Benefits: 1- After the second infusion, the serum CRP, bilirubin and ALT/AST were slightly decreased and the count of white blood cell, neutrophil and the lymphocyte returned to normal. 2- The levels of CD3+ T cell, CD4+ T cell, and CD8+ T cell were raised back normal. 3- Chest CT images revealed the great rescue of the abnormalities. - Outcome: After 2 days of the last infusion, the case was discharged out of ICU, and most of the vital indications improved to normal range with no adverse effects |
0/1 (0%) |
| Shu China [74] |
Determine the efficiency and safety of hUCMSC infusion in the therapy of severe NCP |
41 | 24/17 58.78±16.26 |
1- Control group (n = 29): SOC 2- MSCs-treated group (n = 12): SOC, i.v. hUC-MSCs in 100 ml of normal saline with the total number of 2×106 cells/kg of weight infused in about 1 h |
- Benefits: 1- The duration to clinical recovery in control group was slower than that in MSCs group (median, 14.0 days vs. 9.0 days, P = 0.006). 2- CRP (p < 0.001) and IL-6 (p < 0.010) were notably reduced from day 3 of therapy than in control group with faster normalization of lymphocyte count in MSCs group. - Outcome: In MSCs group, all patients improved and discharged with 28-day mortality of 0% than 10.34% in control group without no adverse reactions. |
1- 3/29 (10.3%) 2- 0/12 (0%) |
| Zhang China [75] |
The therapeutic efficacy of hWJCs on NCP patients | A severe patient |
a 54-year-old man | SOC, dexamethasone 2 mg, the adoptive transfer therapy of hWJCs in 100 mL of normal saline with the total number of 1 × 106 cells/kg of weight infused in 40 min |
- Benefits: The pulmonary signs of the patient were notably recovered in 2 days following transplantation and the case discharged in 7 days after therapy. 2- After treatment, the levels of lymphocyte subsets (CD3+, CD4+, and CD8+ T cell) were enhanced, and the level of IL-6, TNF-α, and CRP was greatly reduced. 3- The intravenous transplantation of hWJCs was safe. | 0/1 (0%) |
SOC: standard of care (antibiotics, anti-virals, oxygen therapy, non-invasive or invasive ventilation, ECMO and supportive care according to severity of disease), NCP: novel coronavirus pneumonia, MP: methylprednisolone, IL-6: interleukin-6, TCZ: tocilizumab, HCQ: hydroxychloroquine, CT: computed tomography, FiO2: fraction of inspired oxygen. PaO2: partial pressure of oxygen, i.v.: intravenous, ICU: intensive care unit, CRP: C-reactive protein, ARDS: acute respiratory distress syndrome, HR: hazard ratio, d: day(s), PK: pharmacokinetic, OR: odds ratio, SRF: severe respiratory failure, MAS: macrophage activation syndrome, HLA-DR: human leukocyte antigen D related, NK: natural killer, TNF-a: Tumor necrosis factor-a, hUCMSC: allogeneic human umbilical cord MSC, CRS: cytokine release syndrome, sHLH: secondary hemophagocytic lymphohistiocytosis, MSCs: Mesenchymal stem cells, ALT: alanine aminotransferase, AST: aspartate aminotransferase, AT2: alveolar type II, hWJCs: human umbilical cord Wharton’s jelly-derived MSCs, s.c.: subcutaneous, IQR: interquartile range, DC: Dendritic cell.
Table 3.
Characteristics of included publications related with passive immunotherapy and blood-purification therapy.
|
Author Country |
Aim of study |
N. cases |
Male/Female Age |
Treatment plan | Main Findings | Mortality |
|---|---|---|---|---|---|---|
| Zhang China [76] |
Explore clinical impacts of CP in one COVID-19 case | One critical patient | a 64-year-old female | 200 mL of CP with high IgG titers (≥1:320) | - Benefits: 1- The case did not require ventilation within 11 days following transfusion and achieved desired outcomes. 2- There was no transfusion-related adverse reaction. | 0/1 (0%) |
| Duan China [77] |
Evaluate the possibility of CP therapy in severe NCP cases | 10 severe patients | 6/4 52.5 (34-78) |
1- Historic control group (N = 10): SOC 2- CP-treated group (N = 10): SOC, one dose of 200 mL of inactivated CP (neutralization activity of >1:640 infused in 4 h), i.v. MP (6/10, 20 mg every 24 h) |
- Benefits: 1- The clinical parameters were significantly improved, including increased lymphocyte counts and oxyhemoglobin saturation and decreased CRP within 3 d of therapy. 2- CP could notably enhance or maintain the neutralizing antibodies at a high level, causing elimination of viral loads in 7 d. 3- Radiological findings displayed various degrees of recovery of lung injuries during 7 d. 4- No intense side effects were reported. - Outcome: Three cases discharged and seven cases ready for discharge in the CP-treated group, as compared to three deaths, six patients in stabilized or recovery conditions in the control group (P < 0.001). |
1- 3/10 (30%) 2- 0/10 (0%) |
| Shen China [78] |
Assess whether the use of CP transfusion is favorable in the cure of critical COVID-19 patients |
5 critically ill patients | 3/2 36-73 |
SOC, 2 infusions of 200-250 mL of CP with an IgG binding titer higher than 1:1000 and a neutralizing antibody titer higher than 40 (10 to 22 days after admission), MP |
- Benefits: 1- Viremia reduced and turned to negative during 12 days. 2- Following therapy, fever normalized during 3 days in most cases, the SOFA score reduced, ARDS resolved, and PAO2/FIO2 improved during 12 days. 3- The inflammation markers CRP, procalcitonin, and IL-6 decreased post-transfusion. 4- Neutralizing antibody titers enhanced following the therapy (range, 40-60 before and 80-320 on day 7) - Outcome: Of the 5 cases, 3 discharged (length of stay: 53, 51, and 55 days), and 2 were stabilized within 37 days following transfusion |
0/5 (0%) |
| Zhang China [79] |
Treatment using CP for critical patients infected with SARS-CoV-2 | 4 critical patients |
2/2 31-73 |
SOC, CP (200-2400 ml depending on severity of disease), continuous renal replacement therapy (CRRT) (2/4) |
- Benefits: 1- The virus load significantly decreased and pO2 increased a short time after transfusion. 2- Chest images showed obvious absorption of interstitial pneumonia. 3- No side effects were reported. - Outcome: All the four cases recovered and 3 of them were discharged day 7-30 after infusion |
0/4 (0%) |
| Ye China [80] |
Evaluate the efficacy of CP therapy in COVID-19 patients | 6 | 3/3 28-75 |
SOC, one to three cycles of CP intervention (200ml for each cycle) |
- Benefits: 1- This therapy had notable importance for clearing SARS-CoV-2, recovering patient’s manifestations and relieving radiologic abnormalities. 2- No adverse effects were reported during and in the next 3 days of transfusion. - Outcome: Patients was discharged or ready to discharge. |
0/6 (0%) |
| Young Ahn Korea [81] |
Evaluate the CP transfusion in COVID-19 cases with ARDS | two severe patients | 1/1 71 and 67 |
SOC, MP (0.5-1 mg/kg/day per day), 500 mL of CP (divided into two doses and used at 12 h apart infused in 1 hour) | - Benefits: 1- Leukocytosis, lymphopenia, and the patient's status were immediately recovered with reduction of IL-6 and CRP to baseline range since day 1 after transfusion 2- Improving of both lung abnormalities during 4 days. 3- There were no adverse effects during and following the therapy. 4- Viremia began to reduce right following the infusion of CP | 0/2 (0%) |
| Cao China [82] |
Therapy with high-dose IVIG at the time of initiation of respiratory distress in COVID-19 cases | 3 Severe patients | 2/1 34-56 |
SOC, high-dose IVIG (started hospital day 7) at 25 g/d for 5 days, MP 40 mg/d for 3 days (1/3) |
