Highlights
-
•
Complex dynamics in a susceptible-infected (SI) model for COVID-19 with multi-drug resistance (MDR) and its fractional-order counterpart are investigated.
-
•
New fractional Routh-Hurwitz (FRH) conditions are introduced and proved for the fractional case (0,2].
-
•
Local stability of the new SIMDR model is investigated for all the multi-drug resistance steady sates using the FRH scheme.
-
•
Chaotic attractors are obtained in both the integer and fractional-order SIMDR models.
-
•
This study helps to understand complex behaviors and predict spread of severe infectious diseases such as COVID-19.
Keywords: Susceptible-infected (SI) model, Multi-drug resistance (MDR), Fractional-order, New FRH stability conditions, Chaos
Abstract
Nowadays, exploring complex dynamic of epidemic models becomes a focal point for research after the outbreak of COVID-19 pandemic which has no vaccine or fully approved drug treatment up till now. Hence, complex dynamics in a susceptible-infected (SI) model for COVID-19 with multi-drug resistance (MDR) and its fractional-order counterpart are investigated. Existence of positive solution in fractional-order model is discussed. Local stability based on the fractional Routh-Hurwitz (FRH) conditions is considered. Also, new FRH conditions are introduced and proved for the fractional case (0,2]. All these FRH conditions are also applied to discuss local stability of the multi-drug resistance steady states. Chaotic attractors are also found in this model for both integer-order and fractional-order cases. Numerical tools such as Lyapunov exponents, Lyapunov spectrum and bifurcation diagrams are employed to confirm existence of these complex dynamics. This study helps to understand complex behaviors and predict spread of severe infectious diseases such as COVID-19.
1. Introduction
Some diseases that were thought to have disappeared have reappeared. The family of Coronaviridae is a strong example to this observation; The outbreak of the Middle East Respiratory Syndrome Corona virus (MERS-CoV) was recorded in Saudi Arabia in 2012 [1]. Recently, the whole world suffers from the outbreak of a newer version of coronavirus disease (COVID-19) which becomes more dangerous and is currently considered to be the most pathogenic virus or a world wide pandemic [2], [3], [4], [5]. The MERS-CoV is transmitted from bats-to-human through camels, while COVID-19 has reptilians as intermediate hosts. Unfortunately, COVID-19 has neither a vaccine nor a fully approved drug treatment up till now. Moreover, COVID-19 is an RNA virus which means that it has high mutation rates and has highly conserved sequences in nucleic acids that is maintained by natural selection. In addition, the coronaviruses have the order Nidovirales which are enveloped, non-segmented positive-sense RNA viruses. Therefore, it is difficult to find its suitable vaccine quickly and the memory effect (or hereditary properties) has a vital role in recognizing its behaviors. However, some drugs such as Remdesivir shows promise to some patients and is granted emergency use authorization from US FDA [6]. In March 2020, the anti-malaria drug known as Hydroxychloroquine shows positive signs to reduce some of the symptoms of COVID-19 for some patients especially with adding Azithromycin [7]. In [8], it was recommended that Remdesivir can be used as the preferred agent, and Hydroxychloroquine could be used for patients who have resistance against Remdesivir or when Remdesivir is not available. However in [9], some kind of biological resistance to Hydroxychloroquine was reported. Recently, it has been recommended that the use of Hydroxychloroquine should be under medical supervision since it has some side effects on the heart [10]. Hence, studies of antiviral resistance, antimicrobial resistance (AMR), multi-drug resistance (MDR) and waning vaccination [11] may be useful in this context.
On the other hand, mathematical models are very useful to understand and predict spread of epidemics. In mathematical epidemiology, the Susceptible-Infected (SI) models, Susceptible-Infected-Recovered (SIR) models and Susceptible-Exposed-Infected-Recovered (SEIR) models are used to study the transmission of virus from human-to-human and the humanitarian diffusion of epidemics [12]. Indeed, the AMR and MDR models belong to the class of SIR epidemic models. In addition, the memory effects are also important for AMR and MDR phenomena since they depend on the exposure time. Recently, new mathematical models for COVID-19 have been introduced and studied by scientists (see for example, Sarkar et al. [13], Kucharski et al. [14], Bozkurt et al. [15], Ben Fredj and Chrif [16], Jiwei et al. [17], Ndaïrou et al. [18], Okuonghae and Omame [19], Aslan et al. [20], Chimmula and Zhang [21], Ivorra et al. [22], Çakan [23], Soukhovolsky et al. [24], Huang et al. [25], Cooper et al. [26], Wang et al. [27], Salgotra et al. [28], Saif Ullah and Khan [29], Asamoah et al. [30], Basu and Campbell [31], Annas et al. [32], Croccolo and Roman [33], Kaxiras et al. [34], Lee et al. [35], Mandal et al. [36], Bekiros and Kouloumpou [37], Reis et al. [38], Mahajan et al. [39], Bagal et al. [40], Papo et al. [41], Džiugys et al. [42], Samui et al. [43], Khan et al. [44], and Ghanbari [45]).
