Abstract
Background
Direct-acting antiviral (DAA) therapy is highly effective in people who inject drugs (PWID); however, rates, specific injection behaviors, and social determinants associated with hepatitis C virus (HCV) reinfection following DAA therapy among PWID on opioid agonist therapy (OAT) are poorly understood.
Methods
PREVAIL was a randomized controlled trial that assessed models of HCV care for 150 PWID on OAT. Those who achieved sustained virologic response (SVR) (n = 141; 94%) were eligible for this extension study. Interviews and assessments of recurrent HCV viremia occurred at 6-month intervals for up to 24 months following PREVAIL. We used survival analysis to analyze variables associated with time to reinfection.
Results
Of 141 who achieved SVR, 114 had a least 1 visit in the extension study (62% male; mean age, 52 years). Injection drug use (IDU) was reported by 19% (n = 22) in the extension study. HCV reinfection was observed in 3 participants. Over 246 person-years of follow-up, the incidence of reinfection was 1.22/100 person-years (95% CI, 0.25–3.57). All reinfections occurred among participants reporting ongoing IDU. The incidence of reinfection in participants reporting ongoing IDU (41 person-years of follow-up) was 7.4/100 person-years (95% CI, 1.5–21.6). Reinfection was associated with reporting ongoing IDU in the follow-up period (P < .001), a lack confidence in the ability to avoid contracting HCV (P < .001), homelessness (P = .002), and living with a PWID (P = .007).
Conclusions
HCV reinfection was low overall, but more common among people with ongoing IDU following DAA therapy on OAT, as well as those who were not confident in the ability to avoid contracting HCV, homeless, or living with a PWID. Interventions to mediate these risk factors following HCV therapy are warranted.
Keywords: HCV, PWID, reinfection, DAA, IDU
The hepatitis C virus reinfection rate was low among people who inject drugs on opioid agonist therapy. Rates were higher among those reporting ongoing injection drug use. Attention should be paid to high-risk behaviors following successful treatment with direct-acting antivirals.
The hepatitis C virus (HCV) treatment paradigm has changed dramatically with direct-acting antiviral (DAA) therapy. Yet, the rate of HCV reinfection and associated risk factors following HCV treatment with DAA therapy remain poorly understood. Given that injection drug use (IDU) is the number one risk factor for HCV in the United States, treatment of people who inject drugs (PWID) is an essential strategy for HCV elimination [1]. High sustained virologic response (SVR) rates with DAA therapy have been demonstrated among PWID [2, 3]. However, access to DAA therapy has been limited, in part, due to concerns about HCV reinfection in the setting of ongoing substance use [4].
Opioid agonist therapy (OAT) can provide a period of relative stability from active opioid use. However, OAT does not curtail use of stimulants, benzodiazepines, or alcohol and its efficacy can be compromised by continued use of these substances. Nevertheless, OAT has been shown to reduce the incidence of primary HCV infection [5, 6], and it holds promise to reduce the risk of HCV reinfection following HCV treatment [7]. Nationwide, over 375 000 patients receive OAT in the form of methadone or buprenorphine from approximately 1500 opioid treatment programs (OTPs) [8], and conservative estimates suggest that over 60% of PWID in OTPs are infected with HCV [9]. Studies conducted in OAT settings have shown high SVR rates [3, 10]. However, few studies have been conducted to assess rates of reinfection among PWID on OAT. Moreover, few studies have identified specific risk factors associated with HCV reinfection following DAA therapy.
There is urgency to better understand rates and risk factors associated with reinfection since HCV causes more than 18 000 deaths annually in the United States [11] and is the leading cause of end-stage liver disease, hepatocellular carcinoma (HCC), and liver transplantation [12–14]. Hepatitis C virus–related deaths have surpassed those from human immunodeficiency virus (HIV) and the disease burden is predicted to increase up to 3-fold over the course of the next 10–20 years [15, 16]. Curing HCV can lead to regression of cirrhosis [17], improve quality of life [18], and reduce HCC risk as well as HCV-related and all-cause mortality [19].
The goals of this study were to assess the rate of HCV reinfection among a cohort of PWID on OAT in the Bronx, New York, and explore risk factors associated with HCV reinfection in this population. We propose that the findings from this study can be used to inform interventions to prevent HCV reinfection among PWID. This may be particularly valuable in resource-limited practice settings where subsets of PWID who exhibit high-risk behaviors may need to be targeted.
METHODS
Participants
This study was an extension of the Prevent Resistance Eliminate Virus and Improve Life (PREVAIL) study, a 3-arm randomized controlled trial to assess the effectiveness of 3 models of HCV care among 150 PWID on OAT (R01DA034086) [10]. As part of the PREVAIL study, participants were followed for 12 weeks post–HCV treatment (PT12) to assess for SVR. A final research visit 24 weeks post–HCV treatment (PT24) was conducted to assess short-term reinfection rates [20]. After completion of the parent study, we recruited all of those who achieved SVR to participate in the extension study to assess long-term reinfection rates.
