Abstract
BACKGROUND
Although spontaneous miscarriage is the most common complication of human pregnancy, potential contributing factors are not fully understood. Advanced maternal age has long been recognised as a major risk factor for miscarriage, being strongly related with fetal chromosomal abnormalities. The relation between paternal age and the risk of miscarriage is less evident, yet it is biologically plausible that an increasing number of genetic and epigenetic sperm abnormalities in older males may contribute to miscarriage. Previous meta-analyses showed associations between advanced paternal age and a broad spectrum of perinatal and paediatric outcomes. This is the first systematic review and meta-analysis on paternal age and spontaneous miscarriage.
OBJECTIVE AND RATIONALE
The aim of this systematic review and meta-analysis is to evaluate the effect of paternal age on the risk of spontaneous miscarriage.
SEARCH METHODS
PubMed, Embase and Cochrane databases were searched to identify relevant studies up to August 2019. The following free text and MeSH terms were used: paternal age, father’s age, male age, husband’s age, spontaneous abortion, spontaneous miscarriage, abortion, miscarriage, pregnancy loss, fetal loss and fetal death. PRISMA guidelines for systematic reviews and meta-analysis were followed. Original research articles in English language addressing the relation between paternal age and spontaneous miscarriage were included. Exclusion criteria were studies that solely focused on pregnancy outcomes following artificial reproductive technology (ART) and studies that did not adjust their effect estimates for at least maternal age. Risk of bias was qualitatively described for three domains: bias due to confounding, information bias and selection bias.
OUTCOMES
The search resulted in 975 original articles. Ten studies met the inclusion criteria and were included in the qualitative synthesis. Nine of these studies were included in the quantitative synthesis (meta-analysis). Advanced paternal age was found to be associated with an increased risk of miscarriage. Pooled risk estimates for miscarriage for age categories 30–34, 35–39, 40–44 and ≥45 years of age were 1.04 (95% CI 0.90, 1.21), 1.15 (0.92, 1.43), 1.23 (1.06, 1.43) and 1.43 (1.13, 1.81) respectively (reference category 25–29 years). A second meta-analysis was performed for the subgroup of studies investigating first trimester miscarriage. This showed similar pooled risk estimates for the first three age categories and a slightly higher pooled risk estimate for age category ≥45 years (1.74; 95% CI 1.26, 2.41).
WIDER IMPLICATIONS
Over the last decades, childbearing at later ages has become more common. It is known that frequencies of adverse reproductive outcomes, including spontaneous miscarriage, are higher in women with advanced age. We show that advanced paternal age is also associated with an increased risk of spontaneous miscarriage. Although the paternal age effect is less pronounced than that observed with advanced maternal age and residual confounding by maternal age cannot be excluded, it may have implications for preconception counselling of couples comprising an older aged male.
Keywords: abortion, andrology, chromosomal abnormalities, counselling, DNA damage, epidemiology, germ cells, male infertility, recurrent miscarriage
Introduction
Advanced maternal age is an extensively studied risk factor for adverse reproductive outcome (Hassold and Chiu, 1985; Aldous and Edmonson, 1993; van Katwijk and Peeters, 1998; Nybo Andersen et al., 2000; Bacak et al., 2005; Cleary-Goldman et al., 2005; Delpisheh et al., 2008; Nelson, Telfer, and Anderson, 2013; Waldenstrom et al., 2017; Lisonkova et al., 2017). The reproductive risks associated with advanced maternal age (usually defined as age ≥ 35 years) form an integral part of preconception counselling and are well known to the general public (Heffner, 2004). Moreover, clinical policy is based on this knowledge, for instance, maternal age-related access criteria for in vitro fertilisation (IVF) treatment (National Collaborating Centre for Women's and Children's Health (UK), 2013). In contrast, less attention has been paid to the potential effect of paternal age. There are, however, studies indicating that this is unjustified. In 2018, Oldereid et al. evaluated the influence of paternal factors on a broad spectrum of perinatal and paediatric outcomes (Oldereid et al., 2018). They found associations between advanced paternal age and adverse outcomes in the offspring, particularly with psychiatric disorders like autism spectrum disorders and schizophrenia but also with stillbirth and several birth defects. The age of the father and the mutation rate in the offspring are found to be strongly related, possibly due to the larger number of germline divisions that have occurred in older males (Crow, 2000; Kong et al., 2012). Next to a higher frequency of point mutations, there is evidence suggesting that increasing paternal age is associated with sperm DNA strand breaks, genetic imprinting errors and chromosomal anomalies, all of which are factors related to miscarriage (Sartorius and Nieschlag, 2010; Robinson et al., 2012; Kobayashi et al., 2017). As such, from a biological point of view, it seems justified to consider paternal age as an independent risk factor for miscarriage.
Spontaneous miscarriage is the most common complication of human pregnancy; it is estimated that at least 30% of all pregnancies and 10–15% of clinically recognised pregnancies end in miscarriage (Wilcox et al., 1988; Nybo Andersen et al., 2000). Miscarriage refers to a spontaneous demise of pregnancy before the fetus reaches viability (before 24 weeks of gestational age); however, in many studies it is defined as a pregnancy loss that occurs before 20 completed weeks of gestational age (Zegers-Hochschild et al., 2009; Bender Atik et al., 2018). The majority of studies on miscarriage and its associated factors are focused on female factors. Cytogenetic and chromosomal microarray analysis studies on miscarriage specimens have shown that genetic abnormalities play a role in 50–70% of cases (Levy et al., 2014; Romero et al., 2015; Soler et al., 2017). The prevalence of genetic abnormalities is highest in miscarriage samples from the first trimester, particularly in miscarriage samples of embryonic stage (Romero et al., 2015). Advanced maternal age is strongly related with fetal chromosomal abnormalities, mainly aneuploid conceptions (Nybo Andersen et al., 2000; Group, 2008; Magnus et al., 2019). Besides maternal age, other factors such as uterine anomalies, poorly controlled diabetes and thyroid autoimmunity are related to miscarriage (Dorman et al., 1999; Saravelos, Cocksedge, and Li, 2008; Maraka et al., 2016; Magnus et al., 2019). In addition, associations have been found with behavioural and environmental factors including maternal obesity, smoking, alcohol and caffeine consumption, the use of non-steroidal anti-inflammatory drugs and acute and chronic stress (Metwally et al., 2008; Pineles et al., 2014; Hahn et al., 2015; Qu et al., 2017; Li et al., 2018; Sundermann et al., 2019).
Despite our current knowledge, the cause of miscarriage is not always well-understood, especially in couples with recurrent miscarriages (Stephenson, 1996; Jaslow, Carney, and Kutteh, 2010). Since the male partner contributes half of the genetic material of the embryo, studying paternal factors will possibly contribute to unravelling the complex aetiology of pregnancy loss. This may help to provide answers to affected couples, of whom many experience a high psychological impact and emotional burden (Farren et al., 2018).
This is the first systematic review and meta-analysis evaluating the effect of paternal age on spontaneous miscarriage. We provide an overview of epidemiological studies evaluating the association between paternal age and spontaneous miscarriage and we discuss possible underlying explanatory mechanisms.
Methods
We have conducted a systematic review and meta-analysis following the PRISMA guidelines (Moher et al., 2009). This systematic review was registered and accepted for inclusion in the international prospective register of systematic reviews PROSPERO (ID CRD42019132886).
Systematic search
A systematic search of PubMed, Embase and Cochrane electronic databases was performed to identify relevant studies from inception until 12 August 2019. We used the following free text and MeSH terms: paternal age, father’s age, male age, husband’s age, spontaneous abortion, spontaneous miscarriage, abortion, miscarriage, pregnancy loss, fetal loss, fetal death. The full electronic search strategy for PubMed is shown in Supplementary Table 1. Additional searches in Google Scholar were conducted, and reference lists of identified articles were manually searched for additional relevant references.
The literature search was performed by two researchers (N.F. and E.L.) and a librarian. The results of the search were exported to a citation manager (EndNote), and duplicates were removed. The screening was performed by two researchers (N.F. and E.L.). There were two stages of screening for study inclusion: in the first stage, titles and abstracts were screened and in the second stage, full manuscripts of the articles identified in the initial screening were retrieved and read in detail. Any discordance on selecting studies and assessing risk of bias (see further) was resolved by consensus. If no agreement was obtained, the opinion of a third observer (M.H.) was sought to gain consensus.
Eligibility criteria
Inclusion criteria were original research articles in English language addressing the relation between paternal age and spontaneous miscarriage. Exclusion criteria were studies that solely focused on pregnancy outcomes after artificial reproductive technology (ART) and studies that did not adjust their effect estimates for at least maternal age.
Data extraction
Two reviewers (N.F. and E.L.) extracted data from all selected articles on study design, country, publication year, study period, population characteristics, inclusion and exclusion criteria, exposure and outcome definitions, outcome ascertainment, sample size, type of effect measures, adjusted effect estimates with 95% confidence interval (CI) or P value, variables adjusted for in the analyses and statistical methods of adjustment for maternal age.
Risk of bias assessment
There is lack of a single obvious candidate tool for assessing quality of observational epidemiological studies (Sanderson, Tatt, and Higgins, 2007). Moreover, as stated by Dekkers et al. in the COSMOS-E (Conducting Systematic Reviews and Meta-Analyses of Observational Studies of Etiology) guideline (Dekkers et al., 2019), a ‘one size fits all’ approach for assessing quality of these studies is probably misguided, considering the large heterogeneity in observational research. Therefore, it has been recommended to develop a set of criteria for each observational systematic review and meta-analysis and to assess risk of bias in a qualitative manner (Dekkers et al., 2019).
For the research question of this systematic review, we distinguished three relevant domains of risk of bias: bias due to confounding, information bias and selection bias (including bias due to loss of follow-up or missing data). Risk of bias was assessed by two reviewers (N.F. and E.L.). For each individual study, risk of bias within domains and across domains was assessed and described.
Statistical analysis
The selected studies reported outcomes in adjusted odds ratios (AORs), adjusted hazard ratios (AHRs) and adjusted rate ratios (ARRs) with 95% confidence intervals (CI) or P values. These effect measures were treated equally as risk measures. When standard errors were not reported, we calculated them from 95% CIs or P values. To assess the effect of paternal age on first trimester miscarriage separately, we performed a second meta-analysis for the subgroup of studies that focused on miscarriage <13 weeks.
Most studies used the age category of 25–29 years as the reference category. Two studies (Slama et al., 2005; Kleinhaus et al., 2006; Xu et al., 2014) used <25 years as reference; for these studies the reported AORs were rescaled by dividing the AOR by the reported AOR in age category 25–29 years.
