Abstract
Purpose
To explore whether targeted next generation sequencing (NGS) of liquid biopsy in advanced non-small cell lung cancer (NSCLC) could potentially overcome the innate problems that arise with standard tissue biopsy, like intratumoral heterogeneity and the inability to obtain adequate samples for analysis.
Methods
The Scopus, Cochrane Library, and MEDLINE (via PubMed) databases were searched for studies with matched tissue and liquid biopsies from advanced NSCLC patients, analyzed with targeted NGS. The number of mutations detected in tissue biopsy only, liquid biopsy only, or both was assessed and the positive percent agreement (PPA) of the two methods was calculated for every clinically relevant gene.
Results
A total of 644 unique relevant articles were retrieved and data were extracted from 38 studies fulfilling the inclusion criteria. The sample size was composed of 2000 mutations tested in matched tissue and liquid biopsies derived from 1141 patients. No studies analyzed circulating tumor cells. The calculated PPA rates were 53.6% (45/84) for ALK, 53.9% (14/26) for BRAF, 56.5% (13/23) for ERBB2, 67.8% (428/631) for EGFR, 64.2% (122/190) for KRAS, 58.6% (17/29) for MET, 54.6% (12/22) for RET, and 53.3% (8/15) for ROS1. We additionally recorded data for 65 genes that are not recommended by current guidelines for mutational testing. An extra category containing results of unspecified genes was added, with a PPA rate of 55.7% (122/219).
Conclusion
Despite many advantages, liquid biopsy might be unable to fully substitute its tissue counterpart in detecting clinically relevant mutations in advanced NSCLC patients. However, it may serve as a helpful tool when making therapeutic decisions. More studies are needed to evaluate its role in everyday clinical practice.
Keywords: NGS, ctDNA, NSCLC, EGFR mutations, ALK fusion gene, ROS1 translocation, BRAF testing
Introduction
The advent of tyrosine kinase inhibitors (TKIs) has been a milestone in the treatment of advanced non-small cell compared to previous standard therapy (Vestergaard et al. 2018). Current guidelines have already adopted their use as first- and second-line treatment options (Hanna et al. 2017; Ettinger et al. 2018; Planchard et al. 2018). TKIs are designed to interrupt specific molecular pathways that promote tumor survival and growth (Levy et al. 2012). Genetic tumor profiling is, therefore, necessary to reveal mutations that enhance those pathways, allowing oncologists to effectively match TKI agents to every patient’s individual tumor molecular landscape. This rapid shift towards personalized medicine is reflected by the most recent diagnostic recommendations. Mutational testing of genes including EGFR, ALK, ROS1, and BRAF is currently considered standard for all patients with advanced NSCLC, regardless of their individual characteristics (Kerr et al. 2014; Kalemkerian et al. 2018; Lindeman et al. 2018). In addition, further testing for genes including ERBB2, RET, MET, and KRAS is also encouraged as part of a more extensive laboratory workup, when available, due to their implications in tumor prognosis and treatment response against newer promising targeted agents (Kerr et al. 2014; Kalemkerian et al. 2018; Lindeman et al. 2018).
The current gold standard method for genetic tumor profiling is tissue biopsy. However, its use remains problematic for numerous reasons. Lung biopsy is an invasive procedure with a notoriously high incidence of both major and minor complications (Overman et al. 2013; Heerink et al. 2017). In addition, tissue biopsy yields inadequate DNA material for genetic analysis at a significant rate (VanderLaan et al. 2014). Intratumoral heterogeneity is also a well-established phenomenon (Vogelstein et al. 2013) that inherently limits its accuracy in capturing a complete snapshot of the tumor’s mutational status, with potentially different results from different biopsy sites. Apart from the initial tumor genetic profiling, frequent monitoring for the emergence of new mutations or genetic modifications is also vital in detecting treatment resistance and managing it accordingly, as in the case of the EGFR T790M mutation and the use of third-line TKIs such as osimertinib (Mok et al. 2016). The aforementioned characteristics among others, make serial tissue biopsies contextual and potentially functionally nonrepresentative of the overall mutational burden. As a consequence, using tissue biopsy as a guiding tool for targeted therapy may limit its full efficacy.
Liquid biopsy is a promising complement/alternative to the classic tissue biopsy. By assessing blood samples for the presence of either circulating tumor cells (CTCs) or circulating tumor DNA (ctDNA), it provides the necessary genetic material to perform tumor mutational analysis (Mayo-de-Las-Casas et al. 2018). Unlike many tissue biopsies, liquid biopsies are far less cumbersome with greatly reduced risk for complications. Performing repeated biopsies over time is, therefore, more feasible and may represent the overall tumor mutational burden accurately (Santarpia et al. 2016). However, liquid biopsy is not yet widely adopted as a standard diagnostic method because of inadequate data supporting its use (Kalemkerian et al. 2018; Lindeman et al. 2018). As a result, guidelines suggest it as an alternative to tissue biopsy only in settings where tissue availability becomes the limiting factor for molecular testing (Kalemkerian et al. 2018; Lindeman et al. 2018).
While current recommendations support mutational testing only for a few key cancer-driver genes, it is already evident that obtaining a more complete mutational profile is most likely the future direction of targeted therapy. NSCLC displays a heterogeneous mutational profile across different patients (Tan et al. 2014). Overall, current NSCLC clinical trials assess the mutational status of over 190 different genes, according to the My Cancer Genome online database (https://www.mycancergenome.org). Next generation sequencing (NGS) is a method of DNA analysis that allows parallel sequencing of numerous small DNA fragments, thus making concurrent testing for a very wide mutational gene panel possible (Sabour et al. 2017). It is currently deemed as an acceptable sequencing method for various instances during NSCLC mutational testing (Lindeman et al. 2018). Therefore, combining liquid biopsy with NGS offers the potential to obtain a comprehensive tumor genetic profile through minimally invasive means. Generally, NGS can either be directed towards specific genes through a predetermined gene panel (targeted NGS) or towards the whole genome or exome of the patient. The former represents a more appealing choice for daily clinical practice, through lower cost, faster results and higher sensitivity with lower detection thresholds (El Achi et al. 2019).
The objective of this systematic review is to examine whether liquid biopsy is a suitable alternative to tissue biopsy mutational testing, by reviewing studies that compare matched targeted NGS-analyzed tissue and liquid biopsy samples of advanced NSCLC patients.
Methods
Search and study selection
We accessed relevant articles by searching through the MEDLINE (via PubMed), Scopus, and Cochrane Library databases. The following search algorithm was applied to the MEDLINE and Scopus electronic databases: “(next generation sequencing) AND (liquid biopsy OR circulating OR cfDNA OR ctDNA OR CTC) AND lung”. Cochrane Library was searched by combining the search terms “next-generation sequencing” and “lung”. No publication date or any other type of filters were used. In addition, we manually searched through the references of eligible articles for any relevant articles not already included in the original search. The last search was performed on April 24th, 2020. Two researchers (S.E. and G.G.) performed the initial screening independently and assessed studies for eligibility. Any disagreements were resolved by reaching a consensus. We initially screened the abstracts of all studies and accepted those that included NSCLC in their objective and contained liquid biopsy and NGS as part of their methodology. Review articles were excluded during the screening process.
In the process of assessing the eligibility of studies that passed screening, we first excluded: (1) case reports, (2) articles written in any language other than English and (3) studies that employed whole-genome NGS instead of targeted NGS. We subsequently included studies that: (1) involved human subjects (and not cell lines or artificial samples), (2) included patients with advanced stage NSCLC (stage IIIb, stage IV according to the AJCC Staging System), (3) included both tissue and liquid biopsies in their methodologies, (4) had matched tissue and liquid biopsies and (5) had NGS performed in both tissue and liquid biopsy samples. Finally, eligible studies were excluded from the final analysis if they presented data in a way that did not allow us to properly extract it. For example, we omitted articles with mixed data from different types of cancers other than the subtypes of NSCLC, different NSCLC stages or results that didn’t take matched samples into account.
Data collection
To collect data from studies included in the final analysis, we created specialized spreadsheets via Microsoft Excel®. Two researchers (S.E. and G.G.) extracted all data independently. Their results were compared for all relative parameters and disagreements were resolved by reaching a consensus. We examined all papers included in the final analysis for the following parameters: (1) number of mutations detected in both tissue and liquid biopsy via targeted NGS, (2) number of mutations detected in tissue but not liquid biopsy via targeted NGS, and (3) number of mutations detected in liquid but not tissue biopsy via targeted NGS and (4) total number of patients that we extracted data from (not necessarily equal to the total of number patients in the study). We also recorded identifying study characteristics, such as (5) the country where each study was conducted and (6) the year of publication, in addition to the technical specifications of the NGS assays employed for the analysis of both liquid and tissue biopsies, including (7) the sequencing platform, (8) the gene panel, (9) the average coverage and (10) any allele frequency or copy number alteration threshold for mutation calling as set by the authors.
