Abstract
Background
Perinatal mortality in beef calves impacts on profitability and animal welfare, but the incidence and causes in UK herds are not well known.
Methods
Data from 11 herds were analysed to establish the risk factors for and incidence of perinatal mortality (full-term calves born dead or died within 48 hours). To establish cause of death, 23 herds in total submitted dead calves for postmortem examination (nine herds submitted all calves, 14 herds submitted calves on an ad hoc basis) and the results were reviewed by a panel.
Results
The incidence of perinatal mortality for all 1059 calvings was 5.1 per cent (range 1.6–12.4 per cent across herds; median 4 per cent). The incidence of stillbirth and neonatal mortality was 3.9 per cent (range 0–10.1 per cent) and 1.2 per cent (range 0–2.6 per cent), respectively. Sex of the calf, plurality and level of calving assistance were associated with significantly greater risk of perinatal loss. Parturition-related deaths (n=20), intrauterine infections (n=13), congenital malformations (n=6) and postpartum infections (n=6) were among the diagnosis recorded from 54 calves investigated. Parturition-related deaths and congenital malformations were recorded more commonly from herds submitting all losses than from those submitting on an ad hoc basis.
Conclusion
Variation in perinatal incidence across herds exists and many fail to reach the 2 per cent target. Some significant risk factors and common causes of death identified have the potential to decrease perinatal mortality rates through improved herd management.
Keywords: calves, cattle, neonatal disease, pathology, perinatal, disease investigation
Introduction
Continuous improvement of production efficiency and protection of animal welfare are key goals of the UK beef industry.1–3 Perinatal mortality of beef-bred calves reduces production efficiency through both increased direct costs (eg, to feed a non-productive cow) and reduced output (fewer calves to sell), and carries a high risk of negative impact on the welfare of the cow and calf.4 The incidence and causes of perinatal losses in the UK beef systems are not well characterised or understood.
As beef calves in the UK are not legally required to be tagged and registered in the central movement database until 20 days of age, perinatal loss data can be difficult to extract from existing records.5 6 An achievable farm level target for perinatal mortality (not clearly defined) in the UK beef industry has been suggested to be less than 2 per cent,7 although the limited available data suggest that actual figures may be considerably higher. Benchmarking figures and farmer survey data (which may be subject to recall bias and error) are published annually in various regions of the UK8 9 and typically report a stillbirth rate alone of around 3–4 per cent (defined as calves born dead). Similar retrospective benchmarking data have been published recently from 48 beef clients of a single UK veterinary practice.10 Perinatal mortality was not accurately recorded, but the difference between total calves born and total calves tagged ranged from 0 per cent to 25 per cent across participating herds, with 16 of the 48 herds having a loss rate above 2 per cent. Slightly more information is available from the UK dairy industry, where losses up to 48 hours of age have been estimated to be 5.3–7.9 per cent,11 12 although a direct comparison with the beef industry should not be made as important factors, such as risk of dystocia, are likely to differ.13 International data also focus almost exclusively on dairy herds.14 15
Published research into the underlying causes of perinatal mortality in the UK beef industry is even more limited. A 2011 review (with no UK data) summarising perinatal loss aetiology included only limited data on beef breeds14 and detailed information on beef cattle only available from a Canadian study.16 The UK has a veterinary laboratory diagnostic surveillance system that reports farm animal diagnoses as recorded by participating institutions, but the data are currently of limited value in assessing the causes of bovine perinatal mortality, as stillborn animals cannot be identified within the current data recording system.
The lack of available information on the incidence and causes of perinatal mortality from the UK beef industry is a significant constraint to developing evidence-based advice on how to optimise performance at the farm or national level. The aim of this study was to accurately record the incidence, associated risk factors and aetiology of perinatal losses in beef herds located in Orkney.
Materials and methods
Definitions
The following definitions were used:
Stillbirth: full-term calf (with a full hair coat and erupted or partially erupted teeth) born dead.
Neonatal loss: full-term calf born alive which died within 48 hours of birth.
Perinatal loss: either a stillbirth or neonatal loss (as defined).
Time at risk for incidence calculations: the entire spring calving season of the participating herds, February 1–June 10, 2016.
Recruitment
All beef clients of a veterinary practice in Orkney (approximately 250) were invited to attend a discussion meeting regarding perinatal losses in December 2015. Attending farmers were informed of the proposed study at the meeting and given the option to participate in the study in two different ways.
