Abstract
Mesaconitine (MA), a diester-diterpenoid alkaloid in aconite roots, is considered to be one of the most important bioactive ingredients. In this review, we summarized its neuropharmacological effects, including analgesic effects and antiepileptiform effects. Mesaconitine can act on the central noradrenergic system and the serotonin system; behaving like the norepinephrine reuptake inhibitors and tricyclic antidepressants that increase norepinephrine levels in stress-induced depression. Therefore, the possible perspectives for future studies on the depression of MA were also discussed as well. The pharmacological effect of MA on depression is worthy of further study.
1. Introduction
Many antidepressants have been developed based on the catecholamine deficiency hypothesis, but these drugs cannot meet people's needs. The latency period in antidepressant efficacy is a problem in Major Depressive Disorder (MDD) treatment because the depressive states are often connected with a higher risk of suicide [1, 2]. Representative antidepressants like NA reuptake inhibitors (NRIs) and selective serotonin reuptake inhibitors (SSRIs) require long-term therapy [3, 4]. Besides, only about 50% of MDD patients who received currently available antidepressants (AD) showed complete remission, including several drug trials with or without concurrent psychotherapy, but up to 80% of patients showed partial response [5]. Tricyclic antidepressants may have cardiotoxicity and atropine-like side effects [6]. Many new antidepressants are variants of classic antidepressants and have similar limitations [7, 8]. Therefore, there is a need to develop better antidepressants.
Mesaconitine (MA) is a predominant and representative component of alkaloids contained in the plant of the genera Aconitum [9]. MA possesses multiple pharmacological activities, such as vaso-relaxing effects [10–12], analgesic effects [13], and antiepileptiform effects [14]. Currently, only Nesterova et al. reported that MA possesses antidepressant activity [15]. However, the mechanisms of MA in analgesia and antiepileptiform effects are similar to that of antidepressants. The biological and pharmaceutical properties of MA can be improved by structural modification (Figure 1). So, the pharmacological effect of MA on depression is worthy of further study. In this review, we summarized the analgesic effects and antiepileptic effects. Besides, the possible perspectives for future studies on the depression of MA were also discussed as well.
Figure 1.
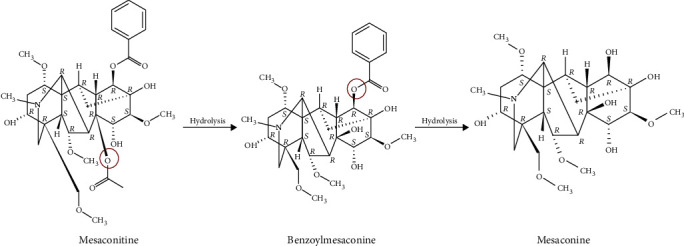
Absolute stereochemistry of mesaconitine and catabolite.
2. Neuropharmacological Effects of MA
2.1. Analgesic Effects of MA
Mesaconitine (MA) possesses antinociceptive activity in nociceptive test models, such as writhing and tail immersion test [16–18]. Also, several components of Fuzi (genera Aconitum) possess analgesic effects, such as hypaconitine, fuziline, neoline, aconitine, songorine, and mesaconitine [18–20]. Benzoylmesaconine, hydrolyte of mesaconitine [21], possesses antinociceptive action in hyperalgesic rats as well [22]. Among these alkaloids, mesaconitine exhibited the strongest analgesic effects [23]. Mesaconitine exerted analgesic effects via the periaqueductal gray (PAG), the nucleus reticularis gigantocellularis (NRGC), the lumbar enlargement, the nucleus raphe magnus (NRM), and the nucleus reticularis paragigantocellularis (NRPG) [17, 22, 24]. Microinjection of mesaconitine into the NRPG, NRM, and PAG produced dose-dependent analgesic activity; The analgesic effect of MA in NRM was more potent and sensitive than PAG and NRPG. [22]. Analgesic activity of the benzoylmesaconine (BM) may be through the activation of the NRM [22]. In the medulla oblongata, NRM is involved in the serotonergic descending inhibitory systems and NRPG acts in noradrenergic descending inhibitory systems. In the mesencephalon, PAG is involved in the descending pain inhibitory systems. Analgesic action of MA and BM appears to be through a descending serotonin system. MA promoted the turnover rate of norepinephrine in the brain stem and spinal cord. Norepinephrine activated adenylate cyclase through β-adrenoceptors, thereby significantly increased the level of cyclic adenosine monophosphate (cAMP), which enhanced the analgesic activity of MA [25, 26]. Murayama et al. reported that in isolated guinea-pig vas deferens, MA promoted the release of norepinephrine through excitatory sympathetic nerve fibers. Its analgesic effect may be the result of the release of noradrenaline from nerve endings and increased receptor sensitivity [26]. The analgesic effect of MA was enhanced by the injection of norepinephrine or isoproterenol intracerebroventricularly (i.c.v.) and attenuated by β-adrenoceptors antagonist [17, 26]. Therefore, the analgesic effect of MA seems to be through the activation of the noradrenergic system and serotonergic descending systems. This information is outlined in Table 1 and Figure 2.
Table 1.
Experimental information of the analgesic effect of MA.
| Animal | Route | Most intense analgesia | ED50 values | Mode of action | Medicine | Method | References |
|---|---|---|---|---|---|---|---|
| Male mice (20-24 g) of the Std:ddY strain Male rats (200-220 g) of the Wistar strain. |
s.c. MA (60 μg/kg) s.c. MA (20 μg/kg) i.c. |
40 min after s.c | 28 μg/kg (95% confidence limit: 11-37) in acetic acid-induced writhing 11 μg/kg (4-28) in tail flick |
Dose-dependent and time-dependent | Mesaconitine hydrobromide | Acetic acid-induced writhing tail-flick | [26].... |
|
| |||||||
| Male Sprague-Dawley rats (250-350 g) | Intracerebral cannulation 10 ng/rats into NRPG 5 ng/rats into NRM 5 ng/rats into PAG |
10 min 5 min 5 min |
Dose-dependent and time-dependent | Mesaconitine hydrobromide | Paw pressure test | [22].... | |
|
| |||||||
| Male rats of the Sprague-Dawley strain (200-250 g) | Intracerebral cannulation 50 and 100 ng/rat into the NRPG 50 ng/rat into the NRGC 50 ng/rat into the PAG 0.5 and 1.0 μg/rat into the lumbar enlargement |
20 to 120 min 20 to 120 min 5 to 120 min 10 to 60 min |
9.1 μg/kg- (95% confidence limits: 5.1-16.2) intravenously | Dose-dependent and time-dependent | Mesaconitine hydrobromide | Tail immersion test | [17].... |
|
| |||||||
| Male NMRI mice (25-30 g) | i.v. | 0.025 (0.021-0.034) | Dose-dependent and time-dependent | Mesaconitine | Formalin test | [18].... | |
Abbreviations: MA: mesaconitine; NRPG: nucleus reticularis paragigantocellularis; NRM: nucleus raphe magnus; NGF: nerve growth factor; PAG: periaqueductal gray.