The mycancergenome.org database was used as a reference point to determine the clinical relevance of each individual gene. Only genes whose mutational status has been an eligibility criterion for clinical trials, regarding either NSCLC or one of its subtypes (e.g. lung adenocar-cinoma, squamous cell lung carcinoma), were selected. In those cases where mutations were clearly indicated as driver or non-driver, we recorded data from the former category only. In studies where the authors did not compile the results from matched tissue and liquid biopsy samples themselves, we extracted data by matching results from the available supplementary data tables. We applied any individual allele frequency confidence thresholds for variant calling set by the authors in cases where it was clearly stated. All available data were grouped by gene only, rather than by each individual mutation and different mutations of the same gene were categorized together.
During our analysis, we only included data derived from advanced NSCLC patients that were the result of targeted NGS. We did not consider data from patients with other malignancies, early stage NSCLC or data derived from any method other than targeted NGS in cases where they were co-presented with relevant results. Moreover, if patients were characterized as stage III instead of IIIa or IIIb, they were not included during data extraction. To avoid introducing bias by recording duplicate data, we skipped data collection from identical patient populations already included in the analysis through a different study. Between studies with identical patient populations, we favored those that presented the greatest amount of extractable data. We also omitted any data where cytologic analysis of pleural fluid was used in lieu of primary tumor biopsy, but accepted tumor biopsies from either primary or metastatic sites. It should be noted that we did not apply any limit to the time difference between tissue and plasma sampling. Finally, studies with extractable data of interest for less than two patients were excluded from the final qualitative synthesis.
Statistical analysis
We compared the performance of tissue biopsy and liquid biopsy, by calculating the positive percent agreement (PPA) between the two. Since tissue NGS analysis was not validated in every study included in the final analysis, it could not be considered as a reference method but rather as a best alternative to liquid biopsy NGS. Therefore, sensitivity, specificity, positive predictive value, and negative predictive value were inappropriate comparison measures in this instance and were not calculated. In addition, data where no mutation was found in both tissue and liquid biopsy NGS were often omitted by the authors or could not be reliably extracted based on the information provided. Thus, negative percent agreement, despite being an appropriate statistical measure, could not be calculated.
Results
Study selection
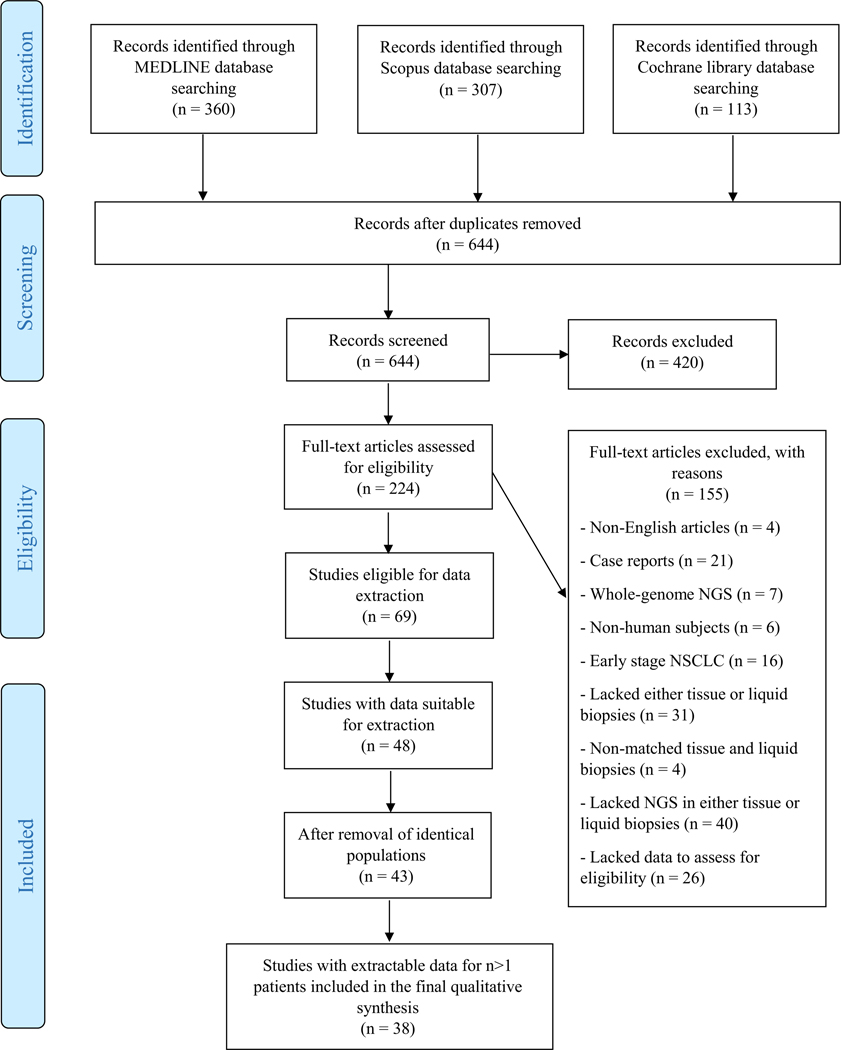
A total of 780 relevant articles were retrieved by searching the literature. The last search was performed on April 24th, 2020. After removing 136 duplicate studies, we screened the titles and abstracts of 644 articles. Of them, 420 were deemed irrelevant, while 224 were determined to be relevant and their full-text articles were assessed for eligibility. After assessment against the preset inclusion and exclusion criteria, 155 articles were excluded: 4 were written in a non-English language, 21 were case reports, 7 employed whole genome instead of targeted NGS, 6 did not involve human subjects, 16 enrolled only early stage NSCLC patients, 31 lacked either tissue or liquid biopsies, 4 had non-matched tissue and liquid biopsy samples, and 40 utilized a method other than NGS for either of the two biopsy types. An additional 26 studies did not provide enough information to unequivocally determine their eligibility and thus had to be excluded as well. The remaining 69 studies fulfilled all eligibility criteria and were selected for the data extraction process. Twenty-one of them presented their data in a manner that did not allow selective extraction of relevant data only. Data were extracted from 48 studies. Five of them were found to enroll populations that had already been included in the analysis through a different study and their results were not counted. Another five studies had extractable data for only n = 1 patient and were also excluded from the data synthesis. The final analysis was performed on the remaining 38 studies. The complete process is summarized in Fig. 1.
Fig. 1.
Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) flow diagram of study selection
Individual data and data synthesis
A total of 38 non-case report studies that compared matched tissue and liquid biopsy samples of advanced NSCLC patients were individually analyzed and their data were subsequently synthesized (Couraud et al. 2014; Thress et al. 2015; Vanni et al. 2015; Kaisaki et al. 2016; Pécuchet et al. 2016; Paweletz et al. 2016; Rachiglio et al. 2016; Villaflor et al. 2016; Schwaederlé et al. 2017; Xu et al. 2017; Yao et al. 2017; Iwama et al. 2017; Dagogo-Jack et al. 2018; Veldore et al. 2018; Yang et al. 2018; Li et al. 2018; Liu et al. 2018; Vollbrecht et al. 2018; Garcia et al. 2018; Toor et al. 2018; McCoach et al. 2018; Guo et al. 2018; Hu et al. 2018; Jin et al. 2018; Sabari et al. 2018; Papadopoulou et al. 2019; Tong et al. 2019; Lam et al. 2019; Aggarwal et al. 2019; Ge et al. 2019; Wu et al. 2019; Chen et al. 2019; Supplee et al. 2019; Tang et al. 2019; Streubel et al. 2019; Horn et al. 2019; Pritchett et al. 2019; Tran et al. 2019). The technical aspects and characteristics of every study included in the final analysis are presented in Table 1. The sample size was composed of 2000 mutations tested in matched biopsies derived from 1141 patients. Data were collected for 74 different gene categories of interest. It should be noted that all 38 eligible studies employed cfDNA/ctDNA analysis as their liquid biopsy method. No studies utilized analysis of CTCs instead, despite being included during the initial screening, as they were deemed ineligible for other reasons.
Table 1.