Full participation: farmers were obliged to accurately record data from all beef calf births and submit all calf perinatal losses for postmortem examination (PME).
Ad hoc participation: farmers could voluntarily submit individual calf perinatal losses for PME on an ad hoc basis at their discretion, with no requirement for further data recording.
Farmers opting for ‘full participation’ had to register their interest by January 1, 2016. This was incentivised by offering an unlimited number of PMEs for a fixed one-off cost of £100, and it was anticipated that an understanding of all causes of perinatal losses could be established from these herds. Farmers opting for ‘ad-hoc’ participation were charged at a commercial rate per PME (approximately £100).
A total of 23 herds were recruited. Eleven herds initially opted for full participation. All 11 submitted calving data, but only nine of these herds submitted all perinatal losses, the other two submitting on an ad hoc basis only. A further 12 herds also submitted on an ad hoc basis, resulting in a total of 14 herds submitting calves ‘ad-hoc’.
All herds participating in the study were beef herds varying in size from 49 to 130 breeding females. Twenty-one herds were located on mainland Orkney, and two herds were located on one of the outer northern isles, accessible by boat. In all cases, breeding cows were housed from November until May on slatted group accommodation, with individual straw-bedded pens available for parturition in most herds. Winter nutrition consisted of a grass silage-based ration, with additional barley and soya supplements given in some herds. The breed of sire varied; Aberdeen Angus, Charolais, Beef Shorthorn and Limousin were the predominant breeds. All herds were actively monitored free of bovine viral diarrhoea virus (BVDV) infection.
Incidence data collection and analysis (11 herds)
The following information was required to be recorded (using paper recording sheets) for every calving during the study period: time and date of parturition, breed of sire, parity of the dam (after calf birth), body condition score of the dam (1–5 scale), number of calves born, sex of calves born, if calf was alive/dead at birth, and the level of assistance provided at parturition. The first author (RN) visited each herd before the start of the study to provide training to relevant personnel on body condition scoring of cows (using the guide available at the Agriculture and Horticulture Development Board17) and to explain the data recording requirements of the study. The level of assistance provided at parturition was recorded on a scale of 0–5, with descriptions of each score provided to farmers: 0, unobserved; 1, observed but not assisted; 2, mild assistance by farmer (not requiring mechanical aid); 3, hard assistance by farmer (requiring use of mechanical aid); 4, veterinary delivery per vaginum; 5, caesarean section.18 For recorded births, data from the British Cattle Movement Service were used to cross-check and complete data on date of parturition, number of calves born and sex of calf, where necessary.
The incidence of stillbirth, neonatal loss and perinatal loss over the study period was calculated for each of the 11 herds, the study population as a whole and for each of the parturition factors. The numerators for stillbirth, neonatal loss and perinatal loss were defined as described in the Definitions section. The denominator for the stillbirth and perinatal loss calculation was the total number of calves born (dead or alive), and the denominator for the neonatal loss calculation was the total number of calves born alive. Statistical evaluation of the risk factors for perinatal loss was conducted using multivariable logistic regression for all losses recorded.19 Herd was considered as a fixed effect in all models, and three levels of calving assistance (hard assistance by farmer, veterinary delivery per vaginum and caesarean section) were grouped for purposes of analysis. Other risk factors were assessed individually in models using herd as a fixed effect. All risk factors found to be significant at a P value of less than 0.10 were included in a multivariable model, and backward selection was used to eliminate risk factors which were not significant at a P value of 0.05 or less to produce the final model. The final multivariable model then included herd as a fixed effect together with other risk factors found to be significant at a P value of 0.05 or less. Model assumptions (the linear relationship between continuous predictors and the logit of the outcome, and the absence of highly correlated predictor variables) were assessed, as was the potential effect of influential observations/outliers.
Establishing time and cause of death
For all perinatal loss submissions, farmers presented the calf for PME within 48 hours of death, in a scavenger-proof box, to a dedicated postmortem facility located at the veterinary practice. A standardised protocol, adapted from previously published guidelines for investigation,20 was applied to each case and included a history questionnaire, gross PME, sample collection and laboratory testing. The history questionnaire asked for information on the management of the cow prepartum, intrapartum and postpartum, the level of assistance at parturition, and the duration of parturition.
