Abstract
PURPOSE
To evaluate the role of diffusion kurtosis and diffusivity as potential imaging biomarkers to predict response to neoadjuvant chemoradiation therapy (CRT) from baseline staging magnetic resonance imaging (MRI) in locally advanced rectal cancer (LARC).
MATERIALS AND METHODS
This retrospective study included 45 consecutive patients (31 male/14 female) who underwent baseline MRI with high b-value sequences (up to 1500 mm/s2) for LARC followed by neoadjuvant chemoradiation and surgical resection. The mean age was 57.4 years (range 34.2–72.9). An abdominal radiologist using open source software manually segmented t2-weighted images. Segmentations were used to derive diffusion kurtosis and diffusivity from diffusion weighted images (DWI) as well as volumetric data. These data were analyzed with regards to tumor regression grade (TRG) using the four-tier American Joint Committee on Cancer (AJCC) classification, TRG0-TRG3. Proportional odds regression was used to analyze the four-level ordinal outcome. A sensitivity analysis was performed using univariable logistic regression for binary TRG groups, TRG0/1 (> 90% response) or TRG2/3 (< 90% response). P < 0.05 was considered significant throughout.
RESULTS
In the univariable proportional odds regression analysis, higher diffusivity summary (Dsum) values were observed to be significantly associated with higher odds of being in one or more favorable TRG group (TRG0 or TRG1). In other words, on average, patients with higher Dsum values were more likely to be in a more favorable TRG group. These results are mostly consistent with the sensitivity analysis, in which higher values for most Dsum values, (all but ROI-max D Median (p=0.08)) were observed to be significantly associated with higher odds of being TRG0 or TRG1. Tumor VOI and ROI volume, ROI kurtosis mean and median, and VOI kurtosis mean and median were not significantly associated with TRG.
CONCLUSION
Diffusivity derived from the baseline staging MRI, but not diffusion kurtosis or volumetric data, is associated with TRG and therefore shows promise as an imaging biomarker to predict the response to neoadjuvant chemotherapy in LARC.
Introduction
Neoadjuvant chemoradiation for locally advanced rectal cancer (LARC) has become standard practice, as a number of trials have shown that its use is associated with decreased rates of local recurrence[1]. Locally advanced rectal cancer includes those cases in which the tumor has spread beyond the wall of the rectum into the surrounding perirectal fat by at least 5 mm (T3c-d), when the tumor has invaded local adjacent structures (T4), or when there is involvement of locoregional lymph nodes (N1 or N2)[2]. Following neoadjuvant chemoradiation and surgical resection, the amount of tumor replaced by fibrosis is quantified at pathology as the tumor regression grade (TRG). Although several scoring systems exist for classifying the TRG of a resected specimen, a significant correlation between the TRG and recurrence free survival is seen across scoring systems[3]. The prognostic value of the TRG seen after neoadjuvant therapy in rectal cancer is significant, and an imaging biomarker that has the ability to predict a patient’s TRG prior to initiating neoadjuvant chemoradiation may have clinical utility.
Diffusion kurtosis imaging (DKI), which reflects non-Gaussian distribution of diffusion values, has been described as a product of tissue microstructures[4]. It has shown promise as a biomarker in a variety of other cancers, where it has correlated with tumor aggressiveness in prostate cancer[5], as well as with histologic grade in breast cancer and gliomas[6–8]. Mean kurtosis values from DKI in gliomas have also been shown to be associated with progression free and overall survival[9]. These findings have contributed to the growing research interest in oncologic applications of DKI.
Several authors have explored the potential role of DKI in imaging of locally advanced rectal cancer. Studies have shown correlations between kurtosis parameters and histologic subtypes and specific imaging features that have prognostic value[10–12], while others have shown associations with specific genetic phenotypes, including KRAS mutational status [13] or mismatch-repair (MMR) gene expression[14]. A handful of papers have also explored whether parameters derived from DKI may be useful in assessing a patient’s response to neoadjuvant therapy[15–17].
In 2017 our institution began routinely acquiring b1500 s/mm2 on all rectal MR studies, as we find it useful in clinical practice. In this study, we examine whether parameters derived from DKI on the baseline MRI are associated with subsequent response to neoadjuvant chemoradiation as measured by the TRG.
