Abstract
Romidepsin (Istodax®, depsipeptide, FR901228, FK228, NSC 630176) is a cyclic peptide, broad-spectrum, potent histone deacetylase inhibitor, with activity mainly against class I histone deacetylase enzymes. In this article, we give an overview of the putative modes of action, such as effects on gene expression, cell cycle regulation, apoptosis induction, DNA repair, protein acetylation and induction of autophagy. Romidepsin has mainly been developed as a therapy for hematologic malignancies and is approved by the US FDA for the treatment of cutaneous T-cell lymphomas. This report outlines the laboratory and clinical development of the compound as a single agent that has more recently been evaluated in combination with other anticancer therapeutics, such as proteasome inhibitors.
Keywords: apoptosis, hematology, histone deacetylase inhibitor, malignancy, romidepsin
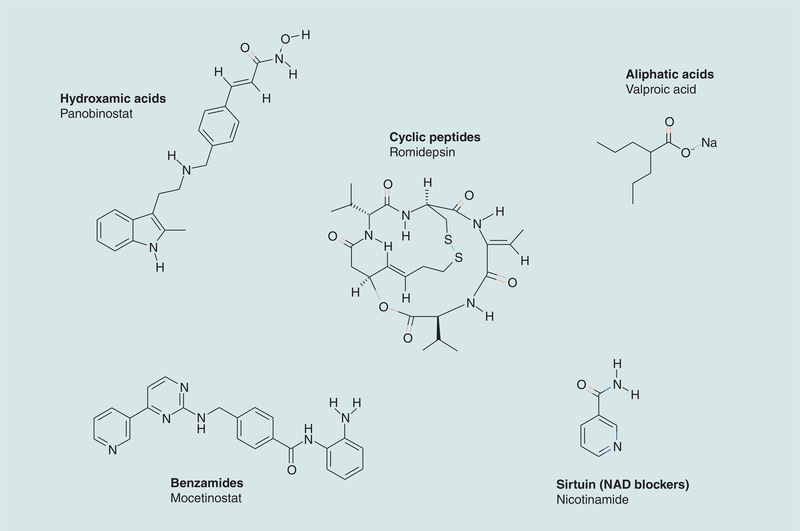
Romidepsin (Istodax®, depsipeptide, FR901228, FK228, NSC 630176) (Figure 1), is a bicyclic tetrapeptide first reported in 1994 as a fermentation product from Chromobacterium violaceum. Romidepsin was subsequently identified in 1998 as a broad-spectrum histone deacetylase (HDAC) inhibitor that induces epigenetic modifications, affecting the acetylation of histones and other proteins, modifying gene expression and altering the status of cancer cells [1].
Figure 1.
Structure of romidepsin and examples from other classes of histone deacetylase inhibitors in clinical use.
Romidepsin is currently being investigated for the treatment of hematologic and solid tumor malignancies. It was given accelerated approved by the US FDA in November 2009 for the treatment of relapsed cutaneous T-cell lymphoma (CTCL) and in June 2011 for the treatment of relapsed/refractory peripheral T-cell lymphoma (PTCL), with both approvals based on efficacy data from two multicenter, single-arm studies [2,3]. In both histologies, comparable response rates were observed in the two studies. For example, in CTCL, objective response rates were 34 and 35%, with an identical complete response (CR) rate of 6% and median response durations of 15 and 11 months, respectively.
This article aims to summarize the development of romidepsin from compound discovery, through preclinical testing and finally into clinical trials.
General background of HDAC inhibitors
Chromatin modification is an important regulator of gene transcription, and the acetylation status of histones plays a critical role in this control mechanism [4]. Histone acetyltransferases (HAT) and HDAC enzymes mediate acetylation and deacetylation of histones, respectively. In general, increased acetylation of specific residues in histones H3 and H4 is associated with open chromatin, allowing active transcription to occur. Acetylation is just one of multiple post-translational modifications of histones that affect gene transcription. Others include methylation, phosphorylation, ubiquitination, sumoylation and so on. Acetylation appears to provide a general ‘go’ signal, while some of the other modifications lend more specificity to gene-expression signals. Regulation of acetylation has been the most widely studied modification and, along with methylation, it has been translated to the clinic. In the 1970s, a number of naturally occurring and synthetic compounds possessing weak HDAC inhibitor activity were discovered. These included sodium butyrate, sodium valproate [5–9] and phenylbutyrate [4] (see [10]). Since then, a range of considerably more potent, structurally diverse HDAC inhibitors have been identified. There are currently 18 known HDACs, grouped into four classes based on their homology to the respective yeast transcriptional control factor sequence [11].
Class I includes HDAC1, 2, 3 and 8 (located within the cell nucleus, isoenzymes homologous to the yeast RPD3 protein); class II includes the HDAC4, 5, 6, 7, 9 and 10 (isoenzymes homologous to the yeast HDA1); and class IV includes HDAC11. Class III HDACs include the NAD+ dependent sirtuin family 1–7 (with homology to yeast Sir2). While the restricted (nuclear or cytoplasmic) localization of HDACs is widely accepted, there is some evidence that the localization of HDACs may not be as tightly regulated as first thought. For example, the class I HDAC1 has been found to also localize to the centrosome during M phase, in the cytosol of axons during demyelination [12,13] and class II shuttles between nucleus and cytoplasm; by contrast, class IV generally reside in the nucleus [11,14]. HDAC inhibitors are generally subdivided into six groups based on their chemical structure and their targets (Figure 1 & Table 1).
Table 1.
Histone deacetylase inhibitor structure and targets.
| HDAC inhibitor group | Examples | HDAC targets |
|---|---|---|
| Hydroxamic acids | Trichostatin A Vorinostat (SAHA) Belinostat (PXD101) Panobinostat (LBH589) |
Class I and II |
| Cyclic peptides | Trapoxin B – tetrapeptide Spiruchostatin A – pentapeptide Romidepsin – pentapeptide (Istodax®– depsipeptide) |
Class I (± class II and IV effects) |
| Benzamides | Entinostat (MS-275, SNDX-275) CI994 Mocetinostat (MGCD0103) |
Class I |
| Electrophilic (heterocyclic) ketones | Trifluoromethyl ketones α-ketoamides |
Class II Class I, II and III? |
| Aliphatic acid | Phenylbutyrate Valproic acid |
Class I |
| NAD blockers | Nicotinamide Derivatives of NAD Dihydrocoumarin Naphthopyranone 2-hydroxynaphaldehydes |
Class III (sirtuin) |
HDAC: Histone deacetylase; SAHA: Suberoylanilide hydroxamic acid.
It is important to recognize that there are likely to be substantial differences between the various HDAC inhibitors, owing not only to individual pharmacokinetic properties, but also on their targets for enzymatic inhibition. Classes I, II and IV require a Zn+ molecule in their active site and are inhibited by HDAC inhibitors, while class III HDACs require NAD+ as a coenzyme, and are not affected by HDAC inhibitors [15].
Currently, clinically available HDAC inhibitors have no action on the sirtuin family, although new molecules such as NAD blockers, cambinol and tenovin 6 have shown promise in preclinical studies. In addition, there is variability in the capacity of HDAC inhibitors to induce hyperacetylation of lysine residues on nuclear (class I) targets as opposed to cytoplasmic (class II) targets, such as tubulin, Hsp90, p53, Ku70, STAT-3, RelA/p65, bcr–abl and HIF-1α, amongst others [16–18].
It remains unclear whether pan-HDAC inhibitors (e.g., panobinostat, vorinostat and romidepsin), which inhibit both class I and II HDACs, are clinically more effective than class I isotype-selective HDAC inhibitors (e.g., MGCD0103, which is class I inhibitor selective). Similarly, toxicity profiles differ across the various HDAC inhibitors, even within a given drug group. The side-effect profile of all HDAC inhibitors includes fatigue, nausea and diarrhea. Thrombocytopenia is the most common myelosuppressive effect.
General pharmacology of romidepsin
Romidepsin is a pentapeptide and a member of the cyclic peptide family. It is a natural product, along with other naturally occurring compounds such as trichostatin A, spiruchostatin A, chlamydocin, and trapoxin A and B [1]. It was first reported in 1994 as a fermentation product from C. violaceum and identified in a screen for novel products that would reverse the malignant phenotype of ras-transformed cells to that of their nontransformed counterparts [19,20]. The National Cancer Institute (NCI) evaluated romidepsin in the NCI 60 cell line panel in the early 1990s, where it was found to have potent activity across a broad spectrum of cancer cell types and, based on its unique profile, was selected for preclinical development [21]. It was subsequently identified as an HDAC inhibitor in 1998 [1,21]. Since that time, the potent anticancer effects of romidepsin have been characterized in numerous in vitro and in vivo biological systems.
Romidepsin is a nonhygroscopic, white crystalline powder with limited aqueous solubility and varying solubility in organic solvents [19]. It is highly bound in human serum (94–95%) and plasma (92–94%). The principal binding protein in human serum is AAG. It was found in a rodent study to be predominantly metabolized by the liver (79.4% eliminated in bile and feces) [19,22]. It is thought to be primarily metabolized by the CYP450 family CYP3A4 isoform. In humans, elimination has yet to be fully characterized and no dedicated hepatic or renal impairment studies have been conducted. The product insert states that, based on a population pharmacokinetic analysis, mild hepatic impairment does not alter pharmacokinetics of romidepsin [23]. Patients with moderate and severe hepatic impairment should be treated with caution. Renal impairment is not expected to significantly influence drug exposure, but the effect of end-stage renal disease on romidepsin pharmacokinetics has not been studied. Phase I PK studies have demonstrated that romidepsin has a time to peak concentration of 4 h after intravenous (iv.) infusion and a terminal half-life of 2.92 h with a curve fit suggesting a two-compartment model [19,22]. In nonhuman primates there is limited CNS penetration (2% of the administered dose), which approaches the IC50 of some tumors [24]. Plasma levels for oral administration were extremely variable, suggesting multiple absorption sites; an orally active preparation is not yet available for use in humans.
Romidepsin requires intracellular disulfide bond reduction to mediate structural changes necessary for its potent activity as an HDAC inhibitor [25]. In contrast to the vorinostat/panobinostat family of HDAC inhibitors, which comprise hydroxamic acids that act by chelating zinc in the catalytic pocket resulting in the inhibition of the enzyme, the functional sulfhydryl group of reduced romidepsin is capable of preferentially interacting with the zinc in the active site of HDAC1 and HDAC2 (class I) enzymes. Romidepsin does inhibit HDAC class II and IV enzymes, albeit at a higher concentration [25,26], and while the Ki of romidepsin with HDAC6 was 0.0095, similar to that of panobinostat at 0.0015 [26], this has not translated into effects on the acetylation status of targets of HDAC6 such as tubulin. Of particular note is that the IC50 is greatly reduced following sulfhydryl reduction/activation (Table 2). This reduction/activation is mediated by glutathione following intracellular uptake and can actually harness one of the main mechanisms by which cancer cells typically inactivate many drugs [25]. This metabolic pathway underlines the need to assess the function of these drugs in vivo where reduction/activation pathways may be different from in vitro effects. However, reduced romidepsin is rapidly inactivated by serum; thus, in vivo circulating romidepsin acts as a stable reservoir of drug, which easily gains entry into cells because of its more hydrophobic properties compared with reduced romidepsin [25].
Table 2.
Increased potency of reduced (activated) romidepsin.
| Enzymes | IC50(nM) | ||
|---|---|---|---|
| Romidepsin | Activated romidepsin | Vorinostat (SAHA) | |
| HDAC1 | 111.4 | 7.0 | 175.8 |
| HDAC2 | 295.8 | 28.4 | 39.4 |
| HDAC3 | 462.4 | 103.0 | 316.2 |
| HDAC4 | 465.6 | 95.7 | 860.3 |
| HDAC5 | 567.0 | 79.6 | 87.8 |
| HDAC6 | 1175.0 | 32.6 | 70.0 |
| HDAC7 | 2472.0 | 278.6 | 107.6 |
| HDAC8 | 413.0 | 34.3 | 437.5 |
| HDAC9 | 6577.0 | 2729.0 | 252.3 |
| HDAC10 | 1172.0 | 368.1 | 1276.0 |
| HDAC11 | 169.0 | 64.1 | 183.2 |
SAHA: Suberoylanilide hydroxamic acid.
