Abstract
BACKGROUND
Gout, caused by hyperuricemia and subsequent deposition of aggregated monosodium urate crystals (MSU) in the joints or extra-articular regions, is the most common inflammatory arthritis. There is increasing evidence that gout is an independent risk factor for hypertension, cardiovascular disease progression and mortality.
AIM
To evaluate if dual energy computed tomography (DECT) could identify MSU within vessel walls of gout patients, and if MSU deposits within the vasculature differed between patients with gout and controls. This study may help elucidate why individuals with gout have increased risk for cardiovascular disease.
METHODS
31 gout patients and 18 controls underwent DECT scans of the chest and abdomen. A material decomposition algorithm was used to distinguish regions of MSU (coded green), and calcifications (coded purple) from soft tissue (uncoded). Volume of green regions was calculated using a semi-automated volume assessment program. Between-group differences were analyzed using Mann-Whitney U exact test and nonparametric rank regression.
RESULTS
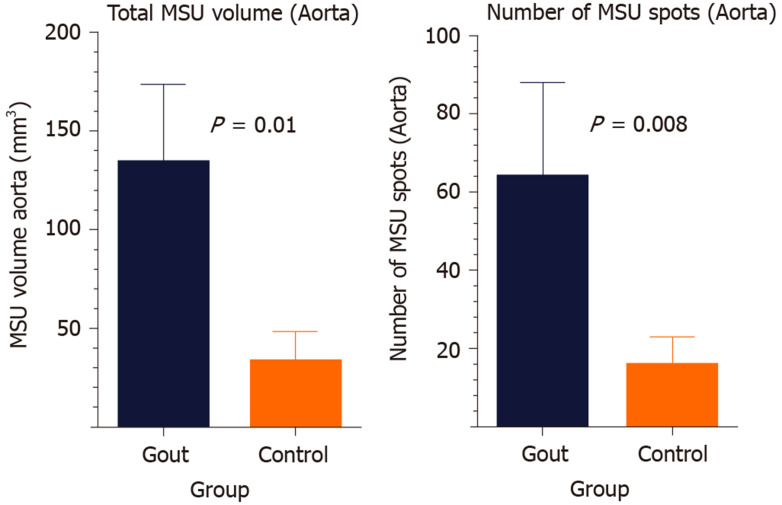
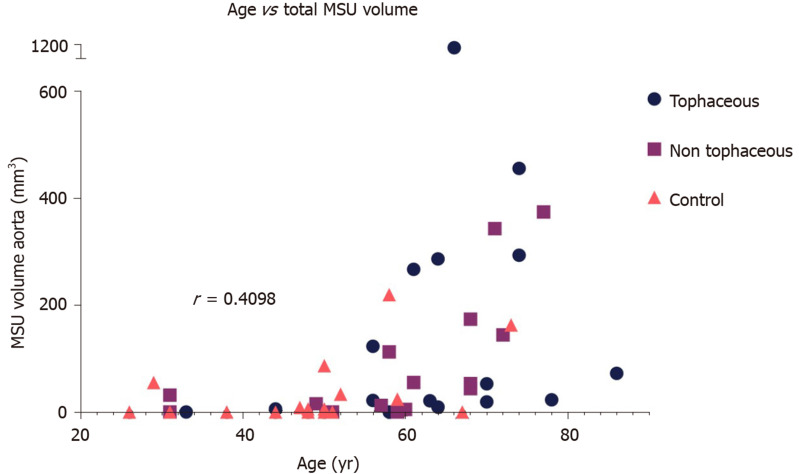
Gout patients had significantly higher volume of MSU within the aorta compared to controls [Median (Min-Max) of 43.9 (0-1113.5) vs 2.9 (0-219.4), P = 0.01]. Number of deposits was higher in gout patients compared to controls [Median (Min-Max) of 20 (0-739) vs 1.5 (0-104), P = 0.008]. However, the difference was insignificant after adjustment for age, gender, history of cardiovascular disease and diabetes. Increased age was positively associated with total urate volume (rs = 0.64; 95% confidence interval: 0.43-0.78).
CONCLUSION
This pilot study showed that DECT can quantify vascular urate deposits with variation across groups, with gout patients possibly having higher deposition. This relationship disappeared when adjusted for age, and there was a positive relationship between age and MSU deposition. While this study does not prove that green coded regions are truly MSU deposition, it corroborates recent studies that show the presence of vascular deposition.
Keywords: Gout, Tophaceous, Dual-energy computed tomography, Hyperuricemia, Monosodium urate, Cardiovascular disease
Core tip: There is increasing evidence that gout is an independent risk factor for cardiovascular disease progression. In hyperuricemic individuals, monosodium urate may be deposited within the vessel wall and possibly lead to an inflammatory cascade. We examine if dual energy computed tomography can quantify monosodium urate depositions in the vasculature of gout patients, and measure deposits volumetrically to see if they are higher in these individuals compared to healthy controls. The study shows that dual energy computed tomography can detect vascular deposits and that gout patients possibly have increased deposition, and corroborates recent studies that show the presence of vascular deposition.