Setting
Research visits for the extension study were conducted at Montefiore General Clinical Research Centers or 1 of 3 OAT clinics in the Bronx, New York. These clinics are comprehensive substance-abuse treatment programs providing pharmacotherapy and related services to approximately 4300 adults (>18 years) with opioid use disorders. All 3 clinics provide onsite primary medical care, including HCV and HIV care.
Study Assessments
In the parent study, we collected baseline demographic data as described elsewhere [20]. Data on substance use within the last 30 days prior to each study visit were collected using the Addiction Severity Index (ASI). In the parent study, IDU was defined as reporting injection of any substance in the ASI in the last 30 days. PREVAIL participants who achieved SVR were offered enrollment in the extension study to assess long-term reinfection rates and risk factors. Research visits were conducted every 6 months following PT24 for up to 24 months. At these visits, participants completed a survey with study coordinators that were entered into REDCap, a Web-based application designed for longitudinal research [21], and underwent HCV RNA testing using the COBAS TaqMan real-time reverse transcriptase–polymerase chain reaction assay version 2.0 (Roche Diagnostics). In the extension study, we evaluated specific risk behaviors such as injection practices; use of cookers, cotton, and rinse water; sharing drugs; injection partners; receptive syringe sharing; “sero-sorting”; and hygiene (eg, how frequently skin was cleaned with alcohol before injecting) within the last 6 months. If blood samples were found to be viremic, an additional sample was collected to be sent to our institution’s biorepository then shipped to the Centers for Disease Control and Prevention in Atlanta for phylogenetic analysis to differentiate reinfections from treatment failures. The hypervariable region (HVR1) of HCV was amplified, and the data acquired by Next Generation Sequencing (NGS).
Analytic Plan
Our primary outcome was reinfection defined as a detectable HCV RNA following an undetectable end-treatment HCV viral load assessment and confirmed using NGS. Since the risk of reinfection begins following treatment completion, we chose the end of treatment time point rather than SVR and used NGS to reduce the risk of misclassifying reinfection and treatment failure [22]. Comparisons of participant characteristics between PREVAIL participants who were and were not retained in the extension study were made using t tests, chi-square tests, or Fisher’s exact tests. The date of reinfection, if it occurred, for all participants was taken as the midpoint between the last undetectable HCV RNA and the first positive HCV RNA. The time to reinfection was computed as the number of days from the end of treatment to the date of reinfection, or to the last date of HCV RNA evaluation during follow-up for those who did not experience a reinfection (ie, right-censored observations). We conducted a subgroup analysis for participants who reported IDU any time during follow-up period. We calculated reinfection incidence rates by dividing the number of reinfections by the total exposure time expressed as the total person-years of exposures. We produced Kaplan–Meier survival curves to assess the proportion of participants who were reinfected over time. We applied log-rank tests or Cox proportional hazards regression models to identify categorical or continuous demographic or risk factors that were significantly associated with incidence of reinfection. Results with a 2-sided significance level of P < .05 were declared statistically significant.
RESULTS
Of the 141 participants in the PREVAIL study who achieved SVR, we enrolled 114 of 141 (81%) in the extension study. The mean follow-up time for all study participants who achieved SVR12 in the parent study (N = 141) was 20.5 (SD = 11.7) months following the end of treatment. There were no significant differences in baseline characteristics including key demographic factors and drug use between participants enrolled in the extension study and those lost to follow-up, with the exception of more cirrhosis among those who were lost to follow-up (P = .015) (Table 1).
Table 1.
Baseline Characteristics of Study Participants in the PREVAIL Extension Study
| Characteristic | Enrolled in PREVAIL Extension Study (N = 114) | Not Enrolled in PREVAIL Extension Study (N = 27) | P |
|---|---|---|---|
| Sex, n (%) | .2738 | ||
| Male | 71 (62) | 20 (74) | |
| Female | 43 (38) | 7 (26) | |
| Age (years) | .1315 | ||
| Mean (SD) | 52 (10) | 48 (12) | |
| Ethnicity, n (%) | .8258 | ||
| Hispanic | 67 (59) | 17 (63) | |
| African American | 29 (25) | 5 (19) | |
| Caucasian | 12 (11) | 5 (19) | |
| Other | 6 (5) | 0 | |
| Education, n (%) | .0825 | ||
| Graduated high school | 62 (54) | 20 (74) | |
| Employment status, n (%) | .4134 | ||
| Employed | 20 (17) | 7 (26) | |
| Unemployed | 94 (83) | 20 (74) | |
| Homeless, n (%) | .6012 | ||
| No | 91 (80) | 20 (74) | |
| Yes | 23 (20) | 7 (26) | |
| HCV genotype, n (%) | .5541 | ||
| 1a | 98 (86) | 22 (82) | |
| 1b | 16 (14) | 5 (18) | |
| Cirrhosis, n (%) | .0151* | ||
| No | 88 (77) | 14 (52) | |
| Yes | 26 (23) | 13 (48) | |
| HIV status, n (%) | .1231 | ||
| HIV/HCV coinfected | 19 (17) | 1 (4) | |
| HCV monoinfected | 95 (83) | 26 (96) | |
| Prior HCV therapy, n (%) | .7370 | ||
| Experienced | 14 (12) | 2 (7) | |
| Naive | 100 (88) | 25 (93) | |
| Injection drug use, n (%) | |||
| Ever IDU | 85 (75) | 22 (82) | .6178 |
| ≤30 days before treatment | 59 (52) | 17 (63) | .2934 |
| ≤30 days before PT12 | 55 (49) | 13 (62) | .2632 |
| ≤30 days before PT24 | 53 (47) | 11 (55) | .5268 |
Significant at P < .05.