Technical specifcations of the next-generation sequencing assays used in all studies included in the analysis, in chronological order, according to the date of frst online publication
| Author | Country | Tissue NGS platform | Tissue NGS panel | # of genes | Coverage | Detection limit | Liquid biopsy NGS platform | Liquid biopsy NGS panel | # of genes | Coverage | Detection limit | # of patients |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Couraud et al. (2014) | France | IonTorrent PGM sequencer | Custom | 5 | 1000× | 5% | IonTorrent PGM sequencer | Custom | 5 | 10,000 × | 0.13% | 38 |
| Thress et al. 2015 | USA | Illumina MiSeq | Vall d’Hebron Institute of Oncology | 57 | 3000 × | 3% | Illumina HiSeq | Qiagen GeneRead Lung Cancer v1 | 20 | 30,000 × | N/A | 2 |
| Paweletz et al. (2016) | USA | N/A | N/A | N/A | N/A | N/A | Illumina | N/A | N/A | N/A | N/A | 31 |
| Vanni et al. 2015 | Italy | IonTorrent PGM sequencer | Ion AmpliSeq Colon and Lung Cancer Panel v1 | 22 | > 500 × | 5% | IonTorrent PGM sequencer | Ion AmpliSeq Colon and Lung Cancer v1 | 22 | > 500 × | 5% | 2 |
| Rachiglio et al. (2016) | Italy | IonTorrent PGM sequencer | Oncomine Solid Tumour DNA kit | 22 | N/A | 2% | IonTorrent PGM sequencer | Oncomine Solid Tumour DNA kit | 22 | N/A | 2% | 19 |
| Villaflor et al. 2016 | USA | N/A | Multiple | < 10 to > 300 | N/A | N/A | Illumina HiSeq 2500 | Guardant360 | 54/68 | 10,000 × | 0.10% | 4 |
| Kaisaki et al. 2016 | UK | IonTorrent PGM sequencer | Ion Ampliseq Cancer Hotspot v1/v2 | 46/50 | N/A | 0.50% | IonTorrent PGM sequencer | Ion | 46/50 | N/A | 0.50% | 3 |
| Iwama et al. 2017 | Japan | Ion Proton Sequencer | Ion AmpliSeq Colon and Lung Cancer Panel v2 | 22 | N/A | N/A | Ion Proton Sequencer | Ion AmpliSeq Colon and Lung Cancer v2 | 22 | N/A | 0.50% | 32 |
| Yao et al. 2017 | China | Illumina HiSeq | N/A | 40 | 855.17 × | 0.20% | Illumina HiSeq | N/A | 40 | 2022.29 × | 0.20% | 25 |
| Pécuchet et al. (2016) | France | Ion Proton Sequencer | Ion AmpliSeq Colon and Lung Cancer v2 | 32 | N/A | 0.1%a, 0.3%b | Ion Proton Sequencer | Ion AmpliSeq Colon and Lung Cancer v2 | 32 | N/A | 0.1%a, 0.3%b | 108 |
| Xu et al. 2017 | China | Illumina HiSeq 2500 | Accu-Act | 61 | > 500 × | N/A | Illumina HiSeq 2500 | Accu-Act | 61 | 7000 × | N/A | 5 |
| Shwaederle et al. (2017) | USA | N/A | Foundation-One CDx | 324 | N/A | N/A | N/A | Guardant360 | 54/68/70 | 15,000 × | N/A | 26 |
| Veldore et al. 2018 | India | Illumina HiSeq 2500 | N/A | N/A | ~ 100,000 × | 0.01% | Illumina HiSeq 2500 | N/A | N/A | ~ 100,000 × | 0.01% | 4 |
| Dagogo-Jack et al. (2018) | USA | HiSeq | Foundation One; Oncopanel | N/A | N/A | N/A | Illumina HiSeq 2500 | PanCancer v3 | 566 | 1195 × | 0.50% | 10 |
| Liu et al. 2018 | China | Ion Proton Sequencer | Beijing Genomics Institute Lung Cancer | 146 | 372 × | 1% | Ion Proton Sequencer | Beijing Genomics Institute Lung Cancer | 146 | 938 × | 0.01% | 25 |
| Toor et al. 2018 | USA | N/A | Caris; Paradigm | 592; 125 | N/A | N/A | HiSeq 2500 | Genomics | 68–73 | N/A | N/A | 8 |
| Hu et al. 2018 | USA | Ion Proton | Genomics | 275–443 | N/A | N/A | Ion Proton | Institute | 275–443 | N/A | N/A | 14 |
| Yang et al. 2018 | China | Illumina NextSeq 500 | Institute | 63 | 500 × | 5% | Illumina NextSeq 500 | Lung | 63 | 3000 × | 0.30% | 56 |
| McCoach et al. (2018) | USA | N/A | N/A | N/A | N/A | N/A | Illumina HiSeq 2500 | Guardant360 | 70a/3b/18d/6c | 10,000 × | 0.04%a, 0.02%b, 0.04%c, 2.12d | 2 |
| Li et al. 2018 | China | Illumina NextSeq 500 | N/A | 168 | 2000 × | N/A | Illumina NextSeq 500 | N/A | 168 | 10,000 × | 2.25d, e, 2.50d /1.75d, e | 13 |
| Vollbrecht et al. (2018) | Germany | IonTorrent S5 XL | Ion AmpliSeq Colon and Lung Cancer v2 | 32 | N/A | N/A | IonTorrent S5 XL | Ion AmpliSeq Colon and Lung Cancer v2; Oncomine Lung cfDNA | 32; 11 | N/A | N/A | 21 |
| Garcia et al. 2018 | France | IonTorrent PGM sequencer | Custom AmpliSeq | N/A | N/A | N/A | Illumina NextSeq 500 | SWIFT-56G Oncology | 56 | > 500 × | 0.5% | 15 |
| Jin et al. 2018 | China | N/A | GeneseeqOne Pan-Cancer | 416 | 600 × | 5% | N/A | GeneseeqOne Pan-Cancer | 416 | 2000 × | 0.1% | 69 |
| Guo et al. 2018 | China | Illumina NextSeq 500 | Illumina NextSeq 500 | 22 | 2000 × | N/A | Illumina NextSeq 500 | Lung Cancer | 17 | 20,000 × | 0.1%e, 0.2% | 10 |
| Lam et al. 2019 | USA | IonTorrent PGM sequencer | IonTorrent AmpliSeq Cancer; Oncomine Cancer | 46; 143 | N/A | N/A | N/A | Guardant360 | 54/68/70 | 8,000–15,000 × | N/A | 2 |
| Aggarwal et al. (2019) | USA | N/A | Center for Personalized Diagnostics; Comprehensive Solid Tumor HaloPlexHS v2.0; Penn Precision | 47; 153; 20 | N/A | N/A | Illumina Hi-Seq 2500 | Guardant360 | 73 | N/A | N/A | 86 |