Meta-analyses were stratified by the following paternal age categories: 30–34, 35–39, 40–44 and ≥45 years (similar to that in Oldereid et al., (2018). If a study reported more subcategories (i.e. 45–49 years and ≥50 years), the effect sizes of these categories were pooled using a within study fixed effect meta-analysis. One study (Baba et al., 2011) reported one odds ratio for the age category 29–39 years. We used the same estimate for both 30–34 and 35–39 years, and we adjusted the standard errors, assuming equal sample sizes in both categories.
Two studies (de la Rochebrochard and Thonneau, 2002; Slama et al., 2003) analysed different combinations of paternal age and maternal age (‘couple age’). To obtain overall AORs and ARRs for paternal age categories adjusted for maternal age, a weighted regression analysis (using fixed effect regression meta-analysis software) was performed with the estimated log AOR as dependent variable and paternal age and maternal age categories as independent variables.
Evidence of publication bias was assessed through qualitative inspection of a funnel plot. Statistical heterogeneity among studies was assessed by inspecting the heterogeneity (I2) statistics. Because of heterogeneity of study populations and study designs, random-effects meta-analysis with DerSimonian and Laird estimation was used for the main analysis (command metan in Stata 14: StataCorp LLC, TX, USA). For sensitivity analysis, fixed-effect estimates were calculated as well. A second sensitivity analysis was conducted to evaluate the influence of the study with the most extreme estimates, by repeating the meta-analysis with exclusion of this study.
Results
Study selection
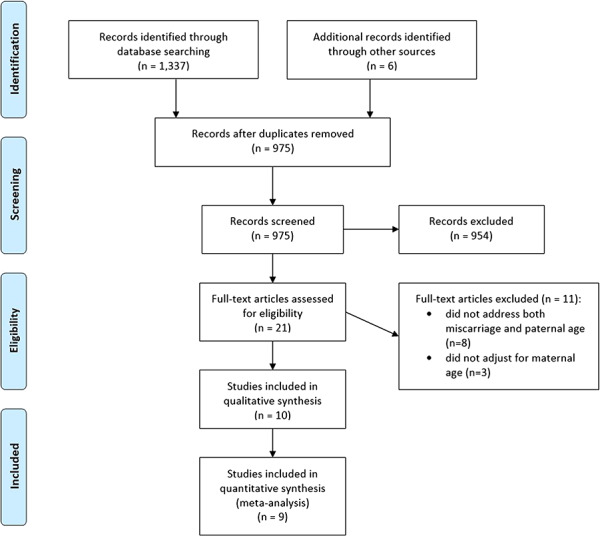
Details of the study selection process are shown in the PRISMA Flow Diagram (Fig. 1). The systematic search retrieved a total of 1343 articles: 1337 were identified by the search strategy and six additional articles were identified by hand searching other sources. After removing duplicates, 975 articles remained for first-stage screening. After first-stage screening by reviewing titles and abstracts, 954 articles were excluded and 21 articles were identified to assess the full text for eligibility. After this second stage of screening, 11 articles were excluded for reasons that are shown in Fig. 1. Finally, 10 articles met all the inclusion criteria. These were included in this review and were potentially appropriate to be included in meta-analysis. One study was excluded from meta-analysis, because of a different reference category and extremely high risk estimates, which is further explained in the narrative synthesis section.
Figure 1. Flow diagram of study selection process. Ten articles met all inclusion criteria and were included in qualitative synthesis. Nine studies were included in the meta-analysis; one study was excluded for reasons explained in the narrative synthesis section.
Study characteristics
Detailed descriptions of key characteristics for all included studies are summarised in Table I. With regard to the study designs of the 10 included studies, four were cohort studies (de la Rochebrochard and Thonneau, 2002; Slama et al., 2003; Nybo Andersen et al., 2004; Slama et al., 2005) and six were case-control studies (Kleinhaus et al., 2006; Maconochie et al., 2007; Baba et al., 2011; Jaleel and Khan, 2013; Xu et al., 2014; Nguyen et al., 2019). Two of the cohort studies (de la Rochebrochard and Thonneau, 2002; Slama et al., 2003) were retrospective studies, and two were prospective studies (Nybo Andersen et al., 2004; Slama et al., 2005). Two of the case-control studies were nested case-control studies (Kleinhaus et al., 2006; Maconochie et al., 2007). As shown in Table I, three studies took place in the USA (Slama et al., 2005; Kleinhaus et al., 2006; Nguyen et al., 2019) (one of these studies used data derived from a historic cohort; the Jerusalem Perinatal Study (Kleinhaus et al., 2006)), two were in France (de la Rochebrochard and Thonneau, 2002; Slama et al., 2003) (one of these studies was based on the European Study of Infertility and Subfecundity, including data from Denmark, Germany, Italy and Spain (de la Rochebrochard and Thonneau, 2002)), and one each was in Denmark (Nybo Andersen et al., 2004), the UK (Maconochie et al., 2007), Japan (Baba et al., 2011), China (Xu et al., 2014) and Pakistan (Jaleel and Khan, 2013). Seven studies were population-based (de la Rochebrochard and Thonneau, 2002; Slama et al., 2003; Nybo Andersen et al., 2004; Slama et al., 2005; Kleinhaus et al., 2006; Maconochie et al., 2007; Nguyen et al., 2019), and three were hospital-based (Baba et al., 2011; Jaleel and Khan, 2013; Xu et al., 2014). The sample sizes varied from 600 participants in a case-control study (Jaleel and Khan, 2013) to 23 821 in the Danish study by Nybo Andersen et al., (2004). Two studies (Kleinhaus et al., 2006; Nguyen et al., 2019) included only spontaneous pregnancies. In three studies (Nybo Andersen et al., 2004; Maconochie et al., 2007; Baba et al., 2011), a specified proportion of pregnancies (the highest proportion being 13% in the study of Baba et al., (2011) were conceived after ART, while in one study (de la Rochebrochard and Thonneau, 2002), it was stated that part of the population had fertility problems but this was not further explained. In four other studies (Slama et al., 2003; Slama et al., 2005; Jaleel and Khan, 2013; Xu et al., 2014), the mode of conception was not stated.
Table I.
Characteristics of included studies.
| Author, year, country | Study period | Study design | Study setting | Number of pregnancies or cases and controls | Proportion of ART pregnancies | Definition of miscarriage | Miscarriage ascertainment | Adjusted risk estimates | Risk factors adjusted for | Methods of adjustment for maternal age | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| De la Rochebrochard and Thonneau (2002) , France | 1991–1993 | Retrospective cohort | Population-based (European Study of Infertility and Subfecundity: Denmark, Germany, Italy, Spain) | 3174 pregnancies | Part of study population had infertility problems, otherwise not stated | Not defined | Self-reports | Paternal age | Maternal age | AOR (95% CI) | Country, number of the pregnancy, time to pregnancy, maternal and paternal smoking, history of miscarriage, history of ectopic pregnancy, history of induced abortion | Logistic regression Definition of new variable ‘couple age’, consisting of classes of maternal and paternal age combinations |
| 20–29 | 20–29 | 1.0 (reference) | ||||||||||
| 30–34 | 20–29 | 1.06 (0.61–1.86) | ||||||||||
| 35–39 | 20–29 | 1.31 (0.56–3.07) | ||||||||||
| 40–64 | 20–29 | 1.80 (0.52–6.24) | ||||||||||
| 20–29 | 30–34 | 1.72 (0.62–4.74) | ||||||||||
| 30–34 | 30–34 | 1.62 (0.93–2.82) | ||||||||||
| 35–39 | 30–34 | 1.06 (0.52–2.17) | ||||||||||
| 40–64 | 30–34 | 2.90 (1.26–6.67) | ||||||||||
| 20–29 | 35–44 | 9.18 (1.80–46.66) | ||||||||||
| 30–34 | 34–44 | 3.87 (1.24–12.02) | ||||||||||
| 35–39 | 35–44 | 3.38 (1.76–6.47) | ||||||||||
| 40–64 | 35–44 | 6.73 (3.50–12.95) | ||||||||||
| 20–29 | 1.0 (reference) | |||||||||||
| 30-34 | 0.93 (0.60–1.4) a | |||||||||||
| 35–39 | 0.68 (0.42–1.12) a | |||||||||||
| 40–64 | 1.31 (0.75–2.28) a | |||||||||||
| Slama et al. (2003), France | 1985–2000 | Retrospective cohort | Population-based | 2414 pregnancies | Not stated | Unplanned termination of pregnancy between 5 and 20 weeks | Self-reports | Paternal age | Maternal age | ARR (p-value or 95% CI) | Area of recruitment | Discrete time survival model Adjusted for maternal age as continuous variable; used age, age squared and age cubed as covariates in the model |
| <25 | <20 | 0.8 (0.64) | ||||||||||
| 25–29 | <20 | 0.7 (0.71) | ||||||||||
| 30–34 | <20 | 2.6 (0.39) | ||||||||||
| <25 | 20–24 | 1.2 (0.52) | ||||||||||
| 25–29 | 20–24 | 1.0 (reference) | ||||||||||
| 30–34 | 20–24 | 0.5 (0.23) | ||||||||||
| 35–39 | 20–24 | 5.3 (0.01) | ||||||||||
| <25 | 25–29 | 1.3 (0.61) | ||||||||||
| 25–29 | 25–29 | 1.1 (0.81) | ||||||||||
| 30–34 | 25–29 | 0.90 (0.70) | ||||||||||
| 35–39 | 25–29 | 1.1 (0.91) | ||||||||||
| >40 | 25–29 | 0.70 (0.77) | ||||||||||
| 25–29 | 30–34 | 1.5 (0.27) | ||||||||||
| 30–34 | 30–34 | 1.2 (0.51) | ||||||||||
| 35–39 | 30–34 | 1.5 (0.28) | ||||||||||
| >40 | 30–34 | 1.5 (0.62) | ||||||||||
| 25–29 | 35–39 | 7.0 (0.03) | ||||||||||
| 30–34 | 35–39 | 3.3. (0.01) | ||||||||||
| 35–39 | 35–39 | 2.2 (0.03) | ||||||||||
| >40 | 35–39 | 1.1 (0.91) | ||||||||||
| 35–39 | >40 | 1.6 (0.68) | ||||||||||
| >40 | >40 | 11.2 (0.00) | ||||||||||