Figure 2.
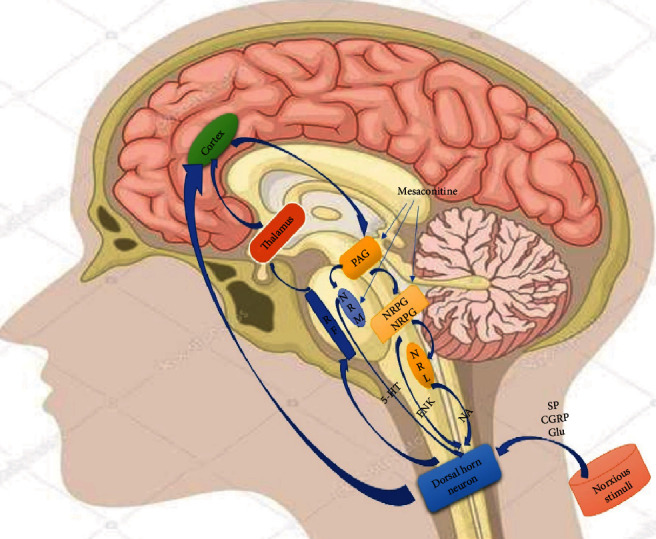
Schematic drawing of the nociceptive system with ascending and descending fibers. RF: reticular formation; PAG: periaqueductal gray; NRM: nucleus raphe magnus; NRGC: nucleus reticularis gigantocellularis; NRPG: nucleus reticularis paragigantocellularis; NRL: nucleus reticularis lateralis; 5-HT: 5-hydroxytryptamine; ENK: enkephalin; NA: noradrenaline.
Some studies have shown that the aromatic ester group of MA bound to site 2 of Na+channels, resulting in sodium ion influx, causing neuronal depolarization and ultimately inhibiting the transmission of pain [18]. Besides, MA has the highest concentration in aconitine-type alkaloids of water extract of Radix Aconiti Carmichaeli (Chuan Wu) as quantified by high-performance liquid chromatography; the analgesic and anti-inflammatory activity of aqueous extracts may be due to high concentration of MA [13]. Heishunpian, Baifupian, and Yan-Fuzi are processed products of Fuzi. Interestingly, compared to Heishunpian and Baifupian, Yan-Fuzi possesses less toxic and antinociceptive activity of Yan-Fuzi is similar to crude Fuzi [27], which may be differences in processing methods that resulted in different alkaloid contents [28].
2.2. Antiepileptiform Effects of MA
From the above statements, we already know that mesaconitine exerts analgesic effects by stimulating the noradrenergic system. Mesaconitine also inhibited epileptic field potentials through α-adrenoceptors in a concentration-dependent manner. Important components of epileptiform discharge include presynaptic fiber peaks, the first postsynaptic population spike, and succeeding spikes, which define epileptiform activity. Stimulation-triggered epileptiform activity (a nominal Mg2+-free perfusate) and spontaneous epileptiform activity (a nominal Mg2+-free perfusate with elevated K+ concentration (5 mM)) are inhibited by MA (30 nM), which was antagonized by the α-adrenoceptors antagonist yohimbine (YOH) [14]. However, MA (300 nM and 1 μM) completely inhibited trigger-induced epileptiform activity and yohimbine cannot antagonize the inhibitory effect of MA [14]. These results indicated that MA (30 nM) activated the α-adrenoceptors when it inhibited experimentally induced epilepsy-like activity in the hippocampus.
Norepinephrine is believed to have both a convulsive and anticonvulsant effect depending on the receptors that are activated [29]. The hippocampus receives a diffuse projection of norepinephrine fibers from the locus coeruleus, and the activation of noradrenergic afferents affect hippocampal neuron activity [24]. The activation of alpha-adrenergic receptors reduced epileptiform discharges, whereas activation of beta-adrenergic receptors increased epileptiform discharges in the hippocampus. The rate of discharges induced by either picrotoxin or elevated extracellular potassium ([K+]o) was slowed by NA(≥10 μM) [29]. Both α1-adrenergic receptors agonists (phenylephrine) and α2-adrenergic receptors antagonists (yohimbine) slowed epileptiform discharge rates [29]. However, some scholars have reported that the anticonvulsant activity of norepinephrine was mediated by α2-adrenergic receptors and the α2-selective agonist inhibited epileptiform discharges [30, 31]. The difference in the antiepileptiform mechanism of NA may be due to the different brain parts and concentrations of applied drugs. Consistently, the antiepileptiform effect is exerted by α-adrenergic receptors, and it is yet to be proven which specific α-adrenergic receptors have worked. It has been reported that mesaconitine induced contractions of the guinea-pig vas deferens by an enhanced neuronal release of noradrenaline [32]. So, MA (like the agonist) may act on α-adrenergic receptors to promote the release of norepinephrine and exert antiepileptiform effects (Figure 3). Besides, the specific molecular mechanism of antiepileptiform effects of MA needs further clarification.
Figure 3.
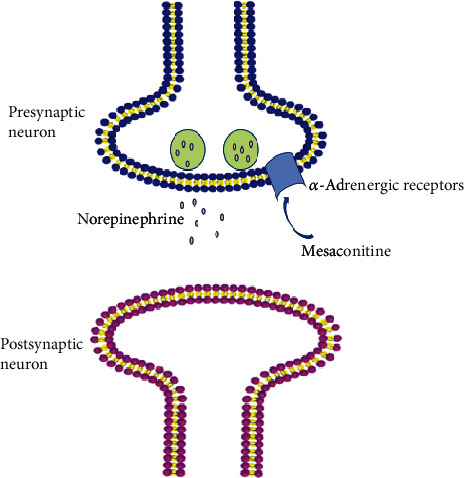
Schematic drawing of the antiepileptiform mechanism of mesaconitine on a noradrenergic neuron in the pyramidal stratum of the hippocampus.
