Abstract
Background
Given the difficulty in preoperatively diagnosing lymph node metastasis, patients with Stage I–III non-small cell lung cancer (NSCLC) are likely to be included in the clinical N1 (cN1) group. However, better treatment options might be selected through further stratification. This study aimed to identify preoperative clinicopathological prognostic and stratification factors for patients with cN1 NSCLC.
Methods
This retrospective study evaluated 60 patients who were diagnosed with NSCLC during 2004–2014. Clinical nodal status had been evaluated using routine chest computed tomography (CT) and/or positron emission tomography (PET). To avoid biasing the fluorodeoxyglucose uptake values based on inter-institution or inter-model differences, we used only two PET systems (one PET system and one PET/CT system). Relapse-free survival (RFS) and overall survival (OS) were the primary study outcomes. The maximum standardized uptake value (SUVmax) was calculated for each tumor and categorized as low or high based on the median value. Patient sex, age, histology, tumor size, and tumor markers were also assessed.
Results
Poor OS was associated with older age (P = 0.0159) and high SUVmax values (P = 0.0142). Poor RFS was associated with positive carcinoembryonic antigen (CEA) expression (P = 0.0035) and high SUVmax values (P = 0.015). Multivariate analyses confirmed that poor OS was independently predicted by older age (hazard ratio [HR] = 2.751, confidence interval [CI]: 1.300–5.822; P = 0.0081) and high SUVmax values (HR = 5.121, 95% CI: 1.759–14.910; P = 0.0027). Furthermore, poor RFS was independently predicted by positive CEA expression (HR = 2.376, 95% CI: 1.056–5.348; P = 0.0366) and high SUVmax values (HR = 2.789, 95% CI: 1.042–7.458; P = 0.0410). The primary tumor’s SUVmax value was also an independent prognostic factor for both OS and RFS.
Conclusions
For patients with cN1 NSCLC, preoperative prognosis and stratification might be performed based on CEA expression, age, and the primary tumor’s SUVmax value. To enhance the prognostic value of the primary tumor’s SUVmax value, minimizing bias between facilities and models could lead to a more accurate prognostication.
Keywords: Clinical N1, Maximum standardized uptake value, Multivariate analysis, Non-small cell lung cancer, Positron emission tomography
Background
Primary lung cancer is the leading cause of cancer-related deaths, with non-small cell lung cancer (NSCLC) accounting for approximately 80% of these cases [1]. Patients with lung cancer are often diagnosed at an advanced stage and experience fatal disease progression, despite improvements in surgical technique and chemoradiotherapy [1]. Thus, preoperative diagnosis and staging are important for lung cancer and greatly affect the selection of treatment strategies [2]. If surgical resection is possible, clinical Stage I NSCLC is typically treated with upfront surgery, while neo-adjuvant chemotherapy is recommended for clinical Stage III NSCLC [3–5]. Therefore, in resectable lung cancer cases, it is important to accurately diagnose the extent of lymph node metastasis, which is the major determinant of clinical staging, although this remains challenging [6–8].
The guidelines recommend primary surgical resection for clinical N1 (cN1) NSCLC, as well as adjuvant chemotherapy if nodal disease is confirmed postoperatively [9]. However, given the difficulty in preoperatively diagnosing lymph node metastasis, all patients with Stage I–III disease are likely to be provisionally included in the cN1 group. This can lead to suboptimal treatment based on a single treatment strategy in at least some of these cases. This approach may also explain the less-than-satisfactory results being reported during the treatment of cN1 cases [10]. The present study aimed to retrospectively evaluate cN1 NSCLC cases in an attempt to identify preoperative clinicopathological factors that might predict the prognosis and guide the selection of optimal treatment strategies.
Patients and methods
Patients
Between January 2000 and December 2014, 908 consecutive patients underwent surgery for NSCLC at our institution. Patients were clinically staged according to the seventh edition of the Union for International Cancer Control TNM classification [11]. Among these patients, we identified 78 patients (8.6%) with cN1 NSCLC based on preoperative computed tomography (CT) and/or positron emission tomography (PET), although 11 patients were excluded because they were diagnosed at other facilities. Among the 67 patients with cN1 NSCLC, we ultimately included 60 patients who had undergone complete anatomical resection of the involved segment, lobe, or lung, with mediastinal and hilar lymph node dissection, but without induction therapy. All patients provided informed consent for the procedure and provided written informed consent for institutional storage of their personal data in a scientific database. The medical ethics committee of the Hokkaido University School of Medicine approved the present study’s retrospective protocol (#019–0346), which was registered at Researchregistry.com (#5246).
Diagnosis of cN1
Clinical nodal status was evaluated using routine chest CT and/or PET. Lymph nodes with a ≥ 10-mm short axis on chest CT or with asymmetric abnormal uptake on 18F-fluorodeoxyglucose (FDG) imaging were defined as being positive for lymph node metastasis. Between 2000 and 2008, we used an FDG-PET Siemens ECAT EXACT HR+ scanner (Siemens/CTI, Knoxville, TN, USA), while after 2009 we used an FDG PET/CT Biograph 64 TruePoint with TrueV PET/CT scanner (Siemens Japan, Tokyo).
Prognostic factors
The relationships between preoperatively evaluable clinicopathological factors and the patients’ outcomes were examined. The relevant factors were considered sex (female vs. male), age (≥70 years vs. < 70 years), histology (adenocarcinoma vs. non-adenocarcinoma), tumor size (> 30 mm vs. ≤30 mm), and tumor marker levels with our institutional cut-off values (carcinoembryonic antigen [CEA, 5.0 ng/mL], squamous cell carcinoma antigen [SCC, 2.0 ng/mL], and CYFRA expression, 3.5 ng/mL). We also considered the prognostic value of the primary tumor’s maximum standardized uptake value (SUVmax).
