Abstract
Background
While the incidence of catastrophic costs due to tuberculosis (TB) remains high, there is little evidence about their impact on TB treatment outcomes and adherence. We assessed their effect on treatment outcomes and adherence in Indonesia.
Methods
We interviewed 282 adult TB patients who underwent TB treatment in urban, suburban and rural districts of Indonesia. One year after the interview, we followed up treatment adherence and outcomes. We applied multivariable analysis using generalized linear mixed models.
Results
Follow-up was complete for 252/282 patients. Eighteen (7%) patients had unsuccessful treatment and 40 (16%) had poor adherence. At a threshold of 30% of annual household income, catastrophic costs negatively impacted treatment outcomes (adjusted odds ratio [aOR] 4.15 [95% confidence interval {CI} 1.15 to 15.01]). At other thresholds, the associations showed a similar pattern but were not statistically significant. The association between catastrophic costs and treatment adherence is complex because of reverse causation. After adjustment, catastrophic costs negatively affected treatment adherence at the 10% and 15% thresholds (aOR 2.11 [95% CI 0.97 to 4.59], p = 0.059 and aOR 2.06 [95% CI 0.95 to 4.46], p = 0.07). There was no evidence of such an effect at other thresholds.
Conclusions
Catastrophic costs negatively affect TB treatment outcomes and treatment adherence. To eliminate TB, it is essential to mitigate catastrophic costs.
Keywords: catastrophic costs, Indonesia, treatment adherence, treatment outcomes, tuberculosis
Introduction
Tuberculosis (TB) often results in severe economic consequences for TB-affected households.1 The latest World Health Organization (WHO) End TB Strategy aims by 2020 to reduce to zero the percentage of TB-affected families that face catastrophic costs.2 However, attaining this target is challenging. In a simulation of eight scenarios, Fuady et al.3 showed that, although the provision of a cash transfer to TB-affected households would reduce the incidence of catastrophic costs due to TB, the incidence would not reach the 0% target. This failure is due to the high variability of costs between TB patients, particularly those due to income loss. If the value of the cash transfer provided were the same for all patients, it would not be possible to eliminate catastrophic costs. This finding raises the question of whether eliminating catastrophic costs, apart from its importance as a proxy of poverty, is a rational target to be achieved.
There are various definitions of catastrophic costs. Given that eliminating the incidence of catastrophic costs is a target of the WHO End TB Strategy, the definition of TB-related catastrophic costs issued by the WHO is the most appropriate to use. The latest TB costs survey handbook of the WHO defines catastrophic costs as the total costs, that is, all direct and indirect costs, including income loss, that exceed a specific threshold of a household's annual income.4 In Peru, Wingfield et al.5 showed that TB-related catastrophic costs were associated with adverse TB outcomes and suggested a threshold of 20% of annual household income to define catastrophic costs. However, there is a paucity of evidence from other countries, especially from high TB burden countries.
Indonesia is such a high TB burden country. Despite the availability of free treatment, Indonesia has a high incidence of TB-related catastrophic costs: 36% in TB-affected households and 83% in multidrug-resistant TB (MDR-TB)-affected households.6 Income loss is the main driver of these costs. Catastrophic costs during the course of treatment may affect treatment adherence and treatment outcomes. Patients may quit treatment altogether or they may interrupt it and thereby extend it, and as a consequence they may fail to be cured or may even die during treatment. Nonetheless, there is no evidence on the impact of catastrophic costs on TB treatment outcomes and treatment adherence from this high-burden country. We aimed to establish the extent to which catastrophic costs in TB-affected Indonesian households affect TB treatment outcomes and adherence.
Methods
Study design
To assess the effect of catastrophic costs on patients’ TB treatment adherence and outcomes, we conducted a cohort study in three districts in Indonesia, each with a different level of urbanization: Jakarta (urban), Depok (suburban) and Tasikmalaya (rural). In each district we identified all primary health centres (PHCs) that delivered TB treatment services and were also linked to the Indonesian National Tuberculosis Program (NTP). For inclusion in our study, we randomly selected five to eight PHCs per district. In total, 19 PHCs were included. In the baseline study (July–September 2016) we interviewed 282 TB patients ≥18 y of age who had undergone TB treatment for at least 1 month or had completed treatment no more than 1 month previously.6 One year after the interview, we followed up their TB treatment adherence and TB treatment outcomes. We excluded TB patients whose treatment had not been evaluated and patients who had been transferred to other health facilities.