- Benefits: All cases were clinically improved quickly following therapy, with their fever turn to normal within 1–2 days and breathing hardness’s alleviation in 3–5 days with normalization of lymphocyte count and CRP levels. 2- There was no adverse reaction - Outcome: all patients discharged in 5 days after the therapy |
0/3 (0%) |
| Mohtadi Iran [83] |
the effects of IVIG use in severely ill COVID-19 cases | Five severe patients | 1/4 50-66 |
SOC, high-dose IVIG (0.3-0.5 g/kg) for 5 consecutive days |
- Benefits: IVIG led to the recovery of the clinical status, O2 saturation and pulmonary lesions. - Outcome: All cases showed a favorable curative response and were discharged from the hospital. |
0/5 (0%) |
| Xie China [84] |
Evaluate the efficacy of IVIG use before (≤48 h group) and after (>48 h group) 48 h of ICU admission in the cure of severe COVID-19 patients. | 58 severe or critical patients | 36/22 63 (29-86) |
SOC, heparin, Thymosin, low dosage of glucocorticoids (1-2 mg/kg) for 5-7 days depending on their condition, IVIG (all cases cured before (≤48 h group) and after (>48 h group) 48 h of ICU admission) |
- Benefits: 1- Beginning of IVIG for pneumonia during 48 h of admission to the ICU decreased the usage of ventilation (p = 0.016), diminished the hospital length of stay (p = 0.0055), and promoted the early recovery of patients. - The increasing dosage of IVIG use over 28 days was notably more in the >48 h group (88.57± 71.14 vs 64.35± 54.74 g, p = 0.006) than ≤48 h group. - Outcome: Within 28 days of admission of the 58 cases, a total of 23 died, 7 in the group used IVIG during 48 h of admission and 16 in the group use of IVIG after 48 h (p = 0.009). |
1- ≤48 h group: 7/58 (12%) 2- >48 h group: 16/58 (27.5%) |
| Shao China [85] |
Effect of IVAG use in curing of critical NCP patients | 325 patients: 222 severe type and 103 critical type |
189/136 58.0 (46-69) |
1- Non-IVIG group (n = 151) 2- IVIG-treated group (n = 174) |
- Outcome: 28-day and 60-day mortality were partly similar between the IVIG group and non-IVIG group (P = 0.872 and P = 0.222, respectively). - Adverse events: Total duration of illness and hospital stay were shorter in non-IVIG group (P < 0.001). - Benefits: 1- Subgroup analysis revealed that only in cases with critical type, IVIG could decrease the 28-day mortality (P = 0.009), reduce the inflammation activity, and mitigate some organ malfunctions (all P < 0.05). 2- Use of IVIG in the early step (admission≤7 days, P = 0.008) with a high dose (>15 g/d, P < 0.0001) showed great diminish of 60-day mortality in the critical type cases. 3-Although IVIG did not show a therapeutic effect on the whole cohort; it can be beneficial to the critical type patients. |
28-day mortality: 42/325 (13%) 60-day mortality: 54/325 (17%) |
| Ma China [23] |
the impact of blood-purification therapy in decreasing cytokine storm of critical NCP patients |
three critical patients | 3 male 56-69 |
SOC, IVIG, MP, blood-purification therapy ((PE) or (CRRT)) using hemoflter |
- Benefits: 1- The inflammation biomarkers were drastically reduced during two days of therapy. 2- patients were favorably discontinued ventilation and achieved stability post-therapy. - Adverse events: one patient had a sudden death. |
1/3 (33%) |
| Shi China [86] |
Therapy of a critical COVID-19 patient using PE followed by IVIG | One critical patient | a 50-year-old woman | SOC, GSF, corticosteroid (i.v. 80 mg and halved to 40mg two days later), four consecutive sessions of PE with 6000 ml plasma followed by IVIG (20g) |
- Benefits: 1- The patient's signs were almost all alleviated, following three sessions of PE therapy. 2- Timely initiating resulted in an avoided ventilator and earned a quick clinical recovery. 3- Oxygenation index increased, the blood pressure restored, and level of enzymes and creatinine recovered without significant adverse reactions. - Outcome: Patient was discharged after 6 days of last use of PE- IVIG. |
0/1 (0%) |
| Yang China [87] |
Assess the effect of CRRT on mortality in cases with COVID-19 undergoing mechanical ventilation |
36 hospitalized patients | 30/6 69.4 (44-86) |
1- Non-CRRT group (n = 14): SOC 2- CRRT group (n = 22): SOC, CRRT (with an ultrafiltration rate of 25-75 mL/kg/hr) |
- Benefits: A higher survival in patients in CRRT group than non-CRRT group (P = 0.032) - Within the average follow-up duration of 10.4 days, the lethality was 54.5% and 78.6% in CRRT group and non-CRRT, respectively. - Outcome: CRRT was independently associated with a lower risk of mortality in COVID-19 patients requiring invasive ventilator (HR 0.283 to 0.424). |
1- 11/14 (78.6%) 2- 12/22 (54.5%) |
| Yang China [88] |
Highlight the clinical course and outcomes of critical NCP patients | 52 critical patients that a total of 9 cases used CRRT | 35/17 59·7 |
SOC, corticosteroid, Immunoglobulin, CRRT (9/52) |
- Outcome: 32 (61·5%) patients had died at 28 days. - Older patients (>65 years) with comorbidities and ARDS are at elevated risk of lethality. - Adverse events: Non-survivors (8/32) used CRRT more than survivors (1/20). |
32/52 |
COVID-19: coronavirus disease 2019, SARS-CoV-2: severe acute respiratory syndrome coronavirus 2, SOC: standard of care (antibiotics, anti-virals, oxygen therapy, non-invasive or invasive ventilation, ECMO and supportive care according to severity of disease), NCP: novel coronavirus pneumonia, CP: convalescent plasma, MP: methylprednisolone, IL-6: interleukin-6, SOFA: sequential organ failure assessment score (range 0–24, with higher scores indicating more severe illness), CT: computed tomography, IVIG: intravenous immunoglobulin, SpO2: percutaneous oxygen saturation, FiO2: fraction of inspired oxygen. PaO2: partial pressure of oxygen, i.v.: intravenous, ICU: intensive care unit, CRP: C-reactive protein, ARDS: acute respiratory distress syndrome, HR: hazard ratio, HCQ: hydroxychloroquine, AZ: azithromycin, CQ: chloroquine, d: day(s), TCM: traditional Chinese medicine, CRRT: continuous renal replacement therapy, PK: pharmacokinetic, ACE2: angiotensin-converting enzyme 2, OR: odds ratio, PE: plasma exchange, CRRT: continuous renal replacement therapy, GSF: human granulocyte stimulating factor, IgG: Immunoglobulin G.
3.2. Findings of the included publications
As described in Table 1, Table 2, Table 3, in 66 relevant publications included in this review, several immunomodulatory drugs, including corticosteroids, hydroxychloroquine or chloroquine, cytokine-targeted therapies (tocilizumab, anakinra, or siltuximab), passive immunotherapy (convalescent plasma and intravenous immunoglobulin), mesenchymal stem cells, and blood-purification therapy were applied for management of the COVID-19 patients, particularly in the severely ill patients. Geographically, most studies (n = 40) were conducted in China, while 26 publications were carried out in France (n = 9), Italy (n = 7), USA (n = 4), New Jersey (n = 1), Brazil (n = 1), Iran (n = 1), Qatar (n = 1), Greece (n = 1), and Korea (n = 1). The number of male patients was more than the female ones in the majority of studies.
In fact, these medicines were developed to regulate the immune system, either by boosting the innate immunity against infection or by suppressing the hyper-inflammatory responses. Majority of these medicines were initially formulated for other pathogens or diseases and were immediately used off-label for management of the current pandemic. Table 1, Table 2, Table 3 show an overview of the included publications investigating the efficacy of immunomodulatory therapies in the patients with COVID-19, and results of the various tested drugs are further explained below in detail.
