ABSTRACT
Purpose
Older adults are more likely to be vitamin D deficient. The aim of the study was to determine whether these patients have worse outcomes with COVID-19.
Methods
We conducted a prospective cohort study between 1 March and 30 April 2020 to assess the importance of vitamin D deficiency in older patients with COVID-19. The cohort consisted of patients aged ≥65 years presenting with symptoms consistent with COVID-19 (n=105). All patients were tested for serum 25-hydroxyvitamin D (25(OH)D) levels during acute illness. Diagnosis of COVID-19 was confirmed via viral reverse transcriptase PCR swab or supporting radiological evidence. COVID-19-positive arm (n=70) was sub-divided into vitamin D-deficient (≤30 nmol/L) (n=39) and -replete groups (n=35). Subgroups were assessed for disease severity using biochemical, radiological and clinical markers. Primary outcome was in-hospital mortality. Secondary outcomes were laboratory features of cytokine storm, thoracic imaging changes and requirement of non-invasive ventilation (NIV).
Results
COVID-19-positive arm demonstrated lower median serum 25(OH)D level of 27 nmol/L (IQR=20–47 nmol/L) compared with COVID-19-negative arm, with median level of 52 nmol/L (IQR=31.5–71.5 nmol/L) (p value=0.0008). Among patients with vitamin D deficiency, there was higher peak D-dimer level (1914.00 μgFEU/L vs 1268.00 μgFEU/L) (p=0.034) and higher incidence of NIV support and high dependency unit admission (30.77% vs 9.68%) (p=0.042). No increased mortality was observed between groups.
Conclusion
Older adults with vitamin D deficiency and COVID-19 may demonstrate worse morbidity outcomes. Vitamin D status may be a useful prognosticator.
Keywords: General medicine (see internal medicine), geriatric medicine, diabetes & endocrinology, calcium & bone
INTRODUCTION
The COVID-19 outbreak, which began in China in late 2019 and then rapidly spread across the world, has spurred a global effort to tackle the disease and establish risk factors and prognostic markers; one such is serum vitamin D deficiency. Vitamin D is a secosteroid with varied immunomodulatory, anti-inflammatory, antifibrotic and antioxidant actions. There is growing evidence that it may play a role in the pathophysiological processes of COVID-19.
The relationship between vitamin D deficiency and adverse prognosis has been suggested by the apparent Northern–Southern latitude gradient, with mortality and hospitalisation rates for COVID-19 seen to be higher in northern latitude countries compared with those closer to the equator.1 Furthermore, research by Alipio and colleagues2 , in a retrospective study, provides evidence of an association between vitamin D deficiency and adverse outcome in patients with COVID-19. Older adults in institutions, such as hospitals and care homes, are particularly likely to be vitamin D deficient as a result of a lack of sun exposure and dietary insufficiency, and may have worse outcomes with COVID-19.
In the UK, The Royal College of Physicians of London Commentary3 reported that patients who died of COVID-19 were severely vitamin D deficient. There is also growing concern that the Black, Asian and Minority Ethnic community who produce less vitamin D as a result of higher skin melanin content are inherently more susceptible to severe presentations of COVID-19.4 Therefore, the British Nutrition Found2ation,5 who applied guidance to the public amidst self-isolation for COVID-19, has suggested that everyone should consider taking a daily supplement of 400 IU of vitamin D.
We investigated serum vitamin D levels in older patients admitted to our institution during the pandemic. The aim of our study was to assess the potential relationship between vitamin D deficiency and COVID-19 severity in hospitalised older adults. We conducted a single-centred prospective cohort study of older patients admitted to a large district general hospital in the UK, with symptoms in keeping with a viral infection. Patients were divided into COVID-19-positive and -negative groups with subsequent assessment of vitamin D status. A subgroup analysis of the COVID-19-positive arm was conducted with analysis of serum markers of infection and clinical markers of disease severity, such as high dependency unit (HDU) admission and non-invasive ventilation (NIV).