Recently, fractional calculus (FC) has been shown to have continuous progress [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58]. Fractional-order modeling provides more adequacies in describing the natural phenomenon. FC can also be used to model complex systems with long term memory, hereditary properties and non-local behaviors since it generalizes the existing classical differentiation to arbitrary real-valued order. Therefore, scientists have recently presented some fractional-order mathematical models for COVID-19 (see for example, Atangana [59], Khan and Atangana [60], Tuan et al. [61], Higazy [62], Alkahtani and Alzaid [63], Zhang [64], Yadav and Verma [65], Baleanu et al. [66], Mohammad and Trounev [67], and Zhang and Jain [68]).
In this work, complex dynamics in Susceptible-Infected (SI) model [69] and its fractional-order counterpart are studied. This Susceptible-Infected (SI) model explores the dynamics of three classes of infected populations by COVID-19 and a susceptible one. The three infected classes are divided into three types; The first type is sensitive to Remdesivir but resistant to Hydroxychloroquine. The second type responds to Hydroxychloroquine but resistant to Remdesivir. The last type is resistant to both of Remdesivir and Hydroxychloroquine. The fractional model is considered in the interval (0,2] which enables us to achieve higher adequacy of modeling the system and higher degrees of freedom. Also, new applicable fractional Routh-Hurwitz (FRH) conditions are introduced and proved for four-dimensional systems in the case (0,2]. Finally, this kind of work helps to understand and predict complex dynamics arising from serious epidemiological diseases such as COVID-19.
2. Fractional calculus
The Caputo fractional differential operator [70] is defined as
| (1) |
| (2) |
where q > 0, p > 0, z is an integer such that When the Caputo fractional differential operator is denoted by Dq. It is clear that is a non-local operator with singular kernel. So it can be used to describe complex dynamics of models involving hereditary properties and long term memory.
According to the well-known Matignon's inequalities, the linearized fractional-order n-dimensional system is locally asymptotically stable (LAS) if
| (3) |
where q ∈ (0, 2] and λi are the eigenvalues of related Jacobian [71]. The associated stability region is illustrated in Fig. 1 .
Fig. 1.
Stability region of linearized system when the fractional parameter q satisfies (a) 0 ≤ q < 1, (b) 1 ≤ q < 2..
Remark 1
It is clear from Fig. 1 that the unstability region for q ∈ [1, 2] contains the entire unstability region for q ∈ (0, 1). So if an equilibrium point lies in the unstable region for q ∈ (0, 1) then it also lies in the unstable region for q ∈ [1, 2]. Therefore, any imperative condition for ensuring the local stability of the case q ∈ (0, 1) is also an imperative condition for ensuring the local stability of the case q ∈ [1, 2].
3. Models’ description
3.1. The integer-order SIMDR model
In [69], Elettreby and Ahmed introduced the following integer-order model for a multi-drug resistance (MDR) which explores the dynamics of the susceptible and three classes of infected populations. The proposed Susceptible-Infected (SIMDR) model is described as
| (4) |
where the state variables x 1, x 2, x 3, x 4 refer, respectively, to the susceptible population, infected population that responding only to Remdesivir, infected population that responding only to Hydroxychloroquine and infected population that shows resistance to both of these drugs. All parameters r, b 1, b 2, b 12, b 4, b 5, μ 1, μ 2 and μ 12 are positive real numbers and their meaning is completely explained in Table 1 .
Table 1.
Meaning of parameters in the SIMDR model.
| Parameters | Meaning |
|---|---|
| μ1 | Natural death rate of the infected population x2 (infected population responding only to Remdesivir) |
| μ2 | Natural death rate of the infected population x3 (infected population responding only to Hydroxychloroquine) |
| μ12 | Natural death rate of the infected population x4 (resisting population to both drugs) |
| b1 | Encounter rate of x1 with x2 per unit time |
| b2 | Encounter rate of x1 with x3 per unit time |
| b12 | Encounter rate of x1 with x4 per unit time |
| b4 | Encounter rate of x2 with x4 per unit time |
| b5 | Encounter rate of x3 with x4 per unit time |
| r | Rate of growth of the population x1 |
3.2. The fractional-order SIMDR model
In fact, involving the Caputo type () into the SIMDR model given by Eq. (4), enables us to achieve more realistic description of natural phenomena. Moreover, the resulting long-term memory effect and hereditary properties of , are better candidate to handle the rich dynamics of the proposed model and give more adequate description of its natural behaviors. Therefore, the fractional-order form of the SIMDR model given by Eq. (4) is described as
| (5) |
where q ∈ (0, 2]. Obviously, the SIMDR model given by Eq. (5) has greater degrees of freedom since the fractional parameter has higher arbitrary real-valued orders.
Both of SIMDR models given by Eqs. (4) and (5) have the same steady states
| (6) |
where
To discuss the existence of positive solution of SIMDR model given by Eq. (5), we prove the following theorem.
Theorem 1
Assume that a closed set where ζ > 0 is very close to zero, and Hence, any solution of the SIMDR system (5) that starts in Ψ remains positive for all t > 0.