Abbreviations: HCV, hepatitis C virus; HIV, human immunodeficiency virus; IDU, injection drug use; PREVAIL, Prevent Resistance Eliminate Virus and Improve Life; PT12, 12 weeks post–HCV treatment; PT24, 24 weeks post–HCV treatment.
All participants had opioid use disorder and were receiving OAT (112 on methadone, 2 on buprenorphine). The majority (75%) reported a history of IDU. Injection drug use was reported in the 30 days prior to HCV treatment, PT12, and PT24 by 59 (52%), 55 (49%), and 53 (47%) participants, respectively. In the extension study cohort, the majority of participants were male (62%), Latino (63%), and unemployed (82%), and 22 (19%) reported ongoing IDU.
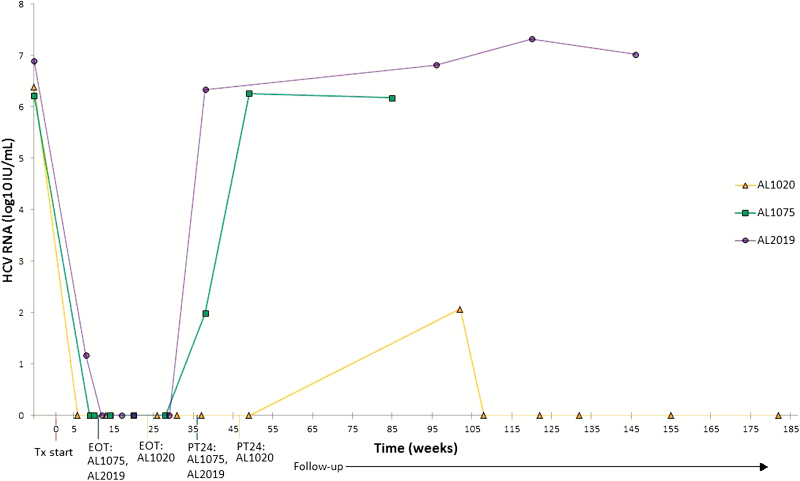
Three participants were identified as cases of reinfection. Two had detectable HCV RNA assessments between PT12 and PT24 and were confirmed to have different phylogenetic strains from baseline using NGS. A third participant was deemed to be reinfected due to transient viremia 17 months after treatment. This participant’s HCV reinfection cleared without retreatment (Figure 1).
Figure 1.
Viral kinetics during and after treatment in participants with HCV reinfection in the PREVAIL extension study. Abbreviations: EOT, end of treatment; HCV, hepatitis C virus; PT24, 24 weeks post HCV treatment; PREVAIL, Prevent Resistance Eliminate Virus and Improve Life; Tx, treatment.
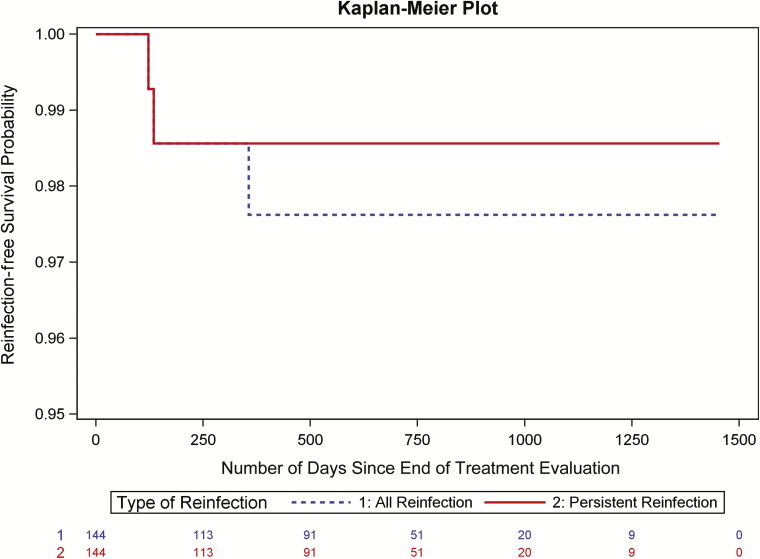
The overall incidence of reinfection was 1.22 per 100 person-years (95% confidence interval [CI], 0.25–3.57) (3 reinfections in 246 person-years of follow-up). Reinfection-free survival rate is depicted in Figure 2 between all and persistent reinfections. Risk factors for reinfection among all participants in the extension study (n = 114) were ongoing IDU in the follow-up period (P < .001), a lack confidence in the ability to avoid contracting HCV (P < .001), homelessness (P = .002), and living with someone who injects drugs (P = .007).