METHODS AND MATERIALS
Population
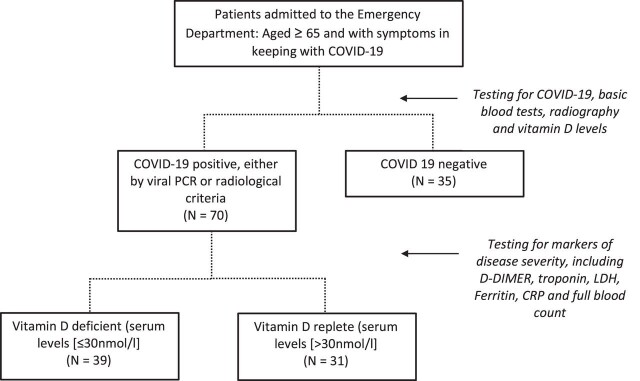
All emergency admissions aged ≥65 years admitted to our hospital with symptoms consistent with COVID-19 including cough, dyspnoea, fever and/or anosmia6 between 1 March and 30 April 2020 were included in the study (figure 1). These patients were investigated with real-time reverse transcriptase-PCR (RT-PCR) assay for severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) on nasopharyngeal swab, blood tests including vitamin D levels (25-hydroxyvitamin D (25(OH)D), basic observations, and chest X-ray (CXR) and/or chest CT.
Figure 1.
Flow chart, depicting recruitment of patients upon admission via the emergency department, with subsequent separation. CRP, C reactive protein; LDH, lactate dehydrogenase.
Diagnosis of active SARS-CoV-2 infection was based on positive viral RT-PCR swab or evidence of COVID-19 on a chest radiograph or chest CT (bilateral peripheral infiltrates/ground-glass opacities, airspace opacification, traction bronchiectasis, inter/intralobular septal thickening and organising pneumonia). Patients who did not meet either of these criteria were enrolled into the COVID-19-negative group.
The COVID-19-positive group was divided into vitamin D-deficient (≤30 nmol/L) and -replete (>30 nmol/L) groups, as per national guidelines and local laboratory standards.7 Vitamin D-deficient patients were supplemented in accordance with national guidelines and all patients received care in line with best practice guidance. In addition, patients were treated with subcutaneous low-molecular-weight heparin venous thromboembolism (VTE) prophylaxis as per national guidance.
Data collection
Data were extracted from medical notes and the local hospital electronic database. These included age, weight, height, ethnicity, smoking status and comorbidities. Ethnicities were self-assigned. Rockwood Clinical Frailty Score8 and Charlson Comorbidity Index9 were calculated retrospectively.
The primary outcome measured was in-hospital mortality secondary to COVID-19. Secondary outcomes were defined as NIV support and admission to HDU, COVID-19 radiographic changes on CXR and laboratory features of cytokine storm. All patients had vitamin D levels checked in keeping with good medical practice. Biochemical/haematological panels (C reactive protein (CRP), D-dimer, ferritin, high sensitivity troponin T, lactate dehydrogenase (LDH) and lymphocyte count) were carried out in accordance with local guidance on COVID-19 diagnostics and prognostication.10 In addition, data for specific features consistent with the COVID-1911 12 were collected from formal reports of CXR and/or chest CT. Causes of death were obtained digitally from mortality reports made by our hospital’s mortality and bereavement office.
Statistical analysis
All statistical analyses were carried out on GraphPad Prism (version 8). For continuous outcome variables, each data set was assessed for normality using Kolmogorov-Smirnov and Shapiro-Wilk tests, and tested for significance with either an unpaired t-test if parametric or a Mann-Whitney test if non-parametric. Possible outcome sets were normalised by logarithmic transformation and tested for significance using a parametric method. For categorical variables, an OR was calculated and tested for significance using a Pearson ᵡ² test. P value <0.05 (two-tailed test) was considered to be statistically significant. In addition, all variables underwent a Pearson and Spearman analysis to gauge linear covariance between the outcome measures and the corresponding serum vitamin D concentration.
For binary outcomes, a receiver operating characteristic (ROC) curve was plotted to assess the prognostic value of serum concentrations of vitamin D. Area under the curve (AUC) values >0.5 were deemed to convey a prognostic value in the measured variable.
Ethics
This survey was approved by the trust audit department with reference FH119 and with clinically collected, non-identifiable data which does not fall under the remit of NHS Research Ethics Committee. All data were collected locally and handled in accordance with European General Data Protection Regulation (GDPR) standards, as well as local and NHS standards on data protection.
