Abstract
Background
Individuals treated for drug-resistant tuberculosis (DR-TB) with aminoglycosides (AGs) in resource-limited settings often experience permanent hearing loss, yet there is no practical method to identify those at higher risk. We sought to develop a clinical prediction model of AG-induced hearing loss among patients initiating DR-TB treatment in South Africa.
Methods
Using nested, prospective data from a cohort of 379 South African adults being treated for confirmed DR-TB with AG-based regimens we developed the prediction model using multiple logistic regression. Predictors were collected from clinical, audiological, and laboratory evaluations conducted at the initiation of DR-TB treatment. The outcome of AG-induced hearing loss was identified from audiometric and clinical evaluation by a worsened hearing threshold compared with baseline during the 6-month intensive phase.
Results
Sixty-three percent of participants (n = 238) developed any level of hearing loss. The model predicting hearing loss at frequencies from 250 to 8000 Hz included weekly AG dose, human immunodeficiency virus status with CD4 count, age, serum albumin, body mass index, and pre-existing hearing loss. This model demonstrated reasonable discrimination (area under the receiver operating characteristic curve [AUC] = 0.71) and calibration (χ2[8] = 6.10, P = .636). Using a cutoff of 80% predicted probability of hearing loss, the positive predictive value of this model was 83% and negative predictive value was 40%. Model discrimination was similar for ultrahigh-frequency hearing loss (frequencies >9000 Hz; AUC = 0.81) but weaker for clinically determined hearing loss (AUC = 0.60).
Conclusions
This model may identify patients with DR-TB who are at highest risk of developing AG-induced ototoxicity and may help prioritize patients for AG-sparing regimens in clinical settings where access is limited.
Keywords: model development, model validation, ototoxicity
This novel prediction model identifies individuals at high risk of aminoglycoside-induced hearing loss during treatment for drug-resistant tuberculosis, based on observable routine clinical characteristics. This model may help to prioritize patients for aminoglycoside-sparing regimens in settings where access is limited.
Tuberculosis (TB) is now the leading cause of infectious mortality worldwide and is particularly common and lethal in human immunodeficiency virus (HIV)–endemic areas such as South Africa [1]. A growing concern in South Africa is drug-resistant TB (DR-TB) [2]. DR-TB includes multidrug-resistant TB (TB resistant to first-line drugs, at least rifampin and isoniazid) and extensively drug-resistant TB (TB resistant to rifampin, isoniazid, and 2 classes of second-line drugs: fluoroquinolones and injectable aminoglycosides [AGs]) [2].
Although treatment outcomes of DR-TB using injectable-containing short-course regimens are improving [3–6], hearing loss remains one of the most common adverse drug effects associated with an AG given during the intensive phase of treatment. AG-induced ototoxicity begins with high-frequency hearing loss and may occur with or without tinnitus, can progress even with discontinuation of AG treatment, and is often permanent [7, 8]. Hearing loss causes social isolation, threatens quality of life, and puts employment stability and family prosperity at risk [9–15]. Although AG-induced hearing loss is a known adverse reaction that occurs in 23% to 69% of patients, AG has been a mainstay of DR-TB treatment as recommended by the World Health Organization [7, 8, 16].
There are several risk factors that may exacerbate AG-induced ototoxicity. High AG plasma concentrations and frequent dosing may increase risk; however, monitoring of drug concentrations is infeasible in most resource-limited settings [7, 8]. The risk of hearing loss is also impacted by HIV coinfection as a result of severe immunosuppression along with antiretroviral therapy (ART) [1, 16, 17]. Both ART and anti-TB drugs may also cause renal impairment, which hastens ototoxicity [18–22]. Clinical manifestations of TB such as malnutrition and severe, disseminated inflammation may be associated with increased incidence of hearing loss [23–29]. Pre-existing hearing loss, prior use of ototoxic drugs for DR-TB treatment, advanced age, and substance use may increase the risk of subsequent hearing loss [30, 31].
Despite these known risks, there is currently no readily available, practical means to quantitatively assess the risk of hearing loss during DR-TB treatment. Such a risk assessment could help to prioritize individuals for AG-sparing regimens and/or more intensive monitoring while being treated with AGs. We therefore aimed to develop a prediction model of AG-induced hearing loss during DR-TB treatment in South Africa.
METHODS
Study Design and Setting
We performed an analysis of a prospective cohort nested within an ongoing cluster-randomized trial in South Africa. The parent study investigated the effects of nurse case management (NCM) in improving treatment outcomes in individuals with DR-TB. Data were collected across 10 public TB hospitals in the Eastern Cape and KwaZulu-Natal provinces. Data for the parent study were collected through medical chart review, patient interviews, and the National Health Laboratory System online laboratory portal from enrollment to the end of multidrug-resistant-TB treatment. Full details regarding the parent study have been reported elsewhere (https://clinicaltrials.gov; NCT02129244) [32].