The gross PME protocol included recording of weight, sex and crown-rump length (measured using a tape from the most dorsal point on the cranium to the tail head), as well as a thorough examination of all organs. A written and photographic record was collected for all investigations. The case history and gross findings were used to categorise the time of death of each calf as prepartum (haemoglobin tissue staining, flaccid muscles, lack of blood clot in umbilical arteries, atelectasis), intrapartum (no haemoglobin staining in tissues, no blood clot in umbilical artery, no milk in abomasum) or postpartum (no haemoglobin staining in tissues, blood clot in the umbilical artery, partial or complete lung inflation).20
Blood, abomasal content and tissue samples (fresh and formalin-fixed) of placenta (when available, n=8), liver, lung, spleen, heart, thyroid and whole brain were collected from all calves. Additional samples were collected based on gross pathology, at the discretion of the first author (RN). Fresh samples were refrigerated until next-day postal delivery to the diagnostic laboratory could be ensured.
In every case, laboratory analysis included microscopy of abomasal content and placenta, culture, and identification of bacteria in abomasal fluid and placenta (for stillbirths) or liver and lung (for neonatal deaths), selective Salmonella, Campylobacter and Brucella culture of abomasal content and placenta (for stillbirths), fungal culture of abomasal content and placenta (for stillbirths), and zinc sulphate turbidity test on calf serum if the calf was over 24 hours of age at the time of death. Furthermore, every case was also screened for the following infectious agents: BVDV, Leptospira hardjo and bovine herpesvirus 1. Additional laboratory analysis (histopathology of fixed tissue, thyroid iodine, etc) was performed depending on the gross pathology findings on a case-by-case basis at the discretion of the first and last authors.
A single diagnosis was recorded for each calf following a retrospective panel discussion (of all authors except AR) held after the end of the study period and once all investigations were completed. All available case details were reviewed for each case in turn, with a diagnosis recorded when the panel unanimously agreed that the cause was ‘beyond reasonable doubt’. Where the criteria of multiple possible causes of death were reached in a single case, clinical reasoning by the authors was used to select a most likely singular cause. The categories and diagnostic criteria used to assign the cause of death are shown in table 1.
Table 1.
Diagnostic criteria used to assign the cause of bovine perinatal mortality in 23 beef herds in Orkney
| Cause of death | Diagnostic criteria |
| Congenital malformations | Gross PME findings identified abnormality considered incompatible with survival beyond the first 48 hours of life. |
| Intrauterine infections | Detection of a significant bacterium or fungus in pure culture from the fetal stomach content of stillborn calves, by detection of other known causative agents by a specific test or by detection of compelling gross (eg, pericarditis) or microscopic (eg, bronchopneumonia) pathology. |
| Parturition-related (anoxia sustained during stage 2 calving) | Case history and some/all of the following PME gross lesions: meconium staining, pulmonary atelectasis, organ congestion (including brain), haemorrhages on serosa (cardiac, thoracic, peritoneal), spleen, thymus, abomasum or conjunctiva. Histopathology indicating inhalation of abundant meconium particles was considered supportive. No other pathology or laboratory evidence considered more likely to have caused the death of the calf. |
| Postpartum infections | Detection of a significant organism in pure culture from the liver and/or lung of calves dying in the neonatal period, supported by consistent gross PME and/or microscopic pathological findings. Zinc sulphate turbidity test of less than 5 units used to define complete failure of passive transfer. |
| Diagnosis not reached | History, PME and further laboratory analysis did not identify a definitive cause of death. |
| Other | Diagnosis reached but does not fit into the categories above, for example, iatrogenic. |
PME, postmortem examination.
Results
Incidence of perinatal mortality
A total of 1059 parturitions were recorded in the 11 data recording herds, with 1101 calves born (including 42 sets of twins, 4 per cent). The number of calves born and the incidence of perinatal loss in these 11 herds (each herd identified by a letter) are shown in figure 1. Of the 1101 calves born, 43 calves were stillborn (3.9 per cent, range 0–10.1 per cent across herds). Of the 1058 calves born alive, 13 calves died in the neonatal period (0–48 hours) (1.2 per cent, range 0–2.6 per cent across herds). The overall incidence of perinatal loss was 5.1 per cent (range 1.6–12.4 per cent across herds; median 4 per cent).
Figure 1.