Proof
Let P(t) be the sum of all the state variables in the population, then we obtain
(7)
Recalling the well-known Mittag–Leffler functions , and applying the Laplace transform to the inequality (7), we get
| (8) |
It is clear that has a bounded positive value. Clearly, when . Hence, Ψ is positive closed invariant set for the fractional-order SIMDR system (5). Furthermore, if one defines a solution X(t) of the model (5) given that , one gets
| (9) |
since Therefore, any solution starts in the closed set Ψ will remain in Ψ. □
4. Local stability
Consider the following general fractional-order nonlinear system
| (10) |
where q ∈ (0, 2],Y ∈ Rn and Ω is a nonlinear vector function. Let be a steady state of Eq. (10) with the following characteristic polynomial:
| (11) |
Here, we will recall the FRH conditions when the degree of polynomial is three and four, respectively [72], [73], [74]. Firstly, let be the discriminant of . Hence, the three-dimensional case of FRH criterion is summarized as follows
-
(i)
is LAS for q ∈ (0, 2] if along with κ 1 > 0, κ 3 > 0 and κ 3 < κ 1 κ 2.
-
(ii)
For , is LAS if in addition to κ 3 > 0, κ 1 ≥ 0 and κ 2 ≥ 0.
-
(iii)
is LAS when q ∈ (0, 1) if and the following conditions κ 1 > 0, κ 2 > 0 and hold.
-
(iv)
If 1 ≤ q < 2, and then the Matignon's conditions (3) do not hold.
-
(v)
If 0 < q ≤ 2 then κ 3 > 0 is an imperative condition for to be LAS.
Also, according to Proposition 1 of [73] and conditions (3), the following four-dimensional FRH conditions are straightforwardly obtained
(1) Let Λ1, Λ2, Λ3, Λ4 be Routh–Hurwitz determinants
| (12) |
then is LAS for 0 < q ≤ 2 if
If q > 2/3, then does not satisfy Matignon's conditions (3).
If 0 < q < 1/3, then is LAS. However, if κ 1 < 0, κ 3 < 0, κ 2 > 0, κ 4 > 0 then does not satisfy Matignon's conditions (3).
If and then is LAS.
(5) If 0 < q ≤ 1 then κ 4 > 0 is an imperative condition for to be LAS.
The following lemma can also be added to extend the above-mentioned FRH conditions.
Lemma 1
Consider the characteristic polynomial (11) with ;
- (a)
If and then the Matignon's inequalities (3) are not satisfied;
- (b)
If 1 < q ≤ 2 then κ 4 > 0 is an imperative condition for to be LAS.
Proof
To prove part (a), we recall that implies the existence of two real roots and a pair of complex conjugate eigenvalues However, the condition implies that So according to the Matignon's conditions (3), the eigenvalues are located in the unstable region for 1 ≤ q ≤ 2 (See Fig. 1b). The part (a) is now proved.
To prove (b), we recall that κ 4 > 0 is an imperative condition for to be LAS when 0 < q ≤ 1. So according to Remark 1, κ 4 > 0 is also an imperative condition for to be LAS when 1 < q ≤ 2. □
4.1. Applications to the SIMDR models
In the following, we will apply the above-mentioned stability results to the SIMDR models given by Eqs. (4) and (5). Although the system has eight equilibrium points, we only focus on the equilibrium states in which the component x 4 is not vanished. Obviously, if the last component of the steady state is vanished then it does not have a multi-drug resistance which contradicts the biological meaning of this model.
Theorem 2
When 0 < q ≤ 2, the steady state of the fractional SIMDR system (5) is LAS if
(13)
Proof
The steady state has the characteristic polynomial
which has the four roots; It is clear that λ 3, 4 satisfy the conditions (3). Moreover, the following condition ensure the existence of :
| (14) |
If conditions (13) hold then λ 1, 2 also satisfy the Matignon's conditions (3) which also imply that is LAS for q ∈ (0, 2]. □
Obviously, the steady state exists only if
| (15) |
Also, has the following eigenvalue equation
| (16) |
where
Obviously, Eq. (16) has negative eigenvalue if
| (17) |
Applying the conditions (3) and the three-dimensional FRH conditions to ϑ(λ), the following lemma is straightforwardly obtained.
Lemma 2
If conditions (15) and (17) hold, the steady state of the fractional SIMDR system (5) is LAS when
- (i)
q ∈ (0, 2] and
- (ii)
q < 2/3 and
- (iii)
q ∈ (0, 1), and
However, if q ∈ [1, 2), and then is not LAS.
It is also clear that, the multi-drug resistance steady state exists only if
| (18) |
Also, has the characteristic equation
| (19) |
where
Eq. (19) has a negative eigenvalue if . Similarly, conditions for local stability of can be obtained by the three-dimensional FRH conditions as shown above.
Indeed, the multi-drug resistance steady state exists only if Also, the Jacobian computed at yields the eigenvalue equation
| (20) |
where
So based on the four-dimensional FRH conditions and Lemma 1, the following lemma is straightforwardly proved.
Lemma 3
If then exists and the following statements hold
- (i)
If the Routh-Hurwitz determinants satisfy the FRH condition (1) then is LAS for 0 < q ≤ 2.
- (ii)
When the steady state is LAS for 0 < q < 1/3.
