Abstract
Background
Total proctocolectomy (TPC) with ileal pouch anal anastomosis (IPAA) is the gold standard surgery for ulcerative colitis (UC) patients with medically refractory disease. The aim of this study was to report the rates and risk factors of inflammatory pouch conditions.
Methods
This was a retrospective review of UC or IBD unspecified (IBDU) patients who underwent TPC with IPAA for refractory disease or dysplasia between 2008 and 2017. Pouchoscopy data were used to calculate rates of inflammatory pouch conditions. Factors associated with outcomes in univariable analysis were investigated in multivariable analysis.
Results
Of the 621 patients more than 18 years of age who underwent TPC with IPAA between January 2008 and December 2017, pouchoscopy data were available for 386 patients during a median follow-up period of 4 years. Acute pouchitis occurred in 205 patients (53%), 60 of whom (30%) progressed to chronic pouchitis. Cuffitis and Crohn's disease–like condition (CDLC) of the pouch occurred in 119 (30%) patients and 46 (12%) patients, respectively. In multivariable analysis, female sex was associated with a decreased risk of acute pouchitis, and pre-operative steroid use and medically refractory disease were associated with an increased risk; IBDU was associated with chronic pouchitis; rectal cuff length ≥2 cm and medically refractory disease were associated with cuffitis; age 45–54 at colectomy was associated with CDLC. Rates of pouch failure were similar in chronic pouchitis and CDLC patients treated with biologics and those who were not.
Conclusions
Inflammatory pouch conditions are common. Biologic use for chronic pouchitis and CDLC does not impact the rate of pouch failure.
Keywords: colectomy, ileal pouch anal anastomosis, pouchitis, cuffitis
Inflammatory pouch conditions are common after ileal pouch anal anastomosis in ulcerative colitis patients. Biologics used to manage chronic inflammatory pouch conditions such as chronic pouchitis and Crohn's disease–like condition, however, may not impact the rate of pouch failure.
INTRODUCTION
Despite advances in medical therapy, approximately 10%–15% of ulcerative colitis (UC) patients will require surgery during their disease course, the most common of which is the staged total proctocolectomy (TPC) with ileal pouch anal anastomosis (IPAA).1 Considered to be the gold standard in surgical treatment for UC patients with medically refractory disease or dysplasia, the pouch is associated with good long-term functional outcomes and quality of life.2–5 Despite it benefits, however, the pouch may be complicated by inflammatory conditions such as acute and chronic pouchitis, cuffitis, and Crohn's disease–like condition (CDLC).
Pouchitis is the most common postoperative inflammatory condition, with reported cumulative incidence rates of 25% at 1 year, 35% at 3 years, and 45% at 5 years.6 Antibiotics are the mainstay of treatment and induce remission rates of 80%; however, approximately 20% of patients progress to chronic pouchitis with an associated 5%–10% risk of pouch failure.3, 7, 8 Cuffitis is increasingly common post-IPAA since the standardization of the stapled anastomosis, with incidence rates as high as 20%.9–11 Cuffitis typically responds to topical mesalamine therapy; however, refractory cases may require surgical intervention with mucosectomy of the rectal cuff and advancement of the pouch.12 Up to 15% of patients with cuffitis will experience pouch failure.10
There is no formal definition for CDLC, and thus incidence rates vary widely from 5%–20%.13–17 The term CDLC has been used to describe a clinical phenotype characterized by inflammation of the pouch or afferent limb, stricturing of the afferent limb or small bowel, and/or fistulizing disease of the perineum or small bowel.18, 19 There is no consensus regarding treatment, and the level of evidence to support medical therapy with 5-aminosalicylic acid products, steroids, immunomodulators, and biologics is relatively weak and largely based on retrospective case series.20 Crohn's disease–like condition is associated with a significant risk of pouch failure with reported frequencies up to 45%.13
There have been major medical and surgical advances since the publication of previous IPAA natural history studies. Minimally invasive, laparoscopic surgical approaches have become standard for TPC and IPAA, and medical therapy with biologics for inflammatory pouch conditions has increased. Recent large studies have reported the short-term postoperative complications and long-term functional outcomes after IPAA; however, none have described the rates of inflammatory conditions.21–23 Herein, we describe the rates and risk factors of inflammatory pouch conditions and their association with pouch failure in a large adult cohort within an inflammatory bowel disease (IBD) tertiary referral center.
METHODS
Study Population
This was a retrospective chart review conducted at a single tertiary-care, high-volume IBD center. All UC or IBD unspecified (IBDU) patients who underwent TPC with IPAA for medically refractory disease or dysplasia between January 2008 and December 2017 followed by pouchoscopy were identified through hospital electronic medical records. Patients younger than 18 years of age at the time of colectomy were excluded.
Data Collection and Variables
Clinical information was abstracted from the medical record using a standardized data collection sheet by 6 investigators (MK, MR, AR, MP, ET, and PT), and all data were reviewed by a single investigator (MK) blinded to outcomes. Study data were managed using Research Electronic Data Capture (REDCap), a secure, web-based application designed to support data capture for research studies.24 Collected patient demographics and disease characteristics included sex, ethnicity, body mass index (BMI) at colectomy, age at colectomy, family history of IBD, disease duration, preoperative diagnosis of UC or IBDU (defined as disease with overlapping features of UC and CD), postoperative diagnosis of indeterminate colitis (defined histopathologically as colectomy specimens with no specific features for UC or CD), disease extent at diagnosis and colectomy defined according to Montreal classification, preoperative medications within 12 weeks of colectomy (steroids, immunomodulators, biologics), history of primary sclerosing cholangitis (PSC), axial or peripheral spondyloarthropathy, backwash ileitis defined histologically on colectomy and ileal specimen, and tobacco use at colectomy.25, 26 Collected surgical details included colectomy indication, surgical procedures and stages, type of anastomosis (handsewn or stapled), and residual rectal cuff length. One-stage surgery was defined as TPC with IPAA construction during 1 procedure. Traditional 2-stage surgery was defined as (1) TPC with immediate IPAA construction and diverting ileostomy with (2) subsequent ileostomy closure. Modified 2-stage surgery was defined as (1) subtotal colectomy with end ileostomy with (2) subsequent completion proctectomy and IPAA creation. Three-stage surgery was defined as (1) subtotal colectomy with end ileostomy followed by (2) completion proctectomy with IPAA construction and diverting ostomy, and (3) subsequent ostomy closure.