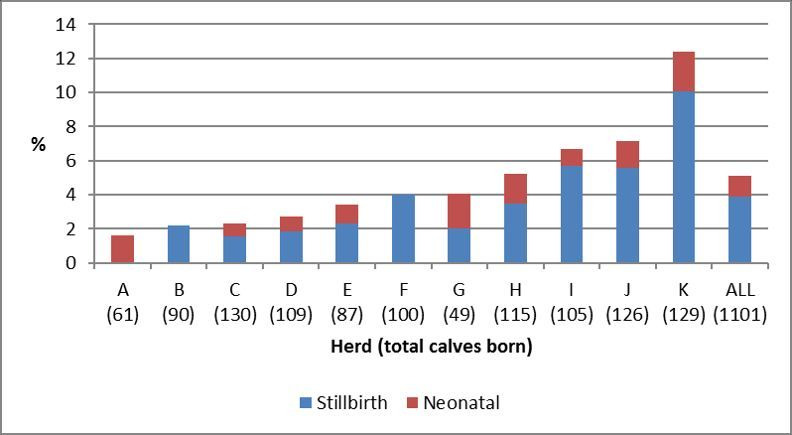
Incidence of perinatal loss in 11 Orkney beef herds during the spring 2016 calving season (the total calves born are shown in parentheses).
Risk factors for perinatal mortality
Data collected on the risk factors for perinatal loss are shown in table 2. Due to missing data, the parity and breed of the dam are not included (502 and 212 missing records, respectively). The risk of perinatal loss varied among the different herds. As these herds were not necessarily representative of the broader population, herd was included as a fixed effect in all subsequent models to control for variability observed among these herds.
Table 2.
Data collected on the risk factors of perinatal mortality in 1101 calves born from 11 Orkney beef herds during the spring 2016 calving season
| Variable | Level | Total calves | Stillborn calves | Neonatal deaths | Perinatal loss (%) |
| Herd | A | 61 | 0 | 1 | 1.6 |
| B | 90 | 2 | 0 | 2.2 | |
| C* | 130 | 2 | 1 | 2.3 | |
| D | 109 | 2 | 1 | 2.8 | |
| E | 87 | 2 | 1 | 3.4 | |
| F | 100 | 4 | 0 | 4.0 | |
| G | 49 | 1 | 1 | 4.1 | |
| H | 115 | 4 | 2 | 5.2 | |
| I | 105 | 6 | 1 | 6.7 | |
| J | 126 | 7 | 2 | 7.1 | |
| K† | 129 | 13 | 3 | 12.4 | |
| Calf sex | Female | 564 | 13 | 3 | 2.8 |
| Male | 537 | 30 | 10 | 7.4 | |
| Plurality | Single | 1017 | 32 | 11 | 4.2 |
| Twin | 84 | 11 | 2 | 15.5 | |
| Dam BCS | <2.5 | 164 | 14 | 4 | 11.0 |
| 2.5–3.5 | 677 | 21 | 6 | 4.0 | |
| >3.5 | 28 | 0 | 0 | 0 | |
| Not recorded | 232 | 8 | 3 | 4.7 | |
| Breed of sire | Aberdeen Angus | 342 | 14 | 6 | 6 |
| Simmental | 203 | 8 | 2 | 5 | |
| Charolais | 191 | 4 | 1 | 3 | |
| Shorthorn† | 142 | 12 | 3 | 11 | |
| Saler | 100 | 1 | 1 | 2 | |
| Limousin | 98 | 3 | 0 | 3 | |
| Stabiliser* | 25 | 1 | 0 | 4 | |
| Month of birth | February | 58 | 1 | 1 | 3.4 |
| March | 282 | 9 | 1 | 3.5 | |
| April | 438 | 15 | 7 | 5 | |
| May | 284 | 16 | 4 | 7 | |
| June | 39 | 2 | 0 | 5.1 | |
| Day of the week | Monday–Friday | 769 | 23 | 10 | 4.3 |
| Weekend | 332 | 20 | 3 | 6.9 | |
| Time of day | 00.00–05.59 | 149 | 4 | 2 | 4 |
| 06.00–11.59 | 239 | 21 | 5 | 10.9 | |
| 12.00–17.59 | 213 | 8 | 5 | 6.1 | |
| 18.00–23.59 | 181 | 7 | 1 | 4.4 | |
| Not recorded | 319 | 3 | 0 | 0.9 | |
| Level of calving assistance | Unobserved | 201 | 17 | 5 | 10.9 |
| Observed, unassisted | 455 | 4 | 2 | 1.3 | |
| Mild assistance | 138 | 9 | 1 | 7.2 | |
| Hard assistance‡ | 71 | 11 | 4 | 21.1 | |
| Vet assistance‡ | 4 | 1 | 0 | 25 | |
| Caesarean‡ | 9 | 1 | 1 | 22.2 | |
| Not recorded | 223 | 0 | 0 | 0 |
*100% of Stabiliser-bred calves were from herd C.