- (iii)
When the steady state is LAS for 0 < q < 1; however it is not LAS for 1 ≤ q ≤ 2.
Remark 2
The healthy steady state has the eigenvalues and then it is LAS for all 0 < q ≤ 2 when μ 1 > b 1,μ 2 > b 2 and μ 12 > b 12.
4.2. Simulation results
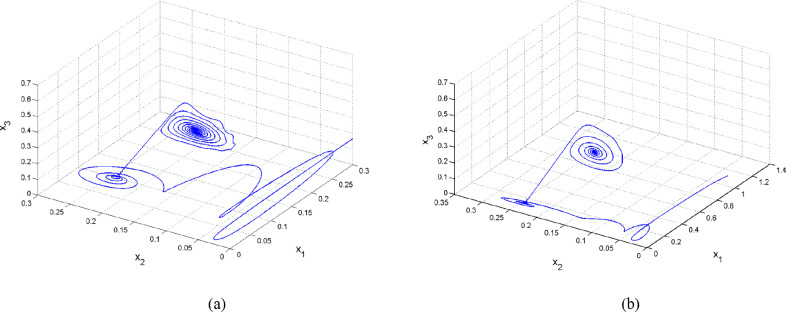
The set satisfies the stability conditions in Remark 2. Simulation results using this data set show that the integer-order SIMDR system (4) converges to the healthy state faster than the corresponding fractional-order counterpart does. Fig. 2 depicts this observation.
Fig. 2.
Plots of susceptible and infective populations of the SIMDR models given by Eqs. (4), (5) with and using different values of q..
The set satisfies the stability conditions of . Simulation results using this data set show that the fractional-order SIMDR system (5) converges to the multi-drug resistance (MDR) steady state faster than the corresponding integer-order counterpart does. Fig. 3 depicts these results.
Fig. 3.
Plots of susceptible and infective populations of the SIMDR models given by Eqns. (4), (5) with and using different values of q..
The set satisfies the stability conditions of given in Lemma 2. Simulation results using this data set show that the fractional-order SIMDR system (5) converges to all the MDR steady state components faster than the corresponding integer-order counterpart does (except ). Fig. 4 illustrates these results.
Fig. 4.
Plots of susceptible and infective populations of the SIMDR models given by Eqns. (4), (5) with and using different values of q..
According to these conditions, the obtained results show that the response of individuals to Hydroxychloroquine is vanishing after a course of time. Also, these results show that the fractional-order SIMDR model exhibits more resistance to both drugs comparing to its integer-order form. The results in the fractional case are more accurate since the memory effect erases the oscillations. Hence, in this case the fractional-order model is better candidate to describe the multi-drug resistance phenomena.
The set satisfies the stability conditions of . Simulation results using this data set show that the fractional-order SIMDR system (5) tends to all components of the multi-drug resistance steady state faster than the corresponding integer-order counterpart does (except ). Fig. 5 summarizes these results. Moreover, the obtained results show that the response of individuals to Remdesivir is vanishing after some time. Also, these results show that the fractional-order SIMDR model exhibits more resistance to both drugs than its integer-order form. Therefore, this case also shows that the fractional-order model given by Eq. (5) is better candidate to describe the multi-drug resistance phenomena.
Fig. 5.
Plots of susceptible and infective populations of the SIMDR models given by Eqns. (4), (5) with and using different values of q..
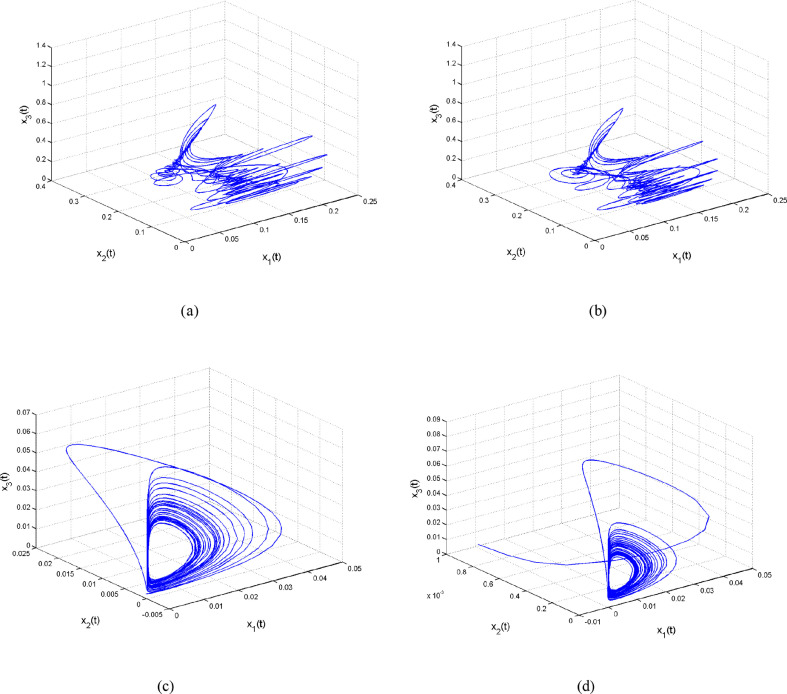
5. Chaos in the integer-order SIMDR model
Chaotic attractors are found in the SIMDR model given by Eq. (4). Calculations of Lyapunov exponents (LEs) corresponding to some chaotic behaviors are given in Table 2 . The calculations are based on Wolf's algorithm [75] and the corresponding chaotic attractors are depicted in Fig. 6 .