Figure 2.
Incidence of reinfection in the PREVAIL extension study. Risk factors for reinfection among all participants in the extension study (n = 114) were ongoing IDU in the follow-up period (P < .001), a lack confidence in the ability to avoid contracting HCV (P < .001), homelessness (P = .002), and living with someone who injects drugs (P = .007). Abbreviations: HCV, hepatitis C virus; IDU, injection drug use; PREVAIL, Prevent Resistance Eliminate Virus and Improve Life.
Among participants who reported ongoing IDU (n = 22) during the extension study period, the incidence of reinfection was 7.4 per 100 person-years (95% CI, 1.5–21.6) based on 41 person-years of follow-up. Most of these participants injected heroin (86%) or cocaine (68%) and nearly half (45%) used a mixture of heroin and cocaine. Characteristics and risk behaviors identified by log-rank tests to be significantly associated with reinfection included injecting heroin combined with stimulants, injecting stimulants alone, and injecting with partners, as well as sharing syringes and drug paraphernalia (Table 2). There was no association between frequency of injection per day and reinfection (data not shown).
Table 2.
Risk Factors Associated With HCV Reinfection in the PREVAIL Extension Study
| Behavior/characteristica | Ongoing IDU | P b | |
|---|---|---|---|
| Reinfected (N = 3) | No Reinfection (N = 19) | ||
| Injection drug use, n (%) | |||
| Heroin with cocaine | 3 (100) | 7 (37) | .0432 |
| Heroin with speed | 2 (67) | 1 (5) | .0007 |
| Speed | 2 (67) | 0 | <.0001 |
| Crack | 2 (67) | 0 | .0002 |
| Injection practices, n (%) | |||
| Inject with partners | 2 (67) | 6 (32) | .0085 |
| Equipment sharing, n (%) | |||
| Share cooker | 2 (67) | 1 (5) | .0026 |
| Backload syringe | 1 (33) | 0 | <.0001 |
| Share syringe | 3 (100) | 1 (5) | <.0001 |
| Share rinse water | 2 (67) | 1 (5) | .0012 |
| Share cotton | 2 (67) | 1 (5) | .0007 |
| Split drug solution with used syringe | 1 (33) | 0 | <.0001 |
| Other characteristics, n (%) | |||
| Not confident in ability to avoid HCV | 1 (33) | 1 (5) | .0160 |
| Not receiving food stamps | 2 (33) | 2 (11) | .0185 |
| Responses to hypothetical questions, n (%) | |||
| Equally likely to share a cooker with someone else enrolled in this research study | 1 (33) | 0 | <.0001 |
| Equally likely to allow another to use their syringe when assuming both parties have the same HCV status | 1 (33) | 0 | .0071 |
| Does not consider others’ HCV status when deciding to use another’s syringe under the assumption that the owner has a different HCV status | 2 (67) | 4 (21) | .0464 |
| Does not consider others’ HCV status when allowing others to use their syringe under the assumption that the borrower has a different HCV status | 2 (67) | 4 (21) | . 0464 |
Abbreviations: HCV, hepatitis C virus; IDU, injection drug use; PREVAIL, .
aResponses taken from first research visit when injection was reported.
bBased on log-rank tests.
The 3 reinfected participants were Latino men (mean age, 44 years), unemployed, homeless, and had a history of IDU spanning more than 10 years. All 3 participants reported actively injecting (range, 3–5 injections/day) heroin with cocaine and/or speed and sharing drug paraphernalia (Table 3).
Table 3.
Characteristics of Participants Who Were Reinfected in the PREVAIL Extension Study
| Characteristic | Study ID | ||
|---|---|---|---|
| AL1020 | AL1075 | AL2019 | |
| Demographic characteristics | |||
| Gender; age, years | Male; 38 | Male; 48 | Male; 47 |
| Ethnicity | Latino | Latino | Latino |
| Genotype | 1a | 1a | 1a |
| Employment status | Unemployed | Unemployed | Unemployed |
| Education | Middle school or less | Some high school | Some high school |
| Homeless | Yes (street) | Yes (shelter) | Yes (shelter) |
| Anxiety/depression | Extremely anxious or depressed | Extremely anxious or depressed | Moderately anxious or depressed |
| Prior HCV therapy | Ribavirin/sofosbuvir | Ledipasvir/sofosbuvir | Simeprevir/sofosbuvir |
| Cirrhosis | No | Yes | Yes |
| Frequency of running out of money for basic necessities | Occasionally | Monthly | Weekly |
| Drug-use behavior | |||
| IDU before treatment | Yes | Yes | Yes |
| Age of first injection, years | 22 | 20 | 22 |
| Duration of IDU, years | 10 | 17 | 17 |
| Overdose history | Never | Yes | Never |
| IDU post-treatment | Yes | Yes | Yes |
| Type | Heroin | Heroin | Heroin |
| Cocaine | Cocaine | Cocaine | |
| Crack | Crack | Speed | |
| Heroin and cocaine | Speed | Heroin and cocaine | |
| Heroin and cocaine | Heroin and speed | ||
| Heroin and speed | |||
| Frequency, times/day | 4 | 3 | 5 |
| Share cooker | Yes | No | Yes |
| Share syringe | Yes | Yes | Yes |
| Split drug solution with used syringe | Yes | Yes | Yes |
| Share rinse water | No | Yes | Yes |
| Number of drug-use partners | 3 | 2 | 1 |
| Drug-use setting | Public or outdoor space | Home of friend or relative | Partner’s home |
| Reported days of methadone use 30 days prior to PT24 | 30 | 30 | 25 |
Abbreviations: HCV, hepatitis C virus; IDU, injection drug use; PREVAIL, ; PT24, 24 weeks post–HCV treatment.