All postoperative pouchoscopy procedure notes were reviewed, and collected data included indication, endoscopic findings as defined by the Pouchitis Disease Activity Index (PDAI) with mucosal edema, granularity, friability, loss of vascular pattern, mucosal exudate and/or ulceration, presence of histologic inflammation, and postprocedure medication prescriptions.27 Biologic therapy was defined as the use of adalimumab, infliximab, certolizumab, vedolizumab, or ustekinumab for chronic pouchitis, cuffitis, or CDLC.
Inflammatory Pouch Conditions and Outcomes
Pouchitis was defined as a total PDAI score ≥7 with symptoms of increased stool frequency from postoperative baseline, hematochezia, fecal urgency, abdominal cramps, or fever (temperature ≥37.8ºCentigrade) in the setting of an abnormal pouchoscopy with mucosal edema, granularity, friability, loss of vascular pattern, mucosal exudate, or ulceration and histologic inflammation.27 Pouchitis was further classified into acute or chronic. Acute pouchitis was defined as symptoms lasting less than 4 weeks and responding to 2-week courses of antibiotics.26 Chronic pouchitis was defined as symptoms lasting greater than 4 weeks and requiring chronic antibiotic therapy, not meeting criteria for CDLC as described later on.28
Cuffitis was defined as symptoms of urgency, increased stool frequency, incontinence, hematochezia, and/or pelvic pain in the setting of an abnormal pouchoscopy with evidence of mucosal edema, erythema, granularity, friability, ulceration, and/or exudate within the rectal cuff in the area between the anastomosis and dentate line.10
Crohn's disease–like condition of the pouch was defined as the presence of severe inflammation of the pouch or afferent limb, strictures of the afferent limb or proximal small bowel, and/or fistulae involving the perineum or proximal small bowel that occurred more than 6 months after final surgical stage.18
Pouch failure was defined as the construction of a permanent end ileostomy, with or without pouch excision.28
Statistical Analysis
Descriptive statistics were used to calculate rates of acute and chronic pouchitis, cuffitis, and CDLC of the pouch. For continuous variables, medians with interquartile range were calculated, and for categorical variables, proportions were reported. Age was divided into the after increments: 18 to 24, 25 to 34, 35 to 44, 45 to 54, 55 to 64, 65+; and age was analyzed as a categorical variable. Body mass index was categorized as <18.5, 18.5 to 24.9, and >25 and analyzed as a categorical variable. Univariable logistic regression was used to assess unadjusted relationships between hypothesized risk factors and all outcomes. Multivariable logistic regression models were then created incorporating all variables that were significant at the P < 0.05 level on univariable analysis. Odds ratios (ORs) and 95% confidence intervals (CIs) are reported. SPSS version 24 (IBM Corp., Armonk, NY) was used for all analyses.
RESULTS
Study Population
Of the 621 patients older than 18 years of age who underwent TPC with IPAA between January 2008 and December 2017, pouchoscopy data were available for 386 patients. Patient demographic, disease characteristics, and surgical characteristics are given in Tables 1 and 2. Preoperative diagnosis was UC in 363 (94.0%) patients and IBDU in 23 (6.0%) patients. The median age at colectomy was 37 (interquartile range [IQR] 28 to 50) years, and median precolectomy disease duration was 60 (IQR 24 to 153) months. Primary sclerosing cholangitis was present in 13 (3.4%) patients. Of the patients with preoperative diagnosis of UC, disease extension defined as progression from proctitis or left-sided colitis at diagnosis to extensive colitis at colectomy occurred in 56 (14.5%) patients. All patients with preoperative IBDU diagnosis were confirmed as indeterminate colitis on histopathologic review.
TABLE 1.
Clinical Characteristics of Pouch Patients, n = 386
| Baseline | n (%) |
|---|---|
| Sex | |
| Male | 206 (53.4%) |
| Female | 180 (46.6%) |
| Race | |
| White | 313 (81.1%) |
| Hispanic | 41(10.6%) |
| Black | 13 (3.4%) |
| Unknown | 19 (4.9%) |
| BMI (kg/m2) at colectomya | 22.7 [21.0–25.9] |
| Age at colectomy, yeara | 37 [28–50] |
| Family history of IBD | 81 (21.0%) |
| Disease duration, montha | 60 [24–153] |
| Preoperative diagnosis | |
| Ulcerative colitis | 363 (94.0%) |
| IBDU | 23 (6.0%) |
| Disease extent at diagnosis | |
| Proctitis | 31 (8.0%) |
| Left-sided | 79 (20.5%) |
| Extensive | 253 (65.5%) |
| Disease extent at colectomy | |
| Left sided | 60 (15.5%) |
| Extensive | 326 (84.5%) |
| Preoperative medications within 12 weeks | |
| Steroids | 286 (74.1%) |
| Immunomodulators | 156 (40.4%) |
| Biologics | 215 (55.7%) |
| PSC | 13 (3.4%) |
| Extraintestinal manifestations | |
| Peripheral spondyloarthropathy | 26 (6.7%) |
| Axial spondyloarthropathy | 3 (0.8%) |
| Episcleritis/uveitis | 5 (1.3%) |
| Pyoderma, psoriasis, alopecia | 11 (2.8%) |
| Backwash ileitis | 33 (8.5%) |
| Tobacco use at colectomy | |
| Current | 18 (4.7%) |
| Former | 79 (20.5%) |
| Never | 240 (62.2%) |
| Unknown | 43 (11.1%) |
aReported as median, IQR
TABLE 2.
