Abstract
BACKGROUND
Perfluoroalkyl and polyfluoroalkyl substances (PFAS) are found widespread in drinking water, foods, food packaging materials and other consumer products. Several PFAS have been identified as endocrine-disrupting chemicals based on their ability to interfere with normal reproductive function and hormonal signalling. Experimental models and epidemiologic studies suggest that PFAS exposures target the ovary and represent major risks for women’s health.
OBJECTIVE AND RATIONALE
This review summarises human population and toxicological studies on the association between PFAS exposure and ovarian function.
SEARCH METHODS
A comprehensive review was performed by searching PubMed. Search terms included an extensive list of PFAS and health terms ranging from general keywords (e.g. ovarian, reproductive, follicle, oocyte) to specific keywords (including menarche, menstrual cycle, menopause, primary ovarian insufficiency/premature ovarian failure, steroid hormones), based on the authors’ knowledge of the topic and key terms.
OUTCOMES
Clinical evidence demonstrates the presence of PFAS in follicular fluid and their ability to pass through the blood–follicle barrier. Although some studies found no evidence associating PFAS exposure with disruption in ovarian function, numerous epidemiologic studies, mostly with cross-sectional study designs, have identified associations of higher PFAS exposure with later menarche, irregular menstrual cycles, longer cycle length, earlier age of menopause and reduced levels of oestrogens and androgens. Adverse effects of PFAS on ovarian folliculogenesis and steroidogenesis have been confirmed in experimental models. Based on laboratory research findings, PFAS could diminish ovarian reserve and reduce endogenous hormone synthesis through activating peroxisome proliferator-activated receptors, disrupting gap junction intercellular communication between oocyte and granulosa cells, inducing thyroid hormone deficiency, antagonising ovarian enzyme activities involved in ovarian steroidogenesis or inhibiting kisspeptin signalling in the hypothalamus.
WIDER IMPLICATIONS
The published literature supports associations between PFAS exposure and adverse reproductive outcomes; however, the evidence remains insufficient to infer a causal relationship between PFAS exposure and ovarian disorders. Thus, more research is warranted. PFAS are of significant concern because these chemicals are ubiquitous and persistent in the environment and in humans. Moreover, susceptible groups, such as foetuses and pregnant women, may be exposed to harmful combinations of chemicals that include PFAS. However, the role environmental exposures play in reproductive disorders has received little attention by the medical community. To better understand the potential risk of PFAS on human ovarian function, additional experimental studies using PFAS doses equivalent to the exposure levels found in the general human population and mixtures of compounds are required. Prospective investigations in human populations are also warranted to ensure the temporality of PFAS exposure and health endpoints and to minimise the possibility of reverse causality.
Keywords: perfluoroalkyl and polyfluoroalkyl substances (PFAS), endocrine-disrupting chemicals (EDCs), ovary, folliculogenesis, steroidogenesis
Introduction
According to the definition adopted by the Endocrine Society Scientific Statement, an endocrine-disrupting chemical (EDC) is ‘a compound, either natural or synthetic, which, through environmental or inappropriate developmental exposures, alters the hormonal and homeostatic systems that enable the organism to communicate with and respond to its environment’ (Diamanti-Kandarakis et al., 2009). A variety of EDCs are used in industrial and consumer applications, such as solvents and lubricants (e.g. polychlorinated biphenyls), flame retardants (e.g. polybrominated diethyl ethers), pesticides (e.g. dichlorodiphenyltrichloroethane and chlorpyrifos) and plasticisers (e.g. phthalates and bisphenol-A) (Burger et al., 2007; Caserta et al., 2011). Among them, perfluoroalkyl and polyfluoroalkyl substances (PFAS) have received unprecedented attention recently due to nationwide drinking water contamination and widespread use that impacts up to 110 million residents in the USA (Environmental Working Group, 2018).
PFAS comprise a large family of manmade fluorinated chemicals that are ubiquitous environmental toxicants to which humans are exposed on a daily basis (Trudel et al., 2008). At least one type of PFAS chemical was detected in the blood of nearly every person sampled in the US National Biomonitoring Program (CDC, 2019). Specific members of this family of chemicals are found in many consumer products, such as non-stick cookware (Teflon) (Bradley et al., 2007; Sinclair et al., 2007), food packaging materials (Begley et al., 2005; Trier et al., 2011; Schaider et al., 2017), stain- and water-resistant coating for clothing, furniture and carpets (Scotchgard and GoreTex) (Hill et al., 2017; Lee et al., 2017) and cosmetics and personal care products (Danish EPA, 2018; Boronow et al., 2019). PFAS are also present in fire-fighting foams (or aqueous film-forming foam, AFFF) widely used in military bases for crash and fire training (Butenhoff et al., 2006; Trudel et al., 2008; Kantiani et al., 2010; Kissa, 2011).
Because PFAS are remarkably widespread in drinking water and groundwater in the USA and globally, especially on and near industrial sites, fire-fighting facilities and military installations, they pose a serious and immediate threat to the communities where the source of drinking water has been contaminated with PFAS. Although government and regulatory bodies have been working towards regulations that limit the production of perfluorooctanesulfonic acid (PFOS) and perfluorooctanoic acid (PFOA), the two primary PFAS compounds that have been the most extensively manufactured (USEPA, 2016a, 2016b), the phase-out and ban of PFOA and PFOS have led to an increased usage of alternative PFAS chemicals (Ateia et al., 2019). Consequently, there is an urgent need to raise the awareness of the potential threat of PFAS to human health.
PFAS have been identified as contaminants of concern for reproductive toxicity (Jensen and Leffers, 2008). Observational studies have shown that PFAS exposure could delay menarche (Lopez-Espinosa et al., 2011), disrupt menstrual cycle regularity (Zhou et al., 2017), cause early menopause (Taylor et al., 2014) and premature ovarian insufficiency (Zhang et al., 2018) and alter the levels of circulating sex steroid hormones (Barrett et al., 2015). The ovary is the site of folliculogenesis and is responsible for the proper maturation of oocytes. It is also the principle site of sex hormone steroidogenesis. Experimental studies have shown that PFAS exposure is associated with the depletion of ovarian reserve (i.e. the number of ovarian follicles and oocytes) (Bellingham et al., 2009; Domínguez et al., 2016; Feng et al., 2015, 2017; Chen et al., 2017; Du et al., 2019; Hallberg et al., 2019; López-Arellano et al., 2019) and inhibition steroidogenic enzyme activities (Shi et al., 2009; Chaparro-Ortega et al., 2018; Wang et al., 2018a). Disruption of this finely controlled network may have physiologic impacts beyond the reproductive system, affecting the overall health of girls and women.
Growing evidence has suggested that the ovaries may be a potential target for PFAS toxicity; however, a comprehensive review of experimental and human studies for the effects of PFAS on normal ovarian function has not previously been reported. In this review, we summarise the sources and pathways of PFAS, describe the processes of ovarian folliculogenesis and steroidogenesis, review the state of the science regarding associations between PFAS exposures and ovarian function in experimental and epidemiological studies, identify gaps in the current data and outline directions for future research.
Methods
A thorough search was carried out for relevant articles in order to ensure a comprehensive review on PFAS exposure and ovarian function. We searched PubMed (https://www.ncbi.nlm.nih.gov/pubmed) through August 2019. The search terms used included PFAS search terms (perfluoroalkyl, polyfluoroalkyl, perfluorinated, fluorocarbons, perfluorobutanoic acid, perfluoropentanoic acid, perfluorohexanoic acid, perfluoroheptanoic acid, perfluorooctanoic acid, perfluorononanoic acid, perfluorodecanoic acid, perfluoroundecanoic acid, perfluorododecanoic acid, perfluorobutane sulfonic acid, perfluorohexane sulfonic acid, perfluoroheptane sulfonic acid, perfluorooctane sulfonic acid, perfluorooctane sulfonamide, PFBA, PFPeA, PFHxA, PFHpA, PFOA, PFNA, PFDA, PFUnDA, PFDoA, PFBS, PFHxS, PFHpS, PFOS, and PFOSA) and outcome search terms (ovary, follicle, oocyte, menarche, menstrual cycle, menopause, primary ovarian insufficiency, premature ovarian failure, steroid hormones, polycystic ovarian syndrome and ovarian cancer). In addition, we manually reviewed the reference lists of identified articles.
Basic Principles of PFAS
Nomenclature
The term PFAS refers to perfluoroalkyl and polyfluoroalkyl substances, a large group of manmade chemicals with the distinguishing structure of a chain of carbon atoms (forming an ‘alkyl’) that has at least one fluorine atom bound to a carbon. Details of PFAS terminology, classification and origins can be found elsewhere (Buck et al., 2011; Interstate Technology Regulatory Council, 2017). Note that use of non-specific acronyms, such as perfluorinated compound (PFC), should be avoided in the scientific publications as it has hampered clear communications in researchers, practitioners, policymakers and the public.
Perfluoroalkyl substances are fully fluorinated molecules in which every hydrogen atom bonded to a carbon in the alkane backbone (carbon chain) is replaced by a fluorine atom, except for the carbon at one end of the chain that has a charged functional group attached. The carbon–fluorine bond is extremely strong and renders these chemicals highly resistant to complete degradation. The basic chemical structure of perfluoroalkyl substances can be written as 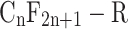 , where ‘
, where ‘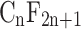 ’ defines the length of the perfluoroalkyl chain tail with n > 2, and ‘
’ defines the length of the perfluoroalkyl chain tail with n > 2, and ‘ ’ represents the attached functional group head (as shown in Fig. 1). PFOA and PFOS (so-called C8 compounds) have been the most extensively produced and studied PFAS homologues. Perfluoroalkyl acids (PFAAs) are some of the most basic PFAS molecules and are essentially non-degradable. PFAAs contain three major groups on the basis of the functional group at the end of the carbon chain: perfluoroalkyl carboxylic acids (PFCAs), perfluoroalkane sulfonic acids (PFSAs) and perfluoroalkyl phosphonates (PFPAs) or perfluoroalkyl phosphinates (PFPiAs).
’ represents the attached functional group head (as shown in Fig. 1). PFOA and PFOS (so-called C8 compounds) have been the most extensively produced and studied PFAS homologues. Perfluoroalkyl acids (PFAAs) are some of the most basic PFAS molecules and are essentially non-degradable. PFAAs contain three major groups on the basis of the functional group at the end of the carbon chain: perfluoroalkyl carboxylic acids (PFCAs), perfluoroalkane sulfonic acids (PFSAs) and perfluoroalkyl phosphonates (PFPAs) or perfluoroalkyl phosphinates (PFPiAs).
Figure 1.
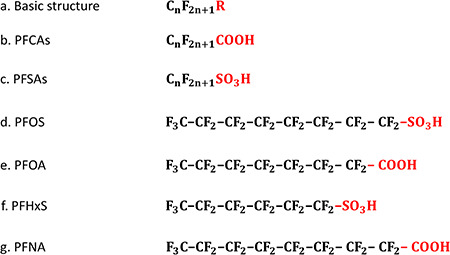
The chemical structures of perfluoroalkyl substances. a. The basic chemical structure of perfluoroalkyl substances, where ‘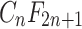 ’ represents the length of the perfluoroalkyl chain and ‘
’ represents the length of the perfluoroalkyl chain and ‘ ’ defines the functional group. c. The general chemical structures of perfluoroalkyl carboxylic acids (PFCAs) with the functional group of –COOH. d. The general chemical structures of perfluoroalkane sulfonic acids (PFSAs) with the functional group of –
’ defines the functional group. c. The general chemical structures of perfluoroalkyl carboxylic acids (PFCAs) with the functional group of –COOH. d. The general chemical structures of perfluoroalkane sulfonic acids (PFSAs) with the functional group of – . d–g. The chemical structures of commonly detected perfluoroalkyl substances, including perfluorooctane sulfonic acid (PFOS), perfluorooctane carboxylic acid (PFOA), perfluohexane sulfonic acid (PFHxS) and perfluorononanoic acid (PFNA).
. d–g. The chemical structures of commonly detected perfluoroalkyl substances, including perfluorooctane sulfonic acid (PFOS), perfluorooctane carboxylic acid (PFOA), perfluohexane sulfonic acid (PFHxS) and perfluorononanoic acid (PFNA).
Polyfluoroalkyl substances differ from perfluoroalkyl substances by the degree of fluorine substitution in the alkane backbone: at least one carbon must not be bound to a fluorine atom and at least two carbons must be fully fluorinated. The fluorotelomer substances are a subset of polyfluoroalkyl substances because they are oligomers with low molecular weight produced by a telomerisation reaction. Some important examples of fluorotelomer substances are fluorotelomer alcohol (FTOH) and perfluorooctane sulfonamidoethanol (FOSE). Since polyfluoroalkyl substances have a carbon that is lacking fluorine substitution, this weaker bond increases potential for degradation (Buck et al., 2011). For example, FTOH and FOSE can be transformed biologically or abiotically to PFOA and PFOS.
In addition to the descriptions above, PFAS can also exist as polymers. These PFAS polymers are large molecules formed by joining many identical small PFAS monomers. Current information indicates that the non-polymer PFAS constitute the greatest risk for environmental contamination and toxicity, although some PFAS polymers can be degradable to basic PFAS.
Sources of human exposure
Previous studies have evaluated daily exposure in populations around the world (Fromme et al., 2007; Tittlemier et al., 2007; Ericson et al., 2008; Trudel et al., 2008; Ostertag et al., 2009; Haug et al., 2010; Zhang et al., 2010; Renzi et al., 2013; Heo et al., 2014). Although it is difficult to compare concentrations among populations because of differences in participant characteristics (e.g. age, sex and geographical locations), the ranges of PFAS serum concentrations are remarkably similar worldwide. Exposure to PFAS in the general population is at lower levels compared to those affected by occupational exposure or local contaminations (ATSDR, 2018). Multiple sources of potential exposure to PFAS have been previously identified in the general population. These sources include diet (Tittlemier et al., 2007; Trudel et al., 2008; Vestergren and Cousins, 2009; Haug et al., 2011a; Domingo and Nadal, 2017), drinking water (Post et al., 2009; Thompson et al., 2011; Hu et al., 2016; Domingo and Nadal, 2019), air and dust (Piekarz et al., 2007; Haug et al., 2011b; Goosey and Harrad, 2012; Fromme et al., 2015; Karásková et al., 2016) and consumer products (Begley et al., 2005; Bradley et al., 2007; Sinclair et al., 2007; Trier et al., 2011; Hill et al., 2017; Lee et al., 2017; Schaider et al., 2017; Boronow et al., 2019). The widespread production of PFAS, their use in common commercial and household products, their improper disposal and their resistance to degradation have led to daily human exposures via oral ingestion, inhalation and dermal contact. Different sources and pathways of human exposure are summarised in Table I.
Table I.
Sources and pathways of human exposure to PFAS.
| Sources | Pathways |
|---|---|
| Dietary sources | |
| Fish and shellfish | Environment/Ingestion |
| Drinking water | Ingestion |
| Food-packaging materials | Ingestion |
| Non-stick cookware | Ingestion |
| Others (including dairy products, eggs, beverages and vegetables) | Ingestion |
| Non-dietary sources | |
| Indoor air | Inhalation |
| Indoor dust | Inhalation/ingestion |
| Soil and sediment | Environment |
| Impregnation spray (for furniture and carpet) | Inhalation/dermal absorption |
| Cosmetics | Dermal absorption |
| Other consumer products (including skin waxes, leather samples andoutdoor textiles) | Dermal absorption |
The highest exposures to PFAS are often from dietary intake, particularly to PFOS and PFOA (Tittlemier et al., 2007; Trudel et al., 2008; Vestergren and Cousins, 2009; Haug et al., 2011a; Domingo and Nadal, 2017). Fish and shellfish generally exhibit the highest PFAS concentrations and detection rates among all types of foodstuffs (Domingo and Nadal, 2017; Jian et al., 2017). Other potential dietary sources of PFAS include dairy products, eggs, beverages and vegetables (Haug et al., 2010; Zhang et al., 2010; Noorlander et al., 2011; Domingo et al., 2012; Eriksson et al., 2013; Herzke et al., 2013; Felizeter et al., 2014; Heo et al., 2014; Gebbink et al., 2015; Vestergren et al., 2012; Chen et al., 2018). However, these foodstuffs have generally low concentrations and low detection frequencies compared to fish and shellfish (Jian et al., 2017). In addition, food can become contaminated with PFAS through transfer from food packaging and/or processing (Schaider et al., 2017) because PFAS are used as in grease- and water-repellent coatings for food-contact materials and non-stick cookware (Begley et al., 2005).
Drinking water is also a common source of PFAS in humans (Domingo and Nadal, 2019). A number of studies have detected PFAS in drinking water samples collected from various countries (Takagi et al., 2008; Jin et al., 2009; Mak et al., 2009; Quinete et al., 2009; Quiñones and Snyder, 2009; Wilhelm et al., 2010; Thompson et al., 2011; Boone et al., 2019). Recently, Boone et al. measured concentrations in source (untreated) and treated drinking water sampled from 24 states across the USA (Boone et al., 2019): Seventeen PFAS analytes were detected in all samples, and summed concentrations ranged from <1–1102 ng/L, with one drinking water treatment plant (DWTP) exceeding the health advisory of 70 ng/L for PFOA and PFOS set by the United States Environmental Protection Agency (U.S. EPA).
Some PFAS polymers such as FTOHs were frequently used for impregnation treatment of furniture and floor coverings and as intermediates in manufacturing various household products (e.g. paints, carpet and cleaning agents). These neutral PFAS, mainly FTOHs, FOSA and FOSEs, are volatile compounds that are easily released into indoor environments (air and dust) due to their low water solubility and high vapour pressure (Langer et al., 2010; Haug et al., 2011b; Yao et al., 2018). Perfluoroalkyls have also been detected in indoor air and dust (Kubwabo et al., 2005; Barber et al., 2007; Strynar and Lindstrom, 2008). In the study of 67 houses in Canada, carpeted homes had higher concentrations of PFOA, PFOS and PFHxS in dust, possibly due to the use of stain-repellent coatings (Kubwabo et al., 2005). The use of aqueous firefighting foams at military installations and the production of fluorochemicals at industrial facilities have resulted in widespread contamination in soil and sediment (Xiao et al., 2015; Anderson et al., 2016). Many consumer products, such as ski waxes, leather samples, outdoor textiles and cosmetics products including hair spray and eyeliner, also contain PFAS (Kotthoff et al., 2015; Danish EPA, 2018).
