Abstract
Purpose:
The New Approaches to Neuroblastoma Therapy Response Criteria (NANTRC) were developed to optimize response assessment in patients with recurrent/refractory neuroblastoma. Response predictors and associations of the NANTRC version 1.0 (NANTRCv1.0) and prognostic factors with outcome were analyzed.
Methods:
A retrospective analysis was performed of patients with recurrent/refractory neuroblastoma enrolled from 2000 to 2009 on 13 NANT Phase 1/2 trials. NANTRC overall response integrated CT/MRI (Response Evaluation Criteria in Solid Tumors [RECIST]), metaiodobenzylguani-dine (MIBG; Curie scoring), and percent bone marrow (BM) tumor (morphology).
Results:
Fourteen (6.9%) complete response (CR) and 14 (6.9%) partial response (PR) occurred among 203 patients evaluable for response. Five-year progression-free survival (PFS) was 16 ± 3%; overall survival (OS) was 27 ± 3%. Disease sites at enrollment included MIBG-avid lesions (100% MIBG trials; 84% non-MIBG trials), measurable CT/MRI lesions (48%), and BM (49%). By multivariable analysis, Curie score of 0 (P < 0.001), lower Curie score (P = 0.003), no measurable CT/MRI lesions (P = 0.044), and treatment on peripheral blood stem cell (PBSC) supported trials (P = 0.005) were associated with achieving CR/PR. Overall response of stable disease (SD) or better was associated with better OS (P < 0.001). In multivariable analysis, MYCN amplification (P = 0.037) was associated with worse PFS; measurable CT/MRI lesions (P = 0.041) were associated with worse OS; prior progressive disease (PD; P < 0.001/P < 0.001), Curie score > 1 (P < 0.001; P = 0.001), higher Curie score (P = 0.048/0.037), and treatment on non-PBSC trials (P = < 0.001/0.003) were associated with worse PFS and OS.
Conclusions:
NANTRCv1.0 response of at least SD is associated with better OS in patients with recurrent/refractory neuroblastoma. Patient and tumor characteristics may predict response and outcome. Identifying these variables can optimize Phase 1/2 trial design to select novel agents for further testing.
Keywords: clinical trials, neuroblastoma, response criteria
1 |. INTRODUCTION
Survival for patients with high-risk neuroblastoma (HR-NB) remains <50%,1 despite improvements with myeloablative therapy, isotretinoin,2,3 and immunotherapy,4 emphasizing the need for more effective therapies. Evaluating response is a critical component of assessing novel agents during Phase 1/2 trials to justify further Phase 3 trials. The Response Evaluation Criteria in Solid Tumors (RECIST),5,6 the accepted standard, require at least one measurable soft tissue site. A retrospective review of Phase 1 adult cancer trials found that change in tumor size by RECIST had a near-linear association with survival7with no significant cutoff points. A retrospective study of Phase 1 pediatric trials8 found that change in tumor size by RECIST had a linear association with response duration, and was associated with overall survival (OS), but not progression-free survival (PFS). RECIST often does not apply to patients with relapsed/refractory HR-NB, since bone and bone marrow (BM) are the most common relapse sites,9,10 and measurable soft tissue disease may not be present. Furthermore, measurable soft tissue lesions may not represent viable tumor. Metaiodobenzylguanidine (MIBG) scans can complement CT/MRI anatomic imaging to assess viable neuroblastoma. Curie scoring11 provides a semiquantitative measure of MIBG-avid lesions, and is prognostic for outcome in HR-NB.12,13
The International Neuroblastoma Response Criteria (INRC) 1993,14 developed with a focus on newly diagnosed neuroblastoma, did not quantitate MIBG scan response, utilized the number of positive BM samples regardless of tumor percentage, and considered a single negative BM followed by a positive BM as progressive disease (PD). The New Approaches to Neuroblastoma Therapy Response Criteria version 1.0 (NANTRCv1.0) were developed in 2000 to optimize response assessment for early-phase trials for recurrent/refractory neuroblastoma. NANTRCv1.0 piloted the inclusion of semiquantitative Curie MIBG scan scoring and a more quantitative BM response assessment that defined intermittent detection of minimal BM tumor as stable disease (SD). The NANTRCv1.0 defined overall response by integrating three categories: modified RECIST for soft tissue (CT/MRI) response, Curie scoring for MIBG response (including bone and soft tissue sites), and morphology (maximal tumor percentage among bilateral aspirates/biopsies) for BM response.
This retrospective analysis of 13 Phase 1/2 NANT trials was performed to determine if the NANTRCv1.0 overall response was associated with PFS or OS of patients with recurrent/refractory neuroblastoma, and to define patient and tumor characteristics associated with response and outcome.
2 |. PATIENTS AND METHODS
2.1 |. Patients
A retrospective review was performed of patients enrolled on 13 NANT therapeutic trials15–25 from March 1, 2000 (first NANT enrollment) through October 19,2009 (per Institutional Review Board [IRB] cutoff date). Trials utilized MIBG-based therapy with peripheral blood stem cell (PBSC) support (n = 5), or novel agents ± chemotherapy with PBSC support (n = 2) or without PBSC support (n = 6) (Supplementary Table S1). Individual patients could enroll serially on multiple trials, however analyses were limited to the first enrollments. Eligibility criteria across trials included HR-NB with one of the following: PD any time before enrollment (prior PD), residual tumor sites after less than a partial response (PR) to at least four induction chemotherapy cycles but no PD (refractory tumor), or residual tumor sites after PR to at least four induction chemotherapy cycles but no PD (persistent tumor). For patients originally diagnosed with localized non-HR-NB, prior PD must have occurred after diagnosis of metastatic HR-NB. MIBG therapy trials required at least one MIBG-avid site. Non-MIBG therapy trials required soft tissue lesion(s) ≥ 1cm in longest diameter (LD) and/or MIBG-avid lesion(s), and/or BM tumor by routine morphology. Patients with persistent tumor required histologic documentation of neuroblastoma and/or ganglioneuroblastoma at enrollment (except N2001–02). Written informed consent was obtained from all patients and/or parents/guardians for the clinical trials. IRB approval was obtained for this retrospective trial and each clinical trial.
2.2 |. Response assessment
Required enrollment evaluations included CT or MRI of the chest, abdomen, and pelvis (plus other sites with history of tumor involvement), MIBG scan, bilateral BM aspirates/biopsies, and urine catecholamines. Repeat evaluations included scans, urine catecholamines, and BM (if positive at enrollment). All patients had a BM evaluation at therapy completion. The first response evaluation was done after one (n = 10 trials) or two (n = 3 trials) courses. The second response evaluation was done after an additional one (n = 1 trial), two (n = 4 trials), or four (n = 1 trial) courses for multicourse protocols, then every three to four courses. Evaluability for response was specified in each protocol, including requiring completion of a minimum amount of protocol therapy, unless PD occurred prior to that time.