| Sabari et al. 2018 | USA | N/A | MSKIMPACT | 468 | 500–1000 × | N/A | Illumina NextSeq | ResBio ctDx-Lung; Archer REVEAL ctDNA 28 | 21; 28 | N/A | 0.10% | 106 |
| Ge et al. 2019 | China | Illumina NextSeq 500, HiSeq 4000, MiSeq, MiniSeq | ddCAP on tissue; OncoAim | 10; 59 | N/A | 5% | Illumina NextSeq 500 | ddCAP | 10 | N/A | 5% | 13 |
| Wu et al. 2019 | China | Illumina HiSeq 4000 | GeneseeqOne | 416 | 800 × | – | Illumina HiSeq 4000 | GeneseeqOne | 416 | 3000 × | 2.00d, 0.65d | 48 |
| Chen et al. 2019 | China | Illumina NovaSeq 6000 | Genecast, Beijing, China | 1407 | 2400 × | 2% | Illumina NovaSeq 6000 | Genecast, Beijing, China | 1407 | 3000 × | 0.20% | 3 |
| Pritchett et al. 2019 | USA | Illumina NextSeq 500, Illumina MiSeq | ArcherDx FusionPlex Solid Tumor | 52 | N/A | N/A | Illumina NextSeq 500 | InVisionFirst | 36 | N/A | N/A | 178 |
| Supplee et al. 2019 | USA | N/A | N/A | N/A | N/A | N/A | Illumina NextSeq 500 | Guardant360 v14.0–17.0; ctDx-Lung kit | -; 20 | N/A | – | 13 |
| Tang et al. 2019 | China | Illumina NextSeq 500 | N/A | 56 | N/A | N/A | Illumina NextSeq 500 | N/A | 56 | N/A | N/A | 47 |
| Tong et al. 2019 | China | Illumina HiSeq 4000 | GeneseeqOne | 416 | 1000 × | 0.50% | Illumina HiSeq 4000 | GeneseeqOne | 416 | 10,000 × | 0.10% | 28 |
| Horn et al. 2019 | USA | Illumina NextSeq | N/A | 12 | N/A | N/A | Illumina NextSeq | N/A | 12 | N/A | N/A | 18 |
| Streubel et al. 2019 | Germany | IonTorrent S5XL | Ion AmpliSeq Colon and Lung Cancer | 32 | N/A | N/A | IonTorrent S5XL | Oncomine Lung cfDNA | 11 | N/A | 0.10% | 10 |
| Tran et al. 2019 | Vietnam | Illumina NextSeq 550 | N/A | 4 | N/A | 1% | Illumina NextSeq | N/A | 4 | 10,000 × | 1% | 21 |
| Papadopoulou et al. 2019 | GreecIonTorrent PGM sequencer4000 | Custom Ion AmpliSeq | 23 | N/A | N/A | IonTorrent PGM sequencer | Custom Ion AmpliSeq | 23 | N/A | N/A | 26 | |
N/A not available; # of genes=number of genes; # of patients= number of patients included in our analysis
Single Nucleotide Variants
Indels
Fusions
Copy Number Alterations
Hotspot mutations
Genes recommended for mutational testing by current guidelines
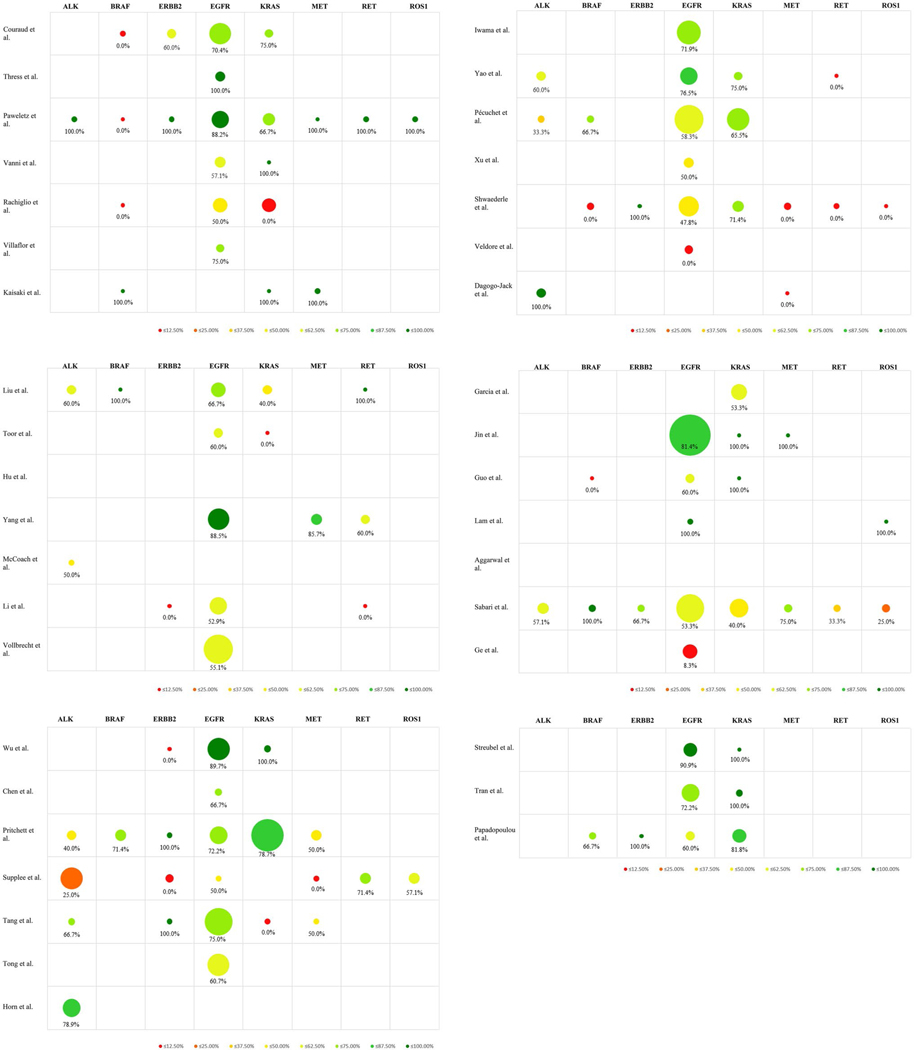
As previously mentioned, the most recent guidelines recommend mutational testing in advanced NSCLC for the genes ALK, BRAF, ERBB2 (HER2), EGFR, KRAS, MET, RET and ROS1(Kerr et al. 2014; Kalemkerian et al. 2018; Lindeman et al. 2018). The sample sizes were 84 samples for ALK, 26 for BRAF, 23 for ERBB2, 631 for EGFR, 190 for KRAS, 29 for MET, 22 for RET, and 15 for ROS1. Cumulative PPA rates were calculated to be as follows: 53.6% (45/84) for ALK, 53.9% (14/26) for BRAF, 56.5% (13/23) for ERBB2, 67.8% (428/631) for EGFR, 64.2% (122/190) for KRAS, 58.6% (17/29) for MET, 54.6% (12/22) for RET, and 53.3% (8/15) for ROS1. The results of every individual study are comprehensively presented in Fig. 2.
Fig. 2.
Positive percent agreement (PPA) rates of genes recommended for mutational testing. A table representation combining a bubble chart of the sample sizes with a heat-map representation of the PPA rates of every individual study included in the analysis, in chronological order, according to the date of first online publication. Only genes recommended for mutational testing by the most recent guidelines are displayed
Genes not recommended for mutational testing by current guidelines
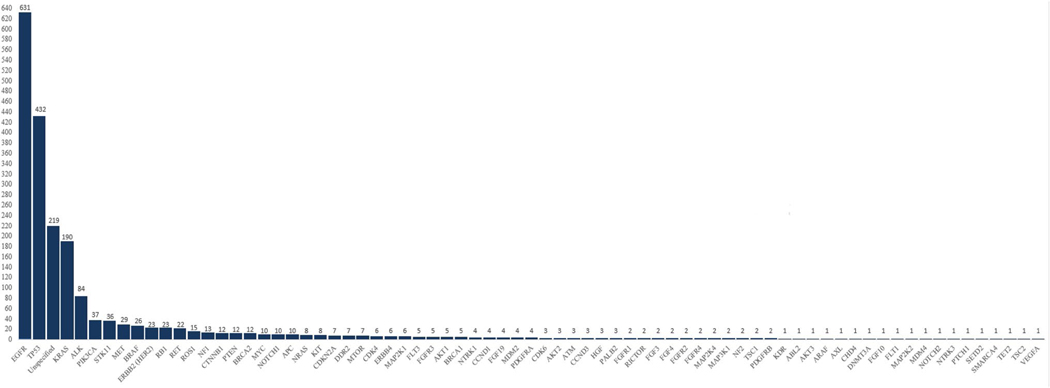
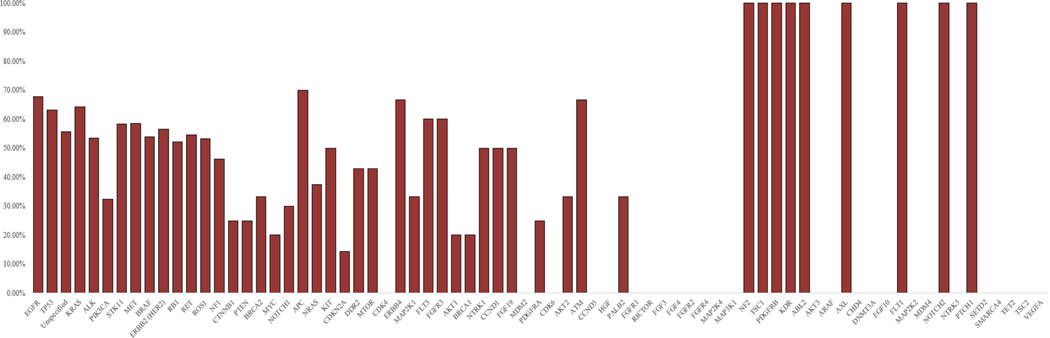
Data were also recorded for 65 genes of clinical interest that are not yet recommended by current guidelines for mutational testing. Total sample sizes for all these genes in addition to the eight genes recommended for testing by the current guidelines are presented in Fig. 3, while cumulative PPA rates are calculated and displayed in Fig. 4. Additional data regarding the PPA results from every individual study can be found in Supplemental Fig. 1.
Fig. 3.
Gene sample sizes. A bar chart representation of the total sample sizes of every gene included in the final analysis
Fig. 4.
Gene cumulative positive percent agreement (PPA) rates. A bar chart representation of the cumulative PPA rates for every gene, when taking into account all studies included in the final analysis
Unspecified genes
An extra category was added for cases where the authors did not provide a detailed breakdown of their results for every gene but their data were otherwise eligible for inclusion and data extraction. This category contained 219 samples, with 132 samples coming from Pritchett et al. (Pritchett et al. 2019), 86 samples coming from Aggarwal et al. (Aggarwal et al. 2019) and 1 sample from Couraud et al. (Couraud et al. 2014). The cumulative PPA for this category was calculated at 55.7% (122/219).
