Abstract
PURPOSE
Cervical cancer (CC) is the most common and second-most deadly cancer among Peruvian women. Access to services is strongly associated with CC screening uptake. This study investigated geospatial features contributing to utilization of screening. We used geolocated data and screening information from a Knowledge, Attitudes, and Practice (KAP) survey implemented in Iquitos, Peru in 2017.
MATERIALS AND METHODS
The KAP collected cross-sectional CC screening history from 619 female interviewees age 18-65 years within 5 communities of varying urbanization levels. We used spatial statistics to determine if screened households tended to cluster together or cluster around facilities offering screening in greater numbers than expected, given the underlying population density.
RESULTS
On the basis of K-functions, screened households displayed greater clustering among each other as compared with clustering among unscreened households. Neighborhood-level factors, such as outreach, communication, or socioeconomic condition, may be functioning to generate pockets of screened households. Cross K-functions showed that screened households are generally located closer to health facilities than unscreened households. The significance of facility access is apparent and demonstrates that travel and time barriers to seeking health services must be addressed.
CONCLUSION
This study highlights the importance of considering geospatial features when determining factors associated with CC screening uptake. Given the observed clustering of screened households, neighborhood-level dynamics should be further studied to understand how they may be influencing screening rates. In addition, results demonstrate that accessibility issues must be carefully considered when designing an effective cancer screening program that includes screening, follow-up, and treatment.
INTRODUCTION
Among women worldwide, cervical cancer (CC) is the fourth-most incident and fourth-most deadly cancer. Low- to middle-income countries (LMICs) shoulder most of the CC burden1; 51% of the incident cases and 59% of the deaths occurred in less-developed regions.2 In Peru, CC is the most incident and second-most deadly cancer in women.3
CONTEXT
Key Objective
Do patterns of cervical cancer screening uptake among Amazonian Peruvian women depend on geospatial factors? We used a unique methodology that combined a traditional Knowledge, Attitudes, and Practice—KAP—survey with detailed person-level geospatial data to answer this question.
Knowledge Generated
Surveyed households where at least 1 screened woman resided tended to be located closer to each other, compared with the proximity of households with no screened women. In addition, women who had received cervical cancer screening generally lived closer to the facilities that perform this procedure, relative to women who had not been screened.
Relevance
Neighborhood-level dynamics should be further studied to understand how they may be influencing screening rates. Accessibility issues must also be carefully considered when designing location-specific, community- and culturally tailored screening programs that include screening, follow-up, and treatment, to assist in the fight against cervical cancer within the Amazonian region of Peru.
The disproportionate burden in LMICs has been attributed to lack of access to effective CC screening and human papillomavirus (HPV) vaccination programs.4 Peruvian national guidelines at the time of this study recommended screening for CC via HPV tests (every 5 years) or visual inspection with acetic acid (VIA) tests (every 3 years) from age 30-49 years, and via Papanicolaou (Pap) smear (every 3 years) from age 50-64 years.5,6 Peruvian women have self-reported that health center distance and accessibility are barriers to preventive service uptake.7,8 In addition, women from coastal Peruvian cities reported greater Pap smear use than urban-dwelling women in the rainforest or highlands.9 These studies demonstrate the importance of location of residence in CC screening uptake among Peruvian women. This prior research, however, was based on self-reports, whether qualitative or obtained in surveys.
We aimed to study the association between quantitative measurements of distance and the utilization of CC screening among Amazonian Peruvian women using unique methodology that combined a traditional Knowledge, Attitudes, and Practice (KAP) survey with detailed person-level geospatial data. By considering geospatial factors, in addition to traditionally explored factors for screening, this research can help advance Peruvian CC prevention programs and more broadly assist in combating the most incident and second-most deadly cancer among women in many LMICs.
MATERIALS AND METHODS
Data were collected from 619 female interviewees age 18-65 years via an interviewer-administered KAP survey in 2017. Although Peruvian CC screening guidelines recommend screening beginning at age 30 years, we chose to also survey 18- to 29-year-old women because Pap smears are a component of national prenatal care,10 and many women get their first screening at that time. KAP questions captured cross-sectional sociodemographic, CC screening, treatment, HPV vaccination history, and geographic coordinate information from interviewees. In addition, CC screening and HPV vaccination history was recorded for all female family members residing in the same household as the interviewees.