Data supplied by Gloucester Pharmaceuticles Inc. and performed by Epigentek 2007.
Overview of HDAC inhibitor mechanisms of action
The precise manner in which HDAC inhibitors exert these biological effects is still under active investigation in a number of preclinical studies. The current opinion is that these agents inhibit, to a greater or lesser extent, the activity of class I, II and IV HDACs, causing chromatin remodeling and altered gene expression, which results in biological effects that are deleterious to tumor cell growth and survival. Biological responses of HDAC inhibitors include inhibition of cell proliferation, induction of apoptosis or autophagy, upregulation of genes related to cellular differentiation, modulation of immune responses and suppression of angiogenensis. In this context, several studies suggest that the lethal effects of HDAC inhibitors toward transformed cells stem from, at least in part, induction of oxidative injury [27,28], interference with DNA repair [29] and the resulting DNA damage [30]. Furthermore, recent evidence implicating alterations in protein acetylation and induction of autophagy [31] provides a link between the latter process and the DNA repair program [32]. These observations provide a rationale for combining HDAC inhibitors with radiation, DNA-damaging chemotherapeutics and proteasome inhibitors, amongst others.
Effects on the cell cycle
HDAC inhibitors can induce cell cycle arrest at the G1/S checkpoint through increased gene expression and upregulation of the CDK inhibitor p21WAF1/CIP1 [33,34]. p21 forms complexes with cyclins D and E, preventing them from coupling with CDK and moving the cell through the G1/S checkpoint. One mechanism suggested for the upregulation of p21 is through loss of p21 promoter binding by the Sp1 transcription factor due to its acetylation, allowing access of Sp3 to the p21 promoter, which could alter gene transcription. This mechanism allows gene expression with upregulation of p21 and the proapoptotic protein Bak, suggesting a common mechanism for cell cycle arrest and apoptosis [35].
Effects of HDAC inhibitors on the G2/M phase of the cell cycle appear to be as important, and perhaps more deleterious than the G1 arrest due to p21 induction. Brazelle et al. demonstrated that HDAC inhibitor treatment decreased levels of total Chk1 proteins, with a decrease in the levels of the inhibitory phosphorylation of key cell cycle regulatory proteins cdc25c and cdc2, with abnormal mitosis and failed cytokinesis [36].
HDAC inhibition results in aberrant mitosis after G1 and, in tumor cells where the G2 checkpoint is defective, these cells are allowed to progress to mitosis. HDAC inhibitors slow the normal cellular progression through G2/M, with the result being that cells stay in mitosis up to two and a half times longer than normal. One study reports downregulation of the cyclin B1 gene, blocking the G2/M transition and suppressing the transcription of Plk1 and survivin as a mechanism of action [37,38]. HDAC inhibitors prevent the formation of the normal chromosomal passenger complex. This is most likely owing to disruption of the normal chromatin structure preventing the binding of HP1, as a pan-specific effect of HDAC inhibitors rather than one mediated by an HDAC6-α-tubulin interaction [39]. At the point of mitosis, the mitotic spindle checkpoint is also defective owing to HDAC inhibitor treatment, which therefore results in cells prematurely exiting mitosis without segregating their chromosomes, signaling apoptosis [39]. Finally, Robbins et al. noted that following romidepsin exposure not only was HP1 decreased on pericentrometic heterochromatin, but that there was also reduced pericentrometic Aurora B kinase, reduced phosphorylation of histone H3S10, and reduced levels of hBUB1, CENP-F and CENP-E in the kinetochores of treated cells [40].
Despite major changes, these effects are short-lived for two reasons. First, the effects are rapidly reversed after drug washout due to rapid histone deacetylation. Second, the disruption of the spindle checkpoint occurs at a higher concentration of HDAC inhibitor and requires higher levels of hyperacetylation. Thus, as the drug levels come down, the spindle checkpoint is re-established and aberrant mitosis alone is not enough to induce apoptosis. This, and the relatively short half-life of less than 3 h (see below), may explain why, in our clinical experience, the side effects of romidepsin are short-lived following drug cessation [41]. This has been born out by in vitro data showing that romidepsin has selective cytotoxicity toward chronic lymphocytic leukemia (CLL) B cells compared with normal cells [35].
One study examined effects on cultured ras 1-mutated cells derived from NIH3T3 fibroblast cells through FACS [20]. Romidepsin arrested the cells in G0/G1 phase, and this effect was rapidly reversed after removal of drug. By contrast, in PC-3 (prostate cancer) cells treated with higher doses of romidepsin, the cell cycle was arrested at the G2/M phase rather than in the G1 phase. The onset of gene-expression changes preceded that of apoptosis, cell cycle arrest and differentiation.
Induction of apoptosis
It is now recognized that a key mechanism of action of HDAC inhibitors is through induction of apoptosis [6,7,8], and a variety of HDAC inhibitors have demonstrated apoptosis and cell death in vitro using several lymphoma models [41–48].
The consequence of histone hyperacetylation in tumor cells is an upregulation of proapoptotic genes (i.e., TRAIL, DR4 and 5, Bak, Bax, Apaf-1, Bmf, Bim and TP2) and downregulation of antiapoptotic genes (i.e., Bcl-2, Bcl-xL, Mcl1, XIAP, Survivin, Akt, c-FLIP and c-RAF) (see [17] and references therein), which are important for HDAC inhibitor-induced changes in cell survival [34,49–53].
Romidepsin has been shown to induce apoptosis in a number of cell lines, especially if combined with other antitumour agents. One study demonstrated that mantle cell lymphoma (MCL) cells and follicular lymphoma cells exposed to romidepsin alone showed minimal apoptosis. However, when the direct apoptosis pathway was activated by coincubation with CD95 or TRAIL, there was marked synergistic induction of apoptosis by facilitating the formation of an active DISC, leading to the rapid activation of caspase-8 [54]. Romidepsin has been shown to cause a dose-dependent increase of caspase-induced apoptosis due to selective involvement of the TNF receptor pathway, initiating caspase 8 and effector caspase 3. Caspase activation is accompanied by downregulation of cFLIP, competitive inhibitor of caspase 8, but with no evidence of upregulation of Fas death ligand expression.
The observation that romidepsin operates via a caspase 8-mediated process in human CLL cells is relevant, as this pathway is not activated by any other therapeutic agents currently used. Other antiapoptotic proteins, including Bcl-2, Mcl-1 and XIAP, showed no change [55].
In CLL cells treated with a combination of romidepsin and proteasome inhibitor bortezomib there was an increase in the expression of BIM, a BH3-only proapoptotic protein, and reduced levels of antiapoptotic proteins, such as Bcl-xL, A1, XIAP, cIAP1, c-FLIP and ICAM-1 [56]. By contrast to the combination of bortezomib with belinostat, bortezomib/romidepsin coadministration did not increase tubulin acetylation (mediated by HDAC6). The combination of either HDAC inhibitor with bortezomib prevented HDAC inhibitor-mediated κB activation by RelA acetylation (NF-κB family transcription factor) and nuclear accumulation of NF-κB and its activation. IKK inhibitors block RelA acetylation in cell exposed to HDAC inhibitor mediated by IkBα, resulting in a significant increase in CLL tumor cell kill. When the myeloma cell line U266 was treated with romidepsin at 0.1 μmol/l for 24 h, there was a similar pattern of decrease in antiapoptotic proteins BCL-2, Bcl-xL and MCL-1 at 24 h [57]. The response of the proapoptotic protein BAX to romidepsin was interesting, in that it was reduced in U266 cell lines and increased in primary myeloma cells.
With respect to T-cell lymphomas, where clinical responses are regularly observed (see below), in vitro studies demonstrate that response to romidepsin in the HUT78 human CTCL cell line is associated with induction of histone acetylation, increased p21WAF1/CIP1 expression and detection of markers of apoptosis, rather than cell cycle arrest [58]. Cotreatment with a caspase inhibitor significantly inhibited apoptosis, although not completely, again implying that both caspase-dependent and -independent processes contribute to the cell death effects of romidepsin.
Effects of romidepsin on gene expression
In an attempt to identify genes involved in the growth suppression effects of romidepsin, total RNA from three romidepsin-sensitive cell lines, lymphoma (U-937), prostate cancer (PC-3) and renal cancer (ACHN), were used in vitro, and analyzed using the Affymetrix FL GeneChip. Of the 7070 genes examined for response to romidepsin, 105 genes were commonly induced and 100 genes were commonly repressed by romidepsin. Genes commonly upregulated included p21WAF1/Cip1, IL-8 and caspase 9; examples of commonly downregulated genes were MAPK and cyclin A2. Many of these genes encode proteins with key regulatory roles in signal transduction, growth arrest and apoptosis [49].
Treatment with romidepsin was also demonstrated to upregulate expression of the IL-2 receptor, the target for denileukin diftitox, a fusion toxin combining the receptor component of IL-2 with diphtheria toxin with clinical activity in CTCL [58]. Romidepsin was indeed shown to increase the sensitivity of HUT78 cell line to denileukin diftitox with a synergistic increase in apoptosis [59]. The same study showed romidepsin to increase expression of the MDR1 gene, and it was noted that cells selected for resistance overexpressed P-gp.
Resistance was reversed with P-gp inhibition, and so theoretically romidepsin may induce its own mechanism of resistance [60]. Increased MDR1 expression and P-gp activity have also been shown in both normal (sixfold increase in MDR1) and malignant (eightfold increase in MDR1) peripheral blood mononuclear cells (PBMCs) in patients receiving romidepsin during clinical trials at the NIH [59]. Cells selected for resistance to romidepsin in the presence of a P-gp inhibitor do not have increased P-gp expression; and alternate methods of resistance must also exist [60].
Post-translational targets of acetylation
There is a growing body of evidence to show that as well as altering histone acetylation and thus gene expression, HDAC inhibitors are able to induce effects via post-translational acetylation of proteins. Choudhary et al. have demonstrated that suberoylanilide hydroxamic acid (SAHA; vorinostat) is able to induce acetylation of a wide variety macromolecular complexes involved in a broad spectrum of cellular process such as autophagy, the cell cycle and nuclear transport [61]. For example, p53 may be activated by the acetylation of at lysine residues 320 and 373, and induces apoptosis via PIG3 and NOXA [62], while inhibition of HDAC6 induces Ku70 acetylation, which releases Bax and again triggers apoptosis [63]. In addition, Robert et al. have attempted to link the effects on the DNA-damage response to autophagy via acetylation protein of multiple proteins such as Sae2, Cdk1, Ku, MRN, Blm2 and Rfa1 [32]. We have shown that complex processes such as proplatelet formation and platelet budding resulting in thrombocyopenia, one of the dose-limiting toxicities of romidepsin and other HDAC inhibitors, are due to increased phosphorylation of the myosin light chain. HDAC inhibition with either romidepsin or panobinostat reduced levels of the Rho-GTPases, CDC42, Rac1 and RhoA [64]. These effects may be mediated via either acetylation of HSP90, which in turn lead to a reduction in the levels of binding partners by increased degradation [65], or hyperacetylation of HSP70, which prevents the assembly of HSP90–client protein complexes [66,67]. These effects on chaperone protein binding may help unravel other downstream effects of HDAC inhibition.
In vitro preclinical studies
Cytotoxic activity
The cytotoxic activity of romidepsin has been explored in a large number of human cancer cell lines including leukemia, lymphoma, renal, colon, stomach, lung, ovarian and prostate cancer cell lines [19,20]. The data in Table 3 demonstrates that romidepsin may have activity in a variety of hematological and solid tumor types. Romidepsin cytotoxicity was evaluated by determining cell viability at the end of a 72-h incubation period and demonstrates antiproliferative activity, with mean IC50 ranging from 0.55–9.19 nM/ml.
