Abstract
Background
Ruminants release the majority of agricultural methane, an important greenhouse gas. Different feeds and additives are used to reduce emissions, but each has its drawbacks. This experiment was conducted to determine the effects of Allium fistulosum L. (A. fistulosum) extract on in vitro ruminal fermentation characteristics, and on methane emission.
Methods
Rumen fluid was taken from two cannulated rumen Hanwoo cow (with mean initial body weight 450 ± 30 kg, standard deviation = 30). Rumen fluid and McDougall’s buffer (1:2; 15 mL) were dispensed anaerobically into 50 mL serum bottles containing 300 mg (DM basis) of timothy substrate and A. fistulosum extracts (based on timothy substrate; 0%, 1%, 3%, 5%, 7%, or 9%). This experiment followed a completely randomized design performed in triplicate, using 126 individual serum bottles (six treatments × seven incubation times × three replicates).
Results
Dry matter degradability was not significantly affected (p-value > 0.05) by any A. fistulosum treatment other than 1% extract at 24 h incubation. Methane emission linearly decreased A. fistulosum extract concentration increased at 12 and 24 h incubation (p-value < 0.0001; p-value = 0.0003, respectively). Acetate concentration linearly decreased (p-value = 0.003) as A. fistulosum extract concentration increased at 12 h incubation. Methanogenic archaea abundance tendency decreased (p-value = 0.055) in the 1%, 7%, and 9% A. fistulosum extract groups compared to that in the 0% group, and quadratically decreased (p-value < 0.0001) as A. fistulosum extract concentration increased at 24 h incubation.
Conclusion
A. fistulosum extract had no apparent effect on ruminal fermentation characteristics or dry matter degradability. However, it reduced methane emission and methanogenic archaea abundance.
Keywords: Allium fistulosum L. extract, In vitro, Methane emission, Methanogenic archaea, Ruminal fermentation characteristics
Introduction
Agricultural greenhouse gas emissions include nitrous oxide (N2O) and methane (CH4), which represent 14.5% of total greenhouse gas emissions in Korea (IPCC, 2017). Approximately 75% of the methane of agricultural origin is released by ruminants and is produced by methanogenic archaea during anaerobic fermentation in the rumen (Johnson & Johnson, 1995). Given that such emissions are also associated with a 2%–15% loss of dietary potential energy, productivity is also reduced (Johnson & Johnson, 1995; Beauchemin et al., 2008). In the past, antibiotics have helped control methanogen populations (Odongo et al., 2007), but their efficacy is limited owing to the development of microbial resistance, and they may leave residual traces in beef products (Kim, 2012). Various plant extracts, including saponins, tannins, essential oils, and organic sulfur compounds have shown some promise (Busquet et al., 2005a; Cardozo et al., 2005), but have thus far achieved only short-term reductions in emissions. Current research is aimed at developing plant extracts with more persistent effects.
Plants such as lilies and Allium species (green welsh onions and garlic) contain high levels of allicin, whose thiosulfinate functional group has antimicrobial effects against various microbes, including ruminal bacteria (enteropathogenic bacteria and archaea), and minor effects on rumen microbial fermentation (Reuter, Koch & Lawson, 1996; Calsamiglia et al., 2007; Kamel et al., 2008). In previous studies using allicin-containing plant extracts, Busquet et al. (2005a) and Busquet et al. (2005b) reported that in vitro methane emission, acetate concentration, and methanogenic archaea population decreased, while propionate, butyrate, and ammonia-N concentration increased. Hart et al. (2008) reported stable ruminal fermentation patterns as well as significant reductions in both methane output and methanogenic bacteria population. Kim, Ha & Song (2012) reported in vivo methane and carbon dioxide reduction in the rumen after treatment with powdered garlic. Medicinal properties of green welsh onions (Allium fistulosum L.), especially antifungal and antioxidant properties, were determined; these properties were due to sulfur-containing compounds, flavonoids, fatty acids, and polyphenolic compounds (Štajner & Milić, 2006; Vlase et al., 2013). Plant secondary metabolites, flavonoids, and polyphenolic compounds have direct effects against methanogens (Bodas et al., 2012); these are considered to be an alternative to suppress methane production and increase milk yield and lactation performance in dairy cows (Tedesco et al., 2004; Oskoueian, Abdullah & Oskoueian, 2013). Kim et al. (2012b) reported that total polyphenol content and DPPH radical activity of Allium fistulosum L. (A. fistulosum) extracts were higher than those of A. scorodoprasum var. viviparum Regel (garlic) extract. Therefore, it is believed that A. fistulosum will have methane reduction effects on ruminants. However, most such studies have focused on garlic (Busquet et al., 2005a; Busquet et al., 2005b; Hart et al., 2008; Kim, Ha & Song, 2012; Kim et al., 2012a), while few investigations have examined methane mitigation in ruminants using A. fistulosum.
The objective of this experiment was to determine the effects of A. fistulosum extract on in vitro ruminal fermentation characteristics, gas profiles (gas production, methane and carbon dioxide emission), volatile fatty acid (VFA) profiles (total VFA, acetate, propionate, and butyrate concentration), and microbial population. Our hypothesis was that A. fistulosum extract decreases methane emission, reduces methanogenic archaea population, and does not affect ruminal fermentation characteristics in vitro experiments.
Materials & Methods
Animal use and all experimental protocols were reviewed and approved by the Committee of Gyeongsang National University Animal Research Ethics (GNU-180130-A0007; Jinju, Republic of Korea).
Sample preparation
A. fistulosum extracts were obtained from the Plant Extract Bank (KRIBB, Daejeon, Korea). A. fistulosum leaves were cut into small pieces and dried naturally under shade. Extraction was performed on the dried pieces (100 g each) were then presented with 99.9% methyl alcohol (1,000 mL) using an ultrasonic cleaner (Branson Ultrasonics Corporation, Danbury, CT, USA) at 20 °C for three days (Hwang et al., 2013). After extraction, the solutions were filtered, and the solvents were evaporated under vacuum conditions. Before in vitro incubation, stock solutions (20 mg/mL) of the extracts were dissolved in dimethyl sulfoxide (Sigma-Aldrich Chemical Co., St. Louis, MO, USA) and diluted using a culture medium immediately.
Experimental design for in vitro incubation
Two cannulated rumen Hanwoo cow (mean initial body weight 450 ± 30 kg, standard deviation = 30) were used for rumen fluid collection. Daily amounts of timothy hay and commercial concentrate (BBVMRO 158; Hafeed, Pusan, Korea) were divided at a 60:40 (w/w) ratio and offered in two meals, at 09:00 and 17:00, at 2% of their body weight. Water and mineral vitamin block were available ad libitum. The % dry matter (DM; #934.01) contents of the commercial timothy hay was 8.9% moisture (#984.20), 13.4% crude protein (CP; #976.05), 2.3% ether extracts (#920.39), 21.9% crude fiber (CF; #962.09), 8.6% crude ash (CA; #942.05) (AOAC, 2003; AOAC, 2005), 53.1% neutral detergent fiber, and 30.6% acid detergent fiber (Van Soest, Robertson & Lewis, 1991). The % DM content of the commercial concentrate was 12% CP, 1.5% crude fat (#920.39), 15% CF, 12% CA, 0.75% calcium (#927.02), 0.9% phosphorus (#3964.06) (AOAC, 2003; AOAC, 2005) and 69% total digestible nutrients (National Research Council, 2001).
The contents of the rumen for each cow were collected two hours before morning feeding and transported to the laboratory within 10 min of collection. Rumen fluid contents were squeezed using four layers of cheesecloth to remove feed particles and obtain a pure rumen fluid. Rumen fluid and McDougall’s buffer (McDougall, 1948) were mixed at a 1:2 ratio and bubbled with O2-free N2 gas. O2-free N2 gas was used in this study because other groups generally use O2-free N2 more than O2-free CO2 gas to reduce oxygen solubility in the serum bottle. Fifteen milliliters of the mixture was dispensed into 50 mL serum bottles containing 300 mg (DM basis) of timothy substrate and A. fistulosum extracts (based on the basis of timothy substrate; 0%, 1%, 3%, 5%, 7%, or 9%) with continuous flushing with O2-free N2 gas. Serum bottles were sealed with a butyl rubber stoppers and aluminum caps and placed in a shaking incubator at 120 rpm (SI-900R: Jeio Tech, Daejeon, Korea) for 3, 6, 9, 12, 24, 48, or 72 h at 39 °C. In vitro, incubation used a completely randomized design, and each sample was duplicated. Replicates were performed on three different days with different rumen fluid (n = 3 for each dose in statistical analyses).