Methods
The institutional review board waived the requirement for informed consent for this Health Insurance Portability and Accountability Act (HIPAA)-compliant, retrospective study.
Patient selection
A total of 45 consecutive patients diagnosed with locally advanced rectal cancer that underwent multiparametric MRI with at least three b-values, including b1500 s/mm2, followed by neoadjuvant chemoradiation and surgical resection, were included for analysis.
Image acquisition
MR imaging was performed with a 3-T whole-body MRI unit (Discovery MR750; GE Medical Systems, Waukesha, WI). A 32-channel phased array coil was employed for signal reception. Multiplanar T2-weighted images, dynamic contrast enhanced (DCE) and diffusion weighted images were acquired. DW-MRI was acquired at multiple b-values up to 1500 s/mm2. Our standard institutional protocol for rectal MR on the 3-T scanner is included in the appendix.
Image segmentation
Axial T2-weighted images from the baseline pre-treatment MRI for all 45 patients were segmented by a board-certified radiologist fellowship trained in abdominal imaging with one year of experience (DB) on ImageJ software created by the National Institutes of Health[18]. The decision to use T2-weighted images was made because, in our experience, the margins of the tumor are most reliably identified on T2 images, as opposed to the DWI sequences. The segmentations were superimposed on axial diffusion weighted images to derive DKI parameters.
Image Analysis
Segmented images were post-processed off-line using code written in MATLAB 7.0.1 (The Mathworks, Natick, MA, USA). To increase the signal-to-noise ratio, a Gaussian filter with a full-width at half-maximum of 3 mm was applied to all diffusion-weighted images. Linear least square DKI fitting was used to solve for the coefficients of diffusivity (Dapp) and apparent kurtosis (Kapp) to the following equation [19]:
Sb and S0 are the signal intensities at b-value b and zero, respectively. Kapp is a dimensionless statistical metric that quantifies the non-Gaussian diffusion behavior where the tissue diffusivity demonstrates a more peaked distribution. When Kapp = 0, the standard mono-exponential model is recovered reflecting Gaussian distribution.
Selection of tumor regression grade (TRG) system
Although a number of TRG models have been proposed, the four-tier American Joint Committee on Cancer (AJCC) rectal cancer TRG system has been shown to be more accurate than other systems[3], and was therefore chosen for this study.
Statistical analysis
Tumors from 45 patients with rectal cancer were analyzed. Patients were categorized into one of four groups according to their approximate TRG values: TRG 0 (TRG = 100%), TRG 1 (90%≥TRG<100%), TRG 2 (50%≥TRG<90%), and TRG 3 (TRG<50%). Considering low frequencies in some of the TRG groups, and TRG’s association with recurrence-free survival[3], a binary outcome variable was created: TRG 0/TRG 1 (TRG≥90%) and TRG 2/TRG 3 (TRG<90%).
The relationships between the AJCC tumor regression grade (TRG) groups were compared with diffusivity (D) and kurtosis (K) summary values, as well as with volumes from each tumor’s volume of interest (VOI) and region of interest (ROI). The parameter D derived from diffusion kurtosis imaging reflects the water molecule true diffusion where the non-Gaussian distribution of values has been incorporated, and reflects complexity of tissue structures. It differs from the parameter D derived from intra-voxel incoherent motion (IVIM), which reflects the water molecular true diffusion, where the microcirculation perfusion, which reflects vascularization of tissue, has been separated. Summary values included mean and median diffusion and kurtosis values for tumor VOI and ROI.
In univariable analysis, a multinomial regression method was used, the proportional odds model, to analyze the four-level, ordinal outcome assuming the same effect of individual imaging value on each level of TRG group. As a sensitivity analysis, the relationships between the binary outcome and the explanatory variables of interest were tested using logistic regression. A significance level of 0.05 was used throughout.
All statistical computations were performed, and all output was generated using SAS Software Version 9.4 (The SAS Institute, Cary, NC).