All responses were graded using the NANTRCv1.0 (Table 1), which integrated three categories (CT/MRI, MIBG, and BM) to define overall responses of complete response (CR), very good PR (VGPR), PR, mixed response (MR), SD, or PD. CR/VGPR required normal catecholamines. Soft tissue lesions ≥1 cm LD (including lymph nodes) were assessed for CT/MRI response using RECIST1.0,5 without consideration of MIBG uptake. Soft tissue components of bone lesions ≥1 cm LD were included in CT/MRI response. Intramedullary bone lesions were evaluated under MIBG response. MIBG response utilized Curie scoring11 of soft tissue and bone lesions. BM response utilized routine morphology with categories limited to CR, SD, or PD. Tumor percentage was defined as the highest percentage among bilateral aspirates and biopsies. A negative enrollment BM with subsequent evaluations having <10% tumor was considered SD.
TABLE 1.
NANT Response Criteria version1.0 (NANTRCv1.0)
| CT/MRI response | |
| Definition of evaluable for response | Soft tissue lesion(s) ≥20 mm (10 mm for spiral CT) longest diameter (not required to be MIBG-avid); all target lesions summed into disease measurement |
| CR | Resolution of all target and nontarget lesions. |
| VGPR | >90% decrease in disease measurement. Nontarget lesions stable to smaller. No new lesions. |
| PR | ≥30% decrease in disease measurement. Nontarget lesions stable to smaller. No new lesions. |
| SD | Insufficient decrease for PR and insufficient increase for PD. Nontarget lesions stable to smaller. No new lesions. |
| PD | ≥20% increase in disease measurement and/or new site. Nontarget lesions stable, smaller, or larger. |
| Not evaluable | CT/MRI scans of inadequate quality or not repeated of all sites with tumor at baseline. May apply only to specific time points, with other time points evaluable. |
| Not involved | No target lesions at baseline and subsequently |
| MIBG response | |
| Definition of evaluable for response | At least one MIBG-avid site (bone and/or soft tissue) on conjugate planar images. SPECT used only to delineate location of lesions. |
| CR | Complete resolution of all MIBG-avid bone and soft tissue sites. |
| PR | Treating NANT site: at least one MIBG-avid lesion resolved or decreased in intensity. Central review: relative Curie score 0.1 to ≤0.5. |
| SD | Treating NANT site: no change in number or intensity of MIBG-avid lesions. Central review: relative Curie score >0.5 to ≤1.0. |
| PD | New lesions or relative Curie score >1.0. |
| Not evaluable | MIBG scan of inadequate quality. |
| Not involved | No MIBG-avid lesions at baseline and subsequently. |
| Bone marrow respons | |
| Definition of evaluable for response | Any amounta of tumor by routine morphology alone. |
| CR | No tumor at two subsequent time points ≥3 weeks apart. |
| CR unconfirmed | No tumor at one subsequent time point. |
| SD | Persistence of any amount of tumor not meeting criteria for either CR or PD |
| PD | If any amount of tumor present at baseline: subsequent time point with ≥25% tumor and a doubling in amount of tumor present at baseline. If no tumor present at baseline: tumor on two consecutive samples ≥3 weeks apart. Treating MD may declare PD after one BM with Study Chair approval. |
| Not evaluable | Evaluation does not include an attempt to obtain bilateral aspirates and biopsies and does not obtain at least one adequate biopsy sample. |
| Not involved | No tumor at baseline and subsequently. |
| Overall response | |
| Definition of evaluable for response | Must have repeat evaluations of all sites with tumor documented at baseline. |
| CR | CR all categories; normal urine catecholamines. |
| VGPR | VGPR for CT/MRI, CR in MIBG-avid bone lesions; may have SD/CR in MIBG-avid soft tissue sites; normal urine catecholamines. |
| PR | PR for CT/MRI; PR for MIBG-avid bone lesions; may have SD/CR in MIBG-avid soft tissue sites; may have elevated urine catecholamines. |
| Mixed response (MR) | PR/VGPR/CR for ≥1 category with ≥1 category with SD; no category with PD; may have elevated urine catecholamines. |
| SD | SD for all categories; may have elevated urine catecholamines. |
| PD | PD for any one category or based on clinical assessment of treating physician. |
| Not evaluable | Not evaluable for at least one category with measurable/evaluable tumor at baseline and no category with PD. |
MIBG, meta-iodobenzylguanidine; NANT, New Approaches to Neuroblastoma Therapy; CR, complete response; VGPR, very good partial response; PR, partial response; SD, stable disease; PD, progressive disease.
Amount (percentage) of bone marrow tumor was calculated using surface area involved for biopsies, and number of tumor cells divided by number of total nucleated cells for aspirates.
All radiologic and BM reports were centrally reviewed by the NANT Operations Center during each trial to confirm responses reported by treating sites. Tabulation of tumor sites at enrollment (Table 2) and statistical analyses of response utilized centrally reviewed response if performed, otherwise the treating site’s assessment was utilized. All enrollment MIBG scans available were centrally reviewed. For MIBG therapy trials, all MIBG scans done during therapy were centrally reviewed. For non-MIBG therapy trials, central review was based on overall response reported by the treating site. For overall response of CR/VGPR/PR, all MIBG scans, CT/MRI scans, and BM biopsy slides from all evaluations were centrally reviewed. For overall response of MR/SD, scans and BM were centrally reviewed if measurable/evaluable disease was present for that category at enrollment. If tumor was seen only on BM aspirates, local BM reports were centrally reviewed and considered in grading BM response.
TABLE 2.