INTRODUCTION
Gout is the most common inflammatory arthritis, caused by hyperuricemia and subsequent deposition of aggregated monosodium urate crystals (MSU) in the joints or extra-articular regions[1,2]. Gout and hyperuricemia have increased in prevalence over the last few decades[3] and are estimated to be present in approximately 4% and 20% of the United states population respectively[4,5]. MSU deposition triggers an acute and intense inflammatory response in the synovium[6-8], with infiltration of neutrophils, monocytes/macrophages, lymphocytes and release of proinflammatory cytokines, ultimately responsible for the development of acute gout, which manifests primarily as an acute intermittent monoarthritis. In approximately 12%-35% of patients, gout progresses to a chronic form due to presence of multiple comorbidities, age, renal dysfunction, or poor diet and lifestyle[9]. This chronic form can exhibit “tophi” — hard aggregates of MSU crystals — in the joints (tophaceous gout), and if untreated, can cause joint erosion and destruction[10].
Dual energy computed tomography (DECT) has been previously used to accurately visualize MSU deposits within the joints[2,11], and to diagnose gout with excellent sensitivity and specificity[12]. DECT allows distinguishing different materials based on their relative absorption of X-ray at different energy spectra[13]. Dual source CT scanners are capable of using two separate sets of X-ray tubes and detectors positioned approximately 90 degrees apart to perform simultaneous acquisitions at different energy levels[14]. By using independent tube current modulation, low electronic noise detectors, and tin filtering of the higher kV tube’s spectra, these scanners produce high resolution images with excellent energy spectral separation, while maintaining radiation doses similar to single energy scanners[15]. After acquisition, gout MSU crystals are separated from calcium by using a two material decomposition algorithm based on the principle that, while both are bright (i.e., exhibit high computed tomography-numbers), calcium is brighter due to having a relatively higher atomic number as compared to MSU. Finally, separated images may be color coded to highlight the MSU (e.g., in green with purple/pink for bone/calcifications)[13].
There is increasing evidence that gout is an independent risk factor for hypertension, cardiovascular disease progression and mortality[16-21]. Gout patients are at higher risk of life-threatening myocardial infarction and stroke[3,22-25]. However, the exact mechanisms by which gout confers this increased cardiovascular risk are still poorly understood[26-29]. MSU deposits identified by DECT in regions other than the joints (such as the vasculature) have been anecdotally treated as artifacts[30]. However, it is possible that in hyperuricemic individuals, MSU may actually be deposited within the vessel wall and possibly lead to an inflammatory cascade. As macrophages uptake uric acid crystals, the NLRP3 inflammasome[31] can be activated and generate a cascade of pro-inflammatory cytokines and progression of the atherosclerotic cascade[32] resulting in increased cardiovascular risk in these individuals. Another mechanism by which uric acid has been shown to contribute to cardiovascular risk is by increased vascular smooth muscle cell proliferation following oxidative stress[33]. Increased oxidative stress could also result in atherosclerotic disease progression and thereby increased cardiovascular risk[34]. A very recent study by Klauser et al[35] has confirmed that vascular signal observed from DECT is indeed from MSU by using autopsy specimens using polarizing light microscopy[35].
The aim of this study was to determine if DECT can potentially quantify deposition of MSU within the vessel wall of individuals with gout and if this deposition is increased in these individuals compared to healthy controls by measuring volume of urate deposits.
MATERIALS AND METHODS
Fifty adult subjects were recruited from a single-center urban academic hospital to participate in this study. One subject dropped out after consent. A total of 31 subjects had gout, and 18 were healthy controls. Of the 31 subjects with gout, 16 subjects had tophaceous gout, and the remaining 15 were non tophaceous. Tophaceous gout was determined by the presence of clinically detectable tophaceous deposits of uric acid crystals in individuals who had had recurrent attacks of gout. Recruitment methods for gout subjects consisted of medical records review and physician referral. Study investigators introduced the study at out-patient clinics, where treating physicians identified eligible patients. Interested parties then contacted study coordinators to schedule consenting appointments and participation. Clinical details were obtained via chart review. Healthy controls were recruited by posting institutional review board approved flyers within the hospital.
Inclusion criteria for gout patients consisted of a confirmed diagnosis of gout by a board-certified rheumatologist. Exclusion criteria for gout patients included: Patients under the age of 18, patients with a history of claustrophobia, patients with implanted pacemakers or spinal metal implants, patients with diagnosed malignancies or lymphoproliferative diseases within the past 5 years, patients with a history of chronic infectious disease, including tuberculosis, severe fungal disease, positive human immunodeficiency virus status, as well as patients diagnosed with chronic hepatitis B or C infection, patients undergoing radiotherapy, and lastly, female patients of child bearing potential or intending to breastfeed. Exclusion criteria for healthy controls consisted of the above and also included patients diagnosed with any chronic infectious, rheumatologic or autoimmune disease, as well as any inflammatory arthritic conditions. Demographic information and clinical details were obtained by chart review for gout patients and healthy controls.
All study participants underwent DECT scans of the chest and abdomen. DECT scans were obtained using a second-generation Siemens somatom Force 2 × 192 slice dual source CT scanner (Siemens Healthineers, Forchheim, Germany). Images were acquired at 90 kVp and 150 kVp with a tin filter, with a pitch of 0.6, and reconstructed to 1 mm isotropic voxels using iterative reconstruction (ADMIRE) with a quantitative kernel (Qr40). Dose modulation (CARE DOSE4D) was used to reduce radiation exposure. The estimated radiation exposure that study participants received was approximately 10.5 mSv.