Surgical Characteristics of Pouch Patients, n = 386
| Surgical Characteristics | n (%) |
|---|---|
| Surgical indicationa | |
| Medically refractory disease | 322 (83.4%) |
| Dysplasia | 68 (17.6%) |
| One stage IPAA | 36 (9.3%) |
| 2-stage IPAA | |
| Modified | 39 (10.1%) |
| Traditional | 108 (28.0%) |
| Three stage IPAA | 203 (52.6%) |
| Anastomosis | |
| Stapled | 275 (71.2%) |
| Handsewn | 111 (28.8%) |
aTwo indications for 4 patients
Colectomy indication was medically refractory disease in 322 (83.4%) patients and dysplasia in 68 (17.6%) patients; 4 patients had both indications. All surgical procedures were performed at The Mount Sinai Hospital by one of 15 surgeons. One-stage IPAA was performed in 36 (9.3%) patients, 2-stage IPAA in 147 (38.0%) patients, and 3-stage IPAA in 203 (52.6%) patients. The majority of 1-stage IPAA procedures were performed between 2008 and 2011; only 2 were performed in 2016 and none since then. A laparoscopic surgical approach was utilized in 281 (72.8%) patients during colectomy and IPAA creation. Anastomosis was stapled in 275 (71.2%) patients and hand sewn in 111 (28.8%) patients. Rectal cuff length was available for 262 (67.9%) patients; median residual rectal cuff length was 2 (IQR 1–3) cm.
First pouchoscopy was performed a median 0.97 (IQR 0.39–1.9) years after final surgical stage. The most common indications for pouchoscopy were acute symptoms (increased stool frequency from postoperative baseline, hematochezia, fecal urgency, abdominal cramps, and fever) and dysplasia surveillance. Median length of follow-up after final surgical stage was 3.9 (IQR 2.0–6.4) years.
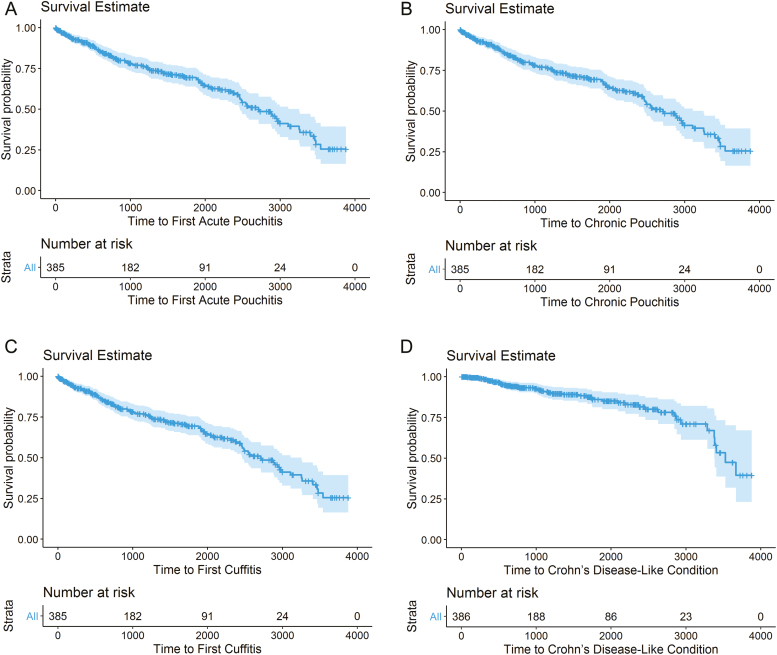
Acute Pouchitis
Acute pouchitis developed in 205 (53.1%) patients a median interval of 1.4 (IQR 0.6–2.9) years after final surgical stage (Fig. 1). The median number of pouchitis episodes experienced by each patient was 1 (IQR 0–1). In univariable analysis, extensive disease (OR 1.9; 95% CI, 1.1–3.3), steroid use within 3 months before colectomy (OR 1.8; 95% CI, 1.2–2.9), and medically refractory disease (OR 2.2; 95% CI, 1.3–4.0) increased the risk of acute pouchitis; but age greater than 65 at colectomy (OR 0.35; 95% CI, 0.14–0.89), female sex (OR 0.59; 95% CI, 0.39–0.88), and family history of IBD (OR 0.57; 95% CI, 0.34–0.97) decreased the risk (Supplementary Table 1). In multivariable analysis, female sex (OR 0.49; 95% CI, 0.32–0.77) decreased the risk of acute pouchitis, whereas steroid use within 3 months before colectomy (OR 1.9; 95% CI, 1.1–3.4) and medically refractory disease (OR 2.1; 95% CI, 1.1–4.2) increased the risk. Tobacco use and PSC were not associated with the development of acute pouchitis in univariable or multivariable analysis.
FIGURE 1.
Kaplan-Meier survival estimates (a) acute pouchitis, (b) chronic pouchitis, (c) cuffitis, and (d) Crohn's disease–like condition.