Statistical analyses
All statistical analyses were performed using JMP software (version 14.0; SAS Institute Inc., NC, USA). Inter-group comparisons were performed using the χ2 test or Fisher’s exact test, as appropriate. Survival outcomes were plotted using the Kaplan-Meier method and compared using the log-rank test. Overall survival was defined as the time from surgery until death, regardless of the cause of death. Relapse-free survival (RFS) was defined as the time from surgery until the first instance of recurrence or death. Univariate and multivariate Cox proportional hazards models were used to analyze the associations between clinicopathological factors and survival. Differences were considered statistically significant at two-sided P values of < 0.05.
Results
Patient characteristics
The patients’ characteristics and clinical findings are summarized in Table 1. The study included 46 men and 14 women with a median age of 70 years (range: 32–81). The tumors were classified according to the World Health Organization International Histological Classification of Tumors [12] as adenocarcinoma in 27 patients (45%), squamous cell carcinoma in 27 patients (45%), pleomorphic carcinoma in 3 patients (5%), adenosquamous carcinoma in 2 patients (3.3%), and large cell carcinoma in 1 patient (1.7%). The median tumor size was 35 mm (range: 13–88 mm), with clinical T classifications of T1 for 16 patients (26.7%), T2 for 29 patients (48.3%), T3 for 14 patients (23.3%), and T4 for 1 patient (1.7%). The preoperative clinical stages were stage IIA for 41 patients (68.3%), stage IIB for 4 patients (6.7%), and stage IIIA for 15 patients (25%). In terms of tumor markers, the median serum CEA value was 4.8 ng/mL (range: 1.5–152.2 ng/mL), the median serum SCC value was 0.8 ng/mL (range: 0.1–19.7 ng/mL), and the median serum CYFRA value was 2.48 ng/mL (range: 0.59–53.08 ng/mL). Given the difference between the FDG-PET instrument models, the original SUVmax values were analyzed without correction, which revealed median SUVmax values of 6 in the FDG-PET group (range: 0–22.78) and 11.45 in the FDG-PET/CT group (range: 1.90–52.60). The surgical approaches were video-assisted thoracic surgery (VATS) for 23 patients (38.3%), standard open thoracic surgery for 20 patients (33.3%), and conversion from VATS to open surgery for 17 patients (28.3%). The resections involved lobectomy for 51 patients (85%), pneumonectomy for 7 patients (11.7%), and segmentectomy for 2 patients. Reconstruction of the pulmonary artery or bronchus was added for 6 of the 51 patients who had undergone lobectomy.
Table 1.
Characteristics of the patients with clinical N1 non-small cell lung cancer
| Number | |
|---|---|
| Sex | |
| Male | 46 |
| Female | 14 |
| Age (years) | |
| Median | 70 |
| Range | 32–81 |
| Histological type | |
| Adenocarcinoma | 27 |
| Squamous cell carcinoma | 27 |
| Pleomorphic carcinoma | 3 |
| Adenosquamous cell carcinoma | 2 |
| Large cell carcinoma | 1 |
| Size of primary tumor (mm) | |
| Median | 35 |
| Range | 13–88 |
| Clinical T status | |
| T1 | 16 |
| T2 | 29 |
| T3 | 14 |
| T4 | 1 |
| CEA (ng/mL) | |
| Median | 4.8 |
| Range | 1.5–152.2 |
| SCC (ng/mL) | |
| Median | 0.8 |
| Range | 0.0–19.7 |
| CYFRA (ng/mL) | |
| Median | 2.48 |
| Range | 0.59–53.08 |
| FDG-PET | |
| Number of patients | 35 |
| Median of SUVmax | 6 |
| Range of SUVmax | 0–22.78 |
| FDG-PET/CT | |
| Number of patients | 25 |
| Median of SUVmax | 11.45 |
| Range of SUVmax | 1.90–52.60 |
| Surgical approach | |
| VATS | 23 |
| Thoracotomy | 20 |
| Conversion (VATS to thoracotomy) | 17 |
| Operative procedure | |
| Pneumonectomy | 7 |
| Lobectomy | 51 |
| + bronchoplasty | 3 |
| + angioplasty | 1 |
| + bronchoplasty & angioplasty | 2 |
| Segmentectomy | 2 |
| Pathological T status | |
| pT1 | 15 |
| pT2 | 29 |
| pT3 | 14 |
| pT4 | 2 |
| Pathological N status | |
| pN0 | 23 |
| pN1 | 22 |
| pN2 | 15 |
| Pathological stage | |
| IA | 4 |
| IB | 12 |
| IIA | 14 |
| IIB | 6 |
| IIIA | 23 |
| IIIB | 1 |
Pathological diagnoses
The postoperative pathological tumor diagnoses were pT1 for 15 patients (25%), pT2 for 29 patients (48.3%), pT3 for 14 patients (23.3%), and pT4 for 2 patients (3.3%). Similar to the clinical diagnoses, the most common pathological diagnosis was pT2. The pathological nodal diagnoses revealed correct preoperative diagnoses of N1 for 22 patients (36.7%), N0 for 23 patients (38.3%), and N2 for 15 patients (25%). Twenty-four patients (40%) were postoperatively diagnosed with stage III disease (Table 1).
