Abstract
Objective
TGF-β2 (TGF-β, transforming growth factor beta), the less-investigated sibling of TGF-β1, is deregulated in rodent and human liver diseases. Former data from bile duct ligated and MDR2 knockout (KO) mouse models for human cholestatic liver disease suggested an involvement of TGF-β2 in biliary-derived liver diseases.
Design
As we also found upregulated TGFB2 in liver tissue of patients with primary sclerosing cholangitis (PSC) and primary biliary cholangitis (PBC), we now fathomed the positive prospects of targeting TGF-β2 in early stage biliary liver disease using the MDR2-KO mice. Specifically, the influence of TgfB2 silencing on the fibrotic and inflammatory niche was analysed on molecular, cellular and tissue levels.
Results
TgfB2-induced expression of fibrotic genes in cholangiocytes and hepatic stellate cellswas detected. TgfB2 expression in MDR2-KO mice was blunted using TgfB2-directed antisense oligonucleotides (AON). Upon AON treatment, reduced collagen deposition, hydroxyproline content and αSMA expression as well as induced PparG expression reflected a significant reduction of fibrogenesis without adverse effects on healthy livers. Expression analyses of fibrotic and inflammatory genes revealed AON-specific regulatory effects on Ccl3, Ccl4, Ccl5, Mki67 and Notch3 expression. Further, AON treatment of MDR2-KO mice increased tissue infiltration by F4/80-positive cells including eosinophils, whereas the number of CD45-positive inflammatory cells decreased. In line, TGFB2 and CD45 expression correlated positively in PSC/PBC patients and localised in similar areas of the diseased liver tissue.
Conclusions
Taken together, our data suggest a new mechanistic explanation for amelioration of fibrogenesis by TGF-β2 silencing and provide a direct rationale for TGF-β2-directed drug development.
Keywords: cholestasis, TGF-beta, fibrosis, primary biliary cirrhosis, primary sclerosing cholangitis
Significance of this study.
What is already known on this subject?
Chronic liver diseases (CLDs) are a major global health issue developing from fibrosis, via cirrhosis to hepatocellular carcinoma, the second deadliest cancer worldwide.
Fibrotic rearrangements are reversible and thus offer a well-druggable disease stage.
We recently described isoform TGF-β2 (TGF-β, transforming growth factor beta) to be deregulated in human and rodent CLDs with a probably special impact in biliary-derived CLDs.
What are the new findings?
We here describe for the first time the antifibrotic and immune-regulative prospects of TgfB2 silencing in multidrug resistance gene 2 knockout (MDR2-KO) mice.
TgfB2-specific antisense oligonucleotides (AONs) target liver sinusoidal endothelial cells as well as activated fibroblasts and macrophages.
TgfB2 silencing by AONs specifically reduces collagen deposition and αSMA (SMA, smooth muscle actin) expression, but induces antifibrotic PparG (PPAR, peroxisome proliferator-activated receptor) expression.
TgfB2-specific AONs reduce CD45-positive immune cell infiltration in MDR2-KO mouse livers.
In patients suffering from primary sclerosing cholangitis (PSC) and primary biliary cholangitis (PBC), rare non-curable CLDs, TGF-β2 levels are elevated and correlate with CD45-positive immune cell infiltration.
Box 2. Significance of this study.
How might it impact on clinical practice in the foreseeable future?
Our data directly suggest the use of TGF-β2 silencing in PSC and PBC patients to ameliorate or reverse fibrogenesis.
Given the general safety of TGFB2/TgfB2-specific AONs for patients (currently phase 1 clinical trial for glaucoma), a clinical trial for fibrotic PSC and PBC patients seems feasible in the near future based on our data.
Introduction
Primary sclerosing cholangitis (PSC) and primary biliary cholangitis (PBC) are rare chronic autoimmune liver diseases, which are initiated by inflammatory attacks towards the biliary system.1 Parenchymal damage, unresolved inflammation, compensatory hepatocyte proliferation and matrix deposition by hepatic stellate cells (HSCs) and portal myofibroblasts lead to fibrosis and subsequent cirrhosis.2 Of note, 15%–25% of PBC patients progress to liver failure,3 4 while PSC patients exhibit a 10%–15% lifetime risk to develop cholangiocarcinoma (CCA).5 6 Besides liver transplantation, curative treatment is not possible so far for both diseases. Hence, lifelong treatment with ursodeoxycholic acid (UDCA) is the gold standard to achieve normalisation of liver serum parameters such as alkaline phosphatase and gamma glutamyltransferase and to minimise disease symptoms such as pruritus and hyperbilirubinaemia.7 Although potential to slow down progression to cirrhosis is discussed, fibrotic rearrangements are not improved from such treatment. Together, this highlights the need for the development of new treatment options in the management of PSC and PBC.
In mammals, three different isoforms of TGF-β are described (TGF-β1, TGF-β2 and TGF-β3; transforming growth factor beta) to regulate apoptosis, proliferation, differentiation, migration and invasion processes utilising overlapping but not redundant mechanisms. All three isoforms are expressed in the liver, but their expression is differentially distributed among liver cell types.8 TGF-β1 has been extensively studied in healthy and chronically injured liver and is proposed as treatment target in multiple clinical studies.9
TGF-β2 expression in different liver cell types was described nearly 30 years ago,10 11 and was also associated with developmental defects and fibrotic diseases in mice.12–14 In human chronic liver diseases (CLDs), few details are known about its functions. TGF-β2 was shown to correlate with bad prognosis in intrahepatic CCAs and hepatocellular carcinoma.15–18 Mechanistically, canonical Smad signalling as well as crosstalk with Yap, Hippo, Wnt and β-catenin signalling have been demonstrated in the liver and other organs.19–24 Recently, we have shown that TGF-β2 expression is significantly dysregulated during progression of CLDs.25 While expression dynamics were mostly similar, a special impact of TGF-β2 in biliary-derived CLDs was obvious. Here, the regulation of TGF-β1 and TGF-β2 differed pronouncedly, suggesting TGF-β2 as a promising target to specifically tackle biliary CLDs, such as PSC or PBC. The multidrug resistance gene 2 knockout (MDR2-KO) mouse model reliably mimicks features of human cholestatic liver disease. Knockout of the multidrug resistance gene 2 (Mdr2) in mice leads to the absence of biliary phospholipids and the accumulation of toxic bile acids resulting in cholestatic liver injury. This injury is accompanied by chronic portal inflammation as well as intrahepatic and extrahepatic fibrosis of bile ducts, finally leading to biliary cirrhosis and cancer.26
Despite many unsolved questions, such as specificity and translation into the clinics, interest in antisense oligonucleotides (AONs) as small compounds with therapeutic potential is still eminent. Major advantages are their easy design, low production costs and the possibility to target nearly any cellular process or ‘gene’ wanted.
In this report, we assessed TGFB2 expression in PSC and PBC patients and determined the potential of TgfB2 silencing to attenuate or inhibit fibrogenesis in cholestatic MDR2-KO mice using specific AONs. Taken together, our data indicate that silencing TgfB2 in non-parenchymal cells (NPC) attenuates fibrogenic and inflammatory responses in MDR2-KO mouse livers and thus provides therapeutic potential against inflamed cholestatic liver fibrosis with increased TGF-β2 levels.
Materials and methods
Human patient samples
The investigation was conducted in accordance with ethical standards, with the Declaration of Helsinki and according to national and international guidelines.
AON treatment of MDR2-KO mice (cholestatic fibrosis model)
MDR2-KO mice were kindly provided by F. Lammert (Homburg),27 and were maintained in a specific pathogen-free environment. The experiments were performed with gender-matched mice at the age of 14 weeks. Of note, 50% male and 50% female mice were used for each experimental setup. Genotyping was done as described elsewhere.28 In the first week, all animals received either 15 mg/kg29 body weight scramble control oligo C3_0047 (Co) or ISTH0047 (AON) each day for 5 days (oligo push). The subsequent treatment was applied once per week for 3 further weeks. Six groups of animals were defined: (1) Balb/c untreated for 4 weeks (n=6), (2) Balb/c control oligo (n=10, received 15 mg/kg body weight of Co); (3) Balb/c AON (n=10, received 15 mg/kg body weight of AON); (4) MDR2-KO untreated (n=10, untreated for 4 weeks); (5) MDR2-KO control oligo (n=10, received 15 mg/kg body weight of Co) and (6) MDR2-KO AON (n=9, received 15 mg/kg body weight of AON). Mice were sacrificed 72 hours after the last treatment and specimens sampled for further analysis.
Statistical analysis
Error bars indicate SD in cell culture experiments. For evaluation of mouse and patient data, error bars indicate SEM. Two-tailed Student-t tests or one-way analysis of variance were used to calculate the p-values. Differences were considered to be significant if the calculated p-value was *p≤0.05, **p≤0.01, ***p≤0.001, ****p≤0.0001; p-values were not significant if not indicated or assigned ns. Significance of differences between Balb/c and MDR2-KO was only calculated for untreated animals. Additionally, Pearson correlation or Fisher’s exact test for clinicopathological data were performed.