Patient characteristics
| Variables | All enrollments (N = 278) |
First enrollment (N = 213) |
First enrollment |
|
|---|---|---|---|---|
| MIBG protocols (N = 114) |
Non-MIBG protocols (N = 99) |
|||
| Age at diagnosis of HR-NB | ||||
| <18 months | 7(3%) | 7(3%) | 2 (2%) | 5(5%) |
| 18 months to 2 years | 51 (18%) | 39 (18%) | 23 (20%) | 16(16%) |
| 3–5 years | 119(43%) | 92 (43%) | 50 (44%) | 42 (42%) |
| 6–9 years | 52 (19%) | 37(17%) | 22(19%) | 15 (15%) |
| 10–20 years | 45 (16%) | 35 (16%) | 16(14%) | 19(19%) |
| ≥21 years | 4(1%) | 3 (1%) | 1 (1%) | 2 (2%) |
| Age at start of treatment (years) | ||||
| <3 | 9 (3%) | 9 (4%) | 6 (5%) | 3(3%) |
| 3–5 | 85 (31%) | 70 (33%) | 43 (38%) | 27(27%) |
| 6–9 | 88 (32%) | 66(31%) | 37 (32%) | 29 (29%) |
| 10–20 | 80 (29%) | 58 (27%) | 27 (24%) | 31(31%) |
| ≥21 | 16 (6%) | 10 (5%) | 1 (1%) | 9(9%) |
| Sex | ||||
| Female | 92 (33%) | 72 (34%) | 38 (33%) | 34 (34%) |
| Male | 186 (67%) | 141 (66%) | 76 (67%) | 65 (66%) |
| MYCN | ||||
| Amplified | 50 (24%) | 39 (24%) | 16(19%) | 23(31%) |
| Not amplified | 159(76%) | 121 (76%) | 69 (81%) | 52 (69%) |
| Unknown | 69 | 53 | 29 | 24 |
| Histopathology | ||||
| Favorable | 7(4%) | 7(5%) | 4(5%) | 3(4%) |
| Unfavorable | 178 (96%) | 135 (95%) | 69 (95%) | 66 (96%) |
| Unknown | 93 | 71 | 41 | 30 |
| Stage at initial diagnosis of NB | ||||
| 1 | 7(3%) | 5(2%) | 4(4%) | 1 (1%) |
| 2 | 12 (4%) | 7 (3%) | 4 (4%) | 3 (3%) |
| 3 | 16 (6%) | 11(5%) | 2 (2%) | 9(9%) |
| 4 | 242 (87%) | 189 (89%) | 103 (91%) | 86 (87%) |
| Unknown | 1 | 1 | 1 | 0 |
| PD prior to NANT enrollment | ||||
| Yes | 208 (75%) | 149 (70%) | 57 (50%) | 92 (93%) |
| No | 70 (25%) | 64 (30%) | 57 (50%) | 7(7%) |
| Baseline BM tumor content based on aspirates and biopsies | ||||
| Negative | 140 (51%) | 109(51%) | 58 (51%) | 51 (52%) |
| Positive | 137 (49%) | 103 (49%) | 56 (49%) | 47 (48%) |
| Unknown | 1 | 1 | 0 | 1 |
| Baseline BM tumor content based on biopsies only | ||||
| 0% | 151 (55%) | 117(55%) | 63 (55%) | 54 (55%) |
| >0 to ≤5% | 50 (18%) | 38(18%) | 21(18%) | 17(17%) |
| >5 to 10% | 6(2%) | 5 (2%) | 3 (3%) | 2 (2%) |
| <10% | 2 (<1%) | 2 (1%) | 0 | 2 (2%) |
| >5% | 1 (<1%) | 1 (<1%) | 1 (<1%) | 0 |
| >10 to 20% | 12 (4%) | 10 (5%) | 5(4%) | 5(5%) |
| >20 to 30% | 4(1%) | 3(1%) | 2 (2%) | 1 (1%) |
| >30% | 22 (8%) | 16 (8%) | 10 (9%) | 6(6%) |
| Positive NOS | 22 (8%) | 14 (7%) | 7(6%) | 7(7%) |
| No report/inadequate specimen | 7(3%) | 6(3%) | 2 (2%) | 4 (4%) |
| Not done | 1 | 1 | 0 | 1 |
| Baseline CT disease | ||||
| Negative/nonmeasurable | 135 (49%) | 110(52%) | 61 (54%) | 49 (49%) |
| Positive/measurable | 141(51%) | 103 (48%) | 53 (46%) | 50 (51%) |
| Unknown | 2 | 0 | 0 | 0 |
| Baseline MIBG scan | ||||
| Negative | 16 (6%) | 15 (7%) | 0 | 15(16%) |
| Positive | 255 (94%) | 194 (93%) | 114(100%) | 80 (84%) |
| Nonavid tumor | 7 | 4 | 0 | 4 |
| Baseline Curie score | ||||
| 0 | 16 (7%) | 15 (8%) | 0 | 15(19%) |
| 1–2 | 58 (25%) | 45 (25%) | 26 (26%) | 19 (24%) |
| 3–4 | 23(10%) | 17 (9%) | 10 (10%) | 7(9%) |
| 5–9 | 51 (22%) | 40 (22%) | 30 (30%) | 10(13%) |
| 10–19 | 60 (26%) | 47 (26%) | 27(27%) | 20 (25%) |
| 20–25 | 23(10%) | 16 (9%) | 8 (8%) | 8 (10%) |
| Nonavid tumor | 7 | 4 | 0 | 4 |
| Unknown | 40 | 29 | 13 | 16 |
| Median (range) | 6 (0, 25) | 6(0,25) | 7(1,25) | 4 (0, 24) |
| Best overall response to first NANT trial | ||||
| Complete response | 14 | 14 | 9 | 5 |
| Partialresponse | 16 | 14 | 13 | 1 |
| Mixed response | 27 | 24 | 22 | 2 |
| Stable disease | 110 | 81 | 43 | 38 |
| Progressive disease | 96 | 70 | 22 | 48 |
| Toxic death | 2 | 2 | 2 | 0 |
| Inevaluable | 13 | 8 | 3 | 5 |
| Follow-up (years) | ||||
| Median (range) | 4.6(0.32,11.6) | 4.6 (0.32,11.6) | 4.3(0.32,11.6) | 5.0 (3.4,6.9) |
HR-NB, high-risk neuroblastoma; MIBG, meta-iodobenzylguanidine; NB, neuroblastoma; PD, progressive disease; NANT, New Approaches to Neuroblastoma Therapy; BM, bone marrow; NOS, not otherwise specified.
2.3 |. Statistics
Study endpoints included overall response, PFS, and OS. Univariate and multivariable logistic regression models were used to examine associations between patient characteristics or trial type and probability of achieving overall CR or CR/PR. Patients inevaluable for response or who died of toxicity were excluded from this analysis. For multivariable analysis, only variables significant in univariate analysis at P ≤ 0.05 were included, then backward selection procedure was used to construct a minimal model including only variables significantly associated with achieving CR or CR/PR (Table 3) at P ≤ 0.05.26
TABLE 3.
Odds ratios (OR), confidence intervals (CI), and P-values from logistic regression models on probability of achieving best overall response of CR or CR/PR in 203 patients (excluding patients inevaluable for response or toxic deaths)
| Variable | #PT | Complete response |
Partial or complete response |
|||
|---|---|---|---|---|---|---|
| OR(95%CI) | P-value | OR (95% CI) | P-value | |||
| Measurable tumor at enrollment on CT/MRI | 0.013 | 0.044 | ||||
| No | 105 | 1.0 | 1.0 | |||
| Yes | 98 | 0.13(0.33,0.65) | 0.38 (0.15,0.98) | |||
| Curie score at enrollment | 0.002a | <0.001a | ||||
| 0 | 12 | 1.0 | 1.0 | |||
| 1–4 | 61 | 0.21 (0.04,1.03) | 0.034b | 0.14 (0.02,0.94) | 0.003b | |
| 5–9 | 39 | 0.080 (0.01,0.59) | 0.071(0.01,0.57) | |||
| 10–19 | 44 | 0.031 (0.003,0.35) | 0.027 (0.003,0.24) | |||
| 20–25 | 16 | No CR | No CR/PR | |||
| Unknown but >0c | 27 | |||||
| Nonavidd | 4 | |||||
| PBSC-supported trial | Excluded | 0.005 | ||||
| No | 84 | 1.0 | ||||
| Yes | 119 | 8.8 (1.9,40) | ||||
Multivariable model includes only variables associated with the response at P ≤ 0.05 in univariate analyses, then a backward selection procedure was used to eliminate variables not significant at P ≤ 0.05. PT: patients; MIBG, meta-iodobenzylguanidine; PBSC, peripheral blood stem cell; CR, complete response; PR, partial response.