The KAP was implemented across 5 communities in Iquitos, Peru. Iquitos is a geographically large and diverse city in the Peruvian Amazon. The 5 communities were selected to reflect representative urban and rural catchment areas of health facilities in the largest health network of Iquitos: the southern district of San Juan Bautista (micro red Iquitos sur). San Juan Bautista had a 2017 population of 42,163 adult women, according to Peruvian National Census estimates.11 The health facilities included in our study are operated through the Peruvian Ministry of Health, where services are free for all individuals holding the Peruvian national health insurance, the Seguro Integral de Salud (SIS). SIS is available to all Peruvians who live in poverty or extreme poverty, for pregnant women, and for children; it is used by approximately 60% of the Peruvian population.12
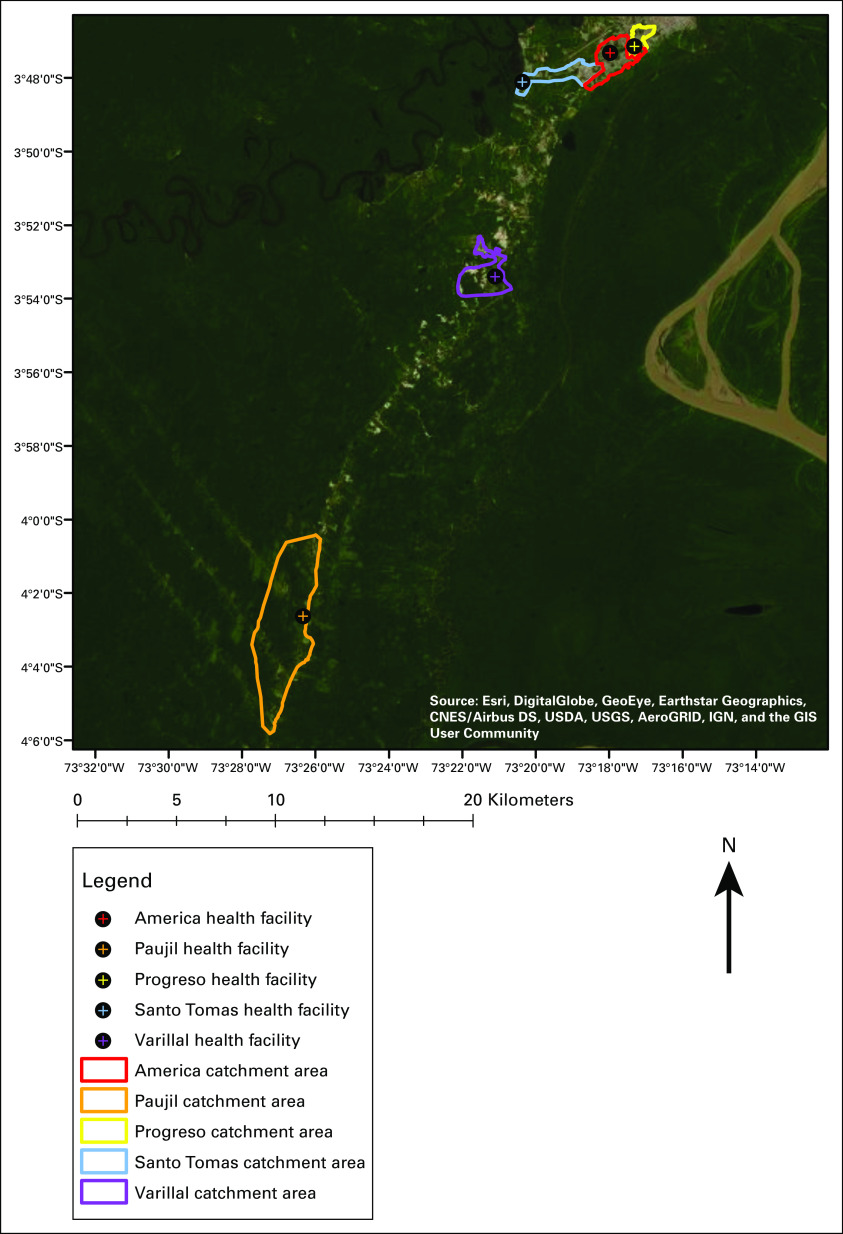
The interview breakdown across the 5 communities, herein referred to by the name of the health facility serving each catchment area, was 205 women in America, 123 in Paujil, 129 in Progreso, 108 in Santo Tomas, and 54 in Varillal (Fig 1).13 The facilities were all the first point of CC care and offered Pap smears, VIA tests, and CC awareness programs. America and Progreso were categorized as urban environments, Santo Tomas peri-urban, and Paujil and Varillal rural.
FIG 1.
Locations of the 5 Iquitos communities selected for study. America and Progreso are urban communities, Santo Tomas is peri-urban, and Paujil and Varillal are rural.
The George Washington University (GWU) Institutional Review Board (IRB) approved this study, and the Johns Hopkins Bloomberg School of Public Health IRB deferred approval to GWU. The IRB of the Peruvian nongovernmental organization, Asociación Benéfica PRISMA, also approved this study, and the Tulane School of Public Health and Tropical Medicine IRB deferred approval to PRISMA.
Descriptive Statistics
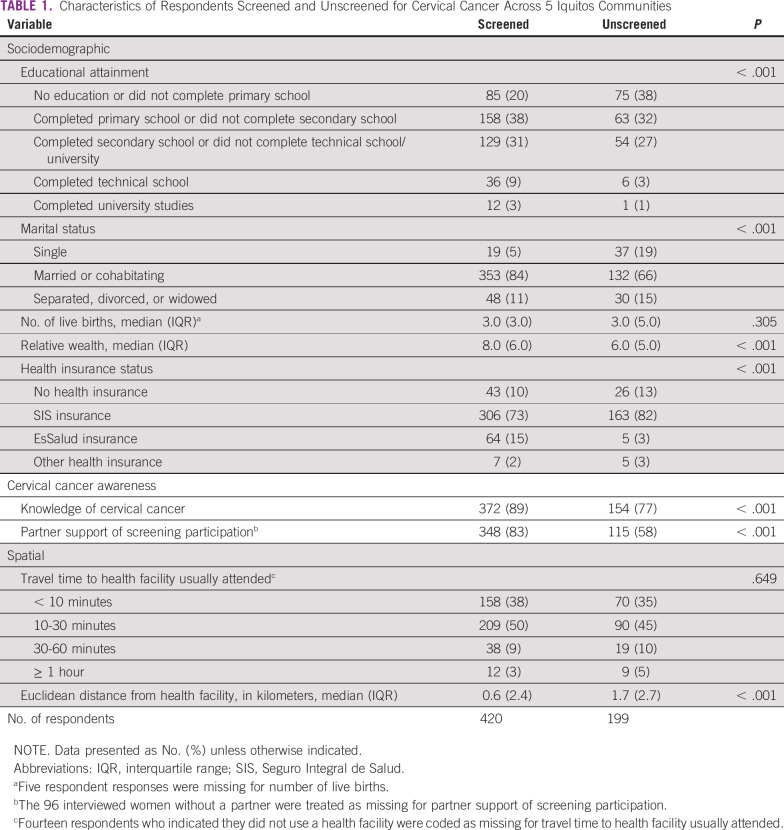
We compared women by CC screening history on a number of exposures with a demonstrated association with CC screening uptake.8,14-18 These variables collected in the KAP included the sociodemographic factors of educational attainment, marital status, number of live births, relative wealth, and health insurance status; the CC awareness factors of knowledge of CC and having a partner who is supportive of screening participation; and the spatial factors of self-reported travel time to health facility usually attended and Euclidean distance from health facility (in kilometers), as measured by collected geocoded data.