| 20 | <35 | 1.36 (0.98–1.90) | Area of recruitment, maternal smoking, maternal alcohol consumption in first trimester, previous history of urogenital disorder | |||||||||
| 25 | <35 | 1.0 (reference) | ||||||||||
| 25 | ≥35 | 1.95 (0.97–3.92) | ||||||||||
| 30 | <35 | 1.12 (0.93–1.35) | ||||||||||
| 30 | ≥35 | 1.32 (0.84–2.07) | ||||||||||
| 35 | <35 | 2.31 (1.42–3.75) | ||||||||||
| 35 | ≥35 | 1.40 (0.89–2.20) | ||||||||||
| 40 | ≥35 | 2.76 (1.51–5.04) | ||||||||||
| 42 | ≥35 | 4.46 (1.90–10.49) | ||||||||||
| Slama et al. (2003), France | 25–29 | 1.0 (reference) | Area of recruitment, maternal age | |||||||||
| 30–34 | 0.92 (0.57–1.52) a | |||||||||||
| 35–39 | 1.21 (0.66–2.22) a | |||||||||||
| >40 | 1.01 (0.35–2.92) a | |||||||||||
| Nybo Andersen et al. (2004), Denmark | 1997–1999 | Prospective cohort | Population-based (Danish National Birth Cohort Recruitment) | 23 821 pregnancies | 6% of total study population | Early fetal death <20 weeks | Hospital diagnosis | Paternal age | AHR (95% CI) | Maternal age, parity, number of previous abortions, maternal alcohol and coffee consumption during pregnancy, maternal and paternal smoking, maternal and paternal occupational status | Cox regression model Maternal age entered in model in three different ways; using age continuously with restricted cubic splines instead of 5-year or 1-year groups yielded similar estimates for paternal age effects |
|
| ≤24 | 1.17 (0.84–1.63) | |||||||||||
| 25–29 | 1 (reference) | |||||||||||
| 30–34 | 0.86 (0.72–1.03) | |||||||||||
| 35–39 | 0.99 (0.79–1.25) | |||||||||||
| 40–44 | 0.77 (0.55–1.09) | |||||||||||
| 45–49 | 0.97 (0.56–1.69) | |||||||||||
| ≥50 | 1.38 (0.66–2.88) | |||||||||||
| Slama et al. (2005) , France | 1990–1991 | Prospective cohort | Population-based (Pregnancy Outcome Study: California) | 5121 pregnancies | Not stated | Spontaneous abortion between 6 and 20 weeks | Hospital diagnosis | Paternal age | AHR (95% CI) | Maternal age, maternal smoking, maternal alcohol consumption, maternal caffeine consumption, paternal smoking in first trimester | Cox regression model Adjusted for maternal age as continuous variable, using a fractional polynomial approach |
|
| <25 | 1 (reference) | |||||||||||
| 25–29 | 1.47 (1.04–2.08) | |||||||||||
| 30–34 | 1.25 (0.84–1.88) | |||||||||||
| 35–39 | 1.74 (1.12–1.72) | |||||||||||
| 40–44 | 1.45 (0.85–2.46) | |||||||||||
| ≥45 | 1.87 (1.01–3.44) | |||||||||||
| 25–29 | 1 (reference) | |||||||||||
| 30–34 | 0.85 (0.57–1.28) b | |||||||||||
| 35–39 | 1.18 (0.76–1.85) b | |||||||||||
| 40–44 | 0.99 (0.58–1.67) b | |||||||||||
| ≥45 | 1.27 (0.69–2.34) b | |||||||||||
| Kleinhaus et al. (2006) , USA | 1964–1976 | Nested case-control | Population-based (Jerusalem Perinatal Study) | Cases: n = 1506 Controls: n = 12 359 (live births) |
Only fertile women, otherwise not stated | Spontaneous abortion <20 weeks | Self-reports | Paternal age | AOR (95% CI) | Maternal age, maternal diabetes, maternal smoking, history of spontaneous abortions, parity, interval from interview to previous pregnancy, maternal and paternal education, history of induced abortions | Unconditional logistic regression Adjusted for maternal age as a continuous variable; used orthogonal coding of parental ages |
|
| <25 | 0.59 (0.45–0.76) | |||||||||||
| 25–29 | 1 (reference) | |||||||||||
| 30–34 | 1.4 (1.2–1.6) | |||||||||||
| 35–39 | 1.9 (1.6–2.3) | |||||||||||
| ≥40 | 1.6 (1.2–2.0) | |||||||||||
| Maconochie et al. (2007) , UK | 2001 | Nested case-control | Population-based (National Women’s Health Study) | Cases: n = 603 Controls: n = 6116 (ongoing pregnancy >12 weeks) |
Cases: 7% Controls: 3% |
Early miscarriage <13 weeks | Self-reports | Paternal age | AOR (95% CI) | Maternal age, year of conception, pregnancy order, history of miscarriage, history of live births | Logistic regression Coding of maternal age not stated |
|
| <25 | 1.18 (0.80–1.73) | |||||||||||
| 25 | 1 (reference) | |||||||||||
| 30 | 1.05 (0.83–1.33) | |||||||||||
| 35 | 1.22 (0.94–1.59) | |||||||||||
| 40 | 1.04 (0.71–1.53) | |||||||||||
| ≥45 | 1.63 (1.08–2.47) | |||||||||||
| Baba et al. (2011) , Japan | 2001–2005 | Matched case-control | Hospital-based | Cases: n = 430 Controls: n = 830 (term delivery) |
Cases: 13% Controls: 12% |
Early miscarriage <12 weeks | Hospital diagnosis | Paternal age | AOR (95% CI) | Maternal agec, year of the event, history of spontaneous abortion, history of induced abortion, treatment of infertility, maternal BMI, maternal smoking, maternal alcohol consumption, maternal employment, paternal smoking | Conditional logistic regression Matched for maternal age ± 3 years |
|
| <29 | 1 (reference) | |||||||||||
| 29–39 | 1.14 (0.75–1.74) | |||||||||||
| ≥40 | 1.65 (0.94–2.88) | |||||||||||
| Jaleel and Khan (2013), Pakistan | 2007–2010 | Case-control | Hospital-based | Cases: n = 200 Controls: n = 400 (ongoing pregnancy >24 weeks) |
Not stated | Early miscarriage (otherwise not defined) | Hospital diagnosis | Paternal age | AOR (95% CI) | Maternal age, paternal genital tract infection | Logistic regression Coding of maternal age not stated |
|
| ≤35 | 1 (reference) | |||||||||||
| 36–40 | 16.44 (6.612–40.896) | |||||||||||
| 41–45 | 13.738 (4.376–43.127) | |||||||||||
| >45 | 7.042 (1.269–39.090) | |||||||||||
| Xu et al. (2014) , China | 2009–2012 | Matched case-control | Hospital-based | Cases: n = 620 Controls: n = 1240 (ongoing pregnancy >12 weeks) |
Not stated | Early miscarriage <13 weeks | Hospital diagnosis | Paternal age | AOR (95% CI) | Maternal agec, history of early miscarriage, history of induced abortion, vitamin supplementation, maternal smoking and alcohol consumption, maternal night shift work, frequent staying up late, physical exercise | Conditional logistic regression Matched for maternal age ± 3 years |
|
| <25 | 1 (reference) | |||||||||||
| 25–29 | 0.94 (0.81–1.28) | |||||||||||
| 30–34 | 1.04 (0.85–1.32) | |||||||||||
| 35–39 | 0.97 (0.79–1.37) | |||||||||||
| ≥40 | 1.16 (0.86–1.42) | |||||||||||
| 25–29 | 1 (reference) | |||||||||||
| 30–34 | 1.11 (0.90–1.40)b | |||||||||||
| 35–39 | 1.03 (0.84–1.46)b | |||||||||||
| ≥40 | 1.23 (0.91–1.51)b | |||||||||||
| Nguyen et al. (2019) , USA | 2011–2015 | Case-control | Population-based (National Survey of Family Growth) | Cases: 2300 pregnancies Controls: 10410 pregnancies (live birth ≥37 weeks) |
Only spontaneous pregnancies | Loss of clinically recognized pregnancy ≤12 weeks and < 20 weeks | Self-reports | Paternal age | AOR (95% CI) | Maternal age, ethnicity, income, marital status, pregnancy intention | Generalized estimating equations logistic regression Maternal age entered in model in four age categories |
|
| <20 weeks | ||||||||||||
| <25 | 1.03 (0.85–1.25) | |||||||||||
| 25–29 | 1 (reference) | |||||||||||
| 30–34 | 1.04 (0.83–1.29) | |||||||||||
| 35–39 | 1.11 (0.81–1.52) | |||||||||||
| 40–44 | 1.10 (0.70–1.74) | |||||||||||
| 45–49 | 1.49 (0.71–3.13) | |||||||||||
| ≥50 | 2.05 (1.06–3.93) | |||||||||||
| ≤12 weeks | ||||||||||||
| <25 | 1.07 (0.86–1.32) | |||||||||||
| 25–29 | 1 (reference) | |||||||||||
| 30–34 | 1.10 (0.86–1.39) | |||||||||||
| 35–39 | 1.08 (0.76–1.52) | |||||||||||
| 40–44 | 1.10 (0.67–1.82) | |||||||||||
| 45–49 | 1.49 (0.65–3.40) | |||||||||||
| ≥50 | 2.30 (1.17–4.52) | |||||||||||
aRecalculated from the risk estimates reported for the combinations of paternal and maternal age, as described in Statistical analysis. bRescaled to reference category 25–29, as described in Statistical analysis. cMatched for maternal age (±3 years)
ART, artificial reproductive technology; AOR, adjusted odds ratio; AHR, adjusted hazard ratio; ARR, adjusted rate ratio; CI, confidence interval
Definition of outcome
Miscarriage is defined as the spontaneous demise of intrauterine pregnancy before 24 weeks of gestational age (Kolte et al., 2014; Bender Atik et al., 2018). In the studies selected for this review, miscarriage was defined by different gestational age ranges. Two studies (Slama et al., 2003; Slama et al., 2005) used a lower threshold for 5 or 6 weeks of gestational age, while a common upper threshold was 20 weeks (Slama et al., 2003; Nybo Andersen et al., 2004; Slama et al., 2005; Kleinhaus et al., 2006; Nguyen, Chang, and Bendikson, 2019). Four studies (Maconochie et al., 2007; Baba et al., 2011; Jaleel and Khan, 2013; Xu et al., 2014) focused on first trimester miscarriages only (<12 or <13 weeks). Two studies (de la Rochebrochard and Thonneau, 2002; Jaleel and Khan, 2013) did not specifically define gestational age ranges for miscarriage.
Risk of bias
Risk of bias assessment was carried out for each included study, and the results of this assessment are shown in Supplementary Table II.