Additional methodology is provided as online supplemental information.
gutjnl-2019-319091supp001.pdf (232.3KB, pdf)
Results
TGFB2 is upregulated in the liver of PSC and PBC patients
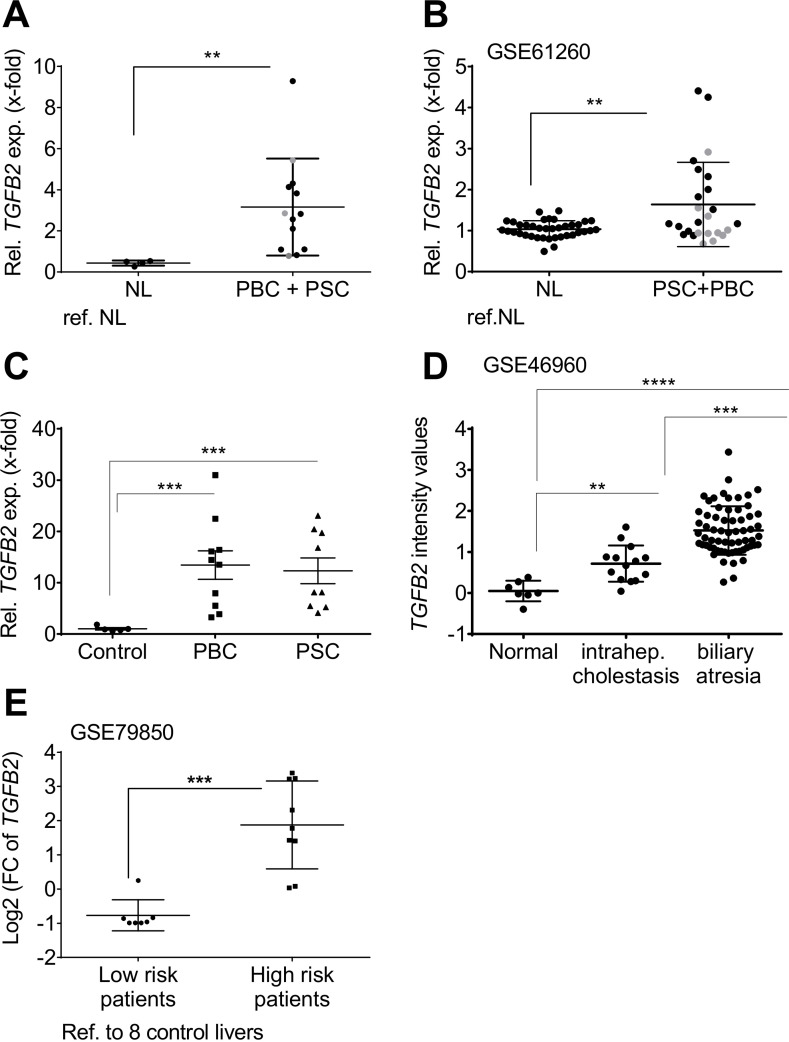
TgfB2 is predominantly upregulated in biliary-derived CLDs in mice,25 but less in other etiologies (online supplementary figure 1). We investigated TGFB2 mRNA expression in human liver tissue samples of PSC and PBC patients, and liver diseases of other etiologies. While TGFB2 was not deregulated in four out of five non-alcoholic fatty liver disease (NAFLD) patient cohorts (online supplementary figure 2), we confirmed upregulation of TGFB2 in biliary-derived liver damage in three PSC and PBC patient cohorts (figure 1A, p=0.0013; figure 1B, p=0.007; figure 1C, pPSC=0.0065 and pPBC=0.0084) and one cohort with intrahepatic cholestasis and biliary atresia patients (figure 1D, p=1,79e-07) compared with normal liver controls; online supplementary figure 3 shows a separate graphical presentation of PSC and PBC patients from the Regensburg cohort and GSE61260. Thus, our data point towards significant TGFB2 upregulation, especially in PSC.
Figure 1.
Patient resolved expression of TGFB2 in tissue of cholestatic liver disease patients compared with healthy individuals. Expression changes of TGFB2 in liver tissue of individual patients represented as single dots in (A) the PSC/PBC cohort from Regensburg, (B) the collective GSE61260, (C) the PBC and PSC cohort from Poland, (D) the cohort GSE46960 and (E) in low-risk and high-risk patients described in GSE79850 as determined in comparison to non-diseased control livers. In (E), low-risk patients responded fully to UDCA treatment. High risk was assigned to the need of liver transplantation in the course of disease. Grey dots: PBC; black dots: PSC; **p≤0.01; ***p≤0.001; ****p≤0.0001; NL, normal liver; PBC, primary biliary cholangitis; PSC, primary sclerosing cholangitis; TGFB, transforming growth factor beta; UDCA, ursodeoxycholic acid.
gutjnl-2019-319091supp002.pdf (526.5KB, pdf)
gutjnl-2019-319091supp003.pdf (125KB, pdf)
gutjnl-2019-319091supp004.pdf (65.5KB, pdf)
Interestingly, TGFB2 expression displayed a significant correlation with the inflammatory grade in the patient cohort from Regensburg (online supplementary table 1, p=0.045). From the GSE61260 cohort, no such clinical data were available to confirm this correlation. TGFB2 was also remarkably upregulated in a cohort of nine ‘high risk’ PBC patients who eventually needed liver transplantation as compared with seven ‘low risk’ patients, who responded to UDCA treatment (GSE79850) (figure 1E, phigh vs co=0.0046, phigh vs low=0.0026, online supplementary tables 2 and 3). These data suggest a predictive ability of TGFB2 expression regarding patients’ treatment response. In GSE79850, high TGFB2 was also significantly associated with higher Scheuer grades (III and IV; p=0.0047), which might explain the worse treatment response of these patients compared with the patients of milder disease stages (online supplementary table 4). High TGFB2 expression was also associated with the diagnosis of ductopenia (p=0.0256), but not with the inflammation grade in this cohort.
As TGFB2 was also upregulated in liver tissue of alcoholic hepatitis patients (online supplementary figure 2), we conclude that TGFB2 plays a predominant, but not exclusive role in biliary liver disease, and may also contribute to other liver diseases, for example, in an inflammatory context.
TgfB2/TGFB2 is localied in areas of ductular reaction and fibrotic rearrangement
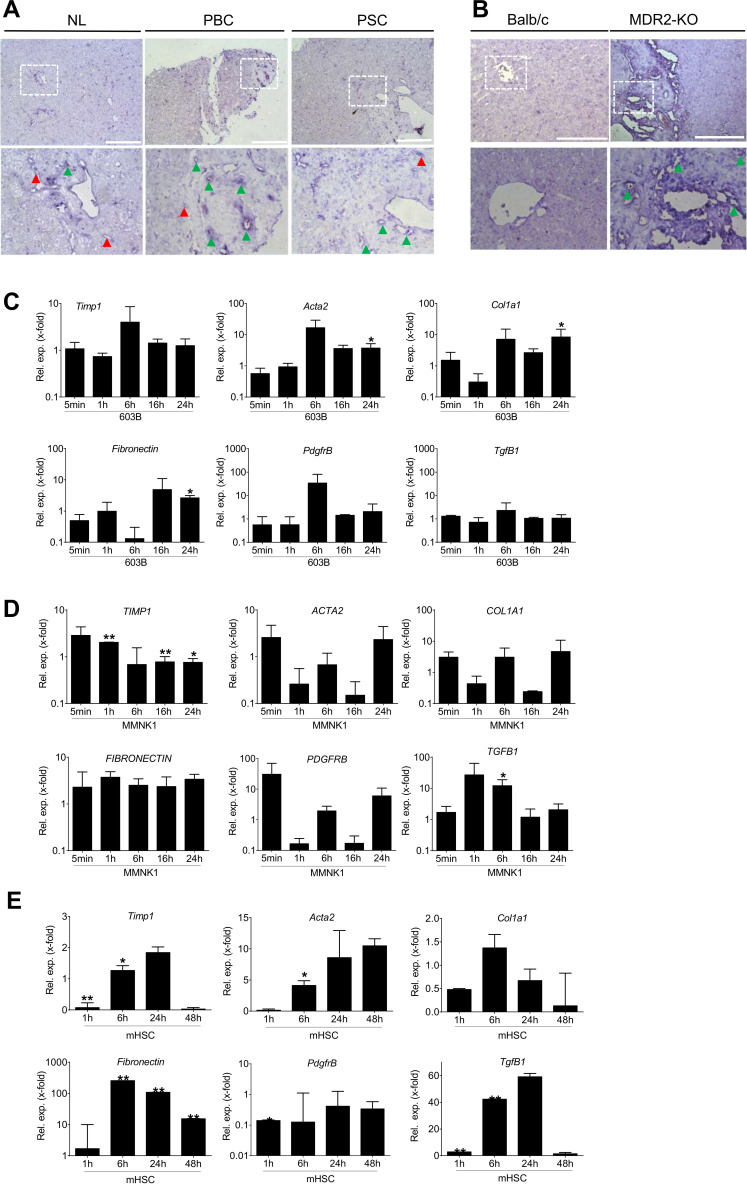
In situ hybridisation of TgfB2/TGFB2 revealed localisation near portal tracts and in areas of fibrogenic rearrangements in PSC and PBC patients, as well as in MDR2-KO mice (figure 2A, B). This is in line with former work describing TgfB2/TGFB2 expression in cirrhosis of undefined origin predominantly in biliary epithelial cells.10 In agreement, treatment of murine or human cholangiocyte cell lines 603B and MMNK1 with TGF-β2 results in upregulation of fibrogenic marker genes. In 603B cells, Acta2 (alpha smooth muscle actin gene), Col1A1 (collagen type I alpha 1), fibronectin and PdgfrB (platelet-derived growth factor receptor beta) were induced as compared with untreated controls (figure 2C). In MMNK1 cells, TGFB1 was significantly induced after 6 hours treatment, while TIMP1 (tissue inhibitor of metalloproteinases) was induced after 1 hour, but reduced thereafter (figure 2D). In MMNK1 cells, some of the targets show a very fast response already after 5 min, suggesting an immediate early and direct response probably to a Smad-dependent signal. ACTA2, COL1A1 and PDGFRB are as well induced transiently, but not significantly.
Figure 2.
Localisation of TgfB2/TGFB2 in human and murine liver tissue and TGF-β2 treatment of primary mouse HSCs as well as mouse and human cholangiocyte cell lines. In situ hybridisation was performed to assess TGFB2/TgfB2 localisation in (A) healthy liver tissue and PSC/PBC patients as well as (B) wild-type and MDR2-KO mice. In PSC and PBC patients as well as MDR2-KO mice, TGFB2 was localised in portal tracts (green arrow heads) and fibrotic rearranged tissue areas. Additional staining was detected in sinusoids (red arrow heads) of the patients’ tissue. Lower panels represent enlarged images of boxed areas in the upper panel. Scale bars indicate 200 µm. Expression of fibrotic marker genes was induced in (C) murine 603B, (D) human MMNK1 cells and (E) primary mouse hepatic stellate cells on treatment with TGF-β2. Fold expression is given as referred to the correlating untreated control of each time point. Error bars indicate SD. *p≤0.05;**p≤0.01. Acta2, actin alpha 2 or alpha smooth muscle actin; Col1a1, collagen type I alpha 1; HSC, hepatic stellate cells; MDR2-KO, multidrug resistance gene 2 knockout; NL, normal liver; PBC, primary biliary cirrhosis; PdgfrB, platelet-derived growth factor receptor beta; PSC, primary sclerosing cholangitis; TGFB, transforming growth factor beta; TIMP, tissue inhibitor of metalloproteinase.