Survival rate
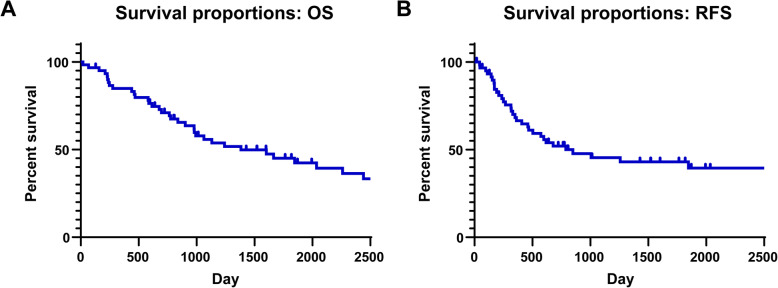
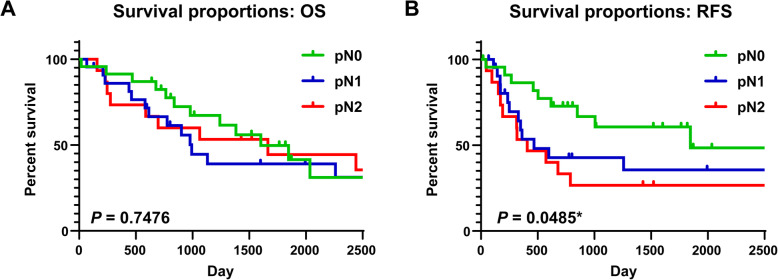
Among the included cN1 cases, the 5-year OS rate was 45.1% and the 5-year RFS rate was 43.0% (Fig. 1). When we considered the pathological diagnoses, no significant difference was observed when we compared the 5-year OS rates between the pN0 (49.8%), pN1 (44.4%), and pN2 groups (44.4%, P = 0.7476). However, we detected a significant difference in the 5-year RFS rates according to the pathological nodal classification (pN0: 60.1%, pN1: 35.6%, pN2: 26.7%, P = 0.0485) (Fig. 2).
Fig. 1.
Overall survival and relapse-free survival curves after complete resection of cN1 non-small cell lung cancer. a The overall survival (OS) curve and 5-year survival rate among all patients (5-year OS rate: 45.1%), b The relapse-free survival (RFS) curve and 5-year survival rate among all patients (5-year RFS rate: 43.0%). *P < 0.05
Fig. 2.
Overall survival and relapse-free survival curves according to pathological N status. a The overall survival (OS) curves and 5-year survival rates according to pathological N status (5-year OS rate: pN0 = 49.8%, pN1 = 39.1%, pN2 = 44.4%), b The relapse-free survival (RFS) curves and 5-year survival rates according to pathological N status (5-year RFS rate: pN0 = 60.1%, pN1 = 35.6%, pN2 = 26.7%). *P < 0.05
Clinicopathological factors for predicting OS and RFS
The prognostic values of the clinicopathological factors are shown in Table 2. We found that there was a large difference in the median SUVmax values that were determined using PET and PET/CT (6.0 vs. 11.45). Thus, we chose to use the raw data without correction because the SUVmax value is influenced by various parameters. Patients with values above the median SUVmax values for PET or PET/CT were assigned to the high-value group, and patients with lower values were assigned to the low-value group.
Table 2.
Clinicopathological factors for predicting disease-free survival
| Variable | Unfavorable/favorable | Number |
|---|---|---|
| Sex | Female vs. male | 14 / 46 |
| Age | ≥70 vs. < 70 years | 32 / 28 |
| Histology | Adenocarcinoma vs. others | 27 / 33 |
| Tumor size | > 30 vs. ≤30 mm | 41 / 19 |
| CEA expression | ≥5.0 vs. < 5.0 ng/mL | 30 / 30 |
| Primary tumor SUVmax | High vs. low | 29 / 31 |
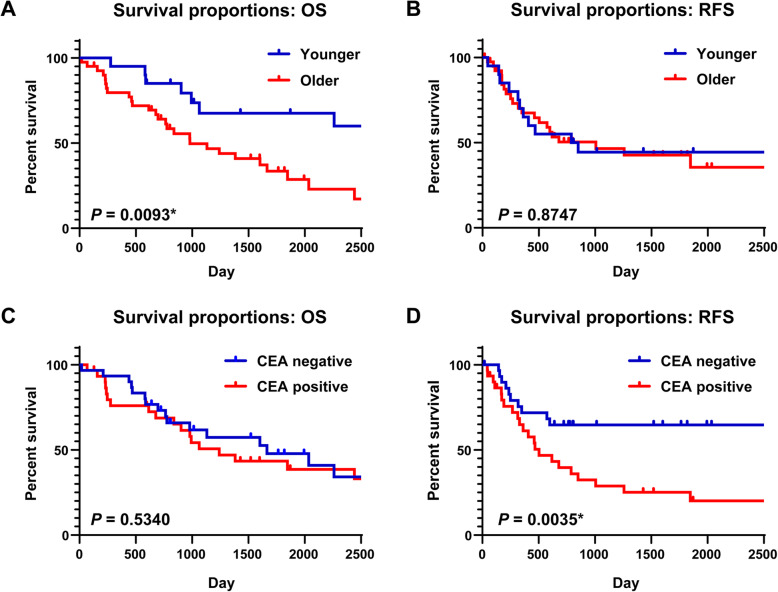
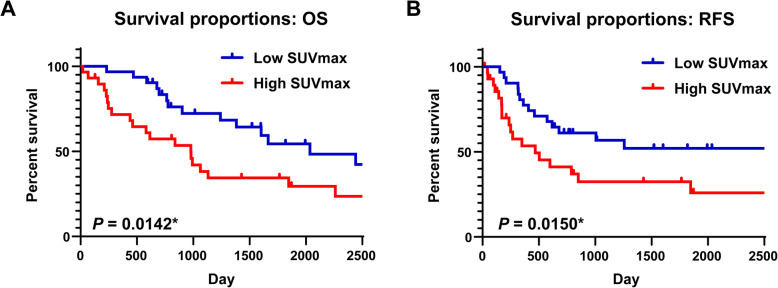
The OS and RFS outcomes were analyzed according to the clinicopathological factors. Based on the log-rank test, age was significantly associated with poor OS (P = 0.0159) but not with poor RFS (P = 0.9543) (Fig. 3a & b). The CEA value was not significantly associated with poor OS (P = 0.534), but was significantly associated with poor RFS (P = 0.0035) (Fig. 3c & d). However, the SCC and CYRA values were not significantly associated with OS or RFS (SCC, OS: P = 0.534, RFS: P = 0.0035; CYFRA, OS: P = 0.534, RFS: P = 0.0035) (Supplementary Fig. 1). The primary tumor’s SUVmax value was significantly associated with both poor OS (P = 0.0142) and poor RFS (P = 0.015) (Fig. 4). No significant differences in OS or RFS were observed when we compared sex (OS: P = 0.8516, RFS: P = 0.5446), histology (OS: P = 0.6415, RFS: P = 0.5027), and tumor size (OS: P = 0.7621, RFS: P = 0.871) (Supplementary Fig. 1).