After acquisition and reconstruction, images were analyzed using a specific segmentation algorithm to differentiate calcium and urate depositions within the arterial vessel wall (aorta, coronaries and iliacs) using the Siemens syngo via software package (Siemens Healthineers, Forchheim, Germany). Total volume of areas coded green by the DECT material decomposition algorithm (indicating MSU deposition) with the entire aorta was computed using a semi-automated volume assessment program from manually drawn regions of interest. The number of distinct green spots (potentially MSU) on images within the aorta were also computed. Other vessels (coronaries, iliacs) were classified as either with the presence or absence of MSU deposits as defined by the presence of green coded spots on analyzed images.
Continuous variables were summarized using medians, minimum, and maximum. Categorical variables were presented with counts and percentages. Unadjusted differences in the total volume of MSU and the number of green spots in the aorta between the gout and healthy control groups were assessed via the Mann-Whitney U exact test. To control for potential confounding of age, gender, history of cardiovascular disease and diabetes, nonparametric rank regression was also used. Presence or absence of MSU within the coronaries and iliacs across the gout and control groups were assessed by means of a Fisher’s exact test. An exploratory subgroup analysis was performed to compare differences in total volume and number of green spots between the tophaceous and non-tophaceous gout groups using the nonparametric methods described above. Hypothesis testing was performed at the 0.05 level (2-sided) using the Rfit package in R 3.4.1 and SAS version 9.4 (SAS Institute Inc.)[36].
RESULTS
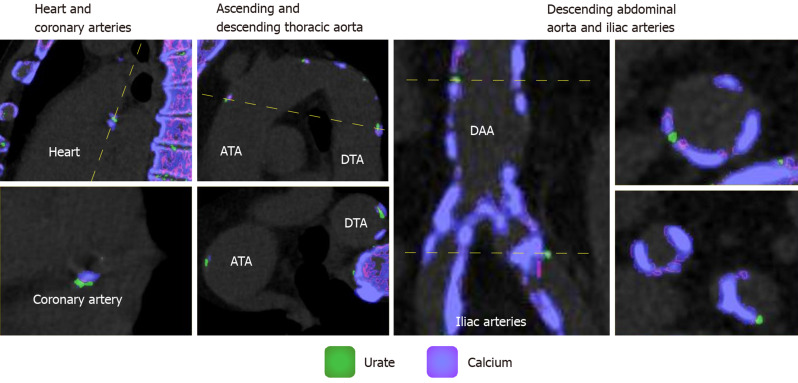
Among the 49 subjects analyzed, the mean age was 56 ± 15 years and the percentage of women was 35% (n = 17). Diabetes was present in 39% (n = 19) of patients, 22% (n = 11) had a history of cardiovascular disease, and 48% (n = 19/40) were current or former smokers. (Table 1) presents demographics and characteristics of the gout and control groups. (Figure 1) shows sample in vivo images of potential MSU uptake (green coded regions) within the different vascular territories. The range of uric acid deposition within the aorta varied, from no deposition to over 1 cm3. Gout subjects had significantly higher volume of MSU within the entire aorta as shown in (Figure 2A) [Median (Min-Max) of 43.9 (0-1113.5) vs 2.9 (0-219.4), respectively for gout and control groups, P = 0.01]. Similarly, the number of deposits (green spots on image) was significantly higher in the gout group compared to the controls as shown in (Figure 2B) [Median (Min-Max) of 20 (0-739) vs 1.5 (0-104) respectively for gout and control groups, P = 0.008]. However, after adjustment for age, gender, history of cardiovascular disease and diabetes, there was no significant difference between the gout and control groups with respect to MSU volume (P = 0.53) and number of green spots (P = 0.39). However, older age was associated with higher total green coded volume on images (P = 0.08) and larger number of green spots (P = 0.03). (Figure 3) shows the relationship between age and MSU volume within the aorta. The estimated Spearman’s rank correlation coefficient for the two variables was 0.64 (95% confidence interval: 0.43-0.78).
Table 1.
Demographics and characteristics of cohort
| Group | Gout (n = 31) | Control (n = 18) |
| Age, median (min-max) (n) | 63 (31-86) (31) | 49 (26-73) (18) |
| Male Gender (n, %) | 26 (83.9) | 6 (33.3) |
| History of CAD (n, %) | 9 (29.0) | 2 (11.1) |
| Diabetes (n, %) | 15 (48.4) | 4 (22.2) |
| Ever Smoked (n, %) | 19/29 (65.5) | 0/11 (0.0) |
| Total Cholesterol, median (min-max) (n) | 165 (114-250) (25) | 192 (145-247) (11) |
| HDL, median (min-max) (n) | 43 (24-88) (25) | 61 (35-88) (11) |
| LDL, median (min-max) (n) | 99 (58-179.2) (25) | 110 (66-139) (11) |
| Hemoglobin A1C, median (min-max) (n) | 5.9 (5.0-10.5) (24) | 5.7 (5.2-11) (9) |
| Systolic BP, median (min-max) (n) | 138 (98-175) (29) | 126.5 (101-157) (12) |
| Diastolic BP, median (min-max) (n) | 80 (61-104) (29) | 79.5 (66-99) (12) |
| Serum urate, median (min-max) (n) | 6 1-13.5) (29) | 4.4 (3.2-4.5) (3) |
| Total MSU volume aorta, mm3 median (min-max) (n) | 43.9 (0-1113.5) (31) | 2.9 (0-219.4) (18) |
| MSU spots Aorta median (min-max) (n) | 20(0-739) (31) | 1.5(0-104) (18) |
CAD: Cardiovascular disease; MSU: Monosodium urate.
Figure 1.