Interviewee CC screening history was operationalized through a respondent replying positively to ever having a CC screening test (Pap smear, VIA, molecular test, or colposcopy/biopsy) done by a health professional. If a surveyed woman indicated that she had not had a prior CC screening test, or did not know, then she was coded as never having been screened.
Educational attainment was defined by the interviewee’s highest level of education completed: no education or did not complete primary school, completed primary school or did not complete secondary school, completed secondary school or did not complete technical school/university, completed technical school, and completed university studies. Marital status was coded as single, marriage or cohabitation, and dissolution via separation, divorce, or spousal death. Number of live births was calculated by subtracting self-reported number of pregnancy losses or abortions from number of pregnancies and gestations. Relative wealth was built by summing the number of household services or items an individual reported owning, including light, landline, cell phone, internet, cable TV, access to the internet by cell phone, refrigerator(s), TV(s), blender(s), computer(s)/laptop(s)/tablet(s), fan(s), radio(s)/sound equipment, electric cooker(s) and/or gas, washing machine(s), microwave(s), bicycle(s)/tricycle(s), motorcycle(s), motorcar(s)/mototaxi(s), and automobile(s). Health insurance status was defined as no health insurance, SIS insurance, EsSalud insurance (formal employer-based insurance, for those on salary), and other form of health insurance (ie, police/military or private).
Knowledge of CC was coded as positive if a respondent reported having seen or heard anything about CC. Having a partner who is supportive of CC screening participation was considered positive if an interviewee stated that their partner supports them getting a CC screening test.
Self-reported travel time to health facility usually attended was defined as < 10 minutes, 10-30 minutes, 30-60 minutes, and ≥ 1 hour. Euclidean distance from health facility was calculated as the number of kilometers each interviewed household was from their assigned SIS health facility.
Significant person-level differences (P < .05) relative to CC screening history between categorical variables were ascertained with χ2 tests, and continuous variables were compared with Mood’s median test.19
Spatial Statistics
We examined clustering of household-level CC screening occurrences, both among other screened households within each community and around health facilities offering the services. Clustering is the occurrence of events within an area that is greater than expected given the underlying population density.20 We were interested in the distance between interviewed households and other households or health facilities and so analyzed CC screening on the household level. A household was defined as screened if the interviewee or a female family member residing in the household had received screening. Furthermore, because spatial variation is influenced by the study area, these analyses were conducted separately for each of the 5 Iquitos communities.
We quantified spatial clustering of household-level CC screening uptake via the (cross) K-function. K-functions examine clustering around an outcome location across various distances from that location, controlling for the expected number of outcome occurrences.21 Cross K-functions assess spatial interaction between different outcome types and were used to search for clustering of households around their respective health facility. This analysis relied on the assumption that ≥ 1 female resident within a screened household had traveled to her health facility for screening.
Separate (cross) K-functions were calculated within each of the 5 Iquitos communities for screened and unscreened households. Then the difference in (cross) K-functions between screened and unscreened households was computed; 95% significance boundaries, determined through Monte Carlo random labeling, indicated if a (cross) K-function difference was statistically significant across a range of point distances, with a null hypothesis of the difference being equal to zero.22 For additional details regarding the analyses presented in this paper, see Appendix.
RESULTS
Descriptive Statistics
A greater proportion of screened respondents completed primary school through university, whereas more than one-third of unscreened women did not complete primary school (P < .001; Table 1). Most women were married or cohabitating. A greater percentage of unscreened interviewees stated they were single (19%), as compared with screened respondents (5%; P < .001). Screened women had a larger median relative wealth score (8.0 for screened v 6.0 for unscreened; P < .001), as well as a greater proportion of respondents with EsSalud health insurance (15% of screened women with EsSalud v 3% of unscreened women; P < .001).
TABLE 1.
Characteristics of Respondents Screened and Unscreened for Cervical Cancer Across 5 Iquitos Communities
A larger proportion of screened respondents had knowledge of CC (89%) compared with unscreened respondents (77%; P < .001). A total of 83% of screened women stated that their partner supported CC screening, relative to only 58% of unscreened women (P < .001). The median distance from a health facility for screened women was 0.6 km, whereas unscreened women were a median distance of 1.7 km away (P < .001).
Spatial Statistics
At approximately 225-550 m from interviewed households in the America community, screened houses clustered more than unscreened houses. These results were not statistically significant (Fig 2A). At all distances from the America health facility, there was not any consistently greater clustering around the health facility among screened or unscreened households. Any observed differences were largely statistically insignificant (Fig 2B).
FIG 2.