Bias due to confounding
When evaluating the effect of paternal age on the risk of miscarriage, maternal age is a major confounding factor, being strongly associated with both the exposure and the outcome. Hence, we decided to include only studies in this review that controlled for maternal age. For other factors, it is less evident whether they are confounding the relation between paternal age and miscarriage or whether they are in the causal pathway. For instance, prior miscarriage is a strong risk factor for a subsequent miscarriage. Six studies (de la Rochebrochard and Thonneau, 2002; Nybo Andersen et al., 2004; Kleinhaus et al., 2006; Maconochie et al., 2007; Baba et al., 2011; Xu et al., 2014) considered this factor as a potential confounder. However, as stated by Slama et al. (Slama et al., 2005; Slama et al., 2014), a previous miscarriage might have been caused by an elevated paternal age during the previous pregnancy. From that perspective, it should be thought of as an intermediate variable (or a proxy for an intermediate variable) instead of a confounder. Other factors controlled for in some of the selected studies were maternal smoking (de la Rochebrochard and Thonneau, 2002; Slama et al., 2003; Nybo Andersen et al., 2004; Slama et al., 2005; Kleinhaus et al., 2006; Baba et al., 2011; Xu et al., 2014) and alcohol consumption (Slama et al., 2003; Nybo Andersen et al., 2004; Slama et al., 2005; Baba et al., 2011; Xu et al., 2014). Furthermore, some authors did adjust for potential confounding factors such as education level (Kleinhaus et al., 2006), occupational status (Nybo Andersen et al., 2004; Baba et al., 2011) and ethnicity (Nguyen et al., 2019).
Information bias and selection bias
The studies in this review can be subdivided into two types of designs: population-based studies and hospital-based studies. An advantage of large population-based studies (de la Rochebrochard and Thonneau, 2002; Slama et al., 2003; Nybo Andersen et al., 2004) is a low risk of selection bias, although as a drawback they often have to rely on self-reports of the women regarding their pregnancy outcomes. This means that miscarriages have not been confirmed. In addition, self-reporting could be subject to recall bias or social desirability bias (Althubaiti, 2016). In hospital-based case-control studies (Baba et al., 2011; Jaleel and Khan, 2013; Xu et al., 2014), miscarriages are ascertained by hospital diagnosis. However, conducting a study in a hospital setting may introduce a selection bias, since only a subset of women that miscarried is recruited and this subset may not be representative for all women experiencing a miscarriage. Risk of selection bias due to loss to follow-up or missing data was low for all studies.
Narrative synthesis
We included 10 studies in this review, and seven studies (de la Rochebrochard and Thonneau, 2002; Slama et al., 2003; Slama et al., 2005; Kleinhaus et al., 2006; Maconochie et al., 2007; Jaleel and Khan, 2013; Nguyen et al., 2019) found a significant effect of paternal age on the risk of miscarriage.
de la Rochebrochard and Thonneau (2002), France analysed data of 3174 couples from four European countries about last planned pregnancies that ended in live birth or miscarriage. They stratified paternal and maternal age in 5-year age classes, with 25–29 years designated as the reference group. Maternal and paternal age were analysed together, defined by the variable ‘couple age’, consisting of a combination of the age classes of both partners. A significant increased AOR for miscarriage was found if the woman was 30–34 years and the man ≥40 years of age, compared to same-aged women and younger men. When we recalculated the reported AORs to obtain AORs for paternal age effects adjusted for maternal age, we found an increased risk for age category 40–64 years, although this was not significant (AOR 1.31; 95% CI 0.75, 2.28).
In a retrospective study by Slama et al. (2003), 1151 randomly selected French women were interviewed about their pregnancy outcomes between 1985 and 2000. The authors developed a survival model to predict the probability of spontaneous miscarriage as a function of the woman’s and man’s age. This model showed an increased ARR of 1.95 (95% CI 0.97, 3.92) for spontaneous miscarriage in women aged 25 years with a partner of 35 years or older, compared to women aged 25 years whose partner was younger than 35 years.
Nybo Andersen et al., (2004) used data of 23 281 pregnancies from a Danish prospective cohort study to assess the association between paternal age and fetal death. They stratified for early (<20 weeks of gestation) and late (≥20 weeks of gestation) fetal death. Paternal age was categorised in 5-year age groups with the last group covering ≥50 years. The authors found an increased hazard ratio for early fetal death for fathers ≥50 years (AHR 1.38; 95% CI 0.66, 2.88), using 25–29 years as the reference group. They entered maternal age in three different ways in the model. Treating maternal age continuously with restricted cubic splines instead of 5- or 1-year age groups yielded similar estimates for paternal age effects, implying that there was no strong residual confounding by maternal age. To ensure that the effect of paternal age was not due to confounding by subfertility or infertility, they performed a second analysis restricted to couples who conceived without fertility treatment and they found comparable AHRs.
A second study of Slama et al., (2005) with a prospective design assessed the risk of spontaneous miscarriage between 6 and 20 weeks of pregnancy in a Cox model. The risk of spontaneous miscarriage was 1.27 times increased for fathers with a paternal age of 35 years and more, compared to fathers younger than 35 years old (AHR 1.27; 95% CI 1.00, 1.60). When they coded paternal age in smaller age groups (and maternal age continuously, using a fractional polynomial approach), they found the highest risk of spontaneous miscarriage for men aged >45 years (AHR 1.87; 95% CI 1.01, 3.44, reference group men aged 18–24 years). We rescaled the AHRs using 25–29 years as the reference category, and this yielded lower AHRs of 0.99 (95% CI 0.58, 67) in category 40–44 and 1.27 (95% CI 0.69, 2.34) in the ≥45-year age group.
In a nested case-control study derived from the Jerusalem Perinatal Study, Kleinhaus et al., (2006) compared 1506 couples with previous pregnancy ending in spontaneous miscarriage with a control group comprising 12 359 couples with prior live birth. They used paternal age categories of 5 years, with 25–29 years being the reference group. The AORs for miscarriage <20 weeks of gestation for the age groups 30–34 (AOR 1.4; 95% CI 1.2, 1.6), 35–39 (AOR 1.9; 95% 1.6–2.3) and ≥40 years (AOR 1.6; 95% CI 1.2–2.0) were all significantly increased.
Maconochie et al., (2007) studied various socio-demographic and behavioural factors in relation to last pregnancy outcomes. Cases consisted of 603 women whose most recent pregnancy was a first trimester (<13 weeks) miscarriage. Controls were 6116 women whose most recent pregnancy had progressed beyond 12 weeks. In fathers ≥45 years of age the AOR for first trimester miscarriage was significantly increased (AOR 1.63; 95% CI 1.08, 2.47; reference group 25–29 years).
Baba et al., (2011) and Xu et al., (2014) conducted similarly designed studies to identify risk factors for first trimester miscarriage. These hospital-based case-control studies were matched for maternal age, with total sample sizes of 1290 and 1860, respectively. For fathers aged ≥40, Baba et al. found an AOR for miscarriage of 1.65 (95% CI 0.94, 2.88) and Xu et al. an AOR of 1.16 (95% CI 0.86, 1.42). In both studies, only women who miscarried and were hospitalised for a medical procedure were selected as cases; women with spontaneous miscarriages without additional treatment were not included. Baba et al. used women who underwent term deliveries in the same hospital as controls. The control group of Xu et al. consisted of women who attended the outpatient clinic for prenatal care and were past 13 weeks of gestation.
In a case-control study conducted in a hospital in Karachi, Pakistan, pregnant women aged 20–35 years were included (Jaleel and Khan, 2013). Cases were women with first trimester miscarriage and controls were those admitted for delivery beyond 24 weeks of gestation. Studied factors were maternal age, paternal age, parental tobacco use and male genital tract infection. The final logistic regression model yielded extremely large effects of paternal age on the risk of first trimester miscarriage compared to all other studies, with AORs of 16.44 (95% CI 6.61, 40.90) in age category 36–40 years, 13.74 (95% CI 4.38, 43.13) in age category 41–45 years and 7.04 (95% CI 1.27, 39.09) in age category >45 years. In contrast to the other studies, paternal age ≤ 35 years and maternal age ≤ 31 years were used as reference categories. The reported data was insufficient to rescale the AORs to reference category 25–29 years, as we did for other studies. Part of the explanation for the deviating risk estimates could be that in this study population, there was less correlation between maternal and paternal ages, meaning there were relatively many couples consisting of older fathers and young mothers. We did not include this study in our meta-analyses, as this study might involve a selected population, reflected by the extreme and potentially unrealistic effects of paternal age that could not be compared to other studies because of the different reference category that was used.
The most recent study of Nguyen et al. (Nguyen et al. 2019) used data of 12 710 pregnancies from the US National Survey of Family Growth and assessed the risk of miscarriage <20 and ≤12 weeks separately. They used pregnancies ending in a live birth ≥37 weeks as controls. Pregnancies resulting in spontaneous miscarriage had 2.05 (95% CI 1.06–3.93) times the odds of being from a father aged ≥50 years. For first trimester miscarriage, the AOR for this age category was 2.30 (95% CI 1.17–4.52).
Quantitative synthesis of paternal age effects
The overall meta-analysis (Fig. 2), including nine studies, showed an increasing risk of miscarriage with advancing paternal age. Significant effects in age categories 40–44 years (pooled estimate 1.23; 95% CI 1.06, 1.43) and ≥45 years (1.43; 95% CI 1.13, 1.81) were found. The reference group was 25–29 years for all studies, except for Baba et al., (2011) (<29 years) and de la Rochebrochard and Thonneau, (2002) (20–29 years).
Figure 2.
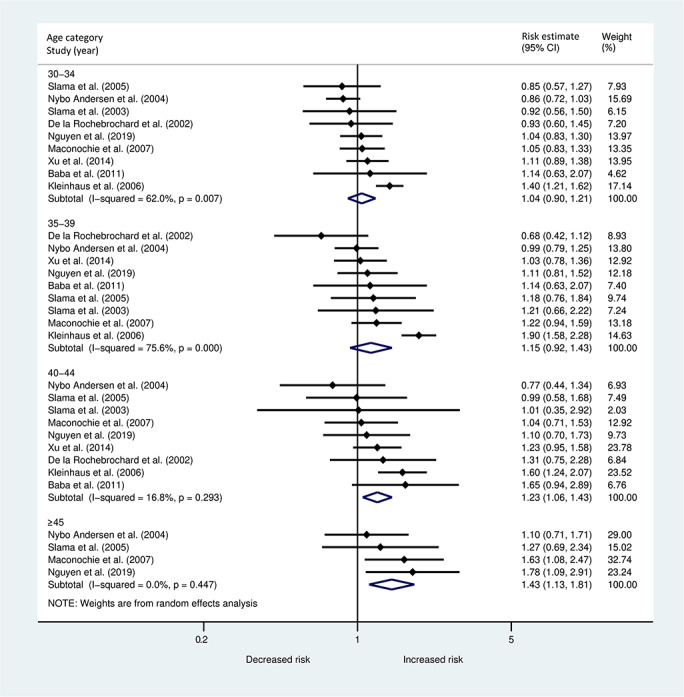
Forest plot describing the association between paternal age in different age categories and the risk of miscarriage <20 weeks.