Treatment outcome and adherence
Since the WHO has set treatment outcome as an essential indicator in TB control programs,7 treatment outcome was the primary outcome measured in this study. To evaluate patients’ treatment outcomes, we examined their medical records (TB 01 forms) and cross-checked the data with the PHCs’ TB records (TB 03 form). We used the definitions and classification of treatment outcome issued by the WHO, defining treatment outcome as successful if a patient had been cured or had completed TB treatment and as unsuccessful if a patient had been lost to follow-up, died or if the treatment had failed (Table 1).8
Table 1.
Primary and secondary outcomes in this study
| Outcomes | Definition |
|---|---|
| Primary outcome: treatment outcomes | |
| Successful treatment | The sum of cured and completed TB cases |
| Cured | A TB patient who had a positive sputum smear or culture at the beginning of TB treatment but had a negative sputum smear or culture in the last month of treatment and on at least one previous occasion |
| Completed | A TB patient who completed treatment but who, in the last month of treatment and on at least one previous occasion, did not have any proof of a negative sputum smear or culture result |
| Unsuccessful treatment | Died, failed or lost to follow-up |
| Died | A TB patient who died for any reason during the course of treatment |
| Failed | A TB patient who still had a positive sputum smear or culture after ≥5 months of TB treatment |
| Lost to follow-up | A TB patient whose treatment had been interrupted for ≥2 consecutive months |
| Secondary outcome: treatment adherence | |
| Good treatment adherence | A patient whose treatment had been successful and whose treatment period had not exceeded the expected end-of-treatment date by ≥14 d |
| Poor treatment adherence | Sum of patients whose treatment period had exceeded the expected end-of-treatment date by ≥14 d and cases lost to follow-up |
Since catastrophic costs are also assumed to reduce treatment adherence, we evaluated treatment adherence as a secondary outcome. However, there is no commonly agreed upon definition of TB non-adherence. By defining non-adherent patients as those who had missed at least one prescribed dose of TB drug, some studies use stringent criteria,9,10 while other criteria are less stringent, such as having interrupted treatment for >1 month or never having been under supervision.11–15 We defined poor adherence as applying to a patient who had been lost to follow-up, indicating that he/she had not adhered to treatment, or to a patient whose treatment had been successful but the treatment period had exceeded the expected end-of-treatment date by ≥14 d. To assess the effect of catastrophic costs on treatment adherence, we excluded patients who had died and those whose treatment had failed.
Catastrophic costs
In the baseline study, we interviewed patients about the total TB-related costs their household had incurred since the pre-diagnostic phase until the they stopped treatment. We used the Tool to Estimate Patient Costs that has been adapted to the Indonesian context.4,16 In compliance with the definition in the WHO handbook, total costs consisted of direct medical costs (i.e. administration costs, laboratory tests, X-ray examinations, drug costs, hospitalization costs and adverse drug effects costs), direct non-medical costs (i.e. transportation, food and the costs of food supplements) and income loss.4 To calculate administration costs and food costs, we multiplied the number of visits by the administration fees and food costs incurred during visits. To measure the total transportation costs, we multiplied single travel costs for a return visit by the number of visits during treatment. The number of visits was recorded in the patient's medical record. If these data were missing or if a patient had become lost to follow-up, we calculated costs on the basis of the patient’s average number of visits to the same PHC.
Income loss was estimated on the basis of the monthly income change reported in the baseline study. The monthly income change was calculated as the difference in income between that received before TB diagnosis and that received at the time of the interview. The monthly income loss was multiplied by the number of months patients had undergone TB treatment. Patients who earned an uncertain monthly income from jobs in the informal sector, such as taxi-bike drivers, often were unable to provide exact information on changes from one month to the next. To avoid underestimating the income loss of these patients, we used the human capital approach to estimate their income loss.4 We collected the self-reported time that patients took to seek and receive healthcare and the hourly rate the patient working in the informal sector normally charged for his/her informal work. We used the following formula to obtain the total income loss: return trip in minutes for a typical visit × patient's income loss per minute×the number of visits over the course of treatment.
Patients may quit treatment due to their actual or expected future financial burden. Some patients may therefore not experience catastrophic costs at the time they quit treatment but would have experienced such costs if they had continued until treatment completion. For patients who were lost to follow-up, we therefore extrapolated the direct and indirect costs by multiplying their direct unit cost by the average number of visits of patients treated in the same PHC. To extrapolate their income loss, we multiplied their monthly income loss by the number of months in a standard period of full treatment.