†85% of Shorthorn-bred calves were from herd K.
‡These categories were grouped for statistical analysis (see table 3).
BCS, body condition score.
The results of the multivariable logistic regression model are shown in table 3 and are based on 878 records for which complete data were available. Herd was included as a fixed effect in order to control for herd-to-herd variability. Three of the risk factors were found to be associated with significantly greater risk of perinatal loss: sex of the calf, plurality and the level of calving assistance. Male calves were 2.5 times more at risk than female calves, and twin calves were 3.5 times more at risk than single calves. Births which were observed but required no assistance were 83 per cent less likely to result in loss of the calf than unobserved births. Births requiring only mild assistance had the same odds of resulting in loss of the calf compared with unobserved births. Births requiring greater levels of assistance were just over three times as likely to result in loss of the calf than unobserved births.
Table 3.
Results of the multivariable logistic regression model showing significant risk factors for perinatal calf mortality
| Variable | Level | Estimate | se | OR (95% CI) | P value |
| Intercept | −4.28 | 1.08 | <0.001 | ||
| Herd* | A | Reference | |||
| B | −0.0885 | 1.27 | 9.2 (0.0072–0.076) | 0.94 | |
| C | 0.477 | 1.19 | 1.6 (0.080–20) | 0.69 | |
| D | 0.391 | 1.20 | 1.5 (0.17–31) | 0.75 | |
| E | 19.5 | 1289 | 2.9† | 0.99 | |
| F | 0.723 | 1.16 | 2.1 (0.27–42) | 0.53 | |
| G | 0.847 | 1.27 | 2.3 (0.21–52) | 0.50 | |
| H | 20.1 | 936 | 5.2† | 0.98 | |
| I | 1.38 | 1.11 | 4.0 (0.62–77) | 0.21 | |
| J | 1.10 | 1.10 | 3.0 (0.49–57) | 0.32 | |
| K | 2.28 | 1.07 | 9.4 (1.8–183) | 0.03 | |
| Calf sex | Female | Reference | |||
| Male | 0.909 | 0.353 | 2.5 (1.3–5.1) | 0.01 | |
| Plurality | Single | Reference | |||
| Twin | 1.25 | 0.427 | 3.5 (1.5–8.0) | 0.003 | |
| Level of calving assistance | Unobserved | Reference | |||
| Observed, unassisted | −1.78 | 0.501 | 0.17 (0.058–0.42) | <0.001 | |
| Mild assistance | −0.325 | 0.510 | 0.72 (0.25–1.9) | 0.52 | |
| Hard assistance, vet assistance or caesarean | 1.17 | 0.438 | 3.2 (1.4–7.7) | 0.007 | |
*Herd was included to control for unmeasured or unobserved differences among herds.
†Too few observations were collected for practical calculation of this confidence interval (CI).
When the body condition score of the dam was considered in isolation of all other risk factors (including herd), it was found to be highly statistically significantly associated with perinatal loss, with lower condition scores associated with higher odds of loss of the calf. However, once differences among herds were accounted for and controlled, body condition score was no longer significant at the 5 per cent level. The effect of body condition score is thus confounded by the effect of herd and cannot be addressed in this study.
None of the predictor variables included in the multivariable logistic regression model was found to be highly correlated, on the basis of their variance inflation factors.21 None of the predictors variables was a continuous variable, so the assumption of linearity was not found to be violated. Three potentially influential observations were identified on the basis of relatively high standardised residual error values.22 The impact of these observations was evaluated and found to be slight, so they were retained in the final model.
Cause of death
Number of calves submitted
A total of 54 calves were submitted for investigation. Thirty-four were from the nine herds that submitted all losses, and 20 were from the 14 herds that submitted on an ad hoc basis.
Characteristics of calves
Of the 54 calves submitted, 19 were female and 35 were male, with a mean weight of 42 kg (range 21–65 kg) and a mean crown rump length (CRL) of 84 cm (range 69–100 cm). Seven calves were categorised as having died prepartum, 29 intrapartum and 18 postpartum.
Diagnosed cause of death
A definitive cause of death was considered ‘beyond reasonable doubt’ by the panel of authors in 85 per cent of all submissions (46 of 54). The complete results are shown in table 4.