Table 3.
Cytotoxic activity of romidepsin in human cell lines.
| Cell type | Cell lines | Mean IC50(nM/ml) |
|---|---|---|
| Leukemia, lymphoma | CCRF-CEM, THP-1, ML-3, HL-60, JOSK-1, K562, JOK-1 and U-937 | 6.07 |
| Renal cell carcinoma | OUR10, ACHN and A-498 | 9.19 |
| Prostate cancer | PC-3 and DU-145 | 2.98 |
| Colon adenocarcinoma | SW-480, Colo201 and HT-29 | 2.98 |
| Lung cancer | PC-10, NCI-H69, A549, PC-9, PC-1, PC-10, ADH and LX-1 | 2.46 |
| Breast cancer | MCF-7 and ZR-75-1 | 1.20 |
| Stomach cancer | MKN28 and MKN74 | 1.67 |
Romidepsin cytotoxicity was evaluated by determining cell viability at the end of a 72-h incubation period.
Data supplied by Gloucester Pharmaceuticals.
Effects on angiogenesis
The ability to suppress angiogenesis by decreasing the expression of VEGF as well as enhancement of the host immune system may also play a role in the therapeutic response of HDAC inhibitors [16–18]. It is currently unclear which of these biological effects predominate for the anticancer responses seen with HDAC inhibitors. The expression of angiogenesis factors, such as VEGF and basic FGF, are important for tumor neovascularization and growth. Romidepsin decreased the expression of VEGF mRNA by 51% after 12 h in PC-3 cancer prostate cells that were sensitive to romidepsin, but not in ACHN renal cancer cells that had been selectively cultured to be insensitive to romidepsin [68]. Under hypoxic conditions (mimicking conditions of angiogenesis in vivo), VEGF transcription is regulated by HIF-1. Activity of HIF-1 is primarily determined by hypoxia-induced stabilization of HIF-1α, a component subunit of HIF-1. Exposure to romidepsin at the onset of hypoxia completely inhibited the time-dependent increase in expression of VEGF mRNA from 4 to 16 h, as seen in control cells. When romidepsin was added 4 h after the onset of hypoxia, the expression level of VEGF mRNA was inhibited at 8 and 16 h. No changes were observed in the expression of HIF-1α mRNA under either treatment schedule. Romidepsin induced accumulation of acetylated histones in chromatin associated with the VEGF gene promoter. The highest level of acetylation of histone H3 and H4 was in the P2 region of the VEGF promoter, a region including the HIF-1 binding site, which plays an important role in regulating the expression of VEGF mRNA. In the I1 region, increased acetylation was observed for histone H3 but not histone H4. No increases were seen for either histone H3 or H4 in the E3 region. These findings suggest that romidepsin causes histone acetylation of VEGF promoter regions, particularly in the P2 region. It remains unclear whether HIF-1α is a direct target for acetylation by romidepsin [69]. However, HDAC inhibitors may also limit angiogenesis by inducing degradation of HIF-1α by altering its interactions with HSP90 and HSP70 mediated by HDAC6 [70] (as discussed in section ‘Post-translational targets of acetylation’).
Autophagy
Autophagy is a primary mechanism through which mammalian cells can capture and degrade protein aggregates, and has been implicated in the pathophysiology of a number of disease process such as Parkinson’s disease, and other neuro- and myo-degenerative disorders [71]. Aggresomes form when the ubiquitin proteasome system is overwhelmed with misfolded protein, such as following proteasome inhibition, and protein aggregates are actively transported on microtubules by a process requiring dynein/dynactin motors and HDAC6 (tubulin deacetylase). Mature autophagosomes form when the aggresome fuses with the lysosome. Autophagy induced by HDAC inhibitors involves inhibition of both HDAC1 and HDAC6 [72,73]. Although activated romidepsin has some direct activity against HDAC6 (Table 2), there is no in vitro evidence that romidepsin can effect intracellular acetylation of tubulin (in contrast to nonselective HDAC inhibitors such as belinostat) [56]. Whether romidepsin can affect HDAC6 in vivo is a subject of ongoing study. It has been suggested that romidepsin can induce autophagy in malignant rhabdoid tumor cells mediated by AIF translocation into the nucleus while also inducing apoptosis and necrosis in cell lines and dose-dependent antitumor responses in tumor-bearing animals [74]. The cytotoxicity was significantly increased by cotreatment with chloroquine, which disrupts autophagy. Whether the activity of romidepsin in this model is because of the observed increase in autophagy, or through nonapoptotic cell death despite the activation of a protective mechanism, remains to be proven.
Synergistic cytotoxicity with other agents
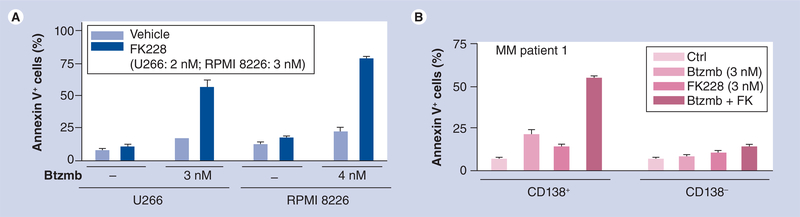
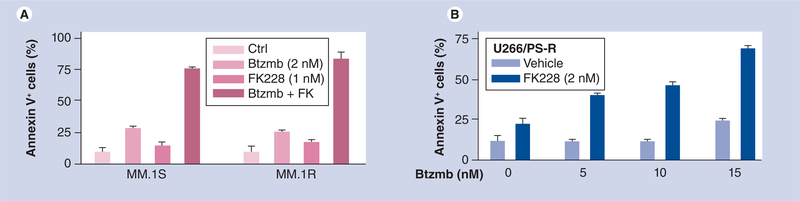
Dai et al. reported that romidepsin interacts in a highly synergistic manner with bortezomib in cultured as well as in primary, patient-derived CLL cells [56]. The mechanism by which HDAC inhibitors interact synergistically with proteasome inhibitors such as bortezomib is likely to be multifactorial, and may involve promotion of oxidative injury [75], disruption of aggresome function [76] and induction of endoplasmic reticulum stress [77]. Notably, bortezomib blocked romidepsin-induced activation of NF-κB in these cells, accompanied by downregulation of the NF-κB-dependent antiapoptotic proteins Bcl-xL and XIAP. As described in the case of CLL cells, synergistic interactions between romidepsin and bortezomib have also been observed in multiple myeloma (MM) cells. For example, as shown in Figure 2A, very low concentrations of romidepsin and bortezomib (e.g., <5 nM), when combined, dramatically increased apoptosis in U266 and 8226 myeloma cells. Similar effects were observed in primary CD138+ myeloma cells, but not in their CD138- counterparts (Figure 2B), suggesting differential effects within subcompartments of the myeloma clone. Notably, the combination displayed marked lethality toward dexamethasone- (Figure 3A) and bortezomib-resistant (Figure 3B) myeloma cells [Grant S, Dai Y, Unpublished data]. In addition, Khan et al. showed similar levels of apoptosis in U266 myeloma cells following treatment with romidepsin 0.01 μmol/l (43%), melphalan 10 μmol/l (44%) versus control (28%), and synergistic apoptosis when treated with both agents (73%) [57].
Figure 2. Romidepsin synergistically interacts with bortezomib to induce apoptosis in human myeloma cells.
(A) Human myeloma U266 and RPMI 8226 cells were exposed (48 h) to 2–3 nM FK228 in the absence or presence of 3–4 nM Btzmb (Valcade), after which the percentage of Annexin V+ (apoptotic) cells were determined by flow cytometry. (B) Primary CD138+ multiple myeloma cells were isolated from bone marrow sample of an patient with multiple myeloma. CD138+ and CD138- cells were then treated for 24 h with 3 nM FK228 ± 3 nM Btzmb, after which apoptosis were assessed by Annexin V-FITC staining and flow cytometry.
Btzmb: Bortezomib; Ctrl: Control; FK228: Romidepsin; MM: Multiple myeloma.
[Grant S, Dai Y, Unpublished data].
Figure 3. The romidepsin–bortezomib regimen overcomes drug-resistance in human myeloma cells.
(A) Dexamethasone-sensitive (MM.1S) and -resistant (MM.1R) human myeloma cells were exposed (24 h) to 1 nM FK228 ± 2 nM Btzmb, after which the percentage of Annexin V+ (apoptotic) cells were determined by flow cytometry. (B) Btzmb-resistant cells (U266/PS-R) were generated by culturing U266 cells in gradually increasing concentrations of Btzmb up to 12 nM. U266/PS-R cells were treated for 48 h with 2 nM FK228 in the absence or presence of 5–15 nM Btzmb, after which apoptosis was assessed by Annexin V-FITC staining and flow cytometry.
Btzmb: Bortezomib; Ctrl: Control; FK228: Romidepsin.
[Grant S, Dai Y, Unpublished data].
In vivo preclinical studies
Effects on apoptosis
It is becoming clear that HDAC inhibitors of different structural classes have very different patterns of activity through varying interactions with the cellular apoptosis machinery. This has been elegantly illustrated by Newbold et al., using the Eμ-Myc lymphoma model system, where romidepsin can induce apoptosis in Eμ-Myc lymphomas in vitro and in vivo [78]. The ability to genetically manipulate Eμ-Myc lymphomas has allowed identification of key apoptotic proteins and pathways necessary for romidepsin-induced apoptosis. Like other HDAC inhibitors tested in this model, romidepsin mediated apoptosis via the intrinsic (mitochondrial) apoptotic pathway rather than the extrinsic (death receptor) pathway. However, unlike other HDAC inhibitors, where their effects in vitro and in vivo were completely suppressed by overexpression of prosurvival Bcl-2 proteins, romidepsin had the unique ability to overcome this resistance mechanism.
Unlike vorinostat, romidepsin was able to kill lymphoma cells that overexpressed the prosurvival protein Bcl-2. In addition, romidepsin improved the survival of mice with both Eμ-Myc and Bcl-2-overexpressing lymphoma [78]. By contrast, overexpression of Bcl-xL blocked romidepsin activity, suggesting that these proteins may have distinct roles in particular physiologic conditions, such as chemotherapy resistance, owing to their complex interactions with other proteins involved in determining the balance between pro- and anti-apoptosis [79].
In other lymphoma mouse xenograft models, romidepsin prolonged survival in severe combined immune deficient mice harboring human lymphoma U-937 tumors; with a median survival of 30.5 and 33 days compared with a median of 20 days for a control group. Furthermore, two out of the 12 treated mice lived past the observation time of 60 days, and associated in vitro studies demonstrated G1 and G2/M arrest with differentiation and apoptosis. The G1 arrest was associated with CDK inhibitor p21WAF1/CIP1 upregulation due to acetylation of the CDKN1A promoter regions [80].
A significant survival advantage following romidepsin treatment has also been demonstrated in Epstein–Barr virus-transformed lymphoblastoid cell tumors in mice, where 90% survival was seen at 30 days compared with 20% in the control group [81]. Romidepsin-mediated apoptosis was associated with a 12-fold increased level of active caspase 3; however, some apoptosis persisted despite z-VAD-fmk treatment to inhibit caspase activity. Romidepsin-resistant cells expressed higher levels of LMP1 and nuclear NF-kβ than romidepsin-sensitive cells. It is of note that apoptosis was inducible by NF-kβ inhibition. These data imply that the apoptosis induced by romidepsin in this model is via caspase-dependent and -independent pathways. Conversely, this was not the case in the Epstein–Barr virus-positive Burkitt lymphoma tumors, where there was no associated improvement in survival [79,81]. In MCL xenograft model lines with t(11,14)(q13,32) translocations, romidepsin was able slow tumor growth, induce H3 hyperacetylation and downregulate levels of Bcl-2, Bcl-xL and Mcl-1, inducing apoptosis via the mitochondrial pathway, in a dose-dependent fashion and demonstrate synergy with bortezomib [82].