In addition to TB-related costs, patients also reported their monthly household income. Per patient, we calculated annual household income in the year before he/she was diagnosed with TB. We calculated total costs as a share of annual household income, which was displayed as a percentage. If the percentage exceeded a specific threshold, e.g. 30%, we defined this as catastrophic costs.17
Statistical analyses
First, we analysed the association between catastrophic costs (as an independent variable) and treatment adherence and treatment outcomes (as dependent variables) using generalized linear mixed models (GLMMs) with a logit link function, generating crude odds ratios (cORs) for these associations. We used random effects to adjust for our cluster sampling design (19 PHCs). To determine the threshold at which catastrophic costs affect treatment outcomes and adherence, we ran the analysis using various thresholds (i.e. 10, 15, 20, 25, 30 and 35%). The threshold at which catastrophic costs were statistically significantly associated with treatment outcomes and treatment adherence was used for further analyses.
Next, we examined whether confounding had produced the association observed in the univariate analysis between catastrophic costs, treatment outcomes and treatment adherence. To assess whether the effect of costs had been confounded by other variables, we decided a priori to include all potential confounders for which we had data (i.e. age, sex, district, education, previous TB treatment, initial sputum result, hospitalization and adverse drug effect). To obtain adjusted ORs (aORs) and 95% confidence intervals (CIs), the potential confounders were analysed simultaneously in a multivariable analysis. Since catastrophic costs incorporate income in their calculation, we did not include household income level, breadwinner and job-loss variables as potential confounders in our multivariable analysis.
To assess the contribution of catastrophic costs to unsuccessful treatment outcomes in the population, we also estimated the population-attributable fraction (PAF) for our study population. This was calculated as [Ppop × (OR−1)]/[Ppop × (OR−1) + 1], where Ppop is the proportion of exposed subjects in the entire study population and OR is the odds ratio of catastrophic costs to unsuccessful treatment outcomes obtained from the multivariable analysis.
Poor treatment adherence in this study included patients who had been lost to follow-up and patients whose treatment period had been prolonged. As prolonged treatment may lead to higher direct and indirect costs (e.g. more healthcare visits, higher income loss), it may be the cause rather than the consequence of catastrophic costs. In our univariate and multivariable analyses, we therefore examined whether the association between catastrophic costs and treatment adherence was due to reverse causation. To do so, we simulated cost data for patients with a prolonged treatment period by adjusting their number of visits to the average number of visits of patients treated in the same PHC and, on the basis of their expected end-of-treatment date, by the number of months they had lost income. After recalculating the costs, we compared the incidence of catastrophic costs between the actual costs and recalculated costs and the effect of catastrophic costs on treatment outcomes. No such reverse effects were expected for patients who had been lost to follow-up, for patients whose treatment had failed or for patients who had died, as these patients’ TB treatment had not been prolonged.
All data were cleaned and analysed using SPSS 21 (IBM, Armonk, NY, USA).18 Costs and income data were entered in Indonesian rupiah (IDR) and were then converted to US dollars (US$) using the average exchange rate for 2016 (US$1 = IDR 13 389.41).19
Results
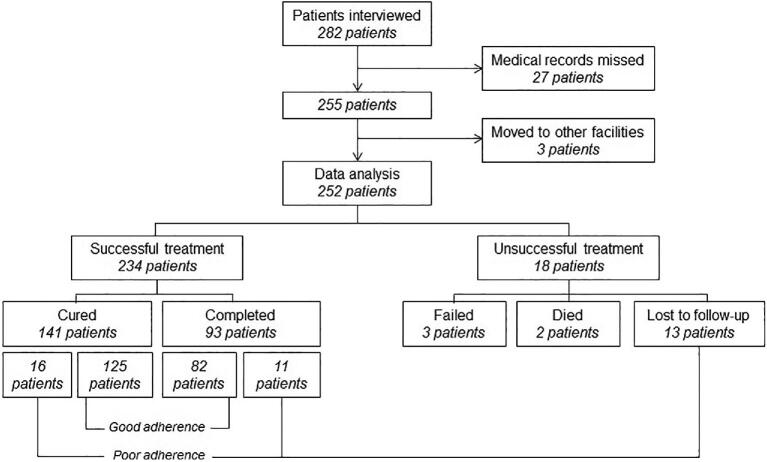
One year after patients had been interviewed, the medical records of 27 patients were missing and could not be tracked for reasons such as storage relocation, the renovation of a PHC building or a change of several persons in charge of the TB program in the PHCs. Three patients had moved to other healthcare facilities. In our analyses, we included 252 (89.4%) of the 282 subjects who had been interviewed in 2016 and whose treatment outcome and adherence had been recorded in 2017 (Figure 1).