Chronic Pouchitis
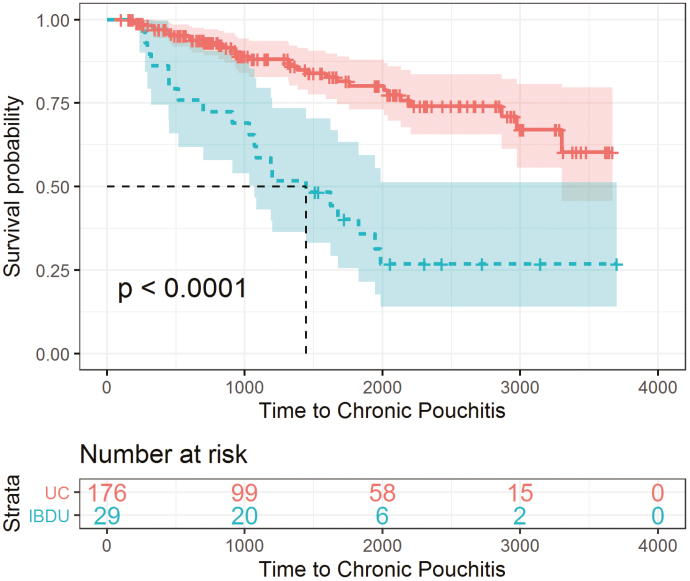
Of the 205 patients who developed acute pouchitis, 60 (29.3%) patients progressed to chronic pouchitis a median interval of 1.1 (IQR 0.27–3.22) years after the first acute pouchitis episode (Fig. 1). In univariable analysis, immunomodulator use within 3 months before colectomy (OR 2.4; 95% CI, 1.2–4.7), preoperative IBDU diagnosis (OR 10.4; 95% CI, 4.3–25.0), and proximal disease extension (OR 2.3; 95% CI, 1.1–5.2) increased the risk of chronic pouchitis (Supplementary Table 1). In multivariable analysis, preoperative IBDU diagnosis (OR 10.0,; 95% CI, 3.9–25.9) increased the risk of chronic pouchitis. The rate of developing chronic pouchitis was significantly greater for IBDU patients (Fig. 2).
FIGURE 2.
Kaplan-Meier survival estimates: chronic pouchitis in patients with IBDU.
Biologic therapy was initiated in 14 of 60 (23.3%) patients with chronic pouchitis a median interval of 10 (IQR 0–135) days after diagnosis—adalimumab in 8 of 14 (57.1%), infliximab in 4 of 14 (28.6%), and ustekinumab in 2 of 14 (14.3%) patients. Pouchoscopy was performed within 12 months of biologic initiation in 8 of 14 (57.1%) patients to assess treatment response. A decrease in the PDAI endoscopic subscore of at least 50% with resolution of mucosal ulcers was noted in 2 of 8 (25.0%) patients, and persistent endoscopic disease was noted in 6 of 8 (75.0%) patients.
Cuffitis
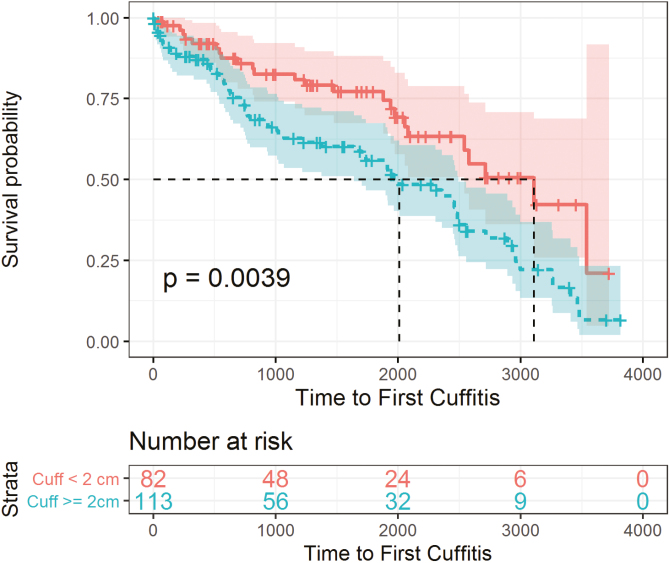
Cuffitis developed in 119 (30.1%) patients a median interval of 1.0 (IQR 0.3–2.4) year after final surgical stage (Fig. 1). The median number of cuffitis episodes experienced by each patient was 1 (IQR 0–1). In univariable analysis, steroid (OR 2.3; 95% CI, 1.3–4.0) or immunomodulator (OR 2.2; 95% CI, 1.4–3.4) use within 3 months before colectomy, medically refractory disease (OR 5.8; 95% CI, 2.3–14.9), 2-stage (OR 3.9; 95% CI, 1.1–13.4) or 3-stage (OR 6.8; 95% CI, 2.0–23.0) restorative proctocolectomy, BMI <18.5 at colectomy (OR 2.5; 95% CI, 1.3–4.7), and greater rectal cuff length (OR 1.3; 95% CI, 1.1–1.6) were associated with an increased risk of cuffitis; although age 55 to 64 at colectomy (OR 0.34; 95% CI, 0.14–0.82) and previous tobacco use (OR 0.54; 95% CI, 0.29–0.97) were associated with a decreased risk (Supplementary Table 1). In multivariable analysis, greater rectal cuff length (OR 1.4,; 95% CI, 1.1–1.8) and medically refractory disease (OR 6.9; 95% CI, 2.1–23.2) increased the risk of cuffitis. The rate of developing cuffitis was significantly greater for patients with rectal cuff length ≥2 cm (Fig. 3).
FIGURE 3.
Kaplan-Meier survival estimates: cuffitis in patients with rectal cuff ≥2 cm and <2 cm.
Infliximab was initiated in 3 patients for persistent cuffitis refractory to topical mesalamine and steroids. Pouchoscopy was performed within 12 months of biologic initiation to assess treatment response. Resolution of rectal cuff ulcers and/or erosions was noted in all patients.