Results
A total of 45 patients were included (31 male/14 female), mean age 57.4 years (range 34.2–72.9). The sample characteristics of the cohort and the frequency of TRG classification are summarized in Table 1 and Table 2, respectively. (TABLE 1 and TABLE 2)
Table 1:
Sample characteristics (N = 45)
| Variable | N | Median (Min-Max) |
|---|---|---|
| TRG Proportion | 45 | 0.7 (0.05–1) |
| ROI-Max Kurtosis Mean | 45 | 0.91837 (0.18113–1.86345) |
| ROI-Max Kurtosis Median | 45 | 0.85896 (1.335327E-10–1.39371) |
| ROI-Max D Mean | 45 | 0.00175 (0.00077–0.003) |
| ROI-Max D Median | 45 | 0.00167 (0.00088–0.00367) |
| ROI-Max Volume (cubic cm) | 45 | 3.96094 (0.82178–17.33643) |
| VOI Kurtosis Mean | 45 | 0.90006 (0.10979–1.61107) |
| VOI Kurtosis Median | 45 | 0.88078 (0.1012–2.06113) |
| VOI D Mean | 45 | 0.00159 (0.00088–0.00257) |
| VOI D Median | 45 | 0.00157 (0.00079–0.00277) |
| VOI Volume (cubic cm) | 45 | 20.14993 (1.69629–84.54684) |
Table 2:
TRG group frequencies
| N (%) | |||
|---|---|---|---|
| Sample size | 45 | ||
| TRG group (ordinal) | TRG 0 | TRG = 100% | 8 (17.8) |
| TRG 1 | 90%≤TRG<100% | 8 (17.8) | |
| TRG 2 | 50%≤TRG<90% | 14 (31.1) | |
| TRG 3 | TRG<50% | 15 (33.3) | |
| TRG group (binary) | TRG 0/TRG 1 | TRG≥90% | 16 (35.6) |
| TRG 2/TRG 3 | TRG<90% | 29 (64.4) | |
In the univariable proportional odds regression analysis, higher diffusivity summary (Dsum) values were observed to be significantly associated with higher odds of being in one or more favorable TRG group (TRG0 or TRG1). This was true for the ROI-max D mean (p = 0.002), ROI-max D median (p = 0.014), VOI D mean (p = 0.002), and VOI D median (p = 0.004). In other words, on average, patients with higher Dsum values were more likely to be in a more favorable TRG group. These results are mostly consistent with the sensitivity analysis, in which higher values for most Dsum values, all except ROI-max D Median (p = 0.08), were observed to be significantly associated with higher odds of being TRG0 or TRG1. (TABLE 3 and TABLE 4)
Table 3: Univariable proportional odds (i.e. Cumulative Logit) regression model results.
Probabilities modeled are cumulated over the groups with lower TRG values
| Variable | N used | OR [95% CI]1 | p-value |
|---|---|---|---|
| ROI-Max Kurtosis Mean2 | 45 | 0.908 [0.779 – 1.058] | 0.22 |
| ROI-Max Kurtosis Median2 | 45 | 0.904 [0.759 – 1.078] | 0.26 |
| ROI-Max D Mean3 | 45 | 1.240 [1.084 – 1.417] | 0.002 |
| ROI-Max D Median3 | 45 | 1.127 [1.025 – 1.239] | 0.014 |
| ROI-Max Volume (cubic cm) | 45 | 1.048 [0.885 – 1.243] | 0.59 |
| VOI Kurtosis Mean2 | 45 | 0.916 [0.768 – 1.093] | 0.33 |
| VOI Kurtosis Median2 | 45 | 0.942 [0.812 – 1.091] | 0.42 |
| VOI D Mean3 | 45 | 1.256 [1.090 – 1.446] | 0.002 |
| VOI D Median3 | 45 | 1.184 [1.055 – 1.329] | 0.004 |
| VOI Volume (cubic cm) | 45 | 1.012 [0.985 – 1.039] | 0.39 |
Note that OR represents the odds ratio of being in one or more favorable TRG group(s) versus being in the rest (less favorable TRG group(s)) associated with a single unit increment in the variable.
Note that the increment unit for OR estimation is 0.1 for all kurtosis summary variables.
Note that the increment unit for OR estimation is 0.0001 for all diffusion summary variables.
Table 4: Univariable logistic regression model results.
