Abstract
Fibroblast-like synoviocytes (FLS) play a crucial role in initiating rheumatoid arthritis. B-cell activating factor (BAFF) plays a role in FLS survival as well as in B cell maturation and maintenance. Here, we investigated whether tumor necrosis factor (TNF)-α-induced BAFF expression controls FLS migration and whether BAFF expression in FLS could be regulated by KR33426 which is the inhibitor of BAFF binding to BAFF receptors (BAFF-R) by using MH7A synovial cells transfected with the SV40 T antigen. More TNF-α-treated cells migrated compared to the control. TNF-α increased BAFF expression in FLS, significantly. FLS migration was inhibited by the transfection with BAFF-siRNA. KR33426 also inhibited BAFF expression increased by TNF-α treatment in FLS as judged by western blotting, PCR, and transcriptional activity assay. Kinases including JNK, p38 and Erk were activated by TNF-α treatment. While JNK and p38 were inhibited by KR33426 treatment, no changes in Erk were observed. Transcription factors including p65, c-Fos, CREB and SP1 were enhanced by TNF-α treatment. Among them, c-Fos was inhibited by KR33426 treatment. Small interference(si)-RNA of c-fos decreased BAFF transcriptional activity. FLS migration induced by TNF-α was inhibited by the transfection with BAFF-siRNA. KR33426 increased Twist, Snail, Cadherin-11 and N-Cadherin. In contrast, KR33426 decreased E-cadherin and TNF-α-enhanced CCL2. Taken together, our results demonstrate that synovial cell migration via CCL2 expression could be regulated by BAFF expression which is decreased by KR33426 and c-Fos-siRNA. It suggests for the first time that the role of BAFF-siRNA on FLS migration might be matched in the effect of KR33426 on BAFF expression.
Keywords: BAFF, Synovial cell, Migration, CCL2, c-Fos, KR33426
INTRODUCTION
Synovial hyperplasia and destruction of cartilage and bone are characteristics of rheumatoid arthritis (RA) (Firestein, 1996). The synovial membrane is thin in a normal joint and consists of only a few cells. However, many cell types, including immune cells and synoviocytes, occur in a rheumatoid synovial membrane (Lipsky, 2007), leading to inflammation (Firestein, 2003) and the abnormal increase in the number of synoviocytes (Ng et al., 2004). Synovial macrophages and synoviocytes produce abundant pro- and anti-inflammatory cytokines, such as tumor necrosis factor (TNF)-α, interleukin (IL)-1, IL-6, and transforming growth factor-β (Lettesjo et al., 1998; Firestein, 2003). TNF-α regulates other cytokines and destroys joint tissue (Di Giovine et al., 1988; Feldmann and Maini, 2001). Certain cytokines may affect synoviocyte invasion (Xing et al., 2016).
Fibroblast-like synoviocytes (FLSs), which are components of the synovial membrane, play a pivotal role in RA pathogenesis through aggressive migration and matrix invasion, leading to progressive cartilage destruction (Xing et al., 2016). FLSs might be another type of fibroblast but could not become myofibroblast. Fibroblasts provide proinflammatory signals and synthesize extracellular matrix including collagens. Fibroblasts can differentiate into the myofibroblast, a specialized contractile cell type responsible for various roles such as wound closure (Abraham et al., 2007). Unlike fibroblasts, FLS also secrete lubricin, a protein crucial for the joint lubrication (Kiener et al., 2010).
RA-FLS develop cancer cell-like characteristics, such as anchorage-independent growth, loss of contact inhibition, and an invasive phenotype (Huber et al., 2006). It has been reported that more than 1 cadherin could be expressed in the synovium. FLS expressed epithelial-mesenchymal transition (EMT) markers such as E-cadherin, N-cadherin, Snail, Twist and cadherin-11 (Trollmo et al., 1996; Agarwal et al., 2008; You et al., 2014; Lauzier et al., 2016; Zhu et al., 2019). Cadherin-11 expressed on FLS plays a key role in normal synovial architecture by the establishment and maintenance of normal synovium through cell-to-cell adhesion, compaction, and extracellular matrix (ECM) production (Lee et al., 2007; Xing et al., 2016). Cadherin-11 has a role in tissue remodelling, cartilage invasion during an inflammatory reaction (Lee et al., 2007). Co-localization of N-cadherin and cadherin 11 was observed onto the same points of cell-cell contact (Agarwal et al., 2008). E-cadherin expression was found in all synovial tissue (Trollmo et al., 1996). Transcription factors incuding Twist1 or Snail are required for migration and invasion of RA-FLSs stimulated with IL-1β (You et al., 2014; Lauzier et al., 2016). Snail is essential for the formation of ECM-degrading invadosomal structures by synovial cells (Lauzier et al., 2016). In addition, monocyte chemotactic protein 1 (MCP-1)/CCL2 is one of the key factors involved in triggering chemotaxis and transendothelial migration of monocytes and lymphocytes to the site of inflammation (O’Hayre et al., 2008; Liu et al., 2013). Synovial tissue and synovial fluid from RA patients contain increased CCL2 concentration (Iwamoto et al., 2008). CCL2 expressed on RA-FLS is involved in the activation of RA-FLS (Nanki et al., 2001).