Analysis of gas profiles and ruminal fermentation characteristics
Total gas production data were obtained, as described in Theodorou et al. (1994). Specifically, a detachable pressure transducer and digital readout voltmeter (Laurel Electronics, Inc., Costa Mesa, CA, USA) were used to measure the gas pressure in the headspace above the culture medium for each fermentation time. The transducer was modified to connect to the inlet of a disposable Luer-lock three-way stopcock. The gas pressure was read from the LED display after hypodermic needle insertion. Methane and carbon dioxide data were obtained, as described in Ørskov & McDonald (1979). Specifically, gas samples for methane and carbon dioxide were analyzed by gas chromatography (HP 5890; Agilent Technologies, Santa Clara, CA, USA) conducted using a thermal conductivity detector with a Carboxen-1006 Plot capillary column (30 × 0.53 mm; Supelco, Bellefonte, PA, USA) (Zafarian & Manafi, 2013).
Serum bottles were then uncapped, and the culture medium was sampled to measure pH (MP230; Mettler-Toledo, Columbus, OH, USA) and VFA content. VFA data were obtained as described in Han, Kim & Shin (2005) and Tabaru et al. (1988). Specifically, the samples were centrifuged at 10,483 × g for 3 min. The resultant supernatants were filtered through a 0.2 µm disposable syringe filter (Whatman Inc., Clifton, NJ, USA) and subjected to high-performance liquid chromatography (HPLC) (Agilent-1200; Agilent Technologies) using a UV/VIS detector with a MetaCarb 87H column (300 × 7.8 mm; Varian, Palo Alto, CA, USA). Samples were eluted isocratically with 0.0085N H2SO4 at a flow rate of 0.6 ml/min and a column temperature of 35 °C.
Individual VFA concentrations were calculated by converting the ppm value of the peak measured at 14.57 min for acetate, 17.31 min for propionate and 21.53 min for butyrate to mmol/L using each standard curve equation. Total VFAs was calculated as the sum of acetate, propionate, and butyrate concentrations.
The in vitro DM degradability rate was measured by following a modified Ørskov, Hovell & Mould (1980) method using nylon-bag digestion. After each of the incubations, a nylon bag containing serum bottles was rinsed in flowing water until the rinse water ran clear and then it was oven-dried at 65 °C to a constant weight. DM degradability was calculated as the difference between nylon bag weight before and after incubation.
Microbial growth rate
Microbial growth rate data was collected as previously in Lee et al. (2011). Specifically, samples obtained from each fermentation period were centrifuged at 655 × g for 3 min to remove feed particles from the supernatants. Next, this was re-centrifuged at 14,269 × g for 3 min, and sodium phosphate buffer (pH 6.5) was added to precipitates, and this was vortexed three times. The total microbial growth rate was estimated based on optical density values obtained at 550 nm using a spectrophotometer (Model 680; Bio-Rad Laboratories Hercules, CA, USA).
Quantitative real-time polymerase chain reaction (PCR) assays
After 12 and 24 h of incubation, 5 mL samples were collected using a 10-mL syringe (before gas analysis) and stored at −80 °C. Samples were placed in screw-capped tubes containing silica beads for DNA extraction with a high-speed reciprocal shaker, following a modified bead-beating protocol with a soil kit (Macherey-Nagel, Düren, Germany). Briefly, a 1 mL aliquot taken from the culture medium using a wide-bore pipette to ensure the collection of a homogeneous sample. Then, it was centrifuged at 655 × g for 3 min. Nucleic acid concentrations were measured using a NanoDrop spectrophotometer (Thermo Scientific, Wilmington, DE USA). Samples for DNA extraction were stored at −80 °C.
The sequences of PCR primer sets (Table 1) used in this study to amplify total bacteria, including Ruminococcus albus, Fibrobacter succinogenes, and Ruminococcus flavefaciens; methanogenic archaea; and ciliate-associated methanogens were obtained from published reports (Koike & Kobayashi, 2001; Denman & McSweeney, 2006; Skillman et al., 2006; Denman, Tomkins & McSweeney, 2007). The amplification efficiency of each primer set was determined by a 4-point standard curve and a non-template control, which run for each sample to test the relative expression levels. The standard curve was generated from genomic DNA, which was serially diluted. The standard curve slope was used to calculate amplification efficiency. The cycle threshold (Ct) was plotted against logarithmic values of different DNA concentrations using the equation: efficiency = 10−1∕slope. The gene “general bacteria” was used as a reference gene.
Table 1. PCR primer sets for real-time PCR assay.
| Target species | Primer sequence (5′ → 3′) | Size (bp)a | Efficiencyb | References |
|---|---|---|---|---|
| Total bacteria | F: CGGCAACGAGCGCAACCC | 130 | 2.31 | Denman & McSweeney (2006) |
| R: CCATTGTAGCACGTGTGTAGCC | ||||
| Ruminococcus albus | F: CCCTAAAAGCAGTCTTAGTTCG | 176 | 1.83 | Koike & Kobayashi (2001) |
| R: CCTCCTTGCGGTTAGAACA | ||||
| Fibrobacter succinogenes | F: GTTCGGAATTACTGGGCGTAAA | 121 | 2.37 | Denman & McSweeney (2006) |
| R: CGCCTGCCCCTGAAC ATC | ||||
| Ruminococcus flavefaciens | F: CGAACGGAGATAATTTGAGTTTACTTAGG | 132 | 1.97 | Denman & McSweeney (2006) |
| R: CGGTCTCTGTATGTTATGAGGTATTACC | ||||
| Methanogenic archaea | F: TTCGGTGGATCDCARAGRGC | 140 | 2.01 | Denman, Tomkins & McSweeney (2007) |
| R: GBARGTCGWAWCCGTAGAATCC | ||||
| Ciliate-associated methanogens | F: GAGCTAATACATGCTAAGGC | 180 | 1.74 | Skillman et al. (2006) |
| R: CCCTCACTACAATCGAGATTTAAGG |
Notes.
Bp, base pair.
Efficiency is calculated as [10−1/slope].
Quantitative real-time PCR assays to enumerate microbes were performed according to the methods described by Denman & McSweeney (2006) and Denman, Tomkins & McSweeney (2007) (Table 2) in a real-time PCR machine (CFX96 Real-Time system; Bio-Rad Laboratories). All quantitative PCR reaction mixtures (final volume of 20 µL) contained forward and reverse primers, SYBR Green Supermix (QPK-201; Toyobo Co., Ltd., Tokyo, Japan), and a DNA template. A negative control without the DNA template was used in every PCR assay. The Ct values obtained from real-time PCR were used to calculate fold changes in different microbial abundance sizes relative to those in control without additives. The abundance of these microbes was determined using the following equation: relative quantification = 2−ΔCt(Target)−ΔCt(Control).
Table 2. PCR condition for real-time PCR assay.
| Target species | Condition | ||||
|---|---|---|---|---|---|
| Initial denaturation | Denaturation | Annealing | Extention | Cycle | |
| Total bacteria | 95 °C | 95 °C | 57 °C | 72 °C | 40 |
| 3:00 | 0:15 | 0:30 | 0:30 | ||
| Ruminococcus albus | 95 °C | 95 °C | 57 °C | 72 °C | 40 |
| 3:00 | 0:30 | 0:15 | 0:30 | ||
| Fibrobacter succinogenes | 95 °C | 95 °C | 55 °C | 72 °C | 40 |
| 9:00 | 0:30 | 0:30 | 0:30 | ||
| Ruminococcus flavefaciens | 95 °C | 95 °C | 57 °C | 72 °C | 40 |
| 3:00 | 0:15 | 0:30 | 0:30 | ||
| Methanogenic archaea | 95 °C | 95 °C | 62 °C | 72 °C | 47 |
| 3:00 | 0:15 | 0:30 | 0:30 | ||
| Ciliate-associated methanogens | 95 °C | 95 °C | 55 °C | 72 °C | 44 |
| 3:00 | 0:30 | 0:30 | 0:30 | ||
Calculations and statistical analysis
To obtain a precise estimate of gas production throughout the fermentation process, the data were fitted to the exponential equation Gp = a + b(1 − exp−c×time), as described by Ørskov & McDonald (1979). Gp represents gas production (mL/g DM of the substrate) at time t; a, b, and c are the scaling factors for the Y-axis intercept (mL/g of DM), potential gas production (mL/g of DM), and the constant rate for gas production per hour, respectively. The gas production rate was fitted to the model using the SAS 9.4 software (SAS Institute, Inc., Cary, NC, USA), and the parameters were estimated using PROC NLIN. Effective gas production (EGp, i.e., substrate availability) from the culture was determined by EGp = a + b(kd∕[kd + kp]), where kd is the constant, and kp is the passage rate constant assumed to be 0.04/h (National Research Council, 1989).