Previous literature has estimated the relative contributions of different exposures routes of PFOA and PFOS in adults (Trudel et al., 2008; Vestergren and Cousins, 2009; Haug et al., 2011a; Lorber and Egeghy, 2011; Gebbink et al., 2015). Oral ingestion from diet and drinking water has been proposed as the largest source of exposure to PFOA and PFOS (around 90%) compared with inhalation or dermal contact (Vestergren and Cousins, 2009; Haug et al., 2011a; Lorber and Egeghy, 2011; Gebbink et al., 2015). For PFOA, Trudel et al. reported ingestion of food from PFOA-containing packaging materials (56%), inhalation of indoor air and dust (14%) and hand-to-mouth transfer of house dust (11%), as significant pathways (Trudel et al., 2008). Other pathways proposed to be less important included ingestion of food prepared with PTFE-coated cookware, dermal contact from clothes and other consumer products (Trudel et al., 2008).
Transport and clearance of PFAS in the human body
Whereas most persistent organic pollutants, such as polychlorinated biphenyls (PCBs) and brominated flame retardants (BFRs), are lipophilic, the substitution of carbon–hydrogen bonds for the strongest carbon–fluorine counterparts coupled with a charged functional group confers unique dual hydrophobic and lipophobic surfactant characteristics to PFAS molecules (Banks and Tatlow, 1994; Kissa, 2011). Most of the available data on transport and clearance of PFAS is based on studies with PFAAs (primarily PFOA and PFOS). In contrast to other persistent organic pollutants, PFAAs are not stored in adipose tissue but undergo extensive enterohepatic circulation. The presence of PFAAs has been confirmed primarily in liver and serum (Pérez et al., 2013; Falk et al., 2015).
The hydrophobic nature of fluorine-containing compounds can also lead to increased affinity for proteins (Jones et al., 2003). Once consumed, PFAAs tend to partition to the tissue of highest protein density, with ~90 to 99% of these compounds in the blood bound to serum albumin (Ylinen and Auriola, 1990; Han et al., 2003). Due to the ability of albumin to pass the blood follicle barrier (Hess et al., 1998; Schweigert et al., 2006), it is suggested that PFAAs can easily be transported into growing follicles. PFAAs have been detected in human follicular fluid and could alter oocyte maturation and follicle development in vivo (Petro et al., 2014; Heffernan et al., 2018).
The primary route of elimination of PFAAs is through the kidney in the urine (Han et al., 2008). Other important clearance pathways include menstruation (Harada et al., 2005; Taylor et al., 2014; Park et al., 2019; Ding et al., 2020), pregnancy (Monroy et al., 2008) and lactation (Bjermo et al., 2013). Sex hormones have been identified as a major factor in determining the renal clearance of PFAAs. One study examined the role of sex hormones and transport proteins on renal clearance and observed that, in ovariectomised female rats, oestradiol could facilitate the transport of PFAAs across the membranes of kidney tubules into the glomerular filtrate, resulting in lower serum concentrations (Kudo et al., 2002).
Serum concentrations of PFOA, PFOS, PFHxS and PFNA appear to be higher in males than in females across all age groups (Calafat et al., 2007). It has been found that ~30% of the PFOS elimination half-life difference between females and males is attributable to menstruation (Wong et al., 2014a). The differences by sex narrows with aging, suggesting that PFAS may reaccumulate after cessation of menstrual bleeding in postmenopausal women (Wong et al., 2014b; Dhingra et al., 2017; Ruark et al., 2017). Decreased serum concentrations have also been shown in premenopausal versus postmenopausal women and, analogously, in men undergoing venesections for medical treatment (Lorber et al., 2015).
PFAAs are considered metabolically inert and remain in the human body for many years. Estimation of human elimination half-lives (or population halving time) for PFOA, PFOS, PFHxS and PFNA have been reported in previous studies (Olsen et al., 2007, 2012; Spliethoff et al., 2008; Bartell et al., 2010; Brede et al., 2010; Glynn et al., 2012; Yeung et al., 2013a, 2013b; Zhang et al., 2013; Wong et al., 2014a; Worley et al., 2017; Eriksson et al., 2017; Li et al., 2018; Ding et al., 2020). Comparing the estimated half-lives of PFAS among populations is difficult as they differ by sampling time intervals, duration of exposure, sex and age of study subjects. Despite these challenges, most of the aforementioned studies have reported that the half-life in humans of PFOA is around 2–3 years and that of PFOS is ~4–5 years.
Mechanistic Evidence for Ovarian Toxicity of PFAS
Effects of PFAS on folliculogenesis
The ovary is the female gonad and an important endocrine organ. The ovaries consist of a surface epithelium surrounding the ovary, a dense underlying connective tissue (tunica albuginea), an outer cortex and an inner medulla. The cortex appears dense and granular due to the presence of ovarian follicles, corpora lutea and stroma. The medulla is highly vascular with abundant blood vessels, lymphatic vessels and nerves. The main functions of the ovary include production, maturation and release of the female gamete (oocyte), and synthesis of female sex steroid and peptide hormones that regulate reproductive and non-reproductive function. Environmental exposures can exhaust the oocyte pool and cause depletion of follicular cells, leading to earlier age at menopause, premature ovarian failure and infertility (Vabre et al., 2017). The processes of oogenesis and follicle development, and the effects of PFAS exposure on folliculogenesis, are summarised in Fig. 2.
Figure 2.
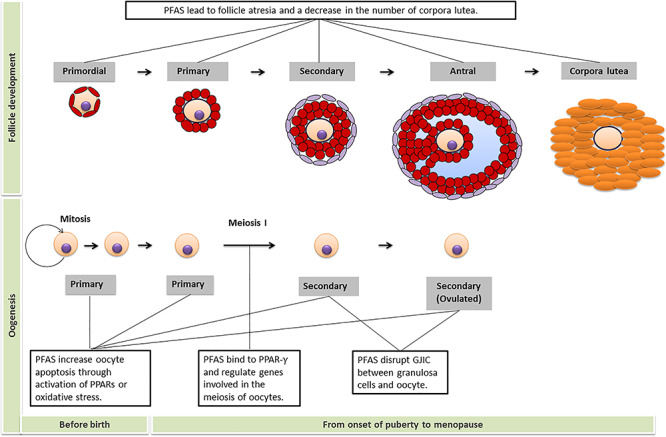
PFAS disrupt folliculogenesis. The upper part of the figure is about follicle development and the text box shows the effects of PFAS on the number of follicles at that stage of development. The part below displays the process of oogenesis and text boxes outline the major effects of PFAS at that stage of oocyte development and maturation. GJIC = gap junction intercellular communication; PPAR = peroxisome proliferator activated receptor.
Effects of PFAS on oogenesis
PFAS exposure has been shown to disrupt the earliest stage of folliculogenesis by altering oocyte development (Domínguez et al., 2016; Hallberg et al., 2019; López-Arellano et al., 2019). The potential mechanisms include activation of peroxisome proliferator-activated receptor (PPAR) signalling pathways, disruption of intercellular communication between oocytes and granulosa cells and induction of oxidative stress.
PPARs are family of nuclear hormone receptors that have been identified as key players in the mode of action for PFAS-induced reproductive toxicity (Desvergne and Wahli, 1999). All three known PPAR family members, α, β/δ and γ, are expressed in the ovary (Dauça et al., 2014). The PPAR-α and PPAR-β/δ isoforms are expressed primarily in thecal and stromal cells in the ovary, while the PPAR-γ isoform is detected strongly in granulosa cells and the corpus lutea (Komar et al., 2001). The ability of PFAS to interact with nuclear PPARs has been put forward as an explanation for metabolic disturbances associated with PFAS exposure, mainly through PPAR-α. In addition, PPAR-γ has been found to inhibit the expression of genes involved in the meiosis of oocytes (e.g. endothelin-1 and nitric oxide synthase) (Komar, 2005), implicating a role in female gamete development.
A recent study reported that administration of 10 μg/mL PFNA on bovine oocytes in vitro for 22 h has a negative effect on oocyte developmental competence during their maturation (Hallberg et al., 2019). This decrease in oocyte survival was attributed to PPAR-α(Hallberg et al., 2019), leading to the disturbance of lipid metabolism and increased lipid accumulation in the ovaries (Bjork and Wallace, 2009; Wang et al., 2012). Lending further support, another study showed that excessive lipids in the ooplasm correlated with impaired oocyte developmental competence and low oocyte survival rates (Prates et al., 2014). Because PFAS can bind and activate PPARs and play an important role in PPAR signalling during ovarian follicle maturation and ovulation, it is plausible that persistent activation of ovarian PPARs through PFAS exposure could disrupt the ovarian cell function and oocyte maturation.
In addition to the impact on PPAR signalling, PFAS exposure could alter cell–cell communication within a follicle. Because the interior of an ovarian follicle is avascular, cell–cell communication among granulosa cells and between granulosa cells and the oocyte is critically dependent on bidirectional transfer of low molecular weight nutrients, signalling molecules and waste products via gap junction intercellular communication (Clark et al., 2018). When treated with an aqueous solution with 0, 12.5, 25 and 59 μM PFOS in vitro for a 44-h maturation period, the number of live oocytes and the percentage of matured oocytes decreased in porcine ovaries (Domínguez et al., 2016). Similarly, foetal murine oocytes exposed to 28.2 and 112.8 μM PFOA in vitro for 7 days exhibited increased apoptosis and necrosis (López-Arellano et al., 2019). These effects are attributed due to inhibition of gap junction intercellular communication between oocytes and granulosa cells (Domínguez et al., 2016; López-Arellano et al., 2019).
PFAS may also induce oxidative stress with increased generation of reactive oxygen species (ROS) production, increased DNA damage and decreased total antioxidant capacity (Wielsøe et al., 2015). Pregnant mice administered 10 mg PFOA/kg/day from gestational days 1–7 or 1–13 exhibited inhibited superoxide dismutase and catalase activity, increased generation of ROS and increased expression of p53 and Bax proteins (important in apoptotic cell death) in the maternal ovaries (Feng et al., 2015; Chen et al., 2017; Xie et al., 2017). Similarly, another study reported significantly increased ROS production in rats exposed to PFOA, which interfered with the activities of complexes I, II and III in the mitochondrial respiratory chain and led to oocyte apoptosis (Mashayekhi et al., 2015; López-Arellano et al., 2019).
Effects of PFAS on follicle development
Studies in laboratory rodents indicate that PFAS alters the formation and/or function of ovarian follicular cells at several stages of development. Adult female mice exposed to 0.1 mg PFOS/kg/day by gavage for 4 months had a decreased number of preovulatory follicles and increased number of atretic follicles (Feng et al., 2015; Chen et al., 2017). Moreover, the PFOS-exposed mice had depressed serum levels of oestradiol and progesterone. Notably, PFOS reduced the mRNA expression of steroidogenic acute regulatory protein (Star), which codes for the StAR protein that transports cholesterol from the outer to the inner mitochondrial membrane, a critical step in steroid hormone biosynthesis: this effect on Star was proposed as the cause of deficits in follicle maturation and ovulation (Feng et al., 2015). Similar findings were reported for pregnant mice exposed to 2.5, 5 and 10 mg PFOA/kg/day from gestational days 1–7 or 1–13, with decreased number of corpora lutea accompanied by decreased mRNA expression of Star in the maternal ovaries (Feng et al., 2015; Chen et al., 2017).
In female rats exposed as neonates to 0.1 and 1 mg PFOA/kg/day or 0.1 and 10 mg PFOS/kg/day, there was a significant reduction in the number of ovarian primordial follicles, growing follicles and corpora lutea (Du et al., 2019). The ovarian effects of the prior study were accompanied by down-regulated mRNA expression of KiSS-1 metastasis-suppressor (Kiss1) and KISS1 receptor (Kiss1r) and a decrease in kisspeptin fibre intensities in the hypothalamus. Because kisspeptin signalling has a critical role in regulation of the ovarian cycle as well as initiation of puberty (Gaytán et al., 2009; Hu et al., 2017), the PFOA and PFOS perturbation of follicular development may have resulted from disruption of kisspeptin signalling in the hypothalamus (Bellingham et al., 2009; Du et al., 2019).
Pregnant mice administered oral doses of 200 and 500 mg/kg/day of perfluorobutane sulfonate (PFBS) on Days 1–20 of gestation gave birth to female offspring that exhibited numerous symptoms of disrupted ovarian function: depressed ovarian size and weight, depressed size and weight, decreased number of ovarian follicles (all stages), delayed vaginal opening, delayed onset of oestrus, prolonged diestrus and reduced serum levels of oestradiol (Feng et al., 2017). In addition, the PFBS exposure disrupted thyroid hormone synthesis consistent with hypothyroxinemia, as indicated by depressed serum levels of the thyroid hormones triiodothyronine (T3) and thyroxine (T4) in the dams on gestation day 20 as well as in the female offspring (Feng et al., 2017). Mounting evidence from animal (Lau et al., 2003; Thibodeaux et al., 2003; Chang et al., 2008) and human studies (Dallaire et al., 2009; Wang et al., 2014) suggests that levels of thyroid hormones decrease with increased PFAS concentrations. Thyroid hormones play a critical role in ovarian follicular development and maturation as well as in the maintenance of other physiological functions (Wakim et al., 1994; Fedail et al., 2014). It is possible that thyroid hormone insufficiency could affect follicle development via an influence on the production of follicular fluid inhibin, oestrogens and other cytokines (Dijkstra et al., 1996; Tamura et al., 1998).
Effects of PFAS on ovarian steroidogenesis
Another primary function of the ovary is ovarian steroidogenesis, i.e. the production and secretion of sex steroid hormones. Ovarian steroidogenesis relies on a strict coordination of both theca cells and granulosa cells and the addition of hypothalamus and anterior pituitary gland (as shown in Fig. 3) (Hillier et al., 1994).
Figure 3.
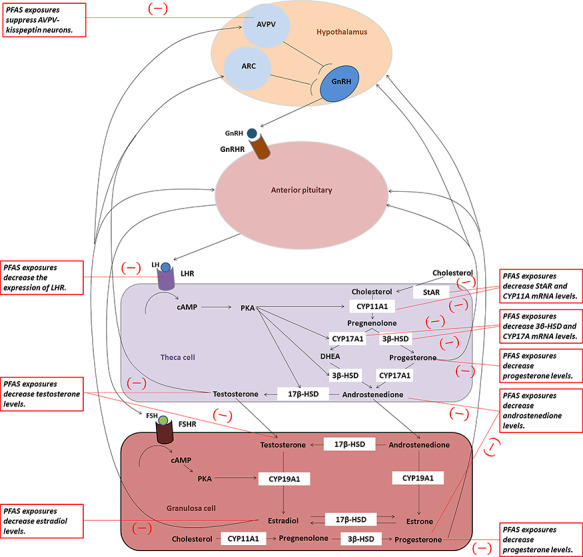
PFAS alter ovarian steroidogenesis. Ovarian steroidogenesis requires the cooperative interactions of the theca and granulosa cells within the follicles. This figure is a simplified overview of the two-cell ovarian steroidogenesis model, with black text boxes indicating PFAS targets from the experimental literature. ARC = arcuate nucleus; AVPV = anteroventral periventricular nucleus; cAMP = cyclic adenosine monophosphate; CYP11A1 = cholesterol side chain cleavage enzyme; CYP17A1 = 17α-hydroxylase-17, 20-desmolase; CYP19A1 = cytochrome P450 aromatase; ERα = oestrogen receptor α; FSH = follicle-stimulating hormone; FSHR = follicle-stimulating hormone receptor; GnRH = gonadotropin-releasing hormone; GnRHR = gonadotropin-releasing hormone receptor; 3β-HSD = 3β-hydroxysteroid dehydrogenase; 17β-HSD = 17β-hydroxysteroid dehydrogenase; LH = luteinising hormone; LHR = luteinising hormone receptor; PKA = protein kinase A; StAR = steroid acute regulatory protein.
Thecal cells produce androgens (androstenedione, A4; and testosterone, T) via the enzyme aromatases. As the precursor to steroidogenesis, cholesterol can be transported to the theca cell cytoplasm via the StAR protein. P450 cholesterol side-chain cleavage enzyme (CYP11A1) then catalyses the conversion of cholesterol to pregnenolone. Pregnenolone is then converted to a precursor androgen, dehydroepiandrosterone (DHEA), which involves the enzyme 17α-hydroxylase-17, 20-desmolase (CYP17A1) or progesterone, via 3β-hydroxysteroid dehydrogenase (3β-HSD). Progesterone and DHEA are then converted to an androgen, A4, via CYP17A1 or 3β-HSD, respectively. The final androgenic steroid produced in the theca cell is T using the enzyme 17β-hydroxysteroid dehydrogenase (17β-HSD).
A4 and T are androgen end-products of theca cell steroidogenesis and migrate across the basal lamina of the follicle to granulosa cells. In preovulatory follicles, granulosa cells proliferate and undergo differentiation to produce increasingly large amounts of 17β-estradiol (E2). Theca cells contain LH receptors (LHRs), and upon receptor binding, LH stimulates the transcription of theca-derived genes that encode the enzymes required for the conversion of cholesterol to androgens. Granulosa cells contain FSH receptors (FSHRs), and in response to FSH binding, the transcription of granulosa-derived genes that encode the enzymes necessary for the conversion of androgens to oestrogens is stimulated.
Endocrine disruption may occur at the molecular and cellular level by interference with steroid hormone biosynthesis in ovaries (Fig. 3). PFAS can modulate the endocrine system by up- or down-regulation of expression of proteins responsible for cholesterol transport and ovarian steroidogenesis. Oral exposure to PFDoA at 3 mg/kg/day from postnatal day 24 for 28 days significantly down-regulated the mRNA expression of ovarian luteinising hormone/choriogonadotropin receptor (Lhcgr), Star, Cyp11a1 and Hsd17b3 in prepubertal female rats, which led to a decrease in E2 production (Shi et al., 2009). Chronic exposure of adult female rats to PFOS (0.1 mg/kg/day) suppresses biosynthesis of E2 possibly through reduced mRNA expression of Star mediated by reduced histone acetylation (Feng et al., 2015). Given that PFAS exposure does not change the substrate (cholesterol) supply in the ovaries (Rebholz et al., 2016), a decrease in Star mRNA levels might account for a reduction in transport of cholesterol as a necessary precursor for ovarian steroidogenesis.