All practices conducted as part of this study were done in accordance with local regulations and best clinical practice protocols. Serum vitamin D levels were tested alongside the hospital routine serum COVID-19 panel and did not require any extra phlebotomy. Patients received care in line with standard practices for the management of COVID-19 throughout the study period.
RESULTS
A total of 105 patients (mean age 81 years, range 65–102; male (n=57):female (n=48)) were recruited to the study, with 70 (66.7%) subsequently allocated to the COVID-19-positive group and 35 (33.3%) allocated to the COVID-19-negative group. Among the COVID-19-positive group, 39 (55.7%) patients were found to have 25(OH)D level ≤30 nmol/L and 31 (44.3%) were found to have a level >30 nmol/L.
Demographics (age, sex, ethnicity, frailty, body mass index, smoking history and comorbidities) between COVID-19-positive and -negative groups were comparable. In addition, the characteristics of vitamin D-replete and -deficient groups in the COVID-19-positive arm were found to be comparable (table 1). Only three patients were admitted from a nursing home with one instance in each of the three study subgroups.
Table 1.
Population characteristics of COVID-19-positive versus -negative groups, subdivided by serum vitamin D concentrations
| Population sample characteristics | ||||||
| COVID-19-positive (N=70) | P value | |||||
| Demographics | Vitamin D ≤30 nmol/L (N=39) | Vitamin D >30 nmol/L (N=31) | COVID-19-negative (N=35) | COVID-19-positive vit D ≤30 nmol/L vs >30 nmol/L | COVID-19-positive versus COVID-19-egative | |
| Mean age (SD) | 79.46 (±9.52) | 81.16 (7.23) | 83.44 (±8.08) | 0.41 | 0.064 | |
| Male:female | 24:15 | 18:13 | 15:20 | 0.77 | 0.075 | |
| Rockwood Clinical Frailty Score Median (IQR) | 6 (6–7) | 5 (5–6) | 5 (5–6) | 0.1 | 0.66 | |
| Median body mass index (IQR) | 25 (23–32) | 24 (20–27) | 25 (22–29) | 0.14 | 0.75 | |
| Smoking status (%) | Current smoker | 1 (2.56) | 5 (16.13) | 4 (11.43) | 0.14 | 0.65 |
| Ex-smoker | 12 (30.77) | 11 (35.48) | 15 (42.86) | 0.66 | 0.13 | |
| Ethnicity (%) | Caucasian | 29 (74.36) | 21 (67.74) | 30 (85.71) | 0.51* | 0.27* |
| South Asian | 8 (20.51) | 10 (32.36) | 3 (8.57) | |||
| East Asian | 2 (5.13) | 0 (0) | 0 (0) | |||
| Afro-Caribbean | 0 (0) | 1 (3.26) | 3 (8.57) | |||
| Comorbidities | N (%) | P value | ||||
| Vitamin D ≤30 nmol/L (N=39) | Vitamin D >30 nmol/L (N=31) | COVID-19-negative (N=35) | COVID-19-positive vit D ≤30 nmol/L vs >30 nmol/L | COVID-19-positive versus COVID-19-negative | ||
| Hypertension | 18 (46.15) | 16 (51.61) | 20 (55.56) | 0.65 | 0.41 | |
| Diabetes mellitus | 17 (43.59) | 9 (29.03) | 8 (22.22) | 0.21 | 0.14 | |
| Ischaemic heart disease | 7 (17.95) | 8 (25.81) | 11 (30.56) | 0.43 | 0.27 | |
| Chronic respiratory disease | 6 (15.38) | 7 (22.58) | 4 (11.11) | 0.44 | 0.35 | |
| Heart failure | 6 (15.38) | 6 (19.35) | 5 (13.89) | 0.66 | 0.71 | |
| Stroke | 6 (15.38) | 3 (9.68) | 3 (8.33) | 0.48 | 0.52 | |
| Dementia | 4 (10.26) | 2 (6.45) | 1 (2.78) | 0.58 | 0.29 | |
| Chronic kidney disease | 10 (25.64) | 6 (19.35) | 6 (16.67) | 0.53 | 0.5 | |
| Atrial fibrillation | 6 (15.38) | 8 (25.81) | 8 (22.22) | 0.28 | 0.73 | |
| Cancer | 2 (5.13) | 1 (3.23) | 2 (5.56) | 0.7 | 0.75 | |
| Endocrinological disease | 1 (7.69) | 2 (6.45) | 2 (5.56) | 0.44 | 0.75 | |
| Median value (IQR) | P value | |||||
| Vitamin D ≤30 nmol/L (N=39) | Vitamin D >30 nmol/L (N=31) | COVID-19-negative (N=35) | COVID-19-positive vit D ≤30 nmol/L vs >30 nmol/L | COVID-19-positive versus COVID-19-negative | ||
| Charlson Comorbidity Index | 5 (3–6) | 4 (4–5) | 4 (4–6) | 0.81 | 0.76 | |
| COVID-19-positive (N=70) | COVID-19-negative (N=35 | Difference | P value | |||
| Vitamin D concentration (nmol/L) | 27.00 (20.00–47.00) | 52.00 (31.50–71.50) | 25 | 0.0008 | ||
P value for ethnicities calculated by comparing the number of Caucasian patients to all other ethnic groups.