3.2.1. Conventional therapy
3.2.1.1. Corticosteroids
Corticosteroids, as a class of steroid hormones, are the conventional therapeutics broadly used to treat the inflammatory conditions. Their anti-inflammatory potential has led to their extensive use, particularly methylprednisolone (MP), in China for treatment of NCP patients, not only to terminate the manifestations of fever and dyspnea but also to alleviate the inflammation associated with the cytokine storm. Notably, the recommended dosage for the treatment of COVID-19 patients with systematic corticosteroid is <1–2 mg/kg/d for 3–5 days [29].
In a retrospective study, a risk-based therapeutic strategy was developed according to the disease severity in the NCP patients, in which severely ill patients received corticosteroid immediately within the first 3 days of hospitalization in addition to the standard treatment. This treatment approach provided considerable mitigation in the clinical manifestations and imaging recovery with the mortality rate of 0% [29], and the reduced mortality rate was also reported in the study by Wu et al. [30]. Another study reported that the early use of corticosteroid in the first 3–5 days of intensive care unit (ICU) admission could appropriately hinder the intense inflammatory storm and improve the oxygen saturation (p = 0.012) [31]. Nevertheless, corticosteroids did not provide survival profit [31], [32]. Even in a case-control analysis, where the cases received the adjuvant corticosteroid (100–800 mg/d) in addition to the standard treatment, elevated corticosteroid dosage led to a considerable increase in the mortality rate (P = 0.003) in the matched cases [33]. Prolonged viral shedding has been reported as one of the main restrictions of corticosteroid therapy. A retrospective cohort study introduced corticosteroid as one of the influential factors for persistent SARS-CoV-2 RNA shedding (p = 0.025) [34]. In contrast, other retrospective studies have shown that early and short-term administration of the low-dose corticosteroid did not prolong the viral clearance in the COVID-19 patients [29], [35]. Other publications also demonstrated that corticosteroid-containing therapy is accompanied by either hopeful or disappointing results (Table 1) [9], [20], [36], [37], [38], [39], [40], [41], [42], [43].
3.2.1.2. Chloroquine (CQ) phosphate and hydroxychloroquine (HCQ) sulfate
CQ and its derivative HCQ, the food and drug administration (FDA)-approved antimalarial and autoimmune disease drugs, have been introduced as the potential broad-spectrum anti-viral chemicals. Both drugs function via modifying the endosomal pH and ACE2 glycosylation. Additionally, these drugs possess an immune-modulatory capacity, which may synergistically reinforce their anti-viral activity in vivo. The safety profile of HCQ has made it a preferred drug for therapy in the clinics [44], [45], [46].
A single-arm non-randomized clinical trial, aimed at evaluating the efficiency of HCQ (200 mg, 3 times daily for 10 days) on 20 SARS-CoV-2-infected patients in comparison with 16 infected control patients, showed that the HCQ administration leads to a considerable decrease in the viral load in the NCP patients within only 3–6 days compared to the controls (p = 0.001) [44], and its effect was augmented by adding azithromycin (AZ) [44], [45], [46]. Other studies have also demonstrated the immediate recovery of the patients through the use of HCQ [47] and CQ [48], [49]. In contrast, some studies have not supported the administration of HCQ for prevention of ICU admission and/or death when used alone [50], [51] or combined with AZ [52], or due to the life-threatening adverse reactions [53], [54]. Even a recent retrospective cohort study found the elevated overall mortality after administration of HCQ alone without AZ [55], as observed in a randomized, phase IIb clinical trial with a higher dosage of CQ (600 mg CQ twice per day during 10 days) [56]. Currently, there are no guidelines for the administration of HCQ. Most studies have applied various doses of HCQ, without an optimized HCQ dosing regimen required for effective treatment and reduction of the side effects. In a study, the pharmacokinetic (PK) characteristics of HCQ at the dose of 600 mg orally were evaluated in the ICU-hospitalized COVID-19 cases and the results showed that this dosing schedule was inadequate to achieve an assumed target blood amount of 1–2 mg/L considered to be therapeutic (Table 1) [57].
3.2.2. Modern therapy
3.2.2.1. Cytokine-targeted therapy
SARS-COV-2-infected patients mostly display the elevated levels of multiple inflammatory ILs, such as IL-6, IL-2, and TNFα, and cytokine antagonists have been suggested for treatment of this pandemic. Tocilizumab (TCZ), an FDA-approved recombinant humanized IL-6 receptor (IL-6R) antagonist, is being applied as an anti-inflammatory therapy for rheumatoid arthritis and, more recently, CRS triggered by the chimeric antigen receptor T cell (CAR-T) immunotherapy. Italian guidelines have also supported the use of TCZ for treatment of COVID-19 (at the dose of 8 mg/kg, with a second dose 12 h after the first one) [17], [19], [58], [59]. A retrospective study on 15 NCP cases reported the effectiveness of TCZ (80–600 mg per time) in the restriction of cytokine storms associated with COVID-19, as majority of the patients achieved a stable condition mirrored by an immediate decline in the CRP levels (P < 0.01) post-TCZ administration [58]. Xu et al., in a study on 21 severe cases of COVID-19, who had been given standard treatment for a week before TCZ administration (4–8 mg/kg body weight) but their condition had been worsened, showed that administration of TCZ led to the discharge of all the patients after, on average, 15.1 days of therapy [17]. Positive findings have also been reported by other recent studies conducted in Italy, France, and Qatar (Table 2) [60], [61], [62], [63], [64], [65]. However, a recent case report study announced the weak outcomes of two cases with COVID-19 after they were given one or two infusions of TCZ [66]. Two Other studies indicated an elevation in the adverse effects, including the acute hypertriglyceridemia [67] and candidaemia [68].
The second IL-6 antagonist, namely siltuximab, approved for treatment of the Castleman’s disease is a chimeric monoclonal antibody (mAb) directly binding to the IL-6 and blocks the IL-6 pathway. In a recent study on 21 COVID-19 patients developed the ARDS, siltuximab (at the mean dose of 900 mg) administration led to the improved clinical conditions in 7 cases and stabilized state in 9 patients; however, 24% (5/21) of them suffered a deteriorating status [69].
Anakinra, as a recombinant IL-1 receptor (IL-1R) blocker and FDA-approved rheumatoid arthritis drug, is another proposed antagonist for management of the patients with COVID-19 (Table 2) [70], [71]. A recent cohort study illustrated that the use of high-dose anakinra (5 mg/kg twice per day, with a mean duration of 9 days), in 29 NCP patients who had developed the ARDS, provided the survival benefit (90%) compared to the historic-control group (56%) (p = 0·009), 21 days after receiving anakinra [72].
3.2.2.2. Mesenchymal stem cells (MSCs)
MSCs, as the powerful immunomodulatory modalities, have been broadly applied to manage the autoimmune diseases, type 2 diabetes, and, more recently, infectious diseases. MSCs act in two ways principally, immunomodulatory functions and tissue repair abilities, making them ideal candidates to treat the diseases related to lung injury [21], [73]. In a case report on a critical NCP case who had advanced liver injury despite receiving the intensive care treatment, the patient received three intravenous infusions of 5 × 107 human umbilical cord MSC (hUCMSC). During 4 days of the second MSC infusion, the case was weaned from the ventilation. All the assessed markers, including CRP, alanine aminotransferase (ALT)/ aspartate aminotransferase (AST), and circulating T cell counts, returned back to the baseline ranges. No distinct adverse effects were monitored [73]. Results of a placebo-controlled trial showed that transplantation of MSCs led to the improvement of lung function and inflammatory indications, reduction of the pro-inflammatory cytokine TNF-α (P < 0.05), and augmentation of anti-inflammatory factor IL-10 (P < 0.05) within a few days compared to the standard of care (SOC)-treated group [21]. In a controlled cohort study, 41 severe NCP patients were randomly divided into 2 groups, SOC-treated group (n = 29) and SOC-hUCMSCs-treated group (n = 12). Within the 3rd to 7th day of therapy, the infusion of hUCMSCs noticeably accelerated the improvement of the clinical symptoms, oxygenation, and lung inflammation absorption, along with a significant reduction in the level of CRP and IL-6. The 28-day mortality was equal to 0% in the SOC-hUCMSCs-treated group, while it was equal to 10.34% for the SOC-treated group [74]. A case report study also described the therapeutic efficacy of the human umbilical cord Wharton’s jelly-derived MSCs (hWJCs) on an NCP patient (Table 2) [75]
3.2.3. Passive immunotherapy
3.2.3.1. Convalescent plasma (CP) therapy
CP therapy, as a classic passive immunotherapy, has been exerted to prohibit and cure several diseases caused by the infections for over one century. The people recovered from a viral disease with a high level of neutralizing antibodies are the useful donor candidates of CP. The China’s national health commission (NHC) recommended the immunoglobulin G (IgG) titers of 1:160 for the COVID-19 patients [76]. In a prospective study, 10 severe COVID-19 cases received a single dosage of 200 mL CP with the neutralizing antibody titers of >1:640 pooled from the recently recovered patients in addition to the SOC. After CP transfusion, the clinical conditions were remarkably ameliorated besides the enhancement of oxyhemoglobin saturation within 3 days, indicating the recovery of lung function. CP immunotherapy was well tolerated and led to neutralizing and disappearance of the viremia in the severe NCP patients within 7 days with a mortality of 0% (0/10) [77]. This intervention has been shown to have distinctive importance for recovering the clinical manifestations, neutralizing the SARS-CoV-2, and mitigating the lung abnormalities in other studies on the severely and critically ill patients who had developed the ARDS or/and multiple organ failure (Table 3) [78], [79], [80], [81].