Discussion
To our knowledge, this is the first systematic review in the literature comparing targeted NGS analysis of plasma and tissue biopsies in advanced NSCLC. Our findings support the notion that targeted NGS in liquid biopsy falls short in detecting mutations compared to NGS in tissue biopsy in these patients. Most genes provided inadequate sample sizes to draw any reliable conclusions. Exceptions were EGFR and TP53, constituting together more than half of the overall sample size (n = 2000) used in the analysis, with 631 and 432 individual-tested mutations respectively. Both displayed similar PPA rates of 67.8% (428/631) for EGFR and 63.2% (273/432) for TP53. On the other hand, genes like ALK, BRAF, ERBB2, KRAS, MET and ROS1 that are currently included in the mutational testing guidelines all had significantly smaller sample sizes and, with the exception of KRAS, all provided unsatisfactory PPA rates of less than 60%. These data seem to be in accordance with the previously reported suboptimal performance of targeted NGS detecting gene translocations in ctDNA, as the breaking point each time might not be included in the limited NGS panels applied for liquid biopsies (Schram et al. 2017). Given the current data, NGS liquid biopsy seems unable to completely substitute its tissue biopsy counterpart, regardless of the gene being tested. Despite this, moderately satisfactory results as in the case of EGFR may solidify its position as a useful tool to complement tissue biopsy in certain clinical scenarios.
As NGS analysis in plasma is generally less sensitive than in tissue biopsy (Rolfo et al. 2018; Li et al. 2019), there was no surprise that a significant number of cases in our review revealed specific mutations during tissue biopsy molecular genotyping and failed to do so in their associated liquid biopsy. Mutation analysis on tissues is a well-standardized procedure where, in contrast to ctDNA, morphologic correlation to enrich tumor DNA is feasible; enrichment is achieved with macro-microdissection of areas containing a high percentage of tumor cells to reach the high initial DNA load necessary for optimal tumor genotyping (Shiau et al. 2014). On the other hand, ctDNA is only a tiny fraction of the total cell-free DNA and negative mutation testing might mean anything from true absence or low release of tumor DNA into the blood (e.g. due to response to therapy), a technical error of the NGS assay itself or a mutation not covered by the selected plasma assay (NGS panels for plasma can be less comprehensive than for tissue molecular analysis) (Weber et al. 2014; Merker et al. 2018; Rolfo et al. 2018; Aggarwal et al. 2019).
In contrast, this review also reported cases that showed rare specific mutations in the blood but not in the associated tissue biopsy, although this finding was less common. Although considered the current standard of care, tissues may fail to provide adequate DNA load for tumor genotyping in a significant number of cases. This may be due to insufficient sampling or sufficient sampling of low tumor cellularity or poor quality caused by chemical degradation (VanderLaan et al. 2014; Hagemann et al. 2015). Such failures are more common when CT-guided transthoracic core biopsies or bone biopsies are performed (VanderLaan et al. 2014). In addition, ctDNA may reflect intratumoral heterogeneity better than tissue-derived DNA, as the former may contain circulating apoptotic/necrotic DNA derived from tumor clones residing in both primary and distant sites, thus could reveal mutation(s) undetected from a tumor biopsy that lacks the relevant clones (spatial heterogeneity). In cases that plasma was collected after tumor biopsy, discordance could also be due to tumor evolution in time reflected in ctDNA but not in the tissue (temporal heterogeneity) (Weber et al. 2014; Siravegna et al. 2017). Of interest, mutations detected during ctDNA analysis might be irrelevant to lung cancer (e.g. clonal hemopoiesis; other benign/premalignant/malignant processes) (Chae and Oh 2019). Similar to false negative, NGS ctDNA assays can also result in false positive results (Rolfo et al. 2018).
When looking at the technical specifications of the NGS assays used for tissue and liquid biopsy analysis, it becomes evident that there was significant heterogeneity, not only across different studies but also within the studies themselves. This finding strongly reflects the lack of standardization in NGS assays (Jennings et al. 2017), which might be hindering its widespread implementation into daily practice. Molecular coverage can be a significant factor affecting the sensitivity of NGS assays (Petrackova et al. 2019). Many studies in our analysis employed coverage of less than 1,000x, especially in the analysis of tissue samples, and while no standard parameters have been established, it may be considered inadequate (Jennings et al. 2017; Petrackova et al. 2019), and could thus explain some of the false-negative results in tissue biopsies. On the other hand, there is a trade-off between the amount of genes that can be covered and the analytical sensitivity achieved by high molecular coverage, which is especially important in liquid biopsy analysis (Karachaliou et al. 2015). Consequently, studies that utilized very wide gene panels may had to compromise depth and sensitivity, while others may have had to limit their gene selection, resulting in liquid biopsy samples with false negative findings in both cases.
In an era where advanced NSCLC patients are mostly diagnosed with small biopsies or cytology rather than surgery or do not undergo biopsy at all for causing discomfort or potential minor/major complications, liquid biopsy incorporation into clinical practice may be exceptionally helpful for patient management (VanderLaan et al. 2014; Travis et al. 2015; Heerink et al. 2017; Siravegna et al. 2017; Mayo-de-Las-Casas et al. 2018). CtDNA tumor genotyping could be a vital supplement or alternative due to its minimally invasive nature, allowing for routine genetic follow-up (Santarpia et al. 2016). In treatment-naive advanced NSCLC patients, ctDNA status has been associated with both survival and response to first- or second-line TKIs (Ai et al. 2016; Pécuchet et al. 2016; Phallen et al. 2019). In advanced NSCLC patients on TKIs that present with disease progression, ctDNA mutational analysis can identify the mechanisms of resistance and predict patient benefit from specific targeted treatments, such as third-line TKIs in the case of T790M mutation (Thress et al. 2015; Vollbrecht et al. 2018; Iwama et al. 2018). In advanced NSCLC patients on immunotherapy, liquid biopsy has also been reported to predict response to treatment (Giroux Leprieur et al. 2018; Rizvi et al. 2018). Besides ctDNA, CTCs could also be of value for monitoring disease, assessing prognosis and predict response to TKIs, chemotherapy or immunotherapy in NSCLC patients (Chinniah et al. 2019; Gallo et al. 2019; Tamminga et al. 2019).
Of interest, several studies have used diverse polymerase chain reaction (PCR) methods (e.g. droplet digital PCR; allele-specific PCR), rather than NGS, on liquid biopsy to assess prognosis, response to treatment and resistance in advanced NSCLC patients and compared plasma-based results with the ones on tissue biopsy (Weber et al. 2014; Karachaliou et al. 2015; Zhu et al. 2015; Lee et al. 2016; Sundaresan et al. 2016). PCR is cheaper, requires less technical expertise (e.g. no bioinformatics support), and has more rapid turnaround time than NGS; it is thus ideal when a selected treatment is urgent, e.g. osimertinib in cases of T790M mutation (Sacher et al. 2016; Postel et al. 2018; Rolfo et al. 2018). However, it is much less comprehensive than NGS, being able to detect only one or just a few mutations; in contrast, NGS can provide a more complete tumor profile detecting single-nucleotide variants, insertions/deletions, translocations, and amplifications (Hagemann et al. 2015; Postel et al. 2018; Rolfo et al. 2018). Although NGS has traditionally been considered less sensitive than PCR, authors in recent reports, which reflect improvement in the NGS technology and standardization, describe similar sensitivity for the detection of driver and resistance mutations (Li et al. 2019) or an even better performance of NGS in the case of T790M (Dono et al. 2019).
This review has several limitations. Most of the studies included were retrospective or heterogeneous in their design. Studies used variable NGS panels which could differ between plasma analyses across different studies, but also between plasma and tissue analysis within the same study. Meanwhile, many of them focused on a limited gene spectrum. As a result, not all genes are covered uniformly in our cumulative results and data for a significant number of genes are extremely limited. Both older and recent studies were included, although the latter reported higher sensitivity/specificity, possibly because of the improvements in NGS technology and standardization. The authors also applied diverse cut-offs to report variants in their NGS experiments. In many studies, plasma and tissue were not collected at the same time (time difference between them ranged from 0 days to many months). Lastly, our total patient population included both treatment-naive and patients under TKI treatment, ranging from adequate responders to first-line treatment to patients progressing after multiple TKI trials. Thus, the results from different studies are not directly comparable to one another.