As a secretory cytokine, TGF-β2 also acts on other liver cell types. Accordingly, we demonstrate induction of fibrogenic genes in HSCs (figure 2E). Together, these data demonstrate profibrogenic activity of TGF-β2 in the liver, including the biliary compartment.
TgfB2-specific AONs target liver tissue without adverse effects on liver parameters
Driven by these findings and the lack of sufficient treatment options for PSC and PBC patients, we aimed to analyse the effect of TgfB2 silencing on CLD parameters in MDR2-KO mice on treatment with TgfB2-specific AONs. These were engineered as 17-mer full phosphorothioate LNA-modified antisense oligodeoxynucleotides ‘4+4’ gapmers (primary sequence in online supplementary figure 4). As published by Huber-Ruano et al,30 after subcutaneous (s.c.) injection, the AONs rapidly translocate from the blood circulation predominantly to livers (37.52% of total signal) and kidneys (38.06% of total signal) of wild-type mice where they are stably present for 2 weeks.
gutjnl-2019-319091supp005.pdf (937.2KB, pdf)
TgfB2-specific AONs downregulate TgfB2 expression in healthy and diseased liver
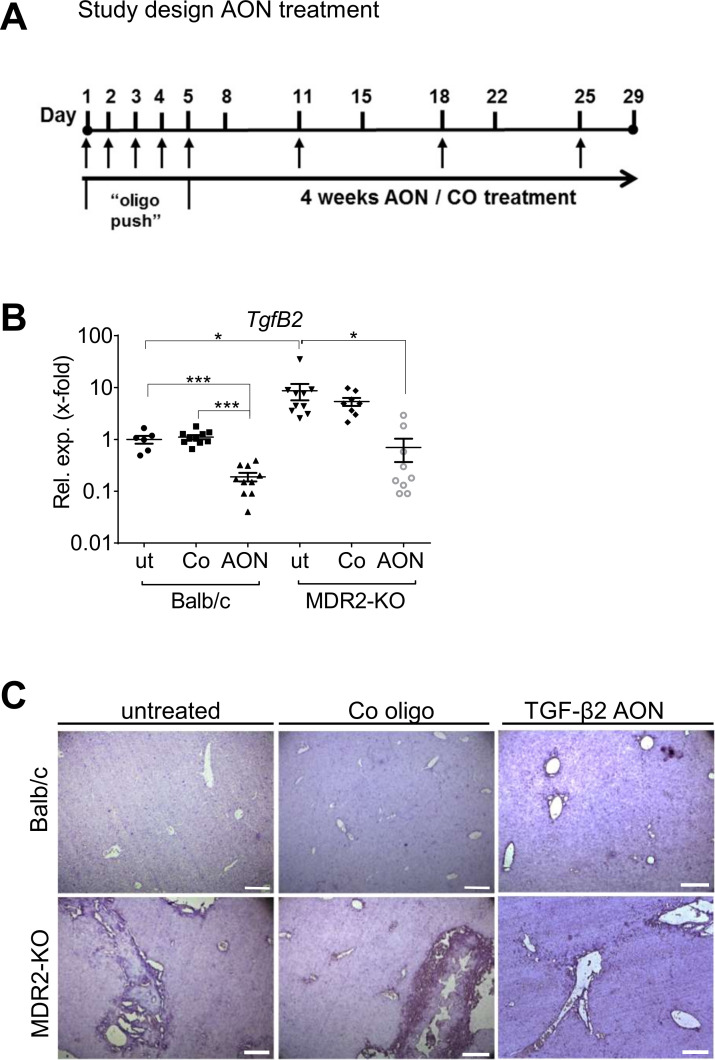
Based on drug delivery and safety testing data, we s.c. applied the AON or Co at a dose of 15 mg/kg body weight,29 30 to 14 weeks old MDR2-KO mice according to the protocol depicted in figure 3A. In untreated MDR2-KO mice, TgfB2 expression was markedly elevated as compared with wild-type animals (~8.7 fold). On AON treatment, significant downregulation of TgfB2 expression was observed in Balb/c wild-type control animals (5.2 fold) as well as in MDR2-KO animals (12.5-fold) (figure 3B) with no negative impact on liver parameters or body weight in both strains (online supplementary figure 5A, B). Remarkably, AON treatment reduced TgfB2 levels in MDR2-KO mice to normal as seen in healthy Balb/c. Upon AON treatment, reduced staining of TgfB2 in MDR2-KO mouse tissue compared with untreated controls was also visualised by in situ hybridisation (figure 3C).
Figure 3.
Study design and AON-mediated TgfB2 downregulation. (A) Schedule of animal treatment with control or TgfB2-specific AONs. TgfB2 expression was significantly downregulated in Balb/c and MDR2-KO animals treated with AONs as (B) analysed by qPCR and (C) visualised by in situ hybridisation. *p≤0.05, ***p≤0.001. Scale bars indicate 200 µm. AON, antisense oligonucleotides; MDR2-KO, multidrug resistance gene 2 knockout; TGFB, transforming growth factor beta.
gutjnl-2019-319091supp006.pdf (599.1KB, pdf)
TgfB2-directed AONs accumulate in NPC
We were interested to identify the target cell type(s) of the AONs in the liver. Immunohistochemical staining of Dig-labelled AONs revealed that they did not localise to hepatocytes or cholangiocytes after s.c. application, but rather accumulated in NPCs located in the space of Disse (online supplementary figure 6A). Using coimmunofluorescence analysis of labelled AONs and marker proteins of different liver cell types, the oligos colocalised with αSMA (SMA, smooth muscle actin), CD31, elastin and S100A4 (FSP1) in MDR2-KO liver tissue, indicating uptake into activated HSCs, liver sinusoidal endothelial cells (LSECs), portal fibroblasts and to a lower extent into macrophages (online supplementary figure 6B, colocalisation indicated by white arrows). Interestingly, in wild-type mice, AONs colocalised with CD31 positive LSECs only for so far unknown reasons. Confirming data from more than 20 years ago, showing TgfB2 mainly in activated biliary epithelial cells, but also in Kupffer cells and other liver cell types in rat and human tissue,8 10 11 we demonstrate by cell isolation TgfB2 expression in the non-parenchymal liver cell fractions HSCs, LSECs and Kupffer cells of Balb/c and MDR2-KO mice (online supplementary figure 6C). We conclude that AON-mediated TgfB2-downregulation in the liver occurs predominantly in NPCs and not in cholangiocytes.
gutjnl-2019-319091supp007.pdf (1.7MB, pdf)
TgfB2-specific AONs decrease ductular reaction and biliary damage markers
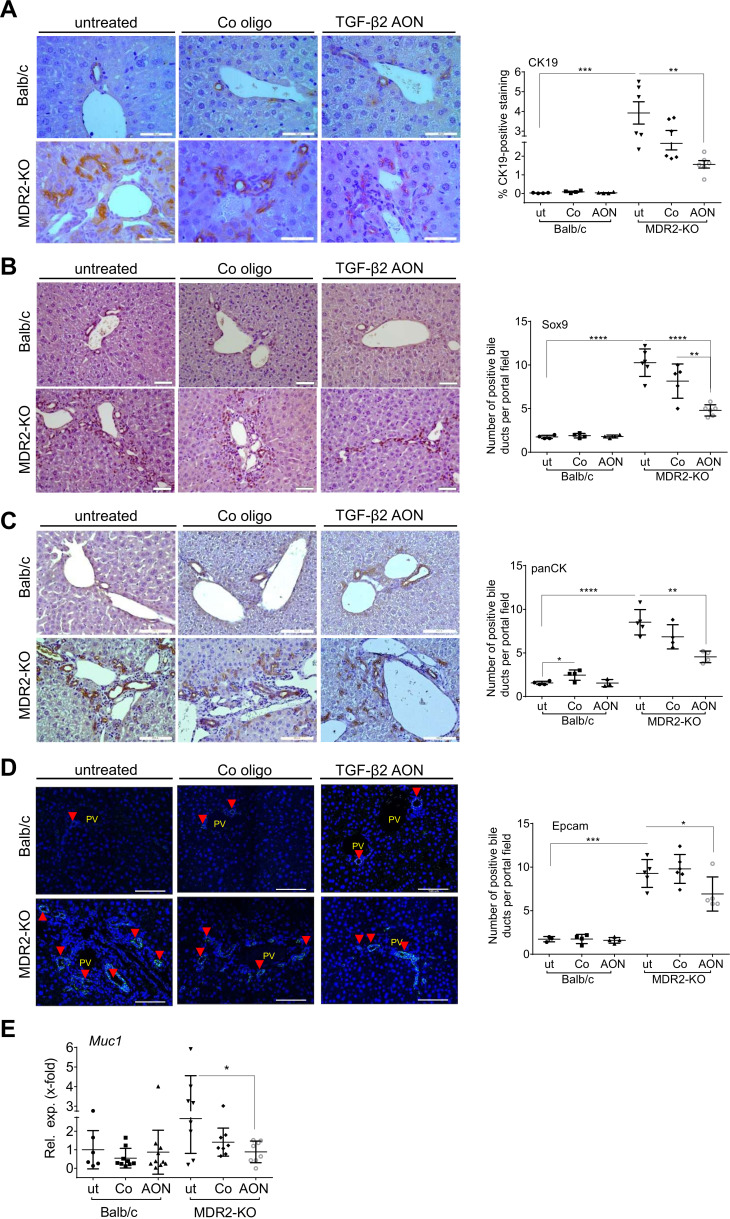
MDR2-KO mice exhibit periductular onion-skin fibrosis and pronounced ductular reactions (DR).31 AON treatment improved biliary damage and reduced DR as demonstrated by significantly downregulated Muc1 (Mucin 1) mRNA expression levels and reduced CK19 (cytokeratin 19), Sox9 (SRY-box 9), panCK and Epcam (epithelial cell adhesion molecule) staining of the liver tissue (figure 4A–E).
Figure 4.
TgfB2-silencing by AONs reduced biliary damage and ductular reactions (DR). Immunohistochemical staining of (A) CK19, (B) Sox9, (C) and panCK, as well as (D) immunofluorescence-based detection of Epcam as a markers of DR and (E) qPCR of Muc1 as a marker of biliary damage revealed significant downregulation in AON-treated MDR2-KO mice. *p≤0.05, **p≤0.01, ***p≤0.001, ****p≤0.0001; scale bars indicate 50 µm for CK19, and 100 µm for Sox9, panCK and Epcam. AON, antisense oligonucleotides; MDR2-KO, multidrug resistance gene 2 knockout; Muc, Mucin; TGFB, transforming growth factor beta.