Test of trend includingall Curie scores.
Test of trend among patients with Curie score ≥ 1.
These patients were included in the model as a missing category.
These patients were excluded from the analyses.
Univariate and multivariable Cox regression models27 were used to assess the associations between PFS/OS and patient/tumor characteristics or NANT trial. PFS was defined as time from therapy start to date of PD or death from tumor (Figs. 1A, 1B, and 2). Patients were censored at the date of diagnosis of the second malignancy (n = 1) or toxic death (n = 2). For patients who died from tumor without a PD date (n = 4), death date was used as event date for PFS. OS was defined as the time from therapy start to date of death from any cause (Figs. 1A, 1B, and 2). Surviving patients were censored at the last known alive date. Follow-up was censored as of October 9,2012 per IRB approval. For multivariate analyses, only variables significant in univariate analysis at P ≤ 0.05 were included, then backward selection procedure was used to construct a minimal model including only variables significantly associated with PFS or OS (Table 4) at P ≤ 0.05.26
FIGURE 1.
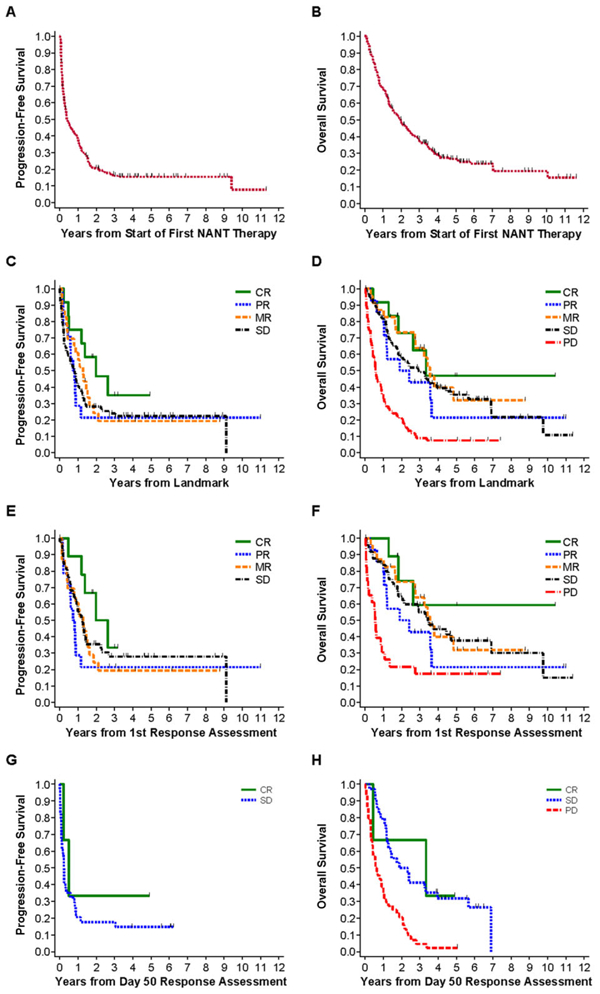
PFS and OS analyses and association with patient’s overall response. PFS/OS are from start of NANT therapy (1A/1B), or from chosen response landmark (1C-H). The overall response assessment used for these analyses was the first overall response for PBSC-supported trials and the Day 50 overall response for non-PBSC supported trials. (A) PFS of all 213 first enrollments on all trials, PFS at 2 years = 21 ± 3%. (B) OS of all 213 first enrollments on all trials, OS at 2 years = 50 ± 3%. (C) PFS of patients treated on all trials grouped by overall response. CR (n = 12) versus PR (n = 14) versus MR (n = 23) versus SD (n = 84), trend: P = 0.40. (D) OS of patients treated on all trials grouped by overall response. For CR (n = 12) versus PR (n = 14) versus MR (n = 23) versus SD (n = 84) versus PD (n = 69), trend: P < 0.001. When PD is excluded, trend: P = 0.73. (E) PFS of patients treated on PBSC trials grouped by overall response. CR (n = 9) versus PR (n = 14) versus MR (n = 23) versus SD (n = 50), trend: P = 0.60. (F) OS of patients treated on PBSC-supported trials grouped by overall response. For CR (n = 9) versus PR (n = 14) versus MR (n = 23) versus SD (n = 50) versus PD (n = 23), trend: P = 0.014. When PD is excluded, trend: P = 0.74. (G) PFS of patients treated on non-PBSC trials grouped by overall response. CR (n = 3) versus SD (n = 34): P = 0.44. (H) OS of patients treated on non-PBSC trials grouped by overall response. For CR (n = 3) versus SD (n = 34) versus PD (n = 44), trend: P < 0.001. When PD is excluded, P = 0.89
FIGURE 2.
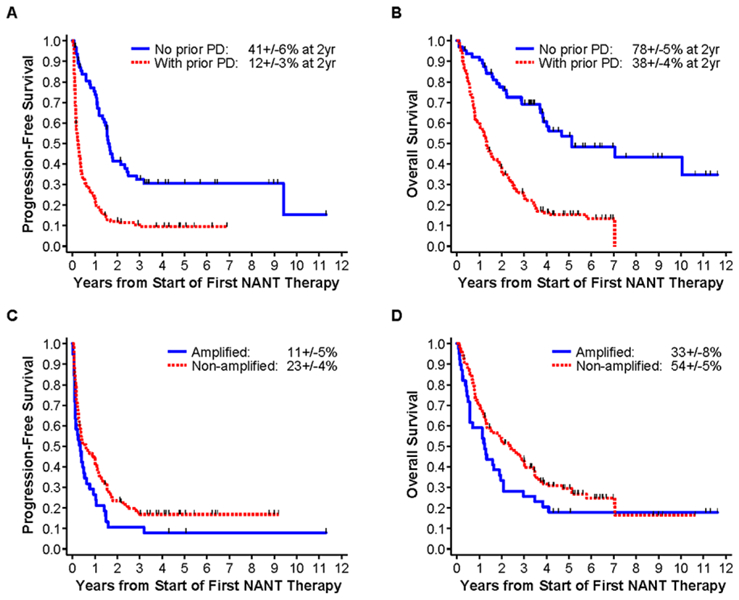
Univariate analysis of association of prior progressive disease (PD) (A and B) and MYCN gene amplification (C and D) with PFS and OS. (A) PFS for no prior PD (n = 64) versus prior PD (n = 149): P < 0.001. (B) OS for no prior PD (n = 64) versus prior PD (n = 149): P < 0.001. (C) PFS for MYCN-amplified (n = 39) versus nonamplified (n = 121): P = 0.047. (D) OS for MYCN-amplified (n = 39) versus nonamplified (n = 121): P = 0.077.
TABLE 4.