TNF-α-stimulated FLS express B-cell activating factor (BAFF), originally known as a B-cell proliferation and survival factor (Mackay and Kalled, 2002; Lee et al., 2017). BAFF is produced by immune cells (monocytes, dendritic cells, and macrophages) (Mackay and Browning, 2002), and also by non-immune cells, such as salivary gland epithelial cells (Ittah et al., 2008), prostate epithelium (Di Carlo et al., 2009), and FLS (Ohata et al., 2005; Lee et al., 2017). Excess levels of BAFF are detected in patients with an autoimmune disease, and particularly in the synovial tissue of patients with RA (Nakajima et al., 2007). Increased B-cell survival in response to BAFF can be detrimental to patients with an autoimmune disorder. An excess B-cell response increases circulating autoantibody levels in patients with certain autoimmune disorders, such as systemic lupus erythematosus, Sjögren’s syndrome, and RA (Wahren-Herlenius and Dorner, 2013; Suurmond and Diamond, 2015). We reported previously that TNF-α-induced BAFF controls RA angiogenesis by regulating vascular endothelial growth factor (VEGF) expression in synoviocytes (Lee et al., 2013). We also reported that chemicals including KR33426 interfere the interaction BAFF and BAFF receptor (BAFF-R) (Moon et al., 2011c) and KR33426 is a novel candidate for anti-autoimmune therapeutics (Lee et al., 2011).
Although BAFF is highly expressed in the joints of patients with RA, the relationship between BAFF and FLS migration is not understood. In this study, we investigated whether TNF-α-induced BAFF expression controls synovial cell migration using MH7A synovial cells and KR33426. We also describe the mechanism of action underlying BAFF expression in TNF-α-stimulated FLS.
MATERIALS AND METHODS
Reagents
Recombinant human TNF-α was purchased from R&D System Inc (Minneapolis, MN, USA). Anti-BAFF antibodies and anti-tubulin antibodies were obtained from Sigma-Aldrich (St. Louis, MO, USA). Antibodies for JNK, phospho-JNK, ERK, phospho-p38, anti-c-Fos, p65, phospho-p65 (serine 536 or serine 276) and SP1 were from Santa Cruz Biotechnology, Inc (Santa Cruz, CA, USA). Antibodies for phospho-p44/42 (ERK1/2) and phospho-CREB (serine 133) were from Cell Signaling Technology (Beverly, MA, USA). Small interference(si) RNA for BAFF or c-Fos was synthesized by Bioneer (Daejeon, Korea). Reporter lysis buffer was obtained from Promega (Madison, WI, USA). KR33426, [2-(2,5-dichlorophenyl)-5-methyloxazol-4yl carbonyl] guanidine (Lee et al., 2009) was kindly provided from Bank of Chemicals, Korea Research Institute of Chemical Technology (KRICT, Daejeon, Korea). Unless indicated, all other materials are obtained from the Sigma chemical company (St. Louis, MO, USA).
Cell cultures
MH7A synovial cells isolated from intra-articular soft tissues of the knee joints of RA patients were obtained from the Riken cell bank (Ibaraki, Japan) through Dr. Ho-Geun Yoon of Yonsei University (Seoul, Korea). Briefly, MH7A is a cell line established by transfection with the SV40 T antigen (Miyazawa et al., 1998). MH7A cells were cultured in RPMI-1640 (Gibco BRL, USA) supplemented with 10% heat-inactivated FBS, penicillin (final concentration, 100 U/mL), streptomycin (P/S, final concentration, 0.1 mg/mL) and L-glutamine at 37°C in an atmosphere of 5% CO2 in air.