Probability modeled is TRG group (binary) = TRG 0/TRG 1 (i.e. TRG ≥90%)
| Variable | N used | OR [95% CI] | p-value |
|---|---|---|---|
| ROI-Max Kurtosis Mean1 | 45 | 0.931 [0.780 – 1.112] | 0.43 |
| ROI-Max Kurtosis Median1 | 45 | 0.917 [0.749 – 1.123] | 0.40 |
| ROI-Max D Mean2 | 45 | 1.253 [1.051 – 1.494] | 0.012 |
| ROI-Max D Median2 | 45 | 1.102 [0.989 – 1.228] | 0.08 |
| ROI-Max Volume (cubic cm) | 45 | 1.091 [0.897 – 1.327] | 0.39 |
| VOI Kurtosis Mean1 | 45 | 0.963 [0.786 – 1.179] | 0.72 |
| VOI Kurtosis Median1 | 45 | 0.980 [0.827 – 1.161] | 0.82 |
| VOI D Mean2 | 45 | 1.231 [1.042 – 1.454] | 0.014 |
| VOI D Median2 | 45 | 1.147 [1.006 – 1.307] | 0.041 |
| VOI Volume (cubic cm) | 45 | 1.025 [0.993 – 1.058] | 0.12 |
Note that the increment unit for OR estimation is 0.1 for all kurtosis summary variables.
Note that the increment unit for OR estimation is 0.0001 for all diffusion summary variables.
Tumor VOI and ROI-max volume, ROI-max kurtosis mean and median, and VOI kurtosis mean and median were not associated with TRG and were therefore not significantly associated with the response to neoadjuvant chemoradiation.
Discussion
In our cohort of 45 patients, we found that diffusivity derived from DKI, but not kurtosis, was significantly associated with AJCC pathologic TRG. Specifically, almost all of the Dsum values derived from DKI were able to distinguish those patients with TRG0 and TRG1, a more favorable response to neoadjuvant chemoradiation, from those with TRG2 or TRG3, those with a less favorable response. This suggests that diffusivity derived from diffusion kurtosis imaging may be able to risk stratify patients with rectal cancer on the baseline staging MRI for their subsequent response to neoadjuvant therapy.
Considering the results of our data in the context of the existing literature on diffusion kurtosis imaging and rectal cancer provides perspective. The significant association Dsum values with a patients subsequent response to neoadjuvant therapy is not entirely unexpected, as diffusivity is a parameter closely aligned with apparent diffusion coefficient values, which have shown promise as an imaging biomarker to predict treatment response to neoadjuvant therapy and recurrence in rectal cancer[20]. Previously, authors have looked at a variety of diffusion and perfusion parameters on MRI before and after neoadjuvant short course radiotherapy (SCR) and found that some of the perfusion parameters, namely variable projection derived from intra-voxel incoherent motion, was a promising biomarker following SCR[15]. In another study, Zhu et al found that kurtosis was more predictive of WHO tumor grade than ADC or diffusivity, and was also able to distinguish N0 from N1–2 disease[12]. Cui et al found that diffusion kurtosis correlated with nodal status, tumor histologic grade, lymphangiovascular invasion and involvement of the circumferential resection margin more than ADC or diffusivity[11]. Thus, our data adds one more piece of information into the growing body of literature around the role of DKI as an imaging biomarker in the assessment of rectal cancer on MRI.
The ability to predict a patient’s response to neoadjuvant therapy for rectal cancer based on data derived from the baseline rectal MRI may be of clinical utility. Diffusion kurtosis has gained considerable interest in recent years, due to its proven associations with tumor aggressiveness, histopathology, and disease-free survival across a range of malignancies[6,7,9,8,5]. Although K itself was not significantly associated with the subsequent response to neoadjuvant therapy in our cohort, D derived from DKI shows promise as an imaging biomarker in this setting. This distinction is not merely an academic question, as it has meaningful prognostic implications for a given patient. In an important study by Trakarnsanga et al [3] that established the AJCC pathologic TRG model as the most accurate, patients who had TRG0 and TRG1 after neoadjuvant therapy had 5-year recurrence free survival rates of 98% and 90%, respectively. This contrasts with those patients who had AJCC TRG2 and TRG3, who exhibited 5-year recurrence free survival rates of 73% and 68%, respectively. Therefore, the Dsum parameters derived from a baseline rectal MRI could presumably stratify patients as being TRG0/TRG1, with significantly better disease free survival rates, before neoadjuvant therapy is given. This information is readily available from baseline staging MRI studies if multiple b-values are acquired, including one high b-value that is at least 1,000 s/mm2, and may help medical oncologists and surgeons stratify patients up front.