2.3. Antidepressant Effects of MA
Our previous studies have demonstrated that Fuzi total alkaloids exerted anticonvulsant and antidepressant effects [33, 34]. Also, the antiepileptiform activity of MA has been reported. Some scholars have demonstrated the antidepressant effect of MA, possibly due to the altering of sensitivity to serotonin [15]. It is similar to the mechanism of the analgesic effects of MA. There are other possible reasons to support further the study of the pharmacological effect of MA on depression.
First, at least half of patients with chronic pain and itching are accompanied by depression and anxiety; chronic pain and itching can be found in as much as 60% of depressed patients. Tricyclic antidepressants have analgesic and antipruritic effects. Drugs treating psychosis can be used for analgesia, and analgesic drugs can also be used for depression [35]. We already knew that MA has an analgesic effect. Second, some scholars have demonstrated that the locus coeruleus noradrenergic neuron α2-adrenergic receptors are functionally blocked in stress-depressed states. Injecting α2-adrenergic receptor agonist intra-clonidine reduced the frequency of neuronal firing and reversed the animals' depressed state [36]. The antiepileptiform effect of MA was blocked by the α2-adrenergic receptor antagonist [14]. So, MA may act on α2-adrenergic receptors like clonidine. Third, dual-acting antidepressants like serotonin and norepinephrine reuptake inhibitors (SNRIs) as well as norepinephrine and dopamine reuptake inhibitors (NDRIs) have better efficacy than one system because of the multisystem monoaminergic pathway. They are the primary choices for clinicians in reducing residual symptoms and remission [37, 38]. MA exerted an analgesic effect through both the adrenergic system and the serotonin system. It is also possible to exert antidepressant effects through these two systems.
Moreover, the lack of monoamines is thought to be the leading cause of major depressive disorder (MDD), and some antidepressants work by increasing monoamine levels in the brain [39]. Representative antidepressants such as reboxetine [40], atomoxetine [41], and nortriptyline [42] inhibit noradrenergic transporters and increase norepinephrine in the brain. It has been reported that MA may promote the release of norepinephrine from neurons [24]. The neurotransmitter norepinephrine plays an important role in cognition, behavior, stress responses, and vigilance [43–45]. When the neurogenesis of the adult animal's hippocampus is destroyed by irradiation, the behavioral effects of antidepressants disappear [46]. Depression leads to atrophy of hippocampal neurons. Antidepressants enhance hippocampal neurogenesis [47–49] and reverse hippocampal volume shrinkage as well as hippocampal neuron loss [39]. NA greatly increased the dentate gyrus-derived neural precursor cells (NPCs) proliferation by activating the β2-adrenergic receptor [50]. Jhaveri et al. reported that an increased amount of NA activated the neurogenic precursors and stem cells via β3-adrenergic receptors [51]. Increasing norepinephrine by antidepressant promotes hippocampal neurogenesis through augmenting the survival and differentiation of new granule cells (DG) [52]. MA may increase hippocampal neurogenesis by norepinephrine acting on β-adrenergic receptors.
Finally, injection of norepinephrine or isoproterenol intracerebroventricularly (i.c.v.) enhanced analgesic effects of MA, and this effect was attenuated by β-adrenoceptors antagonist [17, 26]. MA seems to produce an analgesic effect by activating the β-adrenoceptors. The β-adrenoceptors can activate Gs. And then Gs activates adenylate cyclase (AC) to produce cyclic adenosine monophosphate (cAMP). cAMP further promotes phosphorylation of cAMP response element-binding (CREB), which in turn produces brain-derived neurotrophic factor (BDNF) [53] (Figure 4). Norepinephrine activated AC through β-adrenoceptors and increased the level of cAMP [25]. It is consistent with the previous description that MA promoted the release of norepinephrine. Moreover, some antidepressants act on CREB/BDNF pathway [54–57]. Increased expression of BDNF is considered to be an important mechanism of synaptic plasticity [58–60] and neurogenesis [61–63]. CREB plays an important role in neurogenesis and in reducing depressive symptoms in mice [64]. β3-adrenergic receptor agonists reduced the immobility time of mice in forced swimming tests. The increase of NA by norepinephrine reuptake inhibitor in the synaptic cleft increased BDNF expression in the dentate gyrus (DG) of the hippocampus through β3-adrenoceptor [39]. It seems that the antidepressant effects require the activation of β3-adrenergic receptors. MA may activate β3-adrenoceptor through norepinephrine (Figure 4).
Figure 4.
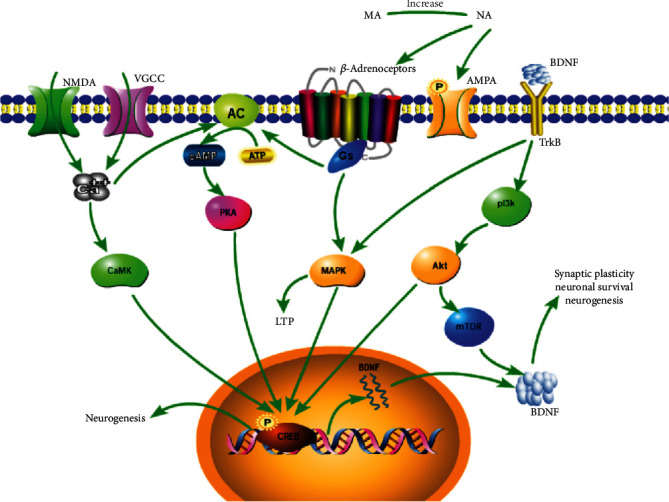
Role of β-adrenoceptors on antidepressant effect. All arrows indicate activation arrows. Gs: stimulating adenylate cyclase g protein; ATP: adenosine triphosphate; AC: adenylate cyclase; cAMP: cyclic adenosine monophosphate; PKA: protein kinase A; CREB: cAMP-response element-binding protein; BDNF: brain-derived neurotrophic factor; LTP: long-term potentiation; AMPA: α-amino-3-hydroxy-5-methyl-4-isoxazole propionate receptor.