Cell migration assay
Cell migration was measured as described previously, with minor modifications (Ryu et al., 2012). Briefly, when MH7A cells reached confluence in a 35-mm culture dish (Corning, NY, USA), three wound lines in the form of a cross were made by scratching the cellular layer with a plastic pipette tip. Floating cells were then washed out, and fresh medium was added. Cells were then incubated under normoxia condition. Narrowing of the wound was then monitored using a phase-contrast microscope beginning 6 h after the scratch. Cells were fixed and stained in a 20% methanol/0.1% crystal violet solution during 1 h at 4°C temperature, followed by washing the cells with water to remove redundant staining. The size of the wound at each time point was then quantified using NIH image analysis software (Image J, version 1.62; National Institutes of Health, MD, USA), and compared with that in the initiation of cell migration.
Transwell cell migration assay
Boyden chamber assay experiments were conducted using a 24-well Transwell system (6.5 mm Transwell (#3422), Corning) with each well separated by a microporous 10 μm thin transparent polycarbonate membrane (8 μm pore size) into an upper(‘insert’) and a lower chamber (‘well’). A volume of 100 uL containing 40,000 cells were plated to each insert and 600 uL medium was added to the wells. Cells in ‘insert’ were allowed to migrate for 24 h. Then, cells were fixed and stained in a 20% methanol/0.1% crystal violet solution during 1 h at 4°C temperature, followed by washing ‘insert’ with water to remove redundant staining. Cells on upper part of ‘insert’ membrane were wiped out. Cells migrated onto lower part of ‘insert’ membrane were photographed, counted and presented as bar graph.
Cloning BAFF gene promoter
Human BAFF (BAFF) promoter (1kb) upstream (AF116456.1) from the starting codon (ATG) was searched from NCBI database (AL157762.13). Primers for various sizes of BAFF promoter were designed from the sequence; forward primer including SacI site (5’-GAG CTC CGA CCT GTT AGG CTG T-3’) and reverse primer including BglII site (5’-GGA GAT CTA TCA CTA CTT GAA CTT TGA AGG-3’). Promoter was cloned into the site between SacI and BglII of pGL3 plasmid that contains firefly luciferase (pGL3-BAFF-Luc). Each product was sequenced and matched to NCBI database.
Measurement of BAFF promoter activity
MH7A cells were transfected with pGL3-BAFF-Luc and pcDNA-lacZ for monitoring transfection efficiency by β-galactosidase assay. Luciferase activity was determined by incubating cell extracts with luciferase substrate (Promega). Luminescence was measured using luminometer (Berthold Technologies, Oak Ridge, TN, USA). Luciferase units of experimental vector were normalized to the control vector in each sample (Lee et al., 2012, 2013; Ryu et al., 2015).
siRNA transfection
siRNA for BAFF and c-Fos was purchased from Bioneer. When cells were 70-80% confluent in 12-well plate, the medium was changed with serum-free RPMI-1640. transfection was performed with lipofectamine® 2000 transfection reagent according to the manufacturer’s protocol (Invitrogen, Carlsbad, CA, USA). Briefly, 20 pmole of each siRNA or 1 μL of lipofectamine 2000 transfection reagent diluted in 50 μL serum-free RPMI-1640 was mixed after the incubation for 5 min. Mixture was incubated for the additional 20 min and carefully added to the cells. Then, cells were incubated for 4 h and the medium was changed again with a complete RPMI-1640 containing 10% FBS, P/S, and L-glutamine. The cells were harvested at 48h for RNA isolation and protein extraction.
Reverse transcriptase polymerase chain reaction (RT-PCR)
Total RNA was extracted from MH7A cells using TRIZOL reagent (Invitrogen). cDNA was synthesized from 1 ug of total RNA using oligo-dT18 primers and reverse transcriptase in a total volume of 21 µL (Bioneer). For standard PCR, 1 µL of the first-strand cDNA product was then used and 10 pmol of specific primers were used as a template for PCR amplification with Taq DNA polymerase (Cosmo Genetech, Seoul, Korea). PCR amplification was performed using primers specific for BAFF (forward; 5’-AAT TCA GAG GAA GAA GGT CC-3’, reverse; 5’-ATG TGA CAT CTC CAT CCA GT-3’) with 36 cycles (95°C for 40 s, 57°C for 30 s and 72°C for 60 s), TNFR1 (forward: 5’-ACC AAG TGC CAC AAA GGA AC-3’, reverse: 5’-CTG CAA TTG AAG CAC TGG AA-3’), TNFR2 (forward: 5’-GGA AAC TCA AGC CTG CAC TC-3’, reverse: 5’-TGC AAA TAT CCG TGG ATG AA-3’) with 30 cycles (95°C for 40 s, 55°C for 30 s and 72°C for 30 s), and GAPDH (forward; 5’-ACA AAC CCG ATA TGG CTG AGA TCG AGA A-3’, reverse; 5’-CTT GCT TCT CCT GTT CAA TC-3’) with 28 cycles (95°C for 30 s, 55°C for 30 s and 75°C for 35 s). PCR products were detected by agarose gel electrophoresis.
