Summary
The roles of obestatin and adropin in paediatric obesity are poorly understood. We compared obestatin and adropin concentrations in younger (n = 21) and older children (n = 14) with Prader-Willi syndrome (PWS) and age and BMI-z-matched controls (n = 31). Fasting plasma obestatin and adropin were higher in younger children with PWS than controls; adropin was also higher in older children with PWS. Growth hormone treatment had no effects on obestatin or adropin in PWS. The ratio of ghrelin to obestatin declined from early to late childhood but was higher in older PWS than older controls. Adropin correlated with fasting glucose in the PWS group only. Changes in the ratio of ghrelin to obestatin may suggest changes in the processing of preproghrelin to ghrelin and obestatin during development and differential processing of preproghrelin in PWS.
Keywords: adropin, obesity, obestatin, Prader-Willi syndrome
1 ∣. INTRODUCTION
Prader-Willi syndrome (PWS) is a genetic disorder characterized by intrauterine growth restriction, hypotonia, and failure to thrive in infancy followed by hyperphagia and obesity during childhood.1 The hyperphagia of PWS has been ascribed to several factors, including defects in hypothalamic signalling2 and paradoxical elevations of the orexigenic hormone ghrelin.3,4
Like ghrelin, obestatin is formed by post-translation modification of preproghrelin but at a different terminus.5 Recent evidence suggests that obestatin augments insulin secretion,6 promotes pancreatic β-cell survival7 and inhibits water intake in animal models.8 The hormone adropin, which is secreted mostly by pancreas as a product of the Energy Homeostasis Association (Enho) gene,9 has similar metabolic effects. For example, adropin promotes carbohydrate oxidation over fat oxidation in skeletal muscle10 and acts in circumventricular organs to mediate the antidipsogenic response to hypoxia in rats.11
Obestatin and adropin are poorly characterized in PWS and nonsyndromic paediatric obesity. Interestingly, the failure to thrive in PWS infants was associated in one-uncontrolled study with reduced water intake,12 while the obesity of older PWS children is associated with relative hypersensitivity to insulin.13 Although it is unclear if obestatin or adropin contribute to these differences in water intake and insulin action, we hypothesized that children with PWS have higher fasting concentrations of obestatin and adropin than children without PWS. To that end, we compared obestatin and adropin concentrations in younger and older children with PWS and controls of similar age, sex, and body mass index (BMI) z score.
2 ∣. METHODS
2.1 ∣. Subjects
The study population consisted of 35 children with PWS and 31 controls who participated in previously described cross-sectional studies of hormonal and metabolic characteristics of children with PWS.13,14 Given that nutritional and hormonal profiles of toddlers with PWS differ from those of older children,1,14 the study population was allocated into two age groups as follows: younger children (<6 y old) and older children (≥6 y old). From the original population, only those children who had data for both obestatin and adropin were included in the present study. Informed written consent from the parents/guardians was obtained. The study protocol was approved by the Institutional Review Board of the Duke University Medical Center.
2.2 ∣. Assays
Plasma fasting obestatin (Yanaihara Institute, Shizuoka, Japan) and adropin (Peninsula Laboratories, San Carlos, California) were measured in duplicate using enzyme-linked immunosorbent assays (ELISAs). Both biomarkers were assayed concurrently on previously unthawed frozen plasma. We calculated the ratio of total ghrelin to obestatin for each participant as a measure of differential processing of preproghrelin. Data from additional hormones were published previously and have been included here.13,14 Homeostatic model assessment-IR (HOMA-IR) was calculated as the product of fasting insulin (μIU/ml) and fasting plasma glucose (mg/dL) divided by 405.15
2.3 ∣. Data analysis
Continuous variables were expressed as medians and interquartile ranges (IQR, 25th and 75th) because of skewed distribution. Comparisons were performed using Independent Student's t tests or Mann-Whitney U test, as appropriate. Differences in obestatin and adropin concentrations were further examined using one-way ANCOVA, with age and sex as covariates. Pearson's or Spearman's correlation coefficient were used to evaluate correlations between variables. In addition, a sensitivity analysis was conducted to test whether obestatin and adropin concentrations differed between older children with PWS and controls with insulin resistance using independent t tests. A significance level of P ≤ 0.05 was used for all analyses. Statistical analyses were performed with Statistical Package for the Social Sciences 24.0 (Chicago, Illinois).