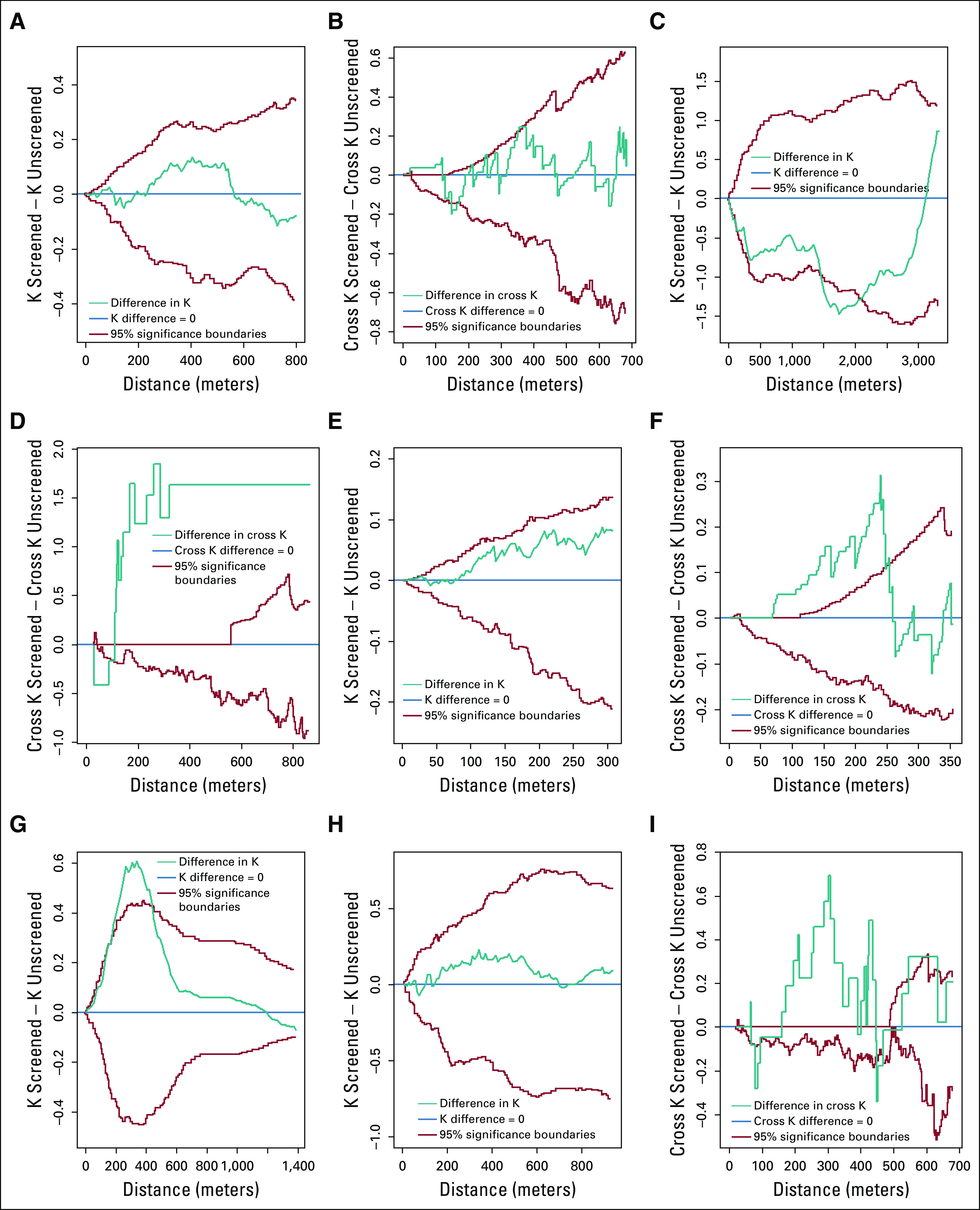
Difference in K-functions between screened and unscreened households, and difference in cross K-functions between screened households and the health facility, and unscreened households and the health facility within the 5 Iquitos communities. Ninety-five percent significance boundaries were obtained through Monte Carlo random labeling. (A) America, K difference. (B) America, cross K difference. (C) Paujil, K difference. (D) Paujil, cross K difference. (E) Progreso, K difference. (F) Progreso, cross K difference. (G) Santo Tomas, K difference. (H) Varillal, K difference. (I) Varillal, cross K difference. Results for Santo Tomas, cross K difference not presented, as the interviewed Santo Tomas households were too far removed from the health facility to allow for meaningful analyses.
In Paujil at a distance of 0-3,000 m from a household, unscreened houses clustered more than screened houses; there was a statistically significant difference in clustering at a distance of approximately 1,500-2,100 m (Fig 2C). Screened households demonstrated statistically significant greater clustering around the health facility than unscreened households at all distances beyond approximately 125 m from the health facility (Fig 2D).
In Progreso, screened households clustered more than unscreened households beyond a distance of roughly 75 m from a house. This clustering was not statistically significant (Fig 2E). At approximately 75-250 m from the Progreso health facility, screened houses clustered around the health facility more than unscreened households, with statistical significance (Fig 2F).
At distances ranging from 0-1,250 m from Santo Tomas households, screened houses clustered more than unscreened houses. This difference was statistically significant at a distance of approximately 150-400 m from a household (Fig 2G).
Screened Varillal households demonstrated greater clustering than unscreened households at approximately 150-650 m beyond a household; this difference was not statistically significant (Fig 2H). Screened Varillal households displayed statistically significant greater clustering around the health facility than unscreened households at a distance of approximately 150-425 m from the health facility (Fig 2I).
DISCUSSION
There are several important findings in this study, each with associated implications on ways to improve rates of CC screening. Households where ≥ 1 screened woman resided tended to be located closer to each other, as compared with the proximity of households with no screened women. Neighborhood-level factors, such as outreach by community health workers,23 community-based educational programs,24,25 and communication among neighbors,26,27 may play a role in creating pockets of screened households within Amazonian Peru. Neighborhood socioeconomic condition may also affect screening participation. Interviews with Peruvian health professionals demonstrated that although the cost of CC screening examinations was not perceived as a barrier to uptake, general socioeconomic deprivation was; impoverished women were much more focused on their daily survival than receiving preventive health care services.28 Women residing in affluent neighborhoods have the opportunity to focus on expenses beyond the day-to-day—such as CC screening—and this may help explain the observed clustering of screened households.
