In SARS-CoV-2 (severe acute respiratory syndrome coronavirus 2) infected patients, hemostasis shows an uncontrolled activation, with a high D-dimer concentration. 1 2 It has been also shown that the SARS-CoV-2 can promote endotheliitis, 3 and in turn, pulmonary endothelial damage fostering thrombogenesis. 4
Thrombotic microangiopathy (TMA) is defined by microangiopathic hemolytic anemia, thrombocytopenia, and organ failure 5 and can be observed in several disorders. 6 Infectious diseases could be one of these disorders. 7 A disintegrin and metalloproteinase with thrombospondin motifs 13 (ADAMTS13) plays a pathophysiological role in the TMA, including that arousing on sepsis. 8 Moreover, a key role of the ADAMTS13 in the coagulopathy of sepsis is also acknowledged. 9 In the same scenario, ADAMTS13 was shown to be one of the endothelium-related markers showing changes, with a trend toward a reduction. 10 Furthermore, in response to the endothelial activation-induced vascular inflammation, reciprocal changes of the ADAMTS13 and its substrate, the von Willebrand factor (vWF), are observed. 11
In septic patients, reduced synthesis of protein C (PC) and its cofactor protein S (PS) in addition to impaired expression of thrombomodulin and endothelial PC receptor on endothelium determines reduced PC levels with an imbalance of thrombin regulation. 12 Furthermore, antithrombin (AT)— the most important inhibitor of thrombin and activated factor X—is reduced as a consequence of its consumption and impaired synthesis and degradation. 13
We aimed at assessing whether ADAMTS13 levels predict mortality in patients with a diagnosis of SARS-CoV-2 infection. The secondary aim was to investigate a possible relationship between ADAMTS13, D-dimer, vWF, and natural anticoagulants levels to have easy-to-handle markers of clinical outcomes. Seventy-seven patients admitted between March 1 and April 30, 2020 at the “Casa Sollievo della Sofferenza” Research Hospital with a laboratory-confirmed diagnosis (i.e., RT-PCR according to the protocol established by the WHO) 14 of the SARS-CoV-2 infection were investigated. Demographic and clinical information and routine laboratory data at the hospital admission were extracted from hospital electronic medical records. In all patients, ADAMTS13, vWF antigen (vWF:Ag)/functional, and natural anticoagulants (AT, PC, and PS) levels were measured on a blood sample obtained within seven hospitalization days. The ADAMTS13 activity levels were measured using a chromogenic enzyme-linked immunosorbent assay (ELISA) method (TECHNOZYM ADAMTS13 Activity ELISA Kit, Technoclone, Austria). Continuous variables are presented as a median and interquartile range, whereas discrete variables as numbers and percentage. After testing for data normality distribution, Mann–Whitney test was used to analyze differences in continuous variables. Fisher's exact test was also performed to estimate the association between D-dimer positive test (> 500 ng/mL) and ADAMTS13 activity levels below median value. Spearman's r was used to explore the reciprocal relationship between ADAMTS13 and D-dimer, vWF, and natural anticoagulants: results are given as r - and p -values. Multiple linear regression analysis, which controlled for D-dimer concentration, age, and sex was performed to evaluate a predictive model for ADAMTS13 activity levels. Kaplan–Meier analysis was performed to assess whether ADAMTS13 can predict mortality. Statistical significance was set at p -values < 0.05. All the analyses were performed using GraphPad Prism version 8.00 for Windows, GraphPad Software (La Jolla, California, United States, www.graphpad.com ). The study was approved by the local ethics committee and was performed according to the Declaration of Helsinki on Ethical Principles for medical research involving human subjects. All patients gave written informed consent.