Solid tumor efficacy
There are some encouraging in vivo mouse model data suggesting that romidepsin has significant effects on the growth of various solid tumors [19,55,58,80,83–85]. The effects that HDAC inhibitors have on gene expression may also impact the interpretation of conventional measurements of tumor response. In a prostate cancer model romidepsin modulated the expression of prostate specific antigen (PSA) mRNA in a biphasic manner in LNCaP cells, with a slight increase (60% relative to control) at concentrations of 0.36 and 0.72 nM. At concentrations of 1.44 nM and above, romidepsin decreased secretion of PSA in a concentration-dependent manner and reached the lowest point at 5.79 nM, approximately the IC50 of romidepsin to induce growth delay. The levels of PSA secreted within 48 h changed in a pattern similar to PSA mRNA expression in response to romidepsin. HDAC inhibitors may change the expression of genes controlling the level of tumor markers, such as serum PSA levels in prostate cancer, which then may not appropriately reflect changes in tumor burden. However, the suppression of PSA secretion was evident prior to the inhibition of cell growth and did not correlate with its cytotoxicity. As previously mentioned, there is evidence of activity against malignant rhabdoid tumors with increased levels of histone acetylation, apoptosis and caspase-independent cell death in vitro at romidepsin concentrations of 0.7–3.7 nM at 72 h [74]. There was also a significant reduction in mean tumor volume in mice bearing human tumors at day 12 when dosed at 1.2, 2.5 and 5 mg/kg on days 0, 4 and 8, respectively.
Early clinical studies
Effect on acetylation
Three Phase I clinical studies described the use of romidepsin in a total of 90 adult patients [22,86,87]. The first two studies investigated escalating doses of a 4-h iv. infusion of romidepsin in 70 patients with advanced refractory solid neoplasms, and confirmed an increase in histone acetylation in circulating mononuclear cells, with maximal acetylation occurring at the end of the infusion [22,86]. Sandor et al. recorded an maximum tolerated dose (MTD) of 17.8 mg/m2 on a day 1 and 5 schedule [22], while Marshall et al. defined the MTD at 13.3 mg/m2 on a day 1, 8 and 15 schedule every 28 days [86]. Subsequent Phase II trials used this latter schedule. In the most recent pharmacodynamic Phase I study, targeting an in vivo dose at which acetylation of histone proteins H3 and H4 increased by 100% in vitro, Marshall et al. demonstrated that the MTD achieved in patients with advanced cancer was 13 mg/m2 as a 4-h iv. infusion on days 1, 8 and 15 of a 28-day cycle [86]. Toxicity prevented repeated dosing, although all patients achieved this minimum effective pharmacological dose. The terminal elimination half-life at this dose was estimated to be 3.67 h. Serial assessment of histone acetylation, using immunohistochemistry correlated with HDAC inhibition, with HDAC activity declining to 24.6% of baseline immediately after treatment and returning to 59% of baseline 24 h after treatment [87]. Romidepsin has subsequently been evaluated in seven other Phase II clinical trials [88–93]. These studies used a dose of 13 or 14 mg/m2 administered as a 4-h iv. infusion on days 1, 8 and 15 of a 28-day cycle in a variety of hematologic and solid malignancies.
Toxicities
In the Phase I clinical studies, the dose-limiting toxicities observed included fatigue, gastrointestinal disturbances (nausea and vomiting) and anorexia [22,86,87]. Cytopenias, most commonly transient thrombocytopenia, were also noted. The mechanism underlying the observed thrombocytopenia is currently unclear, but as with other novel agents such as bortezomib, the pattern of recovery suggests that these are not due to direct myelosuppression and may be caused by defects in platelet release mediated by alterations in phosphorylation of myosin light chain [64].
Early concerns about cardiac toxicity were raised owing to ECG abnormalities observed in preclinical animal studies. These were chiefly an increase in heart rate, thought to be a class effect of HDAC inhibitors. Therefore, clinical trials have included intensive ECG and ejection-fraction monitoring of patients receiving romidepsin along with careful patient selection, excluding those with a significant cardiac history, and the utilization of aggressive electrolyte replacement to reduce risk of QTc prolongation [94]. Cardiac monitoring of 42 patients enrolled and treated with romidepsin in a Phase II trial demonstrated asymptomatic T-wave flattening (grade 1) or ST-segment depression (grade 2) in over 50% of ECGs. Abnormalities were not associated with elevation of cardiac troponin or alterations in left ventricular function on multiple gate acquisition scans or echocardiography. In addition, post-treatment ECGs had a mean corrected QT-interval prolongation of 14.4 ms compared with baseline using the Bazett correction [43]. Furthermore, long-term follow-up has not revealed any late-onset cardiac events in these patients. In a presentation at the 2009 American Society Hematology Annual Meeting by Cabell et al. ECGs from 110 patients with advanced malignancies participating in three clinical studies using romidepsin at 14 mg/m2 on days 1, 8 and 15 of a 28-day cycle were evaluated by blinded, independent assessors [95]. Romidepsin was shown to have only a slight effect on the QT interval corrected heart rate using Fridericia’s formula (QTcF) of 5.0 ms (90% CI: 2.3–7.7) with a mean increase of 2.7 ms (90% CI: 0.2–5.3) in QTcF following the administration of antiemetics alone. There was no relationship between romidepsin concentration and changes in QTcF, but an increase in mean heart rate of 10.1 ± 9.0 bpm after romidepsin administration, with a return to baseline by 24 h was related to romidepsin concentration. The conclusion of this study was that these changes were below the level of clinical and regulatory concern. However, it is prudent to ensure that potassium and magnesium levels are maintained within normal limits prior to treatment with romidepsin, especially in conditions such as CTCL, which are associated with lower levels [96], and to avoid the concomitant use of other drugs that are known to prolong the QTc. These precautions are included in the romidepsin package insert [23].
Disease-specific clinical studies
Lymphoproliferative disorders
T-cell lymphoproliferative disorders
In 2001, responses in four patients with T-cell lymphoma were reported in a Phase I trial conducted at the NCI at dose levels of 12.7 or 17.8 mg/m2 of romidepsin on days 1 and 5 of a 21-day cycle [97]. It is of note that two patients with refractory Sézary syndrome (the leukemic form of CTCL) had a rapid decrease in Sézary cells with improvement in skin erythema and edema. Another patient with extensive tumors had a partial response (PR), and a further patient with refractory PTCL and heavy skin infiltration had a durable CR. Sézary cells isolated from patients after treatment had increased histone acetylation (Table 4).
Table 4.
Clinical trials of single-agent romidepsin in lymphoproliferative disorders.
| Study (year) | Phase | Disease | Patients (n) | Responses | Ref. |
|---|---|---|---|---|---|
| Byrd et al.(2005) | I | CLL | 10 | No PR/CR (see text) | [87] |
| Whittaker et al.(2010) | II | CTCL | 96 | 6 CR; 27 PR; 45 SD | [2] |
| Piekarz et al.(2009) | II | CTCL | 71 | 4 CR; 20 PR; 26 SD | [3] |
| Piekarz et al.(2011) | II | PTCL | 45 | 8 CR; 9 PR; 5 SD | [101] |
| Niesvizky et al.(2011) | II | MM | 12 | 11 SD | [106] |
CLL: Chronic lymphocytic leukemia; CR: Complete response; CTCL: Cutaneous T-cell lymphoma; MM: Multiple myeloma;
PR: Partial response; PTCL; Peripheral T-cell lymphoma; SD: Stable disease.
Subsequently, two Phase II studies investigated romidepsin in patients with CTCL after at least one prior systemic therapy. Piekarz et al. reported final results of 71 patients with advanced CTCL treated in the multicenter NCI study of romidepsin administered as a 4-h infusion on days 1, 8, and 15 of a 28-day cycle with a starting dose of 14 mg/m2 [3]. The overall response rate (ORR) was 34%, with four CRs and 20 PRs, as well as 21 patients with stable disease (SD). The duration of responses improved with increased depth of response, and the median response duration for all patients was 13.7 months. One of the striking features of romidepsin is the very long duration of response extending beyond 3 years in some patients, including some after drug discontinuation. One patient remained in CR after 5.5 years off therapy. Common nonhematologic adverse events included fatigue, nausea, vomiting, anorexia and thrombocytopenia.
Bates et al. found a significant correlation between global H3 histone acetylation at 24 h and Cmax, AUC and clearance [98]. Furthermore, patients with major responses were more likely to have higher levels of acetylated histones at this time point. RNA analysis of both normal and malignant circulating PBMCs in this trial demonstrated increased histone acetylation. Interestingly, increased expression of MDR1 in PBMCs was observed, consistent with preclinical studies, which demonstrated that romidepsin can increase MDR1 gene expression, while cells selected for resistance overexpress P-gp. This would imply that romidepsin may actually induce its own mechanism of resistance, and further studies into this phenomenon are required. However, the induction was transient, and RNA from biopsy samples obtained in patients with resistant disease did not show increased MDR1 expression. Interestingly, a microarray analysis was performed to examine specific CTCL signature genes (which have been recognized to differentiate between Sézary syndrome and leukemic forms of CTCL [99]). Preliminary data suggest that this signature is reversed following romidepsin treatment [100]; confirmatory studies are ongoing.
Favorable responses in CTCL (Figure 4 & 5) were confirmed in a multinational study of 96 patients [2]. The ORR was 34%, as measured by a severity-weighted assessment tool, with CRs observed in six patients and PRs in 24 patients, as well as SD in 26 patients. Romidepsin was active in all disease compartments and across all disease stages. The median time to response was 2 months, median duration of response was 15 months and median time to progression was 8 months. Clinically significant pruritus relief was seen in 43% (28 out of 65) of patients with moderate-to-severe pruritus at baseline, irrespective of objective response. Common adverse events included nausea, asthenia, vomiting, anorexia, hypomagnesemia and pyrexia. Overall, romidepsin has significant and durable single-agent activity in CTCL.
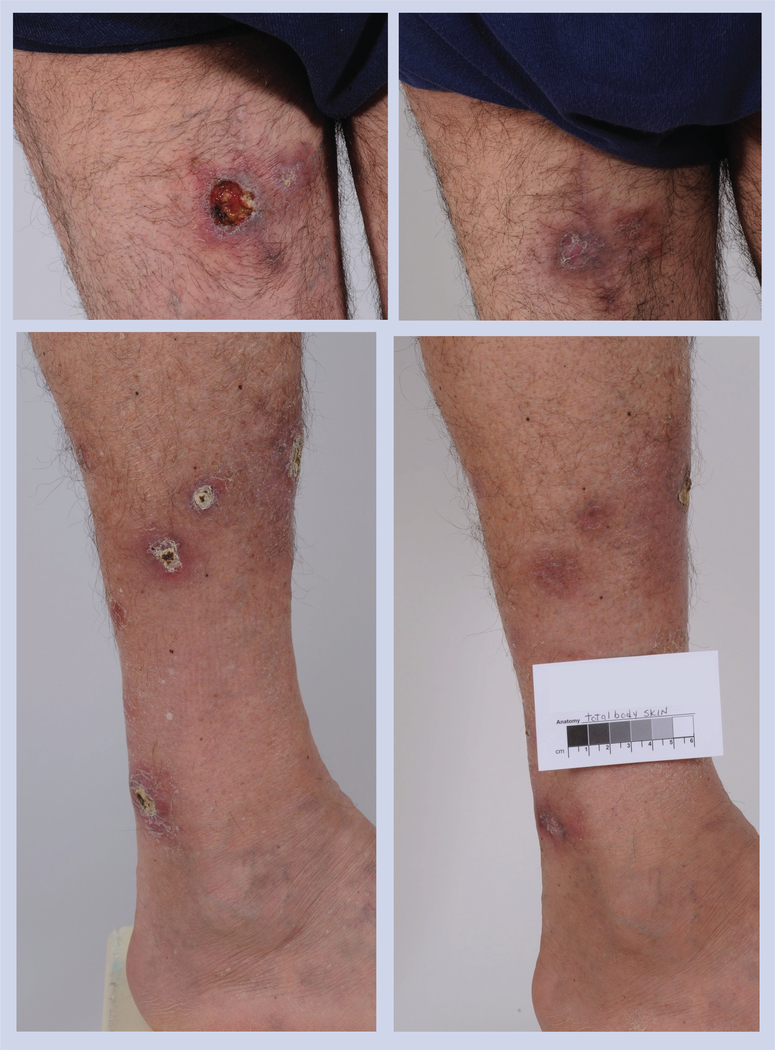
Figure 4. Patient with γ-δ subtype peripheral T-cell lymphoma enrolled in a Phase II trial.