Hazard rations (HR), 95% confidence intervals (CI), and P-values from multivariable Cox regression models for 213 patients’ progression-free survival and overall survival from time of the first NANT enrollment
| Variable | #PT | Progression-free survival |
Overall survival |
||||
|---|---|---|---|---|---|---|---|
| HR(95%CI) | P-value | HR(95%CI) | P-value | ||||
| Age at NANT tx start (years) | Excluded | 0.033 | |||||
| <3 | 9 | 0.54 (0.19,1.5) | |||||
| 3–5 | 70 | 1.0 | |||||
| 6–9 | 66 | 0.88 (0.58,1.3) | |||||
| 10–20 | 58 | 0.72 (0.47,1.1) | |||||
| ≥21 | 10 | 0.27(0.11,0.67) | |||||
| MYCN | 0.037 | Excluded | |||||
| Nonamplified | 121 | 1.0 | |||||
| Amplified | 39 | 1.6(1.03, 2.4) | |||||
| Unknown | 53d | ||||||
| Measurable tumor at enrollment on CT/MRI | Excluded | 0.041 | |||||
| No | 110 | 1.0 | |||||
| Yes | 103 | 1.4 (1.01, 2.0) | |||||
| Curie score at enrollment | <0.001a | 0.001a | |||||
| 0 | 15 | 1.0 | 1.0 | ||||
| 1–4 | 62 | 3.2 (1.6, 6.3) | 0.048b | 2.1(1.01,4.4) | 0.037b | ||
| 5–9 | 40 | 5.9 (2.7,13) | 3.1 (1.4, 6.9) | ||||
| 10–19 | 47 | 5.0(2.4,11) | 2.9 (1.3, 6.3) | ||||
| 20–25 | 16 | 5.1(2.1,12) | 4.6(1.8,12) | ||||
| Unknown but > 0c | 29 | ||||||
| Nonavidd | 4 | ||||||
| PBSC-supported trial | <0.001 | 0.003 | |||||
| No | 86 | 1.0 | 1.0 | ||||
| Yes | 127 | 0.34 (0.24,0.48) | 0.57 (0.40,0.83) | ||||
| Prior PD after dx of HR-NB | <0.001 | <0.001 | |||||
| No | 64 | 1.0 | 1.0 | ||||
| Yes | 149 | 2.9 (2.0,4.3) | 4.1 (2.6, 6.6) | ||||
PT, patients; dx, diagnosis; BM, bone marrow; tx, treatment; HR-NB, high-risk neuroblastoma; MIBG, meta-iodobenzylguanidine; PBSC, peripheral blood stem cell; PD, progressive disease; NANT, New Approaches to Neuroblastoma Therapy. For multivariable analyses, only variables significant in univariate analyses at P ≤ 0.05 were included, then a backward selection procedure was used to eliminate variables not significant at P ≤ 0.05.
Test of trend including all Curie scores.
Test of trend in patients with Curie score ≥1.
These patients included in model as missing category.
These patients excluded from analyses.
Analyses of associations between NANTRC response and PFS/OS utilized the landmark method28 with fixed time points after treatment start selected as landmarks to avoid bias from variability in time-to-response evaluation. For PBSC-supported trials (Supplementary Table S1), the first response assessment was the chosen landmark. For non-PBSC protocols, the chosen landmark was Day 50 from treatment start. Time 0 for PFS/OS analyses was defined as time of the chosen landmark. Patients inevaluable for response or who did not have response assessed by the landmark time were excluded. Patients with response assessments on or before the landmark were grouped based on the most recent response. Patients with PD or death on or before the landmark were excluded from landmark analysis on PFS. Patients who died on or before the landmark were excluded from landmark analysis on OS. Associations between overall response and PFS/OS were assessed separately for trials that did (Figs. 1E and 1F) or did not (Figs. 1G and 1H) include PBSC support, since most PBSC trials allowed only one course versus non-PBSC trials that allowed multiple courses. Figures 1C and 1D show analysis of all trials (both PBSC and non-PBSC), with P values determined from log-rank tests stratified on PBSC versus non-PBSC protocols.
BM tumor percentage (Table 2) utilized central review if done (52/212 [24.5%] of enrollment marrows), otherwise local BM reports were used. For local reports, if tumor was noted as present without specifying percentage, the BM was tabulated as “positive, not otherwise specified” (NOS). When only one of two biopsy reports specified tumor percentage, for percentages >30%, then BM was tabulated as >30%; and if ≤30% BM was tabulated as positive, NOS.
PFS/OS probabilities (Figs. 1 and 2) were based on Kaplan-Meier method29 with Greenwood standard errors.30 Reported P-values are all two-sided. All analyses were performed in STATA.31
3 |. RESULTS
3.1 |. Patient population
A total of 217 patients (Supplementary Fig. S1) diagnosed with HR-NB from 1989 to 2009 had 284 enrollments on 13 NANT trials from March 1, 2000 through October 19, 2009. Excluding 4 patients who never received any protocol therapy, 213 unique patients enrolled serially on 1 (n = 162;76%), 2 (n = 39;18.3%), 3 (n = 10;4.7%), or 4 (n = 2;0.9%) trials. Only their 213 first enrollments were included in PFS/OS analyses. Patient characteristics (Table 2) did not differ between the first and all enrollments. From the first enrollment, PFS and OS for the 213 patients were 38 ± 3% and 69 ± 3%, 21 ± 3% and 50 ± 3%, and 16 ± 3% and 27 ± 3% at 1,2, and 5 years, respectively (Figs. 1A and 1B). Median time from enrollment to PD was 149 days (95% CI: 111–266 days). Median follow-up was 4.6 years (range 0.32–11.6).
3.2 |. Bone marrow assessment
Enrollment BM was positive in 49% (103/212) of patients based on aspirates and biopsies and 43% (89/206) of patients based on biopsies alone (Table 2). Tumor percentage in enrollment BM (including only assessments with specific tumor percentage reported for both biopsies) was ≤5% in 19.8% (38/192) and >5–10% in 2.6% (5/192) of patients. There were 417 BM evaluations done in all 213 patients at enrollment and while on therapy. Only 10 of 359 (2.8%) BM evaluations with positive/negative status known for all four samples had tumor in aspirates only; 61 of 359 (17%) had tumor in biopsies only. Among 56 of 212 patients with ≤20% tumor at enrollment, 12 had at least two subsequent BM assessments and 6 of 12 were intermittently positive. The true incidence of intermittent positivity for patients with <20% tumor could not be determined since not all patients had ≥3 serial BM samples.
3.3 |. Patient/tumor characteristics and response (Table 3)
Ten (4.7%) of 213 patients were inevaluable for response due to the following reasons: not completing minimum required therapy (n = 2), incomplete evaluation (n = 4), no tumor sites at enrollment (n = 2), or toxic death prior to response evaluation (n = 2), leaving 203 patients for analysis of associations with response. Twenty-eight (13.8%) of 203 patients had an overall CR (n = 14) or PR (n = 14); none had a VGPR. Twenty-four (11.8%) patients had an overall MR. Ten of these 24 patients were MR due to SD for MIBG (n = 5), BM/CT-MRI (n = 2), CT-MRI/MIBG (n = 1), CT-MRI (n = 1), or MIBG/BM (n = 1). The remaining 14 patients had at least PR based on CT/MRI and/or MIBG evaluations, but were downgraded to MR because of SD in BM; 10 of 14 patients had <5% residual BM tumor.