Realtime quantitative PCR analysis
To perform realtime quantitative PCR (qPCR), total cellular RNA (5 μg) was reverse transcribed into cDNA as described in RT-PCR. Real-time qPCR was performed using the CFX96 TouchTM Real-Time PCR Detection System (Bio-Rad laboratories, Hercules, CA, USA). The RT reaction product (100 ng) was amplified with ThunderbirdTM SYBR qPCR mix (TOYOBO Co. Ltd., Osaka, Japan) using primers specific for target genes, BAFF primers (forward; 5’-ACA GAA AGG GAG CAG TCA-3’ and reverse; 5’-TGG GAG GAT GGA AAC ACA-3’), Twist primers (forward; 5’-CAA GAT TCA GAC CCT CAA GC-3’ and reverse; 5’- CGC TCG TGA GCC ACA TAG-3’), Snail (forward; 5’-GCT GCA GGA CTC TAA TCC AGA-3’ and reverse; 5’-ATC TCC GGA GGT GGG ATG-3’), E-cadherin (forward; 5’-GCA TTG CCA CAT ACA CTC TC-3’ and reverse; 5’-ATT CGG GCT TGT TGT CAT TC-3’), Cadherin-11 (forward; 5’-CAC AAT CGG CAT CAG GAA-3’ and reverse; 5’-TTG GCT GGT TGG AAA GTG-3’), N-cadherin (forward; 5’-CAT CAT CAT CCT GCT TAT CCT T-3’ and reverse; 5’- TTC TCC TCC ACC TTC TTC AT-3’), CCL2 (forward; 5’- CAG CAA GTG TCC CAA AGA A-3’ and reverse; 5’-GGT TGT GGA GTG AGT GTT C-3’), and β-actin primers (forward; 5’-GCC AGG TCA TCA CCA TTG-3’, reverse; 5’-GTT GAA GGT AGT TTC GTG GAT-3’). Samples were heated to 95°C for 1 min and amplified for 40 cycles (95°C for 10 s, 55°C for 10 s and 72°C for 30 s) followed by a final extension step of 72°C for 10 min. β-actin was used as an internal control. Relative quantification of each mRNA was analyzed by the comparative threshold cycle (CT) method and normalized to β-actin expression using Bio-Rad CFX ManagerTM Software (Bio-Rad laboratories).
Western blot analysis
Western blotting was performed using a standard protocol. Cells were lysed in ice-cold lysis buffer containing 0.5% Nonidet P-40 (vol/vol) in 20 mM Tris-HCl (pH 8.3); 150 mM NaCl; protease inhibitors (2 µg/mL aprotinin, pepstatin, and chymostatin; 1 µg/mL leupeptin and pepstatin; 1 mM phenylmethyl sulfonyl fluoride (PMSF); and 1 mM Na4VO3. Lysates were incubated for 1 h on ice before centrifugation at 13,000 rpm for 10 min at 4oC. Proteins in the supernatant were measured using a protein assay dye reagent (Bio-Rad laboratories) and denatured by boiling for 5 min in sodium dodecyl sulfate(SDS) sample buffer. Proteins were separated by 12% SDS-polyacrylamide gel electrophoresis (SDS-PAGE), and transferred to nitrocellulose membranes by electro-blotting. Following transfer, equal loading of protein was verified by Ponceau staining. The membranes were blocked with 5% skim milk in Tris-buffered saline with Tween 20 (TBST) (10 mM Tris-HCl, pH 7.6; 150 mM NaCl; 0.5% Tween 20) and incubated with the indicated antibodies. Bound antibodies were visualized with HRP-conjugated secondary antibodies with the use of enhanced chemiluminescence (ECL) (Pierce, Rockford, IL, USA). Primary antibodies for each molecule and HRP-labelled secondary anti-IgG antibodies were diluted 1:1,000 and 1:5,000, respectively in TBST containing 0.5% Tween 20. Immunoreactive bands were detected using X-ray film.