The patient had partial remission after two cycles and remained in the study for an additional eight cycles.
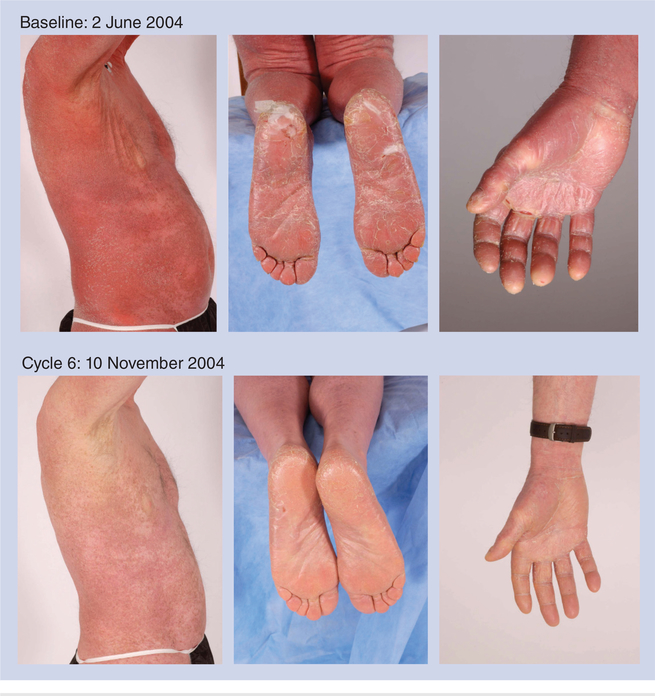
Figure 5. Patient with Sézary syndrome, stage IIIA disease.
This patient was treated in a Phase II clinical study for 3 years, for a total of 35 cycles, best response achieved was partial response.
Very encouraging responses to romidepsin have also occurred in patients with PTCL. Piekarz et al. reported an ORR of 38% with romidepsin as a single agent in 45 patients, including eight CRs and nine PRs. The median duration of response for all patients was 9 months (range: 2–74 months) [101]. Responses were observed independent of prior therapy, including stem cell transplant. In addition, responses were observed in a variety of subtypes of PTCL, including not otherwise specified PTCL, ALK-negative anaplastic large-cell lymphoma, γ-δ T-cell lymphoma and enteropathy-associated T-cell lymphoma. A larger international study in 131 heavily pretreated PTCL patients refractory to at least one prior line reported an ORR of 25%, including 19 (15%) with CRs/complete response unconfirmed and a median duration of response of 17 months (13.4 months in those who achieved CR/complete response unconfirmed). Treatment was complicated by grade ≥3 thrombocytopenia (24%), neutropenia (20%) and infections (19%) [102].
B-cell lymphoproliferative disorders
The pharmacodynamic study in patients with CLL and acute myeloid leukemia (AML) was performed by Byrd et al. with the aim of achieving an in vivo dose that increased acetylation of histones H3 and H4 by 100% in vitro [87]. Although no formal CR or PR occurred in ten CLL patients, antitumor activity was noted, including one patient who experienced tumor lysis syndrome with a drop in peripheral blood lymphocytosis from 303 × 109/l to 125 × 109/l. The seven other patients who had high peripheral blood white cell counts all had a reduction in leukocyte counts with an average reduction of 58% (range: 45–76%), while another patient had a reduction in lymphadenopathy.
These observations are encouraging investigators to examine the activity of romidepsin in combination with other agents in malignancies such as MCL. In a preclinical study Paoluzzi et al. investigated the cytotoxicity of two HDAC inhibitors, romidepsin and belinostat, in combination with bortezomib in HBL2, Granta519 and Jeko1 MCL cell lines [103]. Romidepsin was approximately 100-fold more potent than belinostat and the combination of belinostat (100–1000 nM) or romidepsin (1–40 nM) with bortezomib (3–4 nM) showed synergism in all cell lines. Further investigations suggest that these effects are modulated via cyclin D1 and p27.
Based upon preclinical studies suggesting synergy between romidepsin and bortezomib in CLL, a Phase I trial has recently been initiated (Massy Cancer Centre trial number: MCC-X05240, NCT00963274 [201]) for patients with refractory/progressive CLL, small lymphocytic lymphoma, indolent non-Hodkgin’s lymphoma or PTCL. In this trial, escalating doses of bortezomib, beginning at 0.7 mg/m2 will be administered weekly three-times each month in conjunction with a 4-h iv. infusion of romidepsin beginning at a dose of 5 mg/m2. Parallel correlative studies will be performed to determine if pharmacodynamic effects observed in vitro occur in CLL cells following in vivo administration of these agents.
The combination of romidepsin with standard chemotherapeutic agents is also the subject of ongoing clinical studies. For example, Dupuis et al. are investigating the use of romidepsin in combination with R-CHOP chemotherapy for the treatment of newly diagnosed diffuse large cell lymphoma [104]. The use of romidepsin in conjunction with anti-CD20 antibodies, such as rituximab, is particularly interesting given that HDAC inhibitors increase CD20 expression in mouse lymphoma cells [105].
Multiple myeloma
There is one clinical trial of single-agent romidepsin 13 mg/m2 in 13 MM patients. Although no clinical responses were observed, some patients with SD had demonstrable falls in monoclonal protein [106]. Indeed, several groups have studied HDAC inhibitors preclinically in combination with bortezomib, demonstrating that this combination synergistically induces apoptosis, mitochondrial injury, radical oxygen species generation and oxidative injury in human leukemia and myeloma cells, by targeting the aggresome and proteosome, respectively [75,108–110]. An ongoing Australian Phase I/II trial is examining the combination of romidepsin with bortezomib and dexamethasone in heavily pretreated myeloma patients, and has demonstrated good tolerability in the 25 patients treated to date, with two CRs, seven very good PRs, six PRs, three minimal responses and one SD [40]. Median time to progression was 7.2 (95% CI: 5.5–19.6) months and median overall survival was over 36 months. Seven of these patients had previously been treated with bortezomib.
Berensen et al. have reported interim results from a clinical trial examining the activity of romidepsin and bortezomib without dexamethasone in a Phase II study of a 1-h infusion of romidepsin in patients with MM [110]. Out of the five patients who have been enrolled to date, two that had relapsed following other bortezomib-containing regimens achieved minimal responses after two cycles. Two patients experienced grade 3 thrombocytopenia. Although the numbers are small these studies have shown encouraging safety of the shorter, 1-h romidepsin infusions, with no ECG changes reported in relation to the rapid infusion.
Other combination studies are in progress, including the Phase I/II study of lenalidomide and romidepsin in relapsed and refractory MM, CTCL and Hodgkin’s disease by Foss and Harrison from Yale Cancer Centre and Peter MacCallum Cancer Centre (FDA IND 11541, NCT pending). There is a clear rationale for potential synergies using this combination in that both agents induce cell death through caspase 8-mediated and other mechanisms; both induce p21 and cell cycle arrest; both agents have antiangiogenic effects; and both are likely to interfere with PI3K–Akt signaling. One particularly attractive aspect of this combination is the potential for synergistic immunological effects, particularly related to T-cell polarization, NK cell activation, STAT signaling and cytokine production.
Myeloproliferative disorders
Out of ten patients with AML in the Phase I study by Byrd et al., none achieved CR or PR, although one patient experienced tumor lysis syndrome [87]. Another study with 11 patients with AML/MDS reported one patient with CR and six patients with SD [111]. Correlative studies showed a modest but rapid increase in apoptosis, and changes in myeloid maturation marker expression, although no consistent changes were observed in histone H3 and H4 acetylation levels. In a study reported by Odenike, 20 patients were stratified into two cohorts based on the absence or presence of chromosomal abnormalities known to recruit HDACs, including those involving CBF [112]. Romidepsin 13 mg/m2 was administered iv. over 4 h on days 1, 8 and 15 of a 28-day cycle. Although there were no CRs by standard criteria, antileukemic activity was observed in five out of seven patients, with two patients clearing bone marrow blasts and another three patients achieving a >50% decrease in bone marrow blasts. Furthermore, in the cohort with chromosomal abnormalities known to recruit HDACs, there was a significant increase in MDR1 (p = 0.005), p15 (p = 0.01) and p14 (p < 0.0001) expression at 24 h, suggesting that romidepsin may have differential antileukemic and molecular activity in CBF AML. We await studies examining the use of this agent in combination with other chemotherapeutic, immunomodulatory or demethylating drugs.
Solid tumors
Preclinical data have shown significant activity of romidepsin against solid tumor cell lines. Despite this, clinical responses have been disappointing to date. Schrump et al. reported initial results from a Phase II trial of romidepsin (17.8 mg/m2) on days 1 and 7 of two 21-day cycles in 19 patients with refractory lung cancer [113]. Myelosuppression was dose limiting in one individual and no significant cardiac toxicities were observed. There were no objective responses; however, disease stabilization was noted in nine patients.
Similar observations have been noted in early-phase clinical studies of romidepsin in patients with refractory renal cell carcinoma [114], colorectal cancer (four out of 25 patient achieved SD as best response) and prostate cancer [115,116]. Following these results, and the promise of drug combination studies in hematologic malignancies, a small number of studies have been initiated with romidepsin in combination with traditional chemotherapeutic drugs and newer agents. Doss et al. reported initial results of a Phase I trial of romidepsin in combination with gemcitabine in patients with pancreatic and other advanced solid tumors [117]. The initial romidepsin/gemcitabine dose level was 10/800 mg/m2 on days 1, 8 and 15 of a 28-day cycle; however, owing to excessive hematologic toxicity, the romidepsin dose was decreased to 7 mg/m2 and the day 8 dose was omitted, allowing dose escalation of romidepsin to 12 mg/m2 and gemcitabine 800 mg/m2 on days 1 and 15. Nonhematologic toxicities have been mild to moderate, mainly nausea, vomiting and fatigue. One patient with ovarian cancer achieved a minor response and SD lasting >4 cycles was noted in 12 patients (five pancreatic, four breast cancer, one non-Hodgkin’s lymphoma, one ovarian cancer and one ampullary cancer).
The explanations for the lack of clinical responses in solid tumors are currently unclear, but may include differences in both the cell and the microenvironment. For example, HDAC inhibitors have been demonstrated to exert differential activities against cells that over- or under-express pro- and anti-apoptotic molecules, such as p53 and Bcl-2, among many others. Specific HDAC expression in cancer cells may also affect clinical responses to HDAC inhibitors, with reports suggesting that high class I HDAC expression is associated with advanced, proliferative solid tumors and adverse clinical outcome. Most preclinical work in solid tumors at present aims to identify effective combination therapies. We recently reported synergy between romidepsin (or belinostat) and cisplatin (or etoposide) in small-cell lung cancer cells [118]. Combination therapies may target apoptotic pathways as noted above, DNA damage pathways (HDAC inhibitors interfere with DNA repair), and degradation pathways. In addition therapies may target markers of differentiation, often induced by exposure to HDAC inhibitors. Induction of CD30, CD25 or the sodium iodide symporter would be examples of this strategy.
Conclusion & future perspective
To date we have seen the registration of romidepsin for the treatment of CTCL and PTCL, which has enabled researchers to unravel many potential mechanisms by which HDAC inhibitors exert their therapeutic effects in tumor cells as well as uncover novel insights via investigations in to the mechanisms of toxicity. Over the next 5 years we would hope that romidepsin and other HDAC inhibitors will prove their efficacy alone or in combination with traditional chemotherapeutics and other novel agents in other tumor types, such as PTCL, myeloma Hodgkin’s disease and B-cell lymphomas. The increasing use of correlative studies built into clinical trials will uncover potential biomarkers that may predict response or increase our understanding of the effects of this class of drugs on tumor cells, the microenvironment and the immune system; and how this impacts on therapeutic response. Furthermore, we would hope to finally understand whether selective or global inhibition of HDACs induce preferential therapeutic effects.