Table 4.
Cause of death for 54 beef calves that died in the perinatal period (full term, born dead or died within 48 hours) from nine herds that submitted all perinatal losses and 14 herds that submitted losses on an ad hoc basis
| Cause of death | Calves, n (N=54) | Further details of cause of death | |
| All (n=34) | Ad hoc (n=20) | ||
| Congenital malformations | 5 | 1 | Atresia jejuni (3), schistosomus reflexus (1), complex (cleft palate, VSD, contracted tendons) (1), very low birthweight (21kg) and hypotrichosis following hydrops allantois (1). |
| Intrauterine infections | 8 | 5 | Bacillus licheniformis (3), Aspergillus fumigatus (1), Aspergillus niger (1), Aeromonas hydrophila (2), Escherichia coli (1), Leptospira hardjo (1), non-specific gross or histopathology (4). |
| Parturition-related (anoxia sustained during stage 2 calving) | 14 | 6 | Includes cases with specific evidence of bradytocia (9), trauma (3), malpresentation (3), twin birth (2), arthrogryposis (1) and non-specific evidence of fetal response to infection considered non-life-threatening (3). These are not mutually exclusive. |
| Postpartum infections | 0 | 6 | All due to E coli; all calves more than 24 hours of age at time of death had complete failure of passive transfer (5). |
| Diagnosis not reached | 5 | 2 | Time of death was recorded as prepartum (4) or intrapartum (3). |
| Other | 2 | 0 | Colostrum administered into the lungs (1), abomasal rupture of unknown cause (1). |
VSD, ventricular septal defect.
Congenital malformations and parturition-related deaths were more commonly recorded in herds that submitted all losses than in herds that submitted on an ad hoc basis. Postpartum infection was recorded in six cases (30 per cent) from ‘ad-hoc’ herds, but from none of the herds that submitted all losses (table 4).
Discussion
The incidence of perinatal mortality in this study was 5.1 per cent, with stillbirth rate and neonatal mortality incidence of 3.9 per cent and 1.2 per cent, respectively. Variation in definitions used makes direct comparison with other studies on beef herds difficult,15 although the rate is similar to UK regional benchmarking estimates (calves born dead: 3–4 per cent) and higher than rates reported from Canada (death up to 1-hour-old: 2.6 per cent from 1689 calves born in 203 herds) and the USA (death up to 12-hour-old: 2.5 per cent from 3666 calves born in 10 Colorado herds).8 9 16 23 In dairy herds, the majority of studies report a perinatal mortality incidence of 5–8 per cent, with some as high as 20 per cent.14 24 This wide variation in mortality incidence between herds is similar to that reported from 48 commercial beef farmers in southern England10 and has also been reported here. It is also interesting to note that only one herd in this study achieved the commonly cited 2 per cent target.7
Sex of the calf, twinning and the level of assistance required for calving were significantly associated with perinatal loss. There are no comparable risk factor studies from the UK beef industry, although all of these factors have been reported previously to be significant from UK dairy herds or non-UK beef herds.11 13 23 25 Body condition score may be an important predictor of perinatal loss of calves in beef herds, but given the design of this study the effect of this factor could not be distinguished from other, possibly unobserved, differences at the herd level.
The authors recorded a definitive diagnosis in 85 per cent of the calves investigated. The diagnostic rate is similar to one previous study on beef calves where histopathology was performed on all cases as a routine,16 but is higher than some other studies based on dairy calves where it has been as low as 50 per cent.24 While there is no internationally accepted method for reaching or categorising diagnoses in bovine perinatal mortality, and doing so is highly complex,26 the authors believe the methods reported here are robust and maximised the probability of an accurate diagnosis.
Parturition-related death was the most common diagnosis recorded. This is broadly consistent with findings from studies on beef and dairy calves internationally. If anoxia and difficult calving categories are combined, they account for 40–71 per cent of the diagnoses reached in a review by Mee in 2011.14 This high incidence of parturition-related death is an area of concern for both animal welfare4 and production efficiency. It is also an area that has the potential to be improved through farmer training in breeding management, improved nutritional control and assistance at parturition. Nine (45 per cent) of the calves considered to have parturition-related deaths were more than 50 kg in weight, compared with just six (18 per cent) of all other cases submitted. Intrauterine infections were the next most common cause, with five of the 12 cases being caused by Bacillus licheniformis and fungal infections. These agents are also the most commonly recorded causes of abortion in Scottish beef herds.27 As these agents are commonly found in the animals’ feed, water or environment when conditions are sub-optimal, reduction in calf losses may be possible by focusing on improving the quality of feed and water offered to pregnant beef cattle.