vit, vitamin.
No patients were admitted to the intensive treatment unit, and ceilings of care were set to NIV support on the HDU. There was no difference in the average length of stay between vitamin D subgroups (29 days).
Vitamin D levels in the COVID-19-positive group were overall significantly lower compared with that in the COVID-19-negative group (27.00 nmol/L vs 52.00 nmol/L) (p=0.0008). Among patients with vitamin D deficiency in the COVID-19-positive group, there was a higher average peak in D-dimer level (1914.00 μgFEU/L vs 1268.00 μgFEU/L) (p=0.034) and a higher incidence of NIV support and HDU admission (30.77% vs 9.68%) (p=0.042). The vitamin D-deficient case group demonstrated higher peak CRP, LDH and ferritin levels; lower trough lymphocyte counts; and increased incidence of radiographic changes, although these were not statistically significant. The vitamin D-replete case group demonstrated a higher peak troponin level that was not statistically significant (table 2). There was only one confirmed case of pulmonary thrombosis in the vitamin D-deficient case group. There was no apparent difference in mortality between the two groups. Ethnicity did not influence outcomes in this cohort. The outcome measures did not appear to follow a linear relationship with serum vitamin D concentrations.
Table 2.
Primary and secondary outcome measures, for vitamin D-deficient and -replete groups
| Outcome measures | ||||
| Median value (IQR) | ||||
| Serum markers | Vitamin D ≤30 nmol/L | Vitamin D >30 nmol/L | Difference | P value |
| Peak CRP (mg/L) | 191.00 (108.00–274.00) | 155.00 (96.00–252.00) | −36 | 0.32 |
| Peak LDH (IU/L) | 272.50 (217.25–367.50) | 239.50 (180.50–333.00) | −33 | 0.17* |
| Peak ferritin (μg/L) | 518.50 (894.00–1109.25) | 484.50 (221.00–715.50) | −34 | 0.40* |
| Peak D-dimer (μgFEU/L) | 1914.00 (1323.75–3131.50) | 1268.00 (1003.50–2273.00) | −646 | 0.034 |
| Peak troponin(ng/L) | 37.00 (26.00–95.00) | 42.00 (25.00–88.00) | 5 | 0.83* |
| Trough lymphocyte count (×109/L) | 0.56 (0.44–0.78) | 0.68 (0.54–0.95) | 0.12 | 0.15* |
| N (%) | OR (CI) | P value | ||
| Chest X-ray changes | 11 (28.20) | 8 (25.80) | 1.13 (0.39–3.28) | 0.82 |
| Ventilation requirement | 12 (30.77) | 3 (9.68) | 4.15 (1.05–16.34) | 0.042 |
| Mortality | 6 (15.38) | 4 (12.90) | 1.40 (0.36–5.47) | 0.5 |
P value derived using a parametric technique on logarithmically transformed data.
CRP, C reactive protein; LDH, lactate dehydrogenase.