The latest guidelines support the use of ctDNA mutation analysis in cases when tissue biopsy is not performed or provides inadequate DNA for analysis (Kalemkerian et al. 2018; Lindeman et al. 2018). More evidence is needed to support its use in treatment-naive patients or patients under TKI that undergo progression, e.g. due to EGFR T790M or C797S mutations, and there is the recommendation to follow-up a negative result with reflex tissue-based testing whenever possible (Kalemkerian et al. 2018; Lindeman et al. 2018; Rolfo et al. 2018; Li et al. 2019). In this direction, recent studies have attempted to provide more clinical validity/utility of testing the ctDNA of advanced NSCLC patients with NGS (Laufer-Geva et al. 2018; Sabari et al. 2018; Leighl et al. 2019; Aggarwal et al. 2019; Li et al. 2019). Dual plasma and tissue-based targeted NGS testing detects more mutations than each one separately, thus more patients can be treated with targeted therapies (Leighl et al. 2019; Aggarwal et al. 2019).
An inherent weakness of NGS is the failure to detect epigenetic modifications affecting gene expression without altering the base sequence (Fernandez-Marmiesse et al. 2017). The techniques used to reveal these modifications may significantly degrade the already limited genetic material available for sequencing (Gai and Sun 2019). As a result, choosing sequencing over other applications of the isolated tumor genetic material may still provide an incomplete picture of the mechanisms involved in treatment response and tumor behavior, even if both tissue and liquid biopsy are utilized.
Conclusion
In conclusion, most advanced NSCLC patients are unresectable and are diagnosed with small biopsies or cytology, both of which may have insufficient DNA for molecular analysis. This systematic review showed that targeted NGS in plasma shows inferior performance in detecting mutations compared to tissue biopsy in advanced NSCLC patients. However, given the fact that technology around ctDNA mutation analysis with NGS will most likely continue to be improving the years to come, accumulating evidence in the form of prospective studies, randomized clinical trials and systematic reviews/meta-analyses will soon result in re-evaluation of ctDNA clinical validity and utility in advanced NSCLC. Targeted NGS testing on ctDNA has the potential to become a highly accurate diagnostic modality for the presence of actionable mutations in treatment-naïve and resistant to TKIs patients or for the selection for clinical trials, as shown in the most recent publications on the field. The field is rapidly evolving, but current retrospective data show a synergistic and clinical utility to both methodologies when clinically feasible.
Supplementary Material
Acknowledgments
Funding This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
Data availability Raw data available upon request.
Compliance with ethical standards
Electronic supplementary material The online version of this article (https://doi.org/10.1007/s00432–020-03267-x) contains supplementary material, which is available to authorized users.
Conflict of interest The authors report no conflict of interest.
References
- Aggarwal C, Thompson JC, Black TA et al. (2019) Clinical Implications of Plasma-Based Genotyping with the Delivery of Personalized Therapy in Metastatic Non-Small Cell Lung Cancer. JAMA Oncol 5:173–180. 10.001/jamaoncol.2018.4305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ai B, Liu H, Huang Y, Peng P (2016) Circulating cell-free DNA as a prognostic and predictive biomarker in non-small cell lung cancer. Oncotarget 7:44583–44595. 10.18632/oncotarget.10069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chae YK, Oh MS (2019) Detection of minimal residual disease using ctDNA in lung cancer: current evidence and future directions. J Thorac Oncol 14:16–24. 10.1016/j.jtho.2018.09.022 [DOI] [PubMed] [Google Scholar]
- Chen Y, Han T, Zhou Y et al. (2019) Comparing the efficacy of targeted next-generation sequencing in the identification of somatic mutations in circulating tumor DNA from different stages of lung cancer. Neoplasma 66:652–660. 10.4149/neo_2018_181130N910 [DOI] [PubMed] [Google Scholar]
- Chinniah C, Aguarin L, Cheng P et al. (2019) Early detection of recurrence in patients with locally advanced non–small-cell lung cancer via circulating tumor cell analysis. Clin Lung Cancer. 10.1016/j.cllc.2019.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couraud S, Vaca-Paniagua F, Villar S et al. (2014) Noninvasive diagnosis of actionable mutations by deep sequencing of circulating free DNA in lung cancer from never-smokers: a proof-of-concept study from BioCAST/IFCT-1002. Clin Cancer Res 20:4613–4624. 10.1158/1078-0432.CCR-13-3063 [DOI] [PubMed] [Google Scholar]
- Dagogo-Jack I, Brannon AR, Ferris LA et al. (2018) Tracking the evolution of resistance to ALK tyrosine kinase inhibitors through longitudinal analysis of circulating tumor DNA. JCO Precis Oncol 2018:1–14. 10.1200/po.17.00160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dono M, De Luca G, Lastraioli S et al. (2019) Tag-based next generation sequencing: a feasible and reliable assay for EGFR T790M mutation detection in circulating tumor DNA of non small cell lung cancer patients. Mol Med 25:15 10.1186/s10020-019-0082-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Achi H, Khoury JD, Loghavi S et al. (2019) Liquid biopsy by next-generation sequencing: a multimodality test for management of cancer. Curr Hematol Malig Rep 14:358–367. 10.1007/s11899-019-00532-w [DOI] [PubMed] [Google Scholar]
- Ettinger DS, Aisner DL, Wood DE et al. (2018) NCCN guidelines ® insights non–small cell lung cancer, version 5.2018 featured updates to the NCCN guidelines. J Natl Compr Cancer Netw 16:807–821. 10.6004/jnccn.2018.0062 [DOI] [PubMed] [Google Scholar]
- Fernandez-Marmiesse A, Gouveia S, Couce ML (2017) NGS technologies as a turning point in rare disease research, diagnosis and treatment. Curr Med Chem 25:404–432. 10.2174/0929867324666170718101946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gai W, Sun K (2019) Epigenetic biomarkers in cell-free DNA and applications in liquid biopsy. Genes (Basel). 10.3390/genes10010032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo M, De Luca A, Frezzetti D et al. (2019) The potential of monitoring treatment response in non-small cell lung cancer using circulating tumour cells. Expert Rev Mol Diagn 19:1–12. 10.1080/14737159.2019.1640606 [DOI] [PubMed] [Google Scholar]
- Garcia J, Forestier J, Dusserre E et al. (2018) Cross-platform comparison for the detection of RAS mutations in cfDNA (ddPCR Biorad detection assay, BEAMing assay, and NGS strategy). Oncotarget 9:21122–21131. 10.18632/oncotarget.24950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge M, Zhan Q, Zhang Z et al. (2019) Different next-generation sequencing pipelines based detection of tumor DNA in cerebrospinal fluid of lung adenocarcinoma cancer patients with leptomeningeal metastases. BMC Cancer 19:1–8. 10.1186/s12885-019-5348-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giroux Leprieur E, Herbretau G, Dumenil C et al. (2018) Circulating tumor DNA evaluated by Next-Generation Sequencing is predictive of tumor response and prolonged clinical benefit with nivolumab in advanced non-small cell lung cancer. Oncoimmunology 7:e1424675. 10.1080/2162402X.2018.1424675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Q, Wang J, Xiao J et al. (2018) Heterogeneous mutation pattern in tumor tissue and circulating tumor DNA warrants parallel NGS panel testing. Mol Cancer 17:4–8. 10.1186/s12943-018-0875-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagemann IS, Devarakonda S, Lockwood CM et al. (2015) Clinical next-generation sequencing in patients with non-small cell lung cancer. Cancer 121:631–639. 10.1002/cncr.29089 [DOI] [PubMed] [Google Scholar]
- Hanna N, Johnson D, Temin S et al. (2017) Systemic therapy for stage IV non–small-cell lung cancer: American Society of clinical oncology clinical practice guideline update. J Clin Oncol 35:3484–3515. 10.1200/JCO.2017.74.6065 [DOI] [PubMed] [Google Scholar]
- Heerink WJ, de Bock GH, de Jonge GJ et al. (2017) Complication rates of CT-guided transthoracic lung biopsy: meta-analysis. Eur Radiol 27:138–148. 10.1007/s00330-016-4357-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn L, Whisenant JG, Wakelee H et al. (2019) Monitoring therapeutic response and resistance: analysis of circulating tumor DNA in patients With ALK+ lung cancer. J Thorac Oncol 14:1901–1911. 10.1016/j.jtho.2019.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y, Ulrich BC, Supplee J et al. (2018) False-positive plasma genotyping due to clonal hematopoiesis. Clin Cancer Res 24:4437–4443. 10.1158/1078-0432.CCR-18-0143 [DOI] [PubMed] [Google Scholar]