Sample in vivo images of greed coded regions (monosodium urate deposits) within the different vascular territories. Coronary arteries (left panel), ascending and descending aorta (2nd from left), descending abdominal aorta and iliac arteries (right panels). Monosodium urate deposition is color coded in green whereas calcium is coded in purple/pink. ATA: Ascending thoracic aorta; DTA: Descending thoracic aorta; DAA: Descending abdominal aorta.
Figure 2.
Plots showing total volume of green coded areas (urate deposition) within the aorta (mean ± SD) (Left). Number of green deposits/spots in the aortas of gout and control subjects (mean ± SD) (Right). MSU: Monosodium urate.
Figure 3.
Relationship between age and green coded volumes in the aorta (monosodium urate uptake) across the groups. The estimated Spearman’s rank correlation coefficient for the two variables was 0.64 (95% confidence interval: 0.43-0.78). MSU: Monosodium urate.
Exploratory subgroup analysis of tophaceous and non-tophaceous gout showed no significant differences between the groups for either total MSU volume in the aorta (P = 0.63) or the number of green spots (P = 0.55). This did not change when the models were adjusted for the aforementioned confounders. (Table 2) highlights these results.
Table 2.
Comparison of tophaceous vs non tophaceous gout groups
| Group | Tophaceous gout | Non tophaceous gout |
| Age, median (min-max) (n) | 64 (33-86) (16) | 60 (31-77) (15) |
| Male gender (n, %) | 14 (87.5) | 12 (80.0) |
| History of CAD (n, %) | 4 (25.0) | 5 (33.3) |
| Diabetes (n, %) | 7 (43.8) | 8 (53.3) |
| Ever smoked (n, %) | 8 (50.0) | 11 (73.3) |
| Total cholesterol, median (min-max) (n) | 162 (114-250) (12) | 176 (128-234) (13) |
| HDL, median (min-max) (n) | 40 (24-61) (12) | 52 (26-88) (13) |
| LDL, median (min-max) (n) | 92.5 (61.6-179.2) (12) | 103.4 (58-143) (13) |
| Hemoglobin A1C, median (min-max) (n) | 6 (5.5-10.5) (12) | 5.5 (5-6.4) (12) |
| Systolic BP, median (min-max) (n) | 136 (98-175) (15) | 140 (120-156) (14) |
| Diastolic BP, median (min-max) (n) | 80 (64-104) (15) | 82 (61-101) (14) |
| Serum Urate, median (min-max) (n) | 6.7 (1-13.5) (16) | 5.6 (2.9-12.7) (13) |
| Total MSU volume aorta, mm3 median (min-max) (n) | 38 (0-1113.5) (16) | 43.9 (0-374.2) (15) |
| MSU spots aorta median (min-max) (n) | 24.5(0-739) (16) | 20(0-157) (15) |
CAD: Cardiovascular disease; MSU: Monosodium urate.
A significantly larger proportion of subjects in the gout group had MSU deposits in the coronaries and iliac arteries. Specifically, 55% (n = 16/29) of the subjects in the gout group had potential uric acid deposits in the coronary arteries compared to 0% in the control group (P < 0.001). Uric acid deposition within the iliac arteries was observed in 54% (n = 15/28) of gout subjects compared to 11% (n = 2/18) in controls (P = 0.005).
DISCUSSION
Our results illustrated that patients with gout have increased green coded regions and MSU deposition within their vasculature. The total volume of these regions within the aorta of individuals with gout was significantly higher than the controls. Similarly, the proportion of individuals with MSU deposits within the coronary vessels and iliacs was higher in patients with gout compared to controls. To our knowledge, this is amongst the first few studies to demonstrate such a relationship and may provide some insight into the reasons for increased cardiovascular risk in individuals with gout. Recently, Klauser et al[35] have shown similar findings in gout and control patients and validated their findings in 3 cadaver specimens[35]. They, however, did not measure volume of these MSU deposits in the aorta, but only looked at prevalence.
While the data presented are compelling, there are several major limitations to our study, and the results should be interpreted with caution. The primary limitation of this study is that there is no independent validation of the presence of uric acid deposits within the vasculature either by an alternative method or by histology. It was not possible to obtain histological samples from vasculature of living individuals in this study. While several animal models for gout are available[37-40], most involve injecting MSU crystals within the joints of animals, and to our knowledge, no animal models for vascular urate deposition exist. We have attempted to validate the current approach by scanning ex vivo urinary calculi and in vitro exams of crystalline MSU and calcium with reasonable results (See supplementary material). Future studies should attempt to devise a suitable animal model to perform such in vivo validations. Alternatively, cadavers can be examined as previously performed by Klauser et al[35]. Past studies have typically treated MSU observed by dual energy CT in regions other than the joints as artifacts[30]. However, our study provides compelling evidence of varying distribution across risk groups that may indicate that this may not necessarily be the case. With increasing studies with similar findings, confidence that this is indeed urate signal in the vasculature is growing[35]. Our study also indicated reasonably large potential uric acid deposits (green coded regions of DECT analyzed images) both in close proximity and far away from regions of calcification in the vasculature, lending credence to the fact that the signal may legitimately be from urate deposition.