DISCUSSION
This study is among the first to present a rate of HCV reinfection in the DAA era and, to our knowledge, the first to present specific risk factors for HCV reinfection in a cohort of PWID on OAT. Using long-term, repeated follow-up assessments, we outline some of the most specific data on risk factors for HCV reinfection in the DAA era. The overall rate of reinfection (1.22/100 person-years) was low and consistent with estimates from the DAA era [7, 23]. The risk factors we identified are consistent with known risk factors for primary HCV infection and reinforce the need for targeted education of those who continue to inject drugs and to address social determinants of health such as homelessness.
Early reinfection data from phase 2 and 3 clinical trials have shown rates of 2.6–4.6 reinfections per 100 person-years among PWID on OAT [2, 3]. C-EDGE CO-STAR was a randomized controlled trial to evaluate elbasvir–grazoprevir in treating HCV among PWID on OAT. Through 2 years of interim results from a C-EDGE CO-STAR extension study, the overall incidence of reinfection was 2.3 per 100 person-years, which is closer to the rate we observed [24]. Taken together, these data support that concerns about reinfection should not limit HCV treatment among PWID, particularly in OAT settings. Treatment as prevention has been proven to be an effective strategy to reduce ongoing HCV transmission, particularly when coupled with needle-and-syringe programs and OAT, which argues for treatment irrespective of reinfection rates [25].
Reinfection rates have been demonstrated to be low even among PWID who are not on OAT. The low reinfection rate (2.6/100 person-years) reported by SIMPLIFY is notable given that the study population was composed entirely of participants reporting recent IDU (within 6 months of treatment initiation). Further, 78% of participants reported IDU during therapy [2]. In our study, more than half (52%) of the participants reported IDU during the 30 days preceding treatment initiation and slightly fewer than half reported IDU during follow-up (49% before PT12, 47% before PT24). Still, the reinfection rate observed in the SIMPLIFY study was similar to the rate we observed.
In our study, we found that the incidence of reinfection decreased over time. Two of the 3 reinfection events we observed occurred between PT12 and PT24. The overall reinfection rate in the C-EDGE CO-STAR study also decreased over time: 6 reinfections were observed during the first 24 weeks of follow-up and only 4 additional reinfections were observed in the subsequent 18 months [24]. Similarly, in a recent large-cohort study Rossi et al [7] found that reinfection was more common among recent PWID in the 36 weeks following SVR. Higher incidence of HCV reinfection soon after HCV treatment suggests that the level of risk during this period warrants continued screening (for HCV and other blood-borne pathogens) in concert with overdose prevention and harm-reduction services.
Similar to other existing studies, we demonstrate an increased rate of HCV reinfection among participants who continue to inject drugs [7, 24]. In C-EDGE CO-STAR, the rate of reinfection was 4.2 per 100 person-years among the 37% of participants who reported ongoing IDU (compared with 2.3/100 person-years in the whole population) [24]. Rossi et al. [7] found that reinfection rates were higher among recent (3.1/100 person-years) compared with former (1.4/100 person-years) and non-PWID (0.3/100 person-years).
While our study demonstrated reinfection rates that are similar to those reported elsewhere, we observed a higher incidence among the subpopulation reporting ongoing IDU (6-fold higher than in the overall population). Higher reinfection rates (up to 33/100 person-years) among participants who continue to inject drugs after treatment have been reported in studies of prisoners treated with interferon [26]. Other studies of PWID, both on and not on OAT in the DAA era, are engaged in observational follow-up and, at present, limited interim data are available for comparison [2, 3]. The reinfection rate among participants with ongoing IDU reported by the C-EDGE CO-STAR extension is almost twice the rate observed in the overall population. In addition, 4 of the 6 reinfections observed in the initial study occurred in participants who tested positive for opioids after treatment, and the only reinfection in SIMPLIFY occurred in a participant who was actively injecting. These findings suggest that attention should be focused on those who continue to inject following HCV treatment to reduce the risk of reinfection. This may be particularly relevant to clinicians employing a “consult model” for HCV in which treatment may be followed by discharging patients post-SVR. More data are needed on the frequency of follow-up since visits every 6 months may not be often enough for people with ongoing IDU and risk of reinfection and overdose. Data are also needed on optimal models to promote ongoing care engagement since these individuals are at high risk of loss to follow-up.