Study Population
We included in our analysis all participants who met the following criteria: (1) 13 years of age or older, (2) microbiologically confirmed DR-TB using cartridge-based Xpert (Cepheid) mycobacterium tuberculosis/rifampicinF, (3) enrolled in the parent study from November 2014 to June 2017, and (4) provided informed consent within 7 days of treatment initiation. We excluded patients who did not receive either kanamycin or amikacin intramuscular injection and those whose confirmatory drug susceptibility testing results (within 6 months of treatment initiation) showed either drug-sensitive TB or resistance to either fluoroquinolones or AGs. Participants were not excluded due to their impaired renal function.
Predictors and Measures
The following variables were abstracted from the parent study’s baseline data: (1) demographic characteristics and medical history, including previous TB history, prescribed medications, and substance use; (2) presence of lung cavities on chest radiograph at DR-TB diagnosis; (3) serum creatinine levels to calculate estimated glomerular filtration rate; (4) HIV infection history including use of ART and cluster of differentiation 4 (CD4) count; and (5) body mass index (BMI). Since the parent study did not collect serum albumin levels, baseline albumin results were collected from the South African National Health Laboratory System online portal as a routine laboratory test for DR-TB treatment.
The following variables were abstracted from the parent study’s baseline and monthly follow-up data during the 6-month intensive phase: (1) DR-TB treatment regimen including AG type, AG dose, and frequency; (2) confirmatory TB test results including sputum culture and drug susceptibility testing; and (3) auditory symptoms (ie, hearing loss and tinnitus) and audiometric hearing evaluation results. The initial AG dose was determined based on the patients’ baseline weight and the weight band-dosing table guided selection of dose (milliliters) in practice [33]. The proxy measure of cumulative (or weekly) AG dose per body weight (standardized weekly AG dose) following treatment initiation was calculated as follows:
Audiometric evaluation was conducted by on-site audiologists or trained nurses to establish the lowest intensity of sound (= hearing threshold) in decibels that the person could hear at frequencies ranging from 250 to 8000 Hz [31]. Then, the hearing threshold for each frequency was used to define a degree of hearing loss (Table 1).
Table 1.
Study Variables and Degree of Hearing Loss
| Degree of Hearing Loss | ASHA Hearing-loss Range,dB* | Hearing-loss Range in This Study, dB |
|---|---|---|
| Normal | –10 to 15 | –10 to 25 |
| (Slight) | 16 to 25 | |
| Mild | 26 to 40 | 26 to 40 |
| Moderate | 41 to 55 | 41 to 55 |
| Moderately severe | 56 to 70 | 56 to 70 |
| Severe | 71 to 90 | 71 to 90 |
| Profound | 91+ | 91+ |
Abbreviation: ASHA, American Speech-language-hearing Association.
*Data from reference [39]
This study defined pre-existing composite hearing loss on an a priori basis as either (1) a hearing threshold outside of the normal range (>25 dB) in 1 or both ears at any frequency in the range from 250 to 8000 Hz, tested by either standard audio-booth or computer-based portable audiometer (KUDUwave, Emoyo) at baseline audiometry (ie, pre-existing audiometric hearing loss), or (2) self-reported auditory symptoms at baseline. The outcomes of AG-induced hearing loss were further defined as (1) clinically determined hearing loss resulting in a change in treatment (ie, reduced or stopped AGs) due to ototoxicity confirmed by either audiological evaluation or self-reported auditory symptoms or (2) audiometric hearing loss defined as a deterioration of at least 1 category of hearing loss compared with baseline hearing in the same range of frequencies in 1 or both ears during the intensive phase.
Statistical Analysis for Model Development and Validation
We used multivariable logistic regression to develop prediction models by introducing the following predictors: standardized weekly AG dose, HIV status, use of ART, CD4 count, presence of lung cavities, renal impairment (estimated glomerular filtration rate), BMI, serum albumin, pre-existing composite hearing loss, age, sex, previous TB history, smoking, and alcohol use. We first selected as a development cohort the 265 individuals who had follow-up audiometric data using frequencies from 250 to 8000 Hz but not at ultrahigh frequencies (ie, hearing threshold from 9000 to 16 000 Hz). We then used the 114 participants with full audiometric data (including ultrahigh frequencies) as a validation cohort. We selected the final prediction model as the one that maximized discrimination—measured as the area under receiver operating characteristic (ROC) curve (AUC or c-statistic)—without statistical evidence of poor calibration (ie, P < .05 on the Hosmer-Lemeshow χ2 goodness-of-fit test, grouping the data into 10 equal bins) in the development cohort. We included HIV with CD4 count and weekly AG dose as important predictors on an a priori basis. When addition of an extra variable did not improve the AUC by at least 0.01, we opted for the more parsimonious model. We then report the predictive accuracy of the model, measured as the c-statistic (for discrimination) and Hosmer-Lemeshow χ2 goodness-of-fit statistic (for calibration) after applying the model to the validation cohort. We performed a second validation using data on clinical assessment of AG-induced ototoxicity from 671 participants who did not undergo audiometric evaluation. All statistical tests were conducted at a 2-sided significance level of 0.05 using STATA 15 (StataCorp) [34].
Ethics Approval
The parent study was approved by the Provincial Health Research Committee of the Eastern Cape and KwaZulu-Natal Provincial Departments of Health in South Africa. The parent study and this substudy were both approved by the Biomedical Research and Ethical Committee of the University of KwaZulu-Natal in South Africa and the Institutional Review Board of the Johns Hopkins Medical Institutions (NA_00078899/CIR00024657).