Fig. 3.
Survival curves stratified according to age and carcinoembryonic antigen expression. a, b The overall survival (OS) and relapse-free survival (RFS) curves with 5-year survival rates are shown according to ages of ≥70 years and < 70 years (5-year OS rate: 30.9% vs. 61.5%, log-rank P = 0.0159; 5-year RFS rate: 29.8% vs. 41.7%, log-rank P = 0.9543). c, d The OS and RFS curves with 5-year survival rates according to normal and abnormal expression of carcinoembryonic antigen (CEA; 5-year OS rate: 43.4% vs. 47.8%, log-rank P = 0.534; 5-year RFS rate: 25.2% vs. 64.7%, log-rank P = 0.0035). *P < 0.05
Fig. 4.
Survival curves stratified according to SUVmax values. Kaplan-Meier analysis of survival among cN1 NSCLC patients. a, b The overall survival (OS) and relapse-free survival (RFS) according to high and low SUVmax values for the primary tumor (5-year OS rate: 34.4% vs. 54.4%, log-rank P = 0.0142; 5-year RFS rate: 32.4% vs. 52.0%, log-rank P = 0.015). *P < 0.05
The univariate Cox proportional hazards model revealed similar results to those obtained with the Kaplan-Meier analyses. The expression of CEA was used as a representative tumor marker, because only CEA was significantly associated with prognosis based on the Kaplan-Meier analysis. Poor OS was significantly associated with older age (hazard ratio [HR]: 2.369, 95% confidence interval [CI]: 1.152–4.870; P = 0.019) and high SUVmax values for the primary tumor (HR: 2.284, 95% CI: 1.159–4.502; P = 0.017). Poor RFS was associated with abnormal preoperative serum CEA levels (HR: 2.901, 95% CI: 1.374–6.127; P = 0.005) and high SUVmax values for the primary tumor (HR: 2.312, 95% CI: 1.155–4.630; P = 0.018). The multivariate Cox proportional hazard model also confirmed that a high SUVmax value for the primary tumor independently predicted poor OS (HR: 5.121, 95% CI: 1.759–14.910; P = 0.003) and poor RFS (HR: 2.789, 95% CI: 1.042–7.458; P = 0.041) in patients with cN1 NSCLC. In addition, age independently predicted poor OS (HR: 2.751, 95% CI: 1.300–5.822; P = 0.008) and CEA expression independently predicted poor RFS (HR: 2.376, 95% CI: 1.056–5.348; P = 0.037) (Table 3). We also evaluated whether the SUVmax value was associated with the various clinicopathological factors, including pN1 status. The results revealed that a high SUVmax was significantly associated with non-adenocarcinoma type (P = 0.011) and tumor size of ≥30 mm (P = 0.001), but not pN status (P = 0.211) (Table 4).
Table 3.
Cox proportional hazards analysis of prognostic factors in patients with cN1 disease
| Unfavorable vs. favorable | OS | RFS | |||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P-value | HR | 95% CI | P-value | ||
| Univariate analysis | |||||||
| Sex | Female vs. male | 1.075 | 0.502–2.305 | 0.8517 | 1.269 | 0.586–2.750 | 0.5455 |
| Age | ≥70 vs. < 70 years | 2.369 | 1.152–4.870 | 0.0190* | 1.020 | 0.510–1.960 | 0.9543 |
| Histology | Adenocarcinoma vs. others | 1.171 | 0.602–2.277 | 0.6419 | 1.265 | 0.635–2.520 | 0.5037 |
| Tumor size | > 30 vs. ≤30 mm | 0.892 | 0.427–1.865 | 0.7622 | 1.066 | 0.495–2.296 | 0.8710 |
| CEA | ≥5.0 vs. < 5.0 ng/mL | 1.235 | 0.634–2.405 | 0.5348 | 2.901 | 1.374–6.127 | 0.0052* |
| Primary tumor SUVmax | High vs. low | 2.284 | 1.159–4.502 | 0.0171* | 2.312 | 1.155–4.630 | 0.0180* |
| Multivariate analysis | |||||||
| Sex | Female vs. male | 1.315 | 0.556–3.276 | 0.5564 | 1.018 | 0.430–2.413 | 0.9669 |
| Age | ≥70 vs. < 70 years | 2.751 | 1.300–5.822 | 0.0081* | 1.130 | 0.545–2.343 | 0.7419 |
| Histology | Adenocarcinoma vs. others | 1.970 | 0.754–5.149 | 0.1668 | 1.353 | 0.575–3.181 | 0.4884 |
| Tumor size | > 30 vs. ≤30 mm | 1.989 | 0.603–6.565 | 0.2589 | 0.523 | 0.186–1.475 | 0.2205 |
| CEA | ≥5.0 vs. < 5.0 ng/mL | 1.039 | 0.504–2.143 | 0.9170 | 2.376 | 1.056–5.348 | 0.0366* |
| Primary tumor SUVmax | High vs. low | 5.121 | 1.759–14.910 | 0.0027* | 2.789 | 1.042–7.458 | 0.0410* |
HR Hazard ratio, CI Confidence interval. *P < 0.05
Table 4.
