Abstract
PURPOSE
Novel cancer immunotherapies, called immune checkpoint inhibitors, have demonstrated clinical efficacy in the treatment of squamous cell carcinomas of the head and neck. Tissue expression of programmed cell death 1 ligand 1 (PD-L1) and programmed cell death 1 ligand 2 (PD-L2) has been shown to predict tumor response to these drugs. We examine the expression of prognostic immune biomarkers, PD-L1 and PD-L2, in invasive ocular surface squamous neoplasia.
DESIGN
Retrospective case series.
METHODS
Eighteen cases of ocular surface or ocular adnexal invasive squamous cell carcinomas were identified in pathology case files of the Massachusetts General Hospital/Massachusetts Eye and Ear Infirmary and at the Wills Eye Hospital accessioned between January 1, 2014 and January 1, 2017. Immunohistochemical staining for PD-L1, PD-L2, CD8, and p16 was performed and graded in a standardized fashion.
RESULTS
PD-L1 and PD-L2 were expressed on tumor cells to varying degrees, and also on some stromal cells and endothelial cells. Stromal and endothelial cell expression was also seen in control conjunctival specimens. Tumor expression of PD-L1 and PD-L2 was present on the cell membranes. All 18 (100%) of the tumors expressed PD-L1: 7 (39%) expressed a high level, 3 (17%) expressed a medium level, and 8 (44%) expressed a low level. Only 9 (50%) tumors expressed PD-L2 and it was at a low level. The expression of PD-L1 in tumor cells correlated with the presence of CD8-positive cytotoxic T lymphocytes among tumor cells (P < .01) and with the presence of CD8-positive cells in the surrounding stroma (P = .04).
CONCLUSIONS
A subset of ocular invasive conjunctival squamous carcinomas express high levels of PD-L1 and CD8 and therefore may respond therapeutically to immune checkpoint inhibition.
INVASIVE CONJUNCTIVAL SQUAMOUS CELL CARCINOMA is rare among ocular surface squamous neoplasms.1–3 Despite its infrequency, in certain parts of the world invasive disease is responsible for considerable morbidity and occasional mortality. Improved treatment options for invasive disease are needed for advanced cases. Surgical excision and topical therapies that are used for superficial disease are often unsuccessful for invasive disease.
A class of antibody-based drugs called checkpoint inhibitors4 was introduced into the clinical sphere in the 2000s. Most checkpoint inhibitors target the interactions of the lymphocytic cell surface receptor PD-1 (programmed cell death protein 1) and its 2 ligands, which are present on tumor cells and/or immune cells: PD-L1 (programmed cell death 1 ligand 1) and PD-L2 (programmed cell death 1 ligand 2). Checkpoint inhibitors have had palpable success in the treatment of nonocular head and neck squamous cell carcinomas, and might be expected to be transferable to analogous ophthalmic neoplasia.5–9 While off-target ophthalmic side effects of these drugs have been reported,10 there is only 1 published investigation to date on their potential application in extraocular ophthalmic oncology—specifically, for ocular adnexal sebaceous carcinomas.11
The current study is designed to determine whether there is a role for checkpoint inhibitors in the treatment of conjunctival invasive squamous cell carcinomas. The specific objectives of this study were to (1) determine if PD-L1 and PD-L2 are expressed in invasive conjunctival squamous cell carcinomas; (2) determine if there is a correlation between the expression of PD-L1 and PD-L2 in these tumors; (3) assess whether there is a correlation between the expression of PD-L1 and PD-L2 and the presence of tumor-invading CD8-positive lymphocytes; and (4) evaluate whether the expression of PD-L1 or PD-L2 has any correlation with patient clinical characteristics or outcomes.
METHODS
THIS STUDY IS A RETROSPECTIVE CASE SERIES. IT WAS approved by the Institutional Review Board of the Massachusetts General Hospital (MGH) and Partners Healthcare (IRB #2014P000478) and is compliant with the Declaration of Helsinki and Health Insurance Portability and Accountability Act regulations. A search of the Massachusetts General Hospital, Massachusetts Eye and Ear Infirmary (MEEI), and Wills Eye Hospital’s pathology files using the terms “squamous cell carcinoma” and “conjunctiva” was performed for cases submitted between January 1, 2014 and January 1, 2017. Forty-seven cases were identified at MGH/MEEI and 84 cases identified in the Wills Eye Hospital files, resulting in a total of 131 cases. Pathology reports, existing histopathologic slides, and tissue blocks from the identified cases were retrieved and reviewed to establish the depth of invasion of the carcinoma. Recuts were performed to further clarify the depth of invasion. Cases that were diagnosed as in situ squamous cell carcinoma, or microinvasive squamous cell carcinoma, negative for squamous cell carcinoma, or that had insufficient remaining tissue in the block, were excluded from the study group. Patient clinical charts and clinical photographs were reviewed to ensure that tumors were of conjunctival origin and to glean data pertaining to patient treatment and follow-ups. Two cases that arose in the eyelid margin (Table 1, Patients 9 and 13) were included in this series, since the eyelid margin is a transition zone analogous to the corneal-conjunctival limbus. Two patients included in the current study have been the subjects of earlier case reports (Table 1, Patients 3 and 12); the current study, however, provides additional follow-ups for both.12,13 Eighteen cases of unequivocal conjunctival or eyelid margin invasive squamous cell carcinoma were ultimately selected for further investigations. Three cases of normal conjunctiva and 3 cases of inflamed conjunctiva were selected as controls.
TABLE 1.
Patient Clinical Characteristics and Tumor Histopathologic Characteristics and Scoring
| Pt | Sex/Agea/Race | Smoker | Other PMHx | Laterality/Location | # Prior Biopsies/Partial Excisions | Treatment | IFN Prior to Excisionb | HPV+c | Diff/Kerd | PD-L1: O/T/S/Ee | PD-L2: O/T/S/Ee | CD-8: O/T/Sf | Recurrence (Y/N)/Time to Recur | Follow-up |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F/61/W | Y | Asthma, HTN | L/Inf fornix | 2 | Excision, with margins to frozen section. Hughes flap, sentinel node bx. No cryo. | N | N | WD/+ | 3/4/3/1 | 2/0/2/1 | 3/1/3 | N | 11 mo |
| 2 | F/68/W | Y | DM, CKD, Gout, HTN, CVA, Bell’s Palsy, obese | R/Inf fornix | 2 | Excision, cryo, map biopsies. Postop IFN QID × 2 months | N | N | MD/+ | 2/2/2/1 | 2/0/1/0 | 2/0/2 | Y; 2 mo; repeat excision, cryo, margins to frozen sections, sentinel node bx; no further recurrence | 11 mo |
| 3 | F/82/A | N | HCV (untreated); cervical CA; HLD; HTN | R/Superior conj and cornea | 1 | Subtotal exenteration; margins to frozen section | N | N | PD/+ | 4/5/4/1 | 4/1/1/1 | 3/3/3 | Y; 7 mo; presented with headache, recurrent orbital mass, with bone erosion at the orbital apex and an enlarged parotid lymph node found; pt refuses further testing; died 2 months after recurrence with “fungating mass extending from orbit”; cause of death uncertain | 9 mo |