This study has several limitations. First, this study is retrospective, which inherently limits the broad applicability of this data. Second, it is a relatively small cohort, with 45 patients. Lastly, our data is derived from a cohort of patients imaged at a single institution with a given set of MR parameters. As there is variability in MR scanning parameters across different institutions, this may limit reproducibility of our data.
As clinicians and radiologists investigate imaging biomarkers to help predict subsequent response to therapy in oncology, diffusivity values derived from DKI show potential in our cohort of rectal cancer patients, even though kurtosis values did not. Further investigation is needed in this area to establish the precise role Dsum may fill as a biomarker to stratify patients based on their expected response to neoadjuvant therapy.
Supplementary Material
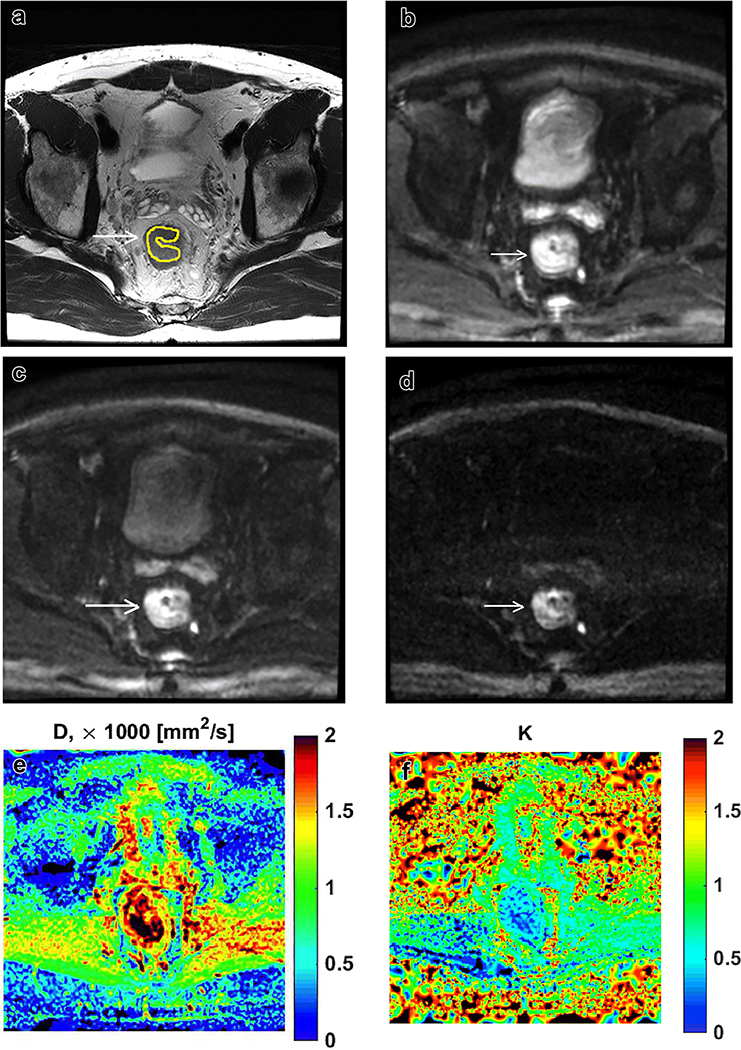
Figure 1:
50-year old male with locally advanced rectal cancer. Axial T2-weighted image through the level of the tumor with segmentation (a), and corresponding diffusion weighted images at b400, b800 and b1500 (b-d). Corresponding D- and K-maps from diffusion kurtosis imaging (e-f).
CLINICAL RELEVANCE STATEMENT.