A second meta-analysis (Fig. 3) was performed including the four studies that were restricted to first trimester miscarriage. A similar pattern of the paternal age effect was found, with a pooled estimate of 1.74 (95% CI 1.26, 2.41) in the highest age category.
Figure 3.
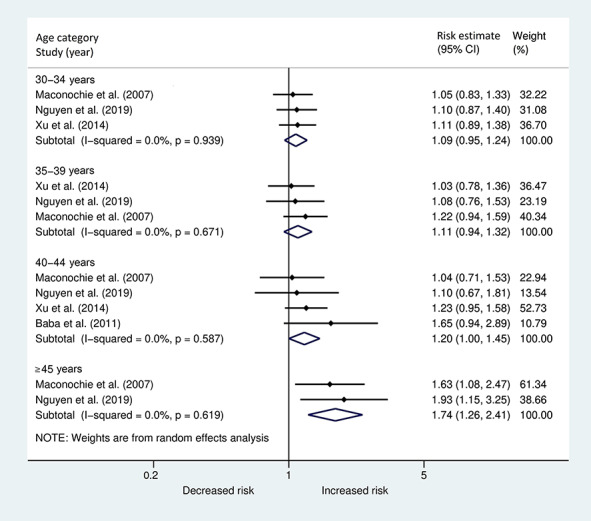
Forest plot describing the association between paternal age in different age categories and the risk of early miscarriage <13 weeks.
In both meta-analyses, there was substantial heterogeneity in the two lower age categories, while in the more advanced age categories the effects across studies were more similar, as indicated by I2. In Supplementary Fig. S1, funnel plots are displayed for each age category separately, including all nine studies. No clear evidence of small study effects or publication bias was found.
Maternal age effects
Besides analysis of the paternal age effect, four of the included studies (Slama et al., 2005; Kleinhaus et al., 2006; Maconochie et al., 2007; Nguyen et al., 2019) evaluated the effect of maternal age on the risk of miscarriage. They reported risk estimates for the maternal age effect, analysed on the same data as used for the paternal age effect. One study (Slama et al., 2005) provided risk estimates for maternal age, adjusted for paternal age. The other studies did not adjust the maternal age effects for paternal age. For two studies (de la Rochebrochard and Thonneau, 2002; Slama et al., 2003) that analysed combinations of paternal and maternal age (couple-age), it was possible to obtain risk estimates for maternal age categories, adjusted for paternal age (in the same way as performed for the paternal age effect, described in the statistical analysis). Maternal risk estimates with a reference category other than 25–29 years were rescaled to reference category 25–29 years when possible. An overview of maternal age effects on the risk of spontaneous miscarriage is shown in Table II.
Table II.
Maternal age effects.
| Author, year, country | Adjusted risk estimates | Risk factors adjusted for | |
|---|---|---|---|
| de la Rochebrochard and Thonneau (2002), France | Maternal age | AOR (95% CI) | Paternal age, country, number of the pregnancy, time to pregnancy, maternal and paternal smoking, history of miscarriage, history of ectopic pregnancy, history of induced abortion |
| 20–29 | 1.0 (reference) | ||
| 30–34 | 1.76 (1.10–2.82) a | ||
| 35–44 | 6.49 (4.43–9.51) a | ||
| Slama et al. (2003) , France | Maternal age | ARR (95% CI) | Paternal age, area of recruitment |
| 25–29 | 1 (reference) | ||
| 30–34 a | 1.34 (0.81–2.20) a , b | ||
| 35–39a | 2.39 (1.21–4.69) a , b | ||
| ≥40a | 6.23 (1.48–26.17) a , b | ||
| Slama et al. (2005), France | Maternal age | AHR (95% CI) | Paternal age, maternal smoking, maternal alcohol consumption, maternal caffeine consumption, paternal smoking in first trimester |
| <22.5 | 1.27 (1.04–1.55) | ||
| 22.5–27.4 | 1 | ||
| 27.5–32.4 | 0.98 (0.84–1.13) | ||
| 32.5–37.4 | 1.30 (1.03–1.66) | ||
| 37.5–42.4 | 2.63 (1.86–3.71) | ||
| ≥42.5 | 8.80 (4.73–16.73) | ||
| Kleinhaus et al. (2006) , USA | Maternal age | AOR (95% CI) | Parity, time interval from index pregnancy to interview, history of miscarriage |
| 25–29 | 1 (reference) | ||
| 30–34 | 2 (1.68–2.36) b | ||
| ≥35 | 3.77 (3.05–4.68) b | ||
| Maconochie et al. (2007) , UK | Maternal age | AOR (95% CI) | Year of conception, history of miscarriage, history of live birth |
| <25 | 1.09 (0.81–1.45) | ||
| 25–29 | 1 (reference) | ||
| 30–34 | 1.06 (0.85–1.31) | ||
| 35–39 | 1.75 (1.37–2.22) | ||
| ≥40 | 5.16 (3.54–7.52) | ||
| Nguyen et al. (2019) , USA | Maternal age | AOR (95% CI) | Ethnicity, income, marital status, pregnancy intention |
| <20 weeks | |||
| <25 | 0.89 (0.72–1.10) | ||
| 25–29 | 1 (reference) | ||
| 30–34 | 0.98 (0.72–1.33) | ||
| ≥35 | 1.52 (1.04–2.20) | ||
| ≤12 weeks | |||
| <25 | 0.86 (0.69–1.09) | ||
| 25–29 | 1 (reference) | ||
| 30–34 | 0.92 (0.68–1.24) | ||
| ≥35 | 1.66 (1.12–2.44) | ||
aRecalculated from the risk estimates reported for the combinations of paternal and maternal age, as described in the statistical analysis. bRescaled to reference category 25–29, as described in the statistical analysis.
Significant effects of maternal age ≥ 35 years were found in all of the above studies, varying from AOR 1.52 (95% CI 1.04–2.20, age category ≥35 years) (Nguyen et al. 2019) to AHR 8.80 (95% CI 4.73–16.73, age category ≥42.5 years) (Slama et al., 2005).
Because of the small number of studies and substantial differences in adjustments of the estimates and used age categories, a meta-analysis of the risk estimates of the maternal age effect was not performed.
Additional analyses
There were no major differences between the pooled estimates of the paternal age effect provided by models with random and fixed effects (Supplementary Fig. S2).
In the sensitivity analysis excluding the study (Kleinhaus et al., 2006) that consequently yielded relatively extreme estimates, the pooled estimates for the paternal age effect in age categories 35–39 and 40–44 years were slightly decreased (−8%). The pattern of the association between paternal age and risk of miscarriage was similar to that observed in the main analysis (Supplementary Fig. S3).
Discussion
In this systematic review and meta-analysis of 10 population-based cohort and case-control studies, advanced paternal age beyond 40 years was found to be significantly associated with an increased risk of spontaneous miscarriage, adjusted for maternal age. This paternal age effect was also observed in a subgroup of studies focusing on first trimester miscarriage.
A major strength of this systematic review and meta-analysis is that we could increase statistical power by combining data of the extreme paternal age categories of different studies. In the individual studies, the analyses were limited by small patient numbers in the more advanced age groups. Often increased risk estimates were found within these categories, although they were not statistically significant. By pooling the effect measures of different studies, we were able to find significant paternal age effects for both the 40–44 and ≥45 age classes.
It is important to mention that investigating a paternal age effect on the risk of miscarriage is challenging, due to the high level of collinearity between paternal and maternal age. To prevent confounding by maternal age, we only selected studies that did control for this variable. However, residual confounding by maternal age may still be present, especially when maternal age is treated as a discrete variable in broad age classes (de la Rochebrochard and Thonneau, 2002; Reijneveld, 2003). We evaluated the methods used for adjustment of maternal age in the included studies. The majority of studies carefully adjusted for maternal age, either by matching cases and controls according to maternal age (Baba et al., 2011; Xu et al., 2014), or treating maternal age as a continuous variable, using orthogonal coding of parental ages (Kleinhaus et al., 2006), a fractional polynomial approach (Slama et al., 2003; Slama et al., 2005) or restricted cubic splines (Nybo Andersen et al., 2004). Two studies (de la Rochebrochard and Thonneau, 2002; Nguyen et al., 2019) entered maternal age in their model as a categorical variable and two other studies (Maconochie et al., 2007; Jaleel and Khan, 2013) did not state how they treated maternal age in their models.
Other factors taken into account by several authors in the statistical adjustments were maternal smoking and alcohol consumption. The association of these maternal behaviours with spontaneous miscarriage is well-established (DiFranza and Lew, 1995; Nielsen et al., 2006; Pineles et al., 2014; Sundermann et al., 2019; Andersen et al., 2012). It is debatable to what extent maternal smoking and alcohol consumption are correlated with paternal age, which is another criterion for considering these factors as confounding factors. When such correlations do indeed exist in a study population, as suggested in some of the articles included in this review (Nybo Andersen et al., 2004; Slama et al., 2005; Kleinhaus et al., 2006), these factors could potentially bias the estimated association between paternal age and miscarriage. However, it is conceivable that some of the included studies controlled for too many variables. If a study adjusts for a variable that is, instead of being a confounder, in the causal pathway between paternal age and miscarriage, the total causal effect cannot be consistently estimated (i.e. the effect will be underestimated) (Schisterman, Cole, and Platt, 2009; Howards et al., 2012; Slama et al., 2014; Ananth and Schisterman, 2017).
In contrast to the risk of overadjustment bias for maternal factors, there might exist residual confounding by paternal factors. Six of the included studies have taken into account at least one paternal factor other than age (de la Rochebrochard and Thonneau, 2002; Nybo Andersen et al., 2004; Slama et al., 2005; Kleinhaus et al., 2006; Baba et al., 2011; Jaleel and Khan, 2013). It is, however, possible that the encountered relation between paternal age and miscarriage is biased by other, unmeasured, paternal characteristics (Henriksen et al., 2004; Venners et al., 2004; Raad et al., 2017).