All experimental data were analyzed based on the general linear model (GLM) procedures using SAS 9.4 software (SAS Institute, Inc., Cary, NC, USA). The model included terms for extract concentration, incubation time, and their interaction. To determine the effective extract concentration data were analyzed according to the following model: yij = μ + ai + eij; where yij is the jth observation in the ith extract concentration, µis the overall mean, ai is the fixed effect of the extract (ai = 0%, 1%, 3%, 5%, 7%, or 9%) and eij is the unexplained random error. Orthogonal contrast was used to assess the treatment, linear, and quadratic relationships between the concentration levels of A. fistulosum, and the dependent variables. Orthogonal coefficients for unequally spaced concentrations were acquired using the interactive matrix language (IML) procedure in SAS 9.4. Differences among treatments were assessed using Duncan’s multiple range tests, in which p-value < 0.05 indicated a statistically significant value, whereas p-value < 0.10 was considered a tendency.
Results
pH linearly increased with A. fistulosum extract concentration at 3 and 24 h incubation (p-value < 0.0001; p-value = 0.0004, respectively; Table 3). Microbial growth rate was not significantly affected (p-value > 0.05) by any concentration of A. fistulosum extract at 12 h incubation. Conversely, microbial growth rate quadratically decreased (p-value = 0.012) as A. fistulosum extract concentration increased at 24 h incubation.
Table 3. Effects of Allium fistulosum L. extracts on in vitro ruminal fermentation characteristics.
| Incubation time (h) | Extract concentratione, % | SEMf | p-valueg | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 3 | 5 | 7 | 9 | T | L | Q | ||
| pH | ||||||||||
| 3 | 7.36d | 7.37cd | 7.44b | 7.40c | 7.48a | 7.47ab | 0.01 | <0.0001 | <0.0001 | 0.195 |
| 6 | 7.34b | 7.36b | 7.39ab | 7.41ab | 7.44a | 7.46a | 0.02 | 0.015 | 0.0005 | 0.771 |
| 9 | 7.24b | 7.31a | 7.34a | 7.32a | 7.33a | 7.32a | 0.02 | 0.042 | 0.029 | 0.031 |
| 12 | 7.11b | 7.26ab | 7.30ab | 7.31ab | 7.33ab | 7.36a | 0.07 | 0.276 | 0.043 | 0.376 |
| 24 | 7.14c | 7.19bc | 7.20bc | 7.25ab | 7.22b | 7.29a | 0.02 | 0.006 | 0.0004 | 0.727 |
| 48 | 6.68d | 6.71cd | 6.74bcd | 6.77abc | 6.81ab | 6.84a | 0.02 | <0.0001 | 0.978 | 0.920 |
| 72 | 6.59b | 6.65b | 6.61b | 6.62b | 6.65b | 6.72a | 0.02 | 0.002 | 0.067 | 0.131 |
| Microbial growth rate, OD at 550 nm | ||||||||||
| 3 | 0.18 | 0.17 | 0.21 | 0.19 | 0.19 | 0.20 | 0.01 | 0.396 | 0.320 | 0.677 |
| 6 | 0.39a | 0.30ab | 0.41a | 0.40a | 0.25b | 0.38a | 0.03 | 0.023 | 0.464 | 0.935 |
| 9 | 0.41 | 0.44 | 0.39 | 0.42 | 0.45 | 0.43 | 0.04 | 0.910 | 0.642 | 0.820 |
| 12 | 0.42 | 0.40 | 0.37 | 0.45 | 0.49 | 0.38 | 0.03 | 0.201 | 0.614 | 0.317 |
| 24 | 0.44a | 0.40ab | 0.37b | 0.36b | 0.38b | 0.39b | 0.02 | 0.053 | 0.049 | 0.012 |
Notes.
Means with different superscript letters in the same row indicate significant differences (P < 0.05).
Allium fistulosum L. extract concentrations are based on quantity of timothy hay (300 mg) substrate.
SEM, standard error of the mean.
T, treatment; L, linear; Q, quadratic effect.
OD, optical density.
n = 3.
Dry matter (DM) degradability was not significantly affected (p-value > 0.05) by any concentration of A. fistulosum extract, except for 24 h incubation, at which it was decreased by a 1% A. fistulosum extract (Table 4). Effective DM degradability rate (EDM) tended to decrease linearly (p-value = 0.051) with increases in A. fistulosum extract concentration.
Table 4. Effects of Allium fistulosum L. extracts on in vitro dry matter degradability.
| Incubation time (h) | Extract concentrationc, % | SEMd | P-valuee | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 3 | 5 | 7 | 9 | T | L | Q | ||
| DM degradability, % | ||||||||||
| 3 | 18.88 | 19.02 | 19.17 | 18.86 | 18.47 | 17.99 | 0.39 | 0.360 | 0.063 | 0.209 |
| 6 | 21.42 | 18.81 | 19.79 | 19.87 | 19.91 | 17.91 | 1.10 | 0.389 | 0.176 | 0.726 |
| 9 | 20.64 | 20.46 | 21.20 | 21.04 | 20.60 | 21.49 | 0.80 | 0.931 | 0.509 | 0.971 |
| 12 | 20.26 | 19.58 | 19.88 | 19.81 | 18.92 | 18.63 | 0.67 | 0.535 | 0.095 | 0.636 |
| 24 | 29.85a | 27.32b | 29.89a | 29.62ab | 28.38ab | 28.55ab | 0.70 | 0.132 | 0.639 | 0.454 |
| 48 | 37.27 | 38.23 | 36.80 | 38.26 | 36.75 | 37.28 | 0.53 | 0.235 | 0.464 | 0.808 |
| 72 | 42.23 | 42.33 | 40.83 | 41.39 | 41.63 | 40.19 | 1.50 | 0.904 | 0.370 | 0.998 |
| DM degradability parametersf | ||||||||||
| a, % | 6.45 | 7.17 | 6.03 | 6.18 | 6.66 | 5.84 | 0.50 | 0.496 | 0.281 | 0.929 |
| b, % | 34.52 | 35.32 | 33.00 | 34.30 | 33.65 | 33.60 | 1.13 | 0.755 | 0.391 | 0.689 |
| a + b, % | 40.97 | 42.49 | 39.04 | 40.46 | 40.31 | 39.44 | 1.56 | 0.690 | 0.333 | 0.774 |
| k, DM/h | 0.063 | 0.046 | 0.066 | 0.060 | 0.054 | 0.058 | 0.01 | 0.575 | 0.974 | 0.846 |
| EDM | 26.76a | 25.95ab | 26.46ab | 26.61ab | 25.92ab | 25.68b | 0.29 | 0.106 | 0.051 | 0.366 |
Notes.
Means with different superscript letters in the same row indicate significant differences (P < 0.05).
Allium fistulosum L. extract concentrations are based on quantity of timothy hay (300 mg) substrate.
SEM, standard error of the mean.
T, treatment; L, linear; Q, quadratic effect.
Potential extent and rate of dry matter degradability were determined using the exponential model: DM = a + b(1 − exp−c×time), where DM is dry matter degradability (%) at time t; a, dry matter degradability from the immediately soluble fraction; b, dry matter degradability from the insoluble fraction; c, the fractional rate of dry matter degradability per hour; a + b, potential extent of dry matter; EDM, effective dry matter degradability rate from cultures, calculated as EDM = a + b[kd∕(kd + kp)], where kd is a dry matter degradability rate constant, and kp is a passage rate constant assumed to be 0.04 h−1.
DM, dry matter.
n = 3.
Cumulative gas production was not significantly affected (p-value > 0.05) by any concentration of A. fistulosum extract at 9 and 12 h incubation (Table 5). However, it increased linearly with the increase in A. fistulosum extract concentration at 48 and 72 h incubation (p-value = 0.0002 and p-value = 0.002, respectively). Potential gas production (a + b) was increased linearly (p-value < 0.0001) with the increase in A. fistulosum extract concentration. However, the effective gas production rate (EGp) was not significantly affected (p- value > 0.05) by any concentration of A. fistulosum extract.