Diffusivity shows promise as an imaging biomarker to predict AJCC TRG following neoadjuvant CRT, which has implications for risk stratification. Patients with TRG0/1 have 5-year disease free survival (DFS) of 90–98%, as opposed to those who are TRG2/3 with 5-year DFS of 68–73%.
Footnotes
Compliance with Ethical Standards
Conflict of Interest: The authors declare that they have no relevant conflicts of interest.
Ethical approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors.
Informed consent: Written informed consent was waived by the Institutional Review Board.
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
- 1.Benson AB 3rd, Venook AP, Al-Hawary MM, Cederquist L, Chen YJ, Ciombor KK, Cohen S, Cooper HS, Deming D, Engstrom PF, Grem JL, Grothey A, Hochster HS, Hoffe S, Hunt S, Kamel A, Kirilcuk N, Krishnamurthi S, Messersmith WA, Meyerhardt J, Mulcahy MF, Murphy JD, Nurkin S, Saltz L, Sharma S, Shibata D, Skibber JM, Sofocleous CT, Stoffel EM, Stotsky-Himelfarb E, Willett CG, Wuthrick E, Gregory KM, Gurski L, Freedman-Cass DA (2018) Rectal Cancer, Version 2.2018, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 16 (7):874–901. doi: 10.6004/jnccn.2018.0061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Horvat N, Carlos Tavares Rocha C, Clemente Oliveira B, Petkovska I, Gollub MJ (2019) MRI of Rectal Cancer: Tumor Staging, Imaging Techniques, and Management. Radiographics 39 (2):367–387. doi: 10.1148/rg.2019180114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Trakarnsanga A, Gonen M, Shia J, Nash GM, Temple LK, Guillem JG, Paty PB, Goodman KA, Wu A, Gollub M, Segal N, Saltz L, Garcia-Aguilar J, Weiser MR (2014) Comparison of tumor regression grade systems for locally advanced rectal cancer after multimodality treatment. J Natl Cancer Inst 106 (10). doi: 10.1093/jnci/dju248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jensen JH, Helpern JA (2010) MRI quantification of non-Gaussian water diffusion by kurtosis analysis. NMR Biomed 23 (7):698–710. doi: 10.1002/nbm.1518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang X, Tu N, Qin T, Xing F, Wang P, Wu G (2018) Diffusion Kurtosis Imaging Combined With DWI at 3-T MRI for Detection and Assessment of Aggressiveness of Prostate Cancer. AJR Am J Roentgenol 211 (4):797–804. doi: 10.2214/AJR.17.19249 [DOI] [PubMed] [Google Scholar]
- 6.Di Trani MG, Nezzo M, Caporale AS, De Feo R, Miano R, Mauriello A, Bove P, Manenti G, Capuani S (2018) Performance of Diffusion Kurtosis Imaging Versus Diffusion Tensor Imaging in Discriminating Between Benign Tissue, Low and High Gleason Grade Prostate Cancer. Acad Radiol. doi: 10.1016/j.acra.2018.11.015 [DOI] [PubMed] [Google Scholar]
- 7.Falk Delgado A, Nilsson M, van Westen D, Falk Delgado A (2018) Glioma Grade Discrimination with MR Diffusion Kurtosis Imaging: A Meta-Analysis of Diagnostic Accuracy. Radiology 287 (1):119–127. doi: 10.1148/radiol.2017171315 [DOI] [PubMed] [Google Scholar]
- 8.Huang Y, Lin Y, Hu W, Ma C, Lin W, Wang Z, Liang J, Ye W, Zhao J, Wu R (2019) Diffusion Kurtosis at 3.0T as an in vivo Imaging Marker for Breast Cancer Characterization: Correlation With Prognostic Factors. J Magn Reson Imaging 49 (3):845–856. doi: 10.1002/jmri.26249 [DOI] [PubMed] [Google Scholar]
- 9.Hempel JM, Brendle C, Bender B, Bier G, Kraus MS, Skardelly M, Richter H, Eckert F, Schittenhelm J, Ernemann U, Klose U (2019) Diffusion kurtosis imaging histogram parameter metrics predicting survival in integrated molecular subtypes of diffuse glioma: An observational cohort study. Eur J Radiol 112:144–152. doi: 10.1016/j.ejrad.2019.01.014 [DOI] [PubMed] [Google Scholar]