Figure 1.
Number of subjects in the 1-y cohort.
TB treatment had been successful for 234 patients (93%). More than half of all patients (n = 141 [56%]) were cured, as indicated from sputum smear conversion from a positive smear to a negative smear at the end of treatment, while 93 patients (37%) had completed the treatment without proof of smear conversion. Treatment had been unsuccessful in 18 patients (7%): 3 patients had failed, 2 patients had died and 13 patients had been lost to follow-up. Most patients (n = 207 [84%]) had good treatment adherence. The treatment period had been prolonged in 27 patients (11%) and 13 patients (5%) had been lost to follow-up. Treatment outcome had been successful in all patients with a prolonged treatment period.
Most patients lived in a poor household (61%), had an income-earning job (74%), had smear-positive TB (66%) and had undergone category 1 TB treatment (the first-line treatment for susceptible TB patients who have not previously received TB treatment; 88%). (Table 2) The median of total costs incurred by the patients was US$118 (interquartile range [IQR] 455). The median of total costs as a share of annual household income was 9% (IQR 25). The incidences of catastrophic costs were 46, 38, 33, 26 and 22% at a threshold of 10, 15, 20, 25 and 30% of annual household income, respectively.
Table 2.
Subject characteristics
| Characteristics | Values |
|---|---|
| Sociodemographic (n = 252) | |
| Age (years), n (%) | |
| 18–40 | 126 (50) |
| 41–60 | 93 (37) |
| >60 | 33 (13) |
| Sex, n (%) | |
| Male | 135 (54) |
| Female | 117 (46) |
| Household income levela, n (%) | |
| Poor | 153 (61) |
| Non-poor | 99 (39) |
| Education level, n (%) | |
| Low | 87 (35) |
| Middle | 154 (61) |
| High | 11 (4) |
| Patient as breadwinner, n (%) | |
| Yes | 114 (45) |
| No | 138 (55) |
| Patient had earned money before diagnosis, n (%) | |
| Yes | 187 (74) |
| Fixed payment | 83 (33) |
| Uncertain | 99 (39) |
| Others | 5 (2) |
| No | 65 (26) |
| Experienced job lossb, n (%) | |
| Yes | 73 (39) |
| No | 114 (61) |
| Clinical characteristics | |
| Category of treatment, n (%) | |
| Category 1 | 222 (88) |
| Category 2 | 30 (12) |
| Result of initial sputum test, n (%) | |
| Positive | 167 (66) |
| Negative | 85 (34) |
| Was hospitalized, n (%) | |
| Yes | 34 (13) |
| No | 218 (87) |
| Experienced adverse drug effect(s) , n (%) | |
| Yes | 4 (2) |
| No | 248 (98) |
| Costs | |
| Total costs (US$), median (IQR) | 118 (455) |
| Costs as a share of annual income (%), median (IQR) | 9 (25) |
| Incidence of catastrophic costs, n (%) | |
| Threshold of 10% | 117 (46) |
| Threshold of 15% | 97 (38) |
| Threshold of 20% | 83 (33) |
| Threshold of 25% | 66 (26) |
| Threshold of 30% | 55 (22) |
| Threshold of 35% | 44 (17) |
Household income was divided into two groups, poor and non-poor, on the basis of the World Bank definition that a household is defined as poor if the income per capita per day is ≤US$1.90.
The percentages were calculated for those who had an income-earning job before diagnosis.
Our univariate analysis suggests that the odds of unsuccessful treatment outcomes were around two to three times higher for patients experiencing catastrophic costs compared with patients not experiencing catastrophic costs at thresholds of 10, 25 and 30% of annual household income (Table 3). Nevertheless, the association was only statistically significant at the 0.05 level when using the 30% threshold (cOR 3.32 [95% CI 1.13 to 9.69], p = 0.03).
Table 3.