Table 5. Effects of Allium fistulosum L. extracts on in vitro cumulative gas production.
| Incubation time (h) | Extract concentrationf, % | SEMg | p-valueh | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 3 | 5 | 7 | 9 | T | L | Q | ||
| Gas production, mL/0.1 g DM | ||||||||||
| 3 | 16.78ab | 17.00a | 16.44bc | 17.03a | 16.27c | 16.48bc | 0.13 | 0.007 | 0.013 | 0.760 |
| 6 | 17.78a | 17.73a | 17.46ab | 17.22b | 17.15b | 17.25b | 0.10 | 0.002 | 0.0001 | 0.037 |
| 9 | 19.13 | 18.58 | 18.72 | 18.73 | 18.93 | 18.65 | 0.21 | 0.507 | 0.557 | 0.634 |
| 12 | 17.27 | 17.34 | 17.31 | 17.31 | 17.25 | 17.49 | 0.09 | 0.561 | 0.334 | 0.342 |
| 24 | 22.01ab | 21.39bc | 22.14a | 21.80abc | 22.06ab | 21.30c | 0.20 | 0.051 | 0.318 | 0.074 |
| 48 | 24.71d | 25.64ab | 25.64ab | 25.53bc | 25.28c | 25.85a | 0.09 | <0.0001 | 0.0002 | 0.053 |
| 72 | 26.75d | 26.99cd | 27.64abc | 28.02ab | 28.13a | 27.38bcd | 0.21 | 0.003 | 0.002 | 0.0009 |
| Gas production parametersi | ||||||||||
| a, mL/0.1 g DM | 0.98b | 1.26b | 1.95a | 1.99a | 2.03a | 1.79a | 0.11 | <0.0001 | <0.0001 | <0.0001 |
| b, mL/0.1 g DM | 21.80de | 21.78e | 22.18ab | 22.00bc | 22.24a | 21.98cd | 0.06 | 0.0008 | 0.002 | 0.002 |
| a + b, mL/0.1 g DM | 22.78c | 23.04c | 24.13a | 23.99ab | 24.27a | 23.77b | 0.11 | <0.0001 | <0.0001 | <0.0001 |
| k, Gp/h | 0.281a | 0.258b | 0.195c | 0.202c | 0.186c | 0.205c | 0.01 | <0.0001 | <0.0001 | <0.0001 |
| EGp | 20.06 | 20.11 | 20.34 | 20.35 | 23.70 | 20.19 | 1.40 | 0.435 | 0.347 | 0.554 |
Notes.
Means with different superscript letters in the same row indicate significant differences (P < 0.05).
Allium fistulosum L. extract concentrations are based on quantity of timothy hay (300 mg) substrate.
SEM, standard error of the mean.
T, treatment; L, linear; Q, quadratic effect.
Potential extent and rate of gas production were determined using the exponential model: Gp = a + b(1 − exp−c×time), where Gp is gas production (mL/g DM) at time t; a, gas production from the immediately soluble fraction; b, gas production from insoluble fraction; c, the fractional rate of gas production per hour; a + b, potential extent of gas production; k, gas production rate constant for the insoluble fraction; EGp, effective gas production rate from the cultures, calculated as EGp = a + b[kd∕(kd + kp)], where kd is a gas production rate constant, and kp is a passage rate constant assumed to be 0.04 h−1.
DM, dry matter.
n = 3.
Methane emission linearly and quadratically decreased (p-value < 0.0001 and p-value = 0.002) as A. fistulosum extract concentration increased at 12 h incubation (Table 6). Moreover, methane emission linearly decreased (p-value = 0.0003) as A. fistulosum extract concentration increased at 24 h incubation. Carbon dioxide emission tendency decreased (p-value = 0.057) with all concentrations of A. fistulosum extract at 12 h incubation.
Table 6. Effects of Allium fistulosum L. extracts on in vitro methane and carbon dioxide emission.
| Incubation time (h) | Extract concentrationd, % | SEMe | p-valuef | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 3 | 5 | 7 | 9 | T | L | Q | ||
| Methane, mL/g DM | ||||||||||
| 3 | 3.55a | 3.04ab | 2.75ab | 2.64ab | 2.09b | 2.66ab | 0.32 | 0.112 | 0.021 | 0.126 |
| 6 | 6.80a | 5.11bc | 4.98bc | 4.25c | 5.71ab | 6.25ab | 0.38 | 0.006 | 0.892 | 0.0005 |
| 9 | 11.04 | 10.85 | 8.97 | 10.37 | 11.03 | 10.82 | 0.80 | 0.468 | 0.833 | 0.207 |
| 12 | 12.36a | 9.83b | 7.87c | 8.43bc | 7.84c | 7.94c | 0.50 | 0.0002 | <0.0001 | 0.002 |
| 24 | 20.06a | 20.78a | 19.23ab | 15.61bc | 15.67bc | 14.04c | 1.20 | 0.015 | 0.0003 | 0.923 |
| Carbon dioxide, mL/g DM | ||||||||||
| 3 | 57.54a | 58.35a | 47.72ab | 42.73b | 38.87b | 42.12b | 3.46 | 0.006 | 0.0004 | 0.084 |
| 6 | 52.91a | 46.17ab | 49.30ab | 42.84b | 55.12a | 54.34a | 2.94 | 0.069 | 0.211 | 0.048 |
| 9 | 65.96 | 65.16 | 61.90 | 70.29 | 72.84 | 70.19 | 4.28 | 0.513 | 0.154 | 0.901 |
| 12 | 63.97a | 52.48b | 51.13b | 55.00b | 50.89b | 52.48b | 2.87 | 0.057 | 0.050 | 0.104 |
| 24 | 89.69 | 94.30 | 94.45 | 87.70 | 137.08 | 86.41 | 17.15 | 0.340 | 0.484 | 0.533 |
Notes.
Means with different superscript letters in the same row indicate significant differences (P < 0.05).
Allium fistulosum L. extract concentrations are based on quantity of timothy hay (300 mg) substrate.
SEM, standard error of the mean.
T, treatment; L, linear; Q, quadratic effect.
DM, dry matter.
n = 3.
Acetate concentration linearly decreased (p-value = 0.003) as A. fistulosum extract concentration increased at 12 h incubation (Table 7). However, total VFA concentration was not significantly affected (p-value > 0.05) by concentration of A. fistulosum extract at 12 h incubation. Propionate concentration linearly decreased (p-value = 0.035) as A. fistulosum extract concentration increased at 24 h incubation. Butyrate concentration linearly increased (p-value < 0.0001) as A. fistulosum extract concentration increased at 24 h incubation. The acetate to propionate (A/P) ratio increased linearly (p-value = 0.003) with the increase in A. fistulosum extract concentration at 24 h incubation.
Table 7. Effects of Allium fistulosum L. extract on VFA profiles.
| Incubation time (h) | Extract concentrationd, % | SEMe | p-valuef | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 3 | 5 | 7 | 9 | T | L | Q | ||
| Total VFA, mmol/L | ||||||||||
| 12 | 62.48 | 58.71 | 55.04 | 56.63 | 56.43 | 55.98 | 2.20 | 0.554 | 0.179 | 0.311 |
| 24 | 73.15ab | 70.64b | 72.42b | 70.23b | 76.02a | 73.44ab | 0.96 | 0.012 | 0.056 | 0.223 |
| Acetate, mmol/L | ||||||||||
| 12 | 41.92a | 39.59ab | 37.33b | 38.17b | 37.48b | 37.31b | 0.88 | 0.018 | 0.003 | 0.053 |
| 24 | 49.51 | 47.67 | 48.96 | 47.60 | 49.40 | 47.70 | 0.59 | 0.101 | 0.385 | 0.903 |
| Propionate, mmol/L | ||||||||||
| 12 | 12.65 | 11.79 | 11.93 | 11.91 | 11.66 | 11.67 | 0.44 | 0.646 | 0.210 | 0.588 |
| 24 | 16.92a | 16.02b | 16.33ab | 15.78b | 16.13ab | 15.88b | 0.25 | 0.076 | 0.035 | 0.227 |
| Butyrate, mmol/L | ||||||||||
| 12 | 7.91a | 7.32ab | 5.78c | 6.55bc | 7.30ab | 7.00ab | 1.07 | 0.012 | 0.284 | 0.005 |
| 24 | 6.72b | 6.95b | 7.13b | 6.85b | 10.48a | 9.86a | 0.28 | <0.0001 | <0.0001 | 0.012 |
| Acetate to propionate ratio | ||||||||||
| 12 | 3.34ab | 3.36a | 3.13b | 3.21ab | 3.22ab | 3.20ab | 0.06 | 0.169 | 0.082 | 0.151 |
| 24 | 2.93c | 2.98bc | 3.00ab | 3.02ab | 3.06a | 3.00ab | 0.02 | 0.013 | 0.003 | 0.024 |
Notes.