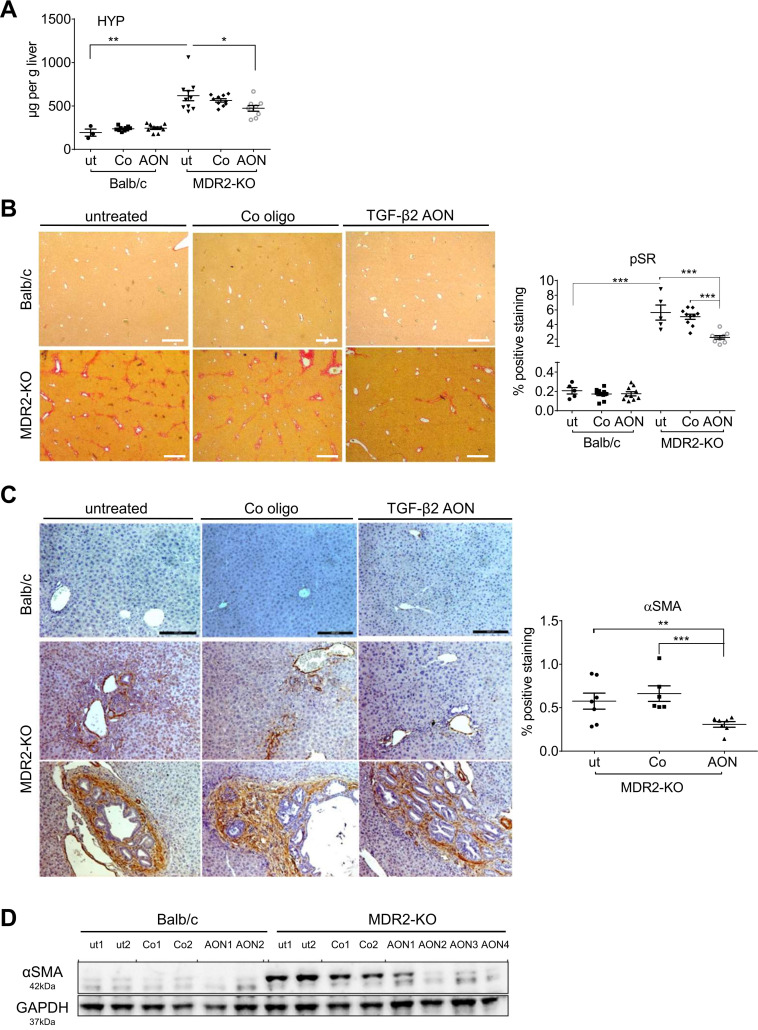
TgfB2-specific AONs decreased hydroxyproline content, collagen deposition and αSMA expression in MDR2-KO mouse livers
In line with data demonstrating that DR correlates with fibrosis in experimental models of liver disease,32 we next analysed the consequences of TGF-β2 downregulation on liver fibrogenesis. In MDR2-KO mice, hydroxyproline content, collagen deposition and αSMA expression were elevated compared with Balb/c controls. AON treatment significantly decreased hydroxyproline content and Sirius Red stained areas of liver tissue (~56%) compared with untreated MDR2-KO mice (figure 5A, B). Further, periductular αSMA staining was significantly reduced (~35%) on AON-treatment in MDR2-KO mice, which was confirmed by immunoblot (figure 5C, D). Hydroxyproline did not change in wild-type Balb/c mice on AON treatment and rather no collagen deposition or αSMA was present.
Figure 5.
Impact of AON-mediated TgfB2 silencing on collagen expression and deposition as well as αSMA expression in MDR2-KO mice. (A) Hydroxyproline (HYP) content in the liver of AON-treated MDR2-KO mice was significantly downregulated compared with untreated and control oligo-treated animals. (B) Sirius red staining (pSR) revealed significant downregulation (~56%) of collagen deposition in the tissue of AON-treated compared with untreated MDR2-KO mice. Scale bars indicate 500 µm. (C) Immunohistochemical staining demonstrated a decrease of αSMA expression in AON-treated animals of 35% compared with untreated animals and 43% compared with animals treated with control oligos. Scale bars indicate 200 µm. (D) Reduction was verified by immunoblot analysis. *p≤0.05, **p≤0.01, ***p≤0.001. AON, antisense oligonucleotides; MDR2-KO, multidrug resistance gene 2 knockout; SMA, smooth muscle actin; TGFB, transforming growth factor beta.
These data suggest for the first time an antifibrotic effect of TgfB2-directed AON treatment in liver.
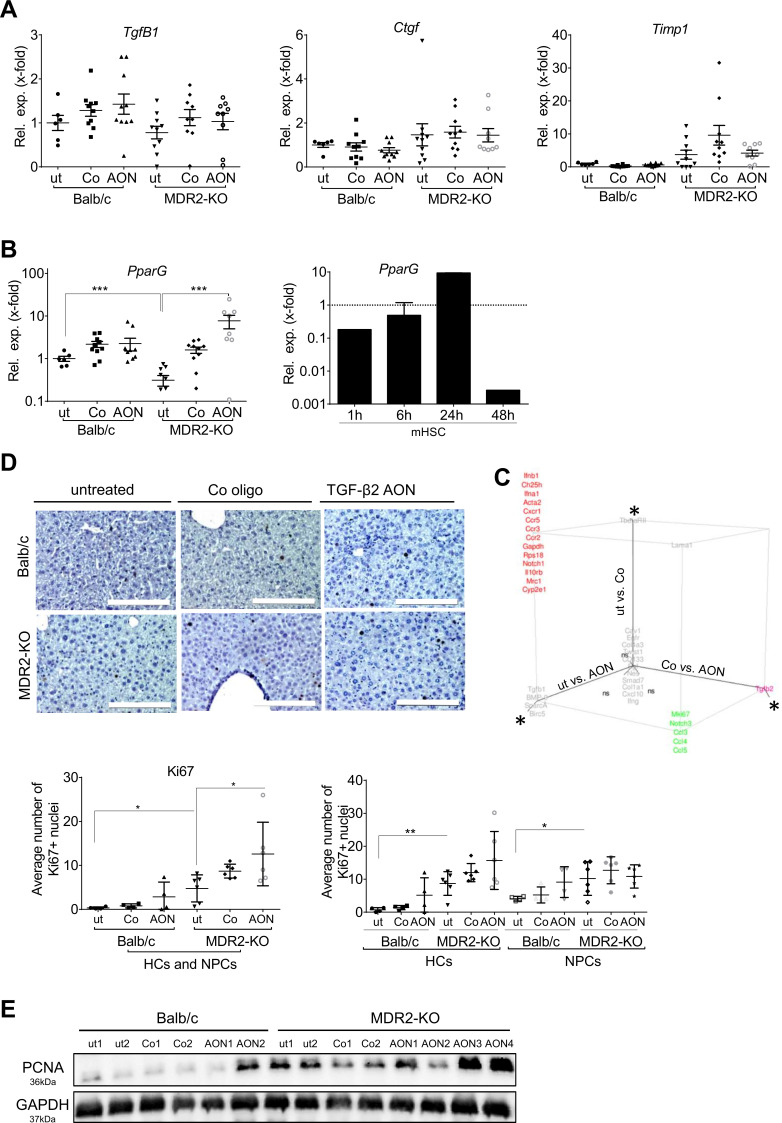
TgfB2-directed AONs elevate PparG and chemokine expression in MDR2-KO mouse livers
We next examined fibrosis marker expression. In contrast to TgfB2, TgfB1 was not induced in MDR2-KO compared with wild-type mice,25 underlining the predominant role of TGF-β2 in this model. AON treatment did not change TgfB1 and Ctgf (connective tissue growth factor) expression (figure 6A). In MDR2-KO animals, Timp1 expression was increased as published before,33 but AON treatment showed no reducing effect (figure 6A). Expression of PparG (inversely associated with fibrosis and inflammation before34 35) was significantly induced by AON-treatment in MDR2-KO mice, supporting an antifibrotic effect of TgfB2 silencing (figure 6B). TGF-β2 treatment reduced PparG expression in mouse JS-1 cells and primary mouse HSCs (online supplementary figure 7, figure 6B) underlining the profibrotic capacity of TGF-β2.
Figure 6.
Regulation of fibrosis marker gene expression by TgfB2 silencing. (A) TgfB1, Ctgf and Timp1 mRNA expression was not considerably changed by TgfB2-directed AON treatment. (B) Expression of antifibrotic and anti-inflammatory PparG was markedly upregulated by AONs in MDR2-KO mice. According to a profibrotic role of TGF-β2, TGF-β2 treatment of primary mHSCs inhibited PparG expression. Error bars represent SD for the mHSC experiment. ***p≤0.001. (C) Fluidigm analysis of fibrotic and inflammation-related marker genes (see online supplementary table 6) revealed specific AON-based effects of TgfB2 downregulation as well as placebo-associated effects of oligo treatment in MDR2-KO mice (also see online supplementary table 5) presented as 3D plot. Log2FC values are shown. Changes were considered significant, if Log2FC was >0.5 or <−0.5 and one-way analysis of variance plus Tukey analysis revealed p-values<0.05, here shown as *; ns=non-significant. (D) Ki67 expression was detected by immunohistochemistry in liver tissue of treated and untreated Balb/c and MDR2-KO mice. Quantification was performed for all cells as well as separated for hepatocytes (HC) and non-parenchymal cells (NPC). *p≤0.05, **p≤0.01. Scale bars indicate 200 µm. (E) Immunoblot analysis of PCNA expression. AON, antisense oligonucleotides; Ctgf, connective tissue growth factor; MDR2-KO, multidrug resistance gene 2 knockout; PCNA, proliferating cell nuclear antigen; Ppar, peroxisome proliferator-activated receptor; TGFB, transforming growth factor beta; Timp, tissue inhibitor of metalloproteinases.
gutjnl-2019-319091supp008.pdf (110KB, pdf)
Since we did not find significant AON effects on genes usually altered in fibrosis despite improvement of collagen deposition and αSMA expression, we attempted to get a broader insight on AON-dependent gene expression changes and performed a fluidigm-based real-time PCR analysis of selected fibrosis and inflammation associated genes (see online supplementary table 6). AON-treatment of MDR2-KO mice upregulated Mki67, Notch3, Ccl3 (C-C motif chemokine ligand), Ccl4 and Ccl5 in liver tissue compared with untreated and oligo-treated MDR2-KO mice (figure 6. online supplementary table 5). Except for Notch3, the AON effect was also present in Balb/c mice, suggesting a context-independent influence of the AON on TGF-β2 target genes.