Secondly, since this was a pilot study, our sample size was limited, especially while looking at subgroups such as tophaceous and non-tophaceous gout. We did not observe any differences between patients with tophaceous or non-tophaceous gout in this study. Additionally, our demographics and patient characteristics were obtained by chart review, and no blood samples were collected as part of the study procedures themselves. This led to some data (such as cardiovascular risk profiles, serum urate levels, etc.) being unavailable for many cases, especially in the control group. Additionally, the healthy control group relied on self-disclosure of any preexisting medical conditions including inflammatory diseases. It is possible that some of the control group had undiagnosed gout that could have confounded our results. Two controls had DECT scans with MSU deposition within joints, suggesting undiagnosed or subclinical gout. Both patients had large aggregates of MSU seen in their fingers and wrists, and were referred to primary care physicians for follow up. However, for the purposes of this study, they were still included as part of the control group. This could account for greater-than-zero green spots in vasculature (MSU vascular urate volume) in some of our control subjects.
Thirdly, the gout and control groups in this study were not balanced with respect to age, gender and other risk factors. Our gout group was predominantly male while there were more women in the control group. Our control group was also younger than the gout group. There were no notable differences between the tophaceous and non-tophaceous gout subgroups. Interestingly, we found a correlation between increased age and MSU deposition (green coded regions) within the aorta. As gout typically progresses with age, it is interesting to note that the MSU volumes seems to follow a similar trend.
Finally, this study would have benefited from the availability of serum inflammatory biomarkers and availability of serum urate data for correlating with the results obtained. Being a pilot and exploratory study, such data was not readily available.
Nevertheless, we feel that this study has provided compelling data regarding vascular deposition of MSU in patients with gout and could lead to future studies that examine whether this finding has implications for the increased cardiovascular disease risk in individuals with gout. Further independent confirmatory studies in newly developed animal models or in human cadavers with histological confirmation may shed light on the sensitivity, specificity, and accuracy of DECT in quantifying vascular urate depositions.
Regions coded green on DECT images using the material decomposition algorithms have lower HU at lower kVp compared to regions coded purple/pink. Materials that exhibit such characteristics are MSU, keratin or some plastics. It is unlikely that we would find plastics or keratin within the vessel wall, and this lends more credence to the material being MSU. However, it is entirely possible that these could be some components of atherosclerotic lesions with similar X-ray attenuation characteristics as MSU. The mere presence of and further differences in green coded regions between the different groups are therefore of importance even if the material cannot be confirmed as MSU.
This pilot study showed that DECT can detect vascular urate deposits and that there is a variation in these deposits across groups, with gout patients possibly having higher MSU deposition within their vasculature. Vascular MSU could potentially be a mechanism by which individuals with gout have higher cardiovascular risk. This relationship, however, disappeared when adjusted for age and there was a positive relationship between age and MSU deposition. While this study does not prove that the green coded regions on DECT are truly MSU deposition, it corroborates other recent studies that show the presence of vascular MSU deposition. Further studies are required to further examine and validate these results.
ARTICLE HIGHLIGHTS
Research background
Gout, caused by hyperuricemia and subsequent deposition of aggregated monosodium urate crystals (MSU) in the joints or extra-articular regions, is the most common inflammatory arthritis. There is increasing evidence that gout is an independent risk factor for hypertension, cardiovascular disease progression and mortality. Gout patients are at higher risk of life-threatening myocardial infarction and stroke. However, the exact mechanisms by which gout confers this increased cardiovascular risk are still poorly understood.
Research motivation
Dual energy computed tomography (DECT) has been previously used to diagnose gout and accurately visualize MSU deposits within the joints. MSU deposits identified by DECT in regions other than the joints, such as the vasculature, have been anecdotally treated as artifacts. However, it is possible that in hyperuricemic individuals, MSU may actually be deposited within the vessel wall and possibly lead to an inflammatory cascade.
Research objectives
The aim of this study was to determine if DECT can quantify deposition of MSU within the vessel wall of individuals with gout and to measure volume of urate deposits to determine if this deposition is increased in these gout patients as compared to healthy controls.
Research methods
Fifty adult subjects were recruited from a single-center urban academic hospital to participate in this study. 31 subjects had gout, and 18 were healthy controls. Of the 31 gout patients, 16 subjects had tophaceous gout, and the remaining 15 were non tophaceous. All study participants underwent dual energy computed tomography (DECT) scans of the chest and abdomen using a second-generation Siemens somatom Force 2 × 192 slice dual source computed tomography scanner. Images were analyzed using a specific segmentation algorithm to differentiate calcium and urate depositions within the arterial vessel wall (aorta, coronaries and iliacs) using the Siemens syngo via software package. Total volume of areas coded green by the DECT material decomposition algorithm (indicating MSU deposition) with the entire aorta was computed using a semi-automated volume assessment program from manually drawn regions of interest. The number of distinct green spots on images within the aorta were also computed. Unadjusted differences in the total volume of MSU and the number of green spots in the aorta between the gout and healthy control groups were assessed via the Mann-Whitney U exact test. An exploratory subgroup analysis was performed to compare differences in total volume and number of green spots between the tophaceous and non-tophaceous gout groups using the nonparametric methods described above.
Research results
Gout subjects had significantly higher volume of MSU within the entire aorta and the number of deposits (green spots on image) was significantly higher in the gout group compared to the controls. However, after adjustment for age, gender, history of cardiovascular disease and diabetes, there was no significant difference between the gout and control groups with respect to MSU volume. Older age was associated with higher total green coded volume on images (P = 0.08) and larger number of green spots (P = 0.03). Exploratory subgroup analysis of tophaceous and non-tophaceous gout showed no significant differences between the groups for either total MSU volume in the aorta (P = 0.63) or the number of green spots (P = 0.55). A significantly larger proportion of subjects in the gout group had MSU deposits in the coronaries and iliac arteries. Specifically, 55% (n = 16/29) of the subjects in the gout group had potential uric acid deposits in the coronary arteries compared to 0% in the control group (P < 0.001).