3.2.3.2. Intravenous immunoglobulin (IVIG) therapy
IVIG, as another passive immunomodulatory therapy, is a highly purified product comprising the polyclonal IgG obtained from the blood of healthy individuals. It binds to and neutralizes the components of the immune response and is clinically used for treatment of the autoimmune diseases. The recommended dosage of IVIG is 0.5 g/kg daily for 5 days [82], [83]. A retrospective study illustrated the importance of timely administration of IVIG for the effective treatment of the severe COVID-19 patients. Treatment with IVIG during 48 h of admission could mitigate the application of mechanical ventilation (p = 0.016), diminish the hospital and ICU length of stay (p = 0.0055), and ultimately ameliorate the 28-day mortality (p = 0.009) compared to administration of IVIG after 48 h of admission [84]. A recent multicenter cohort study showed that early administration (admission ≤ 7 days) of high-dose IVIG (>15 g/d) improved the outcomes of only critical cases with COVID-19 but not severe ones [85]. In several other studies on the severely or critically ill COVID-19 patients, although IVIG was used, it was impossible to determine the efficacy of IVIG administration on the recovery from COVID-19 (Table 1, Table 3) [40], [41], [86].
3.2.4. Blood-purification therapy
Blood-purification treatments can remove the inflammatory factors and clear the pathogenic cytokines achieved using the modalities, including plasma exchange (PE), adsorption, perfusion, blood/plasma filtration, etc. A case series study reported the effectiveness of the blood purification therapy in 3 critically COVID-19 patients transferred to the ICU, who had deteriorated states despite receiving different kinds of treatments. Blood-purification therapy led to the normalization of the inflammatory markers. Eventually, 2 patients were successfully come off the ventilator and reached a stable clinical status [23]. Another case report study described that a critically COVID-19 case, who had failed to respond to the conventional interventions, was recovered promptly after treatment using the intensive PE followed by intravenous IVIG [86]. A retrospective cohort study, in which 22 patients received the continuous renal replacement therapy (CRRT) and 14 cases were given the conventional therapy (non-CRRT group), indicated that the CRRT independently led to a lower rate of mortality in the NCP cases requiring invasive mechanical ventilation [87], unlike the results reported by Yang et al., (Table 3) [88].
3.3. Findings of the registered clinical trials
Totally, 111 registered trials were included in the review that had investigated the therapeutic effects and safety of the immunomodulators, including corticosteroid, MSC, CQ or HCQ, anti-cytokine therapies, passive immunotherapy, and blood purification therapy. The most studied immunomodulators were CP (n = 44), CQ or HCQ (n = 26), MSC (n = 21), and anti-cytokine antagonists (n = 19). They were used alone or in combination with anti-virals or standard of care to treat the SARC-COV-2-infected patients. Most of the trials were short‐term clinical trials. Most of them were ongoing in phase 2, some in phase 3, and rarely in phase 4. The outcomes of trials mainly focused on (1) assessment of the efficacy and safety, (2) as well as investigating the pneumonia improvement, (3) immunological characteristics, (4) negative conversion rate of viremia nucleic acid test, and (5) mortality rate. The ongoing clinical trials that may lead the clinicians, decision-makers and novel investigations in opting the effective drug regime to combat the novel pathogen were all reviewed and summarized (Supporting Information 2).
However, it is worth noting that no clinical trials have been completed, and the results of trials have not been posted yet in the clinical registries. The study findings are intently awaited because they may present valuable data on the application of immunomodulators to treat the patients with mild-to-severe COVID-19. Nevertheless, the results of these trials are likely to be of limited help in the current epidemic with more importance for management and therapy in the future.
4. Discussion
In this systematic review, an overview was presented regarding the medicines having an already known or putative role in the modulation of immunity applied for management of the patients infected with SARS-COV-2. Since the outbreak of COVID-19, there has been a comprehensive attempt to identify an effective clinical treatment scheme for control of this disease. Currently, there is no FDA‐approved therapy for this pneumonia. It seems that drug repurposing demonstrated as a suitable drug discovery approach from available drugs, could partly control the disease until an effective vaccine is identified [89].
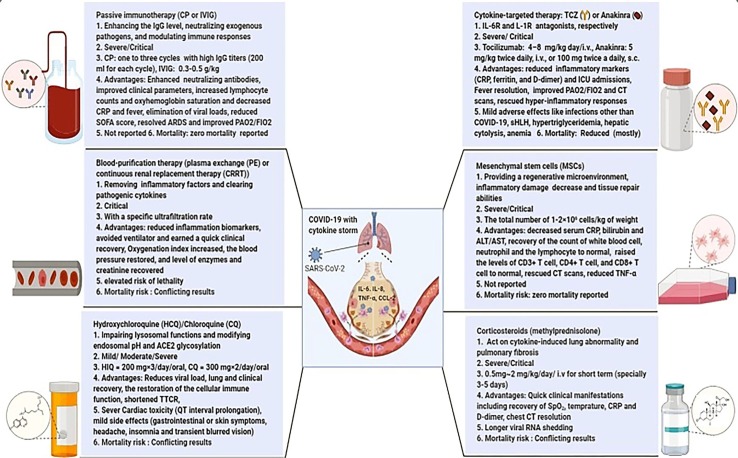
The studies met the inclusion criteria for entering this systematic review had identified several medicines linked to the immunomodulation, i.e., corticosteroid, HCQ or CQ, anti-cytokine therapies, CP, IVIG, MSC, and blood purification therapy. Among these, cytokine antagonists, CP, and MSCs were the immunomodulators that showed more hopeful results. Available clinical experience regarding the immunomodulators for COVID-19 relies on the case reports, case series, cohorts, and case-control studies. These studies are highly heterogeneous in nature; thus, a common conclusion could not be acquired to make strong recommendations regarding application of these medicines in the routine clinical practice. It is essential to underline that although adverse events are mostly regarded as tolerable, long-term follow up remains a serious concern making it challenging to interpret the safety profiles of the drugs. The results indicated the considerable importance of the timing, duration, and dosage of the medicines for effective therapy. Fig. 2 shows a summary of the immunomodulatory therapies discussed in this review.
Fig. 2.
Summary of available therapeutic options to modulate COVID-19 Immunopathology. 1- Mechanism of action, 2- Target population, 3- Main therapeutic regimen, 4- Advantages of immunomodulatory therapies, 5- Main reported safety, 6- Mortality risk (Figure created with BioRender.com).