Apart from the risk of confounding, conducting studies that aim to identify the risk of paternal age on spontaneous miscarriage comes with more challenges. Each study design has its own opportunities and obstacles. Population-based studies typically provide more generalisable results. At the same time, they are prone to information bias since they depend on the women’s declaration of miscarriage; especially early miscarriage is hard to establish. Furthermore, as previously suggested by other authors, some of the reported miscarriages may actually have been induced abortions (de la Rochebrochard and Thonneau, 2002; Slama et al., 2003; Kleinhaus et al., 2006). Hospital-based studies have less of a problem with case ascertainment. Nevertheless, these studies are more susceptible to selection bias since they exclusively recruit women who have received medical service for their miscarriage. From the studies included in this review, the cohort studies appear to have more conservative estimates compared to the case-control studies. This finding does not seem to be clearly related to differences in study setting or patient selection. Some of the case-control studies are population-based and others are hospital-based, while the cohort studies are all population-based. Also, the number of variables adjusted for does not substantially differ between the two clusters of studies. Because of the limited number of studies, especially when stratified per age group, sensitivity analysis on study design or meta-regression was not performed.
Supporting the observed epidemiological associations, it is plausible from a biological perspective that advanced paternal age increases the risk of adverse reproductive outcome. In women, the age-related decline in reproductive capacity is explained by a gradual decrease in ovarian reserve and oocyte integrity (te Velde and Pearson, 2002). More frequent chromosome segregation errors result in oocyte aneuploidy, and this is thought to be primarily responsible for maternal age-related miscarriage. In contrast to the process of oogenesis, where germ cell replication is completed at birth, male germ cells divide continuously throughout a man’s reproductive lifespan. From entering puberty on, spermatogenic stem cells divide approximately 23 times per year and by the age of 50 years, more than 800 replications have occurred (Crow, 2000). Therefore, advancing paternal age most likely increases the probability of replication errors in the germ line, resulting in an accumulation of de novo mutations (Kong et al., 2012). This process is exacerbated when DNA repair mechanisms are also deteriorating with age (Wiener-Megnazi, Auslender, and Dirnfeld, 2012). Kong et al. performed whole genome sequencing on 78 trios of parents and their children and demonstrated a clear association between advanced paternal age and increased number of de novo genetic mutations in the offspring, probably contributing to autosomal dominant disorders and complex disorders such as autism spectrum disorders (Kong et al., 2012; Oldereid et al., 2018). Advanced paternal age may also be linked to increased sperm aneuploidy; however, inconsistent findings have been reported in the literature (Luetjens et al., 2002; Coates et al., 2015; Garcia-Ferreyra et al., 2015). It is suggested that due to continual spermatogenesis, the male gamete is less vulnerable to age-related non-disjunction aneuploidies than its female counterpart (Brandt et al., 2019).
The influence of paternal age on miscarriage is perhaps acting mostly at the level of sperm DNA integrity. Multiple studies have shown elevated levels of sperm DNA fragmentation in older men, with a more than doubling DNA fragmentation index (DFI) between 20 and 60 years old (Plastira et al., 2007; Wyrobek et al., 2006; Schmid et al., 2013). This is probably due to a combination of age-related mechanisms and inherent characteristics of spermatozoa, such as accumulation of reactive oxygen species, absence of antioxidant capacity and paucity of DNA repair mechanisms (Martin et al., 2018). Although conventional sperm parameters such as volume, motility and morphology decline with increasing paternal age (Johnson et al., 2015), they are relatively poor predictors of male fertility potential and miscarriage (Guzick et al., 2001; Keel, 2006). In contrast, sperm DNA fragmentation seems directly associated with reproductive outcome. There is solid evidence that an increased level of sperm DNA fragmentation is associated with (recurrent) pregnancy loss (Robinsonxet al., 2012; Zhao et al., 2014; McQueen, Zhang, and Robins, 2019; Tan et al., 2019). In the case of fertilisation, sperm DNA fragmentation can to some extent be repaired by the oocyte. However, with advancing age, the oocyte quality is deteriorating, together with its repair capacity (Cozzubbo et al., 2014). This supports the hypothesis that the impact of paternal age on miscarriage, mediated by an increased DFI, is more present in interaction with higher maternal age. This is in line with epidemiological studies that demonstrated such an interaction between advanced paternal and maternal age for the risk of miscarriage (de la Rochebrochard and Thonneau, 2002). Furthermore, a recent study in IVF/ICSI couples observed a higher miscarriage rate in women beyond 35 years and partners with high sperm DFI, compared to couples with similarly high sperm DFI and younger women (Liang et al., 2019). It is noteworthy that quality of sperm, measured either by conventional parameters or DNA integrity, has not been taken into account by any of the studies included in this review. An ongoing prospective study is currently investigating the predictive role of sperm DNA damage in recurrent pregnancy loss, as well as the relation with paternal age and lifestyle factors (du Fossé et al., 2019).
In this review, we excluded studies that were restricted to couples who conceived after ART, since we were interested in the association between paternal age and miscarriage in the general population. The relationship between advanced parental age, infertility and miscarriage is complex. In some studies, miscarriage rates appear to be higher among ART pregnancies compared to natural pregnancies (Sunderam et al., 2015); however, this is not easily interpreted. Assisted pregnancies are usually closely monitored and, as a consequence, pregnancy losses, especially from early stages, will probably be detected more often than in the general population. In addition, ART-treated couples are generally of more advanced age, which predisposes them to an increased risk of miscarriage. For these reasons, it is difficult to distinguish whether an increased risk of miscarriage in couples receiving fertility treatment is a consequence of the treatment itself, or due to underlying patient characteristics. Studies investigating the effect of paternal age on miscarriage after different forms of ART reported inconclusive results (Gallardo et al., 1996; Spandorfer et al., 1998; Klonoff-Cohen and Natarajan, 2004; Paulson, Milligan, and Sokol, 2001; Belloc et al., 2008; Bellver et al., 2008; Whitcomb et al., 2011). These contradictory data may be explained by the heterogeneity of these studies, the small proportions of older men they included and the exclusion of women with advanced age or the use of young oocyte donors in some studies (Brandt et al., 2019). Furthermore, studies that did not observe an effect of paternal aging on the risk of pregnancy loss were mainly in IVF/ICSI pregnancies from a very heterogeneous population of men with extensive variations in sperm parameters and cause and severity of infertility, which may have diluted an age effect (Belloc et al., 2014).
While advanced maternal age is generally agreed upon as age ≥ 35, there is currently no consensus for the definition of advanced paternal age. However, ageing is a complex process and it is hard to determine a clear cutoff point, the more because age effects are likely to occur gradually and thresholds are not necessarily the same for all different outcomes that are affected by paternal age. Most studies suggest that infertility and reproductive risks start to increase after the paternal age of 40 (Ramasamy et al., 2015). This is in accordance with the results of our meta-analyses. Based on our findings, it should be considered to counsel couples with older males about the increased risk of miscarriage at preconception visits. Furthermore, our results are of value for patients with recurrent miscarriages. This condition remains unexplained in the majority of cases (Stephenson, 1996; Jaslow et al., 2010), and for a proportion of the idiopathic cases, advanced paternal age could be responsible. Currently, there are no studies that did specifically focus on the relation between paternal age and recurrent miscarriages and this should certainly be addressed in future research. Although it is challenging to distinguish paternal age effects from maternal age effects, most studies included in this review made relevant efforts and collectively they suggest the existence of an, albeit small, independent effect of paternal age on the risk of spontaneous miscarriage. Since there are strong biological hypotheses for this paternal effect, it is likely that future studies will establish it even more. Both large population-based registry studies and hospital-based case-control studies may help to validate the paternal age effect on pregnancy loss, provided that they carefully control for maternal age in their statistical analyses. There is a trend toward delayed childbearing in western societies and it has become more common to father children at older age (Billari et al., 2007). Hence, we consider it important to not merely focus on the effects of maternal aging on reproductive outcome, but to be aware of risks associated with advanced paternal age as well.
Supplementary Material
Acknowledgements
We thank Jan Schoones, librarian at Leiden University Medical Center, for his help in performing the literature search, Olaf Dekkers, professor at the department of Clinical Epidemiology of Leiden University Medical Center, for his advice regarding risk of bias assessments and Michael Hunter for his English language editing service.
Authors’ roles
E.L., M.H. and N.F. contributed to the design of the study. E.L. and N.F. screened and selected articles and performed the data extraction and risk of bias assessment. N.F. and S.C. performed the statistical analyses. E.L., J.M., M.H., N.F. and S.C. interpreted data. N.F. wrote the manuscript. E.L., J.M., M.H. and S.C. contributed to the manuscript revision. All authors contributed to manuscript preparation and have approved the final version.
Funding
E.L. and M.H. received funding from the Leiden University research profile ‘Innovation in Health Strategy and Quality of Care’.
Conflict of interest
The authors report no conflicts of interest in this work.