| 4 | M/62/W | Y | Bladder CA; pancreatic CA; | L/Diffuse inferior fornix, inferior palpebral conj and inferior bulbar conj | 0 | IFN QID × 2 mo without initial confirmatory biopsy | Y (1 mo) | N | WD/+ | 3/4/3/0 | 2/0/1/1 | 3/1/3 | Y; 2 mo; lateral canthal mass; biopsied; then IFN QID restarted × 1 mo; excision with cryotherapy, margins to frozen section; no further recurrence | 42 mo |
| 5 | M/49/W | Y | HTN; pheochromocytoma | R/superonasal palpebral and forniceal conj | 1 | Excision; cryo 2 months postop owing to in situ carcinoma on 1 margin from excision | Unknown | Y | MD/+ | 2/2/1/1 | 1/1/0/0 | 3/1/3 | Y; 54 mo; treated with eyelid wedge excision and map biopsies, followed by another excision 1 week later for positive margins; 4 subsequent excisions over subsequent years for carcinoma in situ, as well as multiple courses of interferon | 87 mo |
| 6 | M/58/W | N; but history of sun exposure | HTN; HLD | R/temporal limbus | 0 | Excision, cryo, EtOH, and corneal epithelial debridement | N | N | MD/+ | 1/2/1/0 | 1/0/1/0 | 1/0/2 | N; only 3 months of ophthalmic follow-up available, although patient alive at 36 mo and without recurrence per his nonophthalmic physicians | 3 mo |
| 7 | F/62/W | Y | Facial BCC; HLD | L/Inf fornix and inf bulbar | 0 | Excision and cryo; postop IFN QID ×2 months, stopped for 2 months, then resumed 2 more months | N | N | MD/+ | 4/5/3/0 | 2/1/2/1 | 3/1/3 | N | 18 mo |
| 8 | M/58/A | Y | Waldenstrom macroglobulinemia | L/Lower eyelid palpebral conj and fornix | Unknown | Excision, without cryo. | Y (1 mo) | N | MD/− | 2/2/2/1 | 1/1/1/0 | 2/1/2 | Y; 89 mo; inferior palpebral, bulbar, and forniceal conj; IFN QID ×1 mo without response, then excision, cryo and map biopsies. Margins positive, so treated with MMC 0.04% QID ×2 weeks. No recurrence since. | 116 mo |
| 9 | M/65/B | Y | Skull fracture; seizures; gastric CA; enucleation | R/eyelid margin and conj of anophthalmic socket (enucleated 40 years earlier for open globe injury) | 1 | Mohs surgery; 4 stages until margins cleared (whole lower eyelid, lateral canthus, all conjunctiva and most upper eyelid removed); first stage of reconstruction | N | N | MD/+ | 4/5/3/1 | 2/1/1/0 | 3/3/3 | N; postoperative radiation recommended, but pt refused; further reconstruction planned, but pt found to have advanced gastric CA and surgery not advised; underwent radiation for gastric CA, but refused chemo or surgery | 21 mo |
| 10 | F/71/W | Y | Breast CA; renal CA; ovarian CA; HTN; HLD; COPD | R/superior bulbar, forniceal and palpebral conjunctiva | 1 | IFN QID × 2 months to shrink tumor, then excision and cryo | Y (2 mo) | Y | MD/− | 5/5/3/1 | 2/1/1/0 | 3/3/3 | Y; 4 mo; nodule near lateral orbital rim, excised, positive for tumor, but with clear margins; 1 mo later 2 nodules above upper eyelid crease, biopsy positive for tumor; treated with 66 Gy photon radiation in 6 fractions with weekly carboplatin ×3 (last 3 doses not given owing to allergy); died 7 mo later from influenza | 16 mo |
| 11 | M/80/W | Y | None | R/superior and nasal limbal bulbar conjunctiva | 0 | IFN QID × 1 mo, then excision, cryo, EtOH and corneal epithelial debridement | Y (1 mo) | N | WD/− | 2/2/1/1 | 0/0/0/0 | 2/3/1 | Y; 28 mo; 2 locations on bulbar conjunctiva; re-excision recommended, but patient preferred IFN; IFN QID ×6 mo, both lesions resolved | 40 mo |
| 12 | M/51/W | Y | Enucleation | R/conj of anophthalmic socket (enucleate 38 years earlier for open globe injury); parotid mass | 1; also FNA of periparotid lymph node positive | Orbital exenteration, right parotidectomy and neck dissection | N | Y | MD/− | 3/3/3/1 | 1/1/0/1 | 1/1/1 | Unknown; postop, treated with 60 Gy photon radiation; last seen at cancer center 1 week postradiation. Followed-up with PCP 14 months postsurgery, nonhealing wounds and granulation tissue noted in orbit; referred to ophthalmology and oncology, but was lost to follow-up | 14 mo |
| 13 | M/48/W | Y | Quadriplegia and brain injury after MVA | R/eyelid margin | 0 | Wedge resection of eyelid margin, margins to frozen section | N | N | MD/+ | 3/5/2/1 | 2/1/2/1 | 3/3/2 | N | 17 mo |
| 14 | F/94/unk | Unknown | CABG, HTN, advanced dementia, pacemaker | L/lower eyelid palpebral conj | 0 | Incisional biopsy; tumor extends to deep and later margins–no further surgery; treatment with IFN drops started | N | N | WD/− | 2/2/3/1 | 3/1/2/1 | 2/0/2 | N/A; tumor incompletely excised and not yet fully treated | 1 mo |
| 15 | F/66/W | N; but exposed to secondhand smoke | Benign breast tumor | L/nasal bulbar conj | 0 | Excisional biospy, cryo, AMT and IFN injection 8 million units | N | N | MD/− | 3/3/3/1 | 2/0/1/0 | 2/0/2 | N | 6 mo |
| 16 | F/67/W | N | HLD, squamous CA on leg and arm | L/nasal limbus | 0 | Excisional biopsy, alcohol, and cryo | N | N | WD/+ | 2/2/2/0 | 0/0/0/0 | 1/0/2 | N | 16 mo |
| 17 | M/78/W | N | Coronary artery disease and stent | L/upper eyelid conj of anophthalmic socket (enucleated owing to old trauma) | 1 | Posterior lamella excision with AMT and IFN injection; postoperatively 3 additional IFN injections (monthly) | N | N | MD/+ | 2/2/2/1 | 2/0/1/1 | 2/1/3 | N | 3 mo |
| 18 | M/53/unk | unknown | Unknown | R/inferotemporal limbus | 0 | Excisional biopsy with cryo and AMT | N | N | MD/+ | 2/3/2/0 | 1/0/1/0 | 2/1/3 | N | 16 mo |
A = Asian; AMT = amniotic membrane transfer; B = black; BCC = basal cell carcinoma; Bx = biopsy; CA = cancer; CABG = coronary artery bypass graft; CKD = chronic kidney disease; COPD = chronic obstructive pulmonary disease; Conj = conjunctiva; Cryo = cryotherapy; CVA = cerebrovascular accident; Diff = differentiation; DM = diabetes mellitus; E = endothelial; EtOH = ethanol; FNA = fine needle aspiration; HCV = hepatitis C virus; HLD = hyperlipidemia; HPV+ = human papilloma virus-positive (by p16 immunostaining); HTN = hypertension; IFN = interferon alpha 2b; Inf = inferior; Ker = keratinization; L = left; MD = moderately differentiated; MMC = mitomycin C; MVA = motor vehicle accident; O = overall; PD = poorly differentiated; PD-L1 = programmed cell death 1 ligand 1; PD-L2 = programmed cell death 1 ligand 2; PMHx = past medical history; Postop = postoperative; Pt = patient; QID = 4× per day; R = right; S = stroma; T = tumor; unk = unknown; W = white; WD = well-differentiated.
Age at presentation.
Interferon treatment prior to tumor excision was assessed as its effects may have altered tumor expression patterns of PD-L1 or PD-L2.
HPV positivity was assessed by >70% of tumor cells staining positively for p16.
Differentiation was graded as well-differentiated (WD), moderately differentiated (MD), or poorly differentiated (PD); keratinization was graded as keratinizing (+) or nonkeratinizing (−).
PD-L1 and PD-L2 expression levels were graded on a 0–5 scale (see Methods section for more detail). The 0–5 scale was used to grade overall expression of the marker, and also expression in tumor cells alone, stromal cells alone, and endothelial cells alone.
CD8-positive T lymphocytes were graded on a scale of 0–3 (see Methods section for more detail). This scale was used to grade overall expression of the marker, as well as expression in tumor cells alone and stromal cells alone.