RESULTS
Population Description
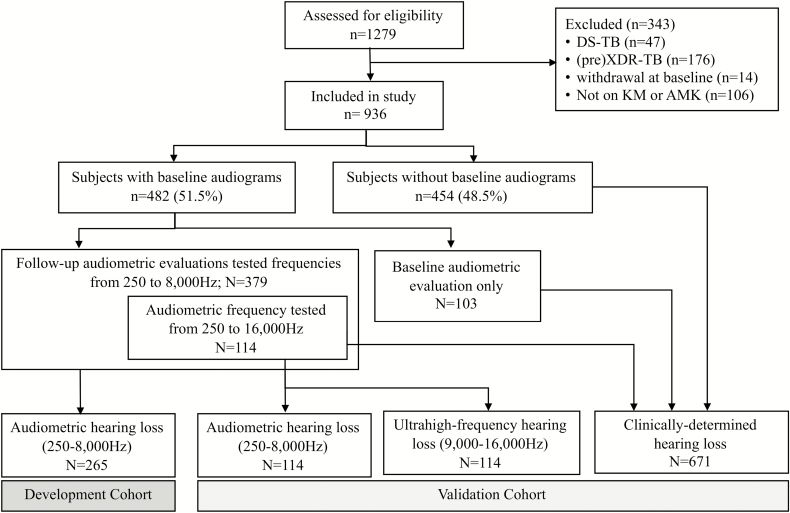
Of the 1279 participants enrolled in the parent study, 936 were eligible for the present analysis (Figure 1). At baseline, the population’s median age was 35 (interquartile range, 29–42) years, 54% were male, 49% were unemployed, 58% were hospitalized at treatment initiation, 75% were HIV coinfected, 41% had a prior history of drug-sensitive TB, and 5% had a prior history of DR-TB infection treated with second-line injectable anti-TB drugs. In terms of nutritional status, 32% (n = 296) were underweight (ie, BMI <18.5 kg/m2) and 59% (n = 551) had hypoalbuminemia (serum albumin <35 g/L). Of 697 HIV-coinfected participants, 46% were taking ART and 34% were severely immunosuppressed (CD4 count <200 cells/mm3). The majority received kanamycin (90%, n = 847), with 9 hospital sites offering kanamycin and 1 offering amikacin.
Figure 1.
Diagram of study flow. Abbreviations: AMK, amikacin; DS-TB, drug-sensitive tuberculosis; KM, kanamycin; XDR-TB, extensively drug-resistant tuberculosis.
Of 936 participants, 51% (n = 481) were tested for baseline hearing by either audio-booth (n = 238) or KUDUwave (n = 243); 60% (n = 289) had at least mild hearing loss (≥26 dB) at any frequency between 250 and 8000 Hz. Additionally, 157 of 481 participants were tested for ultrahigh-frequency hearing (>8000 Hz). Of those, 74% (n = 116) had at least mild hearing loss at frequencies from 250 to 16 000 Hz, and 67% (n = 105) had ultrahigh-frequency hearing loss. One hundred forty-two of 936 (15%) reported auditory symptoms at baseline, and those who had auditory symptoms were more likely to have audiometry-confirmed hearing loss at baseline at any frequencies from 250 to 8000 Hz (prevalence ratio, 1.41; 95% confidence interval [CI], 1.10–1.87).
Among those who were tested for hearing at baseline (n = 481), 379 were tested for follow-up audiometric evaluations during the intensive phase and therefore eligible to contribute to the development or validation of the prediction model. Of those, 114 were tested for ultrahigh-frequency audiometry (ie, for frequencies from 250 to 16 000 Hz) and served as the validation cohort, with the remaining 265 being used for model development. During follow-up, 63% of participants (n = 238) developed any level of hearing loss at frequencies from 250 to 8000 Hz; of those, 56% (n = 134) had their AG either discontinued or reduced in dose due to ototoxicity. The development cohort had a 3 times greater number of hospitalized participants compared with the validation cohort. Table 2 describes participant characteristics according to analytical cohort.
Table 2.