Peruvian health professionals also believed women were aware of the availability of CC screening tests, but did not receive them because of taboo, fear, and embarrassment, lack of knowledge, and negative health care experiences.28 These barriers demonstrate how social networks may also be partially responsible for screening uptake patterns. Prior studies have demonstrated that information sharing and support among family and friends are critical to the utilization of cancer screening.29-31 Procedural knowledge and a subsequent positive CC screening experience can be spread throughout a woman’s social network,32 encouraging others to seek screening. Conversely, a negative experience may discourage others from screening.32
Future research should identify the neighborhood-level dynamics that promote or prevent CC screening in Amazonian Peru, so these can be incorporated into interventions. A cluster detection analysis could identify any local clusters of screened and unscreened households and compare the households falling within these clusters on sociodemographic characteristics and communication behaviors. This would highlight the neighborhood-level factors that may differ between pockets of screened and unscreened households, and health officials could focus on these factors to improve CC screening rates within unscreened neighborhoods.
Women who received CC screening generally lived closer to the facilities that perform this procedure, relative to women who had not been screened. Several factors could account for this observation. The neighborhoods surrounding health facilities may be more socioeconomically developed,33,34 and thus women who live closer to the facilities would have the income and time to allow for screening. Health facility educational outreach programs would most easily reach women who live close by, which could encourage these women to be screened.35,36 Or, proximity to a health facility may increase screening rates simply because of the lower monetary and time sacrifice in traveling to the facility to get screened.7,8 Whether it is because of the effects of community outreach or reduced travel burden, our results demonstrate that geographic access to health facilities is essential for uptake of CC screening. It should be noted, however, that this observation operates on the household level, and individual reasons for participating or not participating in CC screening will vary.
Interventional efforts should focus on decreasing the distance gap to screening, particularly in remote, rural locations. Mobile health campaigns can alleviate travel distance and time barriers. These campaigns have been effective at increasing uptake of CC services internationally, including in Peru,37-40 although the required frequency of the campaigns to ensure sustainable effect is uncertain—particularly because a successful CC screening program must also ensure that women are able to receive any required follow-up diagnostics or treatment. Under the screening strategy used at the time of our study, follow-up would normally be managed in the capital city of Iquitos.
Self-sampling for HPV can also help to improve rates of CC screening in areas with limited access to health facilities.41-43 Pilot programs have demonstrated uptake acceptability and efficacy in Uganda,44 Bolivia,45 India,46 among indigenous New Zealanders,47 and in Peru.48 As the prevalence of HPV self-sampling increases, this procedure will face some of the same challenges encountered by traditional screening methods, such as lack of knowledge, fear, and distrust.49,50 Women have also cited doubt in their ability to correctly perform the self-sample as a barrier to uptake.49,50 Information sharing through social networks may counter these HPV self-sampling obstacles, much in the same way that neighborhood support could have resulted in pockets of screened households observed in this study. Alternative strategies for delivering HPV test results that do not require patient travel or a provider home visit could increase satisfaction with the CC screening system and reduce some of the clustering of screened households around health facilities.51
Although our study had many strengths, including incorporation of geospatial measurement and analytical methods to study CC screening uptake in Amazonian Peru, there are considerations in interpreting the findings. Interviewee CC screening history was self-reported, which may have influenced accuracy. This could have biased findings toward or away from the null, depending on the nature of the relationship between recall of screening history and location of residence.52,53 We also assumed that ≥ 1 female residing in a CC screen–positive household had sought screening at a health facility. This assumption is reasonable since, as of 2004, > 90% of Peruvian pregnant women received prenatal care at a health facility, and a Pap smear is included in the first prenatal visit.10 In 2017, the national average for receipt of prenatal care was 97.5%, with this number ranging between 68.3% and 85.3% in Loreto (the department within which Iquitos is located).10
The health facilities included in our study may have differed in quality of care provided. Varillal was a level 1 primary care facility, meaning that it was staffed entirely by nonmedical health professionals and health technicians. Paujil, Santo Tomas, and Progreso were level 2 facilities, indicating that there was a doctor on duty. America was a level 3 facility, classified as such because of more advanced personnel (specialized doctors, laboratory technicians) available for support and supervision.54 Women who receive care from the health facilities must do so within their community, so the facility level may have uniquely factored into the decision whether to get screened within each community.
Finally, the KAP survey strategy was not specifically designed for a geospatial analysis. Households were selected by interviewing a predetermined number of women within each community to represent relative population density and surveying every 5 households within selected community neighborhoods. The survey strategy was slightly altered in Paujil—as some households are 3-5 hours away from the health facility, combining travel by boat and foot—where every household was approached for interview until the predetermined number of women was surveyed. Had households been selected with greater spread within each community, geospatial analytic results may have shifted.
This study demonstrates that location-specific, community- and culturally tailored interventions are needed in the Amazonian region of Peru to aid in the uptake of CC screening, and to ensure that follow-up and treatment services for women with abnormal screening results are accessible. Availability of follow-up procedures is even more critical than screening, because these are currently only offered at centralized locations and are more challenging to access. Efforts to improve accessibility of vital CC screening and treatment services will assist in the fight against one of the most incident and deadly cancers among Peruvian women.
Appendix
Spatial clustering of screened/unscreened households within each community was examined with K-functions. K-functions are estimated with the equation
| (1) |
where is the estimated K-function at distance h from an arbitrary outcome, si, and n is the total number of outcomes in the study area. dij is the distance between si and any other outcome sj. I is the indicator function, which equals 1 if the distance between point si and sj is less than or equal to the K-function distance h being estimated, and equals 0 if the distance between point si and sj is greater than h. The indicator function is inserted to properly weight points close to the edge of the study area, where the weight, wij, is the proportion of circumference of a circle with center si and radius dij that falls within the study area. Finally, is the constant spatial intensity (expected number of occurrences) of outcomes in the study area (Curriero F: Spatial Point Pattern 2. 2017).