The authors compared the diagnostic outcomes from farmers that submitted all calves for investigation with those from farmers that self-selected which calves to submit. There is an inherent bias when a farmer self-selects calves for investigation, as the decision to investigate will be influenced by multiple motivators (as is currently the case in the UK’s national surveillance system). The observation that congenital malformations and parturition-related deaths were less common from farmers that self-selected which calves to submit is of interest. It is reasonable to presume that, for both these categories, the farmer might feel they already know the cause of death based on their own observations and may therefore be less likely to invest time and money in an investigation. Practitioners should note that investigating mortality in calves that a farmer self-selects (especially if few in number) may not be truly representative of the on-farm picture.
The major limitations of this study were the limited herd sample size and the non-random selection of participants, and the findings are therefore not representative of the wider UK industry. Repeating the study in different areas of the UK and with a greater number of randomly selected participant herds would yield more representative results; however, compliance with data collection can be problematic when study participants are selected randomly.
In conclusion, this study reliably established the incidence and causes of perinatal death in a sample of herds in Orkney and is the first study of its kind in UK beef herds. Although the findings should not be considered representative of the UK beef industry, the study makes a significant contribution to the understanding of perinatal mortality. The large variation in herd perinatal mortality incidence and the only one herd meeting the suggested 2 per cent target rate suggest that some herds in particular could make significant improvements. Some of the significant risk factors (the level of assistance required and body condition score) and most common causes of death identified (parturition-related death and intrauterine infections) have the potential to be mitigated and prevented through improved herd management.
Acknowledgments
The authors would like to thank the Northvet Veterinary Group for allowing use of their facilities and their farm clients to participate in this study.
Footnotes
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Ethics approval: Ethical approval for clinical research involving animal subjects, materials or data was granted by the University of Glasgow, School of Veterinary Medicine Ethics and Welfare Committee.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data are available upon reasonable request from TG (timothy.geraghty@sac.co.uk).
References
- 1. Home | AHDB [Internet]. Available: https://ahdb.org.uk/ [cited 6 Aug 2019].
- 2. Quality Meat Scotland [Internet]. Available: https://www.qmscotland.co.uk/ [cited 6 Aug 2019].
- 3. Scottish Beef Association [Internet]. Available: https://www.scottishbeefassociation.co.uk/ [cited 6 Aug 2019].
- 4. Mellor DJ, Stafford KJ. Animal welfare implications of neonatal mortality and morbidity in farm animals. Vet J 2004;168:118–33. 10.1016/j.tvjl.2003.08.004 [DOI] [PubMed] [Google Scholar]
- 5. The Cattle Identification Regulations 1998 [Internet]. Queen’s Printer of Acts of Parliament. Available: https://www.legislation.gov.uk/uksi/1998/871/regulation/3/made [cited 30 Apr 2019].
- 6. Ortiz-Pelaez A, Pritchard DG, Pfeiffer DU, et al. Calf mortality as a welfare indicator on British cattle farms. Vet J 2008;176:177–81. 10.1016/j.tvjl.2007.02.006 [DOI] [PubMed] [Google Scholar]
- 7. Caldow G, Lowman B, Riddell I. Veterinary intervention in the reproductive management of beef cow herds. In Pract 2005;27:406–11. 10.1136/inpract.27.8.406 [DOI] [Google Scholar]
- 8. AHDB Beef and Lamb Farmbench Costs of Production 2017/18 [Internet]. Available: http://beefandlamb.ahdb.org.uk/wp-content/uploads/2019/05/spring-calving-2018.pdf [cited 6 Aug 2019].
- 9. QMS Cattle and Sheep Enterprise Profitability in Scotland [Internet], 2017. Available: https://www.qmscotland.co.uk/sites/default/files/cattle_and_sheep_enterprise_profitability_in_scotland_2017.pdf [cited 23 Nov 2018].