3 ∣. RESULTS
Fasting concentrations of obestatin were higher in younger children with PWS than controls but similar between the older PWS and control groups (Table 1). Adropin concentrations were higher in both younger and older children with PWS than controls. Adjustments for age and sex abolished the differences in adropin (younger children: P = 0.06, older children: P = 0.08) but not obestatin (P = 0.04). Obestatin and adropin concentrations did not differ between PWS genetic subtypes in both younger and older children and were not affected by growth hormone (GH) treatment of older PWS children (Table S1). Because nearly all the younger children with PWS were treated with GH (85.7%), it was not possible to assess effects of GH on their obestatin and adropin concentrations. Ghrelin and the ratio of ghrelin to obestatin were higher in older children with PWS than controls (all P < 0.0005).
TABLE 1.
Clinical characteristics of younger and older children with Prader-Willi syndrome (PWS) and controls
| Variables | Younger Children |
Older Children |
||||
|---|---|---|---|---|---|---|
| PWS (n = 21) | Controls (n = 17) | P valuec | PWS (n = 14) | Controls (n = 14) | P valuec | |
| Age (months) | 15.5 (10.7, 31.9)a | 27.3 (10.4, 45.6)a | 0.82 | - | - | - |
| Age (years) | - | - | - | 11.4 (7.1, 14.9) | 12.0 (10.3, 14.6) | 0.38 |
| Sex | 12 male, 9 female | 8 male, 9 female | NS | 9 male, 5 female | 6 male, 8 female | NS |
| PWS subtype | ||||||
| Deletion | 11 (52.4%) | - | - | 9 (64.3%) | - | - |
| UPD | 9 (42.9%) | - | - | 4 (28.6%) | - | - |
| Undeterminedd | 1 (4.7%) | - | - | 1 (7.1%) | - | - |
| GH treatment | 18 (85.7%) | - | - | 9 (64.3%) | - | - |
| Weight for age z score | −0.99 (−1.71, 0.98) | 0.16 (−0.46, 0.94) | 0.09 | 2.2 (1.5, 2.6) | 2.3 (1.8, 2.7) | 0.87 |
| Obestatin (pg/mL) | 2691.0 (2049.5, 4288.0)b | 2101.0 (1915.0, 2464.5)b | 0.04 | 3350.0 (2979.3, 3765.0)b | 3510.0 (3003.0, 4185.8)b | 0.75 |
| Adropin (ng/mL) | 3.50 (2.63, 5.23)b | 2.57 (2.13, 3.25)b | 0.05 | 2.69 (2.26, 4.56)b | 1.93 (1.40, 3.00)b | 0.04 |
| Ghrelin (pg/mL) | 2190.0 (1593.5, 3283.5)a | 1980.0 (1644.6, 2240.5)a | 0.06 | 1468.5 (1133.4, 1659.1) | 836.7 (712.9, 918.6) | <0.0005 |
| Ghrelin:Obestatin | 0.91 (0.38, 1.28)a | 0.91 (0.63, 1.09)a | 0.66 | 0.40 (0.33, 0.49)a | 0.23 (0.18, 0.31)a | <0.0005 |
| Leptin (ng/mL) | 6.29 (3.50, 7.09)a | 3.47 (2.64, 5.13)a | 0.01 | 31.45 (12.78, 48.84) | 32.24 (14.92, 54.71) | 0.86 |
| Insulin (μIU/mL) | 7.66 (6.29, 10.20)a | 5.94 (4.45, 7.36)a | 0.01 | 13.49 (10.02, 29.48) | 23.20 (17.08, 35.50) | 0.03 |
| Glucose (mg/dL) | 85.20 (76.40, 88.80)a | 81.00 (77.80, 85.00)a | 0.99 | 87.75 (80.88, 101.69) | 94.88 (88.25, 99.56) | 0.52 |
| HOMA-IR | 1.60 (1.29, 2.13)a | 1.15 (0.93, 1.56)a | 0.03 | 3.20 (2.38, 5.80)a | 5.11 (4.10, 8.92)a | 0.02 |
Abbreviations: GH: growth hormone; HOMA-IR: Homeostatic model assessment-insulin resistance; NS: not significant; PWS: Prader-Willi syndrome; UPD: uniparental disomy.