To quantify spatial interaction between health facilities and surveyed households categorized by cervical cancer screening history, cross K-functions were calculated. Cross K-functions can be represented by the general form
| (2) |
where is the estimated cross K-function between outcomes i and j at distance h from outcome i, E is the number of j outcomes within a distance (h) of i outcomes, and λj is the constant spatial intensity of j outcomes (Curriero F: Spatial Point Pattern 4. 2017). In practice, the cross K-function is calculated in a similar fashion to what is presented in Equation 1; rather than centering a circle at an outcome location and searching for other outcomes of the same type within that circle, a circle is centered at an outcome i location and the number of j outcomes within that circle is ascertained (Curriero F: Spatial Point Pattern 4. 2017). In the application of this research, circles of varying radiuses (h) were centered at a community’s health facility, and the number of screened or unscreened households captured within the circles was determined.
Monte Carlo random labeling was used to generate 95% significance boundaries for the difference in (cross) K-function estimates between screened and unscreened households. Briefly, this method uses the established locations of Knowledge, Attitudes, and Practice–surveyed households (and for cross K-functions, health facility location), and randomly assigns these locations a different label. For example, when calculating cross K-functions, a point would be randomly labeled as a screened household, an unscreened household, or a health facility. Because these labels are random, a geographical layout of spatial independence (or for K-functions, complete spatial randomness) is simulated (Besag J, et al: J R Stat Soc Ser C Appl Stat 26:327-333, 1977). Within each new randomly labeled layout, a (cross) K-function is calculated for each outcome category (in the case of this research, screened and unscreened households). These simulations are repeated a large number of times, and the clustering calculations are ranked. Then, on the basis of the level of significance desired, an m-th ranked (cross) K-function is selected (Diggle PJ, et al: Biometrics 47:1155-1163, 1991). Finally, the difference in the m-th ranked (cross) K-functions is calculated, which provides an upper or lower limit of significance for the difference in clustering functions. If an observed (cross) K-function difference (nonsimulated data) falls outside of these upper and lower limits, this suggests that the difference statistically significantly deviates from a difference that would be seen under spatial independence (cross K-function) or complete spatial randomness (K-function).
All analyses were performed within RStudio version 1.1.423 (Boston, MA; http://www.R-project.org/; http://www.rstudio.com/). Data cleaning and variable creation used the packages ‘measurements’ (https://CRAN.R-project.org/package=measurements), ‘rgdal’ (https://CRAN.R-project.org/package=rgdal), and ‘geosphere’ (https://CRAN.R-project.org/package=geosphere). Geospatial analysis and visualization relied on the packages ‘spatstat’ (http://www.crcpress.com/Spatial-Point-Patterns-Methodology-and-Applications-with-R/Baddeley-Rubak-Turner/9781482210200/), ‘splancs’ (https://CRAN.R-project.org/package=splancs), ‘maptools’ (https://CRAN.R-project.org/package=maptools), ‘maps’ (https://CRAN.R-project.org/package=maps), and ‘sp’ (https://cran.r-project.org/doc/Rnews/).
Presented at the International Papillomavirus Conference, Sydney, Australia, October 2-6, 2018.
SUPPORT
Supported by Grant No. R01 CA190366-01 (V.A.P.-S., P.E.G.) from the National Institutes of Health/National Cancer Institute.
AUTHOR CONTRIBUTIONS
Conception and design: Benjamin W. Barrett, Valerie A. Paz-Soldan, Graciela Meza Sánchez, Jhonny J. Córdova López, Patti E. Gravitt, Anne F. Rositch
Financial support: Patti E. Gravitt, Valerie A. Paz-Soldan, Anne F. Rositch
Administrative support: Patti E. Gravitt, Valerie A. Paz-Soldan, Anne F. Rositch
Provision of study material or patients: Patti E. Gravitt, Valerie A. Paz-Soldan
Collection and assembly of data: All authors
Data analysis and interpretation: Benjamin W. Barrett
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/go/site/misc/authors.html.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Patti E. Gravitt
Research Funding: Cepheid (Inst)
Anne F. Rositch
Consulting or Advisory Role: UE LifeSciences
No other potential conflicts of interest were reported.
REFERENCES
- 1. International Agency for Research on Cancer (IARC): Stewart BW, Wild CP (eds): World Cancer Report 2014. Lyon, France, IARC Press, 2014. [Google Scholar]
- 2. International Agency for Research on Cancer: GLOBOCAN 2018, Population Fact Sheets. Lyon, France. https://gco.iarc.fr/today/data/factsheets/populations/900-world-fact-sheets.pdf.
- 3. http://globocan.iarc.fr Ferlay J, Soerjomataram I, Ervik M, et al: GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11. Lyon, France, International Agency for Research on Cancer, 2013.
- 4.Catarino R, Petignat P, Dongui G, et al. Cervical cancer screening in developing countries at a crossroad: Emerging technologies and policy choices. World J Clin Oncol. 2015;6:281–290. doi: 10.5306/wjco.v6.i6.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. https://www.gob.pe/institucion/minsa/normas-legales/191413-1013-2016-minsa Ministerio de Salud, Republica del Perú: Resolución Ministerial No. 1013-2016.
- 6. http://bvs.minsa.gob.pe/local/MINSA/4232.pdf Ministerio de Salud, Republica del Perú: Plan Nacional de Prevención y Control de Cáncer de Cuello Uterino, 2017-2021.
- 7.Bingham A, Bishop A, Coffey P, et al. Factors affecting utilization of cervical cancer prevention services in low-resource settings. Salud Publica Mex. 2003;45(suppl 3):S408–S416. doi: 10.1590/s0036-36342003000900015. [DOI] [PubMed] [Google Scholar]
- 8.Agurto I, Bishop A, Sánchez G, et al. Perceived barriers and benefits to cervical cancer screening in Latin America. Prev Med. 2004;39:91–98. doi: 10.1016/j.ypmed.2004.03.040. [DOI] [PubMed] [Google Scholar]
- 9.Paz Soldan V.A, Lee FH, Carcamo C, et al. Who is getting Pap smears in urban Peru? Int J Epidemiol. 2008;37:862–869. doi: 10.1093/ije/dyn118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. https://www.inei.gob.pe/media/MenuRecursivo/publicaciones_digitales/Est/Lib1525/index.html Encuestas Demográfica y de Salud Familiar (ENDES), Perú: Encuesta Demográfica y de Salud Familiar 2017: Nacional y Regional.