These data argue for increased (compensatory) proliferation and modulated immune response in AON-treated mice; an AON-dependent increase in number of Ki67-positive nuclei as shown by immunohistochemistry, especially in hepatocytes, as well as PCNA induction as demonstrated by immunoblot confirmed this outcome (figure 6D, E).
We also report an off-target C3-0047 control oligonucleotide effect.36 In MDR2-KO mice, but not Balb/c mice, the control oligo caused downregulation of the chemokine receptors Ccr2 (C-C motif chemokine receptor), Ccr3, Ccr5 and Cxcr1 (C-X-C motif chemokine receptor) similar to the specific oligo. Both also affected the expression of Ifna1 (interferon alpha), Ifnb1, Ch25h, Rps18, Notch1, Il10rb and Mrc1. In this respect, we also mention the control oligo-dependent decrease of Sirius red staining in MDR2-KO mice (figure 5B).
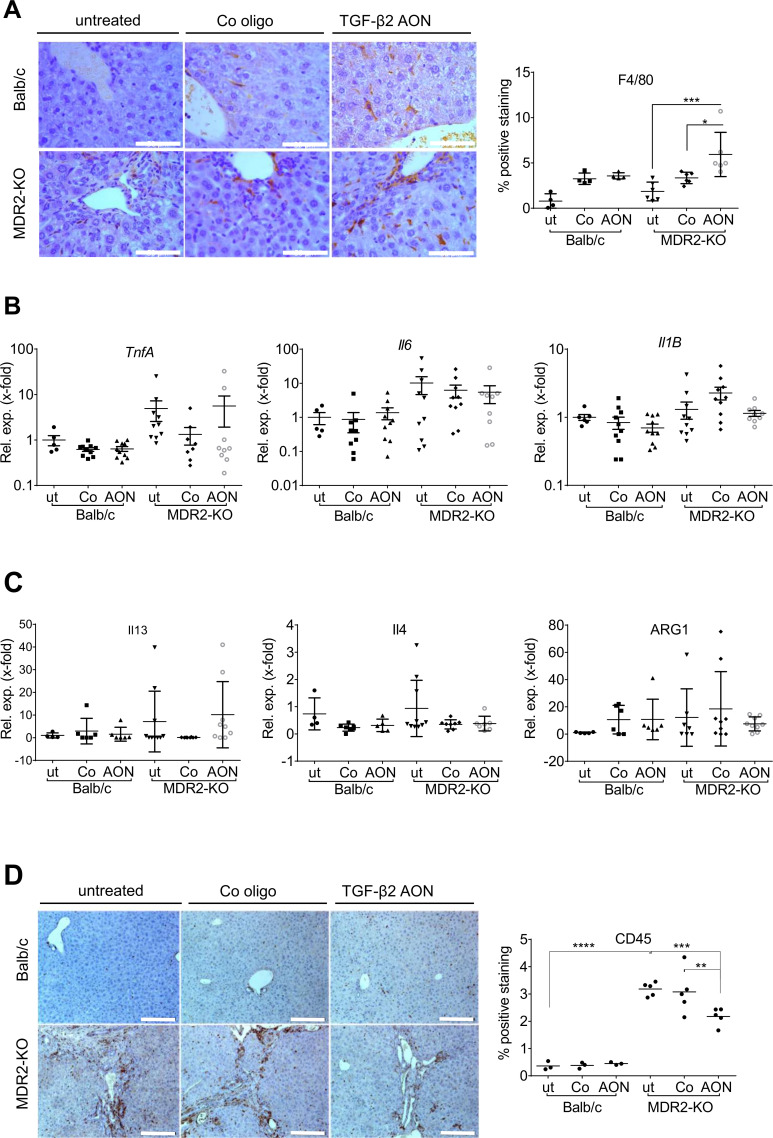
TgfB2-specific AONs modulates the inflammatory niche of MDR2-KO mouse livers
Inflammation contributes to the development of chronic cholestatic liver disease.26 Accordingly, F4/80 positive cell numbers were increased in MDR2-KO mice (figure 7A, online supplementary figure 8A). Interestingly, TgfB2-directed AON-treatment further increased the F4/80-positive cell population in the tissue within the myeloid compartment (CD3/CD19/NKp46/DX5negCD11b+). No significant changes in expression of Il1B (interleukin1 beta), TnfA (tumour necrosis factor alpha) and Il6 (M1 phenotype) or Il13, Il4 and Arg1 (arginase 1) (M2 phenotype) occurred in MDR2-KO mice after AON treatment (figure 7B, C). FACS analysis revealed an AON-dependent enrichment of infiltrating eosinophils (online supplementary figure 8B left column) rather than macrophages in five out of six mice. Eosinophils express F4/80,37 (online supplementary figure 8B middle column), which may explain our findings. The remaining myeloid cells in AON-treated mice mainly comprised Ly6G lymphocyte antigen 6 complex locus G6D)+neutrophils and Ly6C+monocytes, whose numbers did not significantly increase on treatment (online supplementary figure 8B right column).
Figure 7.
Infiltration of immune cells into the liver tissue of AON-treated MDR2-KO mice compared with controls. An anti-inflammatory role of TGF-β2 was suggested. (A) In AON-treated MDR2-KO mice, increased amounts of resident F4/80-positive macrophages were detected. These are probably not polarised to typical M1 or M2 phenotypes as levels of (B) TnfA, Il6 and Il1B and (C) Il13, Il4 and Arg1 were not changed by AON treatment. (D) Immunohistochemical staining of CD45 revealed significant downregulation in AON-treated MDR2-KO mice. *p≤0.05, **p≤0.01, ***p≤0.001. Scale bars indicate (A) 50 µm or (D) 200 µm. AON, antisense oligonucleotides; Arg, arginase; Il, interleucin; MDR2-KO, multidrug resistance gene 2 knockout; TGFB, transforming growth factor beta; Tnfa, tumour necrosis factor-alpha.
gutjnl-2019-319091supp009.pdf (1.7MB, pdf)
We also analysed the inflammatory status of the mice by staining the panleukocyte marker CD45 (figure 7D) and found a significant decrease of infiltrating inflammatory cells in AON-treated MDR2-KO mice. Further analysis of lymphoid cell infiltrates (online supplementary figure 8C) revealed a significant AON-dependent increase in numbers of cytotoxic CD8+ T cells (online supplementary figure 8D). Numbers of other lymphoid immune cells did not change significantly, although in tendency, numbers of CD4+ T cells and Tregs were reduced by AON treatment. These findings in total point towards ameliorated inflammation in the livers of AON-treated MDR2-KO mice.
Which exact cell type can be accounted for the overall downregulation of the CD45+ compartment needs further investigation and is essential to allow detailed mechanistic and functional explanation of our finding.
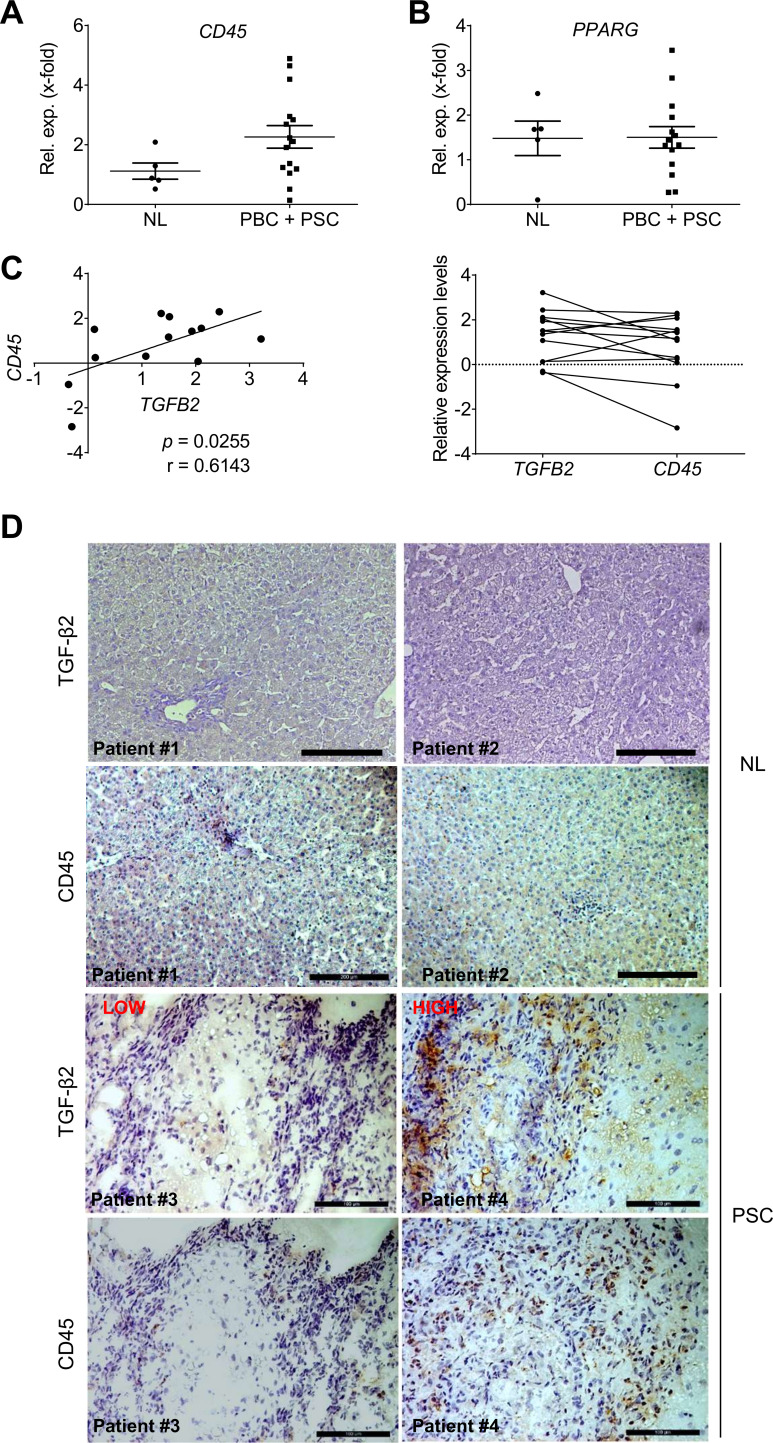
CD45 and TGFB2 expression levels correlate in PSC and pBC patients
In PSC and PBC patients, CD45 mRNA is upregulated by trend in comparison to normal liver (figure 8A), while no significant elevation of PPARG expression was detected (figure 8B). Interestingly, CD45 expression correlated with TGFB2 expression in PSC/PBC patients from the Regensburg cohort (figure 8C, p=0.0255). Immunohistochemical staining confirmed high TGF-β2 expression in liver tissue of patients with high CD45+ cell infiltration (figure 8D). In patients with low numbers of CD45+ cells, only little TGF-β2 was detected. Thus, patients’ data confirmed a possible mechanistic link between the number of CD45+ cells and TGFB2/TGF-β2 levels, as shown in mice.