Five-micrometer paraffin sections were immunostained with the following antibodies: PD-L1 (rabbit monoclonal antibody clone E1L3N, 1:30 dilution; Cell Signaling Technology, Danvers, Massachusetts, USA), PD-L2 (rabbit monoclonal antibody clone D7U8C, 1:100 dilution; Cell Signaling Technology), CD8 (rabbit polyclonal antibody, #ab4055, 1:200 dilution; Abcam, Cambridge, Massachusetts, USA), and p16 (mouse monoclonal antibody clone E6H4, 1:4 dilution; MTM/Ventana Roche, Tucson, Arizona, USA) at the Massachusetts General Hospital’s Immunopathology Laboratory. Two pathologists (W.C.F. and F.A.J.) scored the immunostained slides independently and a third pathologist (N.W.) reviewed any case with a discordant score. Briefly, overall tissue expression of PD-L1, and separately of PD-L2, was graded on a 0–5 scale based on a previously described scale.14 Zero indicated no positive cells; 1 was used for rare individual positive cells or only a very small focus within or directly adjacent to tumor tissue; 2 signified infrequent small clusters of positive cells within or directly adjacent to tumor tissue; 3 indicated a single large cluster, multiple smaller clusters, or a moderately dense diffuse infiltration within or directly adjacent to tumor tissue; 4 indicated a single very large dense cluster, multiple large clusters, or dense diffuse infiltration; and 5 designated staining of coalescing clusters or dense infiltration throughout the tumor tissue.14 For each sample, an assessment was made whether the PD-L1 or PD-L2 immunostaining was present or absent in the tumor cells, stromal cells, or endothelial cells and this was also graded on a scale of 0–5 for each cellular compartment.
Intratumoral CD8 immunostaining was graded on an overall 0–3 scale, with 0 indicating no infiltration; 1 indicated low, 2 moderate, and 3 high levels of infiltration. A separate 0–3 score was given for CD8 staining in the stroma and CD8 staining in the tumor. For p16 immunostaining, positive results were those in which greater than 70% of tumor cells demonstrated a strong diffuse nuclear and cytoplasmic staining; other staining patterns, such as patchy or partial staining, were considered to be negative.
STATISTICS
Pearson and Spearman tests were used to assess for correlations between continuous groups. One-way ANOVA or a t test was performed to assess for differences between means of continuous and categorical variables. Fisher exact or χ2 tests were used to check for relationships between categorical variables. Statistica 13 (TIBCO Software Inc [2017], Statistica data analysis software system, version 13; http://statistica.io.) and GraphPad Prism7 software (La Jolla, California, USA) were used for statistical analyses. Although there are likely preexisting biologic relationships between tissue expression of PD-L1 and PD-L2 and CD8, a correction for this was not applied because the extent of this relationship is unknown and appears to vary greatly among different tissue and tumor types.
RESULTS
CLINICAL DEMOGRAPHIC DATA
Eighteen cases with invasive squamous cell carcinoma were identified. Ten patients (56%) were male and 8 (44%) were female (Table 1). Their median age at presentation was 63.5 years (range 48–94 years). Seven tumors involved the inferior fornix (Figure 1, Top left), 4 tumors the superior fornix, and 5 the bulbar conjunctiva near the limbus; 6 displayed a more diffuse involvement of bulbar and forniceal conjunctiva; and 7 involved either the superior or inferior palpebral conjunctiva or eyelid margin. Twelve patients had a history of cigarette smoking or smoke exposure. In 3 patients (Table 1, Patients 9, 12,13 and 17) malignancy arose in the conjunctiva of an anophthalmic socket (Figure 1, Top right); all 3 had had an enucleation for a remote history of ocular trauma. Only 1 patient (Table 1, Patient 12, who is the subject of a case report13) presented with regional metastases in a parotid lymph node.
FIGURE 1.
Clinical and radiographic findings in patients with invasive squamous cell carcinoma of the conjunctiva. (Top left) Invasive squamous cell carcinoma in the inferior fornix of Patient 1 (Table 1). (Top right) Invasive squamous cell carcinoma that originated in an anophthalmic socket in Patient 17 (Table 1). Three of 18 patients in our series had squamous cell carcinoma in an anophthalmic socket. (Middle left) Subcutaneous tumor recurrences near the eyelid crease (arrows) in Patient 10 (Table 1). (Middle right) Coronal computed tomography (CT) scan section demonstrating intraorbital tumor recurrence 7 months after a limited exenteration in Patient 3 (Table 1). (Bottom left) Axial CT scan sections from the same patient reveal destruction of the sphenoid bone by the tumor (arrow) and tumor involvement of the orbital apex. (Bottom right) The same patient also had evidence of periparotid lymph node metastases (arrow) in an axial CT scan section.
Seventeen patients underwent surgical excision as part of their initial definitive treatment, while 1 patient was initially treated with interferon drops alone. In those patients whose tumors were excised, 4 were treated prior to surgery with interferon alpha 2b (IFN) eye drops in order to shrink the mass. Intraoperatively, cryotherapy was used in 9 patients. Two patients had intraoperative IFN injections. Three patients’ tumors were treated postoperatively with IFN eye drops. Three patients underwent a sentinel lymph node biopsy or a neck dissection—2 at the time of initial treatment (Table 1, Patients 1 and 12), and 1 (Table 1, Patient 2) at the time of recurrence. The patient with metastases to a periparotid lymph node at initial presentation (Table 1, Patient 12) was treated postoperatively with radiotherapy.
Seven patients had a tumor recurrence. Four recurrences occurred within the first year after definitive treatment, while 3 manifested more than 2 years after initial treatment. In 1 patient (Figure 1, Middle left), the tumor recurred as local subcutaneous nodules and was managed with wide local excision and radiotherapy (Table 1, Patient 10). Another patient (Table 1, Patient 3) presented with local intraorbital tumor recurrence with extension to the orbital apex, sphenoid bone erosion, and a regional metastasis to a periparotid lymph node (Figure 2, Middle right, Bottom left, and Bottom right). She refused further medical intervention.
FIGURE 2.
Histopathologic and immunohistochemical features of invasive squamous cell carcinoma of the conjunctiva. (Top left) A well-differentiated, keratinized, invasive squamous cell carcinoma of the conjunctiva from Patient 1 (Table 1), whose tumor was in the inferior fornix. (Top right) A poorly differentiated, keratinizing, invasive squamous cell carcinoma of the conjunctiva from Patient 3 (Table 1), who developed local tumor recurrence and periparotid lymph node metastases 7 months after a limited exenteration. (Middle left) PD-L1 immunostaining, here graded as an overall 5 out of 5, and positive mostly in tumor cells, where it appears on cellular membranes. This tumor is from Patient 10 (Table 1), who had subcutaneous tumor recurrences after initial treatment. (Middle right) PD-L2 immunostaining, here graded as an overall 2 out of 5. Small clusters of positive cells are appreciated. This tumor is from Patient 1 (Table 1), whose tumor was from the inferior conjunctival fornix. (Bottom left) CD-8 immunostaining, here graded as an overall 3 out of 3. The CD8-positive T lymphocytes in this section are infiltrating the tumor, which is from Patient 3 (Table 1). (Bottom right) CD-8 immunostaining, here graded as an overall 1 out of 3. The CD8-positive T lymphocytes in this section are, again, infiltrating the tumor, but their numbers are very low. This tumor is from Patient 12 (Table 1). (For middle left, middle right, bottom left and bottom right, immunoperoxidase reaction, diaminobenzidine chromagen, hematoxylin counterstain, ×40.)
A weak relationship was found between patient age and use of preoperative IFN (Table 2). The mean age of patients treated preoperatively with IFN was 75 years, while the age of those who were not was 60. A weak relationship was also found between patient age and sex, where female patients tended to be older at presentation than male patients. Female patients were on average 71.4 years of age, while male patients were 60.2 years (Table 2). No relationships were otherwise found between history of smoke exposure, preoperative IFN use, tumor recurrence, anophthalmia, patient age, patient sex, or patient race (Supplemental Tables 1 and 2; Supplemental Material available at AJO.com).
TABLE 2.