Differences in clinicopathological factors according to the primary tumor’s SUVmax
| Total (n = 60) | Primary tumor’s SUVmax | P value | ||
|---|---|---|---|---|
| High | Low | |||
| Sex | ||||
| Male | 46 | 23 | 23 | 1.000 |
| Female | 14 | 6 | 8 | |
| Age | ||||
| < 70 years | 28 | 14 | 14 | 0.7961 |
| ≥ 70 years | 32 | 15 | 17 | |
| Histological type | ||||
| Adenocarcinoma | 27 | 8 | 19 | 0.0108* |
| Non-adenocarcinoma | 33 | 21 | 12 | |
| Tumor size | ||||
| ≤ 30 mm | 19 | 3 | 16 | < 0.0001* |
| > 30 mm | 41 | 26 | 15 | |
| CEA | ||||
| < 5.0 ng/mL | 30 | 11 | 19 | 0.1205 |
| ≥ 5.0 ng/mL | 30 | 18 | 12 | |
| Pathological N status ng/mL | ||||
| pN0 | 23 | 9 | 14 | 0.2106 |
| pN1 | 22 | 14 | 8 | |
| pN2 | 15 | 6 | 9 | |
*P < 0.05
Discussion
The present study demonstrated that the prognoses of patients with cN1 NSCLC were associated with age, the CEA value, and the primary tumor’s SUVmax value. The primary tumor’s SUVmax value was also an independent prognostic factor for both OS and RFS, which is consistent with previous reports regarding the associations between FDG uptake and tumor malignancy [13–16]. Clinical and experimental studies have indicated that FDG accumulation during PET examination was correlated with tumor growth rate, cell density, and cell differentiation [17–19]. These results support our finding that a high value for the primary tumor’s SUVmax may predict high-grade disease and a poor prognosis. Furthermore, the associations of SUVmax with histological type and tumor size may reflect the malignant potential of the primary tumor. Nevertheless, it appears that the SUVmax value’s relationship with prognosis is not solely explained by a correlation with the pN status.
There can be some variations in the FDG uptake values based on inter-institution or inter-model differences between the PET instruments, and it is possible that its true effectiveness as a prognostic marker cannot be accurately assessed without considering these differences. For example, it would be appropriate to consider the SUVmax value for a separate group when a phantom is used, and to also consider the PET model or facility [20–23]. Furthermore, SUVmax values can vary according to the instrument model, size of the patient’s body, and the presence or absence of diabetic complications [24–26]. Moreover, different facilities use different PET protocols (e.g., image acquisition timing and the use of one or two scans), which also complicates the analysis of data from multiple facilities. Therefore, we examined the original SUVmax values without correction for each instrument model. It would be ideal to conduct a study using the same PET instruments, although it would be difficult to accumulate an appropriate number of cN1 cases. Thus, we believe that a single-center analysis using only two PET instrument models may be a useful starting point. Finally, because FDG integration was significantly different between the instrument models, we categorized the SUVmax values as high or low using the median values from the time periods when each instrument was used (2000–2008 and 2009–present).
While analyzing the cN1 NSCLC cases, we encountered two problems that should be considered. The first problem was the accuracy of the preoperative diagnosis, and the second problem was the recommended treatment strategy. An accurate preoperative assessment is critical for selecting the most suitable treatment for NSCLC patients [27]. However, despite advances in diagnostic CT and FDG/PET-CT, over-diagnosis and under-diagnosis of nodal metastasis can occur easily because cN1 disease is a marginal stage for surgery. It has been reported that 19–30% of patients with a preoperative diagnosis of cN1 were diagnosed with pN2 disease after the surgery [6, 10, 11, 28], and our findings revealed a similar result (15 patients [25%] with preoperative cN1 disease were found to have pN2 disease). A meta-analysis has indicated that endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) is a potentially useful technique that can provide a sensitivity of 88–94% for mediastinal staging of lung cancer [29]. However, the use of EBUS-TBNA to diagnose lymph node metastasis is relatively new, and would likely be used in only a small proportion of cases, so this factor was not included in the present study.
Several randomized trials and meta-analyses have shown survival benefits for adjuvant chemotherapy after surgery in stage II–III NSCLC patients [30–32]. However, some reports have indicated that induction chemotherapy was not associated with improved survival in cN1 NSCLC patients [33, 34]. Therefore, the current guidelines for patients with cN1 NSCLC recommend a surgery-first strategy, followed by adjuvant chemotherapy for patients who have pathologically confirmed nodal metastasis [35]. Furthermore, the clinical stage is determined based on the tumor size, the lymph node metastasis, and the distant metastasis diagnosis at the time of each examination. However, cN1 NSCLC patients are a heterogeneous population and we believe that a more personalized treatment strategy might be useful in this population. Thus, we hope to guide treatment selection based on preoperatively evaluable parameters, as we believe that malignant potential (e.g., measured based on tumor growth rate) is not reflected in the current clinical staging of NSCLC. We hypothesize that cN1 NSCLC patients might be stratified based on preoperative factors that reflect the tumor’s malignant potential, in additional to other standard clinical staging factors, which might guide treatment selection before the patient undergoes surgery.