- 10.Wen Z, Chen Y, Yang X, Lu B, Liu Y, Shen B, Yu S (2019) Application of magnetic resonance diffusion kurtosis imaging for distinguishing histopathologic subtypes and grades of rectal carcinoma. Cancer Imaging 19 (1):8. doi: 10.1186/s40644-019-0192-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cui Y, Yang X, Du X, Zhuo Z, Xin L, Cheng X (2018) Whole-tumour diffusion kurtosis MR imaging histogram analysis of rectal adenocarcinoma: Correlation with clinical pathologic prognostic factors. Eur Radiol 28 (4):1485–1494. doi: 10.1007/s00330-017-5094-3 [DOI] [PubMed] [Google Scholar]
- 12.Zhu L, Pan Z, Ma Q, Yang W, Shi H, Fu C, Yan X, Du L, Yan F, Zhang H (2017) Diffusion Kurtosis Imaging Study of Rectal Adenocarcinoma Associated with Histopathologic Prognostic Factors: Preliminary Findings. Radiology 284 (1):66–76. doi: 10.1148/radiol.2016160094 [DOI] [PubMed] [Google Scholar]
- 13.Cui Y, Cui X, Yang X, Zhuo Z, Du X, Xin L, Yang Z, Cheng X (2019) Diffusion kurtosis imaging-derived histogram metrics for prediction of KRAS mutation in rectal adenocarcinoma: Preliminary findings. J Magn Reson Imaging. doi: 10.1002/jmri.26653 [DOI] [PubMed] [Google Scholar]
- 14.Feng Q, Yu H, Sun S, Ma Z (2019) The value of diffusion kurtosis imaging in assessing mismatch repair gene expression of rectal carcinoma: Preliminary findings. PLoS One 14 (2):e0211461. doi: 10.1371/journal.pone.0211461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fusco R, Sansone M, Granata V, Grimm R, Pace U, Delrio P, Tatangelo F, Botti G, Avallone A, Pecori B, Petrillo A (2018) Diffusion and perfusion MR parameters to assess preoperative short-course radiotherapy response in locally advanced rectal cancer: a comparative explorative study among Standardized Index of Shape by DCE-MRI, intravoxel incoherent motion- and diffusion kurtosis imaging-derived parameters. Abdom Radiol (NY). doi: 10.1007/s00261-018-1801-z [DOI] [PubMed] [Google Scholar]
- 16.Yu J, Xu Q, Song JC, Li Y, Dai X, Huang DY, Zhang L, Li Y, Shi HB (2017) The value of diffusion kurtosis magnetic resonance imaging for assessing treatment response of neoadjuvant chemoradiotherapy in locally advanced rectal cancer. Eur Radiol 27 (5):1848–1857. doi: 10.1007/s00330-016-4529-6 [DOI] [PubMed] [Google Scholar]
- 17.Hu F, Tang W, Sun Y, Wan D, Cai S, Zhang Z, Grimm R, Yan X, Fu C, Tong T, Peng W (2017) The value of diffusion kurtosis imaging in assessing pathological complete response to neoadjuvant chemoradiation therapy in rectal cancer: a comparison with conventional diffusion-weighted imaging. Oncotarget 8 (43):75597–75606. doi: 10.18632/oncotarget.17491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rasband WS, ImageJ, U. S. National Institutes of Health, Bethesda, Maryland, USA, https://imagej.nih.gov/ij/,1997-2018. [Google Scholar]
- 19.Jensen JH, Helpern JA, Ramani A, Lu H, Kaczynski K (2005) Diffusional kurtosis imaging: the quantification of non-gaussian water diffusion by means of magnetic resonance imaging. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine 53 (6):1432–1440. doi: 10.1002/mrm.20508 [DOI] [PubMed] [Google Scholar]
- 20.Elmi A, Hedgire SS, Covarrubias D, Abtahi SM, Hahn PF, Harisinghani M (2013) Apparent diffusion coefficient as a non-invasive predictor of treatment response and recurrence in locally advanced rectal cancer. Clin Radiol 68 (10):e524–531. doi: 10.1016/j.crad.2013.05.094 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.