Crohn's Disease–like Condition of the Pouch
Crohn's disease–like condition of the pouch developed in 46 (11.9%) patients a median interval of 2.1 (IQR 1.0–4.1) years after final surgical stage (Fig. 1). Colectomy pathology for all patients who developed CDLC was reviewed and confirmed to be consistent with UC. Of these patients, 38 (82.6%) had inflammation of the pouch reservoir, and 18 (39.1%) had inflammation of the afferent limb; 12 (26.1%) had strictures of the pouch, 9 (19.6%) had pouch fistulae involving the proximal small bowel, and 7 (15.2%) had pouch fistulae involving the perineum. A subset of 32 (69.6%) patients had 2 or more features, the most common of which were inflammation of the pouch reservoir and afferent limb. In univariable analysis, age 45 to 54 at colectomy as compared with 18 to 24 (OR 0.17,; 95% CI, 0.04–0.84) was associated with a decreased risk of CDLC. No factors were significant in multivariable analysis.
Biologic therapy was initiated in 37 of 46 (80.4%) patients with CDLC a median interval of 19 (IQR 0–50) days after diagnosis—adalimumab in 17 of 37 (45.9%), infliximab in 9 of 37 (24.3%), ustekinumab in 7 of 37 (18.9%), vedolizumab in 2 of 37 (5.4%), and certolizumab in 2 of 37 (5.4%) patients. Pouchoscopy was performed within 12 months of biologic initiation in 16 of 37 (43.2%) patients to assess treatment response. A decrease in the PDAI endoscopic subscore of at least 50% with resolution of mucosal ulcers and/or erosions in the pouch and/or afferent limb was noted in 6 of 16 (37.5%) patients, and persistent endoscopic disease was noted in 10 of 16 (62.5%) patients.
Pouch Failure
Pouch failure, defined as the creation of a permanent end ileostomy with or without pouch excision, occurred in 26 of 386 (6.7%) patients a median interval of 2.1 (IQR 1.2–4.3) years after final surgical stage. The most common diagnoses that resulted in pouch failure were CDLC (57.7%), followed by chronic pouchitis (19.2%), cuffitis (15.4%), and dysplasia/adenocarcinoma of the rectal cuff (7.7%). In univariable analysis, BMI <18.5 at colectomy as compared with BMI >25 (OR 3.2; 95% CI, 1.1–9.4), greater rectal cuff length (OR 2.1; 95% CI, 1.5–2.9), cuffitis (OR 2.8; 95% CI, 1.3–6.3), chronic pouchitis (OR 2.6; 95% CI, 1.1–6.4), and CDLC (OR 5.6; 95% CI, 2.4–13.3) increased the risk of pouch failure (Supplementary Table 1). In multivariable analysis, greater rectal cuff length (OR 2.2,; 95% CI, 1.5–3.2) and cuffitis (OR 6.6,; 95% CI, 1.2–37.6) increased the risk of pouch failure.
Biologic use significantly increased the risk of pouch failure (OR 3.0; 95% CI, 1.7–7.9) in bivariable analysis but not multivariable analysis (OR 2.9; 95% CI, 0.7–11.1). In chronic pouchitis, the rate of pouch failure was 7.1% (1 of 14) among those treated with biologics compared with 8.7% (4 of 46) in those not. In cuffitis, the rate of pouch failure was 0% (0 of 4) among those treated with biologics compared with 3.5% (4 of 115) in those not. In CDLC, the rate of pouch failure was 21.6% (8 of 37) among those treated with biologics compared with 22.2% (2 of 9) in those not.
DISCUSSION
In this large series of more than 300 UC or IBDU patients who underwent TPC with IPAA and subsequent pouchoscopy between 2008 and 2017, acute pouchitis occurred in 53%, of whom approximately 30% progressed to chronic pouchitis, cuffitis in 30%, and CDLC in 11% during a median follow-up of 4 years. We observed greater rates of inflammatory pouch conditions than reported in the literature because of our strict inclusion of only patients who underwent pouchoscopy and had endoscopic evidence of disease. Rates of pouch failure in chronic pouchitis and CDLC patients were similar among patients who received biologics and those who did not, suggesting that once chronic inflammation has occurred, treatment may be futile.
Previously reported risk factors for acute pouchitis also noted in this cohort include extensive UC, preoperative steroid use, and medically refractory disease.7 We also noted that female sex decreased the risk of acute pouchitis. No specific sex-based differences in the incidence of acute pouchitis have been previously reported; however, male sex has been associated with an increased risk of chronic pouchitis and correlated with severe histologic pouchitis.29–31 Our study is the first to support and extend this sex-based association with acute pouchitis, the prologue to chronic pouchitis. It is unclear precisely why male sex increases the risk of pouchitis; however, one often postulated reason is the narrower male pelvis, which increases the technical complexity of the restorative proctolectomy and the risk of postoperative complications and may therefore predispose to future inflammation.29
Of the 205 patients who developed acute pouchitis, approximately 30% progressed to chronic pouchitis. Previously reported risk factors for chronic pouchitis include extensive preoperative disease and longer pouch duration.32 In our study, preoperative IBDU and proximal disease extension were significantly associated with chronic pouchitis. Patients with disease extension have been shown to have worse outcomes in UC and an increased risk of colectomy.33 Our results are the first to extend these findings to the post-IPAA period with an increased risk of chronic pouchitis in patients with proximal disease extension. Likewise, the observed association and high odds ratio between IBDU and chronic pouchitis is novel and suggests a similar underlying disease process. Chronic pouchitis has historically been considered an inflammatory condition related to microbial disarray that requires chronic antibiotic use; however, it may instead be a continuum of IBDU or CDLC that requires early biologic use.