Research conclusions
The results of this study show that patients with gout have increased green coded regions and MSU deposition within their vasculature and that the total volume of these regions within the aorta of individuals with gout was significantly higher than the controls. Similarly, the proportion of individuals with MSU deposits within the coronary vessels and iliacs was higher in patients with gout compared to controls. To our knowledge, this is amongst the first few studies to demonstrate such a relationship. This study has provided compelling data regarding vascular deposition of MSU in patients with gout and could lead to future studies that examine whether this finding has implications for the increased cardiovascular disease risk in individuals with gout. In the future, this study could benefit from a larger sample size as well as from the availability of serum inflammatory biomarkers and serum urate data for correlating with the results. Further independent confirmatory studies in newly developed animal models or in human cadavers with histological confirmation may also shed light on the sensitivity, specificity, and accuracy of DECT in quantifying vascular urate depositions.
ACKNOWLEDGEMENTS
The authors wish to thank Sonum Naidu, BSE for help with formatting and submission of this manuscript.
Footnotes
Institutional review board statement: The study was reviewed and approved by the Icahn School of Medicine at Mount Sinai, Mount Sinai St, Luke’s, Mount Sinai West.
Informed consent statement: All study participants, or their legal guardian, provided informed written consent prior to study enrollment.
Conflict-of-interest statement: None of the authors have any conflicts of interest with regards to the study.
Data sharing statement: No additional data are available.
STROBE statement: The authors have read the STROBE Statement-checklist of items, and the manuscript was prepared and revised according to the STROBE Statement-checklist of items.
Manuscript source: Unsolicited manuscript
Peer-review started: March 9, 2020
First decision: April 25, 2020
Article in press: July 19, 2020
Specialty type: Radiology, nuclear medicine and medical imaging
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Gheita T S-Editor: Zhang L L-Editor: A P-Editor: Liu JH
Contributor Information
Sharon Hannah Barazani, Department of Radiology, Translational and Molecular Imaging Institute, Icahn School of Medicine at Mount Sinai, New York, NY 10029, United States.
Wei-Wei Chi, Division of Rheumatology, Department of Medicine, Icahn School of Medicine at Mount Sinai, New York, NY 10029, United States.
Renata Pyzik, Department of Radiology, Translational and Molecular Imaging Institute, Icahn School of Medicine at Mount Sinai, New York, NY 10029, United States.
Helena Chang, Center for Biostatistics, Department of Population Health Science and Policy, Icahn School of Medicine at Mount Sinai, New York, NY 10029, United States.
Adam Jacobi, Department of Radiology, Icahn School of Medicine at Mount Sinai, New York, NY 10029, United States.
Tom O’Donnell, Siemens Healthineers, Erlangen 91052, Germany.
Zahi A Fayad, Department of Radiology, Translational and Molecular Imaging Institute, Icahn School of Medicine at Mount Sinai, New York, NY 10029, United States; Department of Radiology, Icahn School of Medicine at Mount Sinai, New York, NY 10029, United States.
Yousaf Ali, Division of Rheumatology, Department of Medicine, Icahn School of Medicine at Mount Sinai, New York, NY 10029, United States.
Venkatesh Mani, Department of Radiology, Translational and Molecular Imaging Institute, Icahn School of Medicine at Mount Sinai, New York, NY 10029, United States; Department of Radiology, Icahn School of Medicine at Mount Sinai, New York, NY 10029, United States. venkatesh.mani@mssm.edu.
References
- 1.Ankli B. [Current epidemiology of gout] Ther Umsch. 2016;73:125–129. doi: 10.1024/0040-5930/a000767. [DOI] [PubMed] [Google Scholar]
- 2.Yu Z, Mao T, Xu Y, Li T, Wang Y, Gao F, Sun W. Diagnostic accuracy of dual-energy CT in gout: a systematic review and meta-analysis. Skeletal Radiol. 2018;47:1587–1593. doi: 10.1007/s00256-018-2948-y. [DOI] [PubMed] [Google Scholar]
- 3.Abeles AM. Hyperuricemia, gout, and cardiovascular disease: an update. Curr Rheumatol Rep. 2015;17:13. doi: 10.1007/s11926-015-0495-2. [DOI] [PubMed] [Google Scholar]
- 4.Zhu Y, Pandya BJ, Choi HK. Prevalence of gout and hyperuricemia in the US general population: the National Health and Nutrition Examination Survey 2007-2008. Arthritis Rheum. 2011;63:3136–3141. doi: 10.1002/art.30520. [DOI] [PubMed] [Google Scholar]