Statistical analyses
Experimental differences were examined using ANOVA and Students’ t-tests, as appropriate. The p values of <0.05 were considered to indicate significance
RESULTS
TNF-α treatment increased MH7A cell migration and BAFF expression
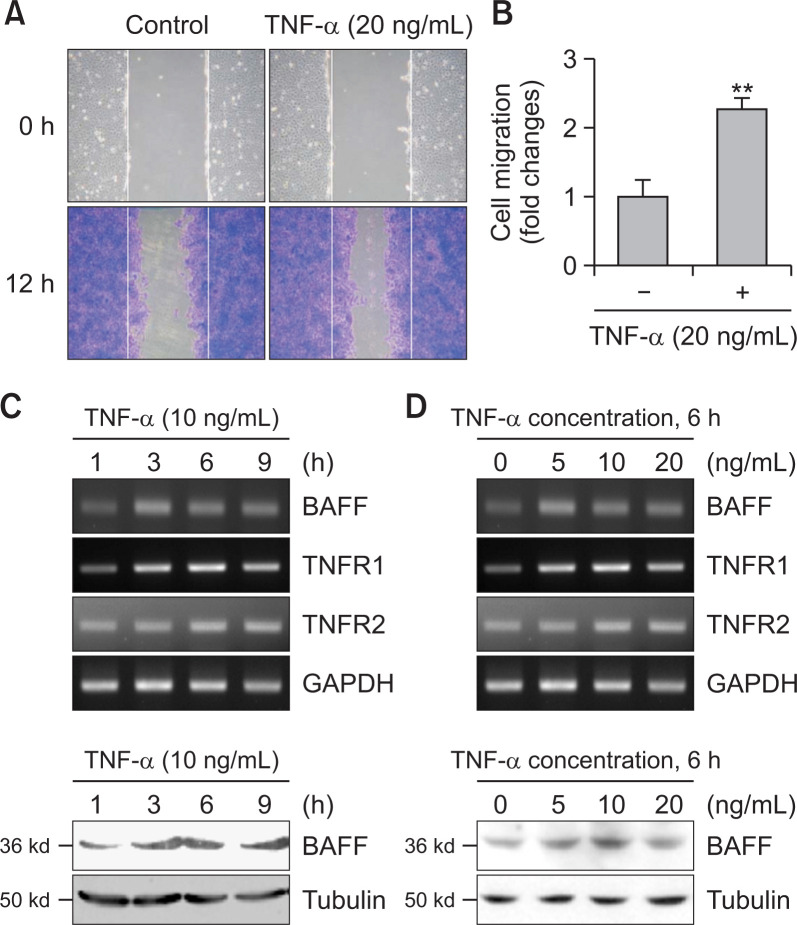
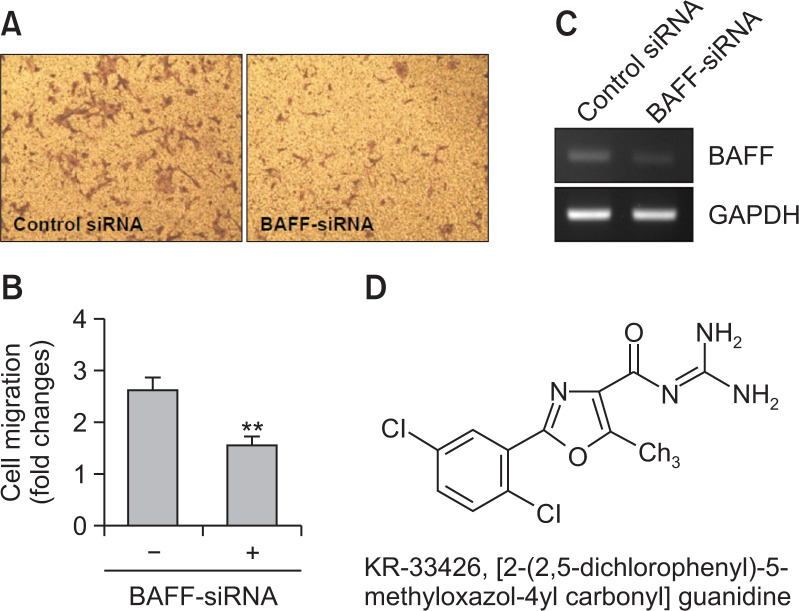
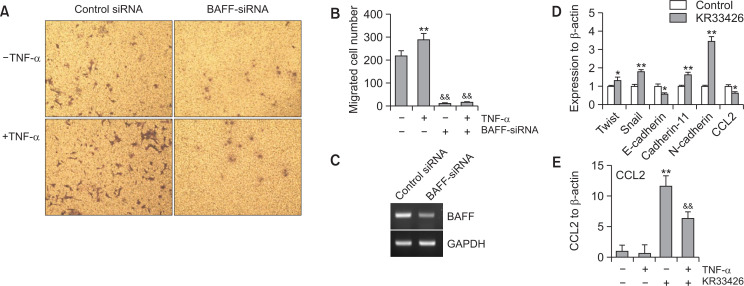
FLS play a role in RA pathogenesis via aggressive migration like cancer cells (Huber et al., 2006). Thus, we examined the migration of synoviocytes using wound healing assay with MH7A cells. MH7A cells were migrated significantly, which is enhanced by the treatment with TNF-α for 24 h (Fig. 1A, 1B). Then, we observed BAFF expression increased by stimulating MH7A synovial cells in response to TNF-α. When cells were treated with TNF-α for various time points, BAFF expression was increased as judged by PCR (Fig. 1C, top) or western blot analysis (Fig. 1C, bottom). When cells were treated with various concentation of TNF-α for 6 h, BAFF expression was increased as judged by RT-PCR (Fig. 1D, top) or western blot analysis (Fig. 1D, bottom). Significant changes were also observed in TNF-α receptor (TNFR) 1 and 2 (Fig. 1C, 1D). To confirm the effect of BAFF on synovial cell migration, MH7A cells were transfected with BAFF-siRNA. Then, cells were plated into insert well of Transwell and incubated for 24 h. As shown in Fig. 2A and 2B, synovial cell migration was inhibited about 40% compared to control. Decrease in BAFF expression was confirmed by PCR (Fig. 2C). As KR33426 interfere BAFF-BAFF-R interaction (Fig. 2D) (Moon et al., 2011c), we will investigate whether KR33426 could regulate TNF-α-treated BAFF expression. From these data, it suggests that BAFF expression could be associated with synovial cell migration.
Fig. 1.
TNF-α increased synovial cell migration and BAFF expression. (A) MH7A cells were plated on 35-mm2 dishes and incubated for 24 h. A confluent monolayer of MH7A cells was then scratched with a sterile pipet tip, and incubated for 24 h. Migration of cells into the space left by the scratch was stained with 0.1% crystal violet solution including 20% methanol and photographed using a phase-contrast microscope at 200× magnification. (B) The empty area remaining at each time point was quantified using NIH image analysis