Another possible mechanism of action of PFAS as endocrine disruptors is through activation of PPARs. Exposure of isolated porcine ovarian cells in vitro to 1.2 μM PFOS or PFOA for 24 h inhibited LH-stimulated and FSH-simulated secretion of progesterone, oestradiol and androstenedione in granulosa cells (Chaparro-Ortega et al., 2018). PPAR-γ can inhibit the expression of aromatase, the enzyme for the conversion of androgens to oestrogens, by disrupting the interaction of nuclear factor-kappa B (NF-κB) (Fan et al., 2005). Rak-Mardyla and Karpeta showed that the activation of PPAR-κ caused lower expression and decreased enzymatic activity of CYP17 and 17β-HSD in porcine ovarian follicles (Rak-Mardyła and Karpeta, 2014) and thus decreased levels of progesterone and A4.
PFAS are also known to have weak oestrogenic activity and, as with other weak oestrogens, exposure to a combination of E2, and these compounds produced anti-estrogenic effects (Liu et al., 2007). Studies have reported contradictory results using in vitro screening systems to assay for hormone activity by ER- or AR-mediated transactivation in the human breast adenocarcinoma cell lines MCF-7 and MVLN (Maras et al., 2006; Wang et al., 2012; Kjeldsen and Bonefeld-Jørgensen, 2013; Behr et al., 2018), human adrenal carcinoma cell H295R (Du et al., 2013a; 2013b; Wang et al., 2015; Behr et al., 2018) and human placental choriocarcinoma cell JEG-3 (Gorrochategui et al., 2014), as well as in in vivo testing (Biegel et al., 1995; Du et al., 2013a; Yao et al., 2014). It remains unclear whether PFAS affect oestrogen or androgen receptor signalling at concentrations relevant to human exposure.
Nonetheless, because gonadotropin (GnRH) neurons in the hypothalamus do not express ER, they are regulated by E2 and T primarily from kisspeptin neurons in the arcuate nucleus (ARC) and anteroventral periventricular nucleus (AVPV) which send projections to GnRH neurons (Roa et al., 2009). E2 and T down-regulate Kiss1 mRNA in the ARC and up-regulate its expression in the AVPV. Therefore, kisspeptin neurons in the ARC may participate in the negative feedback regulation of GnRH secretion, whereas kisspeptin neurons in the AVPV contribute to generating the preovulatory GnRH surge in the female. In vivo evidence demonstrated that exposure of adult female mice to PFOS at 10 mg/kg/day for 2 weeks led to diestrus prolongation and ovulation reduction through suppression of AVPV-kisspeptin neurons, but not via ARC-kisspeptin neurons in the forebrain (Wang et al., 2018a). PFAS may impair ovulation and reproductive capacity through suppression of the activation of ER-mediated AVPV-kisspeptin expression.
Epidemiologic Evidence Linking PFAS Exposure and Ovarian Outcomes
Ovarian folliculogenesis and steroidogenesis are essential processes for normal reproductive health. Increasing evidence suggests that PFAS could adversely affect numerous aspects of these processes. Specifically, exposures to PFOA and PFOS have been shown to impact ovarian steroidogenesis (Table II), delay onset of menarche (Table III), disrupt menstrual cycle regularity (Table IV), accelerate ovarian aging (Table V) and may affect other chronic conditions such as polycystic ovarian syndrome (PCOS) and ovarian cancer (Table VI). Other PFAS homologues may also have an impact on ovarian function (Table VII).
Table II.
Epidemiologic evidence on the associations of exposure to PFOA and PFOS with sex hormones.
| First author, Year | Study design | Population, location, and time period | Sample size | Age, year | PFOA, ng/mL | PFOS, ng/mL | Inclusion/exclusion criteria | Hormones | Measure of association | Results | Covariate adjusted |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Knox 2011 | Cross-sectional | Women enrolled in the C8 Health Project from the Mid-Ohio Valley in the US during 2005–2006 | 25 957 | 18–65 | Median 23.6 | Median 17.6 | Excluding pregnant women, women on hormones or medications affecting hormones, women who had hysterectomy | Serum E2, pmol/L |
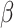 (P value) stratified by age groups (18–42, >42–51 and >51 years) (P value) stratified by age groups (18–42, >42–51 and >51 years) |
PFOA: no association PFOS: only in women >42–51 years,(−) -13.4 (P < 0.0001); and >51 years, (−) −3.0 (P = 0.007) | Age, BMI, alcohol consumption, smoking, exercise |
| Kristensen, 2013 | Birth cohort | Female offspring enrolled in a Danish population-based cohort from 1988–1989 with follow-ups in 2008–2009 | 267 | ~20 | Median (IQR) maternal exposure 3.6 (2.8–4.8) | Median (IQR) maternal exposure 21.1 (16.7–25.5) | Excluding mothers with breastfeeding, or signs of premature ovarian insufficiency | Serum E2, T, SHBG, DHEA, FSH, LH, and AMH, ln(pmol/L); FAI |
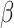 (95%CI) (95%CI) |
No association | Maternal smoking during pregnancy, household income, BMI, smoking status, menstrual cycle phase |
| Barrett, 2015 | Cross-sectional | Healthy women with natural cycling enrolled in the parent Energy Balance and Breast Cancer Aspects study from Norway during 2000–2002 | 178 | 25–35 | Median by parity Nulliparous: 3.4; parous: 2.0 | Median by parity Nulliparous: 14.8; parous: 12.7 | Excluding women with OC use, known histories of infertility gynaecological disorders, or chronic illness (e.g. Type 2 diabetes or hypothyroidism) | Saliva E2 and P, ln(pmol/L) Day −7 to −1 for E2 and day +2 to +10 for P | β (95%CI) stratified by parity | Among nulliparous women, 1) ln(E2) PFOA: no association PFOS:(−) -0.025 (−0.043, −0.007) 2) ln(P) PFOA: no association PFOS:(−) -0.027 (−0.048, −0.007) Among parous women, no association | Age, marital status, BMI, physical activity, history of hormone contraceptives, alcohol consumption, smoking status |
| Lewis, 2015 | Cross-sectional | Women in NHANES 2011–2012 from the US | 824 | 12–80 | Median by age groups 12–<20:1.5; 20–<40:1.5; 40–<60:1.6; 60–<80:2.6 | Median by age groups 12–<20:3.8; 20–<40:4.2; 40–<60:4.9; 60–<80: 9.5 | NA | Serum T, pmol/L | Percent change (95% CI) per doubling increase in PFAS stratified by age groups | No association | Age, BMI, PIR, serum cotinine, race/ethnicity |
| Tsai, 2015 | Cross-sectional | Women recruited from China during 2006–2008 | 330 | 12–30 | Median 3.6 | Median 5.4 | Including adolescent and young adult students | Serum SHBG, FSH, T, and E2 ln(pmol/L) | Predicted mean(SE) in PFAS categories (<median, median-p75, >p75–p90, >p90) stratified by age groups (12–17; 18–30) | Among adolescents, (i) SHBG PFOA: (−) 3.5 (0.2), 3.5 (0.3), 3.4 (0.3), 3.0 (0.3) PFOS: no association (ii) FSH: no association (iii) T PFOA: no association PFOS: (−) 4.0 (0.2), 4.0 (0.2), 3.9 (0.2), 3.6 (0.4) (iv) E2: no association Among young adults, no association | Age, gender, BMI, high-fat diet |
| Lopez-Espinosa, 2016 | Cross-sectional | Girls enrolled in the C8 Health Project during 2005–2006 | 1123 | 6–9 | Median 35 | Median 22 | Excluding girls with menarche | Serum E2 and T, pmol/L | Percent change (95% CI) in the p75 vs. p25 of ln(PFAS) | PFOA: no association PFOS: (−) -6.6% (−10.1%, −2.8%) | Age, time of sampling |
| Zhou, 2016 | Cross-sectional | Girls enrolled in the Genetics and Biomarkers study for Childhood Asthma from China during 2009–2010 | 123 | 13–15 | Median (IQR) 0.5 (0.4–1.2) | Median (IQR) 28.8 (14.8–42.6) | Including girls from seven public schools who had no personal or family history of asthma | Serum E2 and T, pmol/L |
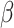 (95%CI) per 1 ng/mL increase in PFAS (95%CI) per 1 ng/mL increase in PFAS |
No association | Age, BMI, ETS exposure, parental education, regular exercise and month of survey |
| McCoy, 2017 | Cross-sectional | Women undergoing IVF in the US during 2013–2014 | 36 | Mean 34 | Plasma, ng/g Mean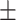 SD 2.4 SD 2.4 0.3 0.3 |
Plasma, ng/g Median SD 6.5 SD 6.5 0.5 0.5 |
Including women at the Coastal Fertility Center in the Mount Pleasant, South Carolina | Plasma E2, pg/mL | Correlation coefficient (P value) | PFOA: no association PFOS: (−) -0.47 (P < 0.05) | NA |
| Heffernan, 2018 | Case–control | Women with PCOS and age- and BMI-matched controls recruited from the UK in 2015 | 59 | 20–45 | GM (range) 2.4 (0.5–8.2) | GM (range) 3.5 (0.9–7.7) | Including women with BMI  35 and undergoing IVF; excluding those with immunological disease, diabetes, renal insufficiency, infections or inflammatory diseases. 35 and undergoing IVF; excluding those with immunological disease, diabetes, renal insufficiency, infections or inflammatory diseases. |
Serum T, and SHBG, ln(pmol/L); FAI; Serum A4 and E2, pmol/L Luteal phase | β (SE) per ln-unit increase in PFAS stratified by cases and controls | Among PCOS cases, no association. Among controls, (i) ln(T) PFOA: (+) 0.52 (0.15) PFOS: no association (ii) SHBG: no association (iii) ln(FAI) PFOA: no association PFOS: (−) -0.61 (0.26) (iv) A4: no association (v) E2: no association | Serum albumin |
| Zhang, 2018 | Case–control | Women with overt POI and 120 healthy controls from China recruited during 2013–2016 | 240 | 20–40 | Median (IQR) 11.1 (7.6–14.5) | Median (IQR) 8.4 (6.3–11.3) | Excluding women with chromosomal abnormalities, a history of radiotherapy or chemotherapy, ovarian surgery, thyroid-related diseases or use of thyroid medications | Serum FSH, LH, PRL, T and E2, ln(ng/mL) Early follicular phase |
 (95%CI) per ln-unit increase in PFAS (95%CI) per ln-unit increase in PFAS |
Among POI cases, (i) ln(FSH) PFOA: no association PFOS: (+) 0.3 (0.2–0.4) (ii) ln(LH): no association (iii) ln(E2) PFOA: no association PFOS: (−) -0.2(−0.4,-0.04) (iv) ln(PRL): PFOA: (+) 0.2 (0.01–0.3) PFOS: (+) 0.2 (0.06–0.3) (v) ln(T): no association Among controls, no association | Age, BMI, education, income, sleep quality, parity |
Abbreviations: AMH, anti-Müllerian hormone; A4, androstenedione; BMI, body mass index; DHEA, dehydroepiandrosterone; ETS, environmental tobacco smoke; E2, estradiol; FAI, free androgen index, was calculated as 100  total T / SHBG; FSH, follicle-stimulating hormone; GM, geometric mean; IQR, interquartile range; NA, not available; NM, not measured; OR, odds ratio; P, progesterone; PCOS, polycystic ovarian syndrome; PFOA, perfluorooctanoic acid; PFOS, perfluorooctane sulfonic acid; POI, premature ovarian insufficiency; PRL, prolactin; p25, 75 and 90, 25th, 75th and 90th percentiles; SHBG, sex hormone-binding globulin; T, testosterone; 95% CI, 95% confidence interval.
total T / SHBG; FSH, follicle-stimulating hormone; GM, geometric mean; IQR, interquartile range; NA, not available; NM, not measured; OR, odds ratio; P, progesterone; PCOS, polycystic ovarian syndrome; PFOA, perfluorooctanoic acid; PFOS, perfluorooctane sulfonic acid; POI, premature ovarian insufficiency; PRL, prolactin; p25, 75 and 90, 25th, 75th and 90th percentiles; SHBG, sex hormone-binding globulin; T, testosterone; 95% CI, 95% confidence interval.
Table III.
Epidemiologic evidence on the associations of exposure to PFOA and PFOS with menarche.
| First author, Year | Study design | Population, location, and time period | Sample size | Age, year | PFOA, ng/mL | PFOS, ng/mL | Outcome | Measure of association | Results | Covariate adjusted |
|---|---|---|---|---|---|---|---|---|---|---|
| Christensen, 2011 | Case–control | Girls with age of menarche before 11.5 years and controls with age of menarche later than 11.5 years from the Avon Longitudinal Study of Parents and Children conducted during 1991–1992 in the UK | 218 cases and 230 controls | 8–13 | Median (IQR) maternal exposure 3.7 (2.8–4.8) | Median (IQR) maternal exposure 19.8 (15.1–24.9) | Early menarche before age 11.5 years | OR (95% CI) by PFAS dichotomous categories | PFOA/PFOS: no association | Birth order, maternal age at delivery |
| Lopez-Espinosa, 2011 | Cross-sectional | Girls enrolled in the C8 Health Project from the Mid-Ohio Valley in the US during 2005–2006 | 2931 | 8–18 | Median (IQR) 28.2 (11–58) | Median (IQR) 20.2 (14–27) | (i) Being postmenarcheal (ii) Delay in age of menarche, day | OR (95%CI) in the highest quartile vs. the lowest (the reference) | (i) Being postmenarcheal PFOA: (−) 0.57 (0.37–0.89). PFOS: (−) 0.55 (0.35–0.87). (ii) Delay in age of menarche PFOA: 130 days PFOS: 138 days | Age |
| Kristensen, 2013 | Birth cohort | Female offspring enrolled in a Danish population-based cohort from 1988–1989 with follow-ups in 2008–2009 | 267 | ~20 | Median (IQR) maternal exposure 3.6 (2.8–4.8) | Median (IQR) maternal exposure 21.1 (16.7–25.5) | Age of menarche, month |
 (95%CI) in the highest tertile vs. the lowest (the reference) (95%CI) in the highest tertile vs. the lowest (the reference) |
PFOA: (+) 5.3 (1.3–9.3) PFOS: no association | Maternal smoking during pregnancy, household income, daughter’s BMI |
Abbreviations: BMI, body mass index; IQR, interquartile range; OR, odds ratio; PFOA, perfluorooctanoic acid; PFOS, perfluorooctane sulfonic acid; 95% CI, 95% confidence interval.
Table IV.
Epidemiologic evidence on the associations of exposure to PFOA and PFOS with menstrual cycle characteristics.
| First author, Year | Study design | Population, location, and time period | Sample size | Age, year | PFOA, ng/mL | PFOS, ng/mL | Outcome | Measure of association | Results | Covariate adjusted |
|---|---|---|---|---|---|---|---|---|---|---|
| Fei, 2009 | Cross-sectional | Women with planned pregnancy enrolled in the Danish National Birth Cohort during 1996–2002 | 1240 | Mean 30.6 | Median (IQR) 5.3 (4.0–7.0) | Median (IQR) 33.7 (26.6–43.5) | Self-reported irregular menses | Proportion of women in the lowest vs. the upper three quartiles | PFOA: 9.0 vs. 15.0% PFOS: 11.6 vs. 14.2% | None |
| Kristensen, 2013 | Birth cohort | Female offspring enrolled in a Danish population-based cohort from 1988–1989 with follow-ups in 2008–2009 | 267 | ~20 | Median (IQR) prenatal exposure 3.6 (2.8–4.8) | Median (IQR) maternal exposure 21.1 (16.7–25.5) | (i) Cycle length, day (ii) Number of follicles per ovary |
 (95% CI) per ln-unit increase stratified by OC use (95% CI) per ln-unit increase stratified by OC use |
PFOA/PFOS: no association | Maternal smoking during pregnancy, social class, BMI, smoking status. |
| Lyngsø, 2014 | Cross-sectional | Women with planned pregnancy enrolled in the Inuit-Endocrine Cohort during 2002–2004 from Greenland, Poland, and Ukraine | 1623 | 19–49 | Median (p10;90) 1.5 (0.7–3.1) | Median (p10;90) 8.0 (3.6–25.6) | (i) Longer cycle with cycle length  32 days (ii) Shorter cycle with cycle length 32 days (ii) Shorter cycle with cycle length  24 days (iii) Irregular cycle with 24 days (iii) Irregular cycle with  7 days in difference between cycles 7 days in difference between cycles |
OR (95% CI) in the highest tertile vs. the lowest (the reference) | (i) Longer cycle PFOA: (+) 1.8 (1.0–3.3). PFOS: no association (ii) Shorter cycle PFOA/PFOS: no association (iii) Irregular cycle PFOA/PFOS: no association | Age at menarche, age at pregnancy, parity, BMI before pregnancy, smoking, and country |
| Lum, 2017 | Cross-sectional | Female attempting pregnancy enrolled in the Longitudinal Investigation of Fertility and Environment Study during 2005–2009 from the US | 501 | 18–40 | Median 3.2 | Median 12.3 | Relative difference in cycle length | AF (95% CI) in the highest tertile vs. the lowest (the reference) | PFOA: (−) 0.98 (0.96–1.00) PFOS: no association | Age, BMI, smoking status |
| Zhou, 2017 | Cross-sectional | Women who were attempting pregnancy recruited during 2013–2015 from China | 950 | Median (IQR) 30 (28–32) | Median (IQR) 13.8 (10.1–18.8) | Median (IQR) 10.5 (7.6–15.4) | (i) Longer periods with cycle length > 35 days (ii) Irregular periods with  7 days in difference between cycles (iii) Menorrhagia as self-reported heavy or very heavy bleeding (iv) Hypomenorrhea as self-reported light bleeding 7 days in difference between cycles (iii) Menorrhagia as self-reported heavy or very heavy bleeding (iv) Hypomenorrhea as self-reported light bleeding |
OR (95% CI) in the highest quartile vs. the lowest (the reference) | (i) Longer periods PFOA: (+) 2.0 (1.2–3.1). PFOS: no association (ii) Irregular periods PFOA: (+) 2.0 (1.2–3.2). PFOS: no association (iii) Menorrhagia PFOA: (−) 0.2 (0.1–0.5). PFOS: (−) 0.3 (0.1–0.6). (iv) Hypomenorrhea PFOA/PFOS: no association | Age, BMI, income, age at menarche, and parity |
Abbreviations: AF, acceleration factor; BMI, body mass index; IQR, interquartile range; OR, odds ratio; PFOA, perfluorooctanoic acid; PFOS, perfluorooctane sulfonic acid; 95% CI, 95% confidence interval.