Many of the knowledge and sociodemographic characteristics that were associated with HCV reinfection in our study have been associated with primary HCV infection. For example, a lack of confidence in remaining uninfected and homelessness have been associated with increased incidence of initial HCV infection [27, 28]. It is not surprising that stressful socioeconomic conditions have deleterious effects on health, including an increased likelihood of primary HCV infection as well as reinfection [29–31].
While all of those who were reinfected in this study were male, we are unable to assess the role of female sex in reinfection due to the small number of overall individuals reporting ongoing IDU. The incidence of HCV among young women in the United States is rising and there may be risk factors specific to this subpopulation to account for this change in epidemiology (eg, receptive syringe sharing, which may be related to gender inequality) [32]. It is currently unclear whether these factors also impact HCV reinfection. Similarly, a large proportion of overdose deaths in the United States occur in people under age 40 years [33]. Given that the majority of our study population was older, future studies are warranted to examine reinfection rates and risk factors among women and individuals younger than those reported here.
Our study is among the first to evaluate specific risk factors among those who report ongoing IDU following HCV treatment in the DAA era. Reinfection was significantly associated with heroin with cocaine or speed, speed alone, or crack alone, suggesting higher-risk IDU among individuals who inject stimulants, which has been reported elsewhere [34, 35]. The best established risk factor for HCV transmission is syringe sharing, which was reported by all of those who were reinfected in this study. It is debated as to whether cookers are true vectors for HCV transmission or a surrogate for transmission resulting from sharing syringes [36, 37]. Until more evidence is available, PWID need to be educated about the risks of sharing all paraphernalia in order to reduce the risk of HCV transmission. In addition, we identified factors related to social determinants of health (such as a lack of food stamps) and certain attitudes regarding equipment sharing—for example, not considering another’s HCV status when using the syringe of an individual with a different HCV status. Despite the exploratory nature of these findings due to the small sample size, they suggest that certain high-risk behaviors may be prioritized. They also re-emphasize the need for a focus on social determinants of health and highlight the need for educational and motivational interventions, particularly for stimulant use due to a lack of medically assisted treatment for these substances.
This study has limitations. First, research visits were conducted every 6 months. While it is possible that some reinfection events occurred between these time intervals, we detected the clinically relevant reinfections that resulted in lasting viremia during the time frame analyzed. In addition, risk behaviors for 2 reinfected participants were ascertained after the reinfection event. However, it is unlikely that there were changes in risk behavior because the risk behavior of all 3 reinfected participants persisted throughout the follow-up period. Further, the majority of the extension study population were not at high risk of reinfection because 19% reported ongoing IDU. For this reason, we present separate reinfection rates for the overall population and the subset of participants reporting ongoing IDU. Of 141 who achieved SVR, 27 (19%) participants were lost to follow-up; however, we demonstrated that there were no significant differences between these participants with the exception of cirrhosis. One participant was deemed to be reinfected due to transient viremia 17 months after treatment. No specimen for this participant was collected for NGS; however, it was deemed unlikely that this was a treatment failure due to the length of time from the end of treatment [38]. Last, we did not perform a multivariate analysis due to the small number of reinfections and a relatively small sample size, leading to unstable estimates with wide CIs.
Conclusions
Our study is among the first to identify a rate of reinfection and specific risk factors for HCV reinfection among PWID on OAT. We provide further evidence that HCV reinfection rates are low among PWID; however, our data strongly indicate that reinfection is driven by ongoing IDU after HCV treatment even among PWID on OAT. For this reason, individuals who have completed HCV treatment should be screened regularly for ongoing IDU. Patients who continue to inject drugs should receive counseling to reduce syringe and paraphernalia sharing, OAT dose evaluation, and an offer of adjunctive pharmacotherapies and/or behavioral interventions for opioids and other substances. Additionally, enhanced treatment for substance use (eg, residential substance-use treatment) may be indicated to prevent HCV reinfection, overdose, and acquisition/spread of other blood-borne infections such as HIV and endocarditis. Future work should focus on further characterizing specific risk factors with consideration of a standardized, reproducible reinfection visit checklist, which may be used for harm-reduction efforts among those at highest risk of HCV reinfection, as well as efforts to address social determinants of health.
Notes
Acknowledgments. The authors gratefully acknowledge Nicole Bjorklund and the staff at the Montefiore/Einstein biorepository for their assistance in sample preparation, as well as the Centers for Disease Control and Prevention, Atlanta, for their assistance with phylogenetic analysis of reinfection specimens obtained in this study.
Financial support. This work was supported by the National Institute of Drug Abuse of the National Insitutes of Health (K99DA043011 and R01DA034086) and a pilot grant from the Albert Einstein College of Medicine Liver Research Center (P30DK41296).