- 5.Kuo CF, Grainge MJ, Zhang W, Doherty M. Global epidemiology of gout: prevalence, incidence and risk factors. Nat Rev Rheumatol. 2015;11:649–662. doi: 10.1038/nrrheum.2015.91. [DOI] [PubMed] [Google Scholar]
- 6.Agudelo CA, Schumacher HR. The synovitis of acute gouty arthritis. A light and electron microscopic study. Hum Pathol. 1973;4:265–279. doi: 10.1016/s0046-8177(73)80013-9. [DOI] [PubMed] [Google Scholar]
- 7.Rock KL, Kataoka H, Lai JJ. Uric acid as a danger signal in gout and its comorbidities. Nat Rev Rheumatol. 2013;9:13–23. doi: 10.1038/nrrheum.2012.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dalbeth N, Haskard DO. Mechanisms of inflammation in gout. Rheumatology (Oxford) 2005;44:1090–1096. doi: 10.1093/rheumatology/keh640. [DOI] [PubMed] [Google Scholar]
- 9.Brook RA, Forsythe A, Smeeding JE, Lawrence Edwards N. Chronic gout: epidemiology, disease progression, treatment and disease burden. Curr Med Res Opin. 2010;26:2813–2821. doi: 10.1185/03007995.2010.533647. [DOI] [PubMed] [Google Scholar]
- 10.Eggebeen AT. Gout: an update. Am Fam Physician. 2007;76:801–808. [PubMed] [Google Scholar]
- 11.Metzger SC, Koehm M, Wichmann JL, Buettner S, Scholtz JE, Beeres M, Kerl JM, Albrecht MH, Hammerstingl R, Vogl TJ, Bauer RW. Dual-Energy CT in Patients with Suspected Gouty Arthritis: Effects on Treatment Regimen and Clinical Outcome. Acad Radiol. 2016;23:267–272. doi: 10.1016/j.acra.2015.10.019. [DOI] [PubMed] [Google Scholar]
- 12.Chhana A, Doyle A, Sevao A, Amirapu S, Riordan P, Dray M, McGlashan S, Cornish J, Dalbeth N. Advanced imaging assessment of gout: comparison of dual-energy CT and MRI with anatomical pathology. Ann Rheum Dis. 2018;77:629–630. doi: 10.1136/annrheumdis-2017-211343. [DOI] [PubMed] [Google Scholar]
- 13.Chou H, Chin TY, Peh WC. Dual-energy CT in gout - A review of current concepts and applications. J Med Radiat Sci. 2017;64:41–51. doi: 10.1002/jmrs.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Flohr TG, McCollough CH, Bruder H, Petersilka M, Gruber K, Süss C, Grasruck M, Stierstorfer K, Krauss B, Raupach R, Primak AN, Küttner A, Achenbach S, Becker C, Kopp A, Ohnesorge BM. First performance evaluation of a dual-source CT (DSCT) system. Eur Radiol. 2006;16:256–268. doi: 10.1007/s00330-005-2919-2. [DOI] [PubMed] [Google Scholar]
- 15.Henzler T, Fink C, Schoenberg SO, Schoepf UJ. Dual-energy CT: radiation dose aspects. AJR Am J Roentgenol. 2012;199:S16–S25. doi: 10.2214/AJR.12.9210. [DOI] [PubMed] [Google Scholar]
- 16.Pontremoli R. The role of urate-lowering treatment on cardiovascular and renal disease: evidence from CARES, FAST, ALL-HEART, and FEATHER studies. Curr Med Res Opin. 2017;33:27–32. doi: 10.1080/03007995.2017.1378523. [DOI] [PubMed] [Google Scholar]
- 17.Singh JA, Ramachandaran R, Yu S, Yang S, Xie F, Yun H, Zhang J, Curtis JR. Is gout a risk equivalent to diabetes for stroke and myocardial infarction? A retrospective claims database study. Arthritis Res Ther. 2017;19:228. doi: 10.1186/s13075-017-1427-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee KA, Ryu SR, Park SJ, Kim HR, Lee SH. Assessment of cardiovascular risk profile based on measurement of tophus volume in patients with gout. Clin Rheumatol. 2018;37:1351–1358. doi: 10.1007/s10067-017-3963-4. [DOI] [PubMed] [Google Scholar]
- 19.Desai RJ, Franklin JM, Spoendlin-Allen J, Solomon DH, Danaei G, Kim SC. An evaluation of longitudinal changes in serum uric acid levels and associated risk of cardio-metabolic events and renal function decline in gout. PLoS One. 2018;13:e0193622. doi: 10.1371/journal.pone.0193622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuwabara M, Borghi C, Cicero AFG, Hisatome I, Niwa K, Ohno M, Johnson RJ, Lanaspa MA. Elevated serum uric acid increases risks for developing high LDL cholesterol and hypertriglyceridemia: A five-year cohort study in Japan. Int J Cardiol. 2018;261:183–188. doi: 10.1016/j.ijcard.2018.03.045. [DOI] [PubMed] [Google Scholar]
- 21.Singh JA, Cleveland JD. Gout and the risk of incident atrial fibrillation in older adults: a study of US Medicare data. RMD Open. 2018;4:e000712. doi: 10.1136/rmdopen-2018-000712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cannon PJ, Stason WB, Demartini FE, Sommers SC, Laragh JH. Hyperuricemia in primary and renal hypertension. N Engl J Med. 1966;275:457–464. doi: 10.1056/NEJM196609012750902. [DOI] [PubMed] [Google Scholar]