software (version 1.62; National Institutes of Health), and compared to that of the 0-h time point. Fold changes in migration were presented as bar graph. Data are presented as means ± SD. **p<0.01, significant difference as compared to TNF-α-untreated control. (C, D) MH7A cells were treated with 10 ng/mL TNF-α for 1, 3, 6, and 9 h (C). MH7A cells were treated with 5, 10 and 20 ng/mL TNF-α for 6 h (D). RNA was isolated with Trizol. BAFF, TNFR1 and TNFR2 transcripts were measured by RT-PCR (C, top and D, top). Cell lysates were prepared and western blotting was used to detect BAFF (C, bottom and D, bottom). Data were the representative of four experiments (A-D).
Fig. 2.
Synovial cell migration was inhibited by BAFF-siRNA. (A-C) MH7A cells were transfected with BAFF-siRNA. Then, 40,000 cells were plated to each ‘insert’ and allowed to migrate for 24 h. Then, cells were fixed, stained, and washed with water to remove redundant staining. Cells migrated onto lower part of ‘insert’ membrane were photographed (A), counted and presented as bar graph (B) after cells on upper part of ‘insert’ membrane were wiped out. Data were the representative of four experiments (A-C). Data in the bar graph represent the means ± SED. **p<0.01, significant difference as compared to scambled siRNA-treated control. RNA was isolated with Trizol. BAFF transcripts were measured by RT-PCR (C). (D) Chemical structure of KR-33426, [2-(2,5-dichlorophenyl)-5-methyloxazol-4yl carbonyl] guanidine.
TNF-α-treated BAFF expression is inhibited by the treatment with KR33426
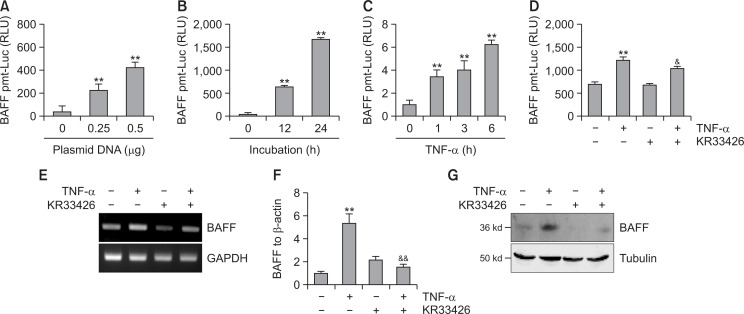
We examined the effect of KR33426 on TNF-α-treated BAFF expression. When MH7A cells were transfected with 0.25 or 0.5 µg pGL3-BAFF-Luc plasmids, transcriptional activity of pGL3-BAFF-Luc was increased, concentration-dependently (Fig. 3A) and time-dependently (Fig. 3B). TNF-α treatment for 1, 3, and 6 h increased BAFF transcriptional activity 3.5, 4.0 and 6.0 times, respectively (Fig. 3C). Transcriptional activity of pGL3-BAFF-Luc was increased about twice by the treatment of TNF-α but it was decreased about 20% by KR33426 treatment (Fig. 3D). BAFF level was increased by the treatment of TNF-α but it was decreased by KR33426 treatment as judged by RT-PCR (Fig. 3E), real-time qPCR (Fig. 3F) and western blot analysis (Fig. 3G).