Descriptive analysis is shown in Table 1 . Most patients (73%) were older than 60 years, with a slightly higher percentage (56 vs. 46%) of women. Almost all the patients (97.5%) showed comorbidities. Fourteen patients (18%) were admitted to the intensive care unit. Median value of ADAMTS13 in the entire sample was 70 U/dL. Kaplan–Meier analysis showed a significantly lower survival in those individuals with ADAMTS13 activity below the median value ( p = 0.025) ( Fig. 1 ). Overall, we recorded four deaths (5.2%), all in patients with hypertension, two of them with associated ischemic cardiomyopathy (one diabetic). We cannot exclude that comorbidities could have contributed to mortality in these patients. Seventy-three patients were discharged ( n = 51) or are still alive ( n = 22) at the end of the study. Since vWF might be directly involved in the pathophysiology of TMA, we performed also the Kaplan–Meier analysis according to vWF ristocetin cofactor (vWF:RCo) levels: no statistically significant difference was found, in terms of mortality prevalence ( p = 0.80). Multiple linear regression showed that D-dimer concentration at admission ( p = 0.0145) and age ( p = 0.0036) were independently associated with ADAMTS13 activity levels. D-dimer concentration above 500 ng/mL at admission was associated with a 2.8-fold higher likelihood of ADAMTS13 activity below the median value (odds ratio: 4.8 [95% confidence interval: 1.3–16.9], p = 0.02). Lastly, D-dimer concentration, vWF:Ag, and natural anticoagulants (AT, PC, and PS) levels were inversely (D-dimer and vWF) or directly (AT, PC, PS) associated with ADAMTS13 levels ( Supplementary Table S1 ). ADAMTS13 activity negatively correlated with D-dimer concentration (Spearman's r : –0.41, p = 0.002 ) and vWF:Ag (Spearman's r : –0.34, p = 0.005) levels, whereas a positive correlation was found with AT (Spearman's r : 0.45, p < 0.001), PC (Spearman's r : 0.33, p = 0.004), and PS (Spearman's r : 0.23, p = 0.04). The main finding of this study is that patients with ADAMTS13 activity below 70 U/dL have a higher risk of in-hospital death. Present findings are consistent with those observed in patients with sepsis, in whom low ADAMTS13 levels are inversely correlated with vWF 15 and a poor prognosis, as well as with sepsis severity. 16 17 Elevated levels of vWF and reduced ADAMTS13 could correlate with endothelial damage and a progressive worsening of the organ function. In our setting, the ratio of vWF:RCo/vWF:Ag is 0.65. This value does not reflect a predominance of ultra-large vWF multimers, which could represent the culprit of microangiopathic thrombosis. We hypothesize that, at variance with other TMA, the underlying pathogenetic mechanism is the endothelial inflammation and damage resulting in a slight reduction of ADAMTS13 levels. Consistent with this possible pathogenetic mechanism is the lack of thrombocytopenia. Indeed, in TMA other than thrombotic thrombocytopenic purpura, the relationship between reduced ADAMTS13 activity and thrombocytopenia is not clearly demonstrated. 18 As reported in other septic patients, the endothelial damage triggers the coagulation cascade, as suggested by elevated D-dimer and decreased natural anticoagulants plasma levels. 19 20 Notably, we observed that D-dimer, a blood parameter routinely investigated in SARS-CoV-2 patients for the possible clinical impact on mortality in these settings, 21 22 strongly predicts ADAMTS13 levels. Thus, it is conceivable that endothelial dysfunction could be reflected in any of the hemostasis phases and variables, including the natural anticoagulants. Endothelial dysfunction is a key finding and major determinant of poor prognosis in SARS-CoV-2 patients. 23 Indeed, direct or indirect activation by SARS-CoV-2 on endothelium would cause intensive vWF secretion from Weibel–Palade bodies, as expected upon perturbations on these vascular cells. 13 Our data are consistent with previous findings suggesting that SARS-CoV-2 patients present a low-grade disseminated coagulopathy and localized pulmonary microangiopathy, resulting in organ dysfunction. 24 Notwithstanding the retrospective nature and limited size of this study, overall, present findings may speculatively suggest that ADAMTS13 would represent a clinic-diagnostic marker predicting SARS-CoV-2-related prognosis.
Table 1. Demographic/clinical information and laboratory data collected in the study population.