Figure 8.
Analysis of inflammation marker expression with respect to TGFB2 expression in PSC and PBC patients (Regensburg cohort). Expression of (A) CD45 and (B) PPARG in liver tissue of PSC and PBC patients was determined. (C) CD45 expression levels correlated with TGFB2 expression levels of the same patients (left: Pearson Correlation p TGFB2/CD45=0.0255; rTGFB2/CD45=0.61). (D) Immunohistochemical staining of TGF-β2 and CD45 in liver tissue of PSC patients with low (patient 3) and high (patient 4) CD45 levels as well as healthy controls (patient 1, 2=NL). Scale bars indicate 200 µm for NL, for PSC scale bars indicate 100 µm. NL, normal liver; PBC, primary biliary cirrhosis; PPAR, peroxisome proliferator-activated receptor; PSC, primary sclerosing cholangitis; TGFB, transforming growth factor beta.
Discussion
The TGF-β superfamily has been shown to be a promising field to tackle human diseases from fibrosis to oncogenesis by experts of all areas including cardiology, nephrology and gastroenterology in research and clinics.38 Accordingly, members of the TGF-β superfamily are currently analysed in clinical and preclinical studies as therapeutic targets for several diseases using different approaches of interference.38 Although in some cases, pan-TGF-β reagents targeting all three isoforms are applied, methodologies specifically directed towards TGF-β2 are rare. Rabbits receiving subconjunctival injections of a TGF-β2-neutralising antibody (CAT-152) after glaucoma surgery display significantly improved outcome, and in accordance with our data in liver, present reduced subconjunctival collagen deposition.39 40
Based on results on TGFB2/TgfB2 deregulation in animal models of biliary-derived diseases and its profibrogenic effects in HSCs and cholangiocytes, we are interested in its potential role in PSC and PBC patients also presenting elevated TGF-β2 levels. To get further insight, we targeted TGF-β2 in early stage biliary liver disease of MDR2 knockout mice in order to attenuate or reverse disease parameters related to fibrogenesis and inflammation.
Clinical trials as well as in vivo studies using AONs delineating the function of specific genes have already been reviewed back in the 90s of the last century.41 42 Despite remaining obstacles, these molecules have been developed into next generations including backbone modifications to optimise, for example, half-life, cellular uptake and binding affinities and have been proven to be valuable tools since then.43–45 Accordingly, AONs used in this study were designed to meet these state-of-the-art requirements and were subsequently approved to be well tolerated with respect to well-being and liver parameters in mice and to selectively downregulate TgfB2 in the livers. Our data argue for increased AON uptake into activated NPC such as fibroblasts and macrophages in damaged livers as compared with quiescent cell populations in healthy livers, where AONs predominantly target LSECs. To this state, we cannot explain the mechanistic background and the physiological meaning of this finding. Control oligo-associated deregulation of chemokine receptors and few other genes (placebo effect) in MDR2-KO but not in Balb/c mice could for example be due to different uptake modalities in wild-type versus disease-activated liver cells.
Tumour growth reducing effects of TgfB2-targeting AONs were reported in malignant mesotheliomas,46 highlighting TGF-β2 as a druggable target. In animal models, fibrotic diseases have been successfully tackled using AONs against TGF-β signalling components and other fibrogenic drivers in the past. For example, Timp2 downregulation by AONs ameliorated fibrogenesis in rats and in line with our model reported here, significantly reduced collagen deposition, while in contrast to us, HSC activation (αSMA expression) remained unchanged.47 Likewise, Uchio et al 48 described mild preventive effects of TgfB1 and Ctgf-directed AON treatment in CCl4-induced liver fibrosis as measured by procollagen mRNA expression.
Smad7, endogenous inhibitor of TGF-β, was also successfully targeted by AONs in different diseases. Oral administration of Smad7-directed AONs ameliorated colitis in mice which presents with high TGF-β1 levels but defective signalling due to high SMAD7 levels.49 Further, higher remission rates and clinical responses were shown in Crohn’s disease patients receiving Smad7-specific AONs.50
Our data demonstrate attenuation of fibrogenesis in MDR2-KO mice by silencing TgfB2 accompanied by significant downregulation of CD45-postive cell infiltration and upregulation of at least one anti-inflammatory component (PparG). A link between TGF-β2 function and inflammation has been described before for different organs and pathologies. However, here it is discussed for the first time in the context of liver fibrosis. Maleszewska et al described that costimulation of human umbilical vein endothelial cell cells with IL-1β and TGF-β2 resulted in EndMT (endothelial to mesenchymal transition) mediated by upregulation of inflammatory NFκB signalling.51 In liver, our data also suggest proinflammatory effects of TGF-β2; if silenced, reduction of inflammatory cells occurred which probably subsequently attenuated fibrosis. Opposing our data, TGF-β2 inhibits LPS-induced cytokine production of macrophages in intestinal tissue characterising the cytokine as an anti-inflammatory factor in this organ.52
Just recently, TgfB2 silencing in mice using the same AONs avoided lung metastasis induced by kidney, breast and lung tumor- derived cell lines.30 It is concluded that this in part would be due to induction of CD86 expression in tumour associated macrophages, influencing the immunological niche. Here, we also found a prominent modulatory effect of TgfB2 (silencing) on the complex fibrotic and immunological fate in MDR2-KO mouse livers.
It seems obvious, that TGF-β2 as other regulatory cytokines has multiple cell-type as well as context-dependent and inflammation modulatory roles. Thus, it remains to be explored for any tissue, cell type and disease stage whether TGF-β2 has beneficial or adverse effects on disease development or progression. Our data indicate that downregulation of inflammatory cell infiltrations by TgfB2-targeting AONs in inflammation-associated biliary fibrosis is of advantage for the diseased animal. Further, TgfB2-silencing modulated F4/80-positive cell numbers. F4/80-positive macrophages can be activated to an M1 phenotype associated with an increased secretion of inflammatory cytokines such as Il1β, Tnfα and Il6,53 or can be polarised to an M2 status with, for example, Il13, Il4 or Arg1 expression taking part in Th2 responses such as inflammation dampening and tissue remodelling.53–55 We could not find any regulation of these markers from AON treatment, indicating that liver resident macrophages rather present with mixed and subsequently context-dependent specialised phenotypes instead of expressing exclusive M1 or M2 marker profiles.56–58 To get detailed insight, a thorough characterisation of the F4/80 positive population in MDR2-KO animals before and after AON treatment could be performed taking into account findings of Guicciardi et al published in May 2018.59 Interestingly, our analysis suggests that within the F4/80+ myeloid compartment rather eosinophils than liver resident macrophages are affected by AON treatment. Eosinophils express CCR3 and based on our data, we suggest that AON treatment may recruit eosinophils via CCR3 by inducing CCL5 expression in the liver tissue. Eosinophils may support liver regeneration,60 which is in accordance with our data showing PCNA and Ki67 upregulation and PparG downregulation on AON treatment.61 We conclude that improved, regenerative processes contribute to the beneficial AON effects in MDR2-KO mice.
When analysing the lymphoid compartment, we demonstrated increased numbers of CD8+ T cells as well as lower numbers of CD4+ T cells and Tregs by trend. The latter two together may account for overall significant reduction of CD45+ cell infiltration which is in line with less CD45-positive staining in healthy human liver tissue and patients with low TGF-β2 levels. According to current knowledge, TGF-β1 is involved in generation/maturation of Tregs and Th17 cell populations. The decrease in Tregs on AON treatment would be in line with a potentially similar function of TGF-β2. How this integrates in detail in the disease-related inflammatory, NPC and parenchymal cell communication,62 and accounts for the net effect on disease parameters warrants further investigations. The specific role of Tregs and Th17 cells is still controversially debated in the field of autoimmune hepatitis, including in PSC and PBC.63 We can speculate that in line with a recent study, Tregs, instead of being anti-inflammatory, may suppress NK cells (M1) Kupffer cells and CD8+ T cells, but not CD4+ cells in fibrotic mouse liver, there being also able to promote a proinflammatory microenvironment and enhance fibrogenesis.64 Antifibrotic effects of TGF-β2-directed AONs could be explained by reduced Treg infiltration rates providing an anti-inflammatory niche. However, final conclusions are difficult to draw at this point and further studies are warranted.
Of note, no gender differences for AON specificity and effectiveness were detected in our mouse model with respect to TgfB2 expression as well as CD45 and αSMA staining quantification (data not shown).
To our knowledge for the first time, we demonstrate beneficial effects of TgfB2 silencing in the development of rodent liver fibrosis. Safe application, tolerability and antifibrotic and immune-modulatory effects of TgfB2-directed AONs in a biliary-derived fibrosis model, as well as the upregulated TGFB2 levels in PSC or PBC patients suggest testing AON efficacy in the treatment of patients with biliary cholestatic fibrosis, especially if associated with increased CD45+ cell infiltration. As no healing treatment for these rare but detrimental diseases are available yet, clinical trials targeting TGF-β2 would possibly offer positive prospect for the patients.
Acknowledgments
We thank Igor Liebermann for experimental assistance with respect to the fluidigm platform. We are grateful to Sophie Alex, Vanessa Nalewaja and Kerry Gould for excellent technical support.
Footnotes
Correction notice: This article has been corrected since it published Online First. Figures 5-8 have been replaced for clarity.