Synaptic plasticity is currently considered to be an important basis for the formation of learning and memory. It is the fastest-growing research field in neuroscience. Synaptic plasticity includes structural plasticity and functional plasticity. The plasticity of the structure refers to the change in synaptic morphology and quantity caused by repeated synaptic activity. Functional plasticity refers to changes in synaptic transmission efficiency, including long-term potentiation (LTP) and long-term depression (LTD) [65]. Various forms of stress impair long-term potentiation and enhance long-term inhibition [66–69]. Tricyclic antidepressants increase synaptic plasticity at different levels [70]. Norepinephrine facilitates or induces LTP of the population spike through the β-adrenergic receptor [53]. Activation of β-adrenergic receptors increases the concentration of the cAMP, followed by activation of protein kinase A(PKA) and mitogen-activated protein kinase (MAPK) [71]. Also, norepinephrine-induced long-term potentiation may require activation of N-methyl-D-aspartic acid receptor (NMDA) receptors [72]. Calcium ions enter the cell through the activated NMDA receptors, then bind to intracellular calmodulin and activate adenylate cyclase to increase cAMP levels [73]. Activation of adenylate cyclase, PKA, and MAPK is involved in long-term potentiation induction [74–77]. Besides, norepinephrine reduces the threshold of LTP through phosphorylation of AMPA receptor subunit GluR1 [78]. Therefore, MA may affect synaptic plasticity through increasing norepinephrine. The possible mechanisms of the antidepressant effects induced by MA are outlined in Figure 4.
3. Side Effects of MA
Mesaconitine, a diester-diterpenoid alkaloid in aconite roots, is considered to be one of the most important bioactive ingredients and toxic ingredients [79]. Understanding the toxicity and toxicokinetic of MA is important for the application of MA and risk control. The therapeutic window of MA is narrow [80]: studies have shown that the median lethal dose (LD50) of a single oral administration MA was 1.9 mg/kg in animal [81] and the half-life of MA was around 2.8–5.8 h [82]. The intravenous LD50 value in mice was 0.068 mg/kg [83]. Data from toxicological tests for MA have been presented by Zhou et al. [84] (Table 2). Although there are few reports on the toxicity of MA, three major diterpenoid alkaloids aconitine (AC), MA, and hypaconitine (HA) may share similar cardiotoxicity and mechanisms because of similar core structures [79]. Aconitine-type alkaloids are unstable and unsafe [28, 84–86]. Studies on the metabolism of MA in organisms have been reported [10, 87]. However, a comprehensive MA metabolism database still needs to be built for future pharmacological studies and clinical use [80]. In order to better understand the toxicity of MA, other advanced methods like an electrocardiogram, histopathology, serum biomarkers, and lipidomic profile changes need to be applied [79].
Table 2.
LD50 of mesaconitine.
| Alkaloid | LD50 (mg/kg) | |||
|---|---|---|---|---|
| p.o. | s.c. | i.p. | i.v. | |
| Mesaconitine | 1.90 (mice) | 0.20–0.38 (mice) 0.204 (rat) |
0.20–0.30 (mice) | 0.068–0.13 (mice) |
The diester diterpene alkaloids (DAs) with acetyl group at the C-8 position and ester group the C-14 benzoyl are toxic [84]. As the ester bond is hydrolyzed, the toxicity of MA is reduced [83, 87]. However, its pharmacological activity has not been affected [84]. Therefore, it is possible to improve the biological and pharmaceutical properties of MA through structural modification [19].
4. Conclusion
MA exerted analgesic effects and antidepressant effects through the serotonin system. Besides, MA exerted analgesic and antiepileptiform effects through the noradrenergic system. Like norepinephrine reuptake inhibitors and tricyclic antidepressants, MA can also increase norepinephrine levels, possibly through norepinephrine acting on related targets to produce multiple neuropharmacological effects. Therefore, the pharmacological effect of MA on depression is worthy of further study. Moreover, a thorough understanding of the toxicity and toxicokinetics of MA is required through advanced methods.
Acknowledgments
This work was supported by grants from the Natural Science Foundation of China (NSFC) (81971276), the National Key R&D Program of China (Grant #2018YFC1311600), and the Jilin Province Medical and Health Talents (2019SCZT007 and 2019SCZT013).
Abbreviations
- BDNF:
Brain-derived neurotrophic factor
- CREB:
cAMP-response element-binding protein
- NRPG:
Nucleus reticularis paragigantocellularis
- NRM:
Nucleus raphe magnus
- NGF:
Nerve growth factor
- PAG:
Periaqueductal gray
- BM:
Benzoylmesaconine
- cAMP:
Cyclic adenosine monophosphate
- LD50:
Median lethal dose
- NA:
Noradrenaline
- PKA:
Protein kinase A
- MAPK:
Mitogen-activated protein kinase
- LTP:
Long-term potentiation
- NMDA:
N-methyl-D-aspartic acid receptor.
Contributor Information
Limin Yang, Email: ylmh777@126.com.
Wei Yang, Email: wyang2002@jlu.edu.cn.
Conflicts of Interest
The authors declare no conflict of interest.
Authors' Contributions
ZHS, LHZ, LMY, and WY contributed to drafting the article. All authors have revised the manuscript critically for important intellectual content and approved the final version to be published.