Means with different superscript letters in the same row indicate significant difference (P < 0.05).
Allium fistulosum L. extract concentrations are based on quantity of timothy hay (300 mg) substrate.
SEM, standard error of the mean.
T, treatment; L, linear Q, quadratic effect.
VFA, volatile fatty acids.
n = 3.
F. succinogenes abundance linearly decreased (p-value = 0.028) with increasing A. fistulosum extract concentration at 12 h incubation (Fig. 1 and Table S1). R. albus abundance linearly increased (p-value = 0.004) with increasing A. fistulosum extract concentration at 24 h incubation. F. succinogenes (p-value = 0.046) and R. flavefaciens abundances (p-value = 0.048) significantly increased in the 7% A. fistulosum extract group compared to the levels in the 0% group at 24 h incubation. Methanogenic archaea abundance tended decrease (p-value = 0.055) in the 1%, 7%, and 9% A. fistulosum extract groups compared to the levels in the 0% group at 12 h incubation. Moreover, methanogenic archaea abundance linearly and quadratically decreased (p-value < 0.0001) with the increase in concentration of A. fistulosum extract at 24 h incubation.
Figure 1. Relative quantification of rumen microbial abundance at 12 h (A) and 24 h (B) incubation at different concentrations of Allium fistulosum L. extract.
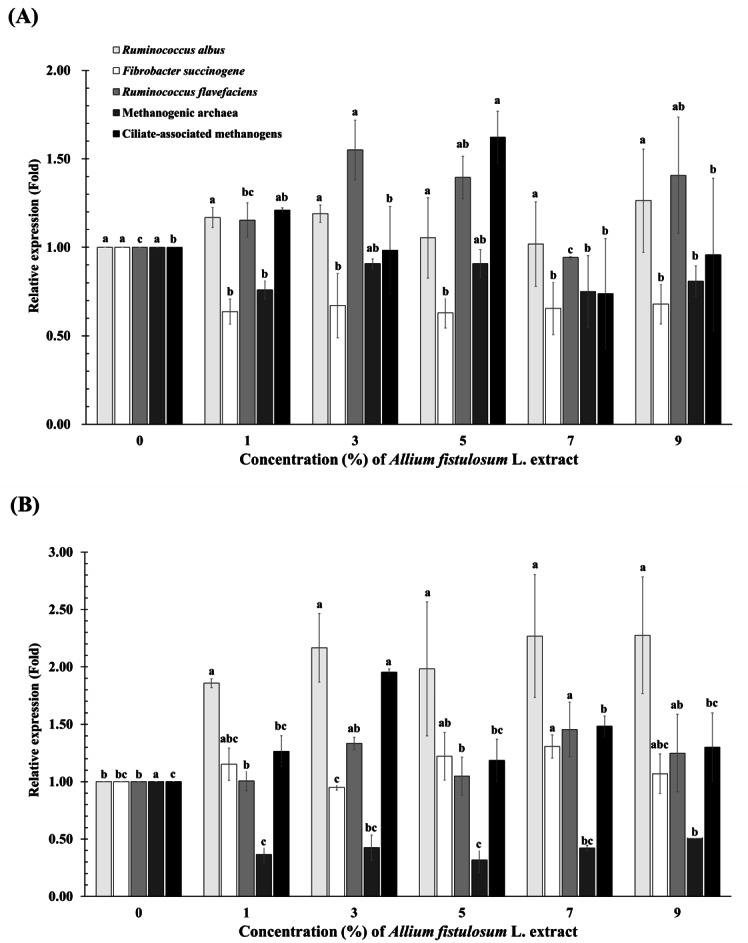
(A–C) Means with different superscript letters in the same row indicate significant differences (P < 0.05).
Discussion
Hiltner & Dehority (1983) reported that a normal ruminal microbial activity pH range was 5.8–7.2. If the pH in the rumen is high, degradability, microbial growth, gas production, and total VFA concentration may decrease. In the current study, pH in groups receiving A. fistulosum extracts was higher than that in the 0% group and consistently remained between 6.59 and 7.48. This result is thought to be influenced by the N2 gas used for anaerobic maintenance, which is similar to the results of pH and dry matter digestibility from Hu et al. (2005) and Chowdhury et al. (2018). Also, these results are similar to those of Chaves et al. (2007), wherein pH was increased with the addition of garlic extract. Ruminal microbial activity is affected by plant extracts and secondary plant metabolites (Busquet et al., 2006). A. fistulosum contains high levels of the secondary plant metabolite allicin, which has previously been shown to have antibacterial activity (Ankri & Mirelman, 1999), and Reuter, Koch & Lawson (1996) reported that it has antimicrobial activity against ruminal bacteria. Kaempferol is a major flavonoid in the green welsh onion, whose antioxidant activity is higher than those of other welsh onion species (Aoyama & Yamanoto, 2007). Kaempferol is also associated with reduced total gas production in ruminants (Oskoueian, Abdullah & Oskoueian, 2013). Ruminal microbial activity is closely correlated with total gas production, which in turn is related to DM degradability (Oskoueian, Abdullah & Oskoueian, 2013; Getachew et al., 2004). In the current study, supplementation with A. fistulosum extract resulted in a significantly decreased microbial growth rate at 24 h incubation. Additionally, gas production at 24 h incubation was significantly decreased by 9% A. fistulosum extract. This result agrees with previous research showing that the ruminal microbial growth rate is closely correlated with gas production. The major substrates used by ruminal methanogens during methanogenesis are hydrogen, carbon dioxide, and methyl groups (Liu & Whitman, 2008), with 80% of total enteric methane emissions generated from these substrates (Whitman, Bowen & Boone, 1992). Acetate and butyrate formation result in additional hydrogen production, while propionate reduces hydrogen available for methanogenesis and inhibits ruminal fermentation (Moss, Jouany & Newbold, 2000; Faniyi et al., 2016; Kumar et al., 2018). Ruminal ciliate protozoa are intimately involved in methanogenesis partly via their abundant hydrogen production. Newbold, Lassalas & Jouany (1995) reported that ciliate protozoa were responsible for 9% to 25% of the methanogenesis in the rumen. Methanogenic archaea can be divided into free-living species (Methanomicrobiales sp. and Methanosarcinales sp.) and species associated with protozoa (Methanobrevibacter sp. and Methanococcales sp.) (Tymensen, Beauchemin & McAllister, 2012; Kim et al., 2013). Their population ratios can change with dietary changes which may lead to decreases protozoal diversity (Hegarty, 1999). These methanogens play a role in methane emission and loss of energy from the ingested feed-in ruminants. Therefore, reducing methane in ruminants will require the control of associated microorganisms. Allicin inhibits thiol enzyme reactivation, and this effect may inhibit methanogenic archaea activity (Ankri & Mirelman, 1999; Busquet et al., 2006). Busquet et al. (2005b) suggested that the essential oil in garlic (Allium sativum species) might inhibit methane emission due to organosulfur compounds, which in turn may inhibit HMG-CoA reductase that catalyzes the synthesis of isoprenoid units in the membranes of methanogenic archaea. Previous studies have shown that flavonoid-rich plant extracts hindered methanogens and reduced ruminal methanogenesis (Patra & Saxena, 2010; Becker et al., 2014). Supplementation with A. fistulosum extract also resulted in linearly decreased methane emission at 12 h incubation, which can be partially explained by decreased methanogenic archaea abundance and acetate concentration. The carbon dioxide can be used as a substrate for methanogenesis in ruminants (Balch, Fox & Magrum, 1979) and is the most highly produced gas in rumen fermentation during metabolism of pyruvic acid in the production of acetate. In the current study, carbon dioxide emission linearly decreased at 12 h incubation. A. fistulosum extracts showed no correlation with methane and carbon dioxide generation. However, it is thought to have a simultaneous reduction effect on methane and carbon dioxide emission. This result was similar to that of an in vivo study by Kim, Ha & Song (2012), who reported that the addition of garlic powder to Hanwoo cow diets increased methane and carbon dioxide emission. At 24 h incubation, methane emission and methanogenic archaea abundance decreased linearly. Therefore, we propose that A. fistulosum extracts used in our study reduced methane emission by direct inhibition of methanogenic archaea abundances. However, the cause underlying the inverse relationship between propionate concentration, ciliate-associated methanogen abundances, and methane emission remained unclear. These results were similar to Busquet et al. (2005b) and Zafarian & Manafi (2013), who used garlic extract in the in vitro study and garlic powder in the in vivo study. Ruminal cellulolysis is conducted primarily by R. albus, F. succinogenes, and R. flavefaciens (Dehority, 1993; Busquet et al., 2006), and their relative abundances can impact the ratios of VFAs available to ruminants (Kim et al., 2013; Kim et al., 2018) as well as hydrogen and carbon dioxide emission (Latham & Wolin, 1977; Ntaikou et al., 2008). Busquet et al. (2006) reported that high concentrations of various plant extracts decreased total VFA concentration, possibly reflecting decreased feed digestion. In addition, reduction of methane emission from the rumen, have been accompanied by reduced fiber digestibility, thus, influencing energy input into the ruminant (Kim et al., 2018). F. succinogenes is involved in the production of acetate and succinate by digestion of polysaccharides in the rumen (Bryant et al., 1958; Dehority, 2003). On the other hand, it produces acetate, which is the precursor (accounting for 72%) for methane emission during anaerobic digestion (Grady, Daigger & Lim, 1999). In addition, garlic extracts also reduced acetate and branched chain fatty acid levels (Busquet et al., 2005b). In the current study, a decrease in ruminal acetate concentration was observed after addition A. fistulosum extracts, which was consistent with the observed decrease in the abundance of the gram-negative F. succinogenes at 12 h incubation. This result was similar to that of in vitro study Lee et al. (2018), who reported that the addition of A. fistulosum powder to decreased in the abundance of gram-negative F. succinogenes. However, total VFA concentration and abundance of R. albus and R. flavefaciens were not significantly different from levels in the 0% extract group. The abundance of cellulolytic bacteria (R. albus, R. flavefaciens, and F. succinogenes) significantly increased with addition of 7% extract at 24 h incubation. Moreover, total VFA concentration was numerically increased and butyrate concentration significantly increased. However, propionate concentration significantly decreased and A/P ratio significantly increased. This result was similar to Ma et al. (2016), who reported that the addition of allicin to the feed provided to ewes increased total VFA, butyrate concentration and abundance of cellulolytic bacteria. In addition, in an in vitro study, Chaves et al. (2007) found decreased propionate concentration and increased A/P ratio by the addition of garlic extracts. Therefore, these findings indicate that A. fistulosum extract reduced methane emission without affecting feed efficiency in ruminants. However, further studies are needed to clarify the relationship between methanogens and A. fistulosum extracts observed in this study.