There are little published data on cuffitis; previous studies have reported incidence rates up to 20% and such preoperative risk factors as biologic use and toxic megacolon or fulminant colitis.9–11 Cuffitis occurred in approximately 30% of patients in our cohort and was associated with greater rectal cuff length and medically refractory disease in multivariable analysis. The majority of patients in our cohort underwent stapled anastomosis, a surgical technique that requires a residual 1.5 to 2 cm cuff, and thereby predisposes to the development of cuffitis, especially in the setting of precolectomy refractory disease. Previous studies have compared hand-sewn and stapled anastomosis and found similar short-term postoperative outcomes but better long-term functional outcomes such as nocturnal continence with stapled anastomosis.34, 35 The benefits of functional outcomes should be weighed against the risks of cuffitis when patients are counseled about restorative proctocolectomy and the options for anastomosis. We emphasize the clinical significance of cuffitis particularly because of its significant association with pouch failure in our cohort.
Crohn's disease–like condition of the pouch developed in approximately 12% of our patients a median interval of 2.1 years after IPAA completion and was significantly more associated with age 45 to 54 years at colectomy as compared with 18 to 24. Our results are similar to those published in a recent meta-analysis by Barnes et al, where the standardized incidence of CDLC of the pouch was 10.3% and median time to diagnosis was 12 to 36 months.36 Endoscopic remission within 12 months of biologic initiation, defined as a decrease in the PDAI endoscopic subscore of at least 50% with resolution of mucosal ulcers and/or erosions in the pouch and/or afferent limb, was noted in roughly 40% of our patients, comparable to previously reported rates of 40%–60%.37–41 Overall, these rates are quite low and suggest limited efficacy of biologics once chronic inflammation has occurred. The etiology and pathogenesis of CDLC of the pouch is unknown, and whether this disease entity represents traditional CD or a new phenotype in the spectrum of IBD remains to be studied.
The overall incidence of pouch failure in our cohort was 6.7% and was driven by inflammatory conditions. Rates of pouch failure in chronic pouchitis and CDLC patients were similar among those who received biologics and those who did not. While biologic use was significantly associated with pouch failure in bivariable analysis, these results are limited by confounding by indication and our inability to retrospectively assess severity of disease. Biologics were likely used in patients with refractory inflammatory conditions at the greatest risk for nonresponse and pouch failure. The underlying pathophysiology of these refractory pouches needs to be further characterized to develop adequate therapies.
Our study has a number of strengths. First, our sample size was large and reflected the updated use of minimally invasive laparoscopic surgical techniques and biologic therapies. Second, all data were reviewed by a single investigator blinded to outcomes to ensure homogeneity. Third, only patients with pouchoscopy data were included, and all outcomes were defined strictly according to clinical and endoscopic data. Symptomatic patients who were empirically treated for inflammatory pouch conditions without pouchoscopy data were not included to preserve the homogeneity of outcomes and avoid patients who might have other noninflammatory, symptomatic pouch conditions. Fourth, we report multiple original findings and risk factors for inflammatory pouch disorders that have not been previously published.
Our study also has a number of limitations. First, it is a retrospective review and therefore subject to selection bias and potential inclusion of patients with complications that require pouchoscopy and ongoing medical care. Second, patients without pouchoscopy follow-up were excluded, and therefore, only 386 (62%) patients of the original 621 patient cohort were included for analysis. As a result, the true rates and risk factors of inflammatory pouch conditions might be different than reported. Reassuringly, however, there were no significant demographic or clinical differences between the included patients with pouchoscopy follow-up and the excluded patients without. Third, our median follow-up was approximately 4 years and may have been inadequate to capture the development of all inflammatory pouch conditions. Fourth, there is significant overlap between chronic pouchitis and CDLC as they represent subtle gradations of pouch inflammation. Many patients who were labeled as chronic pouchitis according to our criteria may have been captured at the early stages of CDLC. We were careful to categorize chronic pouchitis as distinct from CDLC; however, it is difficult to accurately delineate their differences based on retrospective clinical data alone. Fifth, given that our cohort is from a single center, these results may not be broadly generalizable; however, the majority of IPAA surgeries occur at tertiary care centers similar to our study location.42
CONCLUSION
In conclusion, inflammatory pouch conditions are common and are associated with multiple preoperative risk factors. Rates of pouch failure were similar in chronic pouchitis and CDLC patients who received biologics and those who did not. There is significant overlap between chronic pouchitis and CDLC, and certain patients may harbor a type of refractory pouch inflammation that is unresponsive to biologic therapy and results in pouch failure. We do not yet possess the ability to risk stratify patients at the time of colectomy and predict those who will develop chronic inflammatory conditions and pouch failure. Prospective studies are needed to assess the genetic, proteomic, transcriptomic, and microbiota factors that predict inflammatory pouch conditions in order to develop risk stratification algorithms, surveillance protocols, and targeted therapies.
Supplementary Material
Glossary
Abbreviations
- UC
ulcerative colitis
- CD
Crohn’s disease
- CDLC
Crohn’s disease–like condition
- IBDU
inflammatory bowel disease unspecified
- TPC
total proctocolectomy
- IPAA
ileal pouch anal anastomosis
- IQR
interquartile range
- BMI
body mass index
Author Contribution: MK contributed to the study concept and design, acquisition of data, analysis and interpretation of data, drafting of the manuscript, critical revision of the manuscript for important intellectual content, and statistical analysis. MP contributed to the study concept and design, acquisition of data, and critical revision of the manuscript for important intellectual content. ARizvi, MR, ARiggs, CY, ET, and PT acquisition of data, critical revision of the manuscript for important intellectual content. RCU contributed to the analysis and interpretation of data, statistical analysis, and critical revision of the manuscript for important intellectual content. SK, PS, AG, and JFC contributed to the critical revision of the manuscript for important intellectual content. MCD contributed to the study concept and design and critical revision of the manuscript for important intellectual content. All authors approved the final version of the manuscript.