Baseline Characteristics of Participants in the Model
| Development Cohort: Audiometric HL (250–8000 Hz) (n = 265) | Validation Cohort: Audiometric HL (250–16 000 Hz) (n = 114) | Second Validation Cohort: Clinically Determined HL (n = 671) | |
|---|---|---|---|
| Sex | |||
| Male | 145 (55) | 56 (49) | 361 (54) |
| Female | 120 (45) | 58 (51) | 310 (46) |
| Age, mean (SD), y | 35.61 (10.56) | 33.86 (9.39) | 36.43 (11.24) |
| 13–19 years | 14 (5) | 9 (8) | 31 (5) |
| 20–29 years | 69 (26) | 30 (26) | 171 (26) |
| 30–39 years | 104 (39) | 47 (41) | 251 (37) |
| 40–49 years | 44 (17) | 24 (21) | 128 (19) |
| ≥50 years | 34 (13) | 4 (4) | 90 (13) |
| Smoking | |||
| Nonsmoker | 168 (63) | 82 (72) | 453 (68) |
| Light smoker (<10 cigarettes/day) | 58 (22) | 20 (18) | 129 (19) |
| Heavy smoker (≥10 cigarettes/day) | 22 (8) | 10 (9) | 61 (9) |
| Alcohol use | |||
| Nondrinker | 146 (55) | 71 (62) | 406 (61) |
| Less than once per week | 92 (35) | 32 (28) | 198 (30) |
| More than twice per week | 27 (10) | 9 (8) | 56 (8) |
| HIV status and CD4 count (cells/mm3) | |||
| HIV negative | 58 (22) | 28 (25) | 181 (27) |
| HIV positive with CD4 ≥200 | 82 (31) | 45 (39) | 201 (30) |
| HIV positive with CD4 <200 | 100 (38) | 30 (26) | 219 (33) |
| HIV positive; unknown CD4 count | 25 (9) | 11 (10) | 70 (10) |
| ART status, n | 207 | 86 | 490 |
| No ART at baseline | 75 (36) | 32 (37) | 190 (39) |
| On ART at baseline | 132 (64) | 54 (63) | 300 (61) |
| Previous history of DR-TB | |||
| New DR-TB | 134 (50) | 56 (49) | 345 (51) |
| Ever had prior TB | 121 (46) | 56 (49) | 299 (45) |
| Unknown | 10 (4) | 2 (2) | 27 (4) |
| Pre-existing composite HLa | |||
| Normal hearing | 117 (44) | 66 (58) | 451 (67) |
| Baseline HL | 148 (56) | 48 (42) | 218 (33) |
| Unknown | 0 (0) | 0 (0) | 2 (0) |
| BMI (kg/m2) | |||
| Underweight (<18.5) | 109 (41) | 27 (24) | 187 (28) |
| Normal (18.5–24.9) | 109 (41) | 61 (53) | 277 (41) |
| Overweight or obese (≥25) | 27 (10) | 24 (21) | 150 (16) |
| Unknown | 20 (8) | 2 (2) | 101 (15) |
| Serum albumin (g/L) | |||
| Normal (≥35) | 60 (23) | 26 (23) | 133 (20) |
| Hypoalbuminemia (<35) | 135 (51) | 75 (66) | 416 (62) |
| Unknown | 70 (26) | 13 (11) | 122 (18) |
| eGFR (mL/min/1.73 m2) | |||
| ≥90 | 162 (61) | 87 (76) | 427 (64) |
| 60–89 | 63 (24) | 19 (17) | 133 (20) |
| <60 | 16 (6) | 4 (3) | 51 (7) |
| Unknown | 24 (9) | 4 (3) | 60 (9) |
| AG type | |||
| Kanamycin | 226 (85) | 112 (98) | 621 (93) |
| Amikacin | 39 (15) | 2 (2) | 50 (7) |
| Hospitalization at baseline | |||
| Inpatient setting | 206 (78) | 26 (23) | 309 (46) |
| Outpatient setting | 59 (22) | 88 (77) | 362 (54) |
Data are presented as n (%) unless otherwise indicated. Abbreviations: AG, aminoglycoside; ART, antiretroviral therapy; BMI, body mass index; CD4, cluster of differentiation 4; DR-TB, drug-resistant tuberculosis; eGFR, estimated glomerular filtration rate; HIV, human immunodeficiency virus; HL, hearing loss; SD, standard deviation; TB, tuberculosis.
aPre-existing composite HL defined as confirmed by either audiometry or self-reported auditory symptoms.
Model Development and Validation
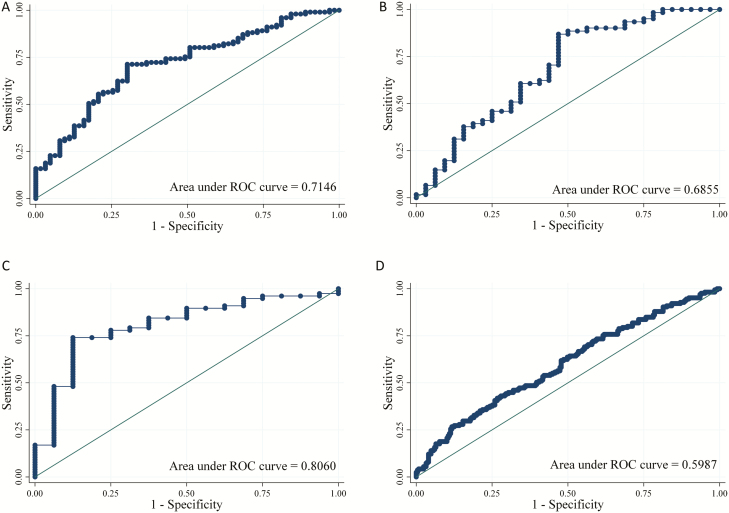
In the model development cohort of 265 participants, 62% (n = 165) developed audiometric hearing loss. We included standardized weekly AG dose and HIV with CD4 count in the model on an a priori basis. In addition, age and pre-existing hearing loss were added based on the findings from our preliminary analysis. In an analysis of ROC curves of hearing loss based on these 4 factors, the AUC was 0.65 (95% CI, 0.58–0.73) in the development cohort. Adding the factors of nutritional status including BMI and serum albumin (final model) led to a better prediction of hearing loss (AUC = 0.71; 95% CI, 0.64–0.79) without evidence of poor calibration (χ2[8] = 6.10; P = .636). The final model of hearing loss with odds ratios is shown in Table 3. Using a cutoff of 80% predicted probability of hearing loss, the positive predictive value of this model for predicting future AG-induced hearing loss was 83%, while the negative predictive value was 40%. Also, using a cutoff of 40% predicted probability, the positive predictive value was 69% and the negative predictive value was 100% (Table 4, Figure 2A).