Conclusions
The results of our study indicate that the addition of A. fistulosum extract does not alter fermentation characteristics (pH, microbial growth rate, gas production, and total VFA concentration) or DM degradability. However, A. fistulosum extract appears to reduce methane emission (decreased methanogenic activity by archaea due to decreased carbon dioxide emission and acetate concentrations). Further research is required to clarify the specific effects of A. fistulosum extracts on feed intake, feed use efficiency, and methane emission.
Supplemental Information
xAllium fistulosum L. extract concentrations are based on quantity of timothy hay (300 mg) substrate.
ySEM, standard error of the mean.
zT: treatment; L: linear; Q: quadratic effect
a–cMeans with different superscript letters in the same row indicate significant differences (P < 0.05).
n = 3.
Funding Statement
This work was carried out with the support of the “Cooperative Research Program for Agriculture Science & Technology Development (Project No. 01266401)”, Rural Development Administration, Republic of Korea. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
The authors declare there are no competing interests.
Author Contributions
Jun Sik Eom and Shin Ja Lee conceived and designed the experiments, performed the experiments, analyzed the data, prepared figures and/or tables, and approved the final draft.
Yejun Lee, Hyun Sang Kim, You Young Choi and Hyeong Suk Kim performed the experiments, analyzed the data, prepared figures and/or tables, and approved the final draft.
Do Hyung Kim conceived and designed the experiments, authored or reviewed drafts of the paper, and approved the final draft.
Sung Sill Lee conceived and designed the experiments, analyzed the data, authored or reviewed drafts of the paper, and approved the final draft.
Animal Ethics
The following information was supplied relating to ethical approvals (i.e., approving body and any reference numbers):
Animal use and all experimental protocols were reviewed and approved by the Committee of Gyeongsang National University Animal Research Ethics (GNU-180130-A0007; Jinju, Republic of Korea)
Data Availability
The following information was supplied regarding data availability:
Raw data is available in Supplemental File.
References
- Ankri & Mirelman (1999).Ankri S, Mirelman D. Antimicrobial properties of allicin from garlic. Microbes and Infection. 1999;1(2):125–129. doi: 10.1016/S1286-4579(99)80003-3. [DOI] [PubMed] [Google Scholar]
- AOAC (2003).AOAC International . Official methods of analysis. Seventeen Edition Association of Official Analytical Chemists; Gaithersburg: 2003. [Google Scholar]
- AOAC (2005).AOAC International . Official methods of analysis. Eighteen Edition Association of Official Analytical Chemists; Gaithersburg: 2005. [Google Scholar]
- Aoyama & Yamanoto (2007).Aoyama S, Yamanoto Y. Antioxidant activity and flavonoid content of welsh onion (Allium fistulosum) and the effect of thermal treatment. Food Science and Technology Research. 2007;13(1):67–72. doi: 10.3136/fstr.13.67. [DOI] [Google Scholar]
- Balch, Fox & Magrum (1979).Balch WE, Fox GE, Magrum LJ. Methanogens: reevaluation of a unique biological group. Microbiology Reviews. 1979;43(2):260–296. doi: 10.1128/MMBR.43.2.260-296.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchemin et al. (2008).Beauchemin KA, Kreuzer M, O’Mara F, McAllister TA. Nutritional management for enteric methane abatement: a review. Australian Journal of Experimental Agriculture. 2008;48(2):21–27. doi: 10.1071/EA07199. [DOI] [Google Scholar]
- Becker et al. (2014).Becker PM, Van Wikselaar PG, Franssen MCR, De Vos RCH, Hall RD, Beekwilder J. Evidence for a hydrogen-sink mechanism of (+) catechin-mediated emission reduction of the ruminant greenhouse gas methane. Metabolomics. 2014;10(2):179–189. doi: 10.1007/s11306-013-0554-5. [DOI] [Google Scholar]
- Bodas et al. (2012).Bodas R, Prieto N, Garcia-Gonzalez R, Andres S, Giraldez FJ, Lopez S. Manipulation of rumen fermentation and methane production with plant secondary metabolites. Animal Feed Science and Technology. 2012;176(1–4):78–93. doi: 10.1016/j.anifeedsci.2012.07.010. [DOI] [Google Scholar]
- Bryant et al. (1958).Bryant MP, Small N, Bouma C, Robinson IM. Studies on the composition of the ruminal flora and fauna of young calves. Journal of Dairy Science. 1958;41(12):1747–1767. doi: 10.3168/jds.S0022-0302(58)91160-3. [DOI] [Google Scholar]
- Busquet et al. (2005b).Busquet M, Caisamiglia S, Ferret A, Cardozo PW, Kamel C. Effect of garlic oil and four of its compounds on rumen microbial fermentation. Journal of Dairy Science. 2005b;145(1–4):351–363. doi: 10.1016/j.anifeedsci.2007.05.050. [DOI] [PubMed] [Google Scholar]
- Busquet et al. (2005a).Busquet M, Caisamiglia S, Ferret A, Kamel C. Screening for effects of plant extracts and active compounds of plants on dairy cattle rumen microbial fermentation in a continuous culture system. Animal Feed Science and Technology. 2005a;123–124(2):597–613. doi: 10.1016/j.anifeedsci.2005.03.008. [DOI] [Google Scholar]
- Busquet et al. (2006).Busquet M, Calsamiglia S, Ferret A, Kamel C. Plant extracts affect in vitro rumen microbial fermentation. Journal of Dairy Science. 2006;89(2):761–771. doi: 10.3168/jds.S0022-0302(06)72137-3. [DOI] [PubMed] [Google Scholar]
- Calsamiglia et al. (2007).Calsamiglia S, Busquest M, Cardozo PW, Castillejos L, Ferret A. Invited review: essential oils as modifiers of rumen microbial fermentation. Journal of Dairy Science. 2007;90(6):2580–2595. doi: 10.3168/jds.2006-644. [DOI] [PubMed] [Google Scholar]
- Cardozo et al. (2005).Cardozo PW, Caisamiglia S, Ferret A, Kamel C. Screening for the effects of natural plant extracts at different pH on in vitro rumen microbial fermentation of a high-concentrate diet for beef cattle. Journal of Animal Science. 2005;83(11):2572–2579. doi: 10.2527/2005.83112572x. [DOI] [PubMed] [Google Scholar]
- Chaves et al. (2007).Chaves AV, He ML, Yang WZ, Hristov AN, McAllister TA, Benchaar C. Effects of essential oils on proteolytic, deaminative and methanogenic activities of mixed ruminal bacteria. Canadian Journal of Animal Science. 2007;88(1):117–122. doi: 10.4141/CJAS07061. [DOI] [Google Scholar]
- Chowdhury et al. (2018).Chowdhury MR, Khan MMH, Mahfuz SU, Baset MA. Effects of dietary supplementation of spices on forage degradability, ruminal fermentation, in vivo digestibility, growth performance and nitrogen balance in Black Bengal goat. Journal of Animal Physiology and Animal Nutrition. 2018;102(2):e591–e598. doi: 10.1111/jpn.12800. [DOI] [PubMed] [Google Scholar]
- Dehority (1993).Dehority BA. Microbial ecology of cell wall fermentation. In: Jung HG, Buxton DR, editors. Forage cell wall structure and digestibility. Hatfield & J Ralph. American Society of Agronomy/Crop Science Society of America/Soil Science Society of America; Madison: 1993. pp. 425–453. [Google Scholar]