Relationships Reaching Statistical Significance, by Degree of Significance
| Variable 1 | Variable 2 | Pearson Correlation (r Value; 95% CI; P Value) | Spearman Correlation (r Value; 95% CI; P Value) |
| PD-L1 overall | PD-L1 tumor | r = 0.8882; CI: 0.7198–0.9579; P < .0001 | r = 0.921; CI: 0.7911–0.9714; P < .0001 |
| PD-L1 overall | PD-L1 stroma | r = 0.7443; CI: 0.4252–0.8988; P = .0004 | r = 0.8033; CI: 0.5214–0.9247; P < .0001 |
| PD-L1 stroma | PD-L2 overall | r = 0.7373; CI: 0.4123–0.8958; P = .0005 | r = 0.7175; CI: 0.3639–0.8903; P = .0008 |
| PD-L1 tumor | CD8 overall | r = 0.7108; CI: 0.365–0.8842; P = .0009 | r = 0.7094; CI: 0.3495–0.8868; P = .0010 |
| CD8 overall | CD8 stroma | r = 0.6789; CI: 0.3104–0.87; P = .0019 | r = 0.7052; CI: 0.3421–0.885; P = .0011 |
| PD-L1 tumor | PD-L1 stroma | r = 0.6582; CI:0.2763–0.8606; P = .0030 | r = 0.6864; CI: 0.3096–0.8769; P = .0017 |
| PD-L1 overall | CD8 overall | r = 0.6448; CI: 0.2546–0.8544; P = .0039 | r = 0.6793; CI: 0.2975–0.8738; P = .0019 |
| PD-L1 tumor | CD8 tumor | r = 0.6442; CI: 0.2536–0.8542; P = .0039 | r = 0.6176; CI: 0.1975–0.8461; P = .0063 |
| PD-L2 overall | PD-L2 stroma | r = 0.6187; CI: 0.2135–0.8423; P = .0062 | r = 0.695; CI: 0.3245–0.8807; P = .0014 |
| PD-L1 overall | CD8 tumor | r = 0.6075; CI: 0.1964–0.837; P = .0075 | r = 0.5792; CI: 0.1393–0.8281; P = .0118 |
| CD8 overall | CD8 tumor | r = 0.5716; CI: 0.1429–0.8197; P = .0132 | r = 0.6228; CI: 0.2056–0.8484; P = .0058 |
| PD-L2 overall | PD-L2 endothelium | r = 0.5534; CI: 0.1167–0.8108; P = .0172 | r = 0.5595; CI: 0.1106–0.8188; P = .0158 |
| PD-L2 overall | CD8 overall | r = 0.5376; CI: 0.09444–0.8029; P = .0214 | r = 0.5505; CI: 0.09779–0.8145; P = .0179 |
| PD-L1 stroma | PD-L2 endothelium | r = 0.5264; CI: 0.079–0.7973; P = .0248 | r = 0.5189; CI: 0.05379–0.799; P = .0273 |
| PD-L1 tumor | PD-L2 overall | r = 0.5251; CI: 0.07718–0.7967; P = .0252 | r = 0.5143; CD: 0.04752–0.7967; P = .0290 |
| PD-L1 overall | PD-L2 tumor | r = 0.5055; CI: 0.05055–0.7867; P = .0324 | r = 0.4771 ; CI: −0.001774–0.778; P = .0453 |
| PD-L2 stroma | PD-L2 endothelium | r = 0.5031; CI: 0.04737–0.7855; P = .0333 | r = 0.5031; CI: 0.03244–0.7911; P = .0333 |
| PD-L1 overall | PD-L2 overall | r = 0.4964; CI:0.03849–0.7821; P = .0361 | r = 0.5424; CI: 0.08636–0.8105; P = .0200 |
| PD-L1 tumor | CD8 stroma | r = 0.4796; CI: 0.01636–0.7733; P = .0440 | r = 0.4853; CI: 0.008844–0.7822; P = .0412 |
| Categorical Variable | Continuous Variable | One-way ANOVA or t Test (Mean [SEM] of Group 1, n; Mean [SEM] of Group 2, n; P Value) | |
| IFN preop (yes/no) | Age | 75.17 (4.693), n = 6; 60.17 (2.793), n = 12; P = .0100 | |
| Differentiation (well/moderate/poor) | PD-L2 overall | 1.4 (0.6), n = 5; 1.583 (0.1486), n = 12; 4 (0), n = 1; P = .0321 | |
| Sex (F/M) | Age | 71.38 (3.964), n = 8; 60.2 (3.583), n = 10; P = .0531 | |
IFN preop = preoperative interferon.
Nonsignificant relationships are reported in the Supplemental Tables (Supplemental Materials available at AJO.com).
PATHOLOGIC FINDINGS
Most tumors were keratinizing (12/18, 67%) (Figure 2, Top left). Five tumors (28%) were well-differentiated (Figure 2, Top left), 12 (67%) were moderately differentiated, and 1 (6%) was poorly differentiated (Figure 2, Top right). Tumors in 3 patients (17%) had strong (greater than 70%) nuclear and cytoplasmic p16 positivity, suggestive of human papillomavirus (HPV) positivity (Table 1, Patients 5, 10, 12). These 3 tumors were moderately differentiated, and 2 of the 3 were nonkeratinizing. Two of the HPV-positive tumors had recurrences (Table 1, Patients 5 and 10), while the third presented with periparotid metastases (Table 1, Patient 12).
PD-L1 and PD-L2 expression on tumor cells, stromal cells, and endothelial cells was assessed. The presence of CD8-positive T lymphocytes in the tumor alone and stroma was also assessed. Tumors displayed a membranous PD-L1 and PD-L2 staining pattern with variable expression levels of PD-L1 and PD-L2 (Figure 2, Middle left and Middle right). In most samples, positively staining stromal cells were present in addition to positive tumor cells; however, there were typically fewer positive stromal than tumor cells (Table 1). Occasional positive vascular endothelial cells were seen in most cases; however, this staining was typically limited to a few sparse cells. All tissues had CD8-positive T lymphocytes, although the lymphocytic infiltrate was more prominent in some tumors than others (Figure 2, Bottom left and Bottom right).
Looking at PD-L1 and PD-L2 expression by cell type, the tumor cells in all 18 cases (100%) expressed PD-L1 (Figure 2, Middle left), while the tumor cells in 9 cases (50%) expressed PD-L2 (Figure 2, Middle right). The extent of PD-L1 expression in tumor cells (mean 3.2 out of 5, range 2–5) was much higher than PD-L2 expression (mean 0.5 out of 5, range 0–1). Seven out of 18 samples (39%) had high (4 or 5 out of 5) PD-L1 expression in tumor cells, 3 out of 18 (17%) had medium (3 out of 5) PD-L1 expression in tumor cells, while 8 out of 18 (44%) had low (1 or 2 out of 5) expression of PD-L1 in tumor cells (Table 1). Tumor cells that did express PD-L2 typically had low (1 out of 5) PD-L2 expression (Table 1).
PD-L1 expression was present in stromal cells in all cases, and in rare endothelial cells in 13 of 18 (72%) cases. PD-L1 stromal and endothelial expression was typically lower than expression in the tumor cells (mean 2.4 out of 5 for stroma, 0.7 out of 5 for endothelial cells, and 3.2 out of 5 for tumor cells). PD-L2 was expressed in the stroma of 14 out of 18 cases (78%), and in the endothelial cells of 8 out of 18 cases (44%). PD-L2 expression was higher in stromal cells than in tumor cells. PD-L2 expression was minimal in endothelial cells (Table 1).
Cytotoxic CD8-positive T lymphocytes were present in all tissues; in 8 tumors (44%) there was an overall high degree (3 out of 3) of CD8 T-lymphocytic infiltration (Figure 2, Bottom left), in 7 (39%) it was moderate (2 out of 3), and in 3 (17%) it was low (1 out of 3) (Figure 2, Bottom right). Cytotoxic T lymphocytes were present and percolated among the tumor cells in 13 out of 18 cases (72%); in all cases they were present in the surrounding stroma.
Control sections of uninflamed normal conjunctiva and conjunctiva with chronic conjunctivitis or primary lymphoid follicles also revealed scattered rare stromal and endothelial cell PD-L1 and PD-L2 positivity, with overall greater PD-L1 positivity than PD-L2 positivity (Supplemental Figure; Supplemental Material available at AJO.com). Rare, basal conjunctival epithelial cells showed positive membranous staining with PD-L1 and PD-L2; weak, nonspecific and nonmembranous staining was occasionally seen in the epithelium. CD8-positive lymphocytes were present immediately beneath the conjunctival epithelium, with greater numbers in the inflamed specimens than the uninflamed ones (Supplemental Figure).