Data are presented as median (25th percentile, 75th percentile). Statistically significant differences are in bold type (P ≤ 0.05).
Variables were transformed logarithmically for statistical analysis but present here as raw values.
Variables were transformed using inverse transformation for statistical analysis but present here as raw values.
P values determined using independent t test.
Prader-Willi syndrome (PWS) confirmed by methylation testing only (undetermined genetic subtype).
Hormonal differences between early and late childhood were analysed in the PWS and control groups (Figure 1). Serum ghrelin concentrations were higher in younger than in older children in both the PWS and control groups, but the magnitude of decline with age was greater in control than in PWS subjects (Figure 1A). Conversely, plasma obestatin concentrations were higher in older than in younger controls, but no age-related differences were observed in the PWS group. Consequently, the ghrelin: obestatin ratio was higher in the older PWS subjects than in the older controls (Figure 1B). No differences in adropin concentrations were found between younger and older children in the PWS or control groups (Figure 1C).
FIGURE 1.
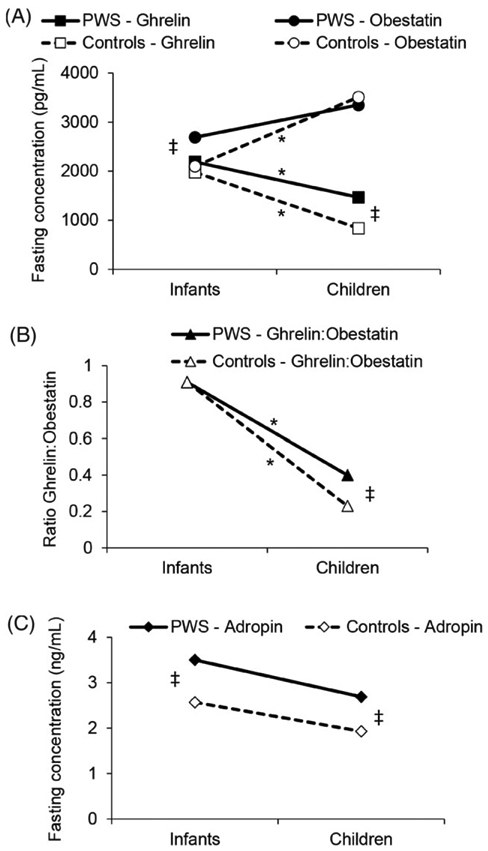
Median changes in fasting (A) ghrelin and obestatin concentrations, (B) ghrelin to obestatin ratio, and (C) adropin concentrations during development in Prader-Willi syndrome (PWS) and controls. *Statistically significant differences between infants and children (P ≤ 0.05). ‡Statistically significant differences between PWS and controls (P ≤ 0.05).
Moderate to strong positive correlations were observed between obestatin and adropin concentrations in all groups (r = 0.54−0.75, all P ≤ 0.05). In older PWS children, there was a positive correlation between adropin and fasting glucose (r = 0.78, P = 0.001). In the older control group, both obestatin and adropin correlated with HOMA-IR (r = 0.69, P = 0.01; r = 0.62, P = 0.02, respectively); obestatin also correlated with leptin (r = 0.66; P = 0.01). Neither obestatin nor adropin correlated with HOMA-IR or leptin in the PWS group. Obestatin correlated positively with BMI z score (r = 0.81, P < 0.0005) in the older control group. In contrast, no significant correlations between obestatin, adropin, and BMI z score were observed in the PWS group.
To explore further the roles of obestatin and adropin in glucose metabolism, we compared hormone levels in older PWS and control subjects with insulin resistance, as defined by HOMA-IR > 3.15.16 Insulin resistant older PWS subjects (n = 7) and insulin resistant older controls (n = 13) had similar levels of obestatin (P = 0.50) and adropin (P = 0.22) (Table S2). Younger subjects were not included because there is no standardized definition of insulin resistance during early childhood.