- Dehority (2003).Dehority BA. Rumen microbiology. Nottingham University Press; Nottingham: 2003. [Google Scholar]
- Denman & McSweeney (2006).Denman SE, McSweeney CS. Development of a real-time PCR assay for monitoring anaerobic fungal and cellulolytic bacterial populations within the rumen. FEMS Microbiology Ecology. 2006;58(3):572–582. doi: 10.1111/j.1574-6941.2006.00190.x. [DOI] [PubMed] [Google Scholar]
- Denman, Tomkins & McSweeney (2007).Denman SE, Tomkins NW, McSweeney CS. Quantitation and diversity analysis of ruminal methanogenic populations in response to the antimethanogenic compound bromochloromethane. FEMS Microbiology Ecology. 2007;62(3):313–322. doi: 10.1111/j.1574-6941.2007.00394.x. [DOI] [PubMed] [Google Scholar]
- Faniyi et al. (2016).Faniyi TO, Prates ER, Adewumi MK, Bankole T. Assessment of herbs and spices extracts/meal on rumen fermentation: review. PUBVET. 2016;10:427–438. doi: 10.22256/pubvet.v10n5. [DOI] [Google Scholar]
- Getachew et al. (2004).Getachew G, Robinson PH, DePeters EJ, Taylor SJ. Relationships between chemical compositions, dry matter degradation and in vitro gas production of several ruminant feeds. Animal Feed Science and Technology. 2004;111(1–4):57–71. doi: 10.1016/S0377-8401(03)00217-7. [DOI] [Google Scholar]
- Grady, Daigger & Lim (1999).Grady CPL, Daigger GT, Lim HC. Biological wastewater treatment. Second Edition. New York: Marcel, Inc.; 1999. pp. 599–604. [Google Scholar]
- Han, Kim & Shin (2005).Han SK, Kim SH, Shin HS. UASB treatment of wastewater with VFA and alcohol generated during hydrogen fermentation of food waste. Process Biochemistry. 2005;40(8):2897–2905. doi: 10.1016/j.procbio.2005.01.005. [DOI] [Google Scholar]
- Hart et al. (2008).Hart KJ, Yáñez Ruiz DR, Duval SM, McEwan NR, Newbold CJ. Plant extracts to manipulate rumen fermentation. Animal Feed Science and Technology. 2008;147(1–3):8–35. doi: 10.1016/j.anifeedsci.2007.09.007. [DOI] [Google Scholar]
- Hegarty (1999).Hegarty RS. Reducing rumen methane emissions through elimination of rumen protozoa. Australian Journal of Agricultural Research. 1999;50(8):1321–1328. doi: 10.1071/AR99008. [DOI] [Google Scholar]
- Hiltner & Dehority (1983).Hiltner P, Dehority B. Effect of soluble carbohydrates on digestion of cellulose by pure culture of rumen bacteria. Applied and Environmental Microbiology. 1983;46(3):642–648. doi: 10.1128/AEM.46.3.642-648.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu et al. (2005).Hu WL, Liu JX, Ye JA, Wu YM, Guo YQ. Effect of tea saponin on rumen fermentation in vitro. Animal Feed Science and Technology. 2005;120(2005):333–339. doi: 10.1016/j.anifeedsci.2005.02.029. [DOI] [Google Scholar]
- Hwang et al. (2013).Hwang HS, Ha DU, Lee SK, Lee ID, Lee SJ, Lee SS. Effects of terpenoids-rich plant extracts on ruminal fermentation and methane production. Korean Journal of Organic agriculture. 2013;21(4):629–646. doi: 10.11625/KJOA.2013.21.4.629. [DOI] [Google Scholar]
- IPCC (2017).IPCC Guidelines for national greenhouse gas inventories 2017.
- Johnson & Johnson (1995).Johnson KA, Johnson DE. Methane emissions from cattle. Journal of Animal Science. 1995;73(8):2483–2492. doi: 10.2527/1995.7382483. [DOI] [PubMed] [Google Scholar]
- Kamel et al. (2008).Kamel C, Greathead HMR, Tejido ML, Ranilla MJ, Carro MD. Effects of allicin and diallyl disulfide on in vitro rumen fermentation of a mixed diet. Animal Feed Science and Technology. 2008;145(1–4):351–363. doi: 10.1016/j.anifeedsci.2007.05.050. [DOI] [Google Scholar]
- Kim (2012).Kim EJ. Reducing greenhouse gas emissions in ruminants: minireview. Korean Journal of Organic Agriculture. 2012;20(2):185–200. [Google Scholar]
- Kim, Ha & Song (2012).Kim DR, Ha JJ, Song YH. Effect of dietary supplementation of garlic and may flower powder on CO2 and CH4 emission by Hanwoo cow. Korean Society of Animal Sciences and Technology. 2012;54(5):363–368. doi: 10.5187/JAST.2012.54.5.363. [DOI] [Google Scholar]
- Kim et al. (2012b).Kim KH, Kim HJ, Byun MW, Yook HS. Antioxidant and antimicrobial activities of ethanol extract from six vegetables containing different sulfur compounds. The Korean Society of Food Science and Nutrition. 2012b;41(5):577–583. doi: 10.3746/jkfn.2012.41.5.577. [DOI] [Google Scholar]
- Kim et al. (2012a).Kim ET, Kim CH, Min KS, Lee SS. Effects of plant extracts on microbial population, methane emission and ruminal fermentation characteristics in vitro. Asian-Australasian Journal of Animal Sciences. 2012a;25(6):806–811. doi: 10.5713/ajas.2011.11447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim et al. (2018).Kim DH, Lee SJ, Oh DS, Lee ID, Eom JS, Park HY, Choi SH, Lee SS. In vitro evaluation of Rhus succedanea extracts for ruminants. Asian-Australasian Journal of Animal Sciences. 2018;31(10):1635–1642. doi: 10.5713/ajas.18.0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim et al. (2013).Kim CH, Moon YS, Beak MK, Seo SW, Lee SS, Lee SS, Lee SY, Lee WS, Chang JS, Cho NJ, Ha JK. Ruminant nutrition and physiology. Seoul National University press; Seoul: 2013. [Google Scholar]
- Koike & Kobayashi (2001).Koike S, Kobayashi Y. Development and use of competitive PCR assays for the rumen cellulolytic bacteria: Fibrobacter succinogenes, Ruminococcus albus and Ruminococcus flavefaciens. FEMS Microbiology Ecology. 2001;204(2):361–366. doi: 10.1111/j.1574-6968.2001.tb10911.x. [DOI] [PubMed] [Google Scholar]
- Kumar et al. (2018).Kumar S, Treloar BP, Teh KH, McKenzie CM, Henderson G, Attwood GT, Waters SM, Patchett ML, Janssen PH. Sharpea and Kandieria are lactic acid producing rumen bacteria that do not change their fermentation products when co-cultured with methanogen. Anaerobe. 2018;54:31–38. doi: 10.1016/j.anaerobe.2018.07.008. [DOI] [PubMed] [Google Scholar]
- Latham & Wolin (1977).Latham MJ, Wolin MJ. Fermentation of cellulose by Ruminococcus flavefaciens in the presence and absence of Methanobacterium ruminantium. Applied and Environmental Microbiology. 1977;34(3):297–301. doi: 10.1128/AEM.34.3.297-301.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee et al. (2018).Lee SJ, Eom JS, Kim HS, Kim HS, Lee SS. Effects of dietary Allium fistulosum L. and tannic acid on in vitro ruminal fermentation characteristics and methane emission. Korean Journal of Organic Agruculture. 2018;26(4):775–787. doi: 10.11625/KJOA.2018.26.4.775. [DOI] [Google Scholar]
- Lee et al. (2011).Lee SJ, Shin NH, Chu GM, Lee SS. Effects of synbiotics containing anaerobic microbes and prebiotics on in vitro fermentation characteristics and in situ disappearance rate of fermented-TMR. Asian-Australasian Journal of Animal Sciences. 2011;24(11):1577–1586. doi: 10.5713/ajas.2011.11057. [DOI] [Google Scholar]