Contributors: AD performed mouse model experiments, immunofluorescence staining, statistical analysis and figure preparation. AD, BD, TD, JW and VH performed the experiments including stainings, immunohistochemistry, qPCR and Western Blot. SN supported determination of plasma parameters. UMZ supported the fluidigm-platform based analyses. AP, TG and TSW provided PSC and PBC patient samples for further analyses by AD. JB and DEJJ contributed clinical data to GSE79850. NB, MM and PM shared the Polish patient cohort including analysis of TGFB2 expression. SH provided the data on non-biliary derived animal models and performed analysis of ductular reaction markers with AD. TD supported statistical analyses; AT and TI supported graphical presentation of fluidigm data. SG and SW performed the in situ hybridisation. AS and AC performed, analysed and interpreted the fluidigm analysis. DS provided the mouse cholangiocyte cell line and supported the interpretation of the data. KW, HK and MJ supported the study with their knowledge about the PK/PD characteristics and dosing regimens of the AON in mice and contributed to the discussion about the data. AD, NMB and SD designed the experiments; NMB and SD organised sample collection; AD, NMB and SD interpreted the data; AD, SD and NMB wrote this manuscript. ME provided infrastructure. All authors contributed with productive discussions and knowledge to the final version of this manuscript.
Funding: This work was supported by the BMBF [Liver Systems Medicine, LiSyM, grant number no. PTJ-031L0043; to SD], Isarna Therapeutics GmbH (to SD and AD), SFB1366 [Project number 394046768-SFB 1366; C02; to AC] and by SPP 1937 [CE 140/2-1 to AC]. NMB was supported by the ESF Baden Württemberg (www.esf-bw.de) and the Ministerium für Wissenschaft, Forschung und Kunst, Baden Württemberg [Margarete von Wrangell Programme], TD was funded by the EU [IT-Liver consortium, Marie Curie Training Network], UMZ by the Robert Bosch Foundation, Stuttgart and AD by Isarna Therapeutics; sponsors did not play a role in study design, data collection, analysis and interpretation of the data.
Competing interests: Isarna Therapeutics GmbH supported this study financially. The company develops TGF-β isoform specific antisense oligonucleotides for therapeutic approaches. However, the company did not influence experimental design and data interpretation. KW, HK and MJ were employed by Isarna Therapeutics at the time of contribution. The position of AD was funded by Isarna Therapeutics.
Patient consent for publication: Informed consent was obtained from all patients.
Ethics approval: Government’s Animal Care Committee: 35-9185.81/G-138/13, Regierungspräsidium Karlsruhe. Tissue procurement was approved by the local Medical Ethics Committees (15-101-0318 Ethikkomission der Universität Regensburg; 2007-011N-MA and 2012-293N-MA Ethikkomission II der Universität Heidelberg, KB/58/A/2016 Bioethical Committee of Medical University of Warsaw).
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data are available in public, open access repositories, directly included in the article, uploaded as supplementary iformation or available upon reasonable request from first or last authors. Data sets were obtained from the following public, open access repositories: https://www.ebi.ac.uk/arrayexpress/, www.ncbi.nlm.nih.gov, GEO datasets.
References
- 1. Jepsen P, Grønbæk L, Vilstrup H. Worldwide incidence of autoimmune liver disease. Dig Dis 2015;33 Suppl 2:2–12. 10.1159/000440705 [DOI] [PubMed] [Google Scholar]
- 2. Liberal R, Grant CR. Cirrhosis and autoimmune liver disease: current understanding. World J Hepatol 2016;8:1157–68. 10.4254/wjh.v8.i28.1157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Prince M, Chetwynd A, Newman W, et al. . Survival and symptom progression in a geographically based cohort of patients with primary biliary cirrhosis: follow-up for up to 28 years. Gastroenterology 2002;123:1044–51. 10.1053/gast.2002.36027 [DOI] [PubMed] [Google Scholar]
- 4. Christensen E, Neuberger J, Crowe J, et al. . Azathioprine and prognosis in primary biliary cirrhosis. Gastroenterology 1986;90:508–9. 10.1016/0016-5085(86)90972-8 [DOI] [PubMed] [Google Scholar]
- 5. Broomé U, Olsson R, Lööf L, et al. . Natural history and prognostic factors in 305 Swedish patients with primary sclerosing cholangitis. Gut 1996;38:610–5. 10.1136/gut.38.4.610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ponsioen CY, Vrouenraets SME, Prawirodirdjo W, et al. . Natural history of primary sclerosing cholangitis and prognostic value of cholangiography in a Dutch population. Gut 2002;51:562–6. 10.1136/gut.51.4.562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Paumgartner G, Beuers U. Ursodeoxycholic acid in cholestatic liver disease: mechanisms of action and therapeutic use revisited. Hepatology 2002;36:525–31. 10.1053/jhep.2002.36088 [DOI] [PubMed] [Google Scholar]
- 8. Bissell DM, Wang SS, Jarnagin WR, et al. . Cell-Specific expression of transforming growth factor-beta in rat liver. Evidence for autocrine regulation of hepatocyte proliferation. J Clin Invest 1995;96:447–55. 10.1172/JCI118055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Herbertz S, Sawyer JS, Stauber AJ, et al. . Clinical development of galunisertib (LY2157299 monohydrate), a small molecule inhibitor of transforming growth factor-beta signaling pathway. Drug Des Devel Ther 2015;9:4479–99. 10.2147/DDDT.S86621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Milani S, Herbst H, Schuppan D, et al. . Transforming growth factors beta 1 and beta 2 are differentially expressed in fibrotic liver disease. Am J Pathol 1991;139:1221–9. [PMC free article] [PubMed] [Google Scholar]
- 11. De Bleser PJ, Niki T, Rogiers V, et al. . Transforming growth factor-beta gene expression in normal and fibrotic rat liver. J Hepatol 1997;26:886–93. 10.1016/S0168-8278(97)80257-7 [DOI] [PubMed] [Google Scholar]
- 12. Coker RK, Laurent GJ, Shahzeidi S, et al. . Transforming growth factors-beta 1, -beta 2, and -beta 3 stimulate fibroblast procollagen production in vitro but are differentially expressed during bleomycin-induced lung fibrosis. Am J Pathol 1997;150:981–91. [PMC free article] [PubMed] [Google Scholar]
- 13. Serini G, Gabbiana G. Modulation of alpha-smooth muscle actin expression in fibroblasts by transforming growth factor-beta isoforms: an in vivo and in vitro study. Wound Repair Regen 1996;4:278–87. 10.1046/j.1524-475X.1996.40217.x [DOI] [PubMed] [Google Scholar]
- 14. Pinzani M, Rombouts K. Liver fibrosis: from the bench to clinical targets. Dig Liver Dis 2004;36:231–42. 10.1016/j.dld.2004.01.003 [DOI] [PubMed] [Google Scholar]
- 15. Abou-Shady M, Baer HU, Friess H, et al. . Transforming growth factor betaS and their signaling receptors in human hepatocellular carcinoma. Am J Surg 1999;177:209–15. 10.1016/S0002-9610(99)00012-4 [DOI] [PubMed] [Google Scholar]
- 16. Coulouarn C, Clément B. Stellate cells and the development of liver cancer: therapeutic potential of targeting the stroma. J Hepatol 2014;60:1306–9. 10.1016/j.jhep.2014.02.003 [DOI] [PubMed] [Google Scholar]
- 17. Coulouarn C, Cavard C, Rubbia-Brandt L, et al. . Combined hepatocellular-cholangiocarcinomas exhibit progenitor features and activation of Wnt and TGFβ signaling pathways. Carcinogenesis 2012;33:1791–6. 10.1093/carcin/bgs208 [DOI] [PubMed] [Google Scholar]
- 18. Sulpice L, Rayar M, Desille M, et al. . Molecular profiling of stroma identifies osteopontin as an independent predictor of poor prognosis in intrahepatic cholangiocarcinoma. Hepatology 2013;58:1992–2000. 10.1002/hep.26577 [DOI] [PubMed] [Google Scholar]
- 19. Shirasaki T, Honda M, Shimakami T, et al. . Impaired interferon signaling in chronic hepatitis C patients with advanced fibrosis via the transforming growth factor beta signaling pathway. Hepatology 2014;60:1519–30. 10.1002/hep.27277 [DOI] [PubMed] [Google Scholar]
- 20. Wang B, Koh P, Winbanks C, et al. . miR-200a prevents renal fibrogenesis through repression of TGF-β2 expression. Diabetes 2011;60:280–7. 10.2337/db10-0892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Biressi S, Miyabara EH, Gopinath SD, et al. . A Wnt-TGFβ2 axis induces a fibrogenic program in muscle stem cells from dystrophic mice. Sci Transl Med 2014;6:267ra176 10.1126/scitranslmed.3008411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sun X, He Y, Ma T-T, et al. . Participation of miR-200a in TGF-β1-mediated hepatic stellate cell activation. Mol Cell Biochem 2014;388:11–23. 10.1007/s11010-013-1895-0 [DOI] [PubMed] [Google Scholar]
- 23. Tschaharganeh DF, Chen X, Latzko P, et al. . Yes-Associated protein up-regulates Jagged-1 and activates the Notch pathway in human hepatocellular carcinoma. Gastroenterology 2013;144:1530–42. e12 10.1053/j.gastro.2013.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dong J, Feldmann G, Huang J, et al. . Elucidation of a universal size-control mechanism in Drosophila and mammals. Cell 2007;130:1120–33. 10.1016/j.cell.2007.07.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dropmann A, Dediulia T, Breitkopf-Heinlein K, et al. . TGF-β1 and TGF-β2 abundance in liver diseases of mice and men. Oncotarget 2016;7:19499–518. 10.18632/oncotarget.6967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mauad TH, van Nieuwkerk CM, Dingemans KP, et al. . Mice with homozygous disruption of the mdr2 P-glycoprotein gene. A novel animal model for studies of nonsuppurative inflammatory cholangitis and hepatocarcinogenesis. Am J Pathol 1994;145:1237–45. [PMC free article] [PubMed] [Google Scholar]