Responders (CR/PR) were treated on MIBG trials with PBSC support (n = 22), non-MIBG trials with PBSC support (n = 1), and non-MIBG trials without PBSC support (n = 5). In multivariable analysis, no measurable CT/MRI disease and Curie score of 0 at enrollment were significantly associated with achieving CR and CR/PR (Table 3). When multivariable analysis was limited to Curie scores of ≥1, there was a significant trend for higher probability of achieving CR and CR/PR with lower Curie scores, with no apparent cut point. Treatment on PBSC-supported trials was associated with higher probability of achieving CR/PR in multivariable analysis, while MIBG therapy trials dropped from the multivariable models due to its strong correlation with treatment on PBSC-supported trials. BM status at enrollment, age at diagnosis or start of treatment, sex, MYCN, histopathology, tumor stage, history of prior PD, time from HR-NB diagnosis to the first PD or NANT treatment start, and time from the first PD to start of NANT treatment were not significantly associated with achieving CR or CR/PR in univariate or multivariate analyses.
3.4 |. Response and PFS/OS
Among the seven PBSC-supported trials, six permitted only one course of therapy (Supplementary Table S1) followed by response evaluation. Median time from therapy start to response evaluation was 77 (range 8–148) days; 50% of patients ranged from 57 to 95 days. For the six non-PBSC trials allowing multiple courses (Supplementary Table S1), all but one patient had a response evaluation by Day 50. OS was significantly better when analyzed for patients with an overall response of SD or better compared to those with a response of PD; regardless of whether the analysis included all trials (Figs. 1C and 1D), PBSC-supported trials only (Figs. 1E and 1F), or non-PBSC trials only (Figs. 1G and 1H). No apparent differences were observed among CR, PR, MR, or SD (Figs. 1C–1H) in either PFS or OS.
3.5 |. Patient/tumor characteristics and PFS/OS
Multivariable analyses results are shown in Table 4. The date of the first PD following diagnosis of HR-NB was known for 147 patients, and occurred at a median of 18 months from diagnosis (range 1.3–160 months). Two-year PFS and OS were 12 ± 3% and 38 ± 4% for patients with prior PD, significantly lower than for patients without prior PD, at 41 ± 6% and 78 ± 5%, respectively (Figs. 2A and 2B). There was no significant difference in PFS (P = 0.46) or OS (P = 0.61) for patients with persistent (n = 8) versus refractory (n = 42) disease, with 2-year PFS/OS of 38 ± 17%; 88 ± 12% and 36 ± 8%; 73 ± 7%, respectively. PFS/OS was 62 ± 13%; 85 ± 10% for the remaining 14 patients with unknown persistent/refractory status.
In multivariable analyses, treatment on PBSC trials was significantly associated with better PFS and OS. Age ≥21 years at enrollment appeared to have better OS in multivariable analyses. In univariate (Figs. 2C/2D) and multivariate analyses, MYCN amplification was significantly associated with lower PFS but not OS.
Higher Curie score was significantly associated with worse PFS/OS for analyses including all trials. This included Curie scores ≥1 versus 0 and for higher scores when analyses were limited to Curie scores ≥1. Curie score ≥1 (vs. 0) was also associated with lower PFS/OS for non-MIBG therapy trials, with no apparent cut point among scores above one. Higher Curie score was not significantly associated with PFS/OS when analyzed for MIBG trials only, where all Curie scores were ≥1 at baseline per eligibility. Measurable tumor on CT/MRI was associated with worse OS but not PFS. LD was not specifically analyzed since it was not captured centrally on all patients. Neither BM-positive/negative status or tumor percentage among positive marrows was associated with PFS/OS.
Age at diagnosis, sex, histopathology, tumor stage, time from diagnosis of HR-NB to the first PD, time from diagnosis of HR-NB to start of NANT treatment, and time from the first PD to start of NANT treatment were not significantly associated with PFS/OS in multivariable analyses.
4 |. DISCUSSION
These analyses of associations between NANTRCv1.0 overall response, patient/tumor characteristics, and PFS/OS in patients with relapsed/refractory/persistent HR-NB were performed in a large series of NANT Phase 1–2 trials with consistent eligibility and response criteria. The combination of standardized trial design and our statistical methodology maximizes the ability to obtain valid assessment of the correlation of response with PFS/OS across trials. The NANTRCv1.0 included more quantitative assessment of bone and BM response than the INRC 1993, and therefore may optimize evaluation of new agent activity against specific tumor sites and more accurately define response. NANTRCv1.0 responses of SD or better were associated with higher OS, validating utilizing the NANTRC to inform selection of agents evaluated in Phase 1 settings for further trials.
There was no significant difference in PFS/OS between overall responses of CR/PR versus MR/SD (Fig. 1). This may be due to small numbers of CR/PR, or to v1.0 criteria for CT/MRI and/or BM response categories, which may have underestimated overall response. The NANTRCv1.0 assessed CT/MRI response based on decrease in soft tissue lesions LD, however LD alone may not identify active neuroblastoma. NANTRCv1.0 did not require MIBG uptake or histologic confirmation to define evaluable soft tissue lesions. Soft tissue masses with resolved MIBG uptake and stable size were considered SD for CT/MRI response category, although MIBG avidity resolution may reflect maturation or residual scar tissue. Subsequent NANTRCv1.2 and v2.0 (Supplementary Table S2) added MIBG uptake or histology to define measurable soft tissue lesions as evaluable for response. NANTRCv1.0 BM response categories were limited to CR and SD. Ten additional patients would have had an overall PR based on CT/MIBG responses, but were downgraded to MR due to ≤5% residual BM tumor. NANTRC v1.2 and v2.0 revised the overall PR definition to include patients with a PR/CR for soft tissue and bone response categories who have <5% tumor in BM. These modifications may better separate overall responses of PR from MR and SD.
NANTRCv1.0 piloted new BM response criteria, which have been challenging in neuroblastoma due to heterogeneity of involvement, sampling errors (particularly with <5% tumor or minimal disease [MD]),32 variable use of immunohistochemistry, and lack of methodology consensus for enumerating tumor percentage. The INRC 1993 utilized14 the number of positive BM samples regardless of tumor percentage, and defined intermittent MD as progression versus the NANTRCv1.0 definition as SD. We found that 19.8% of patients had ≤5% BM tumor at enrollment and provide preliminary data on incidence of intermittent tumor detection in BM for this group. This needs to be evaluated in larger numbers to optimize BM response criteria. We found BM biopsies more accurate than aspirates in determining tumor involvement, similar to Bostrom.33 Cheung32 found no difference between biopsies and aspirates, however their series included many newly diagnosed patients with higher tumor content.
We found no association between baseline BM tumor content and achieving CR/PR or PFS/OS. Higher Curie score and measurable soft tissue disease were significantly associated with worse OS and lower probability of achieving CR/PR; higher Curie score was also associated with lower PFS. This suggests that higher tumor burden is unfavorable.