Relationships between overall tissue expression, tumor cell expression, stromal expression, and endothelial cell expression of PD-L1 and PD-L2 were assessed with respect to patient characteristics, tumor p16 positivity, CD8-positive lymphocyte infiltration, tumor differentiation and keratinization, tumor treatment, and patient outcomes.
A weak relationship was found between tumor differentiation and overall PD-L2 expression (Table 2). No other relationships were found between tumor differentiation, keratinization, PD-L1 expression, PD-L2 expression, or CD8 expression and patient clinical characteristic including age, sex, race, exposure to smoke, anophthalmia, preoperative IFN use, and tumor recurrence (Supplemental Tables 1, 2, 3, and 4; Supplemental Material available at AJO.com). Significant correlations were identified between PD-L1 expression in tumor cells and CD8 expression overall (Table 2). Caution must be exercised in interpreting the identified relationships and correlations, as the study is limited by the small number of patients.
DISCUSSION
INVASIVE OCULAR SURFACE SQUAMOUS CELL CARCINOMA afflicts only a small minority (in most series fewer than 10%) of patients with ocular surface squamous neoplasia in developed countries.1,15,16 The current investigation focuses exclusively on a subset of patients with invasive disease, as treatment options for them are more limited. The major goal of this study was to evaluate whether there is a biologic basis for the use of the novel class of immunotherapeutic drugs called checkpoint inhibitors for treatment of invasive conjunctival squamous cell carcinoma, as this has not been previously investigated.
Although admittedly limited by the number of patients in the study, the current investigation answers certain fundamental questions that could lay the foundation for future research. First, this study demonstrates that PD-L1 and PD-L2 are expressed in invasive conjunctival squamous cell carcinomas. Second, this study reveals that there are gradations in PD-L1 and PD-L2 expression, which may be relevant to clinical response of tumors to treatment. Third, the study reports that PD-L1 is expressed to a greater degree than PD-L2 in invasive conjunctival squamous cell carcinomas. Fourth, correlations were found between expression levels of PD-L1, PD-L2, and the presence of CD8-positive T lymphocytes and invasive conjunctival squamous cell carcinomas. These findings suggest that invasive ocular squamous cell carcinomas may respond to PD-1- or PD-L1-blocking checkpoint inhibitor drugs, meriting further investigation.
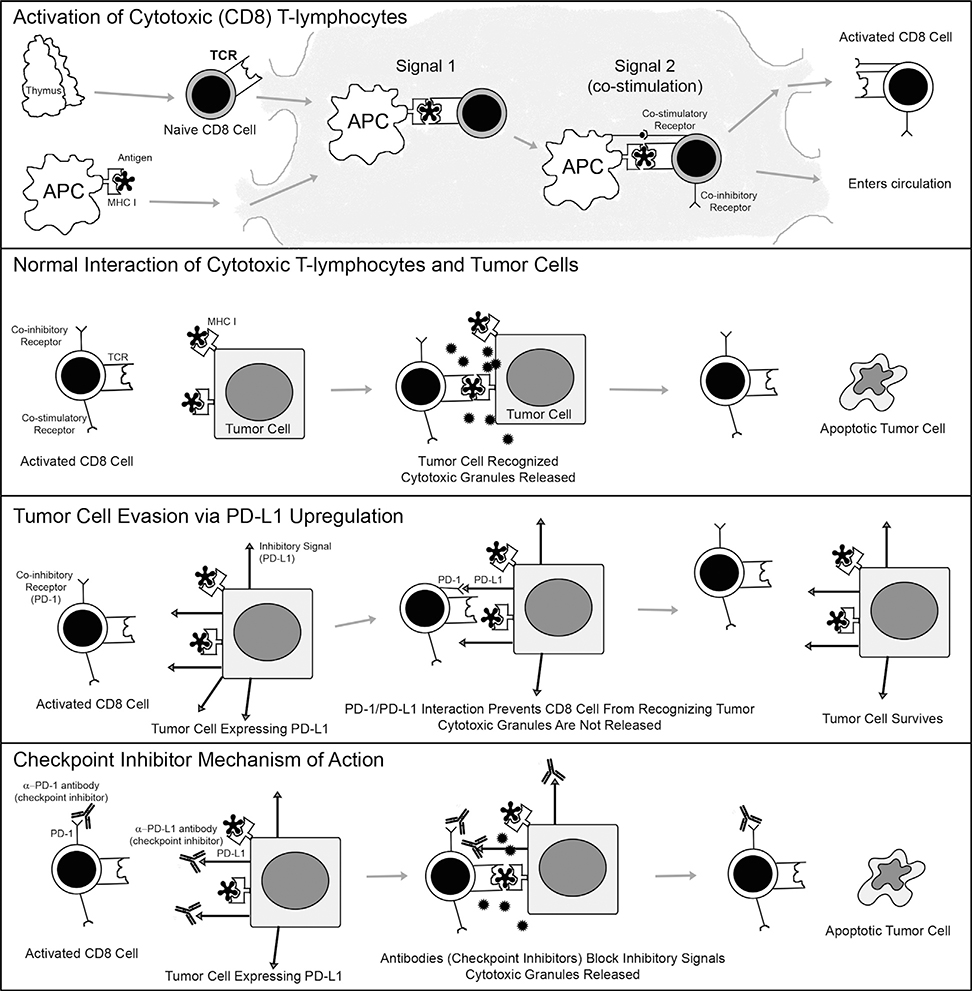
Checkpoint inhibitor drugs are a novel class of drugs being employed in oncology (Figure 3).17,18 Currently available checkpoint inhibitor drugs are monoclonal antibodies that target cell surface receptors and ligands, including PD-1 and PD-L1. Pembrolizumab and nivolumab bind to PD-16,18; atezolizumab, avelumab, and durvalumab block PD-L1.6,18 Several clinical trials have demonstrated that checkpoint inhibitors extend overall survival in patients with recurrent or metastatic head and neck squamous cell carcinoma.7,8,19,20 Pembrolizumab and nivolumab are approved by the US Food and Drug Administration for use in advanced head and neck squamous cell carcinoma.17 Moreover, expression of PD-L1 within the tumor microenvironment has been shown to predict tumor response to checkpoint inhibitor therapy.6,17,18
FIGURE 3.