Table V.
Epidemiologic evidence on the associations of exposure to PFOA and PFOS with ovarian aging.
| First author, Year | Study design | Population, location, and time period | Sample size | Age, year | PFOA, ng/mL | PFOS, ng/mL | Outcome | Measure of association | Results | Covariate adjusted |
|---|---|---|---|---|---|---|---|---|---|---|
| Knox, 2011 | Cross-sectional | Women enrolled in the C8 Health Project from the Mid-Ohio Valley in the US during 2005–2006 | 25 957 | 18–65 | Median 23.6 | Median 17.6 | Natural menopause | OR (95% CI) in the highest quintile vs. the lowest (the reference) stratified by age groups (18–42, >42–51 and >51 years) | 18–42 years PFOA/PFOS: no association >42–51 years PFOA: (+) 1.4 (1.1–1.8) PFOS: (+) 1.4 (1.1–1.8) >51 years PFOA: (+)1.7 (1.3–2.3) PFOS: (+) 2.1 (1.6–2.8) | Age, BMI, alcohol consumption, smoking status, exercise |
| Taylor, 2014 | Retrospective cohort | General adult women in NHANES 1999–2010 from the US | 2732 | 20–65 | Median 3.8 | Median 14.0 | (i) Natural menopause (ii) Hysterectomy | HR (95% CI) in the highest tertile vs. the lowest (the reference) | (i) Natural menopause PFOA: (+) 1.4 (1.1–1.8) PFOS: no association (ii) Hysterectomy PFOA: (+) 2.8 (2.1–3.7) PFOS: (+) 2.6 (1.9–3.4) | Age, race/ethnicity, education, smoking status, parity |
| Dhingra, 2016 | Prospective cohort | Premenopausal women enrolled in the C8 Health Project from the Mid-Ohio Valley in the US during 2005–2006 and followed up during 2008–2011 | 3334 |
 40 40 |
P40, 60 17.8–33.6 | NM | Natural menopause | HR (95% CI) in the highest quintile vs. the lowest (the reference) with hysterectomy censored or excluded | No association | Smoking status, education, BMI, parity |
| Zhang, 2018 | Case–control | Women with overt POI and healthy controls from China recruited during 2013–2016 | 240 | 20–40 | Median (IQR) POI cases, 11.1 (7.6–14.5); Controls, 8.4 (6.3–11.3) | Median (IQR) POI cases, 8.2 (5.5–13.5); Controls, 6.0 (4.2–9.1) | POI as an elevated FSH level >25 IU/L on two occasions >4 weeks apart and oligo/amenorrhea for  4 months 4 months |
OR (95% CI) in the highest tertile vs. the lowest (the reference) | PFOA: (+) 3.8 (1.9–7.5) PFOS: (+) 2.8 (1.5–5.4) | Age, BMI, education, income, sleep quality, parity |
Abbreviations: AF, acceleration factor; IQR, interquartile range; NM, not measured; OR, odds ratio; PFOA, perfluorooctanoic acid; PFOS, perfluorooctane sulfonic acid; POI, premature ovarian insufficiency; 95% CI, 95% confidence interval.
Table VI.
Epidemiologic evidence on the associations of exposure to PFOA and PFOS with other chronic conditions.
| First author, Year | Study design | Population, location, and time period | Sample size | Age, year | PFOA, ng/mL | PFOS, ng/mL | Outcome | Measure of association | Results | Covariate adjusted |
|---|---|---|---|---|---|---|---|---|---|---|
| Barry, 2013 | Retrospective cohort | Female residents enrolled in the C8 Health Project and workers employed at DuPont from the US during 2005–2006 and followed up in 2008–2011 | 17 360 | Mean 53 | Median (range) for residents 24.2 (0.25–4752), and for workers 112.7 (0.25–22 412) | NM | Ovarian cancer | HR (95% CI) per ln-unit increase in PFAS | No association | Smoking, alcohol consumption, sex, education, birth year |
| Vieira, 2013 | Cross-sectional | Cancer patients living near the DuPont plant from the US during 1996–2005 | 25 107 | Median 67 | Range 3.7–655 estimated based on geocoded address | NM | Ovarian cancer | OR (95% CI) in the highest category (110–655 ng/mL) vs. unexposed group (the reference) | No association | Age, race, sex, diagnosis year, insurance provider, smoking status |
| Wang, 2019a | Case–control | Infertile women diagnosed with PCOS and healthy controls from China in 2014 | 367 | 20–40 | Median 5.1 | Median 4.1 | PCOS | OR (95% CI) in the highest tertile vs. the lowest (the reference) | No association | Age, BMI, household income, education, employment, age at menarche, menstrual volume |
Abbreviations: NM, not measured; OR, odds ratio; PCOS, polycystic ovarian syndrome; PFOA, perfluorooctanoic acid; PFOS, perfluorooctane sulfonic acid; 95% CI, 95% confidence interval.
Table VII.
Epidemiologic evidence on the effects of other PFAS homologues.
| First author, Year | Study design | Population, location and time period | Sample size | Age, year | Other PFAS homologues, ng/mL | Outcome | Measure of association | Results | Covariate-adjusted |
|---|---|---|---|---|---|---|---|---|---|
| Menarche | |||||||||
| Christensen, 2011 | Case–control | Girls with age of menarche before 11.5 years and controls with age of menarche later than 11.5 years from the Avon Longitudinal Study of Parents and Children conducted during 1991–1992 in the UK | 448 | 8–13 | Median (IQR) maternal exposure PFOSA, 0.2 (0.2–0.3) EtFOSAA, 0.6 (0.4–0.9) MeFOSAA, 0.4 (0.3–0.8) PFHxS, 1.6 (1.2–2.2) PFNA, 0.6 (0.5–0.8) | Early menarche before age 11.5 years | OR (95% CI) by PFAS dichotomous categories at medians | No association | Birth order, maternal age at delivery |
| Menstrual cycle characteristics | |||||||||
| Zhou, 2017 | Cross-sectional | Women who were attempting pregnancy recruited during 2013–2015 from China | 950 | ~30 | Median (IQR) PFHxS, 0.7 (0.6–0.9) PFNA, 1.4 (1.0–1.9) | (i) Longer cycle with cycle length > 35 days (ii) Irregular cycle with  7 days in difference between cycles (iii) Menorrhagia as self-reported heavy or very heavy bleeding (iv) Hypomenorrhea as self-reported light bleeding 7 days in difference between cycles (iii) Menorrhagia as self-reported heavy or very heavy bleeding (iv) Hypomenorrhea as self-reported light bleeding |
OR (95% CI) in the highest quartile vs. the lowest (the reference) | (i) Longer cycle PFHxS: (+) 2.1 (1.3–3.5) PFNA: (+) 1.7 (1.0–2.7) (ii) Irregular cycle PFHxS: (+) 2.1 (1.3–3.5) PFNA: no association (iii) Menorrhagia PFHxS: (−) 0.3 (0.1–0.7) PFNA: (−) 0.4 (0.2–0.9) (iv) Hypomenorrhea PFHxS: (+) 3.6 (1.5–8.6) PFNA: no association | Age, BMI, income, age at menarche and parity |
| Lum, 2017 | Cross-sectional | Female attempting pregnancy enrolled in the Longitudinal Investigation of Fertility and Environment Study during 2005–2009 from the US | 501 | 18–40 | Median (IQR) among women with normal cycle MeFOSAA, 0.3 (0.1–0.5) PFDA, 0.4 (0.2–0.6) PFNA, 1.2 (0.8–1.7) | Relative difference in menstrual cycle length | AF (95% CI) in the highest tertile vs. the lowest (the reference) | No association | Age, BMI, smoking status |
| Ovarian aging | |||||||||
| Zhang, 2018 | Case–control | Women with overt POI and healthy controls from China recruited during 2013–2016 | 240 | 20–40 | Median (IQR) in controls: PFHpA, 0.2 (0.1–0.3) PFNA, 1.8 (1.3–2.7) PFDA, 1.7 (1.0–2.6) PFDoA, 0.17 (0.1–0.2) PFUnDA, 1.3 (0.8–1.9) PFBS, 0.05 (0.04–0.1) PFHxS, 0.3 (0.2–0.4) | POI as an elevated FSH level > 25 IU/L on two occasions >4 weeks apart and oligo/amenorrhea for  4 months 4 months |
OR (95% CI) in the highest tertile vs. the lowest (the reference) | PFHpA: no association PFNA: no association PFDA: no association PFDoA: no association PFUnDA: no association PFBS: no association PFHxS: (+) 6.6 (3.2–13.7) | Age, BMI, education, income, sleep quality, parity |
| Taylor, 2014 | Retrospective cohort | General adult women in NHANES 1999–2010 from the US | 2732 | 20–65 | Median (IQR) in premenopausal women: PFHxS, 1.0 (0.6–1.8) PFNA, 0.9 (0.6–1.4) | (i) Natural menopause (ii) Hysterectomy | HR (95% CI) in the highest tertile vs. the lowest (the reference) | (i) Natural menopause PFHxS: (+) 1.7 (1.4–2.1) PFNA: (+) 1.5 (1.1–1.9) (ii ) Hysterectomy PFHxS: (+) 3.5 (2.7–4.5) PFNA: (+) 1.8 (1.3–2.4) | Age, race/ethnicity, education, smoking status, parity |
| Sex steroid hormones | |||||||||
| Barrett et al., 2015 | Cross-sectional | Healthy women with natural cycling enrolled in the parent Energy Balance and Breast Cancer Aspects study from Norway during 2000–2002 | 178 | 25–35 | Median (range) in nulliparous women: PFOSA, 0.2 (0.07–1.1) PFHxS, 1.1 (0.3–5.0) PFDA, 0.2 (0.05–2.0) PFUnDA, 0.4 (0.07–1.1) PFNA, 0.6 (0.2–2.9) | Saliva E2 and P, ln(pmol/L) Days −7 to −1 for E2 and Days +2 to +10 for P |
 (95%CI) stratified by parity (95%CI) stratified by parity |
No association | Age, marital status, BMI, physical activity, history of hormone contraceptives, alcohol consumption, smoking status |
| Lewis, 2015 | Cross-sectional | Women in NHANES 2011–2012 from the US | 824 | 12–80 | Median by age groups 12–<20:PFHxS, 0.8; PFNA, 0.7; 20–<40:PFHxS, 0.7; PFNA, 0.7; 40–<60:PFHxS, 0.9; PFNA, 0.8; 60–<80:PFHxS, 1.5; PFNA, 1.1. | Serum T, pmol/L | Percent change (95% CI) per doubling increase in PFAS stratified by age groups | No association | Age, BMI, PIR, serum cotinine, race/ethnicity |
| Tsai, 2015 | Cross-sectional | Women recruited from China during 2006–2008 | 330 | 12–30 | Median (IQR) PFUnDA, 6.5 (1.5–13.4) P60, 90 PFNA, 1.6–6.9 | Serum SHBG, FSH, T, and E2 ln(pmol/L) | Predicted mean(SE) in PFAS categories (<median, median-p75, >p75-p90, >p90) stratified by age groups (12–17; 18–30) | Among adolescents, (i) SHBG: no association (ii) FSH PFNA: no association PFUnDA: only in girls 12–17 years, (−) 1.6 (0.3), 1.6 (0.2), 1.4 (0.2), 1.2 (0.2) (iii) T: no association (iv) E2: no association | Age, gender, BMI, high fat diet |
| Lopez-Espinosa, 2016 | Cross-sectional | Girls enrolled in the C8 Health Project during 2005–2006 | 1123 | 6–9 | Median (IQR) PFHxS, 7.0 (3.8–13.8) PFNA, 1.7 (1.3–2.4) | Serum E2 and T, pmol/L | Percent change (95% CI) in the p75 vs. p25 of ln(PFAS) | No association | Age, time of sampling |
| Zhou, 2016 | Cross-sectional | Girls enrolled in the Genetics and Biomarkers study for Childhood Asthma from China during 2009–2010 | 123 | 13–15 | Median (IQR) PFBS, 0.5 (0.4–0.5) PFHxS, 1.2 (0.5–3.0) PFHxA, 0.2 (0.1–0.3) PFNA, 0.9 (0.6–1.1) PFDA, 1.0 (0.8–1.2) PFDoA, 3.1 (0.9–6.2) PFTeA, 4.5 (0.3–18.4) | Serum E2 and T, pmol/L |
 (95%CI) per 1 ng/mL increase in PFAS (95%CI) per 1 ng/mL increase in PFAS |
(i) ln(T): Only for PFDoA, (−) -0.012 (−0.023–−0.001) (ii) ln(E2): No association | Age, BMI, ETS exposure, parental education, regular exercise, and month of survey |
| McCoy, 2017 | Cross-sectional | Women undergoing IVF in the US during 2013–2014 | 36 | Mean 34 | Plasma, ng/g Mean 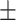 SD PFHxS, 2.2 SD PFHxS, 2.2 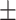 0.4 PFNA, 0.8 0.4 PFNA, 0.8 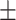 0.1 PFDA, 0.4 0.1 PFDA, 0.4 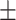 0.05 PFUnDA, 0.3 0.05 PFUnDA, 0.3 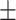 0.03 0.03 |
Plasma E2, pg/mL | Correlation coefficient (P value) | No association | NA |
| Heffernan, 2018 | Case–control | Women with PCOS and age- and BMI-matched controls recruited from the UK in 2015 | 59 | 20–45 | GM (range) PFHxS, 1.0 (0.2–10.2) PFNA, 0.6 (0.2–1.8) | Serum T, and SHBG, ln(pmol/L); FAI; Serum A4 and E2, pmol/L Luteal phase |
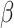 (SE) per ln-unit increase in PFAS stratified by cases and controls (SE) per ln-unit increase in PFAS stratified by cases and controls |
Among PCOS cases, only for A4 and PFNA: (+) 1.71 (0.65) Among controls, only for ln(T), PFHxS: (+) 0.50 (0.17) PFNA: (+) 0.46 (0.21) | Serum albumin |
| Zhang, 2018 | Case–control | Women with overt POI and 120 healthy controls from China recruited during 2013–2016 | 240 | 20–40 | Median (IQR) in controls PFHxS, 0.3 (0.2–0.4) | Serum FSH, LH, PRL, T, and E2, ln(ng/mL) Early follicular phase |
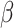 (95%CI) per ln-unit increase in PFAS (95%CI) per ln-unit increase in PFAS |
Among POI cases, (i) ln(FSH) (+) 0.16 (0.04–0.28) (ii) ln(LH): no association (iii) ln(E2) (−) -0.19 (−0.37–−0.02) (iv) ln(PRL): no association (v) ln(T): no association Among controls, no association | Age, BMI, education, income, sleep quality, parity |
| Other chronic conditions | |||||||||
| Wang, 2019a | Case–control | Infertile women diagnosed with PCOS and healthy controls from China in 2014 | 367 | 20–40 | Median (IQR) PFBS, 0.11 (0.1–0.12) PFHxS, 0.24 (0.17–0.3) PFHpA, 0.08 (0.05–0.1) PFNA, 0.5 (0.3–0.9) PFDA, 0.5 (0.3–0.8) PFUnDA, 0.4 (0.3–0.6) PFDoA, 0.24 (0.2–0.27) | PCOS | OR (95% CI) in the highest tertile vs. the lowest (the reference) | PFBS: no association PFHxS: no association PFHpA: no association PFNA: no association PFDA: no association PFUnDA: no association PFDoA: (+) 3.0 (1.2–7.7) | Age, BMI, household income, education, employment, age at menarche, menstrual volume |
Abbreviations: AMH, anti-Müllerian hormone; A4, androstenedione; BMI, body mass index; DHEA, dehydroepiandrosterone; EtFOSAA, 2-(N-ethylperlfuorooctane sulfonamide) acetic acid; E2, estradiol; FAI, free androgen index, was calculated as 100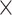 total T/SHBG; FSH, follicle-stimulating hormone; GM, geometric mean; IQR, interquartile range; MeFOSAA, 2-(N-Methylperfluorooctane sulfonamido) acetic acid; NHANES, National Health And Nutrition Examination Survey; NM, not measured; OR, odds ratio; P, progesterone; PCOS, polycystic ovarian syndrome; PFBS, perfluorobutane sulfonic acid; PFDA, perfluorodecanoic acid; PFDoA, perfluorododecanoic acid; PFHpA, perfluoroheptanoic acid; PFHxS, perfluorohexane sulfonic acid; PFNA, perfluorononanoic acid; PFOSA, perfluorooctane sulfonamide; PFUnDA, perfluoroundecanoic acid; POI, premature ovarian insufficiency; PRL, prolactin; p60 and 90, 60th and 90th percentiles; SHBG, sex hormone-binding globulin; T, testosterone; 95% CI, 95% confidence interval.
total T/SHBG; FSH, follicle-stimulating hormone; GM, geometric mean; IQR, interquartile range; MeFOSAA, 2-(N-Methylperfluorooctane sulfonamido) acetic acid; NHANES, National Health And Nutrition Examination Survey; NM, not measured; OR, odds ratio; P, progesterone; PCOS, polycystic ovarian syndrome; PFBS, perfluorobutane sulfonic acid; PFDA, perfluorodecanoic acid; PFDoA, perfluorododecanoic acid; PFHpA, perfluoroheptanoic acid; PFHxS, perfluorohexane sulfonic acid; PFNA, perfluorononanoic acid; PFOSA, perfluorooctane sulfonamide; PFUnDA, perfluoroundecanoic acid; POI, premature ovarian insufficiency; PRL, prolactin; p60 and 90, 60th and 90th percentiles; SHBG, sex hormone-binding globulin; T, testosterone; 95% CI, 95% confidence interval.
Sex hormones
Exposure to PFAS has been shown to disrupt ovarian steroidogenesis and steroidogenic-controlled processes. Although the literature on other PFAS homologues is scant, epidemiologic evidence suggests that exposure to PFOS is associated with steroidogenic defects. Specifically in the C8 Health Project, PFOS exposure had a significant and negative relationship with serum E2 levels among women aged 42–65 years without a history of hormone contraceptive use (Knox et al., 2011). The parent Energy Balance and Breast Cancer Aspects (EBBA-I) study sampled serum from healthy, naturally cycling women aged 25–35 years and found that, among nulliparous women but not parous women, PFOS exposure was negatively associated with serum E2 and progesterone (P) levels (Barrett et al., 2015). Similarly, Zhang et al. suggested that PFOS exposure may lead to decreased serum E2 and prolactin (PRL) levels and increased FSH levels among premature ovarian insufficiency (POI) patients (Zhang et al., 2018). McCoy et al. also found a negative correlation between PFOS concentrations and E2 levels among women undergoing in vitro fertilization (McCoy et al., 2017). Moreover, Heffernan et al. observed a significant and negative association between PFOS exposure and free androgen index (FAI) among healthy women without PCOS (Heffernan et al., 2018).