- 10. Barber B. Benchmarking beef suckler herds in practice: the initial steps. Livestock 2018;23:124–8. 10.12968/live.2018.23.3.124 [DOI] [Google Scholar]
- 11. McGuirk BJ, Going I, Gilmour AR. The genetic evaluation of UK Holstein Friesian sires for calving ease and related traits. Anim. Sci. 1999;68:413–22. 10.1017/S1357729800050414 [DOI] [Google Scholar]
- 12. Brickell JS, McGowan MM, Pfeiffer DU, et al. Mortality in Holstein-Friesian calves and replacement heifers, in relation to body weight and IGF-I concentration, on 19 farms in England. Animal 2009;3:1175–82. 10.1017/S175173110900456X [DOI] [PubMed] [Google Scholar]
- 13. Bleul U. Risk factors and rates of perinatal and postnatal mortality in cattle in Switzerland. Livest Sci 2011;135:257–64. 10.1016/j.livsci.2010.07.022 [DOI] [Google Scholar]
- 14. Mee J. Bovine Neonatal Survival – Is Improvement Possible ? Epidemiology of Bovine Perinatal Mortality Incidence of Perinatal Mortality [Internet]. Vol. 23, WCDS Advances in Dairy Technology, 2011. Available: http://www.wcds.ca/proc/2012/Manuscripts/Mee-2.pdf [cited 22 Nov 2018].
- 15. Cuttance E, Laven R. Estimation of perinatal mortality in dairy calves: a review. Vol. 252 Veterinary Journal. Bailliere Tindall Ltd, 2019: 105367. [DOI] [PubMed] [Google Scholar]
- 16. Waldner CL, Kennedy RI, Rosengren LB, et al. Gross postmortem and histologic examination findings from abortion losses and calf mortalities in Western Canadian beef herds. Can Vet J 2010;51:1227–38. [PMC free article] [PubMed] [Google Scholar]
- 17. EBLEX - Better Returns Programme. Better returns from Body Condition Scoring (BCS) beef cows and heifers [Internet], 2016. Available: https://beefandlamb.ahdb.org.uk/wp-content/uploads/2013/06/Better-returns-from-body-condition-scoring.pdf [cited 23 Nov 2018].
- 18. Lombard JE, Garry FB, Tomlinson SM, et al. Impacts of dystocia on health and survival of dairy calves. J Dairy Sci 2007;90:1751–60. 10.3168/jds.2006-295 [DOI] [PubMed] [Google Scholar]
- 19. R Core Team A language and environment for statistical computing [Internet]. R Foundation for Statistical Computing, Vienna, Austria, 2018. Available: https://www.r-project.org/
- 20. Mee JF. Investigating stillbirths in cattle – What should a practitioner do? | BCVA. Cattle Pract [Internet]. 2015;23(1):114–21. Available: https://www.bcva.eu/cattle-practice/documents/3670 [cited 22 Nov 2018].
- 21. Fox J, Weisberg S. An R Companion to Applied Regression - John Fox, Sanford Weisberg - 375 Google Books [Internet]. Third. Thousand Oaks CA Sage; 2018, 2018. Available: https://socialsciences.mcmaster.ca/jfox/Books/Companion/ [cited 13 Dec 2019].
- 22. Kassambara A. Machine Learning Essentials: Practical Guide in R - Alboukadel Kassambara - Google Books [Internet]. sthda, 2017. Available: http://www.sthda.com [cited 13 Dec 2019].
- 23. Wittum TE, Salman MD, King ME, et al. Individual animal and maternal risk factors for morbidity and mortality of neonatal beef calves in Colorado, USA. Prev Vet Med 1994;19:1–13. 10.1016/0167-5877(94)90010-8 [DOI] [Google Scholar]
- 24. Mee JF. Why do so many calves die on modern dairy farms and what can we do about calf welfare in the future? Animals 2013;3:1036–57. 10.3390/ani3041036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cuttance E, Laven R. Perinatal mortality risk factors in dairy calves. Vol. 253 Veterinary Journal. Bailliere Tindall Ltd, 2019: 105394. [DOI] [PubMed] [Google Scholar]
- 26. Mee JF, Sanchez-Miguel C, Doherty M. An international Delphi study of the causes of death and the criteria used to assign cause of death in bovine perinatal mortality. Reprod Domest Anim 2013;48:651–9. 10.1111/rda.12139 [DOI] [PubMed] [Google Scholar]
- 27. Animal and Plant Health Agency Cattle Dashboard - Surveillance Intelligence Unit | Tableau Public [Internet], 2018. Available: https://public.tableau.com/profile/siu.apha#!/vizhome/CattleDashboard/CattleDashboard [cited 22 Jan 2019].