Executive summary.
Histone deacetylase (HDAC) inhibitors such as romidepsin have shown great promise in initial preclinical and early clinical studies.
HDAC inhibitors target not only internal cell processes such as gene expression, the cell cycle and apoptosis, but also the tumor environment through effects on angiogenesis and surrounding cell infiltrates.
It is clear that the potent HDAC inhibitor romidepsin is an effective agent against T-cell lymphomas in general, cutaneous and peripheral T-cell lymphomas in particular, and has been approved by the US FDA for these indications. Furthermore, complete responses have been observed, with some responses lasting for many months to years.
In myeloid diseases, there is some early evidence that romidepsin may have some disease activity against certain molecular subgroups, and the use of combination strategies appears attractive.
Although clinical responses in solid tumors have been disappointing, there may yet be a role for romidepsin in combination with other agents, and this is currently being investigated.
Synergistic effects with other agents
Preclinical studies have already shown that the exciting prospect of synergy between HDAC inhibitors and chemotherapy appears promising. Future strategies will be needed to explore romidepsin in combination with chemotherapies and other novel agents.
Although there is only modest single-agent activity in myeloma, combination therapy trials with romidepsin and agents such as bortezomib are supported by promising clinical studies.
Romidepsin alone or in combination with bortezomib appears to have clinical activity in B-CLL and further trials are underway.
Toxicity profile
The initial concerns regarding cardiac toxicity, specifically prolongation of the QT interval related to romidepsin, have been addressed by thorough monitoring of patients participating in the early clinical trials.
Recent evidence suggests that if electrolytes are maintained in the high normal range and drugs that are known to prolong the QT interval are avoided, there is no increased risk to patients.
Other toxicities appear to be minimal, with fatigue, gastrointestinal effects and transient myelosuppression, particularly thrombocytopenias, being the most common.
No long-term adverse effects of the clinical use romidepsin have yet been shown; however, the follow-up period remains relatively short, therefore continued caution is advisable until results of prolonged exposures are known.
Acknowledgments
Financial & competing interests disclosure
SJ Harrison and HM Prince have received grant funding and honoraria from Celgene. SE Bates recieves funding via Cooperative Research and Development Agreement between NCI and Celgene. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Footnotes
No writing assistance was utilized in the production of this manuscript.
Ethical conduct of research
The authors state that they have obtained appropriate institutional review board approval or have followed the principles outlined in the Declaration of Helsinki for all human or animal experimental investigations. In addition, for investigations involving human subjects, informed consent has been obtained from the participants involved.
Website
201 Bortezomib and Romidepsin in Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma (NCT00963274). http://clinicaltrials.gov/ct2/show/NCT00963274
References
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- 1.Nakajima H, Kim YB, Terano H, Yoshida M, Horinouchi S. FR901228, a potent antitumor antibiotic, is a novel histone deacetylase inhibitor. Exp. Cell Res. 241(1), 126–133 (1998).▪▪ Initial report of histone deacetylase (HDAC) inhibitor activity of romidepsin.
- 2.Whittaker SJ, Demierre MF, Kim EJ et al. Final results from a multicenter, international, pivotal study of romidepsin in refractory cutaneous T-cell lymphoma. J. Clin. Oncol. 28(29), 4485–4491 (2010).▪▪ Pivotal study for the approval of romidepsin for the treatment of cutaneous T-cell lymphoma.
- 3.Piekarz RL, Frye R, Turner M et al. Phase II multi-institutional trial of the histone deacetylase inhibitor romidepsin as monotherapy for patients with cutaneous T-cell lymphoma. J. Clin. Oncol. 27(32), 5410–5417 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jones PA, Baylin SB. The fundamental role of epigenetic events in cancer. Nat. Rev. Genet. 3(6), 415–428 (2002). [DOI] [PubMed] [Google Scholar]
- 5.Kuendgen A, Strupp C, Aivado M et al. Treatment of myelodysplastic syndromes with valproic acid alone or in combination with all-trans retinoic acid. Blood 104(5), 1266–1269 (2004). [DOI] [PubMed] [Google Scholar]
- 6.Garcia-Manero G, Kantarjian HM, Sanchez-Gonzalez B et al. Phase 1/2 study of the combination of 5-aza-2´-deoxycytidine with valproic acid in patients with leukemia. Blood 108(10), 3271–3279 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gore SD, Weng LJ, Zhai S et al. Impact of the putative differentiating agent sodium phenylbutyrate on myelodysplastic syndromes and acute myeloid leukemia. Clin. Cancer Res. 7(8), 2330–2339 (2001). [PubMed] [Google Scholar]
- 8.Gore SD, Baylin S, Sugar E et al. Combined DNA methyltransferase and histone deacetylase inhibition in the treatment of myeloid neoplasms. Cancer Res. 66(12), 6361–6369 (2006). [DOI] [PubMed] [Google Scholar]
- 9.Bishton M, Kenealy M, Johnstone R, Rasheed W, HM Prince. Epigenetic targets in hematological malignancies: combination therapies with HDACis and demethylating agents. Expert Rev. Anticancer Ther. 7(10), 1439–1449 (2007). [DOI] [PubMed] [Google Scholar]
- 10.Prince HM, Bishton MJ, Harrison SJ. Clinical studies of histone deacetylase inhibitors. Clin. Cancer Res 15(12), 3958–3969 (2009).▪ Reviews the clinical use of HDAC inhibitors.
- 11.Thiagalingam S, Cheng KH, Lee HJ, Mineva N, Thiagalingam A, Ponte JF. Histone deacetylases: unique players in shaping the epigenetic histone code. Ann. NY Acad. Sci. 983, 84–100 (2003). [DOI] [PubMed] [Google Scholar]
- 12.Kim JY, Casaccia P. HDAC1 in axonal degeneration: a matter of subcellular localization. Cell Cycle 9(18), 3680–3684 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim JY, Shen S, Dietz K et al. HDAC1 nuclear export induced by pathological conditions is essential for the onset of axonal damage. Nat. Neurosci. 13(2), 180–189 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sakai H, Urano T, Ookata K et al. MBD3 and HDAC1, two components of the NuRD complex, are localized at aurora-A-positive centrosomes in M phase. J. Biol. Chem. 277(50), 48714–48723 (2002). [DOI] [PubMed] [Google Scholar]
- 15.Frye RA. Characterization of five human cDNAs with homology to the yeast SIR2 gene: Sir2-like proteins (sirtuins) metabolize NAD and may have protein ADP-ribosyltransferase activity. Biochem. Biophys. Res. Commun. 260(1), 273–279 (1999). [DOI] [PubMed] [Google Scholar]
- 16.Bhalla KN. Epigenetic and chromatin modifiers as targeted therapy of hematologic malignancies. J. Clin. Oncol. 23(17), 3971–3993 (2005). [DOI] [PubMed] [Google Scholar]
- 17.Bolden JE, Peart MJ, Johnstone RW. Anticancer activities of histone deacetylase inhibitors. Nat. Rev. Drug Discov. 5(9), 769–784 (2006). [DOI] [PubMed] [Google Scholar]
- 18.Dokmanovic M, Marks PA. Prospects: histone deacetylase inhibitors. J. Cell. Biochem. 96(2), 293–304 (2005). [DOI] [PubMed] [Google Scholar]
- 19.Ueda H, Nakajima H, Hori Y et al. FR901228, a novel antitumor bicyclic depsipeptide produced by Chromobacterium violaceum no. 968. I. Taxonomy, fermentation, isolation, physico-chemical and biological properties, and antitumor activity. J. Antibiot. (Tokyo) 47(3), 301–310 (1994). [DOI] [PubMed] [Google Scholar]
- 20.Ueda H, Nakajima H, Hori Y, Goto T, Okuhara M. Action of FR901228, a novel antitumor bicyclic depsipeptide produced by Chromobacterium violaceum no. 968, on Ha-ras transformed NIH3T3 cells. Biosci. Biotechnol. Biochem. 58(9), 1579–1583 (1994). [DOI] [PubMed] [Google Scholar]
- 21.Lee JS, Paull K, Alvarez M et al. Rhodamine efflux patterns predict P-glycoprotein substrates in the National Cancer Institute drug screen. Mol. Pharmacol. 46(4), 627–638 (1994). [PubMed] [Google Scholar]
- 22.Sandor V, Bakke S, Robey RW et al. Phase I trial of the histone deacetylase inhibitor, depsipeptide (FR901228, NSC 630176), in patients with refractory neoplasms. Clin. Cancer Res. 8(3), 718–728 (2002). [PubMed] [Google Scholar]
- 23.Isodax®, package insert. Celgene Corporation, NJ, USA. [Google Scholar]
- 24.Berg SL, Stone J, Xiao JJ et al. Plasma and cerebrospinal fluid pharmacokinetics of depsipeptide (FR901228) in nonhuman primates. Cancer Chemother. Pharmacol. 54(1), 85–88 (2004). [DOI] [PubMed] [Google Scholar]
- 25.Furumai R, Matsuyama A, Kobashi N et al. FK228 (depsipeptide) as a natural prodrug that inhibits class I histone deacetylases. Cancer Res. 62(17), 4916–4921 (2002).▪▪ Illustrates the requirement for romidepsin to undergo reduction to enhance clinical activity.
- 26.Bradner JE, West N, Grachan ML et al. Chemical phylogenetics of histone deacetylases. Nat. Chem. Biol. 6(3), 238–243 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ruefli AA, Ausserlechner MJ, Bernhard D et al. The histone deacetylase inhibitor and chemotherapeutic agent suberoylanilide hydroxamic acid (SAHA) induces a cell-death pathway characterized by cleavage of Bid and production of reactive oxygen species. Proc. Natl Acad. Sci. USA 98(19), 10833–10838 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rosato RR, Almenara JA, Maggio SC et al. Role of histone deacetylase inhibitor-induced reactive oxygen species and DNA damage in LAQ-824/fludarabine antileukemic interactions. Mol. Cancer Ther. 7(10), 3285–3297 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Subramanian C, Opipari AW Jr, Bian X, Castle VP, Kwok RP. Ku70 acetylation mediates neuroblastoma cell death induced by histone deacetylase inhibitors. Proc. Natl Acad. Sci. USA 102(13), 4842–4847 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rosato RR, Kolla SS, Hock SK et al. Histone deacetylase inhibitors activate NF-kappaB in human leukemia cells through an ATM/NEMO-related pathway. J. Biol. Chem. 285(13), 10064–10077 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 31.Yi C, Ma M, Ran L et al. Function and molecular mechanism of acetylation in autophagy regulation. Science 336(6080), 474–477 (2012). [DOI] [PubMed] [Google Scholar]
- 32.Robert T, Vanoli F, Chiolo I et al. HDACs link the DNA damage response, processing of double-strand breaks and autophagy. Nature 471(7336), 74–79 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vrana JA, Decker RH, Johnson CR et al. Induction of apoptosis in U937 human leukemia cells by suberoylanilide hydroxamic acid (SAHA) proceeds through pathways that are regulated by Bcl-2/Bcl-xL, c-Jun, and p21CIP1, but independent of p53. Oncogene 18(50), 7016–7025 (1999). [DOI] [PubMed] [Google Scholar]
- 34.Richon VM, Sandhoff TW, Rifkind RA, Marks PA. Histone deacetylase inhibitor selectively induces p21WAF1 expression and gene-associated histone acetylation. Proc. Natl Acad. Sci. USA 97(18), 10014–10019 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Waby JS, Chirakkal H, Yu C et al. Sp1 acetylation is associated with loss of DNA binding at promoters associated with cell cycle arrest and cell death in a colon cell line. Mol. Cancer 9, 275 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brazelle W, Kreahling JM, Gemmer J et al. Histone deacetylase inhibitors downregulate checkpoint kinase 1 expression to induce cell death in non-small cell lung cancer cells. PLoS ONE 5(12), e14335 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Noh EJ, Lim DS, Jeong G, Lee JS. An HDAC inhibitor, trichostatin A, induces a delay at G2/M transition, slippage of spindle checkpoint, and cell death in a transcription-dependent manner. Biochem. Biophys. Res. Commun. 378(3), 326–331 (2009). [DOI] [PubMed] [Google Scholar]
- 38.Stevens FE, Beamish H, Warrener R, Gabrielli B. Histone deacetylase inhibitors induce mitotic slippage. Oncogene 27(10), 1345–1354 (2008). [DOI] [PubMed] [Google Scholar]
- 39.Warrener R, Beamish H, Burgess A et al. Tumor cell-selective cytotoxicity by targeting cell cycle checkpoints. FASEB J. 17(11), 1550–1552 (2003).▪▪ Explores the tumor selectivity of HDAC inhibitors and the role of targeting cell cycle checkpoints.