| Variables | Values |
|---|---|
| Demographic and clinical information | |
| Age, median (IQR) | 61.5 (80–62) |
| Female/Male | 43/34 |
| Coexisting morbidities, n (%) | |
| Any | 22 (28.5) |
| Hypertensive disorder | 17 (22) |
| Chronic renal disease | 8 (10.4) |
| Diabetes | 8 (10.3) |
| Dilated cardiomyopathy | 7 (9) |
| Chronic obstructive chronic disease | 7 (9) |
| Atrial fibrillation | 6 (7.8) |
| None | 2 (3) |
| Deaths, n (%) | 4 (5.2) |
| Laboratory data | |
| ADAMTS13 activity (U/dL), median (IQR) | 70 (80–60) |
| vWF:Ag (%), median (IQR) | 231.2 (415.3–205.7) |
| vWF:RCo (%), median (IQR) | 150.0 (334.3–116.9) |
| ADAMTS13/vWF:RCo, median (IQR) | 0.40 (0.50–0.23) |
| vWF:RCo/vWF:Ag, median (IQR) | 0.65 (0.87–0.6) |
| Factor VIII (%), median (IQR) | 95.20 (133.0–69.40) |
| D-dimer (ng/mL) | 882.0 (2250–460.0) |
| Hemoglobin (g/dL), median (IQR) | 11.9 (13.3–9.8) |
| Platelet count (×10 9 /L), median (IQR) | 234 (283–149) |
| White cell count (×10 9 /L), median (IQR) | 6.1 (9.4–4.5) |
| Lactate dehydrogenase (U/L), median (IQR) | 236.0 (314.5–197.0) |
| Creatinine (mg/dL), median (IQR) | 0.9 (1.5–0.6) |
| C-reactive protein (mg/L), median (IQR) | 1.7 (9.3–0.4) |
| Erythrocyte sedimentation rate (mm/h) | 34 (79.5–23.5) |
| Prothrombin time (International Normalized Ratio), median (IQR) | 1.09 (1.18–1.06) |
| Activated partial thromboplastin time (s), median (IQR) | 25.0 (26.5–23.0) |
Abbreviations: ADAMTS13, a disintegrin and metalloproteinase with thrombospondin motifs 13; IQR, interquartile range; vWF:Ag, von Willebrand factor antigen; vWF:RCo, von Willebrand factor ristocetin cofactor.
Fig. 1.
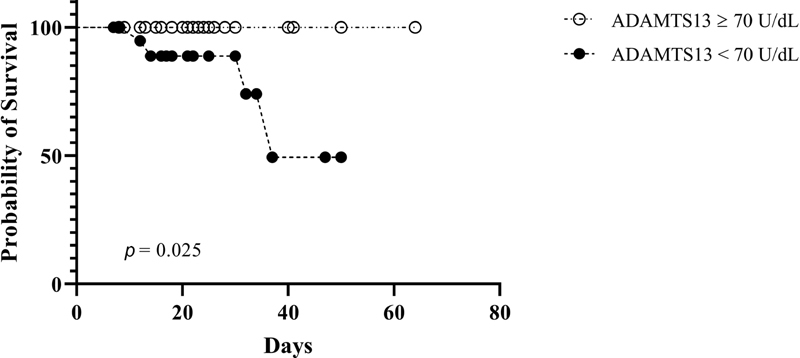
Kaplan–Meier survival analysis according to a disintegrin and metalloproteinase with thrombospondin motifs 13 (ADAMTS13) activity levels: four deaths were observed in the group with values < 70 U/dL.
Conflict of Interest None declared.
Authors' Contributions
G.L.T.: analysis of data and drafting the article; G.F. and E.C.: analysis and interpretation of data; A.D.L.: collection and management of data; F.C. and L.F.: laboratory investigations; L.d.M. and G.M.: collection of data; A.M.: selection of patients; E.G.: conception and design of study, revising the article critically for important intellectual content. All authors critically revised the article and approved the final version.
Members of the CSS COVID-19 Group are provided in the Supplementary Material.