Amidst cytokine storm mediators, IL-6 was the cytokine highly associated with the progression of ARDS and death in the NCP patients. Recently, it has been reported that the IL-6 over-production by the circulating monocytes leads to the low human leukocyte antigen D related (HLA-DR) expression on the CD14 monocytes in the COVID-19 patients with SRF [19]. The use of a particular suppressor of the IL-6 cascade seems to be significantly functional in the treatment of NCP patients. TCZ binds to the IL-6R with high affinity [63], while siltuximab has specificity for IL-6, and blocks the signal transductions [69]. Included clinical studies with 1–63 participants have shown that both antagonists, specially TCZ, are effective in reducing the mortality rate specially in the severely ill patients, improving the symptoms including fever resolution, oxygenation and resolved CT scans, reducing the inflammation markers (ferritin, CRP, and D-dimer), weaning from the ICU hospitalization and ventilation, and dampening the risk of disease progression to ARDS by mitigating the cytokine storm in the NCP patients [60], [62], as applied for CRS controlling in the CAR-T therapy [90]. The recommended dose in most studies was 4–8 mg/kg for TCZ like rheumatoid arthritis, and a dosage of 11 mg/kg/day was reported for siltuximab with the infusion time of more than 1 h [58], [69]. As an IL-1R blocker, anakinra was another proposed antagonist. High dosing of 5 mg/kg twice daily (intravenous) was applied for treatment of the NCP patients resulting in the survival benefit, amelioration of respiratory function, and decreased inflammatory markers, as applied off-label for management of the hyperinflammatory disorders [70], [72]. However, an enhanced risk of opportunistic infections, acute hypertriglyceridemia, and anemia induced by the IL antagonists have been demonstrated among the patients with COVID-19 [68]. Therefore, these antagonists must be prescribed for COVID-19 patients in severe phase, with precise monitoring [12]. The short half-life of anakinra (3 h) provides an immediate withdrawal and clearance from the circulation compared to TCZ with a long half-life (2–3 weeks) [72]. Although these immunological therapies appear to be beneficial to cure NCP cases, safety hazards and substantial costs can limit the wide use of these cytokine antagonists [12].
Improved inflammation situation following receiving the MSC-based therapy was also reported in some limited studies involving 1–41 patients with mild-to-severe COVID-19, providing lower mortality and significant recovery of the pulmonary signs. The factors considered to be vital for effective treatment include the route, timing, dose, volume, source, and duration of the MSC administration [91]. The treatment protocol used in the included publications was transplantation of hUCMSCs, intravenously, with the total number of approximately 1–2 × 106 cells/kg of weight, and the infusions lasted about 40 min to 1 h [21], [73], [74], [75]. MSCs are likely to enter the human body; some of them accumulate in the lungs, leading to improvement in the lung microenvironment and prevention of the pulmonary fibrosis, besides reducing the serum amounts of pro-inflammatory cytokines [73]. Similarly, a study described the improved mortality rate in the cases infected with Influenza A who had developed the ARDS using the menstrual blood-derived MSCs therapy without adverse effects in 5 patients followed-up for 5 years [92]. However, application of MSCs is associated with some limitations, including the diversity in the quality of the MSCs from various donors, transplantation of MSCs into unfavorable environments, or even undergoing the malignant transformation [91], [92]. For overcoming these barriers, an accurate assessment of the safety is needed along with a complete consideration regarding the immunosuppression of MSCs.
Strategies to boost the immune responses, including CP therapy and IVIG are other emergent treatments for serious COVID-19 cases and have been successfully applied for treatment of MERS, SARS, and 2009 H1N1 pandemic with desirable efficacy and safety [81], [93], [94]. Both products, specially CP, can quickly enhance the neutralizing antibodies (Nabs), IgM and IgG levels in the serum, probably neutralizing the SARS-COV-2 and limiting the viral entry and amplification. These interventions also regulate the overactive immune system likely via Fab-mediated and Fc-mediated cascades [85], [95], [96]. While high-dose IVIG (0.3–0.5 g/kg.d), purified from the healthy donors, has been proposed as an adjuvant therapy for severe NCP patients, it is not the particular antibody to any pathogen [95]. There are limited clinical data for its efficacy, and investigators have reported conflicting opinions on IVIG usage [85]. In contrast, recently, the FDA has approved the administration of plasma from the recovered donors to cure seriously ill NCP patients [97]. The key factors associated with the CP therapy are the amounts of neutralizing antibody titer, timely infusion, and suitable plasma volume. In the majority of the included studies, a dosage of 200-mL intravenous CP for each cycle (200–2400 mL depending on the severity of disease) with a high level of neutralizing antibodies could reduce the viremia and the risk of death, and tended to improve the clinical signs and shorten the course of disease without any adverse reactions [77], [93]. A recent meta-analysis study showed that CP therapy is effective for reducing the mortality rate in different types of infectious diseases [98]. Nevertheless, the outcomes of CP therapy are unforeseeable because of the diversity of sera in different donors, its limited supply, and transmission of the potential pathogens [85], [99].
Other pharmacological agents widely used for management of the NCP infection are CQ or its derivative HCQ, and corticosteroids. In the majority of investigations, HCQ had been used orally at the dose of 200 mg, 3 times daily for 10 days alone or in combination with AZ, while the schedules for CQ administration were a bit diverse in different studies, providing quick clearance of viral load, clinical recovery, shortened time to clinical recovery (TTCR), improved pneumonia, and restoration of the immune function [12], [29], [32], [44], [48]. The former unsatisfied experiences on SARS with high-dose corticosteroids have led to the use of low-dose (MP, intravenous, <1–2 mg/kg/d) and short-term (3–5 days) systemic glucocorticoids in the majority of studies, associated with recovery of body temperature, increase of oxygen saturation, reduction of inflammatory markers, and resolution of CT results [29], [41]. Included publications, with 11–1376 participants for CQ or HCQ therapies and 1–244 participants for corticosteroid therapy reported the therapeutic efficacy of all the drugs. However, a number of studies highlighted the unfavorable outcomes concerning the survival benefit of all these drugs in COVID-19 patients, even higher doses led to augmentation of the mortality risk [31], [33], [50], [51], [52], [55], [56]. Limited publications have also described the association of persistent viral RNA shedding with corticosteroid therapy [34] and cardiac toxicity, characterized by prolongation of the QT interval, with administration of CQ and HCQ in SARS-CoV-2-infected patients [54]. HCQ also has particular pharmacokinetic properties requiring certain precautions. It exhibits strong tissue tropism, with a long half-life (50 days) [100]. However, these negative findings may be partly due to the lack of adequate patient selection, along with improper dosing, timing, and duration of the therapy. Outcomes of this intervention may be improved by disease severity profiling of the patients prior to enrollment [29], [30], [31]. Therefore, despite the rapidly pushing of these drugs to clinical testing, all three medicines should be administered with strict rules [95].
Cytokine clearance using blood-purification modules, especially plasma exchange and continuous renal replacement therapy, has also yielded certain survival efficacy on severely and critically ill NCP individuals [23], [86], as formerly used for controlling the cytokine storm in the patients affected with influenza virus [101]. The indications for blood-purification therapy in the patients with COVID-19 include 5-fold increase in the level of plasma inflammatory mediators, quick daily progression of the lung abnormalities, and comorbidities requiring blood-purification therapy [24], [95]. However, a study reported that the NCP non-survivors have a greater proportion of CRRT [88], as seen for those with MERS-CoV [102]. Therefore, the blood-purification therapies are likely not to be feasible, yet, due to the controversial results reported in the literature.
Although most of the included publications have focused on the efficiency of corticosteroids and HCQ and have presented the controversial results, the trials included in the present review seemed to focus mainly on the therapies with MSCs, CP, and cytokine antagonists. Regarding the influence provided by these trials for the academic world, it should be mentioned that the framework of the trials was mostly well-designed, despite small size or the lack of randomization in some trials. It is essential to highlight that the dosing schedules applied in these trials were mostly according to the previous experiments, causing the concern that the side effects may occur in the subjects. Notably, neither these trials have posted the outcomes, nor applicable reports existed, and the trials failed to support the patients and their clinicians. Therefore, no conclusion can be finalized concerning the safety, tolerability, efficacy, pharmacokinetics, and immunomodulatory capacity of these interventions.