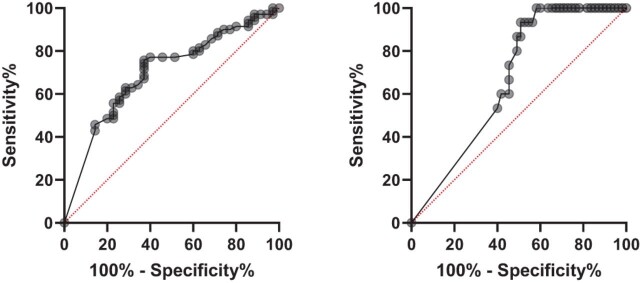
ROC curves were plotted for vitamin D as a distinguisher between COVID-19-positive and -negative states as well as between those requiring and not requiring ventilatory support (figure 2). For the former, AUC was 0.70 (95% CI 0.59 to 0.80) (p value=0.0009). For the latter, AUC was 0.67 (95% CI 0.54 to 0.80) (p value=0.046).
Figure 2.
ROC curves for vitamin D and COVID-19 status (left) and ventilatory support requirement (right). ROC, receiver operating characteristic.
DISCUSSION
The main findings of our study suggest that older patients with lower serum concentrations of 25(OH)D, when compared with aged-matched vitamin D-replete patients, may demonstrate worse outcomes from COVID-19. Markers of cytokine release syndrome were raised in these patients and they were more likely to become hypoxic and require ventilatory support in HDU. There was no difference in mortality between groups.
Evidence of an association between vitamin D deficiency and adverse outcome in COVID-19 is provided by Alipio (2020) and D’Avolio and colleagues.13 The former study (preprint) observed an increased disease severity for patients with vitamin D deficiency, while the latter noted a decreased serum vitamin D concentration between COVID-19-positive and -negative patients. These were both retrospective cohort studies encompassing a sample size of 212 and 120, respectively.
Ilie and colleagues14 performed a meta-analysis to study the association of vitamin D and morbidity and mortality with COVID-19 in 20 European countries and proposed a possible correlation between vitamin D levels, the incidence of SARS-CoV-2 infection and mortality. Hastie and colleagues15 used biobank data on 449 people with confirmed SARS-CoV-2 infection and found no relationship with serum 25(OH)D concentrations. The average age of subjects in this study was 49 years, and a major limitation was utilisation of historical vitamin D levels of patients (between 2006 and 2010) rather than vitamin D status at the time of infection with SARS-CoV-2. In our study, the average age was older at 81 years and we were able to establish vitamin D status during active SARS-CoV-2 infection.
In non-communicable diseases, both viral and bacterial, vitamin D deficiency has been associated with increased morbidity and mortality as well as a higher incidence of acute respiratory distress syndrome in critically unwell patients.16 17 Whether low vitamin D levels are cause or consequence of disease (reverse causality) processes remains unclear. However, a meta-analysis performed in 2017 of 11 321 patients from 25 randomised controlled studies demonstrated that vitamin D supplementation protected against acute respiratory tract infection and patients with very low concentrations of 25(OH)D (<25 nmol/L) benefiting most.18
Cited markers of cytokine storm were elevated in our vitamin D deficiency subset of patients, and the high peak D-dimer concentration was deemed statistically significant (p=0.034). This was found in the absence of in situ pulmonary thrombosis and in the context of standard VTE prophylaxis. Several studies have explored19 20 the pro-thrombotic state induced during the cytokine storm phase of inflammatory lung disease. The coagulation system appears active in critically ill patients, and high D-dimer levels reflect activation of the proinflammatory cytokine cascade (and downregulation of the anti-inflammatory cytokine cascade). Elevated D-dimer levels are associated with amplified risk for multiple organ failure and death.21 This may explain the increased incidence of ventilatory support seen in vitamin D-deficient patients.
Vitamin D has been shown to condition the innate immune reaction against both bacterial and viral infections. Calcitriol (1,25(OH)2D3), the active form of vitamin D, modulates macrophage activity by inhibiting the release of pro-inflammatory cytokines such as interleukin (IL)-1, IL-6, IL-8, IL-12 and tumour necrosis factor-alpha.16 Vitamin D shifts the adaptive immune reaction from a Th1 to a Th2 phenotype,17 22 downregulating differentiation of naïve T cells into pro-inflammatory Th17 cells,23 24 and promotes T regulatory cell induction.25–27 Dysregulation of both innate and adaptive immunity, as a result of vitamin D deficiency, may therefore be central to precipitating the ‘cytokine storm’ seen in COVID-19 infection.