Table 2.
Calculations of LEs (Λi, s) of the SIMDR model given by Eq. (4).
| Parameter Set | Λ1 | Λ2 | Λ3 | Λ4 |
|---|---|---|---|---|
| 0.011391 | 0.005804 | −0.090994 | −0.156050 | |
| 0.014493 | −0.003904 | −0.111544 | −0.130222 | |
| 0.061863 | 0.000212 | −0.498306 | −0.903541 | |
| 0.087525 | −0.124483 | −0.061632 | −1.872964 |
Fig. 6.
Chaotic attractors of SIMDR model given by Eq. (4) exist when (a) ; (b) ; (c) ; (d) .
Computations of the corresponding bifurcation diagrams are performed by varying the dynamical parameter r and using the parameter sets given in Table 2. The results are illustrated in Fig. 7 .
Fig. 7.
Bifurcations diagrams of SIMDR model given by Eq. (4) by varying the dynamical parameter r and using (a) ; (b) ; (c) ; (d) .
6. Chaos in the fractional SIMDR model
Chaotic attractors are found in the SIMDR model given by Eq. (5) when and fractional parameter and These attractors are depicted in Fig. 8 . In addition, computations of Lyapunov spectrum as r and q are varied, are illustrated in Fig. 9 . These computations of Lyapunov spectrum verify the existence of wide range of unpredictable dynamical behaviors in the fractional-order model. Therefore, by studying this kind of complex dynamics, scientists can understand the mechanism of spreading diseases or infections.
Fig. 8.
Chaotic attractors of SIMDR model given by Eq. (5) exist when and (a) (b) .
Fig. 9.
Lyapunov spectrum of SIMDR model given by Eq. (5) with , (a) and varying r, (b) and varying q..
7. Conclusion
A Susceptible-Infected (SI) model describes the dynamics of three classes of infected populations by COVID-19 and a susceptible one has been suggested. The model has been called SIMDR. The infected populations of this model are assumed to have resistance against two drugs; Remdesivir and Hydroxychloroquine such that; The infected first class responds to Remdesivir but is resistant to Hydroxychloroquine. The second infected class responds to Hydroxychloroquine but is resistant to Remdesivir. The third infected class is resistant to both of Remdesivir and Hydroxychloroquine.
Dynamical behaviors in the SIMDR model for COVID-19 and its fractional-order counterpart have been explored. Existence of positive solution in the fractional-order model has been proved. Conditions of local stability based on the FRH criterion have been obtained. In addition, new FRH conditions have been proved for the case (0,2] and have also been applied to the fractional SIMDR model. Chaotic attractors have also been found in this model for both integer-order and fractional-order cases. These results help us to understand and control the spread of serious infectious diseases with multi-drug resistance when the dynamics becomes more complicated.
It has also been shown that the fractional-order SIMDR model is more adequate and realistic in describing natural phenomena than its integer-order counterpart since the former involves fractional differential operator with singular kernel which results in existence of long-term memory effect, hereditary properties. Therefore, it is better choice to handle complex dynamics. Moreover, comparisons between the integer-order and fractional-order SIMDR models show that the fractional model is better candidate to describe the multi-drug resistance phenomena since it exhibits greater resistance of both Remdesivir and Hydroxychloroquine than its integer-order form. These advantages are because of the fractional-order SIMDR has higher degrees of freedom that affect the spread of the disease. Furthermore, the values derived from the fractional model are closer to most of components of the multi-drug resistance steady states due to the existence of memory effect, which erase the oscillations in the fractional model after some time. Thus, it has been shown that the fractional-order SIMDR is more accurate and realistic to describe and explain the data of COVID-19 in some models with multi-drug resistance phenomena. In addition, the simulation results show also that if COVID-19 persists then the fractional-order SIMDR is better in modeling the cases with multi-drug resistance phenomena since it has been shown that the integer-order SIMDR model converges to the healthy state faster than the corresponding fractional-order counterpart does.
Declaration of Competing Interest
No competing of interest.
Acknowledgement
The author would like to thank the Deanship of Scientific Research at Majmaah University for supporting this work under project no. 1439–40. The author thanks anonymous reviewers for their useful comments.