Supplementary Material
References
- 1.Connors J M, Levy J H. COVID-19 and its implications for thrombosis and anticoagulation. Blood. 2020;135(23):2033–2040. doi: 10.1182/blood.2020006000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18(04):844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Varga Z, Flammer A J, Steiger P.Endothelial cell infection and endotheliitis in COVID-19 Lancet 2020395(10234):1417–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marini J J, Gattinoni L. Management of COVID-19 respiratory distress. JAMA [Internet] 2020 doi: 10.1001/jama.2020.6825. [DOI] [PubMed] [Google Scholar]
- 5.Moake J L. Thrombotic microangiopathies. N Engl J Med. 2002;347(08):589–600. doi: 10.1056/NEJMra020528. [DOI] [PubMed] [Google Scholar]
- 6.George J N, Nester C M. Syndromes of thrombotic microangiopathy. N Engl J Med. 2014;371(07):654–666. doi: 10.1056/NEJMra1312353. [DOI] [PubMed] [Google Scholar]
- 7.Coppo P, Adrie C, Azoulay E. Infectious diseases as a trigger in thrombotic microangiopathies in intensive care unit (ICU) patients? Intensive Care Med. 2003;29(04):564–569. doi: 10.1007/s00134-003-1676-4. [DOI] [PubMed] [Google Scholar]
- 8.Masias C, Cataland S R. The role of ADAMTS13 testing in the diagnosis and management of thrombotic microangiopathies and thrombosis. Blood. 2018;132(09):903–910. doi: 10.1182/blood-2018-02-791533. [DOI] [PubMed] [Google Scholar]
- 9.Levi M, Scully M, Singer M. The role of ADAMTS-13 in the coagulopathy of sepsis. J Thromb Haemost. 2018;16(04):646–651. doi: 10.1111/jth.13953. [DOI] [PubMed] [Google Scholar]
- 10.Vasileiadis I, Politou M, Dimopoulos S. Variation of endothelium-related hemostatic factors during sepsis. Microcirculation. 2018;25(08):e12500. doi: 10.1111/micc.12500. [DOI] [PubMed] [Google Scholar]
- 11.Chen J, Chung D W. Inflammation, von Willebrand factor, and ADAMTS13. Blood. 2018;132(02):141–147. doi: 10.1182/blood-2018-02-769000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Levi M, van der Poll T.Inflammation and coagulation Crit Care Med 201038(2, Suppl):S26–S34. [DOI] [PubMed] [Google Scholar]
- 13.Keller T T, Mairuhu A T, de Kruif M D. Infections and endothelial cells. Cardiovasc Res. 2003;60(01):40–48. doi: 10.1016/s0008-6363(03)00354-7. [DOI] [PubMed] [Google Scholar]
- 14.World Health Organization Coronavirus disease (COVID-19) technical guidance: laboratory testing for 2019-nCoV in humansAvailable at:https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/laboratory-guidance. Accessed August 12, 2020
- 15.Kremer Hovinga J A, Zeerleder S, Kessler P. ADAMTS-13, von Willebrand factor and related parameters in severe sepsis and septic shock. J Thromb Haemost. 2007;5(11):2284–2290. doi: 10.1111/j.1538-7836.2007.02743.x. [DOI] [PubMed] [Google Scholar]
- 16.Martin K, Borgel D, Lerolle N. Decreased ADAMTS-13 (a disintegrin-like and metalloprotease with thrombospondin type 1 repeats) is associated with a poor prognosis in sepsis-induced organ failure. Crit Care Med. 2007;35(10):2375–2382. doi: 10.1097/01.ccm.0000284508.05247.b3. [DOI] [PubMed] [Google Scholar]
- 17.Lin J J, Chan O W, Hsiao H J. Decreased ADAMTS 13 activity is associated with disease severity and outcome in pediatric severe sepsis. Medicine (Baltimore) 2016;95(16):e3374. doi: 10.1097/MD.0000000000003374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Song J, Lee K A, Park T S, Park R, Choi J R. Linear relationship between ADAMTS13 activity and platelet dynamics even before severe thrombocytopenia. Ann Clin Lab Sci. 2008;38(04):368–375. [PubMed] [Google Scholar]
- 19.Dhainaut J-F, Shorr A F, Macias W L. Dynamic evolution of coagulopathy in the first day of severe sepsis: relationship with mortality and organ failure. Crit Care Med. 2005;33(02):341–348. doi: 10.1097/01.ccm.0000153520.31562.48. [DOI] [PubMed] [Google Scholar]
- 20.Mathieu J, Boucher S.Sepsis-associated coagulopathy: diagnosis with hematologic biomarkers and a novel hemostatic score that may predict the risk of developing a multiple organ dysfunction syndrome and a higher rate of mortality Chest 2015148340A25927671 [Google Scholar]
- 21.Zhou F, Yu T, Du R.Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study Lancet 2020395(10229):1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang L, Yan X, Fan Q. D-dimer levels on admission to predict in-hospital mortality in patients with Covid-19. J Thromb Haemost. 2020;18(06):1324–1329. doi: 10.1111/jth.14859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sardu C, Gambardella J, Morelli M B, Wang X, Marfella R, Santulli G. Hypertension, thrombosis, kidney failure, and diabetes: is COVID-19 an endothelial disease? A comprehensive evaluation of clinical and basic evidence. J Clin Med. 2020;9(05):1417. doi: 10.3390/jcm9051417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Levi M, Thachil J, Iba T, Levy J H. Coagulation abnormalities and thrombosis in patients with COVID-19. Lancet Haematol. 2020;7(06):e438–e440. doi: 10.1016/S2352-3026(20)30145-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