Potential conflicts of interest. M. J. A. has served on an advisory board for Gilead Sciences outside the submitted work. B. L. N. reports grants from Merck and Co outside the submitted work. A. H. L. has served on advisory boards for Merck Pharmaceuticals, AbbVie, and Gilead Sciences. He has received research grants from Merck Pharmaceuticals and Gilead Sciences. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Day E, Hellard M, Treloar C, et al. ; International Network on Hepatitis in Substance Users (INHSU) Hepatitis C elimination among people who inject drugs: challenges and recommendations for action within a health systems framework. Liver Int 2019; 39:20–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Grebely J, Dalgard O, Conway B, et al. . Sofosbuvir and velpatasvir for hepatitis C virus infection in people with recent injection drug use (SIMPLIFY): an open-label, single-arm, phase 4, multicen tre trial. Lancet Gastroenterol Hepatol. 2018; 3:153–161. doi: 10.1016/S2468-1253(17)30404-1. Epub 2018 Jan 6 [DOI] [PubMed] [Google Scholar]
- 3. Dore GJ, Altice F, Litwin AH, et al. ; C-EDGE CO-STAR Study Group Elbasvir-grazoprevir to treat hepatitis C virus infection in persons receiving opioid agonist therapy: a randomized trial. Ann Intern Med 2016; 165:625–34. [DOI] [PubMed] [Google Scholar]
- 4. Barua S, Greenwald R, Grebely J, Dore GJ, Swan T, Taylor LE. Restrictions for Medicaid reimbursement of sofosbuvir for the treatment of hepatitis C virus infection in the United States. Ann Intern Med 2015; 163:215–23. [DOI] [PubMed] [Google Scholar]
- 5. Tsui JI, Evans JL, Lum PJ, Hahn JA, Page K. Association of opioid agonist therapy with lower incidence of hepatitis C virus infection in young adult injection drug users. JAMA Intern Med 2014; 174:1974–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nolan S, Dias Lima V, Fairbairn N, et al. . The impact of methadone maintenance therapy on hepatitis C incidence among illicit drug users. Addiction 2014; 109:2053–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rossi C, Butt ZA, Wong S, et al. ; BC Hepatitis Testers Cohort Team Hepatitis C virus reinfection after successful treatment with direct-acting antiviral therapy in a large population-based cohort. J Hepatol 2018; 69:1007–14. [DOI] [PubMed] [Google Scholar]
- 8. Alderks C. Trends in the use of methadone, buprenorphine, and extended-release naltrexone at substance abuse treatment facilities: 2003–2015 (update) 2017. Available at: https://www.samhsa.gov/data/sites/default/files/report_3192/ShortReport-3192.html. Accessed 15 March 2019.
- 9. Alter MJ, Kruszon-Moran D, Nainan OV, et al. . The prevalence of hepatitis C virus infection in the United States, 1988 through 1994. N Engl J Med 1999; 341:556–62. [DOI] [PubMed] [Google Scholar]
- 10. Akiyama MJ, Norton B, Arnsten JH, Agyemang L, Heo M, Litwin AH. Intensive models of hepatitis C care for people who inject drugs engaged in opioid agonist therapy. Ann Intern Med. doi:10.7326/M18-1715. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Centers for Disease Control and Prevention. Viral hepatitis surveillance—United States, 2016 Available at: https://www.cdc.gov/hepatitis/statistics/index.htm. Accessed 15 March 2019.
- 12. Verna EC, Brown RS Jr. Hepatitis C virus and liver transplantation. Clin Liver Dis 2006; 10:919–40. [DOI] [PubMed] [Google Scholar]
- 13. US Department of Health and Human Services. Combating the silent epidemic of viral hepatitis (action plan for the prevention, care, and treatment of viral hepatitis). 2011. Available at: https://www.hhs.gov/sites/default/files/action-plan-viral-hepatitis-2011.pdf Accessed 15 March 2019. [Google Scholar]
- 14. Ly KN, Xing J, Klevens RM, Jiles RB, Ward JW, Holmberg SD. The increasing burden of mortality from viral hepatitis in the United States between 1999 and 2007. Ann Intern Med 2012; 156:271–8. [DOI] [PubMed] [Google Scholar]
- 15. Rein DB, Wittenborn JS, Weinbaum CM, Sabin M, Smith BD, Lesesne SB. Forecasting the morbidity and mortality associated with prevalent cases of pre-cirrhotic chronic hepatitis C in the United States. Dig Liver Dis 2011; 43:66–72. [DOI] [PubMed] [Google Scholar]
- 16. Davis GL, Alter MJ, El-Serag H, Poynard T, Jennings LW. Aging of hepatitis C virus (HCV)-infected persons in the United States: a multiple cohort model of HCV prevalence and disease progression. Gastroenterology 2010; 138:513–21, 521.e1–6. [DOI] [PubMed] [Google Scholar]
- 17. Akhtar E, Manne V, Saab S. Cirrhosis regression in hepatitis C patients with sustained virological response after antiviral therapy: a meta-analysis. Liver Int 2015; 35:30–6. [DOI] [PubMed] [Google Scholar]
- 18. Younossi Z, Stepanova M, Gane EJ, et al. . Significant and sustained improvement of Health-Related Quality of Life (HRQL) scores in patients with hepatitis C (HCV) and sustained virologic response (SVR). Presented at American Association for the Study of Liver Diseases. AASLD, 2017. [Google Scholar]