Fig. 3.
BAFF expression was inhibited by the treatment with KR33426. (A-D) MH7A cells were transfected with 0.25 µg (A) or 0.5 µg (A-D) pGL3-BAFF-Luc DNA. Luciferase activity of different DNA concentration of BAFF promoter (A) or each time point (B) was measured by using luminometer. Cells were incubated by the stimulation with TNF-α for various time points and luciferase activity was measured (C). Cells were stimulated with TNF-α in the presence or absence of KR33426 and luciferase activity was measured (D). (E-G) MH7A cells were stimulated with TNF-α in the presence or absence of KR33426 for 6 h. RNA was isolated with Trizol. BAFF transcripts were measured by RT-PCR (E) and realtime qPCR normalized to β-actin expression (F). Cell lysates were prepared and western blotting was used to detect BAFF (G). Data were the representative of four experiments (A-G). Data in the bar graph represent the means ± SED. **p<0.01, significant difference as compared to TNF-α-untreated control. &p<0.05, &&p<0.01, significant difference from TNF-α-treated and KR33426-untreated control (D, F).
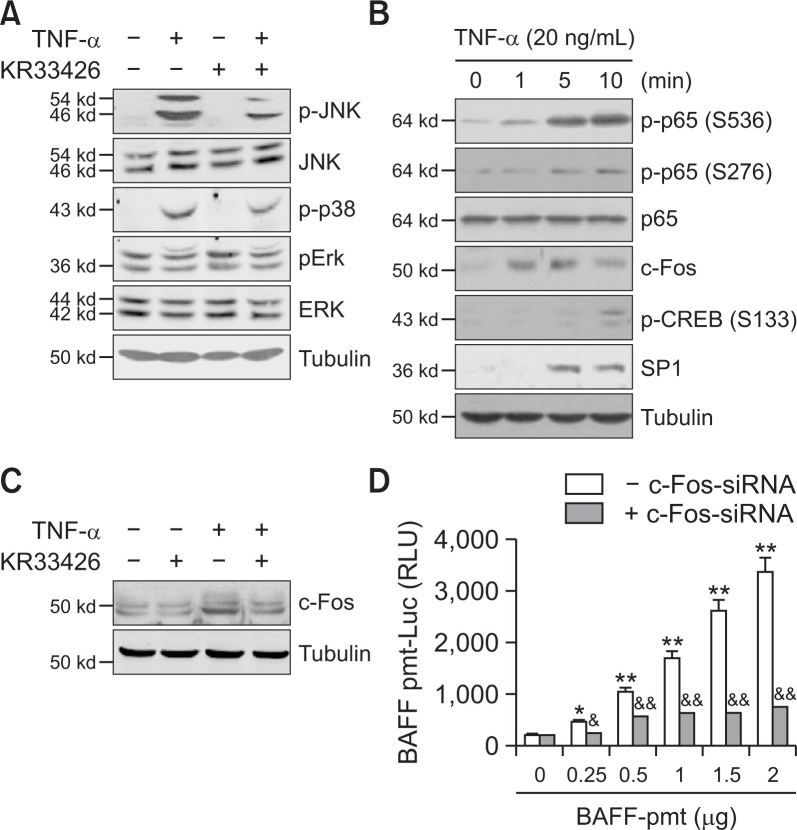
To determine whether kinase and transcription factor control BAFF expression, we assessed various signaling molecules. When MH7A cells were treated with TNF-α in the absence or presence of KR33426, phosphorylation of JNK and p38 was increased significantly in TNF-α-treated cells, which was significantly reduced by KR33426 (Fig. 4A). When MH7A cells were treated with TNF-α for 1, 5 and 10 min, changes in various transcription factors are observed. Among them, the change in c-Fos level was the most rapid at 1 min compared to others (Fig. 4B). c-Fos increased by TNF-α treatment was inhibited by KR33426 treatment (Fig. 4C). In addition, transcriptional activity of pGL3-BAFF-Luc was significantly decreased by the transfection of c-Fos-siRNA (Fig. 4D). Data suggest that KR33426 inhibited BAFF expression via the activation of JNK or p38 and c-Fos.