In addition to anti-inflammatory properties, vitamin D also exerts a protective effect on human alveolar epithelial cells by promoting wound repair.28 Vitamin D has also been shown to preserve endothelial integrity and deficiencies result in increased vascular permeability and leak.29
Vitamin D also increases the expression of ACE-2. While increased ACE-2 expression in the early pandemic was predicted to increase the risk of infection, paradoxically, ACE-2 has also been shown to protect against acute lung injury.14 30 Disruption of one or more of these defensive pathophysiological processes may explain the association we found between vitamin D deficiency and increased requirement of ventilatory support. Whether this actually represents a causal relationship has not yet been elucidated.
Limitations
As a single-centre study at a district general hospital, we cannot generalise our results to other settings. Furthermore, our trust is based in Southern England, where population demographics and socioeconomic status may differ from those elsewhere.
We acknowledge that extracting information from medical notes requires second-hand interpretation and may not be representative of the full clinical picture. Patient outcomes may also have been influenced by current guidelines imposed by the National Institute of Clinical Excellence committee.31
We also acknowledge the role that sunlight exposure may have played in the measured serum levels of 25(OH)D. Unfortunately, this could not reliably be measured in patients; however, efforts were made to account for this. Notably, the study was carried out within a 2-month period which attenuated potential weather influence on sunlight exposure. In addition, the housing status of patients was considered—only three nursing home residents were included in the study, which minimised the risk of institutionalisation and its association with reduced sun exposure bearing influence on the results.
Considerations were made for the utilisation of sample size calculations to ensure the study was powered appropriately. No limit was initially set for sample size; however, the final number was limited by the dwindling numbers of patients with COVID-19. Despite achieving significant results in several outcomes, we acknowledge the risk of a type 2 error occurring with our experimental sample size. Therefore, we were unable to discount an association between vitamin D deficiency and those variables which did not achieve statistical significance with observed effect sizes.
Length of stay was recorded for all patients in the COVID-19 arm of the study, with no difference found between vitamin D subgroups. We emphasise, however, concerns on the reliability of this measure. Given the geriatric population of our study sample, the overwhelming issues affecting hospital discharge were social and housing issues. This was further complicated by infection control measures during the pandemic limiting the ability of some families to receive their relatives back home. Therefore, any firm conclusions should not be drawn from this outcome measure.
Another issue of consideration was vitamin D replacement and the effect this may have on the outcome measures recorded. In our study, vitamin D supplementation was only initiated after the acute phase of illness. Given that steady-state serum concentrations of vitamin D are achieved after 3–6 months with replacement,32 it is unlikely that serum levels would significantly change during the infective and symptomatic phase of admission. Consequently, we do not believe this would have influenced outcome measures. We were also unable to establish whether vitamin D replacement during active SARS-CoV-2 infection results in favourable outcomes.
CONCLUSION
Our study has demonstrated that patients over the age of 65 years presenting with symptoms consistent with COVID-19 are more likely to be vitamin D deficient. There appears to be a clinically relevant association between this and elevated markers of cytokine release syndrome and increased risk of respiratory failure requiring ventilatory support. Although there was no apparent mortality difference between the two groups, this may reflect the overall poor prognosis associated with the higher prevalence of frailty and comorbidities in our older cohort of patients. Vitamin D status may be a prognosticator for COVID-19, and supplementation might improve outcomes. Further studies in all age groups are awaited to validate this.
Main messages.
Older patients with COVID-19 infection and vitamin D deficiency (≤30 nmol/L) have higher peak D-dimer level and higher incidence of NIV support and HDU admission.
Vitamin D deficiency may be associated with worse outcomes from COVID-19, and vitamin D status may be a useful prognosticator.
What is already known on the subject.
There appears to be an association between increased COVID-19 incidence and mortality and countries with an increased prevalence of vitamin D deficiency.
Vitamin D plays an important role in the modulation of the immune system through promotion of anti-inflammatory cytokines and down-regulation of pro-inflammatory T cells.
The occurrence of an inflammatory ‘cytokine storm’ during COVID-19 has been associated with poorer outcomes and increased disease severity.
Footnotes
Contributors: All authors contributed to the manuscript. All were involved in the design of the study. VB, PK and NP collected the data. VB and TH were responsible for the statistical analysis. VB, TH, AKJM, KVdA, SS and CGM wrote the manuscript and all authors were involved in the final approval of the manuscript.