Mechanism of action of the normal PD-1/PD-L1 system, its dysregulation during neoplasia, and its normalization with checkpoint inhibitor drugs. (Top panel) This panel illustrates how normal cytotoxic T lymphocytes become activate. Naïve (unactivated) CD8-positive T lymphocytes expressing T-cell receptors (TCR) on their surfaces exist the thymus and migrate to lymph nodes for activation. Antigen presenting cells (APCs) scavenge antigens throughout the body. They engulf both foreign and endogenous antigens, process them into smaller pieces, and then load them onto major histocompatibility complex class I (MHC I) proteins so that the antigens may be optimally displayed on their surfaces. APCs migrate to lymph nodes. In the lymph nodes, naïve T cells may recognize antigens presented to them by APCs; the TCR interacts with the antigen and MHC I. This type of interaction is known as “signal 1”; a second signal, however, in the form of co-stimulatory signals is needed in order for the naïve T lymphocyte to become activated; without it it will become anergic. Once a co-stimulatory interaction (“signal 2”) is provided, the T lymphocyte is activated. Soon after activation it upregulates co-inhibitory receptors (such as CTLA-4 and PD-1). These co-inhibitory receptors are intended as sites where the activation can be dampened so that autoimmunity is avoided. Activated CD8 lymphocytes, expressing co-inhibitory receptors such as PD-1, then multiply and exit the lymph node into the circulation, in search of cells expressing their target antigens. (Second panel) This panel illustrates the normal action of CD8 T lymphocytes against tumor cells. Activated CD8 T lymphocytes use their TCRs to recognize tumor cells that express MHC I with target antigens on their surfaces. The interaction of the TCR with the MHC I and antigen reads to release of cytotoxic granules from CD8 T lymphocytes, which trigger apoptosis in the tumor cell. The tumor cell is killed, while the CD8 T lymphocyte remains intact and ready to find another target cell. (Third panel) This panel illustrates how tumor cells evade CD8 T lymphocytes by usurping the normal checkpoint PD-1/PD-L1 system. Some tumor cells upregulate inhibitory ligands such as PD-L1 on their surfaces. When an activated T lymphocyte attempts to bind to a tumor cell that expresses the target MHC I and antigen on its surface, PD-L1 interacts with the inhibitory receptor PD-1 on the surface of the T lymphocyte. This PD-1/PD-L1 interaction is called a “checkpoint,” and it prevents the T cells from releasing its cytotoxic granules and from killing the tumor cell. The tumor cell survives, while often the T lymphocyte becomes anergic. (Bottom panel) This panel illustrates how checkpoint inhibitor drugs work to allow CD8 T lymphocytes to find and kill tumor cells, even when they express PD-L1 on their surfaces. Checkpoint inhibitors are monoclonal antibodies that bind to either PD-1 receptors on the surfaces of T lymphocytes, or to PD-L1 on the surfaces of tumor cells. Either drug leads to disruption of the PD-1/PD-L1 interaction, interfering with the inhibitory signal, and thereby allowing the CD8 T lymphocyte to recognize the tumor cell. The CD8 T lymphocyte is able to release cytotoxic granules and once again trigger apoptosis of the targeted tumor cell, as originally intended.
Grading systems and scoring systems of PD-L1 expression across the literature are not uniform.21 Similarly, at least 5 commercially available antibody clones are available for PD-L1 testing.22–24 It is unclear for invasive conjunctival squamous cell carcinomas where to draw the cutoff line for positivity and what level of expression is clinically relevant in assessing response to a PD-L1 inhibitor. With this in mind, a scoring system with gradations rather than a discrete cutoff was selected for the current study and modeled after one that has been recently employed by others, and a well-accepted antibody clone was selected.14,23
A further complicating factor in understanding the relationships and significance of PD-1, PD-L1, and PD-L2 expression is that these markers may be expressed not only on tumor cells, but also on stromal cells. The significance of stromal PD-L1 or PD-L2 expression has not been fully elucidated, although some studies have found that stromal expression of these markers may be predictive of patient survival or response to treatment.25,26 Owing to the growing awareness of tumor microenvironments and importance of the location of the expression of various markers, the expression of markers in the current study was subdivided into tumor, stromal, or endothelial expression.
In the current investigation, we report that PD-L1 and PD-L2 were expressed in tumor cells, stromal cells, and endothelial cells (Table 1). PD-L1 and PD-L2 were also expressed in the stromal cells and endothelial cells of normal and inflamed conjunctiva (Supplemental Figure). Thus, in assessing PD-L1 or PD-L2 expression in invasive conjunctival squamous cell carcinoma, the extent of membranous PD-L1 or PD-L2 tumor expression is likely be a more critical factor than stromal or endothelial expression. Although both PD-L1 and PD-L2 were expressed in tumor cells, PD-L1 was expressed in tumor cells to a much greater extent than PD-L2. Moreover, the proportion of positive tumor cells was much higher for PD-L1 than PD-L2. Perhaps the lower expression of PD-L2 may reflect a lesser role for PD-L2 in invasive conjunctival squamous cell carcinomas. For clinical screening purposes, immunostaining for PD-L1 appears to be more promising in conjunctival squamous cell carcinomas than PD-L2. The degree of PD-L1 expression also varied widely: 39% of patients had a high level of PD-L1 expression, 17% had a medium level and 44% had a low level of PD-L1 tumor expression. It is hypothesized that the subset of patients with tumors expressing greater levels of PD-L1 may respond better to checkpoint inhibitors than those expressing low levels.
The presence of an inflammatory infiltrate composed of CD8-positive T lymphocytes correlates with improved outcomes in head and neck squamous cell carcinomas. This phenomenon may develop because a greater number of CD8 lymphocytes in the local inflammatory infiltrate are available to combat the tumor.6,19 Moreover, a more pronounced CD8 lymphocytic infiltrate in carcinomas of the head and neck has often been found in HPV-positive tumors, which collectively tend to have a better prognosis than HPV-negative tumors.19,27 The expression of checkpoint inhibitory receptors (such as PD-1) is often increased with lymphocytic infiltration of the tumor,19 implying that treatment with a checkpoint inhibitory drug may be more effective in tumors with a more pronounced lymphocytic infiltrate. Although there are many interactions between CD8-positive T lymphocytes and PD-L1-expressing tumor cells, the presence of CD8-positive T lymphocytes has been found to be an independent prognostic factor in certain studies when analyzed with respect to PD-L1.28,29
In the current study, all studied tissues had CD8-positive T lymphocytes. Furthermore, 15 out of 18 tumors in our series had a moderate (2 out of 3) or high (3 out of 3) number of infiltrating CD8-positive T lymphocytes. This finding supplies a strong impetus to introduce a checkpoint inhibitor that could effectively unleash the destructive action of the T lymphocytes on the tumo cells. Both of our patients with parotid lymph node metastases (Table 1, Patients 3 and 12), and the patient with a local subcutaneous tumor recurrence (Table 1, Patient 10), expressed high levels of PD-L1 and had CD8-positive T lymphocytes. They exemplify the kind of cases that could potentially benefit from treatment with a checkpoint inhibitor.
Correlations in the current investigation were identified between expression of PD-L1 in tumor cells and presence of CD8-positive T lymphocytes among the tumor cells and also with CD8-positive T lymphocytes in the surrounding stroma (Table 2). Although our data are limited in regard to drawing firm conclusions, they do suggest that certain conjunctival squamous cell carcinomas with high PD-L1 levels and many CD8 T lymphocytes may be well suited for treatment with checkpoint inhibitors.
HPV tumor positivity has been associated with a better prognosis in head and neck squamous cell cancers; also associated is an increased number of tumor-infiltrating lymphocytes.27 p16 positivity is often used as a marker of HPV infection. In head and neck squamous cell carcinomas treated with checkpoint inhibitors, tumors responded to treatment regardless of p16 expression; however, a greater proportion of p16-positive tumors appeared to respond to therapy than the p16-negative tumors.8,19 Comparable studies have revealed variable HPV positivity in ocular surface squamous neoplasia.30 In contrast, a recent study of 43 cases of ocular surface squamous neoplasias that screened tumors with p16 immunostaining, and confirmed HPV positivity with DNA in situ hybridization, reported a 30% HPV positivity rate.27 In the current cohort, only 3 out of 18 patients (17%) were HPV positive by means of p16 immunostaining (Table 1, Patients 5, 10, 12). This difference in rates of HPV positivity may reflect the selection of a much more aggressive cohort of squamous cell carcinomas in the current study. One of the 3 patients presented with parotid lymph node metastases, while a second patient had recurrent subcutaneous tumor nodules. In these 2 patients, with either a tumor recurrence or a metastasis and concomitant PD-L1 and p16 positivity, an argument can be made for a role for a checkpoint inhibitor if conventional approaches are not sufficient.