References
- 1.Wang J., Jing L., Toledo-Salas J. C., Xu L. Rapid-onset antidepressant efficacy of glutamatergic system modulators: the neural plasticity hypothesis of depression. Neuroscience Bulletin. 2015;31(1):75–86. doi: 10.1007/s12264-014-1484-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li N., Lee B., Liu R. J., et al. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. 2010;329(5994):959–964. doi: 10.1126/science.1190287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Farber G. A., Goldberg J. F. Regarding “combining norepinephrine and serotonin reuptake inhibition mechanisms for treatment of depression: A double-blind randomized study”: Optimizing initial interventions. Biological Psychiatry. 2004;56(7):535–535; author reply 536. doi: 10.1016/j.biopsych.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 4.Aleksandrova L. R., Wang Y. T., Phillips A. G. Evaluation of the Wistar-Kyoto rat model of depression and the role of synaptic plasticity in depression and antidepressant response. Neuroscience and Biobehavioral Reviews. 2019;105:1–23. doi: 10.1016/j.neubiorev.2019.07.007. [DOI] [PubMed] [Google Scholar]
- 5.Haenisch B., Bonisch H. Depression and antidepressants: insights from knockout of dopamine, serotonin or noradrenaline re-uptake transporters. Pharmacology & Therapeutics. 2011;129(3):352–368. doi: 10.1016/j.pharmthera.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 6.Gillman P. K. Tricyclic antidepressant pharmacology and therapeutic drug interactions updated. British Journal of Pharmacology. 2007;151(6):737–748. doi: 10.1038/sj.bjp.0707253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sanacora G., Schatzberg A. F. Ketamine: promising path or false prophecy in the development of novel therapeutics for mood disorders? Neuropsychopharmacology. 2015;40(2):259–267. doi: 10.1038/npp.2014.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Willner P., Scheel-Kruger J., Belzung C. Resistance to antidepressant drugs. Behavioural Pharmacology. 2014;25(5 and 6):352–371. doi: 10.1097/FBP.0000000000000066. [DOI] [PubMed] [Google Scholar]
- 9.Sun B., Zhang M., Zhang Q., et al. Metabonomics study of the effects of pretreatment with glycyrrhetinic acid on mesaconitine-induced toxicity in rats. Journal of Ethnopharmacology. 2014;154(3):839–846. doi: 10.1016/j.jep.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 10.Ye L., Tang L., Gong Y., et al. Characterization of metabolites and human P450 isoforms involved in the microsomal metabolism of mesaconitine. Xenobiotica. 2010;41(1):46–58. doi: 10.3109/00498254.2010.524950. [DOI] [PubMed] [Google Scholar]
- 11.Mitamura M., Boussery K., Horie S., Murayama T., Van de Voorde J. Vasorelaxing effect of mesaconitine, an alkaloid from Aconitum japonicum, on rat small gastric artery: possible involvement of endothelium-derived hyperpolarizing factor. Japanese Journal of Pharmacology. 2002;89(4):380–387. doi: 10.1254/jjp.89.380. [DOI] [PubMed] [Google Scholar]
- 12.Mitamura M., Horie S., Sakaguchi M., et al. Mesaconitine-induced relaxation in rat aorta: involvement of Ca2+ influx and nitric-oxide synthase in the endothelium. European Journal of Pharmacology. 2002;436(3):217–225. doi: 10.1016/S0014-2999(01)01623-5. [DOI] [PubMed] [Google Scholar]
- 13.Lai M. C., Liu I.-M., Liou S.-S., Chang Y.-S. Mesaconitine plays the major role in the antinociceptive and anti-inflammatory activities of Radix Aconiti Carmichaeli (Chuan Wu) Journal of Food and Drug Analysis. 2011;19(3) doi: 10.38212/2224-6614.2182. [DOI] [Google Scholar]
- 14.Ameri A. Inhibition of stimulus-triggered and spontaneous epileptiform activity in rat hippocampal slices by the Aconitum alkaloid mesaconitine. European Journal of Pharmacology. 1998;342(2-3):183–191. doi: 10.1016/S0014-2999(97)01498-2. [DOI] [PubMed] [Google Scholar]
- 15.Nesterova Y. V., Povetieva T. N., Suslov N. I., Semenov A. A., Pushkarskiy S. V. Antidepressant activity of diterpene alkaloids of Aconitum baicalense Turcz. Bulletin of Experimental Biology and Medicine. 2011;151(4):425–428. doi: 10.1007/s10517-011-1347-3. [DOI] [PubMed] [Google Scholar]
- 16.Oyama T., Isono T., Suzuki Y., Hayakawa Y. Anti-nociceptive effects of aconiti tuber and its alkaloids. The American Journal of Chinese Medicine. 2012;22(2):175–182. doi: 10.1142/s0192415x94000218. [DOI] [PubMed] [Google Scholar]
- 17.Hikino H., Murayama M. Mechanism of the antinociceptive action of mesaconitine: participation of brain stem and lumbar enlargement. British Journal of Pharmacology. 1985;85(3):575–580. doi: 10.1111/j.1476-5381.1985.tb10551.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Friese J., Gleitz J., Gutser U. T., et al. Aconitum sp. alkaloids: the modulation of voltage-dependent Na+ channels, toxicity and antinociceptive properties. European Journal of Pharmacology. 1997;337(2-3):165–174. doi: 10.1016/S0014-2999(97)01268-5. [DOI] [PubMed] [Google Scholar]
- 19.Nesterova Y. V., Povet’yeva T. N., Suslov N. I., et al. Analgesic activity of diterpene alkaloids from Aconitum baikalensis. Bulletin of Experimental Biology and Medicine. 2014;157(4):488–491. doi: 10.1007/s10517-014-2598-6. [DOI] [PubMed] [Google Scholar]
- 20.Zhao D., Shi Y., Zhu X., et al. Identification of Potential Biomarkers from Aconitum carmichaelii, a Traditional Chinese Medicine, Using a Metabolomic Approach. Planta Medica. 2018;84(6/7):434–441. doi: 10.1055/s-0043-121708. [DOI] [PubMed] [Google Scholar]
- 21.Liu Y., Chen X., He R., Tan P. Analysis of the metabolites of mesaconitine in rat blood using ultrafast liquid chromatography and electrospray ionization mass spectrometry. Pharmacognosy magazine. 2014;10(38):101–105. doi: 10.4103/0973-1296.131019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Suzuki Y., Oyama T., Ishige A., et al. Antinociceptive mechanism of the Actonitine alkaloids mesaconitine and benzoylmesaconine. Planta Medica. 1994;60(5):391–394. doi: 10.1055/s-2006-959516. [DOI] [PubMed] [Google Scholar]