CR/PR rates were 19.3% for MIBG therapy trials, 6.1% for non-MIBG trials, 17.2%for PBSC trials, and 6.3%for non-PBSC trials. However, in multivariable analyses only treatment on PBSC trials was significantly associated with higher probability of achieving CR/PR and better PFS/OS. PBSC and MIBG trials were highly correlated in our dataset (Supplementary Table S1), thus their individual contribution to achieving CR/PR or PFS/OS cannot be determined. Better results with PBSC trials is likely due to the majority including MIBG therapy given at known active doses, which has a 20–40% response rate in relapsed/refractory neuroblastoma.34 More intensive therapy may also be more effective. For non-MIBG trials, the efficacy and optimal dose for the novel agent(s) was unknown. Additionally, more patients on non-MIBG trials had prior PD, which was strongly associated with worse PFS and OS (P < 0.001).
Time from diagnosis of HR-NB to the first PD was not significantly associated with OS (calculated from NANT treatment start) because of the significant effect of any prior PD. A previous analysis including both HR-NB and non-HR-NB35 found that time from diagnosis to the first PD was associated with worse OS (calculated from the first PD) in the subgroup of MYCN-amplified stage 3 or 4 tumors with the first PD ≤12 months from diagnosis. For patients with HR-NB, longer time to the first PD has been reported to correlate with better survival calculated from diagnosis.36,37 Our series differs from prior reports in being limited to recurrent/refractory HR-NB, identifying the target population at Phase 1/2 trial enrollment rather than at diagnosis, and in the OS starting point. MYCN amplification was associated with worse PFS but not OS. London35 found that MYCN was significant for OS (from time of the first PD) for all neuroblastoma risk groups. Sex was not associated with outcome in our series, similar to a small Argentine series,38despite a 2:1 male/female predominance versus 1.1:1 for newly diagnosed neuroblastoma.39 Male predominance could be due to higher relapse rates, or longer survival after relapse allowing enrollment on salvage trials.
Based on the NANT data, the NANTRCv1.2 were adopted into the INRC 2017 with minor modifications. NANTRCv1.2 was revised as v2.0 (Supplementary Table S2) to harmonize minor differences with the INRC 2017.39 A recent international BM evaluation consensus paper40 also adopted the NANTRC use of percent tumor involvement.
Our results demonstrate that the NANTRCv1.0 response is associated with OS, and suggest that patient/tumor characteristics may predict response and outcome. Identifying significant variables can optimize early-phase trial design to select agents for further testing to ultimately improve outcome. Trials using PFS endpoints may need to stratify for prior PD, MYCN amplification, and tumor sites. Future analyses of NANTRC v1.2/2.0 are needed to verify these results from NANTRCv1.0 in larger patient numbers.
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported by National Cancer Institute grant numbers: R01 CA100895 and P01 CA81403, Friends of Cathryn Foundation, and the Alex’s Lemonade Stand Foundation for Childhood Cancer Center of Excellence Grant
Funding information
Friends of Cathryn Fund
Abbreviations:
- BM
bone marrow
- CR
complete response
- HR-NB
high-risk neuroblastoma
- INRC
International Neuroblastoma Response Criteria
- IRB
Institutional Review Board
- LD
longest diameter
- MD
minimal disease
- MIBG
metaiodobenzylguanidine
- MR
mixed response
- NANT
NewApproaches to Neuroblastoma Therapy
- NANTRCv1.0
NANT Response Criteria version 1.0
- NOS
not otherwise specified
- OS
overall survival
- PBSCs
peripheral blood stem cells
- PD
progressive disease
- PFS
progression-free survival
- PR
partial response
- RECIST
Response Evaluation Criteria in Solid Tumors
- SD
stable disease
- VGPR
verygood PR
Footnotes
SUPPORTING INFORMATION
Additional Supporting Information may be found online in the supporting information tab for this article.
CONFLICT OF INTEREST STATEMENT
The authors have no conflicts of interest or financial disclosures related to this manuscript.
REFERENCES
- 1.Pinto NR, Applebaum MA, Volchenboum SL, et al. Advances in risk classification and treatment strategies for neuroblastoma. J Clin Oncol. 2015;33:3008–3017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Matthay KK, Villablanca JG, Seeger RC, et al. Treatment of high-risk neuroblastoma with intensive chemotherapy, radiotherapy, autologous bone marrow transplantation, and 13-cis-retinoic acid. Children’s Cancer Group.NEngl J Med. 1999;341:1165–1173. [DOI] [PubMed] [Google Scholar]
- 3.Matthay KK, Reynolds CP, Seeger RC, et al. Long-term results for children with high-risk neuroblastoma treated on a randomized trial of myeloablative therapy followed by 13-cis-retinoic acid. A Children’s Oncology Group study. J Clin Oncol. 2009;27:1007–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yu AL, Gilman AL, Ozkaynak MF, et al. Anti-GD2 antibody with GMCSF, interleukin-2, and isotretinoin for neuroblastoma. N Engl J Med. 2010;363:1324–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst. 2000;92:205–216. [DOI] [PubMed] [Google Scholar]
- 6.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–247. [DOI] [PubMed] [Google Scholar]
- 7.Jain RK, Lee JJ, Ng C, et al. Change in tumor size by recist correlates linearly with overall survival in phase I oncology studies. J Clin Oncol. 2012;30:2684–2690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carceller F, Bautista FJ, Fowkes LA, et al. Response assessment in paediatric phase I trials according to RECIST guidelines: survival outcomes, patterns of progression and relevance of changes in tumour measurements. Pediatr Blood Cancer. 2016;63:1400–1406. [DOI] [PubMed] [Google Scholar]
- 9.Matthay KK, Atkinson JB, Stram DO, Selch M, Reynolds CP, Seeger RC. Patterns of relapse after autologous purged bone marrow transplantation for neuroblastoma. A Children’s CancerGroup pilot study. J Clin Oncol. 1993;11:2226–2233. [DOI] [PubMed] [Google Scholar]
- 10.Sibley GS, Mundt AJ, Goldman S, et al. Patterns of failure following total body irradiation and bone marrow transplantation with or without a radiotherapy boost for advanced neuroblastoma. Int J Radiat Oncol Biol Phys. 1995;32:1127–1135. [DOI] [PubMed] [Google Scholar]
- 11.Matthay KK, Shulkin B, Ladenstein R, et al. Criteria for evaluation of disease extent by (123)I-metaiodobenzylguanidine scans in neuroblastoma: a report for the InternationalNeuroblastoma RiskGroup (INRG) task force. Br J Cancer. 2010;102:1319–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matthay KK, Edeline V, Lumbroso J, et al. Correlation of early metastatic response by 123I-metaiodobenzylguanidine scintigraphy with overall response and event-free survival in stage IV neuroblastoma. J Clin Oncol. 2003;21:2486–2491. [DOI] [PubMed] [Google Scholar]