Certain concerns should be addressed if one is considering the use of checkpoint inhibitors for advanced ocular surface squamous neoplasia (OSSN) of the conjunctiva. One is whether checkpoint inhibitors are redundant with the already existent therapies for OSSN. A second pertains to ocular checkpoint inhibitor toxicity. Current treatment for OSSN consists of surgery combined with cryotherapy and/or topical chemotherapy.2,15 Enucleation, exenteration, and radiotherapy are to be resorted to only for advanced disease.1 Three topical chemotherapeutic agents, 5-fluorouracil (5-FU), mitomycin C (MMC), and IFN, are employed; however, IFN has grown in popularity and surpassed the use of 5-FU and MMC owing to its clinical efficacy and lower incidence of side effects.15 IFN is a signaling protein that recruits lymphocytes to the site of application.31 IFN furthermore stimulates tumor and normal tissue cells at the site of application to upregulate the expression of antigen-displaying major histocompatibility complex class I (MHC I) proteins on their surfaces. This allows lymphocytes to more readily recognize tumor cells.31 As an amplifying therapeutic effect, IFN improves the ability of natural killer (NK) T lymphocytes to eliminate target cells.31 Some ophthalmologists treat OSSN primarily with IFN, while others use it as a preoperative or postoperative adjuvant to surgical excision. In the present series, 9 patients were treated with IFN at some point during their treatment course. Although checkpoint inhibitors like IFN modulate the immune system, they act on a separate part of the immune system. Their mechanism of action is not redundant with that of IFN but instead may be synergistic.4,31,32
Ophthalmic side effects occur in approximately 1% of patients treated with checkpoint inhibitors.10 Patients may develop dry eye, uveitis, keratitis, orbital inflammation, optic neuropathy, myasthenia gravis, and cranial nerve palsies. The most common side effects are uveitis and dry eye.10 Many ophthalmic side effects can be medically managed while the patient continues on the systemic checkpoint inhibitor; in some cases, however, cessation of the checkpoint inhibitor may be required. The profile of ophthalmic side effects has been deemed to be acceptable in patients faced with a recurrent invasive carcinoma. Although checkpoint inhibitors have not been used to date for invasive squamous cell carcinoma of the conjunctiva, they have been used in select cases of cutaneous melanoma metastatic to the orbit and invasive conjunctival melanoma, with promising results.10,33 Treatment of uveal melanoma with checkpoint inhibitors, on the other hand, has been disappointing, possibly because metastatic uveal melanoma, unlike cutaneous melanoma, lacks PD-L1 expression.34
In conclusion, invasive conjunctival squamous cell carcinoma with possible metastases is rare in Western countries but not so rare in underdeveloped regions. The detection of a high prevalence of PD-L1 expression in conjunctival invasive squamous cell carcinomas in our series leads to the proposal that checkpoint inhibitors targeting PD-1 or PD-L1 may benefit such patients who have failed other treatments. One should not lose sight of a significant difference between the therapeutic endpoints for head and neck vs those for ocular surface squamous carcinoma. In the former, the prolongation of survival and especially the prevention of fatalities are the desiderata. In ocular invasive squamous cell carcinoma, the prevention of local recurrences, the preservation of vision, and the salvaging of the globe are the endpoints. Regional metastases occur in a distinct minority of cases and death occurs extremely rarely. It is against this background that a role for checkpoint inhibitors will ultimately have to be further investigated and validated.
Supplementary Material
Supplemental Figure. Expression of immunohistochemical markers PD-L1, PD-L2, and CD8 in normal and inflamed conjunctiva. (Top left) Uninflamed normal conjunctiva with non-keratinizing squamous epithelium (Ep). (Top right) Lymphocytic infiltrate (L) of conjunctival epithelium (Ep) containing goblet cells. (Middle left) PD-L1 is not expressed in the epithelium (Ep) of the inflamed conjunctiva. There is thin artifactual surface staining. The arrows point to the epithelial basement membrane region. (Middle right) Left panel: PD-L1 weakly, nonspecifically (nonmembranously) stains the epithelium (Ep) overlying stromal chronic inflammation. H and circles indicate positively staining histiocytes. Right panel: CD8-positive T lymphocytes are abundantly present in the chronic subepithelial infiltrate. (Bottom left) PD-L2 is not expressed in the conjunctival epithelium (Ep) or in the subepithelial lymphocytes (L). (Bottom right) Focally, PD-L2 is very faintly and nonspecifically (nonmembranously) observed in the epithelium (Ep) but more definitively stains the histiocytes (H and circles) within the subepithelial lymphocytic infiltrate.
Acknowledgments
FUNDING/SUPPORT: FUNDING FOR THIS WORK WAS PROVIDED BY THE MASSACHUSETTS GENERAL HOSPITAL DEPARTMENT OF Pathology Austin L. Vickery, Jr Award to William C. Faquin, Natalie Wolkow, Sara I. Pai, and Frederick A. Jakobiec. Frederick A. Jakobiec’s work was also supported by Mass Eye and Ear Departmental Funding. Financial Disclosures: The following authors have no financial disclosures: Natalie Wolkow, Frederick A. Jakobiec, Amir H. Afrogheh, Ralph C. Eagle, Sara I. Pai, and William C. Faquin. All authors attest that they meet the current ICMJE criteria for authorship.
Other Acknowledgments: The authors thank Martin Kidd, PhD, at the Centre for Statistical Consultation, Department of Statistics and Actuarial Sciences, University of Stellenbosch, Stellenbosch, South Africa, for assistance with statistical analyses; Marybeth Cunnane, MD, from the Massachusetts Eye and Ear Infirmary Department of Radiology, for discussions of the radiographic findings; and Lina Ma, MD, from the Massachusetts Eye and Ear Infirmary Department of Eye Pathology, for taking photographs and assembling the image for the Supplemental Figure.
Footnotes
Supplemental Materials available at AJO.com.
Contributor Information
NATALIE WOLKOW, David G. Cogan Ophthalmic Pathology Laboratory, Department of Ophthalmology, Massachusetts Eye and Ear Infirmary, Harvard Medical School, Boston, Massachusetts, USA; Division of Ophthalmic Plastic and Reconstructive Surgery, Department of Ophthalmology, Massachusetts Eye and Ear Infirmary, Harvard Medical School, Boston, Massachusetts, USA.
FREDERICK A. JAKOBIEC, David G. Cogan Ophthalmic Pathology Laboratory, Department of Ophthalmology, Massachusetts Eye and Ear Infirmary, Harvard Medical School, Boston, Massachusetts, USA
AMIR H. AFROGHEH, Department of Oral and Maxillofacial Pathology, National Health Laboratory Service, University of the Western Cape, Cape Town, South Africa
RALPH C. EAGLE, JR, Department of Ophthalmic Pathology, Wills Eye Hospital, Philadelphia, Pennsylvania, USA.