- 11. Instituto Nacional de Estadística e Informática (INEI), Perú: Censos Nacionales de Población y Vivienda 2017. http://censos2017.inei.gob.pe/redatam/
- 12. Lazo-Gonzales O, Alcalde-Rabanal J, Espinosa-Henao O: El Sistema de Salud en Perú: Situación y Desafíos. Lima, Perú, Colegio Médico del Perú, Revistas Especializadas Peruanas, 2016. [Google Scholar]
- 13. Environmental Systems Research Institute (ESRI): ArcGIS Desktop: Release 10.5.1. Redlands, CA, Environmental Systems Research Institute, 2017. [Google Scholar]
- 14.Scarinci IC, Beech BM, Kovach KW, et al. An examination of sociocultural factors associated with cervical cancer screening among low-income Latina immigrants of reproductive age. J Immigr Health. 2003;5:119–128. doi: 10.1023/a:1023939801991. [DOI] [PubMed] [Google Scholar]
- 15.Rodríguez MA, Ward LM, Pérez-Stable EJ. Breast and cervical cancer screening: Impact of health insurance status, ethnicity, and nativity of Latinas. Ann Fam Med. 2005;3:235–241. doi: 10.1370/afm.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Winkler J, Bingham A, Coffey P, et al. Women’s participation in a cervical cancer screening program in northern Peru. Health Educ Res. 2008;23:10–24. doi: 10.1093/her/cyl156. [DOI] [PubMed] [Google Scholar]
- 17.Robles SC, Ferreccio C, Tsu V, et al. Assessing participation of women in a cervical cancer screening program in Peru. Rev Panam Salud Publica. 2009;25:189–195. doi: 10.1590/s1020-49892009000300001. [DOI] [PubMed] [Google Scholar]
- 18.Soneji S, Fukui N. Socioeconomic determinants of cervical cancer screening in Latin America. Rev Panam Salud Publica. 2013;33:174–182. doi: 10.1590/s1020-49892013000300003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hervé M: RVAideMemoire: Testing and Plotting Procedures for Biostatistics. R package version 0.9-69. https://CRAN.R-project.org/package=RVAideMemoire.
- 20.Cuzick J, Edwards R. Spatial clustering for inhomogeneous populations. J R Stat Soc B. 1990;52:73–104. [Google Scholar]
- 21. Curriero F: Spatial Point Pattern 1. Personal Collection of F Curriero, Johns Hopkins Bloomberg School of Public Health, Baltimore MD, 2017. [Google Scholar]
- 22. Curriero F: Spatial Point Pattern 3. Personal Collection of F Curriero, Johns Hopkins Bloomberg School of Public Health, Baltimore MD, 2017. [Google Scholar]
- 23.O’Donovan J, O’Donovan C, Nagraj S. The role of community health workers in cervical cancer screening in low-income and middle-income countries: A systematic scoping review of the literature. BMJ Glob Health. 2019;4:e001452. doi: 10.1136/bmjgh-2019-001452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fang CY, Lee M, Feng Z, et al. Community-based cervical cancer education: Changes in knowledge and beliefs among Vietnamese American women. J Community Health. 2019;44:525–533. doi: 10.1007/s10900-019-00645-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koç Z, Özdeş EK, Topatan S, et al. The impact of education about cervical cancer and human papillomavirus on women’s healthy lifestyle behaviors and beliefs: Using the PRECEDE educational model. Cancer Nurs. 2019;42:106–118. doi: 10.1097/NCC.0000000000000570. [DOI] [PubMed] [Google Scholar]
- 26.Vamos CA, Calvo AE, Daley EM, et al. Knowledge, behavioral, and sociocultural factors related to human papillomavirus infection and cervical cancer screening among inner-city women in Panama. J Community Health. 2015;40:1047–1056. doi: 10.1007/s10900-015-0030-4. [DOI] [PubMed] [Google Scholar]
- 27.Luque JS, Opoku S, Ferris DG, et al. Social network characteristics and cervical cancer screening among Quechua women in Andean Peru. BMC Public Health. 2016;16:181. doi: 10.1186/s12889-016-2878-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Paz-Soldán VA, Bayer AM, Nussbaum L, et al. Structural barriers to screening for and treatment of cervical cancer in Peru. Reprod Health Matters. 2012;20:49–58. doi: 10.1016/S0968-8080(12)40680-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tejeda S, Thompson B, Coronado GD, et al. Barriers and facilitators related to mammography use among lower educated Mexican women in the USA. Soc Sci Med. 2009;68:832–839. doi: 10.1016/j.socscimed.2008.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stafford M, von Wagner C, Perman S, et al. Social connectedness and engagement in preventive health services: An analysis of data from a prospective cohort study. Lancet Public Health. 2018;3:e438–e446. doi: 10.1016/S2468-2667(18)30141-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Adunlin G, Cyrus JW, Asare M, et al. Barriers and facilitators to breast and cervical cancer screening among immigrants in the United States. J Immigr Minor Health. 2019;21:606–658. doi: 10.1007/s10903-018-0794-6. [DOI] [PubMed] [Google Scholar]
- 32.Erwin DO, Johnson VA, Trevino M, et al. A comparison of African American and Latina social networks as indicators for culturally tailoring a breast and cervical cancer education intervention. Cancer. 2007;109(2) suppl:368–377. doi: 10.1002/cncr.22356. [DOI] [PubMed] [Google Scholar]