- 27. Hochrath K, Krawczyk M, Goebel R, et al. . The hepatic phosphatidylcholine transporter Abcb4 as modulator of glucose homeostasis. Faseb J 2012;26:5081–91. 10.1096/fj.12-209379 [DOI] [PubMed] [Google Scholar]
- 28. Pikarsky E, Porat RM, Stein I, et al. . Nf-kappaB functions as a tumour promoter in inflammation-associated cancer. Nature 2004;431:461–6. 10.1038/nature02924 [DOI] [PubMed] [Google Scholar]
- 29. Jaschinski F, Korhonen H, Janicot M. Design and selection of antisense oligonucleotides targeting transforming growth factor beta (TGF-β) isoform mRNAs for the treatment of solid tumors. Methods Mol Biol 2015;1317:137–51. 10.1007/978-1-4939-2727-2_9 [DOI] [PubMed] [Google Scholar]
- 30. Huber-Ruano I, Raventós C, Cuartas I, et al. . An antisense oligonucleotide targeting TGF-β2 inhibits lung metastasis and induces CD86 expression in tumor-associated macrophages. Ann Oncol 2017;28:2278–85. 10.1093/annonc/mdx314 [DOI] [PubMed] [Google Scholar]
- 31. Fickert P, Wagner M, Marschall H-U, et al. . 24-norUrsodeoxycholic acid is superior to ursodeoxycholic acid in the treatment of sclerosing cholangitis in mdr2 (ABCB4) knockout mice. Gastroenterology 2006;130:465–81. 10.1053/j.gastro.2005.10.018 [DOI] [PubMed] [Google Scholar]
- 32. Rókusz A, Veres D, Szücs A, et al. . Ductular reaction correlates with fibrogenesis but does not contribute to liver regeneration in experimental fibrosis models. PLoS One 2017;12:e0176518 10.1371/journal.pone.0176518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ikenaga N, Liu SB, Sverdlov DY, et al. . A new Mdr2(-/-) mouse model of sclerosing cholangitis with rapid fibrosis progression, early-onset portal hypertension, and liver cancer. Am J Pathol 2015;185:325–34. 10.1016/j.ajpath.2014.10.013 [DOI] [PubMed] [Google Scholar]
- 34. Pascual G, Fong AL, Ogawa S, et al. . A SUMOylation-dependent pathway mediates transrepression of inflammatory response genes by PPAR-gamma. Nature 2005;437:759–63. 10.1038/nature03988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Martin H. Role of PPAR-gamma in inflammation. prospects for therapeutic intervention by food components. Mutat Res 2009;669:1–7. 10.1016/j.mrfmmm.2009.06.009 [DOI] [PubMed] [Google Scholar]
- 36. Watts JK, Corey DR. Silencing disease genes in the laboratory and the clinic. J Pathol 2012;226:365–79. 10.1002/path.2993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mowat AM, Bain CC. News & Highlights. Mucosal Immunol 2010;3:420–1. 10.1038/mi.2010.24 [DOI] [Google Scholar]
- 38. Akhurst RJ, Hata A. Targeting the TGFβ signalling pathway in disease. Nat Rev Drug Discov 2012;11:790–811. 10.1038/nrd3810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mead AL, Wong TTL, Cordeiro MF, et al. . Evaluation of anti-TGF-beta2 antibody as a new postoperative anti-scarring agent in glaucoma surgery. Invest Ophthalmol Vis Sci 2003;44:3394–401. 10.1167/iovs.02-0978 [DOI] [PubMed] [Google Scholar]
- 40. Khaw P, Grehn F, Holló G, et al. . A phase III study of subconjunctival human anti-transforming growth factor beta(2) monoclonal antibody (CAT-152) to prevent scarring after first-time trabeculectomy. Ophthalmology 2007;114:1822–30. 10.1016/j.ophtha.2007.03.050 [DOI] [PubMed] [Google Scholar]
- 41. Agrawal S. Antisense oligonucleotides: towards clinical trials. Trends Biotechnol 1996;14:376–87. 10.1016/0167-7799(96)10053-6 [DOI] [PubMed] [Google Scholar]
- 42. Sharma HW, Narayanan R. The therapeutic potential of antisense oligonucleotides. Bioessays 1995;17:1055–63. 10.1002/bies.950171210 [DOI] [PubMed] [Google Scholar]
- 43. Akhtar S, Agrawal S. In vivo studies with antisense oligonucleotides. Trends Pharmacol Sci 1997;18:12–18. 10.1016/S0165-6147(96)01002-4 [DOI] [PubMed] [Google Scholar]
- 44. Fluiter K, ten Asbroek ALMA, de Wissel MB, et al. . In vivo tumor growth inhibition and biodistribution studies of locked nucleic acid (LNA) antisense oligonucleotides. Nucleic Acids Res 2003;31:953–62. 10.1093/nar/gkg185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lorenzer C, Dirin M, Winkler A-M, et al. . Going beyond the liver: progress and challenges of targeted delivery of siRNA therapeutics. J Control Release 2015;203:1–15. 10.1016/j.jconrel.2015.02.003 [DOI] [PubMed] [Google Scholar]
- 46. Marzo AL, Fitzpatrick DR, Robinson BW, et al. . Antisense oligonucleotides specific for transforming growth factor beta2 inhibit the growth of malignant mesothelioma both in vitro and in vivo. Cancer Res 1997;57:3200–7. [PubMed] [Google Scholar]
- 47. Nie Q-H, Zhu C-L, Zhang Y-F, et al. . Inhibitory effect of antisense oligonucleotide targeting TIMP-2 on immune-induced liver fibrosis. Dig Dis Sci 2010;55:1286–95. 10.1007/s10620-009-0858-5 [DOI] [PubMed] [Google Scholar]
- 48. Uchio K, Graham M, Dean NM, et al. . Down-Regulation of connective tissue growth factor and type I collagen mRNA expression by connective tissue growth factor antisense oligonucleotide during experimental liver fibrosis. Wound Repair Regen 2004;12:60–6. 10.1111/j.1067-1927.2004.012112.x-1 [DOI] [PubMed] [Google Scholar]
- 49. Boirivant M, Pallone F, Di Giacinto C, et al. . Inhibition of Smad7 with a specific antisense oligonucleotide facilitates TGF-beta1-mediated suppression of colitis. Gastroenterology 2006;131:1786–98. 10.1053/j.gastro.2006.09.016 [DOI] [PubMed] [Google Scholar]
- 50. Monteleone G, Neurath MF, Ardizzone S, et al. . Mongersen, an oral Smad7 antisense oligonucleotide, and Crohn's disease. N Engl J Med 2015;372:1104–13. 10.1056/NEJMoa1407250 [DOI] [PubMed] [Google Scholar]
- 51. Maleszewska M, Moonen J-RAJ, Huijkman N, et al. . IL-1β and TGFβ2 synergistically induce endothelial to mesenchymal transition in an NFκB-dependent manner. Immunobiology 2013;218:443–54. 10.1016/j.imbio.2012.05.026 [DOI] [PubMed] [Google Scholar]
- 52. Maheshwari A, Kelly DR, Nicola T, et al. . TGF-β2 suppresses macrophage cytokine production and mucosal inflammatory responses in the developing intestine. Gastroenterology 2011;140:242–53. 10.1053/j.gastro.2010.09.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Italiani P, Boraschi D. From monocytes to M1/M2 macrophages: phenotypical vs. functional differentiation. Front Immunol 2014;5:514 10.3389/fimmu.2014.00514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Wynn TA, Chawla A, Pollard JW. Macrophage biology in development, homeostasis and disease. Nature 2013;496:445–55. 10.1038/nature12034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Pesce JT, Ramalingam TR, Mentink-Kane MM, et al. . Arginase-1-expressing macrophages suppress Th2 cytokine-driven inflammation and fibrosis. PLoS Pathog 2009;5:e1000371 10.1371/journal.ppat.1000371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Murray PJ, Allen JE, Biswas SK, et al. . Macrophage activation and polarization: Nomenclature and experimental guidelines. Immunity 2014;41:14–20. 10.1016/j.immuni.2014.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Tacke F. Targeting hepatic macrophages to treat liver diseases. J Hepatol 2017;66:1300–12. 10.1016/j.jhep.2017.02.026 [DOI] [PubMed] [Google Scholar]
- 58. Tacke F, Zimmermann HW. Macrophage heterogeneity in liver injury and fibrosis. J Hepatol 2014;60:1090–6. 10.1016/j.jhep.2013.12.025 [DOI] [PubMed] [Google Scholar]
- 59. Guicciardi ME, Trussoni CE, Krishnan A, et al. . Macrophages contribute to the pathogenesis of sclerosing cholangitis in mice. J Hepatol 2018;69:676–86. 10.1016/j.jhep.2018.05.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Goh YPS, Henderson NC, Heredia JE, et al. . Eosinophils secrete IL-4 to facilitate liver regeneration. Proc Natl Acad Sci U S A 2013;110:9914–9. 10.1073/pnas.1304046110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Gazit V, Huang J, Weymann A, et al. . Analysis of the role of hepatic PPARγ expression during mouse liver regeneration. Hepatology 2012;56:1489–98. 10.1002/hep.25880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Pellicoro A, Ramachandran P, Iredale JP, et al. . Liver fibrosis and repair: immune regulation of wound healing in a solid organ. Nat Rev Immunol 2014;14:181–94. 10.1038/nri3623 [DOI] [PubMed] [Google Scholar]
- 63. Zhang H, Jiang Z, Zhang L. Dual effect of T helper cell 17 (Th17) and regulatory T cell (Treg) in liver pathological process: from occurrence to end stage of disease. Int Immunopharmacol 2019;69:50–9. 10.1016/j.intimp.2019.01.005 [DOI] [PubMed] [Google Scholar]
- 64. Zhang X, Lou J, Bai L, et al. . Immune regulation of intrahepatic regulatory T cells in fibrotic livers of mice. Med Sci Monit 2017;23:1009–16. 10.12659/MSM.899725 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
gutjnl-2019-319091supp001.pdf (232.3KB, pdf)
gutjnl-2019-319091supp002.pdf (526.5KB, pdf)
gutjnl-2019-319091supp003.pdf (125KB, pdf)
gutjnl-2019-319091supp004.pdf (65.5KB, pdf)
gutjnl-2019-319091supp005.pdf (937.2KB, pdf)
gutjnl-2019-319091supp006.pdf (599.1KB, pdf)
gutjnl-2019-319091supp007.pdf (1.7MB, pdf)
gutjnl-2019-319091supp008.pdf (110KB, pdf)
gutjnl-2019-319091supp009.pdf (1.7MB, pdf)