Table 3.
Multivariable Logistic Regression Model Predicting Hearing Loss
| Predictors | Adjusted OR (95% CI) | P Value |
|---|---|---|
| Age (years) | 1.04 (1.01–1.08) | .014 |
| BMI (kg/m2) | ||
| <18.5 | 1 (Reference) | |
| 18.5–24.9 | 0.38 (0.18–0.82) | .014 |
| ≥25 | 0.28 (0.09–0.87) | .028 |
| Standardized weekly AG dose (mg/kg/week) | ||
| <60 | 1 (Reference) | |
| 60–74.9 | 0.66 (0.26–1.69) | .386 |
| ≥75 | 1.31 (0.52–3.33) | .569 |
| HIV status and CD4 count (cells/mm3) | ||
| HIV negative | 1 (Reference) | |
| HIV positive with CD4 ≥200 | 1.69 (0.68–4.22) | .261 |
| HIV positive with CD4 <200 | 2.02 (0.82–5.01) | .127 |
| Serum albumin (g/L) | 1.02 (0.97–1.08) | .486 |
| Pre-existing composite hearing lossa | 1.17 (0.55–2.46) | .685 |
| Full model: log odds of hearing loss = 0.045 (age) – 0.96 (BMI: 18.5–24.9) – 1.27 (BMI: ≥25) – 0.41 (weekly AG dose: 60–74.9) + 0.27 (weekly AG dose: ≥75) + 0.53 (HIV+ with CD4 ≥200) + 0.71 (HIV+ with CD4 <200) + 0.02 (serum albumin) + 0.15 (pre-existing composite hearing loss) – 1.61. |
Abbreviations: AG, aminoglycoside; BMI, body mass index; CD4, cluster of differentiation 4; CI, confidence interval; HIV, human immunodeficiency virus; OR, odds ratio.
aPre-existing composite hearing loss defined as confirmed by either audiometry or self-reported auditory symptoms.
Table 4.
Model Performance in Predicting Aminoglycoside-induced Hearing Loss
| Audiometric HL in Development Cohort (250–8000 Hz) | Audiometric HL in Validation Cohort (250–8000 Hz) | High-frequency HL in Validation Cohort (9000 Hz–16 000 Hz) | Clinically Determined HL Validation Cohort | |
|---|---|---|---|---|
| AUC (95% CI) | 0.71 (0.64–0.79) | 0.69 (0.56–0.81) | 0.81 (0.69–0.92) | 0.60 (0.54–0.66) |
| Hosmer-Lemeshow Goodness-of-fit | χ 2[8] = 6.10 (P = .636) | χ 2[8] = 8.03 (P = .431) | χ 2[8] = 6.48 (P = .593) | χ 2[8] = 4.34 (P = .825) |
| Cutoff of 80% predicted probability, % | ||||
| Sensitivity | 22.77 | 31.15 | 84.42 | 0.00 |
| Specificity | 93.65 | 87.50 | 56.25 | 100.00 |
| PPV | 85.19 | 82.61 | 90.28 | . |
| NPV | 43.07 | 40.00 | 42.86 | 60.10 |
| Cutoff of 40% predicted probability, % | ||||
| Sensitivity | 90.10 | 100.00 | 97.40 | 52.44 |
| Specificity | 23.81 | 12.50 | 0.00 | 58.30 |
| PPV | 65.47 | 68.54 | 82.42 | 45.50 |
| NPV | 60.00 | 100.00 | 0.00 | 64.86 |
Abbreviations: AUC, area under receiver operating characteristic curve; CI, confidence interval; HL, hearing loss; NPV, negative predictive value; PPV, positive predictive value.
Figure 2.
A–D, ROC curves for AG-induced hearing loss. Abbreviations: AG, aminoglycoside; ROC, receiver operating characteristic.
In the audiometric hearing-loss validation cohort (n = 114), 64% (n = 73) developed hearing loss at frequencies from 250 to 8000 Hz and 82% (n = 93) developed hearing loss from 9000 to 16 000 Hz. In this population, the prediction model demonstrated comparable discrimination (AUC = 0.69; 95% CI, 0.56–0.81) and calibration (χ2[8] = 8.03; P = .431) to that of the development cohort (Table 4, Figure 2B). Furthermore, when using ultrahigh-frequency hearing loss as an outcome, the prediction model exhibited higher discrimination (AUC = 0.81; 95% CI, 0.69–0.92) and reasonable calibration (χ2[8] = 6.48; P = .593) (Table 4, Figure 2C). When used to predict clinically assessed (rather than audiometrically confirmed) hearing loss, the model’s discrimination was diminished (AUC, 0.60; 95% CI, 0.54–0.66) (Table 4, Figure 2D).