- 33.Horev T, Pesis-Katz I, Mukamel DB. Trends in geographic disparities in allocation of health care resources in the US. Health Policy. 2004;68:223–232. doi: 10.1016/j.healthpol.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 34.Mayer ML. Disparities in geographic access to pediatric subspecialty care. Matern Child Health J. 2008;12:624–632. doi: 10.1007/s10995-007-0275-3. [DOI] [PubMed] [Google Scholar]
- 35.Paul P, Winkler JL, Bartolini RM, et al. Screen-and-treat approach to cervical cancer prevention using visual inspection with acetic acid and cryotherapy: Experiences, perceptions, and beliefs from demonstration projects in Peru, Uganda, and Vietnam. Oncologist. 2013;18:1278–1284. doi: 10.1634/theoncologist.2013-0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Williamson J, Ramirez R, Wingfield T. Health, healthcare access, and use of traditional versus modern medicine in remote Peruvian Amazon communities: A descriptive study of knowledge, attitudes, and practices. Am J Trop Med Hyg. 2015;92:857–864. doi: 10.4269/ajtmh.14-0536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Swaddiwudhipong W, Chaovakiratipong C, Nguntra P, et al. A mobile unit: An effective service for cervical cancer screening among rural Thai women. Int J Epidemiol. 1999;28:35–39. doi: 10.1093/ije/28.1.35. [DOI] [PubMed] [Google Scholar]
- 38.Guruge S, Hunter J, Barker K, et al. Immigrant women’s experiences of receiving care in a mobile health clinic. J Adv Nurs. 2010;66:350–359. doi: 10.1111/j.1365-2648.2009.05182.x. [DOI] [PubMed] [Google Scholar]
- 39.Kumar Y, Mishra G, Gupta S, et al. Cancer screening for women living in urban slums--acceptance and satisfaction. Asian Pac J Cancer Prev. 2011;12:1681–1685. [PubMed] [Google Scholar]
- 40.Ferris DG, Shapiro J, Fowler C, et al. The impact of accessible cervical cancer screening in Peru—The Día del Mercado Project. J Low Genit Tract Dis. 2015;19:229–233. doi: 10.1097/LGT.0000000000000112. [DOI] [PubMed] [Google Scholar]
- 41.Bansil P, Wittet S, Lim JL, et al. Acceptability of self-collection sampling for HPV-DNA testing in low-resource settings: A mixed methods approach. BMC Public Health. 2014;14:596. doi: 10.1186/1471-2458-14-596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Possati-Resende JC, de Lima Vazquez F, de Paula Pantano N, et al. Implementation of a cervical cancer screening strategy using HPV self-sampling for women living in rural areas. Acta Cytol. 2019;64:7–15. [Google Scholar]
- 43.Yeh PT, Kennedy CE, de Vuyst H, et al. Self-sampling for human papillomavirus (HPV) testing: A systematic review and meta-analysis. BMJ Glob Health. 2019;4:e001351. doi: 10.1136/bmjgh-2018-001351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ogilvie GS, Mitchell S, Sekikubo M, et al. Results of a community-based cervical cancer screening pilot project using human papillomavirus self-sampling in Kampala, Uganda. Int J Gynaecol Obstet. 2013;122:118–123. doi: 10.1016/j.ijgo.2013.03.019. [DOI] [PubMed] [Google Scholar]
- 45.Allende G, Surriabre P, Cáceres L, et al. Evaluation of the self-sampling for cervical cancer screening in Bolivia. BMC Public Health. 2019;19:80. doi: 10.1186/s12889-019-6401-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sowjanya AP, Paul P, Vedantham H, et al. Suitability of self-collected vaginal samples for cervical cancer screening in peri-urban villages in Andhra Pradesh, India. Cancer Epidemiol Biomarkers Prev. 2009;18:1373–1378. doi: 10.1158/1055-9965.EPI-08-1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Adcock A, Cram F, Lawton B, et al. Acceptability of self-taken vaginal HPV sample for cervical screening among an under-screened Indigenous population. Aust N Z J Obstet Gynaecol. 2019;59:301–307. doi: 10.1111/ajo.12933. [DOI] [PubMed] [Google Scholar]
- 48.Levinson KL, Abuelo C, Salmeron J, et al. The Peru Cervical Cancer Prevention Study (PERCAPS): The technology to make screening accessible. Gynecol Oncol. 2013;129:318–323. doi: 10.1016/j.ygyno.2013.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Racey CS, Gesink DC. Barriers and facilitators to cervical cancer screening among women in rural Ontario, Canada: The role of self‐collected HPV testing. J Rural Health. 2016;32:136–145. doi: 10.1111/jrh.12136. [DOI] [PubMed] [Google Scholar]
- 50.Katz ML, Zimmermann BJ, Moore D, et al. Perspectives from health-care providers and women about completing human papillomavirus (HPV) self-testing at home. Women Health. 2017;57:1161–1177. doi: 10.1080/03630242.2016.1243608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sanchez Antelo V, Kohler RE, Curotto M, et al. Developing SMS content to promote Papanicolaou triage among women who performed HPV self- collection test: Qualitative study. JMIR Form Res. 2020;4:e14652. doi: 10.2196/14652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Copeland KT, Checkoway H, McMichael AJ, et al. Bias due to misclassification in the estimation of relative risk. Am J Epidemiol. 1977;105:488–495. doi: 10.1093/oxfordjournals.aje.a112408. [DOI] [PubMed] [Google Scholar]
- 53.Coughlin SS. Recall bias in epidemiologic studies. J Clin Epidemiol. 1990;43:87–91. doi: 10.1016/0895-4356(90)90060-3. [DOI] [PubMed] [Google Scholar]
- 54.ESAN ¿Cómo funciona la categorización en establecimientos de salud? https://www.esan.edu.pe/apuntes-empresariales/2018/03/como-funciona-la-categorizacion-en-establecimientos-de-salud/