5. Limitations
The present study had several limitations. For example, majority of the included studies lacked the proper control groups and were considered to have a moderate to high risk of bias as a consequence of combining the non‐randomized evaluations, poor methodological approach for selection of the participant, small sample sizes, study designs, type of interventions, therapeutic regimens, dosage of drugs, and duration of therapies. This heterogeneous style and, most importantly, the lack of appropriate control groups did not justify us to conduct a meta‐analysis.
6. Conclusion
Results of this systematic review revealed that the immunomodulatory-based therapies are promising and provided a valuable therapeutic strategy to the scientific community and clinicians to fight against this outbreak in real-time situation even though other approaches and studies, including vaccine candidates, would be beneficial, but only in the future. This comprehensive systematic review of the immunomodulatory therapies applied for treatment of COVID-19 patients yielded several important findings. First, based on the consolidated clinical data derived from the included studies, while the interventions including cytokine-targeted therapies, CP and MSC, along with antivirals, have displayed promising evidence on reducing the mortality as the primary outcome, conventional therapies including corticosteroids and HCQ have shown controversial results on the mortality risk causing significant concerns about them. Second, safety is the most important concern for all the new and repurposed therapies. Findings regarding the safety profile of the interventions indicated that CP and MSC therapies are well tolerated providing evidence about their low risk. Cytokine-targeted therapies were associated with the mild to partly severe adverse reactions, and safety of the blood-purification therapy, HCQ, and corticosteroids was not satisfactory. Third, the immunomodulatory therapies had significant benefits regarding improvement of the clinical symptoms, recovery of the pulmonary function, and mitigation of the immune-inflammatory responses, as other outcomes of interest. Forth, the immunomodulatory therapies should be used timely to treat the COVID-19 patients at severe or critical stages of the disease. The reviewed studies underscored the importance of severity profiling of SARS-COV-2-infected patients for the appropriate selection of the patient and therapy. Despite these encouraging findings, to date, a definitive conclusion cannot be drawn on the optimal and reliable therapy for COVID‐19 because of the limited high-quality studies, the lack of control group in the majority of studies, and partly high heterogeneity, reflecting further need for firm investigations from the advanced preclinical researches and well-designed prospective randomized clinical trials.
Declaration of Competing Interest
The authors declare that there are no conflicts of interest.
Acknowledgment
This work was supported by Iran University of Medical Sciences (Grant # 99-1-28-18235).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.intimp.2020.106942.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.WHO, Coronavirus disease 2019 (COVID-19) situation report – 92, (2020).
- 2.Zhang H., Penninger J.M., Li Y., et al. Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: molecular mechanisms and potential therapeutic target. Intensive Care Med. 2020;46:586–590. doi: 10.1007/s00134-020-05985-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Deng S.Q., Peng H.J. Characteristics of and public health responses to the coronavirus disease 2019 outbreak in China. J. Clin. Med. 2020;9 doi: 10.3390/jcm9020575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jin Y., Yang H., Ji W., et al. Virology, epidemiology, pathogenesis, and control of COVID-19. Viruses. 2020;12 doi: 10.3390/v12040372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen N., Zhou M., Dong X., et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ghinai I., McPherson T.D., Hunter J.C., et al. First known person-to-person transmission of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in the USA. Lancet. 2020;395:1137–1144. doi: 10.1016/S0140-6736(20)30607-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferretti L., Wymant C., Kendall M., et al. Quantifying SARS-CoV-2 transmission suggests epidemic control with digital contact tracing. Science. 2020;368 doi: 10.1126/science.abb6936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yuan J., Zou R., Zeng L., et al. The correlation between viral clearance and biochemical outcomes of 94 COVID-19 infected discharged patients. Inflamm. Res. 2020;69:599–606. doi: 10.1007/s00011-020-01342-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang D., Hu B., Hu C., et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected Pneumonia in Wuhan, China. JAMA. 2020 doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cao X. COVID-19: immunopathology and its implications for therapy. Nat. Rev. Immunol. 2020;20:269–270. doi: 10.1038/s41577-020-0308-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou F., Yu T., Du R., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tufan A., Avanoglu Guler A., Matucci-Cerinic M. COVID-19, immune system response, hyperinflammation and repurposing antirheumatic drugs. Turk. J. Med. Sci. 2020;50:620–632. doi: 10.3906/sag-2004-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.L. Fu, J. Fei, H.-X. Xiang, et al., Influence factors of death risk among COVID-19 patients in Wuhan, China: a hospital-based case-cohort study, (2020). doi: 10.1101/2020.03.13.20035329.
- 14.Liu J., Li S., Liu J., et al. Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS-CoV-2 infected patients. EBioMedicine. 2020;55 doi: 10.1016/j.ebiom.2020.102763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang C., Wang Y., Li X., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan. ChinaLancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ruan Q., Yang K., Wang W., et al. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46:846–848. doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu X., Han M., Li T., et al. Effective treatment of severe COVID-19 patients with tocilizumab. Proc. Natl. Acad. Sci. USA. 2020;117:10970–10975. doi: 10.1073/pnas.2005615117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shi Y., Wang Y., Shao C., et al. COVID-19 infection: the perspectives on immune responses. Cell Death Differ. 2020;27:1451–1454. doi: 10.1038/s41418-020-0530-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Giamarellos-Bourboulis E.J., Netea M.G., Rovina N., et al. Complex immune dysregulation in COVID-19 patients with severe respiratory failure. Cell Host Microbe. 2020 doi: 10.1016/j.chom.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wan S., Xiang Y., Fang W., et al. Clinical features and treatment of COVID-19 patients in northeast Chongqing. J. Med. Virol. 2020;92:797–806. doi: 10.1002/jmv.25783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leng Z., Zhu R., Hou W., et al. Transplantation of ACE2(-) mesenchymal stem cells improves the outcome of patients with COVID-19 pneumonia. Aging Dis. 2020;11:216–228. doi: 10.14336/AD.2020.0228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Michot J.M., Albiges L., Chaput N., et al. Tocilizumab, an anti-IL-6 receptor antibody, to treat COVID-19-related respiratory failure: a case report. Ann. Oncol. 2020;31:961–964. doi: 10.1016/j.annonc.2020.03.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ma J., Xia P., Zhou Y., et al. Potential effect of blood purification therapy in reducing cytokine storm as a late complication of critically ill COVID-19. Clin Immunol. 2020;214 doi: 10.1016/j.clim.2020.108408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang Y., Yu L., Tang L., et al. A promising anti-cytokine-storm targeted therapy for COVID-19: The artificial-liver blood-purification system. Engineering (Beijing) 2020 doi: 10.1016/j.eng.2020.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhao M. Cytokine storm and immunomodulatory therapy in COVID-19: Role of chloroquine and anti-IL-6 monoclonal antibodies. Int. J. Antimicrob. Agents. 2020;55 doi: 10.1016/j.ijantimicag.2020.105982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martinez M.A. Compounds with therapeutic potential against novel respiratory 2019 coronavirus. Antimicrob. Agents Chemother. 2020;64 doi: 10.1128/AAC.00399-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mehta P., McAuley D.F., Brown M., et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liberati A., Altman D.G., Tetzlaff J., et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6 doi: 10.1371/journal.pmed.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zheng C., Wang J., Guo H., et al. Risk-adapted treatment strategy for COVID-19 patients. Int J Infect Dis. 2020;94:74–77. doi: 10.1016/j.ijid.2020.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu C., Chen X., Cai Y., et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020 doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhou W., Liu Y., Tian D., et al. Potential benefits of precise corticosteroids therapy for severe 2019-nCoV pneumonia. Signal Transduct Target Ther. 2020;5:18. doi: 10.1038/s41392-020-0127-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Y. Wang, W. Jiang, Q. He, et al., Early low-dose and short-term application of corticosteroid treatment in patients with severe COVID-19 pneumonia: single-center experience from Wuhan, China, (2020). doi: 10.1101/2020.03.06.20032342.