Conflicts of Interest: RCU has served as an advisory board member or consultant for Janssen, Pfizer, and Takeda and has research grants from Abbvie, Boehringer Ingelheim, and Pfizer. JFC has served as a consultant for Abbbie, Amgen, Arena Pharmaceuticals, Boehringer Ingelheim, Celgene Corporation, Celltrion, Eli Lilly, Enterome, Ferring Pharmaceuticals, Genentech, Janssen Pharmaceuticals, Landos, Ipsen, Medimmune, Merck, Novartis, Pfizer, Shire, Takeda, and Tigenix and has research grants from Abbvie, Janssen, and Takeda. MCD has served as a consultant for Janssen, Takeda, Pfizer, Celgene, and Abbvie and has research grants from Pfizer and Abbvie. The authors have no other relevant conflicts of interest or disclosures.
REFERENCES
- 1. Fumery M, Singh S, Dulai PS, et al. . Natural history of adult ulcerative colitis in population-based cohorts: a systematic review. Clin Gastroenterol Hepatol. 2018;16:343–356.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hahnloser D, Pemberton JH, Wolff BG, et al. . Results at up to 20 years after ileal pouch-anal anastomosis for chronic ulcerative colitis. Br J Surg. 2007;94:333–340. [DOI] [PubMed] [Google Scholar]
- 3. Fazio VW, Ziv Y, Church JM, et al. . Ileal pouch-anal anastomoses complications and function in 1005 patients. Ann Surg. 1995;222:120–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Michelassi F, Lee J, Rubin M, et al. . Long-term functional results after ileal pouch anal restorative proctocolectomy for ulcerative colitis: a prospective observational study. Ann Surg. 2003;238:433–441; discussion 442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Berndtsson I, Lindholm E, Oresland T, et al. . Long-term outcome after ileal pouch-anal anastomosis: function and health-related quality of life. Dis Colon Rectum. 2007;50:1545–1552. [DOI] [PubMed] [Google Scholar]
- 6. Ferrante M, Declerck S, De Hertogh G, et al. . Outcome after proctocolectomy with ileal pouch-anal anastomosis for ulcerative colitis. Inflamm Bowel Dis. 2008;14:20–28. [DOI] [PubMed] [Google Scholar]
- 7. Shen B. Acute and chronic pouchitis–pathogenesis, diagnosis and treatment. Nat Rev Gastroenterol Hepatol. 2012;9:323–333. [DOI] [PubMed] [Google Scholar]
- 8. Tulchinsky H, Hawley PR, Nicholls J. Long-term failure after restorative proctocolectomy for ulcerative colitis. Ann Surg. 2003;238:229–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Thompson-Fawcett MW, Mortensen NJ, et al. . “Cuffitis” and inflammatory changes in the columnar cuff, anal transitional zone, and ileal reservoir after stapled pouch-anal anastomosis. Dis Colon Rectum. 1999;42:348–355. [DOI] [PubMed] [Google Scholar]
- 10. Wu B, Lian L, Li Y, et al. . Clinical course of cuffitis in ulcerative colitis patients with restorative proctocolectomy and ileal pouch-anal anastomoses. Inflamm Bowel Dis. 2013;19:404–410. [DOI] [PubMed] [Google Scholar]
- 11. Lavery IC, Sirimarco MT, Ziv Y, et al. . Anal canal inflammation after ileal pouch-anal anastomosis. The need for treatment. Dis Colon Rectum. 1995;38:803–806. [DOI] [PubMed] [Google Scholar]
- 12. Sherman J, Greenstein AJ, Greenstein AJ. Ileal j pouch complications and surgical solutions: a review. Inflamm Bowel Dis. 2014;20:1678–1685. [DOI] [PubMed] [Google Scholar]
- 13. Braveman JM, Schoetz DJ Jr, Marcello PW, et al. . The fate of the ileal pouch in patients developing Crohn's disease. Dis Colon Rectum. 2004;47:1613–1619. [DOI] [PubMed] [Google Scholar]
- 14. Zaghiyan K, Kamiński JP, Barmparas G, et al. . De novo Crohn's disease after ileal pouch-anal anastomosis for ulcerative colitis and inflammatory bowel disease unclassified: long-term follow-up of a prospective inflammatory bowel disease registry. Am Surg. 2016;82:977–981. [PubMed] [Google Scholar]
- 15. Hartley JE, Fazio VW, Remzi FH, et al. . Analysis of the outcome of ileal pouch-anal anastomosis in patients with Crohn's disease. Dis Colon Rectum. 2004;47:1808–1815. [DOI] [PubMed] [Google Scholar]
- 16. Gemlo BT, Wong WD, Rothenberger DA, et al. . Ileal pouch-anal anastomosis. Patterns of failure. Arch Surg. 1992;127:784–786; discussion 787.27. [DOI] [PubMed] [Google Scholar]
- 17. Peyrègne V, Francois Y, Gilly FN, et al. . Outcome of ileal pouch after secondary diagnosis of Crohn's disease. Int J Colorectal Dis. 2000;15:49–53. [DOI] [PubMed] [Google Scholar]
- 18. Lightner AL, Pemberton JH, Loftus EJ Jr. Crohn's Disease of the Ileoanal Pouch. Inflamm Bowel Dis. 2016;22:1502–1508. [DOI] [PubMed] [Google Scholar]
- 19. Shen B, Fazio VW, Remzi FH, et al. . Clinical features and quality of life in patients with different phenotypes of Crohn's disease of the ileal pouch. Dis Colon Rectum. 2007;50:1450–1459. [DOI] [PubMed] [Google Scholar]
- 20. Tulchinsky H, Hawley PR, Nicholls J. Long-term failure after restorative proctocolectomy for ulcerative colitis. Ann Surg. 2003;238:229–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zittan E, Ma GW, Wong-Chong N, et al. . Ileal pouch-anal anastomosis for ulcerative colitis: a Canadian institution's experience. Int J Colorectal Dis. 2017;32:281–285. [DOI] [PubMed] [Google Scholar]