DISCUSSION
We developed and validated a simple prediction tool that can be used to estimate the risk of hearing loss during the first 6 months of AG treatment for DR-TB. Since ototoxicity is dose dependent [35], our model confirmed that initial dosing of AG regimen is a significant predictor. We also found that baseline malnutrition (ie, underweight and hypoalbuminemia) was more strongly associated with hearing loss than was standardized weekly AG dose, perhaps reflecting pharmacokinetic vulnerability of underweight patients to high serum AG doses [36]. Our model validation suggests that, although developed for standard-range hearing loss, it may also predict ultrahigh-frequency hearing loss, which is an early manifestation of AG-induced ototoxicity [7, 37]. Taken together, these findings suggest that readily available clinical data can be used to predict risk of future AG-induced ototoxicity for those at highest risk with reasonable predictive accuracy.
In terms of the practical utility of this prediction model, we found that the model worked more effectively at the extremes—that is, in predicting individuals with very high or very low risk of AG-induced ototoxicity (see the edges of the ROC curves in Figure 2). For example, using a high-specificity cutoff (ie, 80% predicted probability of hearing loss) enabled exclusion of 88% of individuals in the validation cohort who would not develop hearing loss, with a positive predictive value of 83%. Similarly, a high-sensitivity cutoff (ie, 40% predicted probability of hearing loss) enabled prediction of 100% of individuals in the validation cohort who would experience hearing loss while identifying a very-low-risk population of 13% of individuals who could be safely given AGs (Table 4). This model can therefore help providers prioritize patients for AG-sparing regimens in settings where access to such regimens is limited.
This study has certain limitations. First, our sample size for model development and validation was relatively small, reflecting a potentially biased subset of the full cohort on whom audiometric data were available. Since a small sample size in developing a prediction model reduces predictive accuracy and increases variance in the validation of model performance, this model should be validated in other cohorts and used carefully in clinical settings. The concern about sample size is particularly acute for assessment of ultrahigh-frequency hearing loss, which is more clinically useful for early detection of ototoxicity but for which even fewer data were available in this setting. Second, our model did not include all potential predictors of AG-induced hearing loss because the selection of study variables was limited to those collected by the parent study. In practice, this model could be combined with clinical data including conductive hearing loss, objective measures of vestibular dysfunction, noise exposure, and family history to improve clinical decision making. Third, we acknowledge that there might be potential threats of intervention effects because NCM intervention sites may be more likely to facilitate hearing screening and modification of AG regimen since nurse case managers are more involved in patient care. Although the audiometric validation cohort consisted of more NCM intervention sites, this model was not adjusted for assignment of intervention site to maximize generalizability of the model. Fourth, BMI and audiometry data were collected by hospital staff members trained by their respective hospital and thus the quality of measurement is uncertain and may be subject to measurement error. Nevertheless, these programmatic measurements were used by healthcare providers to make clinical decisions including TB medication dosing, so while they may be biased relative to a gold standard, they reflect the data as might be collected if this prediction model were used in clinical practice. Fifth, this study only observed the outcome of hearing loss during the first 6 months of the intensive phase. Since hearing loss may progress even after AG discontinuation [38], it is necessary to follow patients’ hearing beyond the intensive phase of treatment. Finally, we did not perform a formal cost-effectiveness analysis to quantify the economic costs and benefits that could be expected by using this model to prioritize the allocation of more expensive AG-sparing regimens. Future studies performed in larger, well-defined prospective cohorts and that include regular audiometric evaluation and comprehensive history taking by specifically trained personnel, measurement of AG peaks and troughs, as well as cost-effectiveness analyses would be useful to further validate our findings.
Despite these limitations, this is the first study to develop a prediction model of AG-induced hearing loss among individuals being treated for DR-TB. Recently updated World Health Organization treatment guidelines offer, for the first time, an AG-sparing regimen to improve treatment outcomes and patients’ quality of life [39]; however, a number of patients with DR-TB, including those treated with recently recommended standard short-course regimens, will still receive amikacin [40]. Today, the selection of regimen is based solely on availability and clinical expertise; this prediction model offers a validated measure to support those decisions. If the risk of AG-induced hearing loss can be estimated at treatment initiation, healthcare providers can triage high-risk patients to newer, less ototoxic drugs such as bedaquiline that, while more costly, do not have hearing loss as a side effect. Importantly, in July 2017, the South African Department of Health initiated an AG-sparing regimen that includes bedaquiline; because our data collection ended in June 2017, we anticipate that bedaquiline availability had minimal impact on our study findings. In most resource-limited countries, TB programs do not have the financial resources to universally offer an AG-sparing regimen to people suffering from DR-TB. In these settings, our prediction model may be used to guide clinical decision making in the context of constrained resources. We intentionally included only clinical data that would be collected based on South African national guidelines for DR-TB management. Thus, this model can be applied without the need to perform additional laboratory tests or clinical evaluations. We also suggest the need to evaluate provider adherence to national guidelines of audiometric evaluation for those who are receiving injectable-containing regimens. The availability of on-site audiologists and well-functioning audiometers at TB hospitals must be audited regularly to make early detection of AG-induced hearing loss possible.