- 19. Carrat F, Fontaine H, Dorival C, et al. . Clinical outcomes in patients with chronic hepatitis C aft er direct-acting antiviral treatment: a prospective cohort study. Lancet 2019; 393:1453–64. [DOI] [PubMed] [Google Scholar]
- 20. Akiyama MJ, Agyemang L, Arnsten JH, et al. . Rationale, design, and methodology of a trial evaluating three models of care for HCV treatment among injection drug users on opioid agonist therapy. BMC Infect Dis 2018; 18:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009; 42:377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Martinello M, Grebely J, Petoumenos K, et al. . HCV reinfection incidence among individuals treated for recent infection. J Viral Hepat 2017; 24:359–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Falade-Nwulia O, Sulkowski MS, Merkow A, Latkin C, Mehta SH. Understanding and addressing hepatitis C reinfection in the oral direct-acting antiviral era. J Viral Hepat 2018; 25:220–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dore GJ, Grebely J, Altice F, et al. . Hepatitis C virus reinfection and injecting risk behavior following elbasvir/grazoprevir treatment in participants on opiate agonist therapy: C-EDGE Co-Star Part B. Presented at American Association for the Study of Liver Diseases. AASLD, 2017. [Google Scholar]
- 25. Heffernan A, Cooke GS, Nayagam S, Thursz M, Hallett TB. Scaling up prevention and treatment towards the elimination of hepatitis C: a global mathematical model. Lancet 2019; 393:1319–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Marco A, Esteban JI, Solé C, et al. . Hepatitis C virus reinfection among prisoners with sustained virological response after treatment for chronic hepatitis C. J Hepatol 2013; 59:45–51. [DOI] [PubMed] [Google Scholar]
- 27. Craine N, Hickman M, Parry JV, et al. . Incidence of hepatitis C in drug injectors: the role of homelessness, opiate substitution treatment, equipment sharing, and community size. Epidemiol Infect 2009; 137:1255–65. [DOI] [PubMed] [Google Scholar]
- 28. Eckhardt B, Winkelstein ER, Shu MA, et al. . Risk factors for hepatitis C seropositivity among young people who inject drugs in New York City: implications for prevention. PLoS One 2017; 12:e0177341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Page K, Yu M, Cohen J, Evans J, Shumway M, Riley ED. HCV screening in a cohort of HIV infected and uninfected homeless and marginally housed women in San Francisco, California. BMC Public Health 2017; 17:171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Noska AJ, Belperio PS, Loomis TP, O’Toole TP, Backus LI. Prevalence of Human immunodeficiency virus, hepatitis C virus, and hepatitis B virus among homeless and nonhomeless United States veterans. Clin Infect Dis 2017; 65:252–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Strike C, Rudzinski K, Patterson J, Millson M. Frequent food insecurity among injection drug users: correlates and concerns. BMC Public Health 2012; 12:1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Geddes L, Iversen J, Wand H, et al. . Sex discrepancies in the protective effect of opioid agonist therapy on incident hepatitis C infection. Clin Infect Dis 2019. pii: ciz162. doi: 10.1093/cid/ciz162. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jalal H, Buchanich JM, Roberts MS, Balmert LC, Zhang K, Burke DS. Changing dynamics of the drug o verdose epidemic in the United States from 1979 through 2016. Science 2018; 361 pii: eaau1184. doi: 10.1126/science.aau1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ropelewski LR, Mancha BE, Hulbert A, Rudolph AE, Martins SS. Correlates of risky injection practices among past-year injection drug users among the US general population. Drug Alcohol Depend 2011; 116:64–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Booth RE, Lehman WE, Kwiatkowski CF, Brewster JT, Sinitsyna L, Dvoryak S. Stimulant injectors in Ukraine: the next wave of the epidemic? AIDS Behav 2008; 12:652–61. [DOI] [PubMed] [Google Scholar]
- 36. Doerrbecker J, Friesland M, Ciesek S, et al. . Inactivation and survival of hepatitis C virus on inanimate surfaces. J Infect Dis 2011; 204:1830–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Heimer R, Binka M, Koester S, et al. . Recovery of infectious hepatitis C virus from injection paraphernalia: implications for prevention programs serving people who inject drugs. J Infect Dis 2018; 217:466-73. doi: 10.1093/infdis/jix427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yoshida EM, Sulkowski MS, Gane EJ, et al. . Concordance of sustained virological response 4, 12, and 24 weeks post-treatment with sofosbuvir-containing regimens for hepatitis C virus. Hepatology 2015; 61:41–5. [DOI] [PubMed] [Google Scholar]