4 ∣. DISCUSSION
We found that fasting obestatin concentrations were higher in younger but not older PWS children than in age-matched and sex-matched controls. These results are in agreement with findings from two small studies on obestatin in PWS.17,18 One investigation reported higher obestatin levels in five young PWS children than in age-matched (<3 y) controls (P = 0.03) but similar levels in older PWS children (age > 3 y) and control subjects.17 The second described no differences in obestatin concentrations at baseline or during an oral glucose tolerance test in prepubertal children (median age 11.2 y).18 Our study extended these findings by showing that GH treatment had no effects on obestatin or adropin levels in PWS subjects.
Obestatin and ghrelin originate from the same preproghrelin precursor.5 The striking differences in the relative levels of ghrelin and obestatin in younger and older children suggest changes in the processing of preproghrelin to ghrelin and obestatin during development. The ghrelin: obestatin ratio declined less dramatically from early to late childhood in the PWS group than in the control group, suggesting preferential processing of preproghrelin to mature ghrelin in older children with PWS. Alternatively, there may be differences in the rates of clearance of ghrelin and obestatin in PWS or changes in peptide clearance during development. Changes in the relative expression of ghrelin and obestatin might have important implications for regulation of glucose metabolism and pancreatic insulin secretion in children.5,19
Fasting adropin levels were higher in younger PWS than in controls of similar age, sex, and weight-for-age z score. Diverse studies support a role for adropin in glucose and lipid metabolism, suggesting that higher concentrations of adropin might be of benefit for metabolic health.9,10 In our study, adropin levels correlated with glucose levels (all of which were in the normal range) in PWS older children but did not differ in insulin resistant and insulin sensitive subjects.
Infants with PWS have hypotonia and feeding difficulties that usually require support of enteral nutrition.20 Because we did not have detailed growth and weight gain data during infancy, we could not interpret the relationships between obestatin and adropin and failure to thrive. Because the study design was cross sectional, the longitudinal pattern of individual changes in obestatin, adropin, and ghrelin throughout development could not be fully investigated. In addition, the lack of a lean control group limited our ability to interpret fully the physiologic significance of our findings.
In summary, we found that younger children with PWS had higher obestatin concentrations than control subjects of similar age and BMI. Fasting adropin concentrations in older PWS children were related to glucose levels in the higher but normal range but not with other markers of metabolism or insulin resistance. Changes in the ratio of ghrelin to obestatin during childhood suggest developmental changes in the processing of preproghrelin and differential processing in PWS and control subjects.
Supplementary Material
ACKNOWLEDGEMENTS
A. M. H. and M. F. conceived the study design. M. J. M. and H. N. C. assayed blood samples. C. E. O., A. A. B., D. A. R., M. P., M. G. B., C. M. P., A. M. H., and M. F. analysed and interpreted the data. All authors contributed to draft manuscript and have approved the final version.
FUNDING INFORMATION
Funded by Canadian Institutes of Health Research (CIHR) (Grant Nos. 115707 and 119504) (to AMH). C. E. O. is supported by the Alberta Diabetes Institute and Alberta Diabetes Foundation. M. F. is supported by an Atkins Foundation endowed professorship.
Abbreviations:
- BMI z scores
body mass index standard deviation score
- ELISAs
enzyme-linked immunosorbent assays
- Enho
Energy Homeostasis Association
- GH
growth hormone
- HOMA-IR
homeostatic model assessment-insulin resistance
- IQR
interquartile ranges
- PWS
Prader-Willi syndrome
Footnotes
CONFLICTS OF INTEREST
The authors have no conflicts of interest to disclose.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of the article.