- 23.Breckenridge A. Hypertension and hyperuricaemia. Proc R Soc Med. 1966;59:316–319. doi: 10.1177/003591576605900407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim SY, Guevara JP, Kim KM, Choi HK, Heitjan DF, Albert DA. Hyperuricemia and coronary heart disease: a systematic review and meta-analysis. Arthritis Care Res (Hoboken) 2010;62:170–180. doi: 10.1002/acr.20065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Durme C, van Echteld IA, Falzon L, Aletaha D, van der Heijde DM, Landewé RB. Cardiovascular risk factors and comorbidities in patients with hyperuricemia and/or gout: a systematic review of the literature. J Rheumatol Suppl. 2014;92:9–14. doi: 10.3899/jrheum.140457. [DOI] [PubMed] [Google Scholar]
- 26.Koenig W, Khuseyinova N, Baumert J, Meisinger C. Prospective study of high-sensitivity C-reactive protein as a determinant of mortality: results from the MONICA/KORA Augsburg Cohort Study, 1984-1998. Clin Chem. 2008;54:335–342. doi: 10.1373/clinchem.2007.100271. [DOI] [PubMed] [Google Scholar]
- 27.Meisinger C, Koenig W, Baumert J, Döring A. Uric acid levels are associated with all-cause and cardiovascular disease mortality independent of systemic inflammation in men from the general population: the MONICA/KORA cohort study. Arterioscler Thromb Vasc Biol. 2008;28:1186–1192. doi: 10.1161/ATVBAHA.107.160184. [DOI] [PubMed] [Google Scholar]
- 28.Strasak AM, Kelleher CC, Brant LJ, Rapp K, Ruttmann E, Concin H, Diem G, Pfeiffer KP, Ulmer H VHMPP Study Group. Serum uric acid is an independent predictor for all major forms of cardiovascular death in 28,613 elderly women: a prospective 21-year follow-up study. Int J Cardiol. 2008;125:232–239. doi: 10.1016/j.ijcard.2007.11.094. [DOI] [PubMed] [Google Scholar]
- 29.Richette P, Perez-Ruiz F, Doherty M, Jansen TL, Nuki G, Pascual E, Punzi L, So AK, Bardin T. Improving cardiovascular and renal outcomes in gout: what should we target? Nat Rev Rheumatol. 2014;10:654–661. doi: 10.1038/nrrheum.2014.124. [DOI] [PubMed] [Google Scholar]
- 30.Mallinson PI, Coupal T, Reisinger C, Chou H, Munk PL, Nicolaou S, Ouellette H. Artifacts in dual-energy CT gout protocol: a review of 50 suspected cases with an artifact identification guide. AJR Am J Roentgenol. 2014;203:W103–W109. doi: 10.2214/AJR.13.11396. [DOI] [PubMed] [Google Scholar]
- 31.Jo EK, Kim JK, Shin DM, Sasakawa C. Molecular mechanisms regulating NLRP3 inflammasome activation. Cell Mol Immunol. 2016;13:148–159. doi: 10.1038/cmi.2015.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Libby P, Ridker PM, Hansson GK Leducq Transatlantic Network on Atherothrombosis. Inflammation in atherosclerosis: from pathophysiology to practice. J Am Coll Cardiol. 2009;54:2129–2138. doi: 10.1016/j.jacc.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zamudio-Cuevas Y, Martínez-Flores K, Fernández-Torres J, Loissell-Baltazar YA, Medina-Luna D, López-Macay A, Camacho-Galindo J, Hernández-Díaz C, Santamaría-Olmedo MG, López-Villegas EO, Oliviero F, Scanu A, Cerna-Cortés JF, Gutierrez M, Pineda C, López-Reyes A. Monosodium urate crystals induce oxidative stress in human synoviocytes. Arthritis Res Ther. 2016;18:117. doi: 10.1186/s13075-016-1012-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kattoor AJ, Pothineni NVK, Palagiri D, Mehta JL. Oxidative Stress in Atherosclerosis. Curr Atheroscler Rep. 2017;19:42. doi: 10.1007/s11883-017-0678-6. [DOI] [PubMed] [Google Scholar]
- 35.Klauser AS, Halpern EJ, Strobl S, Gruber J, Feuchtner G, Bellmann-Weiler R, Weiss G, Stofferin H, Jaschke W. Dual-Energy Computed Tomography Detection of Cardiovascular Monosodium Urate Deposits in Patients With Gout. JAMA Cardiol. 2019;4:1019–1028. doi: 10.1001/jamacardio.2019.3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kloke JD, McKean JW. Rfit: Rank-based Estimation for Linear Models. The R Journal. 2012;4:57–64. [Google Scholar]
- 37.Hirai A, Kumagai A. [Animal model for gout] Jikken Dobutsu. 1982;31:143–151. [PubMed] [Google Scholar]
- 38.Marcotti A, Miralles A, Dominguez E, Pascual E, Gomis A, Belmonte C, de la Peña E. Joint nociceptor nerve activity and pain in an animal model of acute gout and its modulation by intra-articular hyaluronan. Pain. 2018;159:739–748. doi: 10.1097/j.pain.0000000000001137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pineda C, Fuentes-Gómez AJ, Hernández-Díaz C, Zamudio-Cuevas Y, Fernández-Torres J, López-Macay A, Alba-Sánchez I, Camacho-Galindo J, Ventura L, Gómez-Quiróz LE, Gutiérrez-Ruíz MC, García-Vázquez F, Reginato AM, Gutiérrez M, López-Reyes A. Animal model of acute gout reproduces the inflammatory and ultrasonographic joint changes of human gout. Arthritis Res Ther. 2015;17:37. doi: 10.1186/s13075-015-0550-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Singh N, Parashar P, Tripathi CB, Kanoujia J, Kaithwas G, Saraf SA. Oral delivery of allopurinol niosomes in treatment of gout in animal model. J Liposome Res. 2017;27:130–138. doi: 10.1080/08982104.2016.1174943. [DOI] [PubMed] [Google Scholar]