- 40.Robbins AR, Jablonski SA, Yen TJ et al. Inhibitors of histone deacetylases alter kinetochore assembly by disrupting pericentromeric heterochromatin. Cell Cycle 4(5), 717–726 (2005). [DOI] [PubMed] [Google Scholar]
- 41.Harrison SJ, Quach H, Link E et al. A high rate of durable responses with romidepsin, bortezomib, and dexamethasone in relapsed or refractory multiple myeloma. Blood 118(24), 6274–6283 (2011).▪▪ First study to explore the combination of a proteasome inhibitor (bortezomib) and romidepsin in multiple myeloma, suggesting an increased response rate.
- 42.Mitsiades N, Mitsiades CS, Richardson PG et al. Molecular sequelae of histone deacetylase inhibition in human malignant B cells. Blood 101(10), 4055–4062 (2003). [DOI] [PubMed] [Google Scholar]
- 43.Duan H, Heckman CA, Boxer LM. Histone deacetylase inhibitors down-regulate bcl-2 expression and induce apoptosis in t(14;18) lymphomas. Mol. Cell. Biol. 25(5), 1608–1619 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Witzig TE, Timm M, Stenson M, Svingen PA, Kaufmann SH. Induction of apoptosis in malignant B cells by phenylbutyrate or phenylacetate in combination with chemotherapeutic agents. Clin. Cancer Res. 6(2), 681–692 (2000). [PubMed] [Google Scholar]
- 45.Dhordain P, Lin RJ, Quief S et al. The LAZ3(BCL-6) oncoprotein recruits a SMRT/mSIN3A/histone deacetylase containing complex to mediate transcriptional repression.Nucleic Acids Res 26(20), 4645–4651 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pasqualucci L, Bereschenko O, Niu H et al. Molecular pathogenesis of non-Hodgkin’s lymphoma: the role of Bcl-6. Leuk. Lymphoma 44(Suppl. 3), S5–S12 (2003). [DOI] [PubMed] [Google Scholar]
- 47.Kawamata N, Koeffler HP. Mantle cell lymphoma: SAHA blocks Akt/mTOR pathway and reduces cyclin D1 protein levels by affecting translation. ASH Annual Meeting Abstracts 104(11), 3293 (2004). [Google Scholar]
- 48.Catley L, Weisberg E, Tai Y-T et al. NVP-LAQ824 is a potent novel histone deacetylase inhibitor with significant activity against multiple myeloma. Blood 102(7), 2615–2622 (2003). [DOI] [PubMed] [Google Scholar]
- 49.Peart MJ, Smyth GK, Van Laar RK et al. Identification and functional significance of genes regulated by structurally different histone deacetylase inhibitors. Proc. Natl Acad. Sci. USA 102(10), 3697–3702 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mitsiades CS, Mitsiades NS, McMullan CJ et al. Transcriptional sig nature of histone deacetylase inhibition in multiple myeloma: biological and clinical implications. Proc. Natl Acad. Sci. USA 101(2), 540–545 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nebbioso A, Clarke N, Voltz E et al. Tumor-selective action of HDAC inhibitors involves TRAIL induction in acute myeloid leukemia cells. Nat. Med. 11(1), 77–84 (2005). [DOI] [PubMed] [Google Scholar]
- 52.Maeda T, Towatari M, Kosugi H, Saito H. Up-regulation of costimulatory/adhesion molecules by histone deacetylase inhibitors in acute myeloid leukemia cells. Blood 96(12), 3847–3856 (2000). [PubMed] [Google Scholar]
- 53.Magner WJ, Kazim AL, Stewart C et al. Activation of MHC class I, II, and CD40 gene expression by histone deacetylase inhibitors. J. Immunol. 165(12), 7017–7024 (2000). [DOI] [PubMed] [Google Scholar]
- 54.Inoue S, Macfarlane M, Harper N, Wheat LM, Dyer MJ, Cohen GM. Histone deacetylase inhibitors potentiate TNF-related apoptosis-inducing ligand (TRAIL)-induced apoptosis in lymphoid malignancies. Cell Death Differ. 11(Suppl. 2), S193–S206 (2004). [DOI] [PubMed] [Google Scholar]
- 55.Aron JL, Parthun MR, Marcucci G et al. Depsipeptide (FR901228) induces histone acetylation and inhibition of histone deacetylase in chronic lymphocytic leukemia cells concurrent with activation of caspase 8-mediated apoptosis and down-regulation of c-FLIP protein. Blood 102(2), 652–658 (2003). [DOI] [PubMed] [Google Scholar]
- 56.Dai Y, Chen S, Kramer LB, Funk VL, Dent P, Grant S. Interactions between bortezomib and romidepsin and belinostat in chronic lymphocytic leukemia cells. Clin. Cancer Res. 14(2), 549–558 (2008). [DOI] [PubMed] [Google Scholar]
- 57.Khan SB, Maududi T, Barton K, Ayers J, Alkan S. Analysis of histone deacetylase inhibitor, depsipeptide (FR901228), effect on multiple myeloma. Br. J. Haematol. 125(2), 156–161 (2004). [DOI] [PubMed] [Google Scholar]
- 58.Piekarz RL, Robey RW, Zhan Z et al. T-cell lymphoma as a model for the use of histone deacetylase inhibitors in cancer therapy: impact of depsipeptide on molecular markers, therapeutic targets, and mechanisms of resistance. Blood 103(12), 4636–4643 (2004). [DOI] [PubMed] [Google Scholar]
- 59.Robey RW, Zhan Z, Piekarz RL, Kayastha GL, Fojo T, Bates SE. Increased MDR1 expression in normal and malignant peripheral blood mononuclear cells obtained from patients receiving depsipeptide (FR901228, FK228, NSC630176). Clin. Cancer Res. 12(5), 1547–1555 (2006). [DOI] [PubMed] [Google Scholar]
- 60.Peart MJ, Tainton KM, Ruefli AA et al. Novel mechanisms of apoptosis induced by histone deacetylase inhibitors. Cancer Res. 63(15), 4460–4471 (2003). [PubMed] [Google Scholar]
- 61.Choudhary C, Kumar C, Gnad F et al. Lysine acetylation targets protein complexes and co-regulates major cellular functions. Science 325(5942), 834–840 (2009). [DOI] [PubMed] [Google Scholar]
- 62.Terui T, Murakami K, Takimoto R et al. Induction of PIG3 and NOXA through acetylation of p53 at 320 and 373 lysine residues as a mechanism for apoptotic cell death by histone deacetylase inhibitors. Cancer Res. 63(24), 8948–8954 (2003). [PubMed] [Google Scholar]
- 63.Subramanian C, Jarzembowski JA, Opipari AW Jr, Castle VP, Kwok RP. HDAC6 deacetylates Ku70 and regulates Ku70–Bax binding in neuroblastoma. Neoplasia 13(8), 726–734 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bishton MJ, Harrison SJ, Martin BP et al. Deciphering the molecular and biological processes that mediate histone deacetylase inhibitor-induced thrombocytopenia. Blood 117(13), 3658–3668 (2011).▪▪ Identifies the molecular events leading to HDAC inhibitor-induced thrombocytopenia.
- 65.Yu X, Guo ZS, Marcu MG et al. Modulation of p53, ErbB1, ErbB2, and Raf-1 expression in lung cancer cells by depsipeptide FR901228. J. Natl Cancer Inst. 94(7), 504–513 (2002). [DOI] [PubMed] [Google Scholar]
- 66.To KK, Robey R, Zhan Z, Bangiolo L, Bates SE. Upregulation of ABCG2 by romidepsin via the aryl hydrocarbon receptor pathway. Mol. Cancer Res. 9(4), 516–527 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang Y, Wang SY, Zhang XH et al. FK228 inhibits Hsp90 chaperone function in K562 cells via hyperacetylation of Hsp70. Biochem. Biophys. Res. Commun. 356(4), 998–1003 (2007). [DOI] [PubMed] [Google Scholar]
- 68.Sasakawa Y, Naoe Y, Noto T et al. Antitumor efficacy of FK228, a novel histone deacetylase inhibitor, depends on the effect on expression of angiogenesis factors. Biochem Pharmacol. 66(6), 897–906 (2003). [DOI] [PubMed] [Google Scholar]
- 69.Mie Lee Y, Kim SH, Kim HS et al. Inhibition of hypoxia-induced angiogenesis by FK228, a specific histone deacetylase inhibitor, via suppression of HIF-1alpha activity. Biochem. Biophys. Res. Commun. 300(1), 241–246 (2003). [DOI] [PubMed] [Google Scholar]
- 70.Kong X, Lin Z, Liang D, Fath D, Sang N, Caro J. Histone deacetylase inhibitors induce VHL and ubiquitin-independent proteasomal degradation of hypoxia-inducible factor 1alpha. Mol. Cell. Biol. 26(6), 2019–2028 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Levine B, Kroemer G. Autophagy in the pathogenesis of disease. Cell 132(1), 27–42 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Oh M, Choi IK, Kwon HJ. Inhibition of histone deacetylase1 induces autophagy. Biochem. Biophys. Res. Commun. 369(4), 1179–1183 (2008). [DOI] [PubMed] [Google Scholar]
- 73.Rodriguez-Gonzalez A, Lin T, Ikeda AK, Simms-Waldrip T, Fu C, Sakamoto KM. Role of the aggresome pathway in cancer: targeting histone deacetylase 6-dependent protein degradation. Cancer Res. 68(8), 2557–2560 (2008). [DOI] [PubMed] [Google Scholar]
- 74.Watanabe M, Adachi S, Matsubara H et al. Induction of autophagy in malignant rhabdoid tumor cells by the histone deacetylase inhibitor FK228 through AIF translocation. Int. J. Cancer 124(1), 55–67 (2009). [DOI] [PubMed] [Google Scholar]
- 75.Pei XY, Dai Y, Grant S. Synergistic induction of oxidative injury and apoptosis in human multiple myeloma cells by the proteasome inhibitor bortezomib and histone deacetylase inhibitors. Clin. Cancer Res. 10(11), 3839–3852 (2004). [DOI] [PubMed] [Google Scholar]
- 76.Bali P, Pranpat M, Bradner J et al. Inhibition of histone deacetylase 6 acetylates and disrupts the chaperone function of heat shock protein 90: a novel basis for antileukemia activity of histone deacetylase inhibitors. J. Biol. Chem. 280(29), 26729–26734 (2005). [DOI] [PubMed] [Google Scholar]
- 77.Nawrocki ST, Carew JS, Pino MS et al. Aggresome disruption: a novel strategy to enhance bortezomib-induced apoptosis in pancreatic cancer cells. Cancer Res. 66(7), 3773–3781 (2006). [DOI] [PubMed] [Google Scholar]
- 78.Newbold A, Lindemann RK, Cluse LA, Whitecross KF, Dear AE, Johnstone RW. Characterisation of the novel apoptotic and therapeutic activities of the histone deacetylase inhibitor romidepsin. Mol. Cancer Ther. 7(5), 1066–1079 (2008). [DOI] [PubMed] [Google Scholar]
- 79.Fiebig AA, Zhu W, Hollerbach C, Leber B, Andrews DW. Bcl-xL is qualitatively different from and ten times more effective than Bcl-2 when expressed in a breast cancer cell line. BMC Cancer 6, 213 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sasakawa Y, Naoe Y, Inoue T et al. Effects of FK228, a novel histone deacetylase inhibitor, on human lymphoma U-937 cells in vitro and in vivo. Biochem. Pharmacol. 64(7), 1079–1090 (2002). [DOI] [PubMed] [Google Scholar]