- Iwama E, Sakai K, Azuma K et al. (2017) Monitoring of somatic mutations in circulating cell-free DNA by digital PCR and next-generation sequencing during afatinib treatment in patients with lung adenocarcinoma positive for EGFR activating mutations. Ann Oncol Off J Eur Soc Med Oncol 28:136–141. 10.1093/annonc/mdw531 [DOI] [PubMed] [Google Scholar]
- Iwama E, Sakai K, Azuma K et al. (2018) Exploration of resistance mechanisms for epidermal growth factor receptor-tyrosine kinase inhibitors based on plasma analysis by digital polymerase chain reaction and next-generation sequencing. Cancer Sci 109:3921–3933. 10.1111/cas.13820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings LJ, Arcila ME, Corless C et al. (2017) Guidelines for validation of next-generation sequencing-based oncology panels: a joint consensus recommendation of the Association for Molecular Pathology and College of American Pathologists. J Mol Diagnostics 19:341–365. 10.1016/j.jmoldx.2017.01.011.Guidelines [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Y, Shi X, Zhao J et al. (2018) Mechanisms of primary resistance to EGFR targeted therapy in advanced lung adenocarcinomas. Lung Cancer 124:110–116. 10.1016/j.lungcan.2018.07.039 [DOI] [PubMed] [Google Scholar]
- Kaisaki PJ, Cutts A, Popitsch N et al. (2016) Targeted next-generation sequencing of plasma DNA from cancer patients: Factors influencing consistency with tumour DNA and prospective investigation of its utility for diagnosis. PLoS ONE 11:1–13. 10.1371/journal.pone.0162809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalemkerian GP, Narula N, Kennedy EB et al. (2018) Molecular testing guideline for the selection of patients with lung cancer for treatment with targeted tyrosine kinase inhibitors: American society of clinical oncology endorsement of the college of American pathologists/ international association for the. J Clin Oncol 36:911–919. 10.1200/JCO.2017.76.7293 [DOI] [PubMed] [Google Scholar]
- Karachaliou N, Mayo-De Las Casas C, Queralt C et al. (2015) Association of EGFR L858R mutation in circulating free DNA with survival in the EURTAC trial. JAMA Oncol 1:149–157. 10.1001/jamaoncol.2014.257 [DOI] [PubMed] [Google Scholar]
- Kerr KM, Bubendorf L, Edelman MJ et al. (2014) Second ESMO consensus conference on lung cancer: pathology and molecular biomarkers for non-small-cell lung cancer. Ann Oncol 25:1681–1690. 10.1093/annonc/mdu145 [DOI] [PubMed] [Google Scholar]
- Lam VK, Tran HT, Banks KC et al. (2019) Targeted tissue and cell-free tumor DNA sequencing of advanced lung squamous-cell carcinoma reveals clinically significant prevalence of actionable alterations. Clin Lung Cancer 20:30–36.e3. 10.1016/j.cllc.2018.08.020 [DOI] [PubMed] [Google Scholar]
- Laufer-Geva S, Rozenblum AB, Twito T et al. (2018) The clinical impact of comprehensive genomic testing of circulating cell-free DNA in advanced lung cancer. J Thorac Oncol 13:1705–1716. 10.1016/j.jtho.2018.07.101 [DOI] [PubMed] [Google Scholar]
- Lee JY, Qing X, Xiumin W et al. (2016) Longitudinal monitoring of EGFR mutations in plasma predicts outcomes of NSCLC patients treated with EGFR TKIs: Korean Lung Cancer Consortium (KLCC-12–02). Oncotarget 7:6984–6993. 10.18632/oncotarget.6874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leighl NB, Page RD, Raymond VM et al. (2019) Clinical utility of comprehensive cell-free DNA analysis to identify genomic biomarkers in patients with newly diagnosed metastatic non–small cell lung cancer. Clin Cancer Res 25:4691–4700. 10.1158/1078-0432.ccr-19-0624 [DOI] [PubMed] [Google Scholar]
- Levy MA, Lovly CM, Pao W (2012) Translating genomic information into clinical medicine: Lung cancer as a paradigm. Genome Res 22:2101–2108. 10.1101/gr.131128.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YS, Jiang BY, Yang JJ et al. (2018) Unique genetic profiles from cerebrospinal fluid cell-free DNA in leptomeningeal metastases of EGFR-mutant non-small-cell lung cancer: a new medium of liquid biopsy. Ann Oncol Off J Eur Soc Med Oncol 29:945–952. 10.1093/annonc/mdy009 [DOI] [PubMed] [Google Scholar]
- Li BT, Janku F, Jung B et al. (2019) Ultra-deep next-generation sequencing of plasma cell-free DNA in patients with advanced lung cancers: results from the Actionable Genome Consortium. Ann Oncol Off J Eur Soc Med Oncol 30:597–603. 10.1093/annonc/mdz046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindeman NI, Cagle PT, Aisner DL et al. (2018) Updated molecular testing guideline for the selection of lung cancer patients for treatment with targeted tyrosine kinase inhibitors guideline from the college of American pathologists, the international association for the study of lung cancer, and the a. Arch Pathol Lab Med 142:321–346. 10.5858/arpa.2017-0388-CP [DOI] [PubMed] [Google Scholar]
- Liu L, Liu H, Shao D et al. (2018) Development and clinical validation of a circulating tumor DNA test for the identification of clinically actionable mutations in nonsmall cell lung cancer. Genes Chromosom Cancer 57:211–220. 10.1002/gcc.22522 [DOI] [PubMed] [Google Scholar]
- Mayo-de-Las-Casas C, Garzon Ibanez M, Jordana-Ariza N et al. (2018) An update on liquid biopsy analysis for diagnostic and monitoring applications in non-small cell lung cancer. Expert Rev Mol Diagn 18:35–45. 10.1080/14737159.2018.1407243 [DOI] [PubMed] [Google Scholar]
- McCoach CE, Blakely CM, Banks KC et al. (2018) Clinical utility of cell-free DNA for the detection of ALK fusions and genomic mechanisms of ALK inhibitor resistance in non–small cell lung cancer. Clin Cancer Res 24:2758–2770. 10.1158/1078-0432.CCR-17-588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merker JD, Oxnard GR, Compton C et al. (2018) Circulating tumor DNA analysis in patients with cancer: American society of clinical oncology and college of American pathologists joint review. Arch Pathol Lab Med 142:1242–1253. 10.5858/arpa.2018-0901-SA [DOI] [PubMed] [Google Scholar]
- Mok TS, Wu Y-L, Ahn M-J et al. (2016) Osimertinib or platinum-pemetrexed in EGFR T790M–positive lung cancer. N Engl J Med 376:629–640. 10.1056/nejmoa1612674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overman MJ, Modak J, Kopetz S et al. (2013) Use of research biopsies in clinical trials: are risks and benefits adequately discussed? J Clin Oncol 31:17–22. 10.1200/JCO.2012.43.1718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadopoulou E, Tsoulos N, Tsantikidi K et al. (2019) Clinical feasibility of NGS liquid biopsy analysis in NSCLC patients. PLoS ONE 14:e0226853. 10.1371/journal.pone.0226853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paweletz CP, Sacher AG, Raymond CK et al. (2016) Bias-corrected targeted next-generation sequencing for rapid, multiplexed detection of actionable alterations in cell-free dna from advanced lung cancer patients. Clin Cancer Res 22:915–922. 10.1158/1078-0432.CCR-15-1627-T [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pécuchet N, Zonta E, Didelot A et al. (2016) Base-position error rate analysis of next-generation sequencing applied to circulating tumor DNA in non-small cell lung cancer: a prospective study. PLoS Med 13:1–19. 10.1371/journal.pmed.1002199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrackova A, Vasinek M, Sedlarikova L et al. (2019) Standardization of sequencing coverage depth in NGS: recommendation for detection of clonal and subclonal mutations in cancer diagnostics. Front Oncol 9:1–6. 10.3389/fonc.2019.00851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phallen J, Leal A, Woodward BD et al. (2019) Early noninvasive detection of response to targeted therapy in non–small cell lung cancer. Cancer Res 79:1204–1213. 10.1158/0008-5472.CAN-18-1082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Planchard D, Popat S, Kerr K et al. (2018) Metastatic non-small cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 29:192–237. 10.1093/annonc/mdy275 [DOI] [PubMed] [Google Scholar]
- Postel M, Roosen A, Laurent-Puig P et al. (2018) Droplet-based digital PCR and next generation sequencing for monitoring circulating tumor DNA: a cancer diagnostic perspective. Expert Rev Mol Diagn 18:7–17. 10.1080/14737159.2018.1400384 [DOI] [PubMed] [Google Scholar]