Conclusions
Our model suggests that patients’ initial AG dosing, nutritional status, HIV status, and pre-existing hearing loss at baseline can be used to predict the future development of AG-induced hearing loss. The findings have the potential to inform treatment guidelines using readily accessible data to prioritize patients with DR-TB to receive AG-sparing regimens. This model may therefore improve the management of DR-TB, offering a personalized intervention to prevent drug-induced hearing disability in underserved populations.
Notes
Disclaimer. The content is solely the responsibility of the authors and does not necessarily represent the official views of the organizations or institutions.
Financial support. This work was supported by the National Institute of Allergy and Infectious Disease (grant number R01 AI104488-01A1 to J. E. F.); the National Institute of Nursing Research (grant number F31 NR016910-01A1 to H. H.) of the National Institutes of Health; the Sigma Theta Tau International Global Nursing Research Grant; the Sigma Theta Tau International/Association of Nurses in AIDS Care Grant; Global Korean Nursing Foundation Scientific Award; Dr Scholl Foundation Dissertation Scholarship; and the Johns Hopkins Center for Global Health Established Field Placements Grant.
Potential conflicts of interest. H. W. F. reports personal fees and grants from Advanced Bionics Corporation and personal fees from MedEl Corporation, outside the submitted work. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. World Health Organization. Global tuberculosis report 2016 Available at: http://apps.who.int/iris/bitstream/10665/250441/1/9789241565394-eng.pdf?ua=1. Accessed 7 July 2017.
- 2. World Health Organization. WHO treatment guidelines for drug-resistant tuberculosis, 2016 updates Available at: https://apps.who.int/iris/bitstream/handle/10665/250125/9789241549639-eng.pdf?sequence=1. Accessed 7 July 2017.
- 3. Piubello A, Harouna SH, Souleymane MB, et al. . High cure rate with standardised short-course multidrug-resistant tuberculosis treatment in Niger: no relapses. Int J Tuberc Lung Dis 2014; 18:1188–94. [DOI] [PubMed] [Google Scholar]
- 4. Kuaban C, Noeske J, Rieder HL, Aït-Khaled N, Abena Foe JL, Trébucq A. High effectiveness of a 12-month regimen for MDR-TB patients in Cameroon. Int J Tuberc Lung Dis 2015; 19:517–24. [DOI] [PubMed] [Google Scholar]
- 5. Trébucq A, Schwoebel V, Kashongwe Z, et al. . Treatment outcome with a short multidrug-resistant tuberculosis regimen in nine African countries. Int J Tuberc Lung Dis 2018; 22:17–25. [DOI] [PubMed] [Google Scholar]
- 6. Nunn AJ, Phillips PPJ, Meredith SK, et al. ; STREAM Study Collaborators A trial of a shorter regimen for rifampin-resistant tuberculosis. N Engl J Med 2019; 380:1201–13. [DOI] [PubMed] [Google Scholar]
- 7. Huth ME, Ricci AJ, Cheng AG. Mechanisms of aminoglycoside ototoxicity and targets of hair cell pr otection. Int J Otolaryngol 2011; 2011:937861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gogtay NJ, Kshirsagar NA, Dalvi SS. Therapeutic drug monitoring in a developing country: an overview. Br J Clin Pharmacol 2001; 52(Suppl 1):103–8S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Contrera KJ, Betz J, Li L, et al. . Quality of life after intervention with a cochlear implant or hearing aid. Laryngoscope 2016; 126:2110–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Monteiro E, Shipp D, Chen J, Nedzelski J, Lin V. Cochlear implantation: a personal and societal economic perspective examining the effects of cochlear implantation on personal income. J Otolaryngol Head Neck Surg 2012; 41(Suppl 1):S43–8. [PubMed] [Google Scholar]
- 11. Sataloff RT. Hearing loss: economic impact. Ear Nose Throat J 2012; 91:10–2. [DOI] [PubMed] [Google Scholar]
- 12. Jung D, Bhattacharyya N. Association of hearing loss with decreased employment and income among adults in the United States. Ann Otol Rhinol Laryngol 2012; 121:771–5. [DOI] [PubMed] [Google Scholar]
- 13. Manrique-Huarte R, Calavia D, Huarte Irujo A, Girón L, Manrique-Rodríguez M. Treatment for hearing loss among the elderly: auditory outcomes and impact on quality of life. Audiol Neurootol 2016; 21(Suppl 1):29–35. [DOI] [PubMed] [Google Scholar]
- 14. Peters JPM, Ramakers GGJ, Smit AL, Grolman W. Cochlear implantation in children with unilateral hearing loss: a systematic review. Laryngoscope 2016; 126:713–21. [DOI] [PubMed] [Google Scholar]
- 15. Stika CJ, Hays RD. Development and psychometric evaluation of a health-related quality of life instrument for individuals with adult-onset hearing loss. Int J Audiol 2016; 55:381–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hong H, Budhathoki C, Farley JE. Increased risk of aminoglycoside-induced hearing loss in MDR-TB patients with HIV coinfection. Int J Tuberc Lung Dis 2018; 22:667–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Statistics South Africa. Mid-year population estimates, 2015: HIV prevalence estimates and the number of people living with HIV. Pretoria, South Africa: Statistics South Africa, 2015. [Google Scholar]