- 23.Zhao L., Sun Z., Yang L., Cui R., Yang W., Li B. Neuropharmacological effects of aconiti lateralis radix praeparata. Clinical and Experimental Pharmacology and Physiology. 2020;47(4):531–542. doi: 10.1111/1440-1681.13228. [DOI] [PubMed] [Google Scholar]
- 24.Ameri A. The effects of Aconitum alkaloids on the central nervous system. Progress in Neurobiology. 1998;56(2):211–235. doi: 10.1016/S0301-0082(98)00037-9. [DOI] [PubMed] [Google Scholar]
- 25.Murayama M., Hikino H. Effect of cyclic AMP on mesaconitine-induced analgesia in mice. European Journal of Pharmacology. 1985;108(1):19–23. doi: 10.1016/0014-2999(85)90278-X. [DOI] [PubMed] [Google Scholar]
- 26.Murayama M., Ito T., Konno C., Hikino H. Mechanism of analgesic action of mesaconitine. I. Relationship between analgesic effect and central monoamines or opiate receptors. European Journal of Pharmacology. 1984;101(1-2):29–36. doi: 10.1016/0014-2999(84)90027-X. [DOI] [PubMed] [Google Scholar]
- 27.Liou S. S., Liu I. M., Lai M. C., Cheng J. T. Comparison of the antinociceptive action of crude Fuzei, the root of Aconitum, and its processed products. Journal of Ethnopharmacology. 2005;99(3):379–383. doi: 10.1016/j.jep.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 28.Liu S., Li F., Li Y., Li W., Xu J., Du H. A review of traditional and current methods used to potentially reduce toxicity of Aconitum roots in traditional Chinese medicine. Journal of Ethnopharmacology. 2017;207:237–250. doi: 10.1016/j.jep.2017.06.038. [DOI] [PubMed] [Google Scholar]
- 29.Rutecki P. A. Noradrenergic modulation of epileptiform activity in the hippocampus. Epilepsy Research. 1995;20(2):125–136. doi: 10.1016/0920-1211(94)00078-B. [DOI] [PubMed] [Google Scholar]
- 30.Mueller A. L., Dunwiddie T. V. Anticonvulsant and proconvulsant actions of alpha- and beta-noradrenergic agonists on epileptiform activity in rat hippocampus in vitro. Epilepsia. 1983;24(1):57–64. doi: 10.1111/j.1528-1157.1983.tb04866.x. [DOI] [PubMed] [Google Scholar]
- 31.Shouse M. N., Langer J., Bier M., et al. The alpha 2-adrenoreceptor agonist clonidine suppresses seizures, whereas the alpha 2-adrenoreceptor antagonist idazoxan promotes seizures in amygdala-kindled kittens: a comparison of amygdala and pontine microinfusion effects. Epilepsia. 1996;37(8):709–717. doi: 10.1111/j.1528-1157.1996.tb00640.x. [DOI] [PubMed] [Google Scholar]
- 32.Sato H., Ohizumi Y., Hikino H. Mechanism of mesoconitine-induced contractile response in Guinea pig vas deferens. European Journal of Pharmacology. 1979;55(1):83–92. doi: 10.1016/0014-2999(79)90150-X. [DOI] [PubMed] [Google Scholar]
- 33.Li B., Tang F., Wang L., et al. Anticonvulsant effects of Fuzi total alkaloid on pentylenetetrazole-induced seizure in mice. Journal of Pharmacological Sciences. 2013;123(2):195–198. doi: 10.1254/jphs.13057SC. [DOI] [PubMed] [Google Scholar]
- 34.Liu L., Li B., Zhou Y., et al. Antidepressant-like effect of Fuzi total alkaloid on ovariectomized mice. Journal of Pharmacological Sciences. 2012;120(4):280–287. doi: 10.1254/jphs.12163FP. [DOI] [PubMed] [Google Scholar]
- 35.Belinskaia D. A., Belinskaia M. A., Barygin O. I., Vanchakova N. P., Shestakova N. N. Psychotropic drugs for the management of chronic pain and itch. Pharmaceuticals. 2019;12(2):p. 99. doi: 10.3390/ph12020099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weiss J. M., Simson P. E. Neurochemical and electrophysiological events underlying stress-induced depression in an animal model. Advances in Experimental Medicine and Biology. 1988;245:425–440. doi: 10.1007/978-1-4899-2064-5_33. [DOI] [PubMed] [Google Scholar]
- 37.Nemeroff C. B., Schatzberg A. F., Goldstein D. J., et al. Duloxetine for the treatment of major depressive disorder. Psychopharmacology Bulletin. 2002;36(4):106–132. [PubMed] [Google Scholar]
- 38.Smith D., Dempster C., Glanville J., Freemantle N., Anderson I. Efficacy and tolerability of venlafaxine compared with selective serotonin reuptake inhibitors and other antidepressants: a meta-analysis. The British Journal of Psychiatry. 2002;180(5):396–404. doi: 10.1192/bjp.180.5.396. [DOI] [PubMed] [Google Scholar]
- 39.Seki K., Yoshida S., Jaiswal M. K. Molecular mechanism of noradrenaline during the stress-induced major depressive disorder. Neural Regeneration Research. 2018;13(7):1159–1169. doi: 10.4103/1673-5374.235019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chuluunkhuu G., Nakahara N., Yanagisawa S., Kamae I. The efficacy of reboxetine as an antidepressant, a meta-analysis of both continuous (mean HAM-D score) and dichotomous (response rate) outcomes. The Kobe Journal of Medical Sciences. 2008;54(2):E147–E158. [PubMed] [Google Scholar]
- 41.Kratochvil C. J., Newcorn J. H., Arnold L. E., et al. Atomoxetine alone or combined with fluoxetine for treating ADHD with comorbid depressive or anxiety symptoms. Journal of the American Academy of Child and Adolescent Psychiatry. 2005;44(9):915–924. doi: 10.1097/01.chi.0000169012.81536.38. [DOI] [PubMed] [Google Scholar]
- 42.Reynolds C. F., III, Frank E., Perel J. M., et al. Nortriptyline and interpersonal psychotherapy as maintenance therapies for recurrent major depression. JAMA. 1999;281(1):p. 39. doi: 10.1001/jama.281.1.39. [DOI] [PubMed] [Google Scholar]
- 43.Bouret S. Locus Coeruleus, noradrenaline, and behavior: network effect, network effects? Neuron. 2019;103(4):554–556. doi: 10.1016/j.neuron.2019.07.033. [DOI] [PubMed] [Google Scholar]
- 44.Goddard A. W., Ball S. G., Martinez J., et al. Current perspectives of the roles of the central norepinephrine system in anxiety and depression. Depression and Anxiety. 2010;27(4):339–350. doi: 10.1002/da.20642. [DOI] [PubMed] [Google Scholar]