- Pritchett MA, Camidge DR, Patel M et al. (2019) Prospective clinical validation of the invisionfirst-lung circulating tumor dna assay for molecular profiling of patients with advanced nonsquamous non–small-cell lung cancer. JCO Precis Oncol. 10.1200/po.18.00299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rachiglio AM, Esposito Abate R, Sacco A et al. (2016) Limits and potential of targeted sequencing analysis of liquid biopsy in patients with lung and colon carcinoma. Oncotarget 7:66595–66605. 10.18632/oncotarget.10704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizvi H, Sanchez-Vega F, La K et al. (2018) Molecular determinants of response to anti-programmed cell death (PD)-1 and anti-programmed death-ligand 1 (PD-L1) blockade in patients with non-small-cell lung cancer profiled with targeted next-generation sequencing. J Clin Oncol 36:633–641. 10.1200/JCO.2017.75.3384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolfo C, Mack PC, Scagliotti GV et al. (2018) Liquid biopsy for advanced non-small cell lung cancer (NSCLC): a statement paper from the IASLC. J Thorac Oncol 13:1248–1268. 10.1016/j.jtho.2018.05.030 [DOI] [PubMed] [Google Scholar]
- Sabari JK, Offin M, Stephens D et al. (2018) A prospective study of circulating tumor dna to guide matched targeted therapy in lung cancers. J Natl Cancer Inst 111:575–583. 10.1093/jnci/djy156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabour L, Sabour M, Ghorbian S (2017) Clinical applications of next-generation sequencing in cancer diagnosis. Pathol Oncol Res 23:225–234. 10.1007/s12253-016-0124-z [DOI] [PubMed] [Google Scholar]
- Sacher AG, Paweletz C, Dahlberg SE et al. (2016) Prospective validation of rapid plasma genotyping for the detection of EGFR and kras mutations in advanced lung cancer. JAMA Oncol 2:1014–1022. 10.1001/jamaoncol.2016.0173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santarpia M, Karachaliou N, González-Cao M et al. (2016) Feasibility of cell-free circulating tumor DNA testing for lung cancer. Biomark Med 10:417–430. 10.2217/bmm.16.6 [DOI] [PubMed] [Google Scholar]
- Schram AM, Chang MT, Jonsson P, Drilon A (2017) Fusions in solid tumours: diagnostic strategies, targeted therapy, and acquired resistance. Nat Rev Clin Oncol 14:735–748. 10.1038/nrclinonc.2017.127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwaederlé MC, Patel SP, Husain H et al. (2017) Utility of genomic assessment of blood-derived circulating tumor DNA (ctDNA) in patients with advanced lung adenocarcinoma. Clin Cancer Res 23:5101–5111. 10.1158/1078-0432.CCR-16-2497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiau CJ, Babwah JP, Da Cunha SG et al. (2014) Sample features associated with success rates in population-based EGFR mutation testing. J Thorac Oncol 9:947–956. 10.1097/JTO.0000000000000196 [DOI] [PubMed] [Google Scholar]
- Siravegna G, Marsoni S, Siena S, Bardelli A (2017) Integrating liquid biopsies into the management of cancer. Nat Rev Clin Oncol 14:531–548. 10.1038/nrclinonc.2017.14 [DOI] [PubMed] [Google Scholar]
- Streubel A, Stenzinger A, Stephan-Falkenau S et al. (2019) Comparison of different semi-automated cfDNA extraction methods in combination with UMI-based targeted sequencing. Oncotarget 10:5690–5702. 10.18632/oncotarget.27183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundaresan TK, Sequist LV, Heymach JV et al. (2016) Detection of T790M, the acquired resistance EGFR mutation, by tumor biopsy versus noninvasive blood-based analyses. Clin Cancer Res 22:1103–1110. 10.1158/1078-0432.CCR-15-1031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supplee JG, Milan MSDD, Lim LP et al. (2019) Sensitivity of next-generation sequencing assays detecting oncogenic fusions in plasma cell-free DNA. Lung Cancer 134:96–99. 10.1016/j.lungcan.2019.06.004 [DOI] [PubMed] [Google Scholar]
- Tamminga M, De Wit S, Hiltermann TJN et al. (2019) Circulating tumor cells in advanced non-small cell lung cancer patients are associated with worse tumor response to checkpoint inhibitors. J Immunother Cancer 7:1–9. 10.1186/s40425-019-0649-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan DSW, Camilleri-Broët S, Tan EH et al. (2014) Intertumor heterogeneity of non-small-cell lung carcinomas revealed by multiplexed mutation profiling and integrative genomics. Int J Cancer 135:1092–1100. 10.1002/ijc.28750 [DOI] [PubMed] [Google Scholar]
- Tang Y, Liu X, Ou Z et al. (2019) Maximum allele frequency observed in plasma: a potential indicator of liquid biopsy sensitivity. Oncol Lett 18:2118–2124. 10.3892/ol.2019.10490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thress KS, Paweletz CP, Felip E et al. (2015) Acquired EGFR C797S mediates resistance to AZD9291 in advanced non-small cell lung cancer harboring EGFR T790M HHS Public Access Author manuscript. Nat Med 21:560–562. 10.1038/nm.3854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong L, Ding N, Tong X et al. (2019) Tumor-derived DNA from pleural effusion supernatant as a promising alternative to tumor tissue in genomic profiling of advanced lung cancer. Theranostics 9:5532–5541. 10.7150/thno.34070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toor OM, Ahmed Z, Bahaj W et al. (2018) Correlation of somatic genomic alterations between tissue genomics and ctdna employing next-generation sequencing: analysis of lung and gastrointestinal cancers. Mol Cancer Ther 17:1123–1132. 10.1158/1535-7163.MCT-17-1015 [DOI] [PubMed] [Google Scholar]
- Tran LS, Pham H-ATAT, Tran V-UU et al. (2019) Ultra-deep massively parallel sequencing with unique molecular identifier tagging achieves comparable performance to droplet digital PCR for detection and quantification of circulating tumor DNA from lung cancer patients. PLoS ONE 14:e0226193. 10.1371/journal.pone.0226193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travis WD, Brambilla E, Nicholson AG et al. (2015) The 2015 World Health Organization classification of lung tumors: impact of genetic, clinical and radiologic advances since the 2004 classification. J Thorac Oncol 10:1243–1260. 10.1097/JTO.0000000000000630 [DOI] [PubMed] [Google Scholar]
- VanderLaan PA, Yamaguchi N, Folch E et al. (2014) Success and failure rates of tumor genotyping techniques in routine pathological samples with non-small-cell lung cancer. Lung Cancer 84:39–44. 10.1016/j.lungcan.2014.01013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanni I, Coco S, Truini A et al. (2015) Next-generation sequencing workflow for NSCLC critical samples using a targeted sequencing approach by ion torrent PGM ™ platform. Int J Mol Sci 16:28765–28782. 10.3390/ijms161226129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldore VH, Choughule A, Routhu T et al. (2018) Validation of liquid biopsy: plasma cell-free DNA testing in clinical management of advanced non-small cell lung cancer. Lung Cancer Targets Ther 9:1–11. 10.2147/LCTT.S147841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vestergaard HH, Christensen MR, Lassen UN (2018) A systematic review of targeted agents for non-small cell lung cancer. Acta Oncol (Madr) 57:176–186 [DOI] [PubMed] [Google Scholar]
- Villaflor V, Won B, Nagy R et al. (2016) Biopsy-free circulating tumor DNA assay identifies actionable mutations in lung cancer. Oncotarget 7:66880–66891. 10.18632/oncotarget.11801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogelstein B, Papadopoulos N, Velculescu VE et al. (2013) Cancer genome landscapes NIH public access. Science 339:1546–1558. 10.1126/science.1235122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollbrecht C, Lehmann A, Lenze D, Hummel M (2018) Validation and comparison of two NGS assays for the detection of EGFR T790M resistance mutation in liquid biopsies of NSCLC patients. Oncotarget 9:18529–18539. 10.18632/oncotarget.24908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber B, Meldgaard P, Hager H et al. (2014) Detection of EGFR mutations in plasma and biopsies from non-small cell lung cancer patients by allele-specific PCR assays. BMC Cancer. 10.1186/1471-2407-14-294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z, Yang Z, Li CS et al. (2019) Differences in the genomic profiles of cell-free DNA between plasma, sputum, urine, and tumor tissue in advanced NSCLC. Cancer Med 8:910–919. 10.1002/cam4.1935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu T, Kang X, You X et al. (2017) Cross-platform comparison of four leading technologies for detecting EGFR mutations in circulating tumor DNA from non-small cell lung carcinoma patient plasma. Theranostics 7:1437–1446. 10.7150/thno.16558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang N, Li Y, Liu Z et al. (2018) The characteristics of ctDNA reveal the high complexity in matching the corresponding tumor tissues. BMC Cancer 18:1–12. 10.1186/s12885-018-4199-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Y, Liu J, Li L et al. (2017) Detection of circulating tumor DNA in patients with advanced non-small cell lung cancer. Oncotarget 8:2130–2140. 10.18632/oncotarget.12883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu G, Ye X, Dong Z et al. (2015) Highly sensitive droplet digital PCR method for detection of EGFR-activating mutations in plasma cell-free DNA from patients with advanced non-small cell lung cancer. J Mol Diagnostics 17:265–272. 10.1016/j.jmoldx.2015.01.004 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.