The present study has several limitations that should be considered. First, the small sample size and retrospective nature of the study are prone to bias. However, we hope to collect additional cases and potentially incorporate more accurate preoperative diagnoses using the EBUS-TBNA technique. Second, the cut-off value for SUVmax varied according to the PET instrument model, although previous studies have also used values from multiple facilities/models, which might have obscured the association between SUVmax and prognosis.
Conclusion
We retrospectively reviewed the clinicopathological characteristics of patients with cN1 NSCLC who underwent surgical resection. Although it is difficult to accurately and preoperatively diagnose nodal metastasis using CT and/or PET, our results suggest that the primary tumor’s SUVmax value may be preoperatively used for prognostication in cN1 NSCLC cases. Thus, the SUVmax value might be useful for patient stratification and treatment selection. However, further studies are needed to clarify the differences in FDG accumulation between facilities and/or instrument models, in order to confirm whether this marker is reliable in this setting. Nevertheless, if FDG accumulation is found to be a useful prognostic factor, it might help preoperatively guide the selection of newer therapeutic strategies, including adjuvant chemotherapy, for patients with cN1 NSCLC.
Supplementary information
Additional file 1: Figure S1. Survival curves stratified according to various clinicopathological factors. a, b The overall survival (OS) and relapse-free survival (RFS) curves with 5-year survival rates according to female and male sex (5-year OS rate: 42.9% vs. 45.5%, log-rank P = 0.8516; 5-year RFS rate: 32.1% vs. 46.4%, log rank P = 0.5446). c, d The OS and RFS curves with 5-year survival rates according to adenocarcinoma and non-adenocarcinoma classification (5-year OS rate: 43.4% vs. 46.4%, log-rank P = 0.6415; 5-year RFS rate: 37.1% vs. 48.7%, log-rank P = 0.5027). e, f The OS and RFS curves with 5-year survival rates according to tumor diameters of ≥30 mm and < 30 mm (5-year OS rate: 47.3% vs. 38.9%, log-rank P = 0.7621; 5-year RFS rate: 44.6% vs. 37.5%, log-rank P = 0.871). g, h The OS and RFS curves with 5-year survival rates according to positive and negative expression of squamous cell carcinoma antigen (SCC; 5-year OS rate: 28.1% vs. 48.0%, log-rank P = 0.1254; 5-year RFS rate: 34.2% vs. 35.8%, log-rank P = 0.2338). i, j The OS and RFS curves and 5-year survival rates according to positive and negative expression of CYFRA (5-year OS rate: 49.2% vs. 43.0%, log-rank P = 0.7879; 5-year RFS rate: 50.4% vs. 28.7%, log-rank P = 0.5767). *: P < 0.05.
Acknowledgements
The authors thank Editage (www.editage.com) for English language editing.
Abbreviations
- NSCLC
Non-small cell lung cancer
- cN1
Clinical N1
- CT
Computed tomography
- PET
Positron emission tomography
- RFS
Relapse-free survival
- OS
Overall survival
- SUVmax
Maximum standardized uptake value
- CEA
Carcinoembryonic antigen
- HR
Hazard ratio
- FDG
18F-fluorodeoxyglucose
- VATS
Video-assisted thoracic surgery
- EBUS-TBNA
Endobronchial ultrasound-guided transbronchial needle aspiration
Authors’ contributions
MA contributed to the conception and design of the study. TK and MA analyzed and interpreted the patient data. AF independently analyzed the data. MA performed the statistical analysis. TK, YH, KK, SW, and MA were responsible for follow-up, data collection, and dataset maintenance. All authors read and approved the final manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Availability of data and materials
The study protocol was registered at Researchregistry.com (#5246). The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Ethics approval and consent to participate
All patients provided informed consent for the procedure and provided written informed consent for institutional storage of their personal data in a scientific database. The medical ethics committee of the Hokkaido University School of Medicine approved the present study’s retrospective protocol (#019–0346), which was registered at Researchregistry.com (#5246).
Consent for publication
The requirement for patient consent for publication was waived based on the use of de-identified data.