Associations between catastrophic costs and unsuccessful TB treatment outcome
| Variables | Unsuccessful treatmenta, n/N (%) | cOR (95% CI) | p-Value | aOR (95% CI)b | p-Value |
|---|---|---|---|---|---|
| Catastrophic costs, threshold of 10% | |||||
| Yes | 12/117 (10.3) | 2.60 (0.87 to 7.80) | 0.09 | 2.59 (0.78 to 8.63) | 0.12 |
| No | 6/135 (4.4) | 1 | 1 | ||
| Catastrophic costs, threshold of 15% | |||||
| Yes | 10/97 (10.3) | 2.21 (0.76 to 6.38) | 0.14 | 2.31 (0.72 to 7.47) | 0.16 |
| No | 8/155 (5.2) | 1 | 1 | ||
| Catastrophic costs, threshold of 20% | |||||
| Yes | 8/83 (9.6) | 1.74 (0.61 to 4.97) | 0.30 | 1.80 (0.55 to 5.88) | 0.33 |
| No | 10/169 (5.9) | 1 | 1 | ||
| Catastrophic costs, threshold of 25% | |||||
| Yes | 8/66 (12.1) | 2.65 (0.92 to 7.69) | 0.07 | 3.03 (0.89 to 10.31) | 0.08 |
| No | 10/186 (5.4) | 1 | 1 | ||
| Catastrophic costs, threshold of 30% | |||||
| Yes | 8/55 (14.5) | 3.32 (1.13 to 9.69) | 0.03 | 3.86 (1.11 to 13.38) | 0.03 |
| No | 10/197 (5.1) | 1 | 1 | ||
| Catastrophic costs, threshold of 35% | |||||
| Yes | 2/44 (4.5) | 0.53 (0.11 to 2.51) | 0.42 | 0.53 (0.10 to 2.86) | 0.46 |
| No | 16/208 (7.7) | 1 | 1 | ||
The number (%) of patients with unsuccessful treatment among those who experienced or did not experience catastrophic costs.
aORs of unsuccessful treatment comparing patients with and without catastrophic costs after adjustment for all potential confounders for which we had information (i.e. age, sex, district, education, previous TB treatment, initial sputum result, hospitalization and adverse drug effect).
After adjustment for potential confounders in the multivariable analysis, catastrophic costs at a threshold of 30% remained statistically significantly associated with unsuccessful treatment outcomes. At this threshold, the odds of unsuccessful treatment outcomes were 3.86 times higher (95% CI 1.11 to 13.38, p = 0.03) in patients who had experienced catastrophic costs than in patients who had not. Using this aOR, the PAF was 38.6%, meaning that 38.6% of unsuccessful treatment outcome cases were attributable to catastrophic costs. At the 10–25% thresholds, the pattern of associations was similar, with estimated ORs of around 2 to 3, but these associations were not statistically significant at conventional levels.
Our univariate analysis also suggests that catastrophic costs (at thresholds of 10–25%) were associated with poor treatment adherence: the odds of poor adherence were approximately twice as high in patients who had experienced catastrophic costs as in those who had not (Table 4). However, these findings were statistically significant at the 0.05 level only when using the 15% threshold. At this threshold, the odds of poor adherence among patients who had experienced catastrophic costs were 2.12 times higher (95% CI 1.01 to 4.45, p = 0.046) than in those who had not. None of the other variables were statistically significantly associated with poor treatment adherence.
Table 4.
Associations between catastrophic costs and poor treatment adherence in actual calculation and recalculation adjusted to normal treatment period
| Univariate analysis | Multivariable analysis | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Actual | Recalculationa | Actual | Recalculation | |||||||
| Catastrophic costs | Poor adherenceb, n/N (%) | cOR (95% CI) | p-Value | Poor adherenceb, n/N (%) | cOR (95% CI) | p-Value | aOR (95% CI)c | p-Value | aOR (95% CI)c | p-Value |
| Threshold of 10% | ||||||||||
| Yes | 23/114 (20.2) | 1.94 (0.94 to 4.02) | 0.08 | 23/114 (20.2) | 1.94 (0.94 to 4.02) | 0.08 | 2.11 (0.97 to 4.59) | 0.059 | 2.11 (0.97 to 4.59) | 0.059 |
| No | 17/133 (12.8) | 1 | 17/133 (12.8) | 1 | 1 | 1 | ||||
| Threshold of 15% | ||||||||||
| Yes | 20/94 (21.3) | 2.12 (1.01 to 4.45) | 0.046 | 19/93 (20.4) | 1.87 (0.89 to 3.91) | 0.09 | 2.35 (1.08 to 5.14) | 0.03 | 2.06 (0.95 to 4.46) | 0.07 |
| No | 20/153 (13.1) | 1 | 21/154 (13.6) | 1 | 1 | 1 | ||||
| Threshold of 20% | ||||||||||
| Yes | 17/80 (21.3) | 1.88 (0.90 to 3.94) | 0.09 | 15/78 (19.2) | 1.47 (0.70 to 3.09) | 0.31 | 2.03 (0.93 to 4.42) | 0.08 | 1.55 (0.71 to 3.38) | 0.27 |
| No | 23/167 (13.8) | 1 | 25/169 (14.8) | 1 | 1 | 1 | ||||
| Threshold of 25% | ||||||||||
| Yes | 14/63 (22.2) | 1.94 (0.90 to 4.16) | 0.09 | 11/60 (18.3) | 1.35 (0.690 to 3.01) | 0.46 | 2.08 (0.93 to 4.65) | 0.08 | 1.40 (0.60 to 3.26) | 0.44 |
| No | 26/184 (14.1) | 1 | 29/187 (15.5) | 1 | 1 | 1 | ||||
| Threshold of 30% | ||||||||||
| Yes | 11/52 (21.2) | 1.65 (0.73 to 3.70) | 0.23 | 9/50 (18.0) | 1.26 (0.54 to 2.97) | 0.59 | 1.71 (0.73 to 4.00) | 0.22 | 1.29 (0.53 to 3.15) | 0.58 |