- 13.Yanik GA, Parisi MT, Shulkin BL, et al. Semiquantitative MIBG scoring as a prognostic indicator in patients with stage 4 neuroblastoma: a report from the Children’s OncologyGroup.JNucl Med. 2013;54:541–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brodeur GM, Pritchard J, Berthold F, et al. Revisions of the international criteria for neuroblastoma diagnosis, staging, and response to treatment. J Clin Oncol. 1993;11:1466–1477. [DOI] [PubMed] [Google Scholar]
- 15.Matthay KK, Tan JC, Villablanca JG, et al. Phase I dose escalation of iodine-131-metaiodobenzylguanidine with myeloablative chemotherapy and autologous stem-cell transplantation in refractory neuroblastoma: a New Approaches to Neuroblastoma Therapy consortium study. J Clin Oncol. 2006;24:500–506. [DOI] [PubMed] [Google Scholar]
- 16.Matthay KK, Quach A, Huberty J, et al. Iodine-131-metaiodobenzylguanidine double infusion with autologous stem-cell rescue for neuroblastoma: a New Approaches to Neuroblastoma Therapy Phase I study. J Clin Oncol. 2009;27:1020–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wagner LM, Villablanca JG, Stewart CF, et al. Phase I trial of oral irinotecan and temozolomide for childrenwith relapsed high-risk neuroblastoma: a New Approach to Neuroblastoma Therapy consortium study. J Clin Oncol. 2009;27:1290–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Russell HV, Groshen SG, Ara T, et al. A phase I study of zoledronic acid and low-dose cyclophosphamide in recurrent/refractory neuroblastoma: aNew Approaches to Neuroblastoma Therapy (NANT) study. Pediatr Blood Cancer. 2011;57:275–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Minturn JE, Evans AE, Villablanca JG, et al. Phase I trial of lestaurtinib for children with refractory neuroblastoma: a New Approaches to Neuroblastoma Therapy consortium study. Cancer Chemother Pharmacol. 2011;68:1057–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matthay KK, Weiss B, Villablanca JG, et al. Dose escalation study of no-carrier-added 131I-metaiodobenzylguanidine for relapsed or refractory neuroblastoma: New Approaches to Neuroblastoma Therapy consortium trial. J Nucl Med. 2012;53:1155–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.DuBois SG, Chesler L, Groshen S, et al. Phase I study of vincristine, irinotecan, and (1)(3)(1)I-metaiodobenzylguanidine for patients with relapsed or refractory neuroblastoma: a New Approaches to Neuroblastoma Therapy trial. Clin Cancer Res. 2012;18:2679–2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maurer BJ, Kang MH, Villablanca JG, et al. Phase I trial of fenretinide delivered orally in a novel organized lipid complex in patients with relapsed/refractory neuroblastoma: a report from the New Approaches to Neuroblastoma Therapy (NANT) consortium. Pediatr Blood Cancer. 2013;60:1801–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kang MH, Villablanca JG, Glade Bender JL, et al. Probable fatal drug interaction between intravenous fenretinide, ceftriaxone, and acetaminophen: a case report from a New Approaches to Neuroblastoma (NANT) phase I study. BMC Res Notes. 2014;7:256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yanik GA, Villablanca JG, Maris JM, et al. 131Imetaiodobenzylguanidine with intensive chemotherapy and autologous stem cell transplantation for high-risk neuroblastoma. A New Approaches to Neuroblastoma Therapy (NANT) Phase II study. Biol Blood Marrow Transplant. 2015;21:673–681. [DOI] [PubMed] [Google Scholar]
- 25.Villablanca JG, Volchenboum SL, Cho H, et al. A Phase I New Approaches to Neuroblastoma Therapy study of buthionine sul-foximine and melphalan with autologous stem cells for recurrent/refractory high-risk neuroblastoma. Pediatr Blood Cancer. 2016;63:1349–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hocking RR. The analysis and selection of variables in linear regression. Biometrics. 1976;32:1–49. [Google Scholar]
- 27.Cox D, Oakes D, Analysis of survival data. London, New York: Chapman and Hall; 1984:208. [Google Scholar]
- 28.Anderson JR, Cain KC, Gelber RD. Analysis of survival by tumor response. J Clin Oncol. 1983;1:710–719. [DOI] [PubMed] [Google Scholar]
- 29.Kaplan EL, Meier P. Nonparametric-estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 30.Greenwood M The natural duration of cancer, Report on Public Health and Medical Subjects No. 33 London; 1926:1–26. [Google Scholar]
- 31.StataCorp. Stata Statistical Software: Release 11. College Station, TX: StataCorp LP; 2009. [Google Scholar]
- 32.Cheung NK, Heller G, Kushner BH, Liu C, Cheung IY. Detection of metastatic neuroblastoma in bone marrow: when is routine marrow histology insensitive. J Clin Oncol. 1997;15:2807–2817. [DOI] [PubMed] [Google Scholar]
- 33.Bostrom B, Nesbit ME Jr, Brunning RD. The value of bone marrow trephine biopsy in the diagnosis of metastatic neuroblastoma. Am J Pediatr Hematol Oncol. 1985;7:303–305. [PubMed] [Google Scholar]
- 34.DuBois SG, Matthay KK. 131I-metaiodobenzylguanidine therapy in children with advanced neuroblastoma. Q J Nucl Med Mol Imag. 2013;57:53–65. [PubMed] [Google Scholar]
- 35.London WB, Castel V, Monclair T, et al. Clinical and biologic features predictive of survival after relapse of neuroblastoma: a report from the InternationalNeuroblastoma RiskGroup project. J ClinOncol. 2011;29:3286–3292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Santana VM, Furman WL, McGregor LM, Billups CA. Disease control intervals in high-risk neuroblastoma. Cancer. 2008;112:2796–2801. [DOI] [PubMed] [Google Scholar]
- 37.Garaventa A, Parodi S, De Bernardi B, et al. Outcome of children with neuroblastoma after progression or relapse. A retrospective study of the Italian Neuroblastoma Registry. Eur J Cancer. 2009;45: 2835–2842. [DOI] [PubMed] [Google Scholar]
- 38.Moreno F, Lopez Marti J, Palladino M, Lobos P, Gualtieri A, Cac-ciavillano W. Childhood neuroblastoma: incidence and survival in Argentina. Report from the National Pediatric Cancer Registry, ROHA Network 2000–2012. Pediatr Blood Cancer. 2016;63:1362–1367. [DOI] [PubMed] [Google Scholar]
- 39.Ries LAG, Smith MA, Gurney JG, Linet M, Tamra T, Young JL, Bunin GR, eds. Cancer Incidence and Survival Among Children and Adolescents: United States SEER Program 1975–1995. Bethesda, MD: National Cancer Institute SEER Program; 1999. [Google Scholar]
- 40.Park JR, Bagatell R, Cohn SL, et al. Revisions to the International Neuroblastoma Response Criteria: a consensus statement from the National Cancer Institute Clinical Trials Planning Meeting. J Clin Oncol. 2017;35:2580–2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Burchill SA, Beiske K, Shimada H, et al. Recommendations for the standardization of bone marrow disease assessment and reporting in children with neuroblastoma on behalf of the International Neuroblastoma Response Criteria Bone Marrow Working Group. Cancer. 2017;123:1095–1105. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