- 22. Baek SJ, Dozois EJ, Mathis KL, et al. . Safety, feasibility, and short-term outcomes in 588 patients undergoing minimally invasive ileal pouch-anal anastomosis: a single-institution experience. Tech Coloproctol. 2016;20:369–374. [DOI] [PubMed] [Google Scholar]
- 23. Baek SJ, Lightner AL, Boostrom SY, et al. . Functional outcomes after laparoscopic ileal pouch-anal anastomosis in patients with chronic ulcerative colitis: long-term follow-up of a case-matched study. J Gastrointest Surg. 2017;21:1304–1308. [DOI] [PubMed] [Google Scholar]
- 24. Harris PA, Taylor R, Thielke R, et al. . Research electronic data capture (REDCap) – a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Silverberg MS, Satsangi J, Ahmad T, et al. . Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: report of a Working Party of the 2005 Montreal World Congress of Gastroenterology. Can J Gastroenterol. 2005;19(Suppl A):5A–36A. [DOI] [PubMed] [Google Scholar]
- 26. Martland GT, Shepherd NA. Indeterminate colitis: definition, diagnosis, implications and a plea for nosological sanity. Histopathology. 2007;50:83–96. [DOI] [PubMed] [Google Scholar]
- 27. Sandborn WJ, Tremaine WJ, Batts KP, et al. . Pouchitis after ileal pouch-anal anastomosis: a pouchitis disease activity index. Mayo Clin Proc. 1994;69:409–415. [DOI] [PubMed] [Google Scholar]
- 28. Fazio VW, Kiran RP, Remzi FH, et al. . Ileal pouch anal anastomosis: analysis of outcome and quality of life in 3707 patients. Ann Surg. 2013;257:679–685. [DOI] [PubMed] [Google Scholar]
- 29. Wu XR, Ashburn J, Remzi FH, et al. . Male gender is associated with a high risk for chronic antibiotic-refractory pouchitis and ileal pouch anastomotic sinus. J Gastrointest Surg. 2016;20:631–639. [DOI] [PubMed] [Google Scholar]
- 30. Joelsson M, Benoni C, Oresland T. Does smoking influence the risk of pouchitis after ileal pouch anal anastomosis for ulcerative colitis? Scand J Gastroenterol. 2006;41:929–933. [DOI] [PubMed] [Google Scholar]
- 31. Setti Carraro P, Talbot IC, Nicholls RJ. Longterm appraisal of the histological appearances of the ileal reservoir mucosa after restorative proctocolectomy for ulcerative colitis. Gut. 1994;35:1721–1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hashavia E, Dotan I, Rabau M, et al. . Risk factors for chronic pouchitis after ileal pouch-anal anastomosis: a prospective cohort study. Colorectal Dis. 2012;14:1365–1371. [DOI] [PubMed] [Google Scholar]
- 33. Burisch J, Ungaro R, Vind I, et al. . Proximal disease extension in patients with limited ulcerative colitis: a Danish population-based inception cohort. J Crohns Colitis. 2017;11:1200–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lovegrove RE, Constantinides VA, Heriot AG, et al. . A comparison of hand-sewn versus stapled ileal pouch anal anastomosis (IPAA) following proctocolectomy: a meta-analysis of 4183 patients. Ann Surg. 2006;244:18–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Saigusa N, Kurahashi T, Nakamura T, et al. . Functional outcome of stapled ileal pouch-anal canal anastomosis versus handsewn pouch-anal anastomosis. Surg Today. 2000;30:575–581. [DOI] [PubMed] [Google Scholar]
- 36. Barnes EL, Kochar B, Jessup HR, et al. . The incidence and definition of Crohn's disease of the pouch: a systematic review and meta-analysis. Inflamm Bowel Dis. 2019;25:1474–1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ferrante M, D'Haens G, Dewit O, et al. ; Belgian IBD Research Group Efficacy of infliximab in refractory pouchitis and Crohn's disease-related complications of the pouch: a Belgian case series. Inflamm Bowel Dis. 2010;16:243–249. [DOI] [PubMed] [Google Scholar]
- 38. Bär F, Kühbacher T, Dietrich NA, et al. ; German IBD Study Group Vedolizumab in the treatment of chronic, antibiotic-dependent or refractory pouchitis. Aliment Pharmacol Ther. 2018;47:581–587. [DOI] [PubMed] [Google Scholar]
- 39. Colombel JF, Ricart E, Loftus EV Jr, et al. . Management of Crohn's disease of the ileoanal pouch with infliximab. Am J Gastroenterol. 2003;98:2239–2244. [DOI] [PubMed] [Google Scholar]
- 40. Weaver KN, Gregory M, Syal G, et al. . Ustekinumab is effective for the treatment of Crohn's disease of the pouch in a multicenter cohort. Inflamm Bowel Dis. 2019;25:767–774. [DOI] [PubMed] [Google Scholar]
- 41. Gregory M, Weaver KN, Hoversten P, et al. . Efficacy of vedolizumab for refractory pouchitis of the ileo-anal pouch: results from a multicenter US cohort. Inflamm Bowel Dis. 2019;25:1569–1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hoang C, Davids K, Wyman A, et al. . A steady trend but a general re-distribution of elective IPAA for ulcerative colitis. In: The American Society of Colon and Rectal Surgeons Annual Meeting Abstracts, May 19–23, 2018, Nashville, Tennessee. Abstract S34.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.