| No | 29/195 (14.9) | 1 | 31/197 (15.7) | 1 | 1 | 1 | ||||
| Threshold of 35% | ||||||||||
| Yes | 8/44 (18.2) | 1.27 (0.52 to 3.08) | 0.59 | 5/41 (12.2) | 1.75 (0.27 to 2.10) | 0.58 | 1.35 (0.54 to 3.37) | 0.52 | 0.80 (0.28 to 2.31) | 0.68 |
| No | 32/203 (15.8) | 1 | 35/206 (17.0) | 1 | 1 | 1 | ||||
Recalculating costs if a patient with a prolonged treatment period finished his/her treatment on time.
Number (%) of patients with poor treatment adherence among those who experienced or did not experience catastrophic costs.
aORs of poor comparing patients with and without catastrophic costs after adjustment for all potential confounders for which we had information (i.e. age, sex, district, education, previous TB treatment, initial sputum result and hospitalization).
The association between catastrophic costs and poor treatment adherence was partly due to reverse causation. In other words, prolonged treatment had contributed to catastrophic costs. We evaluated the extent of reverse causation by conducting a simulation analysis for patients with prolonged treatment period. When we assumed that patients with extended treatment duration had finished their treatment on time, the strength of the association between catastrophic costs and treatment adherence decreased at the 15% threshold from a cOR of 2.12 to 1.87, at the 25% threshold from a cOR of 1.94 to 1.35 and at the 20% threshold from a cOR of 1.88 to 1.47. When also adjusting for potential confounders, catastrophic costs at the 10 and 15% thresholds were associated with a two times higher odds of poor adherence compared with patients without catastrophic costs (aOR 2.11 [95% CI 0.97 to 4.59], p = 0.059 and aOR 2.06 [95% CI 0.95 to 4.46], p = 0.07). At other thresholds, we have no evidence of such an effect.
Discussion
Our study shows that catastrophic costs can have a negative impact on treatment outcome. Generally the odds of an unsuccessful treatment outcome were around two to four times higher among patients who experienced catastrophic costs compared with those who had not. While our findings were statistically significant at a threshold for catastrophic costs of 30%, there was an indication that this may also be the case at thresholds between 10 and 25%. The association between catastrophic costs and poor treatment adherence was more complex. Poor adherence can lead to higher costs. After adjustment for such reverse causation, we found that catastrophic costs at the 10 and 15% thresholds were associated with an around two times higher odds of poor treatment adherence compared with patients who had not experienced such costs, with no effect at other thresholds.
While there is currently no agreement on the specific threshold of catastrophic costs for research and policymaking, various studies have defined this threshold as 20% of annual household income, as suggested by the WHO through their task force.3,16,20 However, there is little evidence on whether the use of this threshold accurately reflects the effect of catastrophic costs on treatment outcomes and treatment adherence. Until now, the only study that assessed the association between catastrophic costs and poor treatment outcome was a Peruvian study (2014) that suggested a threshold of 20%.5 The study included MDR-TB patients who incurred higher costs; also in that study, MDR-TB was found to be one of the determinants of poor outcomes. Different from the Peruvian study, our study focused on susceptible TB patients. We found that at a 30% threshold, catastrophic costs lead to poor treatment outcomes, which, in our study, mostly consisted of patients who were lost to follow-up.
At a lower threshold (15%), catastrophic costs were associated with poor treatment adherence (prolonged treatment period or lost to follow-up). This association was due partly to reverse causation. Patients who did not adhere to their treatment course had to catch up with it, either under the orders of the PHC health staff (in order to comply with NTP guidelines) or because they were self-motivated and wished to minimize the risk of recurrence. The additional visits needed to complete the full treatment course led to higher costs and greater income loss, which in some cases led to catastrophic costs. At the same time, even after accounting for such reverse causation, catastrophic costs still negatively affected treatment adherence at thresholds of 10–15%. Due to the size of our study and lack of statistical power, although there is an indication of a similar effect at thresholds of 20–30%, we need to be cautious to draw firm conclusions with respect to these levels.