In contrast, no associations with hormone levels have been reported for PFOA exposure (Knox et al., 2011; Barrett et al., 2015; McCoy et al., 2017; Heffernan et al., 2018; Zhang et al., 2018). PFOS and PFOA were also not related to serum T levels among women 12–80 years of age from the NHANES 2011–2012 (Lewis et al., 2015). In utero exposure to PFOA and PFOS had no impact on serum levels of total T, sex hormone-binding globulin (SHBG), FAI, DHEA, FSH, LH, E2 or anti-Müllerian hormone (AMH) in female adult offspring (Kristensen et al., 2013).
Other PFAS homologues may also have the potential to disturb homeostasis of the endocrine system, although the evidence remains inconclusive. Heffernan et al. found a positive association between PFNA exposure and A4 in both PCOS cases and controls, and a positive association between PFHxS exposure and total T in healthy women (Heffernan et al., 2018). Zhang et al. indicated that PFHxS exposure may increase FSH levels and decrease E2 levels in POI patients (Zhang et al., 2018). No significant associations were observed in cross-sectional studies conducted among naturally cycling women in the EBBA-I study (Barrett et al., 2015), general women in NHANES (Lewis et al., 2015) or women receiving in vitro fertilisation (IVF) (McCoy et al., 2017).
Compared to adults, adolescents may be more susceptible to PFAS toxicity. Serum concentrations of PFOA, PFUnDA and PFOS were inversely associated with serum levels of SHBG, FSH and T, respectively, in adolescents aged 12–17 years but not in young adults (Tsai et al., 2015). Similarly, girls aged 6–9 years who enrolled in the C8 Health Project also had lower serum T levels with higher exposure to PFOS (Lopez-Espinosa et al., 2016). Although no association was observed for PFOA and PFOS exposures with E2 or T in Chinese adolescent girls, serum T levels decreased by 1.2% (95% CI: −2.2%, −0.1%) with an 1-ng/mL increase in serum PFDoA concentrations (Zhou et al., 2016).
Onset age of menarche
Delayed menarche is a common condition defined as the absence of physical signs of puberty by an age 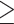 2–2.5 standard deviations above the population mean age of menarche (typically 13 years in girls) (Palmert and Dunkel, 2012). Emerging evidence suggests that later menarche may be linked to negative physiological outcomes and cardiovascular disease in adulthood (Zhu and Chan, 2017). Previous studies examining the associations between exposure to PFAS and timing of menarche have yielded inconsistent results with some of the studies finding no association (Christensen et al., 2011; Lopez-Espinosa et al., 2011; Kristensen et al., 2013). The latter study, a cross-sectional study of 2931 girls 8–18 years of age from the C8 Health Project reported that PFOA and PFOS serum concentrations were associated with later age at menarche, specifically 130 and 138 days of delay when comparing the highest quartile of concentrations versus the lowest quartile, respectively (Lopez-Espinosa et al., 2011).
2–2.5 standard deviations above the population mean age of menarche (typically 13 years in girls) (Palmert and Dunkel, 2012). Emerging evidence suggests that later menarche may be linked to negative physiological outcomes and cardiovascular disease in adulthood (Zhu and Chan, 2017). Previous studies examining the associations between exposure to PFAS and timing of menarche have yielded inconsistent results with some of the studies finding no association (Christensen et al., 2011; Lopez-Espinosa et al., 2011; Kristensen et al., 2013). The latter study, a cross-sectional study of 2931 girls 8–18 years of age from the C8 Health Project reported that PFOA and PFOS serum concentrations were associated with later age at menarche, specifically 130 and 138 days of delay when comparing the highest quartile of concentrations versus the lowest quartile, respectively (Lopez-Espinosa et al., 2011).
In addition, concern exists regarding in utero exposure to PFAS due to high vulnerability in this early-life stage. A Danish birth cohort established in 1988–1989 followed up 267 female offspring when they were ~20 years of age in 2008–2009. The study found that women with in utero exposure to higher concentrations of PFOA reached menarche 5.3 (95% CI: 1.3, 9.3) months later compared with the reference group of lower PFOA concentrations, while no associations were observed for PFOS (Kristensen et al., 2013). In contrast, a study of 218 girls reporting early menarche (before age 11.5 years) and 230 controls (at or after age 11.5 years) born between 1991 and 1992 in the UK showed no association of earlier age at menarche with exposure to PFOSA, EtFOSAA, MeFOSAA, PFOS, PFHxS, PFOA or PFNA (Christensen et al., 2011).
Menstrual cycle characteristics
Disturbances of menstrual cycle manifest in a wide range of presentations. The key characteristics include menstrual cycle regularity, cycle length and the amount of flow, but each of these may exhibit considerable variability. Epidemiologic data on the possible effects of PFAS on menstrual cycle regularity originate primarily from cross-sectional studies (Lyngsø et al., 2014; Zhou et al., 2017). Lyngsø et al. reported a statistically significant association between PFOA exposure and longer cycles (cycle length  32 days) with an odds ratio (OR) of 1.8 (95% CI: 1.0–3.3) when comparing the highest tertile of exposure with the lowest, in 1623 fertile women enrolled in the Inuit-endocrine (INUENDO) cohort from three countries (Greenland, Poland and Ukraine), whereas no significant results were detected for PFOS (Lyngsø et al., 2014). Moreover, a cross-sectional analysis of 950 Chinese women revealed that increased exposures to PFOA, PFOS, PFNA and PFHxS were associated with higher odds of irregular and longer menstrual cycle but lower odds of menorrhagia (Zhou et al., 2017). Interestingly, women with higher concentrations of PFOA, PFNA and PFHxS were more likely to experience hypomenorrhea (Zhou et al., 2017).
32 days) with an odds ratio (OR) of 1.8 (95% CI: 1.0–3.3) when comparing the highest tertile of exposure with the lowest, in 1623 fertile women enrolled in the Inuit-endocrine (INUENDO) cohort from three countries (Greenland, Poland and Ukraine), whereas no significant results were detected for PFOS (Lyngsø et al., 2014). Moreover, a cross-sectional analysis of 950 Chinese women revealed that increased exposures to PFOA, PFOS, PFNA and PFHxS were associated with higher odds of irregular and longer menstrual cycle but lower odds of menorrhagia (Zhou et al., 2017). Interestingly, women with higher concentrations of PFOA, PFNA and PFHxS were more likely to experience hypomenorrhea (Zhou et al., 2017).
The relationship of PFOA and PFOS with menstrual irregularity was detected in a subset of 1240 pregnant women randomly selected from the Danish National Birth Cohort (DNBC); women had higher exposure to PFOA and PFOS tended to report having irregular periods (Fei et al., 2009). Lum et al. used data from 501 couples from Michigan and Texas, who upon their discontinuing contraception for purposes of becoming pregnant enrolled in a prospective cohort, the Longitudinal Investigation of Fertility and the Environment (LIFE) Study (Lum et al., 2017). Menstrual cycles were 3% longer among women in the second versus the lowest tertile of PFDA serum concentrations, but 2% shorter for women in the highest versus the lowest tertile of PFOA concentrations, while no associations were observed with PFOS (Lum et al., 2017). When examining the effects of prenatal exposure, a recent prospective study found no associations between maternal exposure to PFOA and PFOS and menstrual cycle length or number of ovarian follicles in their offspring (Kristensen et al., 2013).
Ovarian aging
POI represents a gynaecological disorder characterised by the absence of normal ovarian function due to depletion of the follicle pool before age 40 years with the presence of oligo/amenorrhea for at least 4 months in combination with elevated FSH levels. It should be noted that POI is the transitional stage from normal ovarian function to complete loss of ovarian function. A case–control study of 240 Chinese women found that high exposures to PFOA, PFOS and PFHxS were associated with increased risks of POI; however, no associations were observed for PFNA, PFDA, PFUnDA, PFDoA, PFHpA and PFBS (Zhang et al., 2018).
Beyond the problem of infertility in POI patients, diminished ovarian reserve and extended steroid hormone deficiency during ovarian aging have far-reaching health implications. Earlier age at natural menopause has been associated with an increased risk of overall mortality (Jacobsen et al., 2003; Mondul et al., 2005; Ossewaarde et al., 2005), cardiovascular disease (Hu et al., 1999; Atsma et al., 2006) and cardiovascular death (van der Schouw et al., 1996; de Kleijn et al., 2002; Mondul et al., 2005), low bone mineral density (Parazzini et al., 1996) and osteoporosis (Kritz-Silverstein and Barrett-Connor, 1993) and other chronic conditions (Shuster et al., 2010). Quality of life may be significantly decreased while risks of sexual dysfunction and neurological disease may be increased later in life (McEwen and Alves, 1999; Van Der Stege et al., 2008; Rocca et al., 2009).
A study of the National Health and Nutrition Examination Survey (NHANES) participants found that higher PFAS concentrations were associated with earlier menopause: the hazard ratio (HR) of natural menopause was 1.42 (95% CI: 1.08, 1.87) comparing PFHxS serum concentrations in tertile 2 versus tertile 1, and 1.70 (95% CI: 1.36, 2.12) in tertile 3 versus tertile 1; positive dose–response relationships were also detected for PFOA, PFOS, PFNA and PFHxS with hysterectomy (Taylor et al., 2014). Additionally, a cross-sectional study of the C8 Health Project participants found that the odds of having already experienced natural menopause increased with increasing exposure quintiles of PFOA and PFOS, particularly in women aged 42–65 years (Knox et al., 2011).
These epidemiologic studies of PFAS and age at menopause were cross-sectional analyses in which the outcome was ascertained through an interview at the same time as a blood sample was collected to determine serum PFAS concentrations. It raises the question of reverse causation, in that measured PFAS concentrations increased with years since menopause, possibly due to the cessation of PFAS excretion via menstruation (Taylor et al., 2014). Using a retrospective cohort of women recruited during 2005–2006, Dhingra et al. found no significant association between PFOA exposure (using either estimated year-specific serum concentrations during 1951 and 2011, or measured serum concentrations) and natural menopause incidence (Dhingra et al., 2016).
Other conditions
PCOS is a common endocrine disorder among women of reproductive age, leading to several health complications including menstrual dysfunction, infertility, hirsutism, acne, obesity, metabolic syndrome and an increased risk of Type 2 diabetes and cardiovascular disease (Norman et al., 2007). A study of 180 infertile PCOS cases and 180 healthy controls showed a significant and positive dose–response relationship between PFDoA serum concentrations and risks of PCOS-related infertility; however, no significant associations were observed for PFBS, PFHpA, PFHxS, PFOA, PFOS, PFNA, PFDA or PFUnDA (Wang et al., 2019a). In regard to cancer, only a few studies have evaluated associations between PFAS exposure and increased risks of ovarian cancer (Barry et al., 2013; Vieira et al., 2013). Neither of these studies observed a significant association but the number of cases in each study was small. Thus, the evidence is insufficient to assess risk of ovarian carcinogenicity.
Discussion
Summary of findings
Findings from in vitro and in vivo studies suggest that PFAS exposure can target the ovary to adversely affect its two essential functional processes, i.e. folliculogenesis and steroidogenesis. PFAS exposure may alter follicle and oocyte development and diminish ovarian reserve (Bellingham et al., 2009; Feng et al., 2015, 2017; Domínguez et al., 2016; Chen et al., 2017; Du et al., 2019; Hallberg et al., 2019; López-Arellano et al., 2019). Potential mechanisms include PPAR activation, disruption of gap junction intercellular communication, oxidative stress and thyroid hormone disruption. Limited experimental evidence published to date also suggests PFAS can be a disruptor of ovarian steroidogenesis with independent actions on both theca and granulosa cells (Chaparro-Ortega et al., 2018; Shi et al., 2009; Wang et al., 2018a). In addition to PPAR signalling pathways, endocrine disruption may also be facilitated by acting directly on gene coding for enzymes responsible for cholesterol transport and ovarian steroidogenesis, and a loss of kisspeptin signalling in the hypothalamus that can impact ovarian function.
In general, experimental studies were limited by the use of doses that exceed the range of estimated human exposure. For example, it is calculated that North American and European consumers had a daily uptake dose of PFOA in the range of 3–220 ng/kg bw and PFOS of 1 to 130 ng/kg bw (Trudel et al., 2008). The lowest concentrations of PFOS and PFOA used in studies of animal models investigating follicle development was 0.1 mg/kg bw/day (=105 ng/kg bw/day) (Feng et al., 2015; Chen et al., 2017).
In epidemiologic studies, the associations between PFAS exposure and ovarian function across different populations and difference ranges of exposure levels were inconsistent. Despite that, most epidemiologic studies have found that exposure to PFOA, PFOS or other PFAS homologues is associated with later menarche (Lopez-Espinosa et al., 2011; Kristensen et al., 2013), irregular and longer menstrual cycle (Fei et al., 2009; Lyngsø et al., 2014; Chen et al., 2017; Lum et al., 2017; Zhou et al., 2017), increased risks of POI (Zhang et al., 2018) and earlier onset of menopause (Knox et al., 2011; Taylor et al., 2014).
Methodologic problems, however, limit the causal interpretation of these findings. The observed associations between PFAS exposure and delayed menarche could be explained by reverse causation rather than a toxic effect of these substances, in that the physiological changes during reproductive growth and maturation in girls may have a considerable influence on serum PFAS concentrations (Wu et al., 2015). It is also possible that the observed associations of PFAS and early onset of menopause in cross-sectional studies might be due to reverse causation related to the presence or volume of menstrual bleeding. Furthermore, information on the timing of menarche, menstrual cycle length and age at menopause were based on self-reports, and recalled data may have been imprecise particularly for users of hormonal contraceptives (Must et al., 2002; Small et al., 2007). Relationships between PFAS exposure and menstrual cycle length among contraceptive users may have been blurred by actions of exogenous hormones (Lum et al., 2017).
Evidence from epidemiologic studies also suggests associations of PFAS exposure with lower E2 levels and higher FSH levels in female adults (Knox et al., 2011; Barrett et al., 2015; McCoy et al., 2017; Heffernan et al., 2018; Zhang et al., 2018). This is consistent with the role of PFAS in accelerating ovarian aging. Compared to adults, girls may be more vulnerable because exposures to PFAS may lead to decreased serum levels of SHBG, FSH and total T (Tsai et al., 2015; Lopez-Espinosa et al., 2016; Zhou et al., 2016). However, the associations have not been confirmed by longitudinal cohort studies. Results from cross-sectional studies are also probably subject to reverse causation because sex steroid hormones could affect rates of renal clearance (Kudo et al., 2002). Higher ovarian hormone levels also tend to have a more proliferative endometrial lining (Clancy, 2009) and, by extension, heavier menstrual bleeding, which could contribute to greater clearance of PFAS in menstrual blood. Therefore, we cannot rule out the possibility that fluctuations in hormone levels might impact PFAS serum concentrations in women. Given the inconsistency in previous findings and lack of longitudinal evidence, no causal inferences can be drawn at this time based on this body of literature.
Future directions
An important conclusion we derived from this review is the limited quality of the evidence base for PFAS effects on ovarian function. This is of significant concern for public health because PFAS exposure is ubiquitous, and PFAS exert biological effects at low doses, causing disruption of ovarian function. PFAS exposure can have lasting effects on reproductive and non-reproductive health, including disruption of fertility, reproductive lifespan and regulation of skeletal, cardiovascular and brain functions. Several research questions and improvements for future studies are proposed.
Expansion of the dose ranges
Because most laboratory studies have utilised doses that exceed the range of estimated human exposure, future research should expand the dose ranges to span known human exposure levels. Similarly, uncertainties in the epidemiologic evidence could be reduced by carefully planned, enriched sampling of a wide range of PFAS exposures including highly exposed communities from locally contaminated areas. For example, the C8 Health Project with a large cohort of Mid-Ohio Valley residents and workers exposed to PFOA from a chemical plant provided extensive and high-quality research findings on PFOA and to a lesser extent, PFOS. There is scant information on the health effects of occupational or high exposure to other PFAS such as PFHxS and PFNA.
Need for prospective cohort designs
There are very few longitudinal studies to support causal relationships between PFAS exposure and ovarian function. Prospective studies are required to fully characterise the impact of PFAS exposure on ovarian function and to minimise potential reverse causation. Such studies should include long-term follow-up of participants, repeated measurements and prospective assessment of ovarian function.
Measurement of emerging PFAS homologues
The phase out of PFOA and PFOS has led to an increasing usage of alternative compounds (Ateia et al., 2019). For example, GenX chemicals are used to make high-performance fluoropolymers and non-stick coatings without the use of PFOA, and PFBS is a replacement chemical for PFOS (USEPA, 2018). However, there is inadequate evidence of general toxicity as well as ovarian toxicity of such alternative compounds. Future studies should fill these gaps with regard to sources and pathway of exposure and toxic effects of emerging PFAS on reproductive outcomes and their related chronic conditions.
Analysis of EDC mixtures
Previous studies have linked phthalates, PCBs, polybrominated diphenyl esters (PBDEs), and other EDCs to impaired ovarian function (Grindler et al., 2015; Craig and Ziv-Gal, 2018; Harley et al., 2019). It is increasingly recognised that environmental endocrine disruption is most often not due to the effect of a single compound, but rather due to co-exposure to mixtures of chemicals at low concentrations (Alyea and Watson, 2009; Braun et al., 2016; Wang et al., 2018b; 2019b). Thus, future research should examine the effects of exposure to a mixture of persistent and non-persistent EDCs on ovarian health.
Conclusion
The possibility of an association between PFAS exposure and abnormal ovarian function has important implications for research and public health. The ovary is a primary regulator of reproductive and endocrine function as well as general health in the female. Because millions of people worldwide are exposed to PFAS-contaminated drinking water, the public health consequences of a causal relationship could be serious. Methodological problems limit the causal interpretation of associations between PFAS exposure and menstrual disorders in epidemiological studies. Overall, there is insufficient evidence to determine a causal relationship between PFAS exposure and ovarian function. Experimental studies with doses relevant to human exposure and epidemiologic research with prospective study designs should be future research priorities.