References
- Aldous MB, Edmonson MB. Maternal age at first childbirth and risk of low birth weight and preterm delivery in Washington State. JAMA 1993;270:2574–2577. [PubMed] [Google Scholar]
- Althubaiti A. Information bias in health research: definition, pitfalls, and adjustment methods. J Multidiscip Healthc 2016;9:211–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ananth CV, Schisterman EF. Confounding, causality, and confusion: the role of intermediate variables in interpreting observational studies in obstetrics. Am J Obstet Gynecol 2017;217:167–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen AM, Andersen PK, Olsen J, Gronbaek M, Strandberg-Larsen K. Moderate alcohol intake during pregnancy and risk of fetal death. Int J Epidemiol 2012;41:405–413. [DOI] [PubMed] [Google Scholar]
- Baba S, Noda H, Nakayama M, Waguri M, Mitsuda N, Iso H. Risk factors of early spontaneous abortions among Japanese: a matched case-control study. Hum Reprod 2011;26:466–472. [DOI] [PubMed] [Google Scholar]
- Bacak SJ, Callaghan WM, Dietz PM, Crouse C. Pregnancy-associated hospitalizations in the United States, 1999-2000. Am J Obstet Gynecol 2005;192:592–597. [DOI] [PubMed] [Google Scholar]
- Belloc S, Cohen-Bacrie P, Benkhalifa M, Cohen-Bacrie M, De Mouzon J, Hazout A, Menezo Y. Effect of maternal and paternal age on pregnancy and miscarriage rates after intrauterine insemination. Reprod Biomed Online 2008;17:392–397. [DOI] [PubMed] [Google Scholar]
- Belloc S, Hazout A, Zini A, Merviel P, Cabry R, Chahine H, Copin H, Benkhalifa M. How to overcome male infertility after 40: influence of paternal age on fertility. Maturitas 2014;78:22–29. [DOI] [PubMed] [Google Scholar]
- Bellver J, Garrido N, Remohi J, Pellicer A, Meseguer M. Influence of paternal age on assisted reproduction outcome. Reprod Biomed Online 2008;17:595–604. [DOI] [PubMed] [Google Scholar]
- Bender Atik R, Christiansen OB, Elson J, Kolte AM, Lewis S, Middeldorp S, Nelen W, Peramo B, Quenby S, Vermeulen N et al. ESHRE guideline: recurrent pregnancy loss. Hum Rep Open 2018;2. doi: 10.1093/hropen/hoy004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billari FC, Kohler H-P, Andersson G, Lundström H. Approaching the limit: long-term trends in late and very late fertility. Popul Dev Rev 2007;33:149–170. [Google Scholar]
- Brandt JS, Cruz Ithier MA, Rosen T, Ashkinadze E. Advanced paternal age, infertility, and reproductive risks: a review of the literature. Prenat Diagn 2019;39:81–87. [DOI] [PubMed] [Google Scholar]
- Cleary-Goldman J, Malone FD, Vidaver J, Ball RH, Nyberg DA, Comstock CH, Saade GR, Eddleman KA, Klugman S, Dugoff L et al. Impact of maternal age on obstetric outcome. Obstet Gynecol 2005;105:983–990. [DOI] [PubMed] [Google Scholar]
- Coates A, Hesla JS, Hurliman A, Coate B, Holmes E, Matthews R, Mounts EL, Turner KJ, Thornhill AR, Griffin DK. Use of suboptimal sperm increases the risk of aneuploidy of the sex chromosomes in preimplantation blastocyst embryos. Fertil Steril 2015;104:866–872. [DOI] [PubMed] [Google Scholar]
- Cozzubbo T, Neri QV, Rosenwaks Z, Palermo GD. To what extent can oocytes repair sperm DNA fragmentation? Fertil Steril 2014;102:e61. [DOI] [PubMed] [Google Scholar]
- Crow JF. The origins, patterns and implications of human spontaneous mutation. Nat Rev Genet 2000;1:40–47. [DOI] [PubMed] [Google Scholar]
- Dekkers OM, Vandenbroucke JP, Cevallos M, Renehan AG, Altman DG, Egger M. COSMOS-E: Guidance on conducting systematic reviews and meta-analyses of observational studies of etiology. PLoS Med 2019;16:e1002742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delpisheh A, Brabin L, Attia E, Brabin BJ. Pregnancy late in life: a hospital-based study of birth outcomes. J Womens Health (Larchmt) 2008;17:965–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiFranza JR, Lew RA. Effect of maternal cigarette smoking on pregnancy complications and sudden infant death syndrome. J Fam Pract 1995;40:385–394. [PubMed] [Google Scholar]
- Dorman JS, Burke JP, McCarthy BJ, Norris JM, Steenkiste AR, Aarons JH, Schmeltz R, Cruickshanks KJ. Temporal trends in spontaneous abortion associated with type 1 diabetes. Diabetes Res Clin Pract 1999;43:41–47. [DOI] [PubMed] [Google Scholar]
- Farren J, Mitchell-Jones N, Verbakel JY, Timmerman D, Jalmbrant M, Bourne T. The psychological impact of early pregnancy loss. Hum Reprod Update 2018;24:731–749. [DOI] [PubMed] [Google Scholar]
- du Fossé N, van der Hoorn ML, Eikmans M, Heidt S, le Cessie S, Mulders A, van Lith J, Lashley E. Evaluating the role of paternal factors in etiology and prognosis of recurrent pregnancy loss: study protocol for a multicenter case-control study and cohort study (the REMI III project). BMJ Open 2019;9:e033095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallardo E, Simon C, Levy M, Guanes PP, Remohi J, Pellicer A. Effect of age on sperm fertility potential: oocyte donation as a model. Fertil Steril 1996;66:260–264. [DOI] [PubMed] [Google Scholar]
- Garcia-Ferreyra J, Luna D, Villegas L, Romero R, Zavala P, Hilario R, Duenas-Chacon J. High aneuploidy rates observed in embryos derived from donated oocytes are related to male aging and high percentages of sperm DNA fragmentation. Clin Med Insights Reprod Health 2015;9:21–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Group TECW. Genetic aspects of female reproduction. Hum Reprod Update 2008;14:293–307. [DOI] [PubMed] [Google Scholar]
- Guzick DS, Overstreet JW, Factor-Litvak P, Brazil CK, Nakajima ST, Coutifaris C, Carson SA, Cisneros P, Steinkampf MP, Hill JA et al. Sperm morphology, motility, and concentration in fertile and infertile men. N Engl J Med 2001;345:1388–1393. [DOI] [PubMed] [Google Scholar]
- Hahn KA, Wise LA, Rothman KJ, Mikkelsen EM, Brogly SB, Sorensen HT, Riis AH, Hatch EE. Caffeine and caffeinated beverage consumption and risk of spontaneous abortion. Hum Reprod 2015;30:1246–1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassold T, Chiu D. Maternal age-specific rates of numerical chromosome abnormalities with special reference to trisomy. Hum Genet 1985;70:11–17. [DOI] [PubMed] [Google Scholar]
- Heffner LJ. Advanced maternal age - how old is too old? N Engl J Med 2004;351:1927–1929. [DOI] [PubMed] [Google Scholar]
- Henriksen TB, Hjollund NH, Jensen TK, Bonde JP, Andersson AM, Kolstad H, Ernst E, Giwercman A, Skakkebaek NE, Olsen J. Alcohol consumption at the time of conception and spontaneous abortion. Am J Epidemiol 2004;160:661–667. [DOI] [PubMed] [Google Scholar]
- Howards PP, Schisterman EF, Poole C, Kaufman JS, Weinberg CR. “Toward a clearer definition of confounding” revisited with directed acyclic graphs. Am J Epidemiol 2012;176:506–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaleel R, Khan A. Paternal factors in spontaneous first trimester miscarriage. Pak J Med Sci 2013;29:748–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaslow CR, Carney JL, Kutteh WH. Diagnostic factors identified in 1020 women with two versus three or more recurrent pregnancy losses. Fertil Steril 2010;93:1234–1243. [DOI] [PubMed] [Google Scholar]
- Johnson SL, Dunleavy J, Gemmell NJ, Nakagawa S. Consistent age-dependent declines in human semen quality: a systematic review and meta-analysis. Ageing Res Rev 2015;19:22–33. [DOI] [PubMed] [Google Scholar]
- van Katwijk C, Peeters LL. Clinical aspects of pregnancy after the age of 35 years: a review of the literature. Hum Reprod Update 1998;4:185–194. [DOI] [PubMed] [Google Scholar]
- Keel BA. Within- and between-subject variation in semen parameters in infertile men and normal semen donors. Fertil Steril 2006;85:128–134. [DOI] [PubMed] [Google Scholar]
- Kleinhaus K, Perrin M, Friedlander Y, Paltiel O, Malaspina D, Harlap S. Paternal age and spontaneous abortion. Obstet Gynecol 2006;108:369–377. [DOI] [PubMed] [Google Scholar]
- Klonoff-Cohen HS, Natarajan L. The effect of advancing paternal age on pregnancy and live birth rates in couples undergoing in vitro fertilization or gamete intrafallopian transfer. Am J Obstet Gynecol 2004;191:507–514. [DOI] [PubMed] [Google Scholar]
- Kobayashi N, Miyauchi N, Tatsuta N, Kitamura A, Okae H, Hiura H, Sato A, Utsunomiya T, Yaegashi N, Nakai K et al. Factors associated with aberrant imprint methylation and oligozoospermia. Sci Rep 2017;7:42336–42336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolte AM, on behalf of the ESHRE Special Interest Group EP, Bernardi LA, on behalf of the ESHRE Special Interest Group EP, Christiansen OB, on behalf of the ESHRE Special Interest Group EP, Quenby S, on behalf of the ESHRE Special Interest Group EP, Farquharson RG, on behalf of the ESHRE Special Interest Group EP et al. Terminology for pregnancy loss prior to viability: a consensus statement from the ESHRE early pregnancy special interest group. Hum Reprod 2014;30:495–498. [DOI] [PubMed] [Google Scholar]
- Kong A, Frigge ML, Masson G, Besenbacher S, Sulem P, Magnusson G, Gudjonsson SA, Sigurdsson A, Jonasdottir A, Jonasdottir A et al. Rate of de novo mutations and the importance of father's age to disease risk. Nature 2012;488:471–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy B, Sigurjonsson S, Pettersen B, Maisenbacher MK, Hall MP, Demko Z, Lathi RB, Tao R, Aggarwal V, Rabinowitz M. Genomic imbalance in products of conception: single-nucleotide polymorphism chromosomal microarray analysis. Obstet Gynecol 2014;124:202–209. [DOI] [PubMed] [Google Scholar]
- Li DK, Ferber JR, Odouli R, Quesenberry C. Use of nonsteroidal antiinflammatory drugs during pregnancy and the risk of miscarriage. Am J Obstet Gynecol 2018;219:275.e271–275.e278. [DOI] [PubMed] [Google Scholar]
- Liang X, Mao Y, Wang Y, Liu S, and Yan J. Female age affects the utility of sperm DNA fragmentation in predicting the outcomes of in vitro fertilization and intracytoplasmic sperm injection. Reprod Biomed Onlin e 2019;39:955–962. [DOI] [PubMed] [Google Scholar]
- Lisonkova S, Potts J, Muraca GM, Razaz N, Sabr Y, Chan WS, Kramer MS. Maternal age and severe maternal morbidity: a population-based retrospective cohort study. PLoS Med 2017;14:e1002307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luetjens CM, Rolf C, Gassner P, Werny JE, Nieschlag E. Sperm aneuploidy rates in younger and older men. Hum Reprod 2002;17:1826–1832. [DOI] [PubMed] [Google Scholar]
- Maconochie N, Doyle P, Prior S, Simmons R. Risk factors for first trimester miscarriage--results from a UK-population-based case-control study. BJOG 2007;114:170–186. [DOI] [PubMed] [Google Scholar]