SARA I. PAI, Division of Surgical Oncology, Department of Surgery, Massachusetts General Hospital, Harvard Medical School, Boston, Massachusetts, USA
WILLIAM C. FAQUIN, Division of Head and Neck Pathology, Department of Pathology, Massachusetts General Hospital, Harvard Medical School, Boston, Massachusetts, USA
REFERENCES
- 1.Arepalli S, Kaliki S, Shields CL, Emrich J, Komarnicky L, Shields JA. Plaque radiotherapy in the management of scleral-invasive conjunctival squamous cell carcinoma: an analysis of 15 eyes. JAMA Ophthalmol 2014;132(6):691–696. [DOI] [PubMed] [Google Scholar]
- 2.Harissi-Dagher M, Colby K. Chapter 58: Tumors of the cornea and conjunctiva In: Albert DM, Miller JW, Azar DT, eds. Albert & Jakobiec’s Principles and Practice of Ophthalmology. 3rd ed. Philadelphia: Saunders/Elsevier; 2008:792–796. [Google Scholar]
- 3.Zimmerman LE. Chapter 2: Squamous cell carcinoma and the related lesions of the bulbar conjunctiva In: Boniuk M, ed. Ocular and Adnexal Tumors: New and Controversial Aspects. Saint Louis, MO: The C. V. Mosby Company; 1964:49–74. [Google Scholar]
- 4.Abbas AK, Lichtman AH, Pillai S. Chapter 18: Immunity to tumors. In: Abbas AK, Lichtman AH, Pillai S, Baker DL, Baker A, eds. Cellular and Molecular Immunology. Ninth ed. Philadelphia, PA: Elsevier; 2018:397–416. [Google Scholar]
- 5.Outh-Gauer S, Alt M, Le Tourneau C, et al. Immunotherapy in head and neck cancers: a new challenge for immunologists, pathologists and clinicians. Cancer Treat Rev 2018;65:54–64. [DOI] [PubMed] [Google Scholar]
- 6.Pai SI, Zandberg DP, Strome SE. The role of antagonists of the PD-1:PD-L1/PD-L2 axis in head and neck cancer treatment. Oral Oncol 2016;61:152–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saleh K, Eid R, Haddad FG, Khalife-Saleh N, Kourie HR. New developments in the management of head and neck cancer - impact of pembrolizumab. Ther Clin Risk Manag 2018;14: 295–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferris RL, Blumenschein G Jr, Fayette J, et al. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med 2016;375(19):1856–1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seiwert TY, Burtness B, Mehra R, et al. Safety and clinical activity of pembrolizumab for treatment of recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-012): an open-label, multicentre, phase 1b trial. Lancet Oncol 2016;17(7):956–965. [DOI] [PubMed] [Google Scholar]
- 10.Dalvin LA, Shields CL, Orloff M, Sato T, Shields JA. Checkpoint inhibitor immune therapy: systemic indications and ophthalmic side effects. Retina 2018;38(6):1063–1078. [DOI] [PubMed] [Google Scholar]
- 11.Xu S, Yu H, Fu G, Fan X, Jia R. Programmed death receptor ligand 1 expression in eyelid sebaceous carcinoma: a consecutive case series of 41 patients. Acta Ophthalmol 2018; 10.1111/aos.13833 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 12.Choi CJ, Jakobiec FA, Zakka FR, Sanchez AV, Lee NG. Ocular surface squamous neoplasia in a patient with hepatitis C. JAMA Ophthalmol 2017;135(10):1121–1123. [DOI] [PubMed] [Google Scholar]
- 13.Gaier ED, Jakobiec FA, Stagner AM, Emerick K, Yoon MK. PV16-positive invasive conjunctival squamous cell carcinoma in an anophthalmic socket. Ophthalmic Plast Reconstr Surg 2017;33(3S Suppl 1):S2–S4. [DOI] [PubMed] [Google Scholar]
- 14.Yearley JH, Gibson C, Yu N, et al. PD-L2 expression in human tumors: relevance to anti-PD-1 therapy in cancer. Clin Cancer Res 2017;23(12):3158–3167. [DOI] [PubMed] [Google Scholar]
- 15.Sayed-Ahmed IO, Palioura S, Galor A, Karp CL. Diagnosis and medical management of ocular surface squamous neoplasia. Expert Rev Ophthalmol 2017;12(1):11–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shields CL, Chien JL, Surakiatchanukul T, Sioufi K, Lally SE, Shields JA. Conjunctival tumors: review of clinical features, risks, biomarkers, and outcomes–The 2017 J. Donald M. Gass Lecture. Asia Pac J Ophthalmol (Phila) 2017;6(2): 109–120. [DOI] [PubMed] [Google Scholar]
- 17.Pai SI, Faquin WC. Programmed cell death ligand 1 as a biomarker in head and neck cancer. Cancer Cytopathol 2017;125(7):529–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sun C, Mezzadra R, Schumacher TN. Regulation and function of the PD-L1 checkpoint. Immunity 2018;48(3): 434–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rothschild U, Muller L, Lechner A, et al. Immunotherapy in head and neck cancer - scientific rationale, current treatment options and future directions. Swiss Med Wkly 2018;148: w14625. [DOI] [PubMed] [Google Scholar]
- 20.Harrington KJ, Ferris RL, Blumenschein G Jr, et al. Nivolumab versus standard, single-agent therapy of investigator’s choice in recurrent or metastatic squamous cell carcinoma of the head and neck (CheckMate 141): health-related quality-of-life results from a randomised, phase 3 trial. Lancet Oncol 2017;18(8):1104–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu W, Chen S, Yang W. The diverse cutoff of PD-L1 positivity and negativity in studies regarding head and neck squamous cell carcinoma. Oral Oncol 2018;87:199–200. [DOI] [PubMed] [Google Scholar]
- 22.Hodgson A, Slodkowska E, Jungbluth A, et al. PD-L1 immunohistochemistry assay concordance in urothelial carcinoma of the bladder and hypopharyngeal squamous cell carcinoma. Am J Surg Pathol 2018;42(8):1059–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rimm D, Han G, Taube JM, et al. ORAL01.01: a prospective, multi-institutional assessment of four assays for PD-L1 expression in NSCLC by immunohistochemistry: topic: pathology. J Thorac Oncol 2016;11(11S):S249. [Google Scholar]
- 24.Soo RA, Yun Lim JS, Asuncion BR, et al. Determinants of variability of five programmed death ligand-1 immunohistochemistry assays in non-small cell lung cancer samples. Oncotarget 2018;9(6):6841–6851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li X, Wetherilt CS, Krishnamurti U, et al. Stromal PD-L1 expression is associated with better disease-free survival in triple-negative breast cancer. Am J Clin Pathol 2016;146(4):496–502. [DOI] [PubMed] [Google Scholar]
- 26.Wyss J, Dislich B, Koelzer VH, et al. Stromal PD-1/PD-L1 expression predicts outcome in colon cancer patients. Clin Colorectal Cancer 2018; 10.1016/j.clcc.2018.09.007. [DOI] [PubMed] [Google Scholar]
- 27.Afrogheh AH, Jakobiec FA, Hammon R, et al. Evaluation for high-risk HPV in squamous cell carcinomas and precursor lesions arising in the conjunctiva and lacrimal sac. Am J Surg Pathol 2016;40(4):519–528. [DOI] [PubMed] [Google Scholar]
- 28.De Meulenaere A, Vermassen T, Aspeslagh S, et al. Tumor PD-L1 status and CD8(+) tumor-infiltrating T cells: markers of improved prognosis in oropharyngeal cancer. Oncotarget 2017;8(46):80443–80452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang H, Li Z, Dong B, et al. Prognostic significance of PD-L1 expression and CD8+ T cell infiltration in pulmonary neuroendocrine tumors. Diagn Pathol 2018;13(1):30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hanbazazh M, Gyure KA. Ocular human papillomavirus infections. Arch Pathol Lab Med 2018;142(6):706–710. [DOI] [PubMed] [Google Scholar]
- 31.Murphy K, Weaver C. Chapter 3: The induced responses of innate immunity In: Murphy K, Weaver C, eds. Janeway’s Immunobiology. 9th ed. New York, NY: Garland Science/Taylor & Francis Group, LLC; 2016:121–126. [Google Scholar]
- 32.Murphy K, Weaver C. Chapter 16: Manipulation of the immune response In: Murphy K, Weaver C, eds. Janeway’s Immunobiology. 9th ed. New York, NY: Garland Science/Taylor & Francis Group, LLC; 2016:727–729. [Google Scholar]
- 33.Kini A, Fu R, Compton C, Miller DM, Ramasubramanian A. Pembrolizumab for recurrent conjunctival melanoma. JAMA Ophthalmol 2017;135(8):891–892. [DOI] [PubMed] [Google Scholar]
- 34.Javed A, Arguello D, Johnston C, et al. PD-L1 expression in tumor metastasis is different between uveal melanoma and cutaneous melanoma. Immunotherapy 2017;9(16): 1323–1330. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure. Expression of immunohistochemical markers PD-L1, PD-L2, and CD8 in normal and inflamed conjunctiva. (Top left) Uninflamed normal conjunctiva with non-keratinizing squamous epithelium (Ep). (Top right) Lymphocytic infiltrate (L) of conjunctival epithelium (Ep) containing goblet cells. (Middle left) PD-L1 is not expressed in the epithelium (Ep) of the inflamed conjunctiva. There is thin artifactual surface staining. The arrows point to the epithelial basement membrane region. (Middle right) Left panel: PD-L1 weakly, nonspecifically (nonmembranously) stains the epithelium (Ep) overlying stromal chronic inflammation. H and circles indicate positively staining histiocytes. Right panel: CD8-positive T lymphocytes are abundantly present in the chronic subepithelial infiltrate. (Bottom left) PD-L2 is not expressed in the conjunctival epithelium (Ep) or in the subepithelial lymphocytes (L). (Bottom right) Focally, PD-L2 is very faintly and nonspecifically (nonmembranously) observed in the epithelium (Ep) but more definitively stains the histiocytes (H and circles) within the subepithelial lymphocytic infiltrate.