Authors’ roles
N.D. and S.K.P.: contributions to conception, study design, literature analysis, manuscript drafting and revising; S.D.H. and J.F.R..: contributions to critical reading and editing; R.L.-C.: contributions to literature analysis, critical reading and editing.
Funding
National Institute of Environmental Health Sciences (NIEHS); National Institutes of Health (NIH, grants R01 ES026578, R01 ES026964 and P30 ES017885); National Institute for Occupational Safety and Health (NIOSH), Centers for Disease Control and Prevention (CDC, with grant T42 OH008455). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIEHS, NIH, CDC or NIOSH.
Conflict of interest
All authors confirm that they have no conflict of interest.
References
- Agency for Toxic Substances and Disease Registry (ATSDR). Toxicological profile for perfluoroalkyls. 2018. https://www.atsdr.cdc.gov/sites/peer_review/tox_profile_perfluoroalkyls.html [PubMed] [Google Scholar]
- Alyea RA, Watson CS. Differential regulation of dopamine transporter function and location by low concentrations of environmental estrogens and 17β-estradiol. Environ Health Perspect 2009;117:778–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson RH, Long GC, Porter RC, Anderson JK. Occurrence of select perfluoroalkyl substances at U.S. Air Force aqueous film-forming foam release sites other than fire-training areas: field-validation of critical fate and transport properties. Chemosphere 2016;150:678–685. [DOI] [PubMed] [Google Scholar]
- Ateia M, Maroli A, Tharayil N, Karanfil T. The overlooked short- and ultrashort-chain poly- and perfluorinated substances: a review. Chemosphere 2019;220:866–882. [DOI] [PubMed] [Google Scholar]
- Atsma F, Bartelink M-LEL, Grobbee DE, van der Schouw YT. Postmenopausal status and early menopause as independent risk factors for cardiovascular disease: a meta-analysis. Menopause 2006;13:265–279. [DOI] [PubMed] [Google Scholar]
- Banks RE, Tatlow JC. Organofluorine chemistry: nomenclature and historical landmarks In: Organofluorine Chemistry. Boston: Springer US, 1994,1–24 [Google Scholar]
- Barber JL, Berger U, Chaemfa C, Huber S, Jahnke A, Temme C, Jones KC. Analysis of per- and polyfluorinated alkyl substances in air samples from Northwest Europe. J Environ Monit 2007;9:530–541. [DOI] [PubMed] [Google Scholar]
- Barrett ES, Chen C, Thurston SW, Haug LS, Sabaredzovic A, Fjeldheim FN, Frydenberg H, Lipson SF, Ellison PT, Thune I. Perfluoroalkyl substances and ovarian hormone concentrations in naturally cycling women. Fertil Steril 2015;103:1261–1270.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry V, Winquist A, Steenland K. Perfluorooctanoic acid (PFOA) exposures and incident cancers among adults living near a chemical plant. Environ Health Perspect 2013;121:1313–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartell SM, Calafat AM, Lyu C, Kato K, Ryan PB, Steenland K. Rate of decline in serum PFOA concentrations after granular activated carbon filtration at two public water systems in Ohio and West Virginia. Environ Health Perspect 2010;118:222–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begley TH, White K, Honigfort P, Twaroski ML, Neches R, Walker RA. Perfluorochemicals: potential sources of and migration from food packaging. Food Addit Contam 2005;22:1023–1031. [DOI] [PubMed] [Google Scholar]
- Behr A-C, Lichtenstein D, Braeuning A, Lampen A, Buhrke T. Perfluoroalkylated substances (PFAS) affect neither estrogen and androgen receptor activity nor steroidogenesis in human cells in vitro. Toxicol Lett 2018;291:51–60. [DOI] [PubMed] [Google Scholar]
- Bellingham M, Fowler PA, Amezaga MR, Rhind SM, Cotinot C, Mandon-Pepin B, Sharpe RM, Evans NP. Exposure to a complex cocktail of environmental endocrine-disrupting compounds disturbs the kisspeptin/GPR54 system in ovine hypothalamus and pituitary gland. Environ Health Perspect 2009;117:1556–1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biegel LB, Liu RCM, Hurtt ME, Cook JC. Effects of ammonium perfluorooctanoate on Leydig-cell function: in vitro, in vivo, and ex vivo studies. Toxicol Appl Pharmacol 1995;134:18–25. [DOI] [PubMed] [Google Scholar]
- Bjermo H, Darnerud PO, Pearson M, Barbieri HE, Lindroos AK, Nälsén C, Lindh CH, Jönsson BAG, Glynn A. Serum concentrations of perfluorinated alkyl acids and their associations with diet and personal characteristics among Swedish adults. Mol Nutr Food Res 2013;57:2206–2215. [DOI] [PubMed] [Google Scholar]
- Bjork JA, Wallace KB. Structure-activity relationships and human relevance for perfluoroalkyl acid–induced transcriptional activation of peroxisome proliferation in liver cell cultures. Toxicol Sci 2009;111:89–99. [DOI] [PubMed] [Google Scholar]
- Boone JS, Vigo C, Boone T, Byrne C, Ferrario J, Benson R, Donohue J, Simmons JE, Kolpin DW, Furlong ET et al. Per- and polyfluoroalkyl substances in source and treated drinking waters of the United States. Sci Total Environ 2019;653:359–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boronow KE, Brody JG, Schaider LA, Peaslee GF, Havas L, Cohn BA. Serum concentrations of PFASs and exposure-related behaviors in African American and non-Hispanic white women. J Expo Sci Environ Epidemiol 2019;29:206–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley EL, Read WA, Castle L. Investigation into the migration potential of coating materials from cookware products. Food Addit Contam 2007;24:326–335. [DOI] [PubMed] [Google Scholar]
- Braun JM, Gennings C, Hauser R, Webster TF. What can epidemiological studies tell us about the impact of chemical mixtures on human health? Environ Health Perspect 2016;124:A6–A9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brede E, Wilhelm M, Göen T, Müller J, Rauchfuss K, Kraft M, Hölzer J. Two-year follow-up biomonitoring pilot study of residents’ and controls’ PFC plasma levels after PFOA reduction in public water system in Arnsberg, Germany. Int J Hyg Environ Health 2010;213:217–223. [DOI] [PubMed] [Google Scholar]
- Buck RC, Franklin J, Berger U, Conder JM, Cousins IT, de Voogt P, Jensen AA, Kannan K, Mabury SA, Leeuwen SPJ. Perfluoroalkyl and polyfluoroalkyl substances in the environment: terminology, classification, and origins. Integr Environ Assess Manag 2011;7:513–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger HG, Hale GE, Robertson DM, Dennerstein L. A review of hormonal changes during the menopausal transition: focus on findings from the Melbourne Women’s Midlife Health Project. Hum Reprod Update 2007;13:559–565. [DOI] [PubMed] [Google Scholar]
- Butenhoff JL, Olsen GW, Pfahles-Hutchens A. The applicability of biomonitoring data for perfluorooctanesulfonate to the environmental public health continuum. Environ Health Perspect 2006;114:1776–1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calafat AM, Wong LY, Kuklenyik Z, Reidy JA, Needham LL. Polyfluoroalkyl chemicals in the U.S. population: data from the national health and nutrition examination survey (NHANES) 2003-2004 and comparisons with NHANES 1999-2000. Environ Health Perspect 2007;115:1596–1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caserta D, Mantovani A, Marci R, Fazi A, Ciardo F, La Rocca C, Maranghi F, Moscarini M. Environment and women’s reproductive health. Hum Reprod Update 2011;17:418–433. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control. National Report on Human Exposure to Environmental Chemicals, 2019. https://www.cdc.gov/exposurereport/index.html [Google Scholar]
- Centers for Disease Control and Prevention. Fourth Report on Human Exposure to Environmental Chemicals. Atlanta: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, 2009. https://www.cdc.gov/exposurereport/ [Google Scholar]
- Chang S-C, Thibodeaux JR, Eastvold ML, Ehresman DJ, Bjork JA, Froehlich JW, Lau C, Singh RJ, Wallace KB, Butenhoff JL. Thyroid hormone status and pituitary function in adult rats given oral doses of perfluorooctanesulfonate (PFOS). Toxicology 2008;243:330–339. [DOI] [PubMed] [Google Scholar]
- Chaparro-Ortega A, Betancourt M, Rosas P, Vázquez-Cuevas FG, Chavira R, Bonilla E, Casas E, Ducolomb Y. Endocrine disruptor effect of perfluorooctane sulfonic acid (PFOS) and perfluorooctanoic acid (PFOA) on porcine ovarian cell steroidogenesis. Toxicol Vitr 2018;46:86–93. [DOI] [PubMed] [Google Scholar]
- Chen W-L, Bai F-Y, Chang Y-C, Chen P-C, Chen C-Y. Concentrations of perfluoroalkyl substances in foods and the dietary exposure among Taiwan general population and pregnant women. J Food Drug Anal 2018;26:994–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Zhou L, Xu J, Zhang L, Li M, Xie X, Xie Y, Luo D, Zhang D, Yu X et al. Maternal exposure to perfluorooctanoic acid inhibits luteal function via oxidative stress and apoptosis in pregnant mice. Reprod Toxicol 2017;69:159–166. [DOI] [PubMed] [Google Scholar]
- Christensen KY, Maisonet M, Rubin C, Holmes A, Calafat AM, Kato K, Flanders WD, Heron J, McGeehin MA, Marcus M. Exposure to polyfluoroalkyl chemicals during pregnancy is not associated with offspring age at menarche in a contemporary British cohort. Environ Int 2011;37:129–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clancy KBH. Reproductive ecology and the endometrium: physiology, variation, and new directions. Am J Phys Anthropol 2009;140:137–154. [DOI] [PubMed] [Google Scholar]
- Clark KL, Ganesan S, Keating AF. Impact of toxicant exposures on ovarian gap junctions. Reprod Toxicol 2018;81:140–146. [DOI] [PubMed] [Google Scholar]
- Craig ZR, Ziv-Gal A. Pretty good or pretty bad? The ovary and chemicals in personal care products. Toxicol Sci 2018;162:349–360. [DOI] [PubMed] [Google Scholar]
- Dallaire R, Dewailly E, Pereg D, Dery S, Ayotte P. Thyroid function and plasma concentrations of polyhalogenated compounds in Inuit adults. Environ Health Perspect 2009;117:1380–1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danish EPA. Risk assessment of fluorinated substances in cosmetic products. 2018. https://mst.dk/service/publikationer/publikationsarkiv/2018/nov/risk-assessment-of-fluorinated-substances-in-cosmetic-products/
- Dauça M, Wahli W, Foufelle F, Braissant O, Scotto C. Differential expression of peroxisome proliferator-activated receptors (PPARs): tissue distribution of PPAR-alpha, -beta, and -gamma in the adult rat. Endocrinology 2014;137:354–366. [DOI] [PubMed] [Google Scholar]
- Desvergne B, Wahli W. Peroxisome proliferator-activated receptors: nuclear control of metabolism. Endocr Rev 1999;20:649–688. [DOI] [PubMed] [Google Scholar]
- Dhingra R, Darrow LA, Klein M, Winquist A, Steenland K. Perfluorooctanoic acid exposure and natural menopause: a longitudinal study in a community cohort. Environ Res 2016;146:323–330. [DOI] [PubMed] [Google Scholar]
- Dhingra R, Winquist A, Darrow LA, Klein M, Steenland K. A study of reverse causation: examining the associations of perfluorooctanoic acid serum levels with two outcomes. Environ Health Perspect 2017;125:416–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamanti-Kandarakis E, Bourguignon J-P, Giudice LC, Hauser R, Prins GS, Soto AM, Zoeller RT, Gore AC. Endocrine-disrupting chemicals: An Endocrine Society scientific statement. Endocr Rev 2009;30:293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijkstra G, de Rooij DG, de Jong FH, van den Hurk R. Effect of hypothyroidism on ovarian follicular development, granulosa cell proliferation and peripheral hormone levels in the prepubertal rat. Eur J Endocrinol 1996;134:649–654. [DOI] [PubMed] [Google Scholar]
- Ding N, Harlow SD, Batterman S, Mukherjee B, Park SK. Longitudinal trends in perfluoroalkyl and polyfluoroalkyl substances among multiethnic midlife women from 1999 to 2011: the Study of Women’s Health Across the Nation. Environ Int 2020;135:105381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domingo JL, Jogsten IE, Eriksson U, Martorell I, Perelló G, Nadal M, Bavel van B. Human dietary exposure to perfluoroalkyl substances in Catalonia. Spain Temporal trend Food Chem 2012;135:1575–1582. [DOI] [PubMed] [Google Scholar]
- Domingo JL, Nadal M. Per- and polyfluoroalkyl substances (PFASs) in food and human dietary intake: a review of the recent scientific literature. J Agric Food Chem 2017;65:533–543. [DOI] [PubMed] [Google Scholar]
- Domingo JL, Nadal M. Human exposure to per- and polyfluoroalkyl substances (PFAS) through drinking water: a review of the recent scientific literature. Environ Res 2019;177:108648. [DOI] [PubMed] [Google Scholar]
- Domínguez A, Salazar Z, Arenas E, Betancourt M, Ducolomb Y, González-Márquez H, Casas E, Teteltitla M, Bonilla E. Effect of perfluorooctane sulfonate on viability, maturation and gap junctional intercellular communication of porcine oocytes in vitro. Toxicol Vitr 2016;35:93–99. [DOI] [PubMed] [Google Scholar]
- Du G, Hu J, Huang H, Qin Y, Han X, Wu D, Song L, Xia Y, Wang X. Perfluorooctane sulfonate (PFOS) affects hormone receptor activity, steroidogenesis, and expression of endocrine-related genes in vitro and in vivo. Environ Toxicol Chem 2013a;32:353–360. [DOI] [PubMed] [Google Scholar]
- Du G, Hu J, Huang Z, Yu M, Lu C, Wang X, Wu D. Neonatal and juvenile exposure to perfluorooctanoate (PFOA) and perfluorooctane sulfonate (PFOS): advance puberty onset and kisspeptin system disturbance in female rats. Ecotoxicol Environ Saf 2019;167:412–421. [DOI] [PubMed] [Google Scholar]
- Du G, Huang H, Hu J, Qin Y, Wu D, Song L, Xia Y, Wang X. Endocrine-related effects of perfluorooctanoic acid (PFOA) in zebrafish, H295R steroidogenesis and receptor reporter gene assays. Chemosphere 2013b;91:1099–1106. [DOI] [PubMed] [Google Scholar]
- Environmental Working Group. Report: up to 110 million Americans could have PFAS-contaminated drinking water. 2018.