References
- 1.Zaki A.M., Van Boheemen S., Bestebroer T.M., Osterhaus A.D., Fouchier R.A. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med. 2012;367:1814–1820. doi: 10.1056/NEJMoa1211721. [DOI] [PubMed] [Google Scholar]
- 2.Wu F. A new coronavirus associated with human respiratory disease in China. Nature. 2020:1–10. doi: 10.1038/s41586-02-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lin Q. A conceptual model for the coronavirus disease 2019 (COVID-19) outbreak in Wuhan, China with individual reaction and governmental action. Int J Inf Dis. 2020;93:211–216. doi: 10.1016/j.ijid.2020.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.WHO . WHO; 2020. Coronavirus disease 2019 (COVID-19): situation report 126. [Google Scholar]
- 5.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72,314 cases from the Chinese center for disease control and prevention. JAMA. 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 6.https://www.fda.gov/media/137564/
- 7.Philippe Gautreta Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an openlabel non-randomized clinical trial. Int J Antimicrob Agents. 2020 Mar 20 doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 8.Chiotos K., et al. Multicenter initial guidance on use of antivirals for children with COVID-19/SARS- COV-2. J Pediatric Infect Dis Soc., piaa045. doi: 10.1093/jpids/piaa045. [DOI] [PMC free article] [PubMed]
- 9.Lee S.W. Biological resistance of hydroxychloroquine for Plasmodium vivax malaria in the Republic of Korea. Am J Trop Med Hyg. 2009;81(4):600–604. doi: 10.4269/ajtmh.2009.09-0102. [DOI] [PubMed] [Google Scholar]
- 10.Capel R.A. Hydroxychloroquine reduces heart rate by modulating the hyperpolarization- activated current IF: novel electrophysiological insights and therapeutic potential. Heart Rhythm. 2015;12(10):2186–2194. doi: 10.1016/j.hrthm.2015.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.WHO Emergencies, https://www.who.int/emergencies/ten-threats-to-global-health-in-2019.
- 12.Ameen I. The effect of vaccination and treatment of measles disease described by a fractional order model. World J Modell Simul. 2018;14:30–38. [Google Scholar]
- 13.Sarkar K., Khajanchi S., Nieto J.J. Modeling and forecasting the COVID-19 pandemic in India. Chaos Solitons Fractals. 2020 doi: 10.1016/j.chaos.2020.110049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kucharski A.J. Early dynamics of transmission and control of COVID-19: a mathematical modelling study. Lancet Global Health. 2020;3099(20):30144. doi: 10.1016/S1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bozkurt F., Yousef A., Baleanu D., Alzabut J. A mathematical model of the evolution and spread of pathogenic coronaviruses from natural host to human host. Chaos Solitons Fractals. 2020 doi: 10.1016/j.chaos.2020.109931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ben Fredj H., Chrif F. Novel Corona virus disease infection in Tunisia: mathematical model and the impact of the quarantine strategy. Chaos Solitons Fractals. 2020 doi: 10.1016/j.chaos.2020.109969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jiwei J., Jian D., Siyu L., Guidong L., Jingzhi L., Ben D. Modeling the control of COVID-19: impact of policy interventions and meteorological Factors. Electronic J Differ Equ. 2020;2020(23):1–24. [Google Scholar]
- 18.Ndaïrou F., Area I., Nieto J.J., Torres Delfim F.M. Mathematical modeling of COVID-19 transmission dynamics with a case study of Wuhan. Chaos Solitons Fractals. 2020;135(June 2020) doi: 10.1016/j.chaos.2020.109846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Okuonghae D., Omame A. Analysis of a mathematical model for COVID-19 population dynamics in Lagos, Nigeria. Chaos Solitons Fractals. 2020 doi: 10.1016/j.chaos.2020.110032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aslan I., Demir M., Wise M.G., Lenhart S. Modeling COVID-19: forecasting and analysing the dynamics of the outbreak in Hubei and Turkey. medRxiv preprint2020. doi: 10.1101/2020.04.11.20061952. [DOI]
- 21.Chimmula V.K.R., Zhang L. Time series forecasting of COVID-19 transmission in Canada using LSTM networks. Chaos Solitons Fractals. 2020;135 doi: 10.1016/j.chaos.2020.109864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ivorra B., Ferrandez M.R., Vela-Perez M., Ramos A.M. Mathematical modeling of the spread of the coronavirus disease 2019 (COVID-19) taking into account the undetected infections. The case of China. Commun Nonlinear Sci Numer Simulat. 2020;88 doi: 10.1016/j.cnsns.2020.105303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Çakan S. Dynamic analysis of a mathematical model with health care capacity for COVID-19 pandemic. Chaos Solitons Fractals. 2020;139 doi: 10.1016/j.chaos.2020.110033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Soukhovolsky V., Kovalev A., Pitt A., Kessel B. A new modelling of the COVID 19 pandemic. Chaos Solitons Fractals. 2020 doi: 10.1016/j.chaos.2020.110039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang Y., Wu Y., Zhang W. Comprehensive identification and isolation policies have effectively suppressed the spread of COVID-19. Chaos Solitons Fractals. 2020 doi: 10.1016/j.chaos.2020.110041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cooper I., Mondal A., Antonopoulos C.G. A SIR model assumption for the spread of COVID-19 in different communities. Chaos Solitons Fractals. 