- 33.Lu X., Chen T., Wang Y., et al. Adjuvant corticosteroid therapy for critically ill patients with COVID-19. Crit. Care. 2020;24:241. doi: 10.1186/s13054-020-02964-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu K., Chen Y., Yuan J., et al. Factors associated with prolonged viral RNA shedding in patients with COVID-19. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fang X., Mei Q., Yang T., et al. Low-dose corticosteroid therapy does not delay viral clearance in patients with COVID-19. J. Infect. 2020;81:147–178. doi: 10.1016/j.jinf.2020.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mo P., Xing Y., Xiao Y., et al. Clinical characteristics of refractory COVID-19 pneumonia in Wuhan, China. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zheng F., Liao C., Fan Q.H., et al. Clinical characteristics of children with coronavirus disease 2019 in Hubei, China. Curr. Med. Sci. 2020;40:275–280. doi: 10.1007/s11596-020-2172-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sun D., Li H., Lu X.X., et al. Clinical features of severe pediatric patients with coronavirus disease 2019 in Wuhan: a single center's observational study. World J. Pediatr. 2020;16:251–259. doi: 10.1007/s12519-020-00354-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xu X.W., Wu X.X., Jiang X.G., et al. Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS-Cov-2) outside of Wuhan, China: retrospective case series. BMJ. 2020;368 doi: 10.1136/bmj.m606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang R., Pan M., Zhang X., et al. Epidemiological and clinical features of 125 hospitalized patients with COVID-19 in Fuyang, Anhui, China. Int J Infect Dis. 2020;95:421–428. doi: 10.1016/j.ijid.2020.03.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu K., Fang Y.Y., Deng Y., et al. Clinical characteristics of novel coronavirus cases in tertiary hospitals in Hubei Province. Chin Med J (Engl). 2020;133:1025–1031. doi: 10.1097/CM9.0000000000000744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhou C., Gao C., Xie Y., et al. COVID-19 with spontaneous pneumomediastinum. Lancet Infect. Dis. 2020;20:510. doi: 10.1016/S1473-3099(20)30156-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zha L., Li S., Pan L., et al. Corticosteroid treatment of patients with coronavirus disease 2019 (COVID-19) Med. J. Aust. 2020;212:416–420. doi: 10.5694/mja2.50577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gautret P., Lagier J.C., Parola P., et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int. J. Antimicrob. Agents. 2020 doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gautret P., Lagier J.C., Parola P., et al. Clinical and microbiological effect of a combination of hydroxychloroquine and azithromycin in 80 COVID-19 patients with at least a six-day follow up: A pilot observational study. Travel Med. Infect. Dis. 2020;34 doi: 10.1016/j.tmaid.2020.101663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Million M., Lagier J.C., Gautret P., et al. Early treatment of COVID-19 patients with hydroxychloroquine and azithromycin: A retrospective analysis of 1061 cases in Marseille, France. Travel Med. Infect. Dis. 2020;35 doi: 10.1016/j.tmaid.2020.101738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Z. Chen, J. Hu, Z. Zhang, et al., Efficacy of hydroxychloroquine in patients with COVID-19: results of a randomized clinical trial, (2020). doi: 10.1101/2020.03.22.20040758.
- 48.Huang M., Tang T., Pang P., et al. Treating COVID-19 with Chloroquine. J. Mol. Cell. Biol. 2020;12:322–325. doi: 10.1093/jmcb/mjaa014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wan S., Yi Q., Fan S., et al. Relationships among lymphocyte subsets, cytokines, and the pulmonary inflammation index in coronavirus (COVID-19) infected patients. Br. J. Haematol. 2020;189:428–437. doi: 10.1111/bjh.16659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.M. Mahevas, V.-T. Tran, M. Roumier, et al., No evidence of clinical efficacy of hydroxychloroquine in patients hospitalised for COVID-19 infection and requiring oxygen: results of a study using routinely collected data to emulate a target trial, (2020). doi: 10.1101/2020.04.10.20060699.
- 51.Geleris J., Sun Y., Platt J., et al. Observational study of hydroxychloroquine in hospitalized patients with Covid-19. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2012410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Molina J.M., Delaugerre C., Le Goff J., et al. No evidence of rapid antiviral clearance or clinical benefit with the combination of hydroxychloroquine and azithromycin in patients with severe COVID-19 infection. Med Mal Infect. 2020;50:384. doi: 10.1016/j.medmal.2020.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.W. Tang, Z. Cao, M. Han, et al., Hydroxychloroquine in patients mainly with mild to moderate COVID–19: an open–label, randomized, controlled trial, (2020). doi: 10.1101/2020.04.10.20060558. [DOI] [PMC free article] [PubMed]
- 54.Chorin E., Wadhwani L., Magnani S., et al. QT interval prolongation and torsade de pointes in patients with COVID-19 treated with Hydroxychloroquine/Azithromycin. Heart Rhythm. 2020 doi: 10.1016/j.hrthm.2020.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.J. Magagnoli, S. Narendran, F. Pereira, et al., Outcomes of hydroxychloroquine usage in United States veterans hospitalized with Covid-19, medRxiv (2020). doi: 10.1101/2020.04.16.20065920. [DOI] [PMC free article] [PubMed]
- 56.Borba M.G.S., Val F.F.A., Sampaio V.S., et al. Effect of high vs low doses of chloroquine diphosphate as adjunctive therapy for patients hospitalized with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. JAMA Network Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.8857. [DOI] [PubMed] [Google Scholar]
- 57.Perinel S., Launay M., Botelho-Nevers E., et al. Towards optimization of hydroxychloroquine dosing in intensive care unit COVID-19 patients. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Luo P., Liu Y., Qiu L., et al. Tocilizumab treatment in COVID-19: A single center experience. J. Med. Virol. 2020;92:814–818. doi: 10.1002/jmv.25801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.C. Guo, B. Li, H. Ma, et al., Tocilizumab treatment in severe COVID-19 patients attenuates the inflammatory storm incited by monocyte centric immune interactions revealed by single-cell analysis, (2020). doi: 10.1101/2020.04.08.029769.
- 60.Cellina M., Orsi M., Bombaci F., et al. Favorable changes of CT findings in a patient with COVID-19 pneumonia after treatment with tocilizumab. Diagn Interv Imaging. 2020;101:323–324. doi: 10.1016/j.diii.2020.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Di Giambenedetto S., Ciccullo A., Borghetti A., et al. Off-label use of tocilizumab in patients with SARS-CoV-2 infection. J. Med. Virol. 2020 doi: 10.1002/jmv.25897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sciascia F.A. S., Baffa A., Baldovino S., et al. Pilot prospective open, single-arm multicentre study on off-label use of tocilizumab in severe patients with COVID-19. Clin. Exp. Rheumatol. 2020:529–532. [PubMed] [Google Scholar]
- 63.Klopfenstein T., Zayet S., Lohse A., et al. Tocilizumab therapy reduced intensive care unit admissions and/or mortality in COVID-19 patients. Med Mal Infect. 2020 doi: 10.1016/j.medmal.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.M. Roumier, R. Paule, M. Groh, et al., Interleukin-6 blockade for severe COVID-19, (2020). doi: 10.1101/2020.04.20.20061861.
- 65.Alattar R., Ibrahim T.B.H., Shaar S.H., et al. Tocilizumab for the treatment of severe coronavirus disease 2019. J. Med. Virol. 2020 doi: 10.1002/jmv.25964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Radbel J., Narayanan N., Bhatt P.J. Use of tocilizumab for COVID-19-induced cytokine release syndrome: a cautionary case report. Chest. 2020 doi: 10.1016/j.chest.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Morrison A.R., Johnson J.M., Ramesh M., et al. Acute hypertriglyceridemia in patients with COVID-19 receiving tocilizumab. J. Med. Virol. 2020 doi: 10.1002/jmv.25907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Antinori S., Bonazzetti C., Gubertini G., et al. Tocilizumab for cytokine storm syndrome in COVID-19 pneumonia: an increased risk for candidemia? Autoimmun. Rev. 2020;19 doi: 10.1016/j.autrev.2020.102564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.G. Gritti, F. Raimondi, D. Ripamonti, et al., Use of siltuximab in patients with COVID-19 pneumonia requiring ventilatory support, medRxiv (2020). doi: 10.1101/2020.04.01.20048561.