- Eriksson U, Kärrman A, Rotander A, Mikkelsen B, Dam M. Perfluoroalkyl substances (PFASs) in food and water from Faroe Islands. Environ Sci Pollut Res 2013;20:7940–7948. [DOI] [PubMed] [Google Scholar]
- Eriksson U, Mueller JF, Toms L-ML, Hobson P, Kärrman A. Temporal trends of PFSAs, PFCAs and selected precursors in Australian serum from 2002 to 2013. Environ Pollut 2017;220:168–177. [DOI] [PubMed] [Google Scholar]
- Ericson I, Martí-Cid R, Nadal M, Van Bavel B, Lindström G, Domingo JL. Human Exposure to Perfluorinated Chemicals through the Diet: Intake of Perfluorinated Compounds in Foods from the Catalan (Spain) Market. J Agric Food Chem 2008;56:1787–94. [DOI] [PubMed] [Google Scholar]
- Falk EB, O’Donnell MB, Cascio CN, Tinney F, Kang Y, Lieberman MD, Taylor SE, An L, Resnicow K, Strecher VJ. Self-affirmation alters the brain’s response to health messages and subsequent behavior change. Proc Natl Acad Sci U S A 2015;112:1977–1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan W, Yanase T, Morinaga H, Mu Y-M, Nomura M, Okabe T, Goto K, Harada N, Nawata H. Activation of peroxisome proliferator-activated receptor-γ and retinoid X receptor inhibits aromatase transcription via nuclear factor-κB. Endocrinology 2005;146:85–92. [DOI] [PubMed] [Google Scholar]
- Fedail JS, Zheng K, Wei Q, Kong L, Shi F. Roles of thyroid hormones in follicular development in the ovary of neonatal and immature rats. Endocrine 2014;46:594–604. [DOI] [PubMed] [Google Scholar]
- Fei C, McLaughlin JK, Lipworth L, Olsen J. Maternal levels of perfluorinated chemicals and subfecundity. Hum Reprod 2009;24:1200–1205. [DOI] [PubMed] [Google Scholar]
- Felizeter S, McLachlan MS, De Voogt P. Root uptake and translocation of perfluorinated alkyl acids by three hydroponically grown crops. J Agric Food Chem 2014;62:3334–3342. [DOI] [PubMed] [Google Scholar]
- Feng X, Cao X, Zhao S, Wang X, Hua X, Chen L, Chen L. Exposure of pregnant mice to perfluorobutanesulfonate causes hypothyroxinemia and developmental abnormalities in female offspring. Toxicol Sci 2017;155:409–419. [DOI] [PubMed] [Google Scholar]
- Feng X, Wang X, Cao X, Xia Y, Zhou R, Chen L. Chronic exposure of female mice to an environmental level of perfluorooctane sulfonate suppresses estrogen synthesis through reduced histone H3K14 acetylation of the StAR promoter leading to deficits in follicular development and ovulation. Toxicol Sci 2015;148:368–379. [DOI] [PubMed] [Google Scholar]
- Fromme H, Schlummer M, Möller A, et al. Exposure of an adult population to perfluorinated substances using duplicate diet portions and biomonitoring data. Environ Sci Technol 2007;41:7928–33. [DOI] [PubMed] [Google Scholar]
- Fromme H, Dreyer A, Dietrich S, Fembacher L, Lahrz T, Völkel W. Neutral polyfluorinated compounds in indoor air in Germany – the LUPE 4 study. Chemosphere 2015;139:572–578. [DOI] [PubMed] [Google Scholar]
- Gaytán F, Gaytán M, Castellano JM, Romero M, Roa J, Aparicio B, Garrido N, Sánchez-Criado JE, Millar RP, Pellicer A et al. KiSS-1 in the mammalian ovary: distribution of kisspeptin in human and marmoset and alterations in KiSS-1 mRNA levels in a rat model of ovulatory dysfunction. Am J Physiol Metab 2009;296:E520–E531. [DOI] [PubMed] [Google Scholar]
- Gebbink WA, Glynn A, Darnerud PO, Berger U. Perfluoroalkyl acids and their precursors in Swedish food: the relative importance of direct and indirect dietary exposure. Environ Pollut 2015;198:108–115. [DOI] [PubMed] [Google Scholar]
- Glynn A, Berger U, Bignert A, Ullah S, Aune M, Lignell S, Darnerud PO. Perfluorinated alkyl acids in blood serum from primiparous women in Sweden: serial sampling during pregnancy and nursing, and temporal trends 1996–2010. Environ Sci Technol 2012;46:9071–9079. [DOI] [PubMed] [Google Scholar]
- Goosey E, Harrad S. Perfluoroalkyl substances in UK indoor and outdoor air: spatial and seasonal variation, and implications for human exposure. Environ Int 2012;45:86–90. [DOI] [PubMed] [Google Scholar]
- Gorrochategui E, Pérez-Albaladejo E, Casas J, Lacorte S, Porte C. Perfluorinated chemicals: differential toxicity, inhibition of aromatase activity and alteration of cellular lipids in human placental cells. Toxicol Appl Pharmacol 2014;277:124–130. [DOI] [PubMed] [Google Scholar]
- Grindler NM, Allsworth JE, Macones GA, Kannan K, Roehl KA, Cooper AR. Persistent organic pollutants and early menopause in U.S. women. Rosenfeld CS (ed) PLoS One 2015;10:e0116057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallberg I, Kjellgren J, Persson S, Örn S, Sjunnesson Y. Perfluorononanoic acid (PFNA) alters lipid accumulation in bovine blastocysts after oocyte exposure during in vitro maturation. Reprod Toxicol 2019;84:1–8. [DOI] [PubMed] [Google Scholar]
- Han X, Mingoia R, Snajdr S, Yang C, Nabb D. Uptake of perfluorooctanoate in freshly isolated hepatocytes from male and female rats. Toxicol Lett 2008;181:81–86. [DOI] [PubMed] [Google Scholar]
- Han X, Snow TA, Kemper RA, Jepson GW. Binding of perfluorooctanoic acid to rat and human plasma proteins. Chem Res Toxicol 2003;16:775–781. [DOI] [PubMed] [Google Scholar]
- Harada K, Inoue K, Morikawa A, Yoshinaga T, Saito N, Koizumi A. Renal clearance of perfluorooctane sulfonate and perfluorooctanoate in humans and their species-specific excretion. Environ Res 2005;99:253–261. [DOI] [PubMed] [Google Scholar]
- Harley KG, Berger KP, Kogut K, Parra K, Lustig RH, Greenspan LC, Calafat AM, Ye X, Eskenazi B. Association of phthalates, parabens and phenols found in personal care products with pubertal timing in girls and boys. Hum Reprod 2019;34:109–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haug LS, Huber S, Becher G, Thomsen C. Characterisation of human exposure pathways to perfluorinated compounds - comparing exposure estimates with biomarkers of exposure. Environ Int 2011a;37:687–693. [DOI] [PubMed] [Google Scholar]
- Haug LS, Huber S, Schlabach M, Becher G, Thomsen C. Investigation on per- and polyfluorinated compounds in paired samples of house dust and indoor air from Norwegian homes. Environ Sci Technol 2011b;45:7991–7998. [DOI] [PubMed] [Google Scholar]
- Haug LS, Salihovic S, Jogsten IE, Thomsen C, van Bavel B, Lindström G, Becher G. Levels in food and beverages and daily intake of perfluorinated compounds in Norway. Chemosphere 2010;80:1137–1143. [DOI] [PubMed] [Google Scholar]
- Heffernan AL, Cunningham TK, Drage DS, Aylward LL, Thompson K, Vijayasarathy S, Mueller JF, Atkin SL, Sathyapalan T. Perfluorinated alkyl acids in the serum and follicular fluid of UK women with and without polycystic ovarian syndrome undergoing fertility treatment and associations with hormonal and metabolic parameters. Int J Hyg Environ Health 2018;221:1068–1075. [DOI] [PubMed] [Google Scholar]
- Heo J-J, Lee J-W, Kim S-K, Oh J-E. Foodstuff analyses show that seafood and water are major perfluoroalkyl acids (PFAAs) sources to humans in Korea. J Hazard Mater 2014;279:402–409. [DOI] [PubMed] [Google Scholar]
- Herzke D, Huber S, Bervoets L, D’Hollander W, Hajslova J, Pulkrabova J, Brambilla G, De Filippis SP, Klenow S, Heinemeyer G et al. Perfluorinated alkylated substances in vegetables collected in four European countries; occurrence and human exposure estimations. Environ Sci Pollut Res 2013;20:7930–7939. [DOI] [PubMed] [Google Scholar]
- Hess KA, Chen L, Larsen WJ. The ovarian blood follicle barrier is both charge- and size-selective in mice. Biol Reprod 1998;58:705–711. [DOI] [PubMed] [Google Scholar]
- Hill PJ, Taylor M, Goswami P, Blackburn RS. Substitution of PFAS chemistry in outdoor apparel and the impact on repellency performance. Chemosphere 2017;181:500–507. [DOI] [PubMed] [Google Scholar]
- Hillier SG, Whitelaw PF, Smyth CD. Follicular oestrogen synthesis: the “two-cell, two-gonadotrophin” model revisited. Mol Cell Endocrinol 1994;100:51–54. [DOI] [PubMed] [Google Scholar]
- Hu FB, Grodstein F, Hennekens CH, Colditz GA, Johnson M, Manson JE, Rosner B, Stampfer MJ. Age at natural menopause and risk of cardiovascular disease. Arch Intern Med 1999;159:1061–1066. [DOI] [PubMed] [Google Scholar]
- Hu K-L, Zhao H, Chang H-M, Yu Y, Qiao J. Kisspeptin/kisspeptin receptor system in the ovary. Front Endocrinol (Lausanne) 2017;8:365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu XC, Andrews DQ, Lindstrom AB, Bruton TA, Schaider LA, Grandjean P, Lohmann R, Carignan CC, Blum A, Balan SA et al. Detection of poly- and perfluoroalkyl substances (PFASs) in U.S. drinking water linked to industrial sites, military fire training areas, and wastewater treatment plants. Environ Sci Technol Lett 2016;3:344–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Interstate Technology Regulatory Council. PFAS Fact Sheets. 2020. https://pfas-1.itrcweb.org/.
- Jacobsen BK, Heuch I, Kvåle G. Age at natural menopause and all-cause mortality: a 37-year follow-up of 19,731 Norwegian women. Am J Epidemiol 2003;157:923–929. [DOI] [PubMed] [Google Scholar]
- Jensen AA, Leffers H. Emerging endocrine disrupters: perfluoroalkylated substances. Int J Androl 2008;31:161–169. [DOI] [PubMed] [Google Scholar]
- Jian J-M, Guo Y, Zeng L, Liang-Ying L, Lu X, Wang F, Zeng EY. Global distribution of perfluorochemicals (PFCs) in potential human exposure source–a review. Environ Int 2017;108:51–62. [DOI] [PubMed] [Google Scholar]
- Jin YH, Liu W, Sato I, Nakayama SF, Sasaki K, Saito N, Tsuda S. PFOS and PFOA in environmental and tap water in China. Chemosphere 2009;77:605–611. [DOI] [PubMed] [Google Scholar]
- Jones PD, Hu W, De Coen W, Newsted JL, Giesy JP. Binding of perfluorinated fatty acids to serum proteins. Environ Toxicol Chem 2003;22:2639–2649. [DOI] [PubMed] [Google Scholar]
- Kantiani L, Llorca M, Sanchís J, Farré M, Barceló D. Emerging food contaminants: a review. Anal Bioanal Chem 2010;398:2413–2427. [DOI] [PubMed] [Google Scholar]
- Karásková P, Venier M, Melymuk L, Bečanová J, Vojta Š, Prokeš R, Diamond ML, Klánová J. Perfluorinated alkyl substances (PFASs) in household dust in Central Europe and North America. Environ Int 2016;94:315–324. [DOI] [PubMed] [Google Scholar]
- Kissa E. Fluorinated Surfactants and Repellents. Marcel Dekker, 2011 [Google Scholar]
- Kjeldsen LS, Bonefeld-Jørgensen EC. Perfluorinated compounds affect the function of sex hormone receptors. Environ Sci Pollut Res 2013;20:8031–8044. [DOI] [PubMed] [Google Scholar]
- de Kleijn MJJ, van der Schouw YT, Verbeek ALM, Peeters PHM, Banga J-D, van der Graaf Y. Endogenous estrogen exposure and cardiovascular mortality risk in postmenopausal women. Am J Epidemiol 2002;155:339–345. [DOI] [PubMed] [Google Scholar]
- Knox JT, Javins B, Frisbee SJ, Shankar A, Ducatman AM. Implications of early menopause in women exposed to perfluorocarbons. J Clin Endocrinol Metab 2011;96:1747–1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komar CM. Peroxisome proliferator-activated receptors (PPARs) and ovarian function – implications for regulating steroidogenesis, differentiation, and tissue remodeling. Reprod Biol Endocrinol 2005;3:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komar CM, Braissant O, Wahli W, Curry TE. Expression and localization of PPARs in the rat ovary during follicular development and the periovulatory period. Endocrinology 2001;142:4831–4838. [DOI] [PubMed] [Google Scholar]
- Kotthoff M, Müller J, Jürling H, Schlummer M, Fiedler D. Perfluoroalkyl and polyfluoroalkyl substances in consumer products. Environ Sci Pollut Res 2015;22:14546–14559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristensen SL, Ramlau-Hansen CH, Ernst E, Olsen SF, Bonde JP, Vested A, Halldorsson TI, Becher G, Haug LS, Toft G. Long-term effects of prenatal exposure to perfluoroalkyl substances on female reproduction. Hum Reprod 2013;28:3337–3348. [DOI] [PubMed] [Google Scholar]
- Kritz-Silverstein D, Barrett-Connor E. Early menopause, number of reproductive years, and bone mineral density in postmenopausal women. Am J Public Health 1993;83:983–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubwabo C, Stewart B, Zhu J, Marro L. Occurrence of perfluorosulfonates and other perfluorochemicals in dust from selected homes in the city of Ottawa, Canada. J Environ Monit 2005;7:1074–1078. [DOI] [PubMed] [Google Scholar]
- Kudo N, Katakura M, Sato Y, Kawashima Y. Sex hormone-regulated renal transport of perfluorooctanoic acid. Chem Biol Interact 2002;139:301–316. [DOI] [PubMed] [Google Scholar]
- Langer V, Dreyer A, Ebinghaus R. Polyfluorinated compounds in residential and nonresidential indoor air. Environ Sci Technol 2010;44:8075–8081. [DOI] [PubMed] [Google Scholar]
- Lau C, Thibodeaux JR, Hanson RG, Rogers JM, Grey BE, Stanton ME, Butenhoff JL, Stevenson LA. Exposure to perfluorooctane sulfonate during pregnancy in rat and mouse. II: Postnatal Evaluation Toxicol Sci 2003;74:382–392. [DOI] [PubMed] [Google Scholar]
- Lee JH, Lee CK, Suh C-H, Kang H-S, Hong C-P, Choi S-N. Serum concentrations of per- and poly-fluoroalkyl substances and factors associated with exposure in the general adult population in South Korea. Int J Hyg Environ Health 2017;220:1046–1054. [DOI] [PubMed] [Google Scholar]
- Lewis RC, Johns LE, Meeker JD. Serum biomarkers of exposure to perfluoroalkyl substances in relation to serum testosterone and measures of thyroid function among adults and adolescents from NHANES 2011-2012. Int J Environ Res Public Health 2015;12:6098–6114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Fletcher T, Mucs D, Scott K, Lindh CH, Tallving P, Jakobsson K. Half-lives of PFOS, PFHxS and PFOA after end of exposure to contaminated drinking water. Occup Environ Med 2018;75:46–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Du Y, Zhou B. Evaluation of estrogenic activities and mechanism of action of perfluorinated chemicals determined by vitellogenin induction in primary cultured tilapia hepatocytes. Aquat Toxicol 2007;85:267–277. [DOI] [PubMed] [Google Scholar]
- López-Arellano P, López-Arellano K, Luna J, Flores D, Jiménez-Salazar J, Gavia G, Teteltitla M, Rodríguez JJ, Domínguez A, Casas E et al. Perfluorooctanoic acid disrupts gap junction intercellular communication and induces reactive oxygen species formation and apoptosis in mouse ovaries. Environ Toxicol 2019;34:92–98. [DOI] [PubMed] [Google Scholar]
- Lopez-Espinosa M-J, Fletcher T, Armstrong B, Genser B, Dhatariya K, Mondal D, Ducatman A, Leonardi G. Association of perfluorooctanoic acid (PFOA) and perfluorooctane sulfonate (PFOS) with age of puberty among children living near a chemical plant. Environ Sci Technol 2011;45:8160–8166. [DOI] [PubMed] [Google Scholar]
- Lopez-Espinosa M-J, Mondal D, Armstrong BG, Eskenazi B, Fletcher T. Perfluoroalkyl substances, sex hormones, and insulin-like growth factor-1 at 6-9 years of age: a cross-sectional analysis within the C8 health project. Environ Health Perspect 2016;124:1269–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorber M, Eaglesham GE, Hobson P, Toms L-ML, Mueller JF, Thompson JS. The effect of ongoing blood loss on human serum concentrations of perfluorinated acids. Chemosphere 2015;118:170–177. [DOI] [PubMed] [Google Scholar]
- Lorber M, Egeghy PP. Simple intake and pharmacokinetic modeling to characterize exposure of Americans to perfluoroctanoic acid, PFOA. Environ Sci Technol 2011;45:8006–8014. [DOI] [PubMed] [Google Scholar]
- Lum KJ, Sundaram R, Barr DB, Louis TA, Buck Louis GM. Perfluoroalkyl chemicals, menstrual cycle length, and fecundity. Epidemiology 2017;28:90–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyngsø J, Ramlau-Hansen CH, Høyer BB, Støvring H, Bonde JP, Jönsson BAG, Lindh CH, Pedersen HS, Ludwicki JK, Zviezdai V et al. Menstrual cycle characteristics in fertile women from Greenland, Poland and Ukraine exposed to perfluorinated chemicals: a cross-sectional study. Hum Reprod 2014;29:359–367. [DOI] [PubMed] [Google Scholar]
- Mak YL, Taniyasu S, Yeung LWY, Lu G, Jin L, Yang Y, Lam PKS, Kannan K, Yamashita N. Perfluorinated compounds in tap water from China and several other countries. Environ Sci Technol 2009;43:4824–4829. [DOI] [PubMed] [Google Scholar]
- Maras M, Vanparys C, Muylle F, Robbens J, Berger U, Barber JL, Blust R, De Coen W. Estrogen-like properties of fluorotelomer alcohols as revealed by MCF-7 breast cancer cell proliferation. Environ Health Perspect 2006;114:100–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashayekhi V, Tehrani KHME, Hashemzaei M, Tabrizian K, Shahraki J, Hosseini M-J. Mechanistic approach for the toxic effects of perfluorooctanoic acid on isolated rat liver and brain mitochondria. Hum Exp Toxicol 2015;34:985–996. [DOI] [PubMed] [Google Scholar]
- McCoy JA, Bangma JT, Reiner JL, Bowden JA, Schnorr J, Slowey M, O’Leary T, Guillette LJ, Parrott BB. Associations between perfluorinated alkyl acids in blood and ovarian follicular fluid and ovarian function in women undergoing assisted reproductive treatment. Sci Total Environ 2017;605–606:9–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Alves SE. Estrogen actions in the central nervous system. Endocr Rev 1999;20:279–307. [DOI] [PubMed] [Google Scholar]
- Mondul AM, Rodriguez C, Jacobs EJ, Calle EE. Age at natural menopause and cause-specific mortality. Am J Epidemiol 2005;162:1089–1097. [DOI] [PubMed] [Google Scholar]
- Monroy R, Morrison K, Teo K, Atkinson S, Kubwabo C, Stewart B, Foster WG. Serum levels of perfluoroalkyl compounds in human maternal and umbilical cord blood samples. Environ Res 2008;108:56–62. [DOI] [PubMed] [Google Scholar]
- Must A, Phillips SM, Naumova EN, Blum M, Harris S, Dawson-Hughes B, Rand WM. Recall of early menstrual history and menarcheal body size: after 30 years, how well do women remember? Am J Epidemiol 2002;155:672–679. [DOI] [PubMed] [Google Scholar]