- 81.Roychowdhury S, Baiocchi RA, Vourganti S et al. Selective efficacy of depsipeptide in a xenograft model of Epstein–Barr virus-positive lymphoproliferative disorder. J. Natl Cancer. Inst. 96(19), 1447–1457 (2004). [DOI] [PubMed] [Google Scholar]
- 82.Paoluzzi L, Scotto L, Marchi E, Zain J, Seshan VE, O’Connor OA. Romidepsin and belinostat synergize the antineoplastic effect of bortezomib in mantle cell lymphoma. Clin. Cancer Res. 16(2), 554–565. [DOI] [PubMed] [Google Scholar]
- 83.Byrd JC, Shinn C, Ravi R et al. Depsipeptide (FR901228): a novel therapeutic agent with selective, in vitro activity against human B-cell chronic lymphocytic leukemia cells. Blood 94(4), 1401–1408 (1999). [PubMed] [Google Scholar]
- 84.Klisovic DD, Katz SE, Effron D et al. Depsipeptide (FR901228) inhibits proliferation and induces apoptosis in primary and metastatic human uveal melanoma cell lines. Invest. Ophthalmol. Vis. Sci. 44(6), 2390–2398 (2003). [DOI] [PubMed] [Google Scholar]
- 85.Sawa H, Murakami H, Kumagai M et al. Histone deacetylase inhibitor, FK228, induces apoptosis and suppresses cell proliferation of human glioblastoma cells in vitro and in vivo. Acta Neuropathol. (Berl.) 107(6), 523–531 (2004). [DOI] [PubMed] [Google Scholar]
- 86.Marshall JL, Rizvi N, Kauh J et al. A Phase I trial of depsipeptide (FR901228) in patients with advanced cancer. J. Exp. Ther. Oncol. 2(6), 325–332 (2002). [DOI] [PubMed] [Google Scholar]
- 87.Byrd JC, Marcucci G, Parthun MR et al. A Phase 1 and pharmacodynamic study of depsipeptide (FK228) in chronic lymphocytic leukemia and acute myeloid leukemia. Blood 105(3), 959–967 (2005). [DOI] [PubMed] [Google Scholar]
- 88.Piekarz RL, Frye R, Turner M et al. Completion of the first cohort of patients with cutaneous T-cell lymphoma enrolled on a Phase II trial of depsipeptide. ASH Annual Meeting Abstracts 106(11), 231 (2005). [Google Scholar]
- 89.Niesvizky R, Ely S, Diliberto M et al. Multicenter Phase II trial of the histone deacetylase inhibitor depsipeptide (FK228) for the treatment of relapsed or refractory multiple myeloma (MM). ASH Annual Meeting Abstracts 106(11), 2574 (2005). [Google Scholar]
- 90.Whitehead RP, Mccoy S, Wollner IS et al. Phase II trial of depsipeptide (NSC-630176) in colorectal cancer patients who have received either one or two prior chemotherapy regimens for metastatic or locally advanced, unresectable disease: a Southwest Oncology Group study. J. Clin. Oncol. (Meeting Abstracts) 24(Suppl. 18), 3598 (2006). [Google Scholar]
- 91.Su YB, Tuttle RM, Fury M et al. A Phase II study of single agent depsipeptide (DEP) in patients (pts) with radioactive iodine (RAI)-refractory, metastatic, thyroid carcinoma: preliminary toxicity and efficacy experience. J. Clin. Oncol. (Meeting Abstracts) 24(Suppl. 18), 5554 (2006). [Google Scholar]
- 92.Molife R, Patterson S, Riggs C et al. Phase II study of FK228 in patients with hormone refractory prostate cancer (HRPC). J. Clin. Oncol. (Meeting Abstracts) 24(Suppl. 18), 14554 (2006). [Google Scholar]
- 93.Whittaker S, McCulloch W, Robak T, Baran E, Prentice A; All Investigators. International multicenter Phase II study of the HDAC inhibitor (HDACi) depsipeptide (FK228) in cutaneous T-cell lymphoma (CTCL): interim report. J. Clin. Oncol. (Meeting Abstracts) 24(Suppl. 18), 3063 (2006). [Google Scholar]
- 94.Piekarz RL, Frye AR, Wright JJ et al. Cardiac studies in patients treated with depsipeptide, FK228, in a Phase II trial for T-cell lymphoma. Clin. Cancer Res. 12(12), 3762–3773 (2006).▪▪ Discusses the fact that there are no significant QTcF changes to associate cardiac events and romidepsin therapy with careful management of electrolytes.
- 95.Cabell C, Bates S, Piekarz R et al. Systematic assessment of potential cardiac effects of the novel histone deacetylase (HDAC) inhibitor romidepsin. ASH Annual Meeting Abstracts 114(22), 3709 (2009).▪▪ Discusses the fact that there are no significant QTcF changes to associate cardiac events and romidepsin therapy with careful management of electrolytes.
- 96.Morgan M, Maloney D, Duvic M. Hypomagnesemia and hypocalcemia in mycosis fungoides: a retrospective case series. Leuk. Lymphoma 43(6), 1297–1302 (2002). [DOI] [PubMed] [Google Scholar]
- 97.Piekarz RL, Robey R, Sandor V et al. Inhibitor of histone deacetylation, depsipeptide (FR901228), in the treatment of peripheral and cutaneous T-cell lymphoma: a case report. Blood 98(9), 2865–2868 (2001). [DOI] [PubMed] [Google Scholar]
- 98.Bates SE, Zhan Z, Steadman K et al. Laboratory correlates for a Phase II trial of romidepsin in cutaneous and peripheral T-cell lymphoma. Br. J. Haematol. 148(2), 256–267 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Nebozhyn M, Loboda A, Kari L et al. Quantitative PCR on 5 genes reliably identifies CTCL patients with 5% to 99% circulating tumor cells with 90% accuracy. Blood 107(8), 3189–3196 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Piekarz R, Frye R, Wright J et al. Update of the NCI multiinstitutional phase II trial of romidepsin, FK228, for patients with cutaneous or peripheral T-cell lymphoma. J. Clin. Oncol. (Meeting Abstracts) 25(Suppl. 18), 8027- (2007). [Google Scholar]
- 101.Piekarz RL, Frye R, Prince HM et al. Phase 2 trial of romidepsin in patients with peripheral T-cell lymphoma. Blood 117(22), 5827–5834 (2011).▪ Led to the approval of romidepsin for the treatment of peripheral T-cell lymphoma.
- 102.Coiffier B, Pro B, Prince HM et al. Results from a pivotal, open-label, Phase II study of romidepsin in relapsed or refractory peripheral T-cell lymphoma after prior systemic therapy. J. Clin. Oncol. 30(6), 631–636 (2012). [DOI] [PubMed] [Google Scholar]
- 103.Paoluzzi L, Scotto L, Marchi E, Zain J, Seshan VE, O’Connor OA. Romidepsin and belinostat synergize the antineoplastic effect of bortezomib in mantle cell lymphoma. Clin. Cancer Res. 16(2), 554–565 (2010). [DOI] [PubMed] [Google Scholar]
- 104.Dupuis J, Casasnovas RO, Morschhauser F et al. Early results of a Phase Ib/II dose-escalation trial of romidepsin in association with CHOP in patients with peripheral T-cell lymphomas (PTCL). ASH Annual Meeting Abstracts 118(21), 2673 (2011). [Google Scholar]
- 105.Shimizu R, Kikuchi J, Wada T, Ozawa K, Kano Y, Furukawa Y. HDAC inhibitors augment cytotoxic activity of rituximab by upregulating CD20 expression on lymphoma cells. Leukemia 24(10), 1760–1768 (2010). [DOI] [PubMed] [Google Scholar]
- 106.Niesvizky R, Ely S, Mark T et al. Phase 2 trial of the histone deacetylase inhibitor romidepsin for the treatment of refractory multiple myeloma. Cancer 117(2), 336–342 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yu C, Rahmani M, Conrad D, Subler M, Dent P, Grant S. The proteasome inhibitor bortezomib interacts synergistically with histone deacetylase inhibitors to induce apoptosis in Bcr/Abl+ cells sensitive and resistant to STI571. Blood 102(10), 3765–3774 (2003). [DOI] [PubMed] [Google Scholar]
- 108.Catley L, Weisberg E, Kiziltepe T et al. Aggresome induction by proteasome inhibitor bortezomib and alpha-tubulin hyperacetylation by tubulin deacetylase (TDAC) inhibitor LBH589 are synergistic in myeloma cells. Blood 108(10), 3441–3449 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sutheesophon K, Kobayashi Y, Takatoku MA et al. Histone deacetylase inhibitor depsipeptide (FK228) induces apoptosis in leukemic cells by facilitating mitochondrial translocation of Bax, which is enhanced by the proteasome inhibitor bortezomib. Acta Haematol. 115(1–2), 78–90 (2006). [DOI] [PubMed] [Google Scholar]
- 110.Berenson JR, Yellin O, Mapes R et al. A Phase II study of a 1-hour infusion of romidepsin combined with bortezomib for multiple myeloma (MM) patients with relapsed or refractory disease. J. Clin. Oncol. (Meeting Abstracts) 27(15S), e19508 (2009).▪ Illustrates the safety of a 1-h romidepsin infusion.
- 111.Klimek VM, Fircanis S, Maslak P et al. Tolerability, pharmacodynamics, and pharmacokinetics studies of depsipeptide (romidepsin) in patients with acute myelogenous leukemia or advanced myelodysplastic syndromes. Clin. Cancer Res. 14(3), 826–832 (2008). [DOI] [PubMed] [Google Scholar]
- 112.Odenike OM, Alkan S, Sher D et al. Histone deacetylase inhibitor romidepsin has differential activity in core binding factor acute myeloid leukemia. Clin. Cancer Res. 14(21), 7095–7101 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Schrump DS, Fischette MR, Nguyen DM et al. Clinical and molecular responses in lung cancer patients receiving romidepsin. Clin. Cancer Res. 14(1), 188–198 (2008). [DOI] [PubMed] [Google Scholar]
- 114.Stadler WM, Margolin K, Ferber S, McCulloch W, Thompson JA. A Phase II study of depsipeptide in refractory metastatic renal cell cancer. Clin. Genitourin. Cancer 5(1), 57–60 (2006). [DOI] [PubMed] [Google Scholar]
- 115.Whitehead RP, Rankin C, Hoff PM et al. Phase II trial of romidepsin (NSC-630176) in previously treated colorectal cancer patients with advanced disease: a Southwest Oncology Group study (S0336). Invest. New Drugs 27(5), 469–475 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Molife LR, Attard G, Fong PC et al. Phase II, two-stage, single-arm trial of the histone deacetylase inhibitor (HDACi) romidepsin in metastatic castration-resistant prostate cancer (CRPC). Ann. Oncol. 21(1), 109–113 (2010). [DOI] [PubMed] [Google Scholar]
- 117.Doss HH, Jones SF, Infante JR et al. A Phase I trial of romidepsin in combination with gemcitabine in patients with pancreatic and other advanced solid tumors. J. Clin. Oncol. 26(Suppl.), Abstract 2567 (2008. ). [Google Scholar]
- 118.Luchenko VL, Salcido CD, Zhang Y et al. Schedule-dependent synergy of histone deacetylase inhibitors with DNA damaging agents in small cell lung cancer. Cell Cycle 10(18), 3119–3128 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]