- 45.Kuehl L. K., Deuter C. E., Hellmann-Regen J., Kaczmarczyk M., Otte C., Wingenfeld K. Enhanced noradrenergic activity by yohimbine and differential fear conditioning in patients with major depression with and without adverse childhood experiences. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2020;96:p. 109751. doi: 10.1016/j.pnpbp.2019.109751. [DOI] [PubMed] [Google Scholar]
- 46.Santarelli L., Saxe M., Gross C., et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301(5634):805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- 47.Park S. C. Neurogenesis and antidepressant action. Cell and Tissue Research. 2019;377(1):95–106. doi: 10.1007/s00441-019-03043-5. [DOI] [PubMed] [Google Scholar]
- 48.Micheli L., Ceccarelli M., D’Andrea G., Tirone F. Depression and adult neurogenesis: Positive effects of the antidepressant fluoxetine and of physical exercise. Brain Research Bulletin. 2018;143:181–193. doi: 10.1016/j.brainresbull.2018.09.002. [DOI] [PubMed] [Google Scholar]
- 49.Tunc-Ozcan E., Peng C. Y., Zhu Y., Dunlop S. R., Contractor A., Kessler J. A. Activating newborn neurons suppresses depression and anxiety-like behaviors. Nature Communications. 2019;10(1):p. 3768. doi: 10.1038/s41467-019-11641-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Masuda T., Nakagawa S., Boku S., et al. Noradrenaline increases neural precursor cells derived from adult rat dentate gyrus through beta2 receptor. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2012;36(1):44–51. doi: 10.1016/j.pnpbp.2011.08.019. [DOI] [PubMed] [Google Scholar]
- 51.Jhaveri D. J., Mackay E. W., Hamlin A. S., et al. Norepinephrine directly activates adult hippocampal precursors via beta3-adrenergic receptors. The Journal of Neuroscience. 2010;30(7):2795–2806. doi: 10.1523/JNEUROSCI.3780-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rizk P., Salazar J., Raisman-Vozari R., et al. The alpha2-adrenoceptor antagonist dexefaroxan enhances hippocampal neurogenesis by increasing the survival and differentiation of new granule cells. Neuropsychopharmacology. 2006;31(6):1146–1157. doi: 10.1038/sj.npp.1300954. [DOI] [PubMed] [Google Scholar]
- 53.Marzo A., Bai J., Otani S. Neuroplasticity regulation by noradrenaline in mammalian brain. Current Neuropharmacology. 2009;7(4):286–295. doi: 10.2174/157015909790031193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang P., Li B., Fan J., et al. Additive antidepressant-like effects of fasting with β‐estradiol in mice. Journal of Cellular and Molecular Medicine. 2019;23(8):5508–5517. doi: 10.1111/jcmm.14434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Keshavarzi S., Kermanshahi S., Karami L., Motaghinejad M., Motevalian M., Sadr S. Protective role of metformin against methamphetamine induced anxiety, depression, cognition impairment and neurodegeneration in rat: the role of CREB/BDNF and Akt/GSK3 signaling pathways. Neurotoxicology. 2019;72:74–84. doi: 10.1016/j.neuro.2019.02.004. [DOI] [PubMed] [Google Scholar]
- 56.Li J., Luo Y., Zhang R., Shi H., Zhu W., Shi J. Neuropeptide trefoil factor 3 reverses depressive-like behaviors by activation of BDNF-ERK-CREB signaling in olfactory Bulbectomized rats. International Journal of Molecular Sciences. 2015;16(12):28386–28400. doi: 10.3390/ijms161226105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li Y., Liu J., Liu X., et al. Antidepressant-like action of single facial injection of Botulinum neurotoxin a is associated with augmented 5-HT levels and BDNF/ERK/CREB pathways in mouse brain. Neuroscience Bulletin. 2019;35(4):661–672. doi: 10.1007/s12264-019-00367-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bjorkholm C., Monteggia L. M. BDNF - a key transducer of antidepressant effects. Neuropharmacology. 2016;102:72–79. doi: 10.1016/j.neuropharm.2015.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Radecki D. T., Brown L. M., Martinez J., Teyler T. J. BDNF protects against stress-induced impairments in spatial learning and memory and LTP. Hippocampus. 2005;15(2):246–253. doi: 10.1002/hipo.20048. [DOI] [PubMed] [Google Scholar]
- 60.Kavalali E. T., Monteggia L. M. Synaptic mechanisms underlying rapid antidepressant action of ketamine. The American Journal of Psychiatry. 2012;169(11):1150–1156. doi: 10.1176/appi.ajp.2012.12040531. [DOI] [PubMed] [Google Scholar]
- 61.Malberg J. E., Eisch A. J., Nestler E. J., Duman R. S. Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. The Journal of Neuroscience. 2000;20(24):9104–9110. doi: 10.1523/JNEUROSCI.20-24-09104.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen B., Dowlatshahi D., MacQueen G. M., Wang J.-F., Young L. T. Increased hippocampal BDNF immunoreactivity in subjects treated with antidepressant medication. Biological Psychiatry. 2001;50(4):260–265. doi: 10.1016/S0006-3223(01)01083-6. [DOI] [PubMed] [Google Scholar]
- 63.Jin Y., Cui R., Zhao L., Fan J., Li B. Mechanisms of Panax ginseng action as an antidepressant. Cell Proliferation. 2019;52(6):p. e12696. doi: 10.1111/cpr.12696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gascon S., Ortega F., Gotz M. Transient CREB-mediated transcription is key in direct neuronal reprogramming. Neurogenesis. 2017;4(1):p. e1285383. doi: 10.1080/23262133.2017.1285383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jedrzejewska-Szmek J., Blackwell K. T. From membrane receptors to protein synthesis and actin cytoskeleton: mechanisms underlying long lasting forms of synaptic plasticity. Seminars in Cell & Developmental Biology. 2019;95:120–129. doi: 10.1016/j.semcdb.2019.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chattarji S., Tomar A., Suvrathan A., Ghosh S., Rahman M. M. Neighborhood matters: divergent patterns of stress-induced plasticity across the brain. Nature Neuroscience. 2015;18(10):1364–1375. doi: 10.1038/nn.4115. [DOI] [PubMed] [Google Scholar]