- Noorlander CW, van Leeuwen SPJ, Biesebeek JD, Mengelers MJB, Zeilmaker MJ. Levels of perfluorinated compounds in food and dietary intake of PFOS and PFOA in the Netherlands. J Agric Food Chem 2011;59:7496–7505. [DOI] [PubMed] [Google Scholar]
- Norman RJ, Dewailly D, Legro RS, Hickey TE. Polycystic ovary syndrome. Lancet (London, England) 2007;370, 97:685. [DOI] [PubMed] [Google Scholar]
- Olsen GW, Burris JM, Ehresman DJ, Froelich JW, Seacat AM, Butenhoff JL, Zobel LR. Half-life of serum elimination of perfluorooctanesulfonate, perfluorohexanesulfonate, and perfluorooctanoate in retired fluorochemical production workers. Environ Health Perspect 2007;115:1298–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen GW, Lange CC, Ellefson ME, Mair DC, Church TR, Goldberg CL, Herron RM, Medhdizadehkashi Z, Nobiletti JB, Rios JA et al. Temporal trends of perfluoroalkyl concentrations in American Red Cross adult blood donors, 2000–2010. Environ Sci Technol 2012;46:6330–6338. [DOI] [PubMed] [Google Scholar]
- Ossewaarde ME, Bots ML, Verbeek ALM, Peeters PHM, van der Graaf Y, Grobbee DE, van der Schouw YT. Age at menopause, cause-specific mortality and total life expectancy. Epidemiology 2005;16:556–562. [DOI] [PubMed] [Google Scholar]
- Ostertag SK, Tague BA, Humphries MM, Tittlemier SA, Chan HM. Estimated dietary exposure to fluorinated compounds from traditional foods among Inuit in Nunavut, Canada. Chemosphere 2009;75:1165–72. [DOI] [PubMed] [Google Scholar]
- Palmert MR, Dunkel L. Delayed puberty. N Engl J Med 2012;366:443–453. [DOI] [PubMed] [Google Scholar]
- Parazzini F, Bidoli E, Franceschi S, Schinella D, Tesio F, La Vecchia C, Zecchin R. Menopause, menstrual and reproductive history, and bone density in northern Italy. J Epidemiol Community Health 1996;50:519–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SK, Peng Q, Ding N, Mukherjee B, Harlow SD. Determinants of per- and polyfluoroalkyl substances (PFAS) in midlife women: evidence of racial/ethnic and geographic differences in PFAS exposure. Environ Res 2019;175:186–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez F, Nadal M, Navarro-Ortega A, Fàbrega F, Domingo JL, Barceló D, Farré M. Accumulation of perfluoroalkyl substances in human tissues. Environ Int 2013;59:354–362. [DOI] [PubMed] [Google Scholar]
- Petro EML, D’Hollander W, Covaci A, Bervoets L, Fransen E, De Neubourg D, De Pauw I, Leroy JLMR, Jorssen EPA, Bols PEJ. Perfluoroalkyl acid contamination of follicular fluid and its consequence for in vitro oocyte developmental competence. Sci Total Environ 2014;496:282–288. [DOI] [PubMed] [Google Scholar]
- Piekarz AM, Primbs T, Field JA, Barofsky DF, Simonich S. Semivolatile fluorinated organic compounds in Asian and Western U.S. air masses. Environ Sci Technol 2007;41:8248–8255. [DOI] [PubMed] [Google Scholar]
- Post GB, Louis JB, Cooper KR, Boros-Russo BJ, Lippincott RL. Occurrence and potential significance of perfluorooctanoic acid (PFOA) detected in New Jersey public drinking water systems. Environ Sci Technol 2009;43:4547–4554. [DOI] [PubMed] [Google Scholar]
- Prates EG, Nunes JT, Pereira RM. A role of lipid metabolism during cumulus-oocyte complex maturation: impact of lipid modulators to improve embryo production. Mediators Inflamm 2014;2014:692067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinete N, Wu Q, Zhang T, Yun SH, Moreira I, Kannan K. Specific profiles of perfluorinated compounds in surface and drinking waters and accumulation in mussels, fish, and dolphins from southeastern Brazil. Chemosphere 2009;77:863–869. [DOI] [PubMed] [Google Scholar]
- Quiñones O, Snyder SA. Occurrence of perfluoroalkyl carboxylates and sulfonates in drinking water utilities and related waters from the United States. Environ Sci Technol 2009;43:9089–9095. [DOI] [PubMed] [Google Scholar]
- Rak-Mardyła A, Karpeta A. Rosiglitazone stimulates peroxisome proliferator-activated receptor gamma expression and directly affects in vitro steroidogenesis in porcine ovarian follicles. Theriogenology 2014;82:1–9. [DOI] [PubMed] [Google Scholar]
- Rebholz SL, Jones T, Herrick RL, Xie C, Calafat AM, Pinney SM, Woollett LA. Hypercholesterolemia with consumption of PFOA-laced Western diets is dependent on strain and sex of mice. Toxicol Reports 2016;3:46–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renzi M, Guerranti C, Giovani A, Perra G, Focardi SE. Perfluorinated compounds: Levels, trophic web enrichments and human dietary intakes in transitional water ecosystems. Mar Pollut Bull 2013;76:146–57. [DOI] [PubMed] [Google Scholar]
- Roa J, Castellano JM, Navarro VM, Handelsman DJ, Pinilla L, Tena-Sempere M. Kisspeptins and the control of gonadotropin secretion in male and female rodents. Peptides 2009;30:57–66. [DOI] [PubMed] [Google Scholar]
- Rocca WA, Shuster LT, Grossardt BR, Maraganore DM, Gostout BS, Geda YE, Melton LJ. Long-term effects of bilateral oophorectomy on brain aging: unanswered questions from the Mayo Clinic Cohort Study of Oophorectomy and Aging. Women’s Heal 2009;5:39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruark CD, Song G, Yoon M, Verner M-A, Andersen ME, Clewell HJ, Longnecker MP. Quantitative bias analysis for epidemiological associations of perfluoroalkyl substance serum concentrations and early onset of menopause. Environ Int 2017;99:245–254. [DOI] [PubMed] [Google Scholar]
- Schaider LA, Balan SA, Blum A, Andrews DQ, Strynar MJ, Dickinson ME, Lunderberg DM, Lang JR, Peaslee GF. Fluorinated compounds in U.S. fast food packaging. Environ Sci Technol Lett 2017;4:105–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweigert FJ, Gericke B, Wolfram W, Kaisers U, Dudenhausen JW. Peptide and protein profiles in serum and follicular fluid of women undergoing IVF. Hum Reprod 2006;21:2960–2968. [DOI] [PubMed] [Google Scholar]
- Shi Z, Zhang H, Ding L, Feng Y, Xu M, Dai J. The effect of perfluorododecanonic acid on endocrine status, sex hormones and expression of steroidogenic genes in pubertal female rats. Reprod Toxicol 2009;27:352–359. [DOI] [PubMed] [Google Scholar]
- Shuster LT, Rhodes DJ, Gostout BS, Grossardt BR, Rocca WA. Premature menopause or early menopause: Long-term health consequences. Maturitas 2010;65:161–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair E, Kim SK, Akinleye HB and Kannan K. Quantitation of gas-phase perfluoroalkyl surfactants and fluorotelomer alcohols released from nonstick cookware and microwave popcorn bags. 2007. [DOI] [PubMed]
- Small CM, Manatunga AK, Marcus M. Validity of self-reported menstrual cycle length. Ann Epidemiol 2007;17:163–170. [DOI] [PubMed] [Google Scholar]
- Spliethoff HM, Tao L, Shaver SM, Aldous KM, Pass KA, Kannan K, Eadon GA. Use of newborn screening program blood spots for exposure assessment: declining levels of perfluorinated compounds in New York state infants. Environ Sci Technol 2008;42:5361–5367. [DOI] [PubMed] [Google Scholar]
- Strynar MJ, Lindstrom AB. Perfluorinated compounds in house dust from Ohio and North Carolina, USA. Environ Sci Technol 2008;42:3751–3756. [DOI] [PubMed] [Google Scholar]
- Takagi S, Adachi F, Miyano K, Koizumi Y, Tanaka H, Mimura M, Watanabe I, Tanabe S, Kannan K. Perfluorooctanesulfonate and perfluorooctanoate in raw and treated tap water from Osaka, Japan. Chemosphere 2008;72:1409–1412. [DOI] [PubMed] [Google Scholar]
- Tamura K, Hatsuta M, Watanabe G, Taya K, Kogo H. Blockage of gonadotropin-induced first ovulation caused by thyroidectomy and its possible mechanisms in rats. Am J Physiol Metab 1998;275:E380–E385. [DOI] [PubMed] [Google Scholar]
- Taylor KW, Hoffman K, Thayer KA, Daniels JL. Polyfluoroalkyl chemicals and menopause among women 20-65 years of age (NHANES). Environ Health Perspect 2014;122:145–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibodeaux JR, Hanson RG, Rogers JM, Grey BE, Barbee BD, Richards JH, Butenhoff JL, Stevenson LA, Lau C. Exposure to perfluorooctane sulfonate during pregnancy in rat and mouse. I: Maternal and prenatal evaluations. Toxicol Sci 2003;74:369–381. [DOI] [PubMed] [Google Scholar]
- Thompson J, Eaglesham G, Mueller J. Concentrations of PFOS, PFOA and other perfluorinated alkyl acids in Australian drinking water. Chemosphere 2011;83:1320–1325. [DOI] [PubMed] [Google Scholar]
- Tittlemier SA, Pepper K, Seymour C, Moisey J, Bronson R, Cao X-L, Dabeka RW. Dietary exposure of Canadians to perfluorinated carboxylates and perfluorooctane sulfonate via consumption of meat, fish, fast foods, and food items prepared in their packaging. J Agric Food Chem 2007;55:3203–3210. [DOI] [PubMed] [Google Scholar]
- Trier X, Granby K, Christensen JH. Polyfluorinated surfactants (PFS) in paper and board coatings for food packaging. Environ Sci Pollut Res 2011;18:1108–1120. [DOI] [PubMed] [Google Scholar]
- Trudel D, Horowitz L, Wormuth M, Scheringer M, Cousins IT, Hungerbühler K. Estimating consumer exposure to PFOS and PFOA. Risk Anal, 2008b;28:251–269. [DOI] [PubMed] [Google Scholar]
- Tsai M-S, Lin C-Y, Lin C-C, Chen M-H, Hsu SHJ, Chien K-L, Sung F-C, Chen P-C, Su T-C. Association between perfluoroalkyl substances and reproductive hormones in adolescents and young adults. Int J Hyg Environ Health 2015;218:437–443. [DOI] [PubMed] [Google Scholar]
- USEPA. Health effects support document for perfluorooctane sulfonate (PFOS). 2016a.
- USEPA. Health effects support document for perfluorooctanoic acid (PFOA). 2016b.
- USEPA. Fact sheet: toxicity assessments for GenX chemicals and PFBS. 2018.
- Vabre P, Gatimel N, Moreau J, Gayrard V, Picard-Hagen N, Parinaud J, Leandri RD. Environmental pollutants, a possible etiology for premature ovarian insufficiency: a narrative review of animal and human data. Environ Health 2017;16:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Schouw YT, van der Graaf Y, Steyerberg EW, Eijkemans JC, Banga JD. Age at menopause as a risk factor for cardiovascular mortality. Lancet (London, England) 1996;347:714–718. [DOI] [PubMed] [Google Scholar]
- Van Der Stege JG, Groen H, Van Zadelhoff SJN, et al. Decreased androgen concentrations and diminished general and sexual well-being in women with premature ovarian failure. Menopause 2008;15:23–31. [DOI] [PubMed] [Google Scholar]
- Vestergren R, Berger U, Glynn A, Cousins IT. Dietary exposure to perfluoroalkyl acids for the Swedish population in 1999, 2005 and 2010. Environ Int 2012;49:120–127. [DOI] [PubMed] [Google Scholar]
- Vestergren R, Cousins IT. Tracking the pathways of human exposure to perfluorocarboxylates. Environ Sci Technol 2009;43:5565–5575. [DOI] [PubMed] [Google Scholar]
- Vieira VM, Hoffman K, Shin H-M, Weinberg JM, Webster TF, Fletcher T. Perfluorooctanoic acid exposure and cancer outcomes in a contaminated community: a geographic analysis. Environ Health Perspect 2013;121:318–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakim AN, Paljug WR, Jasnosz KM, Alhakim N, Brown AB, Burholt DR. Thyroid hormone receptor messenger ribonucleic acid in human granulosa and ovarian stromal cells. Fertil Steril 1994;62:531–534. [DOI] [PubMed] [Google Scholar]
- Wan HT, Zhao YG, Wei X, Hui KY, Giesy JP, Wong CKC. PFOS-induced hepatic steatosis, the mechanistic actions on β-oxidation and lipid transport. Biochim Biophys Acta - Gen Subj 1820;2012:1092–1101. [DOI] [PubMed] [Google Scholar]
- Wang C, Ruan T, Liu J, He B, Zhou Q, Jiang G. Perfluorooctyl iodide stimulates steroidogenesis in H295R cells via a cyclic adenosine monophosphate signaling pathway. Chem Res Toxicol 2015;28:848–854. [DOI] [PubMed] [Google Scholar]
- Wang C, Wang T, Liu W, Ruan T, Zhou Q, Liu J, Zhang A, Zhao B, Jiang G. The in vitro estrogenic activities of polyfluorinated iodine alkanes. Environ Health Perspect 2012;120:119–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Zhou W, Wu S, Liang F, Li Y, Zhang J, Cui L, Feng Y, Wang Y. Perfluoroalkyl substances exposure and risk of polycystic ovarian syndrome related infertility in Chinese women. Environ Pollut 2019a;247:824–831. [DOI] [PubMed] [Google Scholar]
- Wang X, Bai Y, Tang C, Cao X, Chang F, Chen L. Impact of perfluorooctane sulfonate on reproductive ability of female mice through suppression of estrogen receptor α-activated kisspeptin neurons. Toxicol Sci 2018a;165:475–486. [DOI] [PubMed] [Google Scholar]
- Wang X, Mukherjee B, Park SK. Associations of cumulative exposure to heavy metal mixtures with obesity and its comorbidities among U.S. adults in NHANES 2003–2014. Environ Int 2018b;121:683–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Mukherjee B, Park SK. Does information on blood heavy metals improve cardiovascular mortality prediction? J Am Heart Assoc 2019b;8:e013571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Rogan WJ, Chen P-C, Lien G-W, Chen H-Y, Tseng Y-C, Longnecker MP, Wang S-L. Association between maternal serum perfluoroalkyl substances during pregnancy and maternal and cord thyroid hormones: Taiwan Maternal and Infant Cohort Study. Environ Health Perspect 2014;122:529–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wielsøe M, Long M, Ghisari M, Bonefeld-Jørgensen EC. Perfluoroalkylated substances (PFAS) affect oxidative stress biomarkers in vitro. Chemosphere 2015;129:239–245. [DOI] [PubMed] [Google Scholar]
- Wilhelm M, Bergmann S, Dieter HH. Occurrence of perfluorinated compounds (PFCs) in drinking water of North Rhine-Westphalia, Germany and new approach to assess drinking water contamination by shorter-chained C4–C7 PFCs. Int J Hyg Environ Health 2010;213:224–232. [DOI] [PubMed] [Google Scholar]
- Wong F, MacLeod M, Mueller JF, Cousins IT. Enhanced elimination of perfluorooctane sulfonic acid by menstruating women: evidence from population-based pharmacokinetic modeling. Environ Sci Technol 2014a;48:8807–8814. [DOI] [PubMed] [Google Scholar]
- Wong F, MacLeod M, Mueller JF, Cousins IT. Enhanced elimination of perfluorooctane sulfonic acid by menstruating women: evidence from population-based pharmacokinetic modeling. Environ Sci Technol 2014b;48:8807–8814. [DOI] [PubMed] [Google Scholar]
- Worley RR, Moore SM, Tierney BC, Ye X, Calafat AM, Campbell S, Woudneh MB, Fisher J. Per- and polyfluoroalkyl substances in human serum and urine samples from a residentially exposed community. Environ Int 2017;106:135–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Yoon M, Verner M-A, Xue J, Luo M, Andersen ME, Longnecker MP, Clewell HJ. Can the observed association between serum perfluoroalkyl substances and delayed menarche be explained on the basis of puberty-related changes in physiology and pharmacokinetics? Environ Int 2015;82:61–68. [DOI] [PubMed] [Google Scholar]
- Xiao F, Simcik MF, Halbach TR, Gulliver JS. Perfluorooctane sulfonate (PFOS) and perfluorooctanoate (PFOA) in soils and groundwater of a U.S. metropolitan area: migration and implications for human exposure. Water Res 2015;72:64–74. [DOI] [PubMed] [Google Scholar]
- Xie Y, Luo D, Xie X, Zhang D, Xu J, Zhou L, Yu X, Li M, Zhang L, Yang B et al. Maternal exposure to perfluorooctanoic acid inhibits luteal function via oxidative stress and apoptosis in pregnant mice. Reprod Toxicol 2017;69:159–166. [DOI] [PubMed] [Google Scholar]
- Yao P-L, Ehresman DJ, Rae JMC, Chang S-C, Frame SR, Butenhoff JL, Kennedy GL, Peters JM. Comparative in vivo and in vitro analysis of possible estrogenic effects of perfluorooctanoic acid. Toxicology 2014;326:62–73. [DOI] [PubMed] [Google Scholar]
- Yao Y, Zhao Y, Sun H, Chang S, Zhu L, Alder AC, Kannan K. Per- and polyfluoroalkyl substances (PFASs) in indoor air and dust from homes and various microenvironments in China: implications for human exposure. Environ Sci Technol 2018;52:3156–3166. [DOI] [PubMed] [Google Scholar]
- Yeung LWY, Robinson SJ, Koschorreck J, Mabury SA. Part I. a temporal study of PFCAs and their precursors in human plasma from two German cities 1982–2009. Environ Sci Technol 2013a;47:3865–3874. [DOI] [PubMed] [Google Scholar]
- Yeung LWY, Robinson SJ, Koschorreck J, Mabury SA. Part II. A temporal study of PFOS and its precursors in human plasma from two German cities in 1982–2009. Environ Sci Technol 2013b;47:3875–3882. [DOI] [PubMed] [Google Scholar]
- Ylinen M, Auriola S. Tissue distribution and elimination of perfluorodecanoic acid in the rat after single intraperitoneal administration. Pharmacol Toxicol 1990;66:45–48. [DOI] [PubMed] [Google Scholar]
- Zhang S, Tan R, Pan R, Xiong J, Tian Y, Wu J, Chen L. Association of perfluoroalkyl and polyfluoroalkyl substances with premature ovarian insufficiency in Chinese women. J Clin Endocrinol Metab 2018;103:2543–2551. [DOI] [PubMed] [Google Scholar]
- Zhang T, Sun HW, Wu Q, Zhang XZ, Yun SH, Kannan K. Perfluorochemicals in meat, eggs and indoor dust in China: assessment of sources and pathways of human exposure to perfluorochemicals. Environ Sci Technol 2010;44:3572–3579. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Beesoon S, Zhu L, Martin JW. Biomonitoring of perfluoroalkyl acids in human urine and estimates of biological half-life. Environ Sci Technol 2013;47:10619–10627. [DOI] [PubMed] [Google Scholar]
- Zhou W, Zhang L, Tong C, Fang F, Zhao S, Tian Y, Tao Y, Zhang J. Shanghai Birth Cohort Study. Plasma perfluoroalkyl and polyfluoroalkyl substances concentration and menstrual cycle characteristics in preconception women. Environ Health Perspect 2017;125:067012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Hu L-W, Qian Z, Chang J-J, King C, Paul G, Lin S, Chen P-C, Lee YL, Dong G-H. Association of perfluoroalkyl substances exposure with reproductive hormone levels in adolescents: by sex status. Environ Int 2016;94:189–195. [DOI] [PubMed] [Google Scholar]
- Zhu J, Chan Y-M. Adult consequences of self-limited delayed puberty. Pediatrics 2017;139:e20163177. [DOI] [PMC free article] [PubMed] [Google Scholar]