With regard to future global policy, our study provides evidence that can inform a review of the threshold at which catastrophic costs should be measured. If these catastrophic costs are thought to affect patient adherence, the threshold of 15% of annual household income might be considered to define catastrophic costs. When considering TB treatment outcomes, the threshold of 30% of annual household income might be used to define catastrophic costs. Nevertheless, as also suggested by the WHO,20 the threshold for defining catastrophic costs may vary between countries and should be carefully assessed from one setting to the next.
This study is the first cohort study on TB-related catastrophic costs, treatment adherence and treatment outcomes in Indonesia. Although we collected data from urban, suburban and rural areas of Indonesia, all these areas were located on the island of Java, which is home to 60% of the Indonesian population. Therefore our findings may not apply directly to eastern Indonesia and to the country's other remote areas, where healthcare facilities are scarcer—a factor that may affect treatment outcomes and treatment adherence. Similarly, as our study was conducted only in PHCs, its findings may underestimate the costs of the treatment given by private providers (which are assumed to be higher), while simultaneously overestimating treatment adherence, which may be lower among patients who are treated by private providers.21 It is also uncertain whether our findings will apply to high TB burden countries in which TB service delivery is different. Finally, the precise effect estimates in our study remain uncertain due to the limited sample size of the study. While our study is the first to provide evidence for Indonesia on the effects of catastrophic costs on treatment outcomes and treatment adherence, and one of the very few studies that do so for high TB burden countries more generally, larger studies are warranted.
We conclude that catastrophic costs negatively impact TB treatment outcomes and TB adherence at the various thresholds of annual household income. This highlights the need for TB control interventions to properly address both the clinical and socio-economic aspects of the disease.
Acknowledgements
We would like to thank the following: the head of the Jakarta Provincial Health Office, the head of the Tasikmalaya District Health Office and the head of the Depok District Health Office for issuing permits for data collection in their institutions and areas; Dedi Suhendar, the TB program officers in the PHCs and all the enumerators for their support with data collection; the Indonesian Endowment Fund for Education (Lembaga Pengelola Dana Pendidikan) for funding this project and the US Agency for International Development, Sustainable Higher Education Research Alliance, Center for Collaborative Research Scientific Modeling, Application, Research and Training City-centered Innovation and Technology led by the University of Indonesia for support through the SMART CITY Project.
Contributor Information
Ahmad Fuady, Department of Public Health, Erasmus MC, University Medical Center Rotterdam, P.O. Box 2040, 3000CA Rotterdam, The Netherlands; Department of Community Medicine, Faculty of Medicine, Universitas Indonesia, Jl Pegangsaan Timur No. 16 Jakarta 10310, Indonesia.
Tanja A J Houweling, Department of Public Health, Erasmus MC, University Medical Center Rotterdam, P.O. Box 2040, 3000CA Rotterdam, The Netherlands.
Muchtaruddin Mansyur, Department of Community Medicine, Faculty of Medicine, Universitas Indonesia, Jl Pegangsaan Timur No. 16 Jakarta 10310, Indonesia.
Erlina Burhan, Department of Respiratory and Pulmonology, Persahabatan Hospital – Faculty of Medicine, Universitas Indonesia, Jl Persahabatan No. 1 Jakarta 13230, Indonesia.
Jan Hendrik Richardus, Department of Public Health, Erasmus MC, University Medical Center Rotterdam, P.O. Box 2040, 3000CA Rotterdam, The Netherlands.
Authors’ contributions
AF, TAJH, and JHR conceived and developed the study design. AF led the data collection, ran data analysis and wrote the manuscript. TAJH and JHR supervised and contributed to data interpretation and manuscript writing. MM and EB supervised and contributed to data collection and manuscript writing. All authors read and approved the final manuscript.
Funding
This study was funded by the Indonesian Endowment Fund for Education (Lembaga Pengelola Dana Pendidikan [LPDP]) Indonesia. The LPDP granted funding for the study but was not involved in designing the study protocol, conducting data collection and data analysis or finalizing the manuscript.
Competing interests
None declared.
Ethics approval
Ethical clearance for this study was provided by the Ethical Committee at the Faculty of Medicine of Universitas Indonesia–Cipto Mangunkusumo Hospital, Jakarta, Indonesia (416/UN2.F1/ETIK/VI/2016). We provided written and oral explanations to selected TB patients before they decided to sign the informed consent form.
