Van der Heijde D, Gensler LS, Deodhar, et al. Dual neutralisation of interleukin-17A and interleukin-17F with bimekizumab in patients with active ankylosing spondylitis: results from a 48-week phase llb, randomised, double blind, placebo-controlled, dose-ranging study. Ann of Rheum Dis 2020;79:595–4.
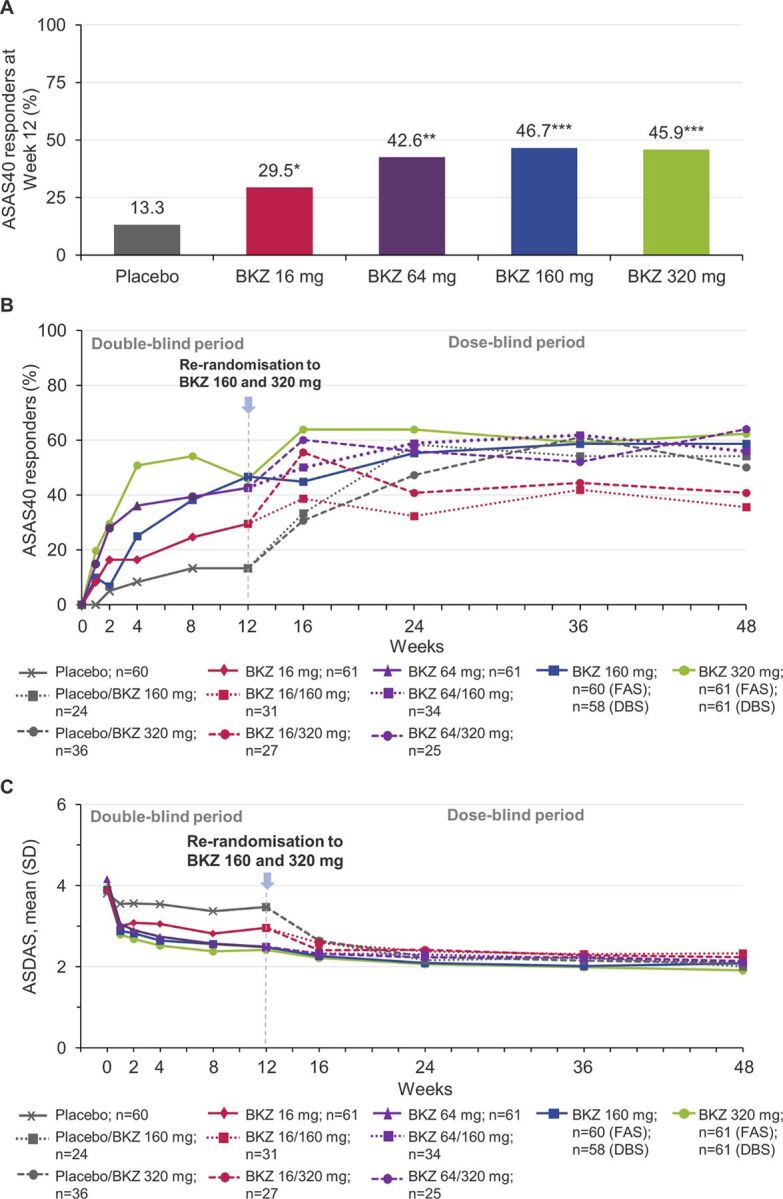
The legend for the Figure 1B, C labels should read ‘Placebo/BKZ 320 mg; n=36’ instead of ‘Placebo/BKZ 160 mg; n=36’; and ‘BKZ 320 mg; n=61 (FAS); n=61 (DBS)’ instead of ‘BKZ 160 mg; n=61 (FAS); n=61 (DBS)’.
A citation to the following paper should be included after first mention of the copyrighted SPARCC instrument: Maksymowych WP, et al. Spondyloarthritis Research Consortium of Canada MRI index for assessment of sacroiliac joint inflammation in ankylosing spondylitis. Arthritis Rheum 2005;53(5):703–9. doi: 10.1002/art.21445.