- 70.Filocamo G., Mangioni D., Tagliabue P., et al. Use of anakinra in severe COVID-19: a case report. Int J Infect Dis. 2020 doi: 10.1016/j.ijid.2020.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Aouba A., Baldolli A., Geffray L., et al. Targeting the inflammatory cascade with anakinra in moderate to severe COVID-19 pneumonia: case series. Ann. Rheum. Dis. 2020 doi: 10.1136/annrheumdis-2020-217706. [DOI] [PubMed] [Google Scholar]
- 72.Cavalli G., De Luca G., Campochiaro C., et al. Interleukin-1 blockade with high-dose anakinra in patients with COVID-19, acute respiratory distress syndrome, and hyperinflammation: a retrospective cohort study. Lancet Rheumatol. 2020;2:e325–e331. doi: 10.1016/S2665-9913(20)30127-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.B. Liang, J. Chen, T. Li, et al., Clinical remission of a critically ill COVID-19 patient treated by human umbilical cord mesenchymal stem cells, (2020). doi: http://chinaxiv.org/abs/202002.00084. [DOI] [PMC free article] [PubMed]
- 74.Shu L., Niu C., Li R., et al. Treatment of severe COVID-19 with human umbilical cord mesenchymal. Stem Cells. 2020 doi: 10.21203/rs.3.rs-23696/v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang Y., Ding J., Ren S., et al. Intravenous infusion of human umbilical cord Wharton's jelly-derived mesenchymal stem cells as a potential treatment for patients with COVID-19 pneumonia. Stem Cell Res. Ther. 2020;11:207. doi: 10.1186/s13287-020-01725-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhang L., Pang R., Xue X., et al. Anti-SARS-CoV-2 virus antibody levels in convalescent plasma of six donors who have recovered from COVID-19. Aging (Albany NY). 2020;12:6536–6542. doi: 10.18632/aging.103102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Duan K., Liu B., Li C., et al. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc Natl Acad Sci USA. 2020;117:9490–9496. doi: 10.1073/pnas.2004168117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shen C., Wang Z., Zhao F., et al. Treatment of 5 critically ill patients with COVID-19 with convalescent plasma. JAMA. 2020 doi: 10.1001/jama.2020.4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhang B., Liu S., Tan T., et al. Treatment with convalescent plasma for critically ill patients with severe acute respiratory syndrome coronavirus 2 infection. Chest. 2020 doi: 10.1016/j.chest.2020.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ye M., Fu D., Ren Y., et al. Treatment with convalescent plasma for COVID-19 patients in Wuhan, China. J. Med. Virol. 2020 doi: 10.1002/jmv.25882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ahn J.Y., Sohn Y., Lee S.H., et al. Use of convalescent plasma therapy in two COVID-19 patients with acute respiratory distress syndrome in Korea. J. Korean Med. Sci. 2020;35 doi: 10.3346/jkms.2020.35.e149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cao W., Liu X., Bai T., et al. High-dose intravenous immunoglobulin as a therapeutic option for deteriorating patients with coronavirus disease 2019, open forum. Infect Dis. 2020;7:ofaa102. doi: 10.1093/ofid/ofaa102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mohtadi N., Ghaysouri A., Shirazi S., et al. Recovery of severely ill COVID-19 patients by intravenous immunoglobulin (IVIG) treatment: A case series. Virology. 2020;548:1–5. doi: 10.1016/j.virol.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Xie Y., Cao S., Dong H., et al. Effect of regular intravenous immunoglobulin therapy on prognosis of severe pneumonia in patients with COVID-19. J. Infect. 2020 doi: 10.1016/j.jinf.2020.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Z. Shao, Y. Feng, L. Zhong, et al., Clinical efficacy of intravenous immunoglobulin therapy in critical patients with COVID-19: A Multicenter Retrospective Cohort Study, (2020). doi: 10.1101/2020.04.11.20061739. [DOI] [PMC free article] [PubMed]
- 86.Shi H., Zhou C., He P., et al. Successful treatment with plasma exchange followed by intravenous immunoglobulin in a critically ill patient with COVID-19. Int. J. Antimicrob. Agents. 2020 doi: 10.1016/j.ijantimicag.2020.105974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Y. Yang, J. Shi, S. Ge, et al., Effect of continuous renal replacement therapy on all-cause mortality in COVID-19 patients undergoing invasive mechanical ventilation: a retrospective cohort study, (2020). doi: 10.1101/2020.03.16.20036780. [DOI] [PMC free article] [PubMed]
- 88.Yang X., Yu Y., Xu J., et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respiratory Med. 2020;8:475–481. doi: 10.1016/s2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhou Y., Hou Y., Shen J., et al. Network-based drug repurposing for novel coronavirus 2019-nCoV/SARS-CoV-2. Cell Discov. 2020;6:14. doi: 10.1038/s41421-020-0153-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Le R.Q., Li L., Yuan W., et al. FDA approval summary: Tocilizumab for treatment of chimeric antigen receptor T cell-induced severe or life-threatening cytokine release syndrome. Oncologist. 2018;23:943–947. doi: 10.1634/theoncologist.2018-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wang J., Cen P., Chen J., et al. Role of mesenchymal stem cells, their derived factors, and extracellular vesicles in liver failure. Stem Cell Res. Ther. 2017;8:137. doi: 10.1186/s13287-017-0576-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chen J., Hu C., Chen L., et al. Clinical study of mesenchymal stem cell treating acute respiratory distress syndrome induced by epidemic Influenza A (H7N9) infection, a hint for COVID-19 treatment. Engineering (Beijing). 2020 doi: 10.1016/j.eng.2020.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhao Q., He Y. Challenges of convalescent plasma therapy on COVID-19. J. Clin. Virol. 2020;127 doi: 10.1016/j.jcv.2020.104358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Arabi Y.M., Hajeer A.H., Luke T., et al. Feasibility of using convalescent plasma immunotherapy for MERS-CoV infection, Saudi Arabia. Emerg. Infect. Dis. 2016;22:1554–1561. doi: 10.3201/eid2209.151164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ye Q., Wang B., Mao J. The pathogenesis and treatment of the ‘Cytokine Storm' in COVID-19. J. Infect. 2020;80:607–613. doi: 10.1016/j.jinf.2020.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rojas M., Rodriguez Y., Monsalve D.M., et al. Convalescent plasma in Covid-19: Possible mechanisms of action. Autoimmun. Rev. 2020 doi: 10.1016/j.autrev.2020.102554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Langhi D.M.J., Santis G.C., Bordin J.O. COVID-19 convalescent plasma transfusion. Hematol Transfus Cell Ther. 2020;42:113–115. doi: 10.1016/j.htct.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sun M., Xu Y., He H., et al. Potential effective treatment for COVID-19: systematic review and meta-analysis of the severe infectious disease with convalescent plasma therapy. Int J Infect Dis. 2020 doi: 10.1016/j.ijid.2020.06.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhou G., Zhao Q. Perspectives on therapeutic neutralizing antibodies against the Novel Coronavirus SARS-CoV-2. Int J Biol Sci. 2020;16:1718–1723. doi: 10.7150/ijbs.45123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yao X., Ye F., Zhang M., et al. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Liu X., Zhang Y., Xu X., et al. Evaluation of plasma exchange and continuous veno-venous hemofiltration for the treatment of severe avian influenza A (H7N9): a cohort study. Ther Apher Dial. 2015;19:178–184. doi: 10.1111/1744-9987.12240. [DOI] [PubMed] [Google Scholar]
- 102.Alfaraj S.H., Al-Tawfiq J.A., Assiri A.Y., et al. Clinical predictors of mortality of Middle East Respiratory Syndrome Coronavirus (MERS-CoV) infection: A cohort study. Travel Med. Infect. Dis. 2019;29:48–50. doi: 10.1016/j.tmaid.2019.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.