Fig. 4.
KR33426 reduced c-Fos leading to a decrease in BAFF expression. (A) MH7A cells were treated with TNF-α in the presence or absence of KR33426. Cell lysates were prepared and western blotting was used to detect JNK, phospho-JNK, phospho-p38, ERK and phospho-ERK. (B) MH7A cells were treated with TNF-α. Cell lysates were prepared and western blotting was used to detect p65, phospho-p65 (serine 536 or 276), c-Fos, phospho-CREB (serine 133) and SP1. (C) MH7A cells were treated with TNF-α in the presence or absence of KR33426. Cell lysates were prepared and western blotting was used to detect c-Fos. (D) MH7A cells were co-transfected with pGL3-BAFF-Luc plasmids and c-Fos-siRNA. Luciferase activity of BAFF promoter was measured by using luminometer. Data were the representative of four experiments (A-D). Data in the bar graph represent the means ± SED. *p<0.05; **p<0.01, significant difference as compared to pGL3-BAFF-Luc plasmids-untransfected control. &p<0.05; &&p<0.01, significant difference from scambled siRNA-treated control at each DNA concentration of pGL3-BAFF-Luc plasmids.
BAFF-siRNA and KR33426 inhibited MH7A cell migration increased by TNF-α treatment
To confirm whether BAFF control synovial cell migration induced by TNF-α treatment, MH7A cells were transfected with BAFF-siRNA. Cells were plated into insert well of Transwell and treated with TNF-α. Then, cells were incubated for 12 h and stained with crystal violet solution. As shown in Fig. 5A and 5B, synovial cell migration was increased about 30% compared to control, which was inhibited about 90% by BAFF-siRNA. Decrease in BAFF expression was confirmed by PCR (Fig. 5C). Data suggest that BAFF expression may be associated with synovial cell migration by TNF-α. We also examined whether synovial cell migration is associated with EMT markers and CCL2 expressed on RA-FLS (Trollmo et al., 1996; Nanki et al., 2001; Agarwal et al., 2008; You et al., 2014; Lauzier et al., 2016; Zhu et al., 2019). As shown in Fig. 5D, KR33426 enhanced the expression of Twist, Snail, cadherin-11 and N-cadherin about 1.2, 1.8, 1.6 and 3.3 times, respectively. In contrast, KR33426 reduced the expression of E-cadherin and CCL2 about 35 and 40%, respectively. Among those factors, CCL2 expression was increased about 12 times by the treatment of TNFα but it was decreased about 40% by KR33426 treatment (Fig. 5E). Data suggest that BAFF may directly or indirectly regulate synovial cell migration through binding to BAFF-R and CCL2 expression.
Fig. 5.
TNF-α–induced synovial cell migration was associated with BAFF expression. (A-C) MH7A cells were transfected with BAFF-siRNA. 40,000 cells were plated to each ‘insert’ and treated with TNF-α. Cells were allowed to migrate for 24 h. Then, cells were fixed, stained, and washed with water to remove redundant staining. Cells migrated onto lower part of ‘insert’ membrane were photographed (A), counted and presented as bar graph after cells on upper part of ‘insert’ membrane were wiped out (B). RNA was isolated with Trizol. BAFF transcripts were measured by RT-PCR (C). (D) MH7A cells were treated with KR33426. RNA was isolated with Trizol. Transcripts for Twist, Snail, E-cadherin, cadherin-11, N-cadherin and CCL2 were measured by real-time qPCR normalized to β-actin expression. (E) MH7A cells were treated with TNF-α in the presence or absence of KR33426. RNA was isolated with Trizol. CCL2 transcripts were measured by real-time qPCR normalized to β-actin expression. Data were the representative of four experiments (A-E). Data in the bar graph represent the means ± SED. *p<0.05; **p<0.01, significant difference as compared to TNF-α-untreated control (B, D, E) for each molecule (D). &&p<0.05, significant difference from TNF-α-treated and BAFF-siRNA-untreated control (B) or TNF-α-treated and KR33426-untreated control (E).
DISCUSSION
Synovial hyperplasia in RA is characterized by abnormal increase in the number of synoviocytes which are