References
- 1. Tanimura T, Jaramillo E, Weil D et al. . Financial burden for tuberculosis patients in low- and middle-income countries: a systematic review. Eur Respir J. 2014;43(6):1763–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. World Health Organization Global strategy and targets for tuberculosis prevention, care and control after 2015. Geneva: World Health Organization; 2013. [Google Scholar]
- 3. Fuady A, Houweling TAJ, Mansyur M et al. . Effect of financial support on reducing the incidence of catastrophic costs among tuberculosis-affected households in Indonesia: eight simulated scenarios. Infect Dis Poverty. 2019;8(1):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. World Health Organization Tuberculosis patient cost surveys: a handbook. Geneva: World Health Organization; 2017. [Google Scholar]
- 5. Wingfield T, Boccia D, Tovar M et al. . Defining catastrophic costs and comparing their importance for adverse tuberculosis outcome with multi-drug resistance: a prospective cohort study, Peru. PLoS Med. 2014;11(7):e1001675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fuady A, Houweling TAJ, Mansyur M et al. . Catastrophic total costs in tuberculosis-affected households and their determinants since Indonesia's implementation of universal health coverage. Infect Dis Poverty. 2018;7(1):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. World Health Organization Treatment of tuberculosis: guidelines. Geneva: World Health Organization; 2010. [Google Scholar]
- 8. World Health Organization Definitions and reporting framework for tuberculosis—2013 revision Geneva: World Health Organization; 2013. [Google Scholar]
- 9. Adane AA, Alene KA, Koye DN et al. . Non-adherence to anti-tuberculosis treatment and determinant factors among patients with tuberculosis in northwest Ethiopia. PLoS One. 2013;8(11):e78791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tang Y, Zhao M, Wang Y et al. . Non-adherence to anti-tuberculosis treatment among internal migrants with pulmonary tuberculosis in Shenzhen, China: a cross-sectional study. BMC Public Health. 2015;15:474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gopi PG, Vasantha M, Muniyandi M et al. . Risk factors for non-adherence to directly observed treatment (DOT) in a rural tuberculosis unit, South India. Indian J Tuberc. 2007;54(2):66–70. [PubMed] [Google Scholar]
- 12. Awofeso N. Anti-tuberculosis medication side-effects constitute major factor for poor adherence to tuberculosis treatment. Bull World Health Org. 2008;86(3):B–D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kulkarni PY, Akarte SV, Mankeshwar RM et al. . Non-adherence of new pulmonary tuberculosis patients to anti-tuberculosis treatment. Ann Med Health Sci Res. 2013;3(1):67–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Woimo TT, Yimer WK, Bati T et al. . The prevalence and factors associated for anti-tuberculosis treatment non-adherence among pulmonary tuberculosis patients in public health care facilities in South Ethiopia: a cross-sectional study. BMC Public Health. 2017;17:269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Herrero MB, Ramos S, Arrossi S. Determinants of non adherence to tuberculosis treatment in Argentina: barriers related to access to treatment. Rev Bras Epidemiol. 2015;18(2):287–98. [DOI] [PubMed] [Google Scholar]
- 16. Fuady A, Houweling TA, Mansyur M et al. . Adaptation of the tool to estimate patient costs questionnaire into Indonesian context for tuberculosis-affected households. Acta Med Indones. 2018;50(1):3–10. [PubMed] [Google Scholar]
- 17. World Health Organization Protocol for survey to determine direct and indirect costs due to TB and to estimate proportion of TB-affected households experiencing catastrophic total costs due to TB. Geneva: World Health Organization; 2015. [Google Scholar]
- 18. IBM IBM SPSS Statistics for Windows, Version 24.0. Armonk, NY: IBM; 2016. [Google Scholar]
- 19. World Bank Official exchange rate. http://databank.worldbank.org/data/reports.aspx?source=2&series=PA.NUS.FCRF&country. [Google Scholar]
- 20. Pedrazzoli D, Borghi J, Viney K et al. . Measuring the economic burden for TB patients in the End TB Strategy and universal health coverage frameworks. Int J Tuberc Lung Dis. 2019;23(1):5–11. [DOI] [PubMed] [Google Scholar]
- 21. Rondags A, Himawan AB, Metsemakers JFM et al. . Factors influencing non-adherence to tuberculosis treatment in Jepara, Central Java, Indonesia. Southeast Asian J Trop Med Public Health. 2014;45(4):859–68. [PubMed] [Google Scholar]