- Magnus MC, Wilcox AJ, Morken NH, Weinberg CR, Haberg SE. Role of maternal age and pregnancy history in risk of miscarriage: prospective register based study. BMJ 2019;364:l869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maraka S, Ospina NM, O'Keeffe DT, Espinosa De Ycaza AE, Gionfriddo MR, Erwin PJ, Coddington CC 3rd, Stan MN, Murad MH, Montori VM. Subclinical hypothyroidism in pregnancy: a systematic review and meta-analysis. Thyroid 2016;26:580–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin JH, Aitken RJ, Bromfield EG, Nixon B. DNA damage and repair in the female germline: contributions to ART. Hum Reprod Update 2018;25:180–201. [DOI] [PubMed] [Google Scholar]
- McQueen DB, Zhang J, Robins JC. Sperm DNA fragmentation and recurrent pregnancy loss: a systematic review and meta-analysis. Fertil Steril 2019;112:54, e53–60. [DOI] [PubMed] [Google Scholar]
- Metwally M, Ong KJ, Ledger WL, Li TC. Does high body mass index increase the risk of miscarriage after spontaneous and assisted conception? A meta-analysis of the evidence. Fertil Steril 2008;90:714–726. [DOI] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Collaborating Centre for Ws and Children's H. National Institute for Health and Clinical Excellence: Guidance. Fertility: Assessment and Treatment for People with Fertility Problems. In: Royal College of Obstetricians & Gynaecologists National Collaborating Centre for Women’s and Children’s Health. London: Royal College of Obstetricians & Gynaecologists, 2013. https://www.ncbi.nlm.nih.gov/books/NBK247932/ [Google Scholar]
- Nelson SM, Telfer EE, Anderson RA. The ageing ovary and uterus: new biological insights. Hum Reprod Update 2013;19:67–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen BT, Chang EJ, Bendikson KA. Advanced paternal age and the risk of spontaneous abortion: an analysis of the combined 2011-2013 and 2013-2015 National Survey of Family Growth. Am J Obstet Gynecol 2019;221:476.e1–476.e7. [DOI] [PubMed] [Google Scholar]
- Nielsen A, Hannibal CG, Lindekilde BE, Tolstrup J, Frederiksen K, Munk C, Bergholt T, Buss L, Ottesen B, Gronbaek M et al. Maternal smoking predicts the risk of spontaneous abortion. Acta Obstet Gynecol Scand 2006;85:1057–1065. [DOI] [PubMed] [Google Scholar]
- Nybo Andersen AM, Hansen KD, Andersen PK, Davey SG. Advanced paternal age and risk of fetal death: a cohort study. Am J Epidemiol 2004;160:1214–1222. [DOI] [PubMed] [Google Scholar]
- Nybo Andersen AM, Wohlfahrt J, Christens P, Olsen J, Melbye M. Maternal age and fetal loss: population based register linkage study. BMJ 2000;320:1708–1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldereid NB, Wennerholm UB, Pinborg A, Loft A, Laivuori H, Petzold M, Romundstad LB, Soderstrom-Anttila V, Bergh C. The effect of paternal factors on perinatal and paediatric outcomes: a systematic review and meta-analysis. Hum Reprod Update 2018;24:320–389. [DOI] [PubMed] [Google Scholar]
- Paulson RJ, Milligan RC, Sokol RZ. The lack of influence of age on male fertility. Am J Obstet Gynecol 2001;184:818–822 discussion 822-814. [DOI] [PubMed] [Google Scholar]
- Pineles BL, Park E, Samet JM. Systematic review and meta-analysis of miscarriage and maternal exposure to tobacco smoke during pregnancy. Am J Epidemiol 2014;179:807–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plastira K, Msaouel P, Angelopoulou R, Zanioti K, Plastiras A, Pothos A, Bolaris S, Paparisteidis N, Mantas D. The effects of age on DNA fragmentation, chromatin packaging and conventional semen parameters in spermatozoa of oligoasthenoteratozoospermic patients. J Assist Reprod Genet 2007;24:437–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu F, Wu Y, Zhu YH, Barry J, Ding T, Baio G, Muscat R, Todd BK, Wang FF, Hardiman PJ. The association between psychological stress and miscarriage: a systematic review and meta-analysis. Sci Rep 2017;7:1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raad G, Hazzouri M, Bottini S, Trabucchi M, Azoury J, Grandjean V. Paternal obesity: how bad is it for sperm quality and progeny health? Bas Clin Androl 2017;27:20–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramasamy R, Chiba K, Butler P, Lamb DJ. Male biological clock: a critical analysis of advanced paternal age. Fertil Steril 2015;103:1402–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reijneveld SA. Age in epidemiological analysis. J Epidemiol Community Health 2003;57:397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson L, Gallos ID, Conner SJ, Rajkhowa M, Miller D, Lewis S, Kirkman-Brown J, Coomarasamy A. The effect of sperm DNA fragmentation on miscarriage rates: a systematic review and meta-analysis. Hum Reprod 2012;27:2908–2917. [DOI] [PubMed] [Google Scholar]
- de la Rochebrochard E, Thonneau P. Paternal age and maternal age are risk factors for miscarriage; results of a multicentre European study. Hum Reprod 2002;17:1649–1656. [DOI] [PubMed] [Google Scholar]
- Romero ST, Geiersbach KB, Paxton CN, Rose NC, Schisterman EF, Branch DW, Silver RM. Differentiation of genetic abnormalities in early pregnancy loss. Ultrasound Obstet Gynecol 2015;45:89–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson S, Tatt ID, Higgins JP. Tools for assessing quality and susceptibility to bias in observational studies in epidemiology: a systematic review and annotated bibliography. Int J Epidemiol 2007;36:666–676. [DOI] [PubMed] [Google Scholar]
- Saravelos SH, Cocksedge KA, Li TC. Prevalence and diagnosis of congenital uterine anomalies in women with reproductive failure: a critical appraisal. Hum Reprod Update 2008;14:415–429. [DOI] [PubMed] [Google Scholar]
- Sartorius GA, Nieschlag E. Paternal age and reproduction. Hum Reprod Update 2010;16:65–79. [DOI] [PubMed] [Google Scholar]
- Schisterman EF, Cole SR, Platt RW. Overadjustment bias and unnecessary adjustment in epidemiologic studies. Epidimiology 2009;20:488–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid TE, Grant PG, Marchetti F, Weldon RH, Eskenazi B, Wyrobek AJ. Elemental composition of human semen is associated with motility and genomic sperm defects among older men. Hum Reprod 2013;28:274–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slama R, Ballester F, Casas M, Cordier S, Eggesbo M, Iniguez C, Nieuwenhuijsen M, Philippat C, Rey S, Vandentorren S et al. Epidemiologic tools to study the influence of environmental factors on fecundity and pregnancy-related outcomes. Epidemiol Rev 2014;36:148–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slama R, Bouyer J, Windham G, Fenster L, Werwatz A, Swan SH. Influence of paternal age on the risk of spontaneous abortion. Am J Epidemiol 2005;161:816–823. [DOI] [PubMed] [Google Scholar]
- Slama R, Werwatz A, Boutou O, Ducot B, Spira A, Hardle W. Does male age affect the risk of spontaneous abortion? An approach using semiparametric regression. Am J Epidemiol 2003;157:815–824. [DOI] [PubMed] [Google Scholar]
- Soler A, Morales C, Mademont-Soler I, Margarit E, Borrell A, Borobio V, Munoz M, Sanchez A. Overview of chromosome abnormalities in first trimester miscarriages: a series of 1,011 consecutive chorionic villi sample karyotypes. Cytogenet Genome Res 2017;152:81–89. [DOI] [PubMed] [Google Scholar]
- Spandorfer SD, Avrech OM, Colombero LT, Palermo GD, Rosenwaks Z. Effect of parental age on fertilization and pregnancy characteristics in couples treated by intracytoplasmic sperm injection. Hum Reprod 1998;13:334–338. [DOI] [PubMed] [Google Scholar]
- Stephenson MD. Frequency of factors associated with habitual abortion in 197 couples. Fertil Steril 1996;66:24–29. [PubMed] [Google Scholar]
- Sunderam S, Kissin DM, Crawford SB, Folger SG, Jamieson DJ, Warner L, Barfield WD. Assisted reproductive technology surveillance - United States, 2013. MMWR Surveill Summ 2015;64:1–25. [DOI] [PubMed] [Google Scholar]
- Sundermann AC, Zhao S, Young CL, Lam L, Jones SH, Velez Edwards DR, Hartmann KE. Alcohol use in pregnancy and miscarriage: a systematic review and meta-analysis. Alcohol Clin Exp Res 2019;43:1606–1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan J, Taskin O, Albert A, Bedaiwy MA. Association between sperm DNA fragmentation and idiopathic recurrent pregnancy loss: a systematic review and meta-analysis. Reprod Biomed Online 2019;38:951–960. [DOI] [PubMed] [Google Scholar]
- te Velde ER, Pearson PL. The variability of female reproductive ageing. Hum Reprod Update 2002;8:141–154. [DOI] [PubMed] [Google Scholar]
- Venners SA, Wang X, Chen C, Wang L, Chen D, Guang W, Huang A, Ryan L, O'Connor J, Lasley B et al. Paternal smoking and pregnancy loss: a prospective study using a biomarker of pregnancy. Am J Epidemiol 2004;159:993–1001. [DOI] [PubMed] [Google Scholar]
- Waldenstrom U, Cnattingius S, Vixner L, Norman M. Advanced maternal age increases the risk of very preterm birth, irrespective of parity: a population-based register study. BJOG 2017;124:1235–1244. [DOI] [PubMed] [Google Scholar]
- Whitcomb BW, Turzanski-Fortner R, Richter KS, Kipersztok S, Stillman RJ, Levy MJ, Levens ED. Contribution of male age to outcomes in assisted reproductive technologies. Fertil Steril 2011;95:147–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiener-Megnazi Z, Auslender R, Dirnfeld M. Advanced paternal age and reproductive outcome. Asian J Androl 2012;14:69–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox AJ, Weinberg CR, O'Connor JF, Baird DD, Schlatterer JP, Canfield RE, Armstrong EG, Nisula BC. Incidence of early loss of pregnancy. N Engl J Med 1988;319:189–194. [DOI] [PubMed] [Google Scholar]
- Wyrobek AJ, Eskenazi B, Young S, Arnheim N, Tiemann-Boege I, Jabs EW, Glaser RL, Pearson FS, Evenson D. Advancing age has differential effects on DNA damage, chromatin integrity, gene mutations, and aneuploidies in sperm. Proc Natl Acad Sci U S A 2006;103:9601–9606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu G, Wu Y, Yang L, Yuan L, Guo H, Zhang F, Guan Y, Yao W. Risk factors for early miscarriage among Chinese: a hospital-based case-control study. Fertil Steril 2014;101:1663–1670. [DOI] [PubMed] [Google Scholar]
- Zegers-Hochschild F, Adamson GD, de Mouzon J, Ishihara O, Mansour R, Nygren K, Sullivan E, van der Poel S. The International Committee for Monitoring Assisted Reproductive Technology (ICMART) and the World Health Organization (WHO) Revised Glossary on ART Terminology, 2009. Hum Reprod 2009;24:2683–2687. [DOI] [PubMed] [Google Scholar]
- Zhao J, Zhang Q, Wang Y, Li Y. Whether sperm deoxyribonucleic acid fragmentation has an effect on pregnancy and miscarriage after in vitro fertilization/intracytoplasmic sperm injection: a systematic review and meta-analysis. Fertil Steril 2014;102:998, e1008–1005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