Funding: This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: All authors understand the policy of declaration of interests. CGM is a former member of the Fellowship of Postgraduate Medicine (FPM) council and is currently an FPM Fellow. VB, TH, NP, SS, PK, KVdA, AKJM all declare that they have no competing interests.
Patient consent for publication: Not required.
Ethics approval: As an audit using clinically collected, non-identifiable data, this work does not fall under the remit of National Health Service Research Ethics Committees. This statement is also present in the ‘Methods and material’ section of our manuscript.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data are available upon reasonable request.
Contributor Information
Vadir Baktash, Department of Medicine, Frimley Health NHS Foundation Trust, Wexham Park Hospital, Slough, UK.
Tom Hosack, Department of Medicine, Frimley Health NHS Foundation Trust, Wexham Park Hospital, Slough, UK.
Nishil Patel, Department of Medicine, Frimley Health NHS Foundation Trust, Wexham Park Hospital, Slough, UK.
Shital Shah, Department of Medicine, Frimley Health NHS Foundation Trust, Wexham Park Hospital, Slough, UK.
Pirabakaran Kandiah, Department of Medicine, Frimley Health NHS Foundation Trust, Wexham Park Hospital, Slough, UK.
Koenraad Van den Abbeele, Department of Medicine, Frimley Health NHS Foundation Trust, Wexham Park Hospital, Slough, UK.
Amit K J Mandal, Department of Medicine, Frimley Health NHS Foundation Trust, Wexham Park Hospital, Slough, UK.
Constantinos G Missouris, Department of Medicine, Frimley Health NHS Foundation Trust, Wexham Park Hospital, Slough, UK; Department of Cardiology, University of Cyprus Medical School, Nicosia, Cyprus.
REFERENCES
- 1. Panarese A, Shahini E. COVID-19, and vitamin D. Aliment Pharm Ther 2020;51:993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Alipio M. Vitamin D Supplementation Could Possibly Improve Clinical Outcomes of Patients Infected with Coronavirus-2019 (COVID-2019). SSRN Journal.
- 3. Cantorna MT. Mechanisms underlying the effect of vitamin D on the immune system. Proc Nutr Soc 2010;69:286–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. GOV.UK . SACN vitamin D and health report. 2016. Available https://www.gov.uk/government/publications/sacn-vitamin-d-and-health-report (accessed 24th Jun 2020)
- 5. British Nutrition Foundation . BNF busts the myths on nutrition and COVID-19. 2020. Available https://www.nutrition.org.uk/press-office/pressreleases/covid (accessed 24th Jun 2020)
- 6. GOV.UK . COVID-19: investigation and initial clinical management of possible cases. 2020. Available https://www.gov.uk/government/publications/wuhan-novel-coronavirus-initial-investigation-of-possible-cases (accessed 26th Jun 2020)
- 7. Giustina A, Adler RA, Binkley N, et al. Controversies in vitamin D: summary statement from an international conference. J Clin Endocrinol Metab 2019;104:234–40. [DOI] [PubMed] [Google Scholar]
- 8. Rockwood K, Song X, MacKnight C, et al. Global clinical measure of fitness and frailty in elderly people. CMAJ 2005;173:489–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373–83. [DOI] [PubMed] [Google Scholar]
- 10. Lippi G, Plebani M. Laboratory abnormalities in patients with COVID-2019 infection. Clin Chem Lab Med 2020;58:1131–4. [DOI] [PubMed] [Google Scholar]
- 11. Wong HYF, Lam HYS, Fong AHT, et al. Frequency and distribution of chest radiographic findings in COVID-19 positive patients. Radiology 2020;296:E72–E78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shi H, Han X, Jiang N, et al. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect Dis 2020;20:425–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. D’Avolio A, Avataneo V, Manca A, et al. 25-hydroxyvitamin D concentrations are lower in patients with positive PCR for SARS-CoV-2. Nutrients 2020;12:1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ilie PC, Stefanescu S, Smith L. The role of vitamin D in the prevention of coronavirus disease 2019 infection and mortality. Aging Clin Exp Res 2020;32:1195–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hastie CE, Mackay DF, Ho F, et al. Vitamin D concentrations and COVID-19 infection in UK Biobank. Diabetes Metab Syndr 2020;14:561–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Almerighi C, Sinistro A, Cavazza A, et al. 1Alpha,25-dihydroxyvitamin D3 inhibits CD40L-induced pro-inflammatory and immunomodulatory activity in human monocytes. Cytokine 2009;45:190–7. [DOI] [PubMed] [Google Scholar]