Competing interests
The authors declare no conflicts of interest in association with the present study.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s13019-020-01272-2.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Silvestri GA, Gonzalez AV, Jantz MA, Margolis ML, Gould MK, Tanoue LT, et al. Methods for staging non-small cell lung cancer: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143:e211S–e250S. doi: 10.1378/chest.12-2355. [DOI] [PubMed] [Google Scholar]
- 3.Asamura H, Goya T, Koshiishi Y, Sohara Y, Eguchi K, Mori M, et al. A Japanese lung cancer registry study: prognosis of 13,010 resected lung cancers. J Thorac Oncol. 2008;3:46–52. doi: 10.1097/JTO.0b013e31815e8577. [DOI] [PubMed] [Google Scholar]
- 4.Sawabata N, Miyaoka E, Asamura H, Nakanishi Y, Eguchi K, Mori M, et al. Japanese lung cancer registry study of 11,663 surgical cases in 2004: demographic and prognosis changes over decade. J Thorac Oncol. 2011;6:1229–1235. doi: 10.1097/JTO.0b013e318219aae2. [DOI] [PubMed] [Google Scholar]
- 5.Hancock J, Rosen J, Moreno A, Kim AW, Detterbeck FC, Boffa DJ. Management of clinical stage IIIA primary lung cancers in the National Cancer Database. Ann Thorac Surg. 2014;98:424–432. doi: 10.1016/j.athoracsur.2014.04.067. [DOI] [PubMed] [Google Scholar]
- 6.Watanabe S, Asamura H, Suzuki K, Tsuchiya R. Problems in diagnosis and surgical management of clinical N1 non-small cell lung cancer. Ann Thorac Surg. 2005;79:1682–1685. doi: 10.1016/j.athoracsur.2004.11.025. [DOI] [PubMed] [Google Scholar]
- 7.Hishida T, Yoshida J, Nishimura M, Nishiwaki Y, Nagai K. Problems in the current diagnostic standards of clinical N1 non-small cell lung cancer. Thorax. 2008;63:526–531. doi: 10.1136/thx.2006.062760. [DOI] [PubMed] [Google Scholar]
- 8.Kim D, Choi YS, Kim HK, Kim K, Kim J, Shim YM. Heterogeneity of clinical n1 non-small cell lung cancer. Thorac Cardiovasc Surg. 2014;62:103–108. doi: 10.1055/s-0032-1333067. [DOI] [PubMed] [Google Scholar]
- 9.Howington JA, Blum MG, Chang AC, Balekian AA, Murthy SC. Treatment of stage I and II non-small cell lung cancer: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143:e278S–e313S. doi: 10.1378/chest.12-2359. [DOI] [PubMed] [Google Scholar]
- 10.Miyoshi K, Mimura T, Iwanaga K, Adachi S, Tsubota N, Okada M. Surgical treatment of clinical N1 non-small cell lung cancer: ongoing controversy over diagnosis and prognosis. Surg Today. 2010;40:428–432. doi: 10.1007/s00595-008-4072-4. [DOI] [PubMed] [Google Scholar]
- 11.Sobin LH, Gospodarowicz MK, Wittekind C. TNM classification of malignant Tumours. 7. New York: Wiley-Liss; 2009. [Google Scholar]
- 12.World Health Organization . International histological classification of tumours. 2. Berlin: Springer; 1988. [Google Scholar]
- 13.Umakoshi H, Iwano S, Yokoi K, Ito S, Ito R, Kawaguchi K, et al. FDG PET/CT overcomes discordance between clinical and pathologic TNM classification of small-size primary lung cancer: influence on postoperative prognosis. Clin Lung Cancer. 2018;9:e37–e45. doi: 10.1016/j.cllc.2017.05.021. [DOI] [PubMed] [Google Scholar]
- 14.Kishimoto M, Iwano S, Ito S, Kato K, Ito R, Naganawa S. Prognostic evaluations of small size lung cancers by 18F-FDG PET/CT and thin-section CT. Lung Cancer. 2014;86:180–184. doi: 10.1016/j.lungcan.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 15.Ito R, Iwano S, Kishimoto M, Ito S, Kato K, Naganawa S. Correlation between FDG-PET/CT findings and solid type non-small cell cancer prognostic factors: are there differences between adenocarcinoma and squamous cell carcinoma? Ann Nucl Med. 2015;29:897–905. doi: 10.1007/s12149-015-1025-z. [DOI] [PubMed] [Google Scholar]
- 16.Kosaka T, Yamaki E, Tanaka S, Mogi A, Kuwano H. Preoperative 18F-fluorodeoxyglucose positron emission tomography can predict the tumor malignancy of small peripheral lung cancer. Ann Thorac Cardiovasc Surg. 2014;20:968–973. doi: 10.5761/atcs.oa.13-00155. [DOI] [PubMed] [Google Scholar]
- 17.Higashi K, Ueda Y, Seki H, Yuasa K, Oguchi M, Noguchi T, et al. Fluorine-18-FDG PET imaging is negative in bronchioloalveolar lung carcinoma. J Nucl Med. 1998;39:1016–1020. [PubMed] [Google Scholar]
- 18.Kubota K, Ishiwata K, Kubota R, Yamada S, Tada M, Sato T, et al. Tracer feasibility for monitoring tumor radiotherapy: a quadruple tracer study with fluorine-18-fluorodeoxyglucose or fluorine-18-fluorodeoxyuridine, L-[methyl-14C] methionine, [6-3H] thymidine, and gallium-67. J Nucl Med. 1991;32:2118–2123. [PubMed] [Google Scholar]
- 19.Higashi T, Saga T, Nakamoto Y, Ishimori T, Mamede MH, Wada M, et al. Relationship between retention index in dual-phase (18)F-FDG PET, and hexokinase-II and glucose transporter-1 expression in pancreatic cancer. J Nucl Med. 2002;43:173–180. [PubMed] [Google Scholar]
- 20.Makris NE, Huisman MC, Kinahan PE, Lammertsma AA, Boellaard R. Evaluation of strategies towards harmonization of FDG PET/CT studies in multicentre trials: comparison of scanner validation phantoms and data analysis procedures. Eur J Nucl Med Mol Imaging. 2013;40:1507–1515. doi: 10.1007/s00259-013-2465-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lasnon C, Desmonts C, Quak E, Gervais R, Do P, Dubos-Arvis C, et al. Harmonizing SUVs in multicentre trials when using different generation PET systems: prospective validation in non-small cell lung cancer patients. Eur J Nucl Med Mol Imaging. 2013;40:985–996. doi: 10.1007/s00259-013-2391-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Akamatsu G, Ishikawa K, Mitsumoto K, Taniguchi T, Ohya N, Baba S, et al. Improvement in PET/CT image quality with a combination of point-spread function and time-of-flight in relation to reconstruction parameters. J Nucl Med. 2012;53:1716–1722. doi: 10.2967/jnumed.112.103861. [DOI] [PubMed] [Google Scholar]