- Liu & Whitman (2008).Liu Y, Whitman WB. Metabolic, phylogenetic, and ecological diversity of the methanogenic archaea. Annals of the New York Academy of Sciences. 2008;1125(1):171–189. doi: 10.1196/annals.1419.019. [DOI] [PubMed] [Google Scholar]
- Ma et al. (2016).Ma T, Chen D, Tu Y, Zhang N, Si B, Deng K, Diao Q. Effect of supplementation of allicin on methanogenesis and ruminal microbial flora in Dorper crossbred ewes. Journal of Animal Science and Biotechnology. 2016;7:1. doi: 10.1186/s40104-015-0057-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDougall (1948).McDougall EI. Studies on ruminant saliva. 1. The composition and output of sheep’s saliva. Biochemical Journal. 1948;43(1):99–109. doi: 10.1042/bj0430099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss, Jouany & Newbold (2000).Moss AR, Jouany JP, Newbold J. Methane production by ruminants: its contribution to global warming. Annales De Zootechnie. 2000;49(3):231–253. doi: 10.1051/animres:2000119. [DOI] [Google Scholar]
- National Research Council (1989).National Research Council . Nutrient requirements of dairy cattle. Sixth rev. ed National Academy Press; Washington, D.C.: 1989. [Google Scholar]
- National Research Council (2001).National Research Council . Nutrient requirements of dairy cattle. Seventh rev. ed National Academy Press; Washington, D.C.: 2001. [Google Scholar]
- Newbold, Lassalas & Jouany (1995).Newbold CJ, Lassalas B, Jouany JP. The importance of methanogens associated with ciliate protozoa in ruminal methane production in vitro. Letters in Applied Microbiology. 1995;21(4):230–234. doi: 10.1111/j.1472-765X.1995.tb01048.x. [DOI] [PubMed] [Google Scholar]
- Ntaikou et al. (2008).Ntaikou I, Gavala HN, Kornaros M, Lyberatos G. Hydrogen production from sugars and sweet sorghum biomass using Ruminococcus albus. International Journal of Hydrogen Energy. 2008;33(4):1153–1163. doi: 10.1016/j.ijhydene.2007.10.053. [DOI] [Google Scholar]
- Odongo et al. (2007).Odongo NE, Bagg R, Vessie G, Dick P, Or-Rashid MM, Hook SE, Gray JT, Kebreab E, France J, McBride BW. Long-term effects of feeding monensin on methane production in lactating dairy cows. Journal of Dairy Science. 2007;90:1781–1788. doi: 10.3168/jds.2006-708. [DOI] [PubMed] [Google Scholar]
- Orskov, Hovell & Mould (1980).Ørskov ER, Hovell FDB, Mould F. The use of the nylon bag technique for the evaluation of feedstuffs. Tropical Animal Production. 1980;5(3):195–213. [Google Scholar]
- Orskov & McDonald (1979).Ørskov ER, McDonald I. The estimation of protein degradability in the rumen from incubation measurements weighted according to rate of passage. The Journal of Agricultural Science. 1979;90:499–503. doi: 10.1017/S0021859600063048. [DOI] [Google Scholar]
- Oskoueian, Abdullah & Oskoueian (2013).Oskoueian E, Abdullah N, Oskoueian A. Effects of flavonoids on rumen fermentation activitiy, methane production, and microbial population. BioMed Research Intermational. 2013;2013:1–8. doi: 10.1155/2013/249129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patra & Saxena (2010).Patra AK, Saxena J. A new perspective on the use of plant secondary metabolites to inhibit methanogenesis in the rumen. Phytochemistry. 2010;71(11–12):1198–1122. doi: 10.1016/j.phytochem.2010.05.010. [DOI] [PubMed] [Google Scholar]
- Reuter, Koch & Lawson (1996).Reuter HD, Koch HP, Lawson LD. Therapeutic effects and applications of garlic and its preparations. In: Koch HP, Lawson LD, editors. garlic: The science and therapeutic application of Allium sativum L. and related species. Williams and Wilkins; Baltimore: 1996. pp. 135–213. [Google Scholar]
- Skillman et al. (2006).Skillman LC, Toovey AF, Williams AJ, Wright ADG. Development and validation of a real-time PCR method to quantify rumen protozoa and examination of variability between Entodinium populations in sheep offered a hay-based diet. Applied and Environmental Microbiology. 2006;72(1):200–206. doi: 10.1128/AEM.72.1.200-206.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Štajner & Milić (2006).Štajner D, Milić N. Exploring Allium species as a source of potential medicinal agents. Phytotherapy Research. 2006;20(7):581–584. doi: 10.1002/ptr.1917. [DOI] [PubMed] [Google Scholar]
- Tabaru et al. (1988).Tabaru H, Kadota E, Yamada H, Sasaki N, Takeuchi A. Determination of volatile fatty acids and lactic acids in bovine plasma and ruminal fluid by high performance liquid chromatography. The Japanese Journal of Veterinary Science. 1988;50(5):1124–1126. doi: 10.1292/jvms1939.50.1124. [DOI] [PubMed] [Google Scholar]
- Tedesco et al. (2004).Tedesco D, Tava A, Galletti S, Tameni M, Varisco G, Costa A, Steidler S. Effects of silymarin, a natural hepatoprotector, in periparturient dairy cows. Journal of Dairy Science. 2004;87(7):2239–2247. doi: 10.3168/jds.S0022-0302(04)70044-2. [DOI] [PubMed] [Google Scholar]
- Theodorou et al. (1994).Theodorou MK, Williams BA, Dhanoa MS, McAllan AB, France J. A simple gas production method using a pressure transducer to determine the fermentation kinetics of ruminant feeds. Animal Feed Science Technology. 1994;48(3–4):185–197. doi: 10.1016/0377-8401(94)90171-6. [DOI] [Google Scholar]
- Tymensen, Beauchemin & McAllister (2012).Tymensen LD, Beauchemin KA, McAllister TA. Structures of free-living and protozoa-associated methanogen communities in the bovine rumen differ according to comparative analysis of 16S rRNA and mcrA genes. Microbiology. 2012;158:1808–1817. doi: 10.1099/mic.0.057984-0. [DOI] [PubMed] [Google Scholar]
- Van Soest, Robertson & Lewis (1991).Van Soest PJ, Robertson JB, Lewis BA. Methods for fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. Journal of Dairy Science. 1991;74(10):3583–3597. doi: 10.3168/jds.S0022-0302(91)78551-2. [DOI] [PubMed] [Google Scholar]
- Vlase et al. (2013).Vlase L, Parvu M, Parvu EA, Toiu A. Chemical constituents of three Allium species from Romania. Molecules. 2013;18(1):114–127. doi: 10.3390/molecules18010114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitman, Bowen & Boone (1992).Whitman WB, Bowen TL, Boone DR. The Prokaryotes, 2nd ed. A handbook on the biology of bacteria. Springer-Verlag; New York: 1992. The methanogenic bacteria; pp. 719–767. [Google Scholar]
- Zafarian & Manafi (2013).Zafarian R, Manafi M. Effects of garlic powder on methane production, rumen fermentation and milk production of Buffaloes. Annual Review & Research in Biology. 2013;3(4):1013–1019. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
xAllium fistulosum L. extract concentrations are based on quantity of timothy hay (300 mg) substrate.
ySEM, standard error of the mean.
zT: treatment; L: linear; Q: quadratic effect
a–cMeans with different superscript letters in the same row indicate significant differences (P < 0.05).
n = 3.
Data Availability Statement
The following information was supplied regarding data availability:
Raw data is available in Supplemental File.