- 67.Marsden W. N. Synaptic plasticity in depression: molecular, cellular and functional correlates. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2013;43:168–184. doi: 10.1016/j.pnpbp.2012.12.012. [DOI] [PubMed] [Google Scholar]
- 68.Maggio N., Segal M. Persistent changes in ability to express long-term potentiation/depression in the rat hippocampus after juvenile/adult stress. Biological Psychiatry. 2011;69(8):748–753. doi: 10.1016/j.biopsych.2010.11.026. [DOI] [PubMed] [Google Scholar]
- 69.Holderbach R., Clark K., Moreau J. L., Bischofberger J., Normann C. Enhanced long-term synaptic depression in an animal model of depression. Biological Psychiatry. 2007;62(1):92–100. doi: 10.1016/j.biopsych.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 70.Duman R. S., Aghajanian G. K., Sanacora G., Krystal J. H. Synaptic plasticity and depression: new insights from stress and rapid-acting antidepressants. Nature Medicine. 2016;22(3):238–249. doi: 10.1038/nm.4050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lin Y. W., Min M. Y., Chiu T. H., Yang H. W. Enhancement of associative long-term potentiation by activation of beta-adrenergic receptors at CA1 synapses in rat hippocampal slices. The Journal of Neuroscience. 2003;23(10):4173–4181. doi: 10.1523/JNEUROSCI.23-10-04173.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Burgard E. C., Decker G., Sarvey J. M. NMDA receptor antagonists block norepinephrine-induced long-lasting potentiation and long-term potentiation in rat dentate gyrus. Brain Research. 1989;482(2):351–355. doi: 10.1016/0006-8993(89)91199-2. [DOI] [PubMed] [Google Scholar]
- 73.Chetkovich D. M., Sweatt J. D. nMDA receptor activation increases cyclic AMP in area CA1 of the hippocampus via calcium/calmodulin stimulation of adenylyl cyclase. Journal of Neurochemistry. 1993;61(5):1933–1942. doi: 10.1111/j.1471-4159.1993.tb09836.x. [DOI] [PubMed] [Google Scholar]
- 74.Otmakhova N. A., Otmakhov N., Mortenson L. H., Lisman J. E. Inhibition of the cAMP pathway decreases early long-term potentiation at CA1 hippocampal synapses. The Journal of Neuroscience. 2000;20(12):4446–4451. doi: 10.1523/JNEUROSCI.20-12-04446.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Makhinson M., Chotiner J. K., Watson J. B., O’Dell T. J. Adenylyl cyclase activation modulates activity-dependent changes in synaptic strength and Ca2+/calmodulin-dependent kinase II autophosphorylation. The Journal of Neuroscience. 1999;19(7):2500–2510. doi: 10.1523/jneurosci.19-07-02500.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Huang Y. Y., Martin K. C., Kandel E. R. Both protein kinase a and mitogen-activated protein kinase are required in the amygdala for the macromolecular synthesis-dependent late phase of long-term potentiation. The Journal of Neuroscience. 2000;20(17):6317–6325. doi: 10.1523/JNEUROSCI.20-17-06317.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Giovannini M. G., Blitzer R. D., Wong T., et al. Mitogen-activated protein kinase regulates early phosphorylation and delayed expression of Ca2+/calmodulin-dependent protein kinase II in long-term potentiation. The Journal of Neuroscience. 2001;21(18):7053–7062. doi: 10.1523/JNEUROSCI.21-18-07053.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hu H., Real E., Takamiya K., et al. Emotion enhances learning via norepinephrine regulation of AMPA-receptor trafficking. Cell. 2007;131(1):160–173. doi: 10.1016/j.cell.2007.09.017. [DOI] [PubMed] [Google Scholar]
- 79.Yang M., Ji X., Zuo Z. Relationships between the toxicities of Radix Aconiti Lateralis Preparata (Fuzi) and the toxicokinetics of its main diester-diterpenoid alkaloids. Toxins. 2018;10(10):p. 391. doi: 10.3390/toxins10100391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Liu L., Liu C., Wang Y., Wang P., Li Y., Li B. Herbal medicine for anxiety, depression and insomnia. Current Neuropharmacology. 2015;13(4):481–493. doi: 10.2174/1570159x1304150831122734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Singhuber J., Zhu M., Prinz S., Kopp B. Aconitum in traditional Chinese medicine: a valuable drug or an unpredictable risk? Journal of Ethnopharmacology. 2009;126(1):18–30. doi: 10.1016/j.jep.2009.07.031. [DOI] [PubMed] [Google Scholar]
- 82.Fujita Y., Terui K., Fujita M., et al. Five cases of aconite poisoning: toxicokinetics of aconitines. Journal of Analytical Toxicology. 2007;31(3):132–137. doi: 10.1093/jat/31.3.132. [DOI] [PubMed] [Google Scholar]
- 83.Ye L., Gao S., Feng Q., et al. Development and validation of a highly sensitive UPLC-MS/MS method for simultaneous determination of aconitine, mesaconitine, hypaconitine, and five of their metabolites in rat blood and its application to a pharmacokinetics study of aconitine, mesaconitine, and hypaconitine. Xenobiotica. 2011;42(6):518–525. doi: 10.3109/00498254.2011.641608. [DOI] [PubMed] [Google Scholar]
- 84.Zhou G., Tang L., Zhou X., Wang T., Kou Z., Wang Z. A review on phytochemistry and pharmacological activities of the processed lateral root of Aconitum carmichaelii Debeaux. Journal of Ethnopharmacology. 2015;160:173–193. doi: 10.1016/j.jep.2014.11.043. [DOI] [PubMed] [Google Scholar]
- 85.Chan T. Y. K. Aconite poisoning. Clinical Toxicology. 2009;47(4):279–285. doi: 10.1080/15563650902904407. [DOI] [PubMed] [Google Scholar]
- 86.Wu J. J., Guo Z. Z., Zhu Y. F., et al. A systematic review of pharmacokinetic studies on herbal drug Fuzi: implications for Fuzi as personalized medicine. Phytomedicine. 2018;44:187–203. doi: 10.1016/j.phymed.2018.03.001. [DOI] [PubMed] [Google Scholar]
- 87.Ye L., Yang X. S., Yang Z., et al. The role of efflux transporters on the transport of highly toxic aconitine, mesaconitine, hypaconitine, and their hydrolysates, as determined in cultured Caco-2 and transfected MDCKII cells. Toxicology Letters. 2013;216(2-3):86–99. doi: 10.1016/j.toxlet.2012.11.011. [DOI] [PubMed] [Google Scholar]