REFERENCES
- 1.Miller JL, Lynn CH, Driscoll DC, et al. Nutritional phases in Prader-Willi syndrome. Am J Med Genet Part a 2011; 155: 1040–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mercer RE, Michaelson SD, Chee MJS, Atallah TA, Wevrick R, Colmers WF. Magel2 is required for leptin-mediated depolarization of POMC neurons in the hypothalamic arcuate nucleus in mice. PLoS Genet 2013; 9: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Irizarry KA, Bain J, Butler MG, et al. Metabolic profiling in Prader-Willi syndrome and nonsyndromic obesity: sex differences and the role of growth hormone. Clin Endocrinol (Oxf) 2015; 83: 797–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ge X, Yang H, Bednarek MA, et al. LEAP2 is an endogenous antagonist of the Ghrelin receptor. Cell Metab 2018; 27: 461–469. [DOI] [PubMed] [Google Scholar]
- 5.Cowan E, Burch KJ, Green BD, Grieve DJ. Obestatin as a key regulator of metabolism and cardiovascular function with emerging therapeutic potential for diabetes. Br J Pharmacol 2016; 173: 2165–2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pradhan G, Wu CS, Lee JH, et al. Obestatin stimulates glucose-induced insulin secretion through ghrelin receptor GHS-R. Sci Rep 2017; 7:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Granata R, Settanni F, Gallo D, et al. Obestatin promotes survival of pancreatic β-cells and human islets and induces expression of genes involved in the regulation of β-cell mass and function. Diabetes 2008; 57: 967–979. [DOI] [PubMed] [Google Scholar]
- 8.Samson WK, White MM, Price C, Ferguson AV. Obestatin acts in brain to inhibit thirst. Am J Physiol - Regul Integr Comp Physiol 2006; 292: R637–R643. [DOI] [PubMed] [Google Scholar]
- 9.Kumar KG, Trevaskis JL, Lam DD, et al. Identification of adropin as a secreted factor linking dietary macronutrient intake with energy homeostasis and lipid metabolism. Cell Metab 2008; 8: 468–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gao S, McMillan RP, Jacas J, et al. Regulation of substrate oxidation preferences in muscle by the peptide hormone adropin. Diabetes 2014; 63: 3242–3252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang F, Zhou L, Qian X, et al. Adropin is a key mediator of hypoxia induced anti-dipsogenic effects via TRPV4-CamKK-AMPK signaling in the circumventricular organs of rats. Front Mol Neurosci 2017; 10:105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Åkefeldt A Water intake and risk of hyponatraemia in Prader-Willi syndrome. J Intellect Disabil Res 2009; 53: 521–528. [DOI] [PubMed] [Google Scholar]
- 13.Haqq AM, Muehlbauer MJ, Newgard CB, Grambow S, Freemark M. The metabolic phenotype of Prader-Willi syndrome (PWS) in childhood: heightened insulin sensitivity relative to body mass index. J Clin Endocrinol Metab 2011; 96: 225–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haqq AM, Grambow SC, Muehlbauer M, et al. Ghrelin concentrations in Prader-Willi syndrome (PWS) infants and children: changes during development. Clin Endocrinol (Oxf) 2008; 69: 911–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Conwell LS, Trost SG, Brown WJ, Batch JA. Indexes of insulin resistance and secretion in obese children and adolescents: a validation study. Diabetes Care 2004; 27: 314–319. [DOI] [PubMed] [Google Scholar]
- 16.Keskin M, Kurtoglu S, Kendirci M, Atabek ME, Yazici C. Homeostasis model assessment is more reliable than the fasting glucose/insulin ratio and quantitative insulin sensitivity check index for assessing insulin resistance among obese children and adolescents. Pediatrics 2005; 115: e500–e503. [DOI] [PubMed] [Google Scholar]
- 17.Butler MG, Bittel DC. Plasma obestatin and ghrelin levels in subjects with Prader-Willi syndrome. Am J Med Genet Part A 2007; 143: 415–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Park WH, Oh YJ, Kim GY, et al. Obestatin is not elevated or correlated with insulin in children with Prader-Willi syndrome. J Clin Endocrinol Metab 2007; 92: 229–234. [DOI] [PubMed] [Google Scholar]
- 19.Chabot F Interrelationships between ghrelin, insulin and glucose homeostasis: physiological relevance. World J Diabetes 2014; 5: 328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Singh P, Mahmoud R, Gold JA, et al. Multicentre study of maternal and neonatal outcomes in individuals with Prader-Willi syndrome. J Med Genet 2018: 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