- 17. Mattner F, Smiroldo S, Galbiati F, et al. Inhibition of Th1 development and treatment of chronic-relapsing experimental allergic encephalomyelitis by a non-hypercalcemic analogue of 1,25-dihydroxyvitamin D(3). Eur J Immunol 2000;30:498–508. [DOI] [PubMed] [Google Scholar]
- 18. Martineau AR, Jolliffe DA, Hooper RL, et al. Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta-analysis of individuals participant data. BMJ 2017;i6583. [DOI] [PMC free article] [PubMed]
- 19. Levi M, Thachil J, Iba T, Levy JH. (2020). Coagulation abnormalities and thrombosis in patients with COVID-19. Lancet Haematol. 7(6), e438–e440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jose RJ, Manuel A. (2020). COVID-19 cytokine storm: the interplay between inflammation and coagulation. The Lancet Respiratory Medicine. 8(6), e46–e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Shorr AF, Thomas SJ, Alkins SA, et al. D-dimer correlates with proinflammatory cytokine levels and outcomes in critically ill patients. Chest 2002;121:1262–8. [DOI] [PubMed] [Google Scholar]
- 22. Boonstra A, Barrat FJ, Crain C, et al. 1alpha,25-Dihydroxyvitamin d3 has a direct effect on naive CD4(+) T cells to enhance the development of Th2 cells. J Immunol 2001;167:4974–80. [DOI] [PubMed] [Google Scholar]
- 23. Tang J, Zhou R, Luger D, et al. Calcitriol suppresses antiretinal autoimmunity through inhibitory effects on the Th17 effector response. J Immunol 2009;182:4624–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Daniel C, Sartory NA, Zahn N, et al. Immune modulatory treatment of trinitrobenzene sulfonic acid colitis with calcitriol is associated with a change of a T helper (Th) 1/Th17 to a Th2 and regulatory T cell profile. J Pharmacol Exp Ther 2008;324:23–33. [DOI] [PubMed] [Google Scholar]
- 25. Barrat FJ, Cua DJ, Boonstra A, et al. In vitro generation of interleukin 10-producing regulatory CD4(+) T cells is induced by immunosuppressive drugs and inhibited by T helper type 1 (Th1)- and Th2-inducing cytokines. J Exp Med 2002;195:603–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gorman S, Kuritzky LA, Judge MA, et al. Topically applied 1,25-dihydroxyvitamin D3 enhances the suppressive activity of CD4+CD25+ cells in the draining lymph nodes. J Immunol 2007;179:6273–83. [DOI] [PubMed] [Google Scholar]
- 27. Penna G, Roncari A, Amuchastegui S, et al. Expression of the inhibitory receptor ILT3 on dendritic cells is dispensable for induction of CD4+Foxp3+ regulatory T cells by 1,25-dihydroxyvitamin D3. Blood 2005;106:3490–7. [DOI] [PubMed] [Google Scholar]
- 28. Dancer RCA, Parekh D, Lax S, et al. Vitamin D deficiency contributes directly to the acute respiratory distress syndrome (ARDS). Thorax 2015;70:617–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gibson CC, Davis CT, Zhu W, et al. Dietary vitamin D and its metabolites non-genomically stabilize the endothelium. PLoS One 2015;10:e0140370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kuka K, Imai Y, Penninger JM. Angiotensin-converting enzyme 2 in lung diseases. Curr Opin Pharmacol 2006;6:271–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. National Institute for Health and Care Excellence (NICE) . COVID-19 rapid guideline: critical care in adults [NG159]. 2020. Available https://www.nice.org.uk/guidance/ng159 (accessed 30 Jun 2020) [PubMed]
- 32. Francis RM, Aspray TJ, Bowring CE, et al. National osteoporosis society practical clinical guideline on vitamin D and bone health. Maturitas 2015;80:119–21. [DOI] [PubMed] [Google Scholar]