2020 doi: 10.1016/j.chaos.2020.110057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang P., Zheng X., Li J., Zhu B. Prediction of epidemic trends in COVID-19 with Logistic model and machine lLearning technics. Chaos Solitons Fractals. 2020 doi: 10.1016/j.chaos.2020.110058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Salgotra R., Gandomi M., Gandomi A.H. Evolutionary modelling of the COVID-19 pandemic in fifteen most affected countries. Chaos Solitons Fractals. 2020 doi: 10.1016/j.chaos.2020.110118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ullah Saif, MA. Khan. Modeling the impact of nonpharmaceutical interventions on the dynamics of novel coronavirus with optimal control analysis with a case study. Chaos Solitons Fractals. 2020 doi: 10.1016/j.chaos.2020.110075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Asamoah J.K.K., Owusu M.A., Jin Z., Oduro F.T., Abidemi A., Esther Gyasi E.O. Global stability and cost-effectiveness analysis of COVID-19 considering the impact of the environment:using data from Ghana. Chaos Solitons Fractals. 2020 doi: 10.1016/j.chaos.2020.110103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Basu S., Campbell R.H. Going by the numbers: learning and modeling COVID-19 disease dynamics. Chaos Solitons Fractals. 2020 doi: 10.1016/j.chaos.2020.110140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Annas S., Pratama M.I., Rifandi M., Sanusi W., Side S. Stability analysis and numerical simulation of SEIR model for pandemic COVID-19 spread in Indonesia. Chaos Solitons Fractals. 2020 doi: 10.1016/j.chaos.2020.110072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Croccolo F., Roman H.E. Spreading of infections on random graphs: a percolation-type model for COVID-19. Chaos Solitons Fractals. 2020 doi: 10.1016/j.chaos.2020.110077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kaxiras E., Neofotistos G., Angelaki E. The first 100 days: modeling the evolution of the COVID-19 pandemic. Chaos Solitons Fractals. 2020 doi: 10.1016/j.chaos.2020.110114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee C., Li Y., Kim J. The Susceptible-Unidentified infected-Confirmed (SUC) epidemic model for estimating unidentified infected population for COVID-19. Chaos Solitons Fractals. 2020 doi: 10.1016/j.chaos.2020.110090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mandal M., Jana S., Nandi S.K., Khatua A., Adak S., Kar T.K. A model based study on the dynamics of COVID-19: prediction and control. Chaos Solitons Fractals. 2020 doi: 10.1016/j.chaos.2020.109889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bekiros S., Kouloumpou D. SBDiEM. A new mathematical model of infectious disease dynamics. Chaos Solitons Fractals. 2020 doi: 10.1016/j.chaos.2020.109828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reis R.F. Characterization of the COVID-19 pandemic and the impact of uncertainties, mitigation strategies, and underreporting of cases in South Korea, Italy, and Brazil. Chaos Solitons Fractals. 2020 doi: 10.1016/j.chaos.2020.109888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mahajan A., Sivadas N.A., Solanki R. An epidemic model SIPHERD and its application for prediction of the spread of COVID-19 infection in India. Chaos Solitons Fractals. 2020;140 doi: 10.1016/j.chaos.2020.110156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bagal D.K., Rath A., Barua A., Patnaik D. Estimating the parameters of susceptible-infected-recovered model of COVID-19 cases in India during lockdown periods. Chaos Solitons Fractals. 2020;140 doi: 10.1016/j.chaos.2020.110154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Papo A minimal model of hospital patients’ dynamics in COVID-19. Chaos Solitons Fractals. 2020;140 doi: 10.1016/j.chaos.2020.110157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Džiugys Simplified model of Covid-19 epidemic prognosis under quarantine and estimation of quarantine effectiveness. Chaos Solitons Fractals. 2020;140 doi: 10.1016/j.chaos.2020.110162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Samui P., Mondal J., Khajanchi S. A mathematical model for COVID-19 transmission dynamics with a case study of India. Chaos Solitons Fractals. 2020 doi: 10.1016/j.chaos.2020.110173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Khan F., Saeed A., Ali S. Modelling and forecasting of new cases, deaths and recover cases of COVID-19 by using vector autoregressive model in Pakistan. Chaos Solitons Fractals. 2020 doi: 10.1016/j.chaos.2020.110189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ghanbari B. On forecasting the spread of the COVID-19 in Iran: the second wave. Chaos Solitons Fractals. 2020;140 doi: 10.1016/j.chaos.2020.110176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Matouk A.E. Advanced Applications of Fractional Differential Operators to Science and Technology. IGI Global. 2020 doi: 10.4018/978-1-7998-3122-8. [DOI] [Google Scholar]
- 47.Matouk A.E. Chaos synchronization of a fractional-order modified van der pol–duffing system via new linear control, backstepping control and Takagi-Sugeno fuzzy approaches. Complexity. 2016;21:116–124. [Google Scholar]
- 48.Matouk A.E. Chaos synchronization between two different fractional systems of Lorenz family. Math Probl Eng. 2009;2009 [Google Scholar]
- 49.Matouk A.E. Chaos, feedback control and synchronization of a fractional-order modified autonomous van der pol-duffing circuit. Commun Nonlinear Sci Numer Simulat. 2011;16:975–986. [Google Scholar]