- 23.Boellaard R. Need for standardization of 18F-FDG PET/CT for treatment response assessments. J Nucl Med. 2011;52:93S–100S. doi: 10.2967/jnumed.110.085662. [DOI] [PubMed] [Google Scholar]
- 24.Adams MC, Turkington TG, Wilson JM, Wong TZ. A systematic review of the factors affecting accuracy of SUV measurements. Am J Roentgenol. 2010;195:310–320. doi: 10.2214/AJR.10.4923. [DOI] [PubMed] [Google Scholar]
- 25.Büsing KA, Schönberg SO, Brade J, Wasser K. Impact of blood glucose, diabetes, insulin, and obesity on standardized uptake values in tumors and healthy organs on 18F-FDG PET/CT. Nucl Med Biol. 2013;40:206–213. doi: 10.1016/j.nucmedbio.2012.10.014. [DOI] [PubMed] [Google Scholar]
- 26.Ohtaka K, Hida Y, Kaga K, Okamoto S, Shiga T, Tamaki N, et al. Outcome analysis of (18)F-fluorodeoxyglucose positron-emission tomography in patients with lung cancer after partial volume correction. Anticancer Res. 2013;33:5193–5198. [PubMed] [Google Scholar]
- 27.Mountain CF. Revisions in the international system for staging lung cancer. Chest. 1997;111:1710–1717. doi: 10.1378/chest.111.6.1710. [DOI] [PubMed] [Google Scholar]
- 28.Cerfolio RJ, Bryant AS, Ojha B, Eloubeidi M. Improving the inaccuracies of clinical staging of patients with NSCLC: a prospective trial. Ann Thorac Surg. 2005;80:1207–1214. doi: 10.1016/j.athoracsur.2005.04.019. [DOI] [PubMed] [Google Scholar]
- 29.De Leyn P, Dooms C, Kuzdzal J, Lardinois D, Passlick B, Rami-Porta R, et al. Preoperative mediastinal lymph node staging for non-small cell lung cancer: 2014 update of the 2007 ESTS guidelines. Transl Lung Cancer Res. 2014;3:225–233. doi: 10.3978/j.issn.2218-6751.2014.08.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Douillard JY, Rosell R, De Lena M, Carpagnano F, Ramlau R, Gonzáles-Larriba JL, et al. Adjuvant vinorelbine plus cisplatin versus observation in patients with completely resected stage IB-IIIA non-small-cell lung cancer (adjuvant Navelbine international Trialist association [ANITA]): a randomised controlled trial. Lancet Oncol. 2006;7:719–727. doi: 10.1016/S1470-2045(06)70804-X. [DOI] [PubMed] [Google Scholar]
- 31.Winton T, Livingston R, Johnson D, Rigas J, Johnston M, Butts C, et al. Vinorelbine plus cisplatin vs. observation in resected non-small-cell lung cancer. N Engl J Med. 2005;352:2589–2597. doi: 10.1056/NEJMoa043623. [DOI] [PubMed] [Google Scholar]
- 32.Pisters KM, Evans WK, Azzoli CG, Kris MG, Smith CA, Desch CE, et al. Cancer Care Ontario and American Society of Clinical Oncology adjuvant chemotherapy and adjuvant radiation therapy for stages I-IIIA resectable non small-cell lung cancer guideline. J Clin Oncol. 2007;25:5506–5518. doi: 10.1200/JCO.2007.14.1226. [DOI] [PubMed] [Google Scholar]
- 33.Speicher PJ, Fitch ZW, Gulack BC, Yang CF, Tong BC, Harpole DH, et al. Induction chemotherapy is not superior to a surgery-first strategy for clinical N1 non-small cell lung cancer. Ann Thorac Surg. 2016;102:884–894. doi: 10.1016/j.athoracsur.2016.05.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gilligan D, Nicolson M, Smith I, Groen H, Dalesio O, Goldstraw P, et al. Preoperative chemotherapy in patients with resectable non-small cell lung cancer: results of the MRC LU22/NVALT 2/EORTC 08012 multicentre randomised trial and update of systematic review. Lancet. 2007;369:1929–1937. doi: 10.1016/S0140-6736(07)60714-4. [DOI] [PubMed] [Google Scholar]
- 35.Ettinger DS, Wood DE, Aggarwal C, Chang AC, Cheney RT, Chirieac LR, et al. National Comprehensive Cancer Network. Non-Small Cell Lung Cancer NCCN.org NCCN Evidence Blocks ® version 1. 2020. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Figure S1. Survival curves stratified according to various clinicopathological factors. a, b The overall survival (OS) and relapse-free survival (RFS) curves with 5-year survival rates according to female and male sex (5-year OS rate: 42.9% vs. 45.5%, log-rank P = 0.8516; 5-year RFS rate: 32.1% vs. 46.4%, log rank P = 0.5446). c, d The OS and RFS curves with 5-year survival rates according to adenocarcinoma and non-adenocarcinoma classification (5-year OS rate: 43.4% vs. 46.4%, log-rank P = 0.6415; 5-year RFS rate: 37.1% vs. 48.7%, log-rank P = 0.5027). e, f The OS and RFS curves with 5-year survival rates according to tumor diameters of ≥30 mm and < 30 mm (5-year OS rate: 47.3% vs. 38.9%, log-rank P = 0.7621; 5-year RFS rate: 44.6% vs. 37.5%, log-rank P = 0.871). g, h The OS and RFS curves with 5-year survival rates according to positive and negative expression of squamous cell carcinoma antigen (SCC; 5-year OS rate: 28.1% vs. 48.0%, log-rank P = 0.1254; 5-year RFS rate: 34.2% vs. 35.8%, log-rank P = 0.2338). i, j The OS and RFS curves and 5-year survival rates according to positive and negative expression of CYFRA (5-year OS rate: 49.2% vs. 43.0%, log-rank P = 0.7879; 5-year RFS rate: 50.4% vs. 28.7%, log-rank P = 0.5767). *: P < 0.05.
Data Availability Statement
The study protocol was registered at Researchregistry.com (#5246). The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.