- 18. Crass RE. Gentamicin-induced ototoxicity in a carefully monitored renal-failure patient. Am J Hosp Pharm 1981; 38:540–5. [PubMed] [Google Scholar]
- 19. Prayle A, Watson A, Fortnum H, Smyth A. Side effects of aminoglycosides on the kidney, ear and balance in cystic fibrosis. Thorax 2010; 65:654–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jin S, Kim MH, Park JH, et al. . The incidence and clinical characteristics of acute serum creatinine elevation more than 1.5 mg/dL among the patients treated with tenofovir/emtricitabine-containing HAART regimens. Infect Chemother 2015; 47:239–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. De Waal R, Cohen K, Fox MP, et al. . Clinician compliance with laboratory monitoring and prescribing guidelines in HIV-1-infected patients receiving tenofovir. S Afr Med J 2016; 106:52–3. [DOI] [PubMed] [Google Scholar]
- 22. Kenyon C, Wearne N, Burton R, Meintjes G. The risks of concurrent treatment with tenofovir and aminoglycosides in patients with HIV-associated tuberculosis. South Afr J HIV Med 2011; 12:43–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hunter RL. Tuberculosis as a three-act play: a new paradigm for the pathogenesis of pulmonary tuberculosis. Tuberculosis (Edinb) 2016; 97:8–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Oshikoya KA, Senbanjo IO. Pathophysiological changes that affect drug disposition in protein-energy malnourished children. Nutr Metab (Lond) 2009; 6:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Traynor AM, Nafziger AN, Bertino JS Jr. Aminoglycoside dosing weight correction factors for patients of various body sizes. Antimicrob Agents Chemother 1995; 39:545–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Oshikoya KA, Sammons HM, Choonara I. A systematic review of pharmacokinetics studies in children with protein-energy malnutrition. Eur J Clin Pharmacol 2010; 66:1025–35. [DOI] [PubMed] [Google Scholar]
- 27. Blot SI, Pea F, Lipman J. The effect of pathophysiology on pharmacokinetics in the critically ill patient—concepts appraised by the example of antimicrobial agents. Adv Drug Deliv Rev 2014; 77:3–11. [DOI] [PubMed] [Google Scholar]
- 28. Ashour MN, Salem SI, El-Gadban HM, Elwan NM, Basu TK. Antioxidant status in children with protein-energy malnutrition (PEM) living in Cairo, Egypt. Eur J Clin Nutr 1999; 53:669–73. [DOI] [PubMed] [Google Scholar]
- 29. Khare M, Mohanty C, Das BK, Jyoti A, Mukhopadhyay B, Mishra SP. Free radica ls and antioxidant status in protein energy malnutrition. Int J Pediatr 2014; 2014:254396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Schacht J, Talaska AE, Rybak LP. Cisplatin and aminoglycoside antibiotics: hearing loss and its prevention. Anat Rec (Hoboken) 2012; 295:1837–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tysome JR KR. Heari ng: an introduction & practical guide. Boca Raton, FL: CRC Press, Taylor & Francis Group LLC, 2016. [Google Scholar]
- 32. Farley JE, Kelly AM, Reiser K, et al. . Development and evaluation of a pilot nurse case management model to address multidrug-resistant tuberculosis (MDR-TB) and HIV in South Africa. PLoS One 2014; 9:e111702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Republic of South Africa Department of Health. Management of drug-resistant tuberculosis: policy guidelines. Vol. 161 Pretoria, South Africa: Department of Health, 2013. [Google Scholar]
- 34. StataCorp. Stata statistical software: release 15. College Station, TX: StataCorp LLC,2017. [Google Scholar]
- 35. Gonzalez LS 3rd, Spencer JP. Aminoglycosides: a practical review. Am Fam Physician 1998; 58:1811–20. [PubMed] [Google Scholar]
- 36. Pai MP, Nafziger AN, Bertino JS Jr. Simplified estimation of aminoglycoside pharmacokinetics in underweight and obese adult patients. Antimicrob Agents Chemother 2011; 55:4006–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Jiang M, Karasawa T, Steyger PS. Aminoglycoside-induced cochleotoxicity: a review. Front Cell Neurosci 2017; 11:308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Marcotti W, van Netten SM, Kros CJ. The aminoglycoside antibiotic dihydrostreptomycin rapidly enters mouse outer hair cells through the mechano-electrical transducer channels. J Physiol 2005; 567:505–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. World Health Organization. WHO treatment guidelines for multidrug- and rifampicin-resistant tuberculosis, 2018 update. Geneva, Switzerland: World Health Organization, 2018. [Google Scholar]
- 40. World Health Organization. Rapid communications: key changes to treatment of multidrug- and rifampicin-resistant tuberculosis (MDR/RR-TB) Available at: http://www.who.int/tb/publications/2018/WHO_RapidCommunicationMDRTB.pdf?ua=1. Accessed 23 December 2018.