- 50.Hegazi A.S., Matouk A.E. Dynamical behaviors and synchronization in the fractional order hyperchaotic chen system. Appl Math Lett. 2011;24:1938–1944. [Google Scholar]
- 51.Al-khedhairi A., Matouk A.E., Khan I. Chaotic dynamics and chaos control for the fractional-order geomagnetic field model. Chaos Solitons Fractals. 2019;128:390–401. [Google Scholar]
- 52.Al-khedhairi A., Matouk Askar SS. Computations of synchronization conditions in some fractional-order chaotic and hyperchaotic systems. Pramana – J Phys. 2019;92(72):11. [Google Scholar]
- 53.Atangana A., Gómez-Aguilar J.F. Fractional derivatives with no-index law property: application to chaos and statistics. Chaos Solitons Fractals. 2018;114:516–535. [Google Scholar]
- 54.Atangana A., Baleanu D. Caputo-Fabrizio derivative applied to groundwater flow within confined aquifer. J Eng Mech. 2017;143(5) [Google Scholar]
- 55.Atangana A., Baleanu D. New fractional derivatives with non-local and non-singular kernel: theory and application to heat transfer model. Therm Sci. 2016;20:763–769. [Google Scholar]
- 56.Atangana A. Non validity of index law in fractional calculus: a fractional differential operator with Markovian and non-Markovian properties. Physica A. 2018;505:688–706. [Google Scholar]
- 57.Hristov J. Derivatives with Non-Singular kernels from the Caputo-Fabrizio definition and beyond: appraising analysis with emphasis on diffusion models. Frontiers (Boulder) 2017;1:270–342. [Google Scholar]
- 58.Yang X.-.J. Methods and applications. CRC Press; New York: 2019. General Fractional Derivatives: theory. [Google Scholar]
- 59.Atangana A. Modelling the spread of COVID-19 with new fractal-fractional operators: can the lockdown save mankind before vaccination? Chaos Solitons Fractals. 2020;136 doi: 10.1016/j.chaos.2020.109860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Khan M.A., Atangana A. Modeling the dynamics of novel coronavirus (2019- nCoV) with fractional derivative. Alexandria Eng J. 2020 doi: 10.1016/j.aej.2020.02.033. [DOI] [Google Scholar]
- 61.Tuan N.H., Mohammadi H., Rezapour S. A mathematical model for COVID-19 transmission by using the Caputo fractional derivative. Chaos Solitons Fractals. 2020;140 doi: 10.1016/j.chaos.2020.110107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Higazy M. Novel fractional order SIDARTHE mathematical model of the COVID-19 pandemic. Chaos Solitons Fractals. 2020 doi: 10.1016/j.chaos.2020.110007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Alkahtani B.S.T., Alzaid S.S. A novel mathematics model of covid-19 with fractional derivative. Stability and numerical analysis. Chaos Solitons Fractals. 2020 doi: 10.1016/j.chaos.2020.110006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang Z. A novel covid-19 mathematical model with fractional derivatives: singular and nonsingular kernels. Chaos Solitons Fractals. 2020 doi: 10.1016/j.chaos.2020.110060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yadav R.P., Verma R. A numerical simulation of fractional order mathematical modeling of COVID-19 disease in case of Wuhan China. Chaos Solitons Fractals. 2020;140 doi: 10.1016/j.chaos.2020.110124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Baleanu D., Mohammadi H., Rezapour S. A fractional differential equation model for the COVID-19 transmission by using the Caputo–Fabrizio derivative. Adv Differ Equ. 2020;2020:299. doi: 10.1186/s13662-020-02762-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mohammad M., Trounev A. On the dynamical modeling of COVID-19 involving Atangana–Baleanu fractional derivative and based on Daubechies framelet simulations. Chaos Solitons Fractals. 2020;140 doi: 10.1016/j.chaos.2020.110171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang Z., Jain S. Mathematical model of Ebola and Covid-19 with fractional differential operators: non-Markovian process and class for virus pathogen in the environment. Chaos Solitons Fractals. 2020;140 doi: 10.1016/j.chaos.2020.110175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Elettreby M.F., Ahmed E. Multi-drug antimicrobial resistance model. Math Meth Appl Sci. 2020:1–12. doi: 10.1002/mma.6433. [DOI] [Google Scholar]
- 70.Podlubny I. Academic Press; New York: 1999. Fractional differential equations. [Google Scholar]
- 71.Matignon D. IMACS, IEEE-SMC Proceedings. Vol. 2. 1996. Stability results for fractional differential equations with ap- plications to control processing, in: computational engineering in systems and application multi-conference; pp. 963–968. July. [Google Scholar]
- 72.Ahmed E., Elgazzar A.S. On fractional order differential equations model for non-local epidemics. Physica A. 2007;379:607–614. doi: 10.1016/j.physa.2007.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Matouk A.E. Stability conditions, hyperchaos and control in a novel fractional order hyperchaotic system. Phys Lett A. 2009;373:2166–2173. [Google Scholar]
- 74.Matouk A.E., Khan I. Complex dynamics and control of a novel physical model using non-local fractional differential operator with singular kernel. J Adv Res. 2020;24:463–474. doi: 10.1016/j.jare.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wolf A., Swift J.B., Swinney H.L., Vastano J.A. Determining Lyapunov exponents from a time series. Physica D. 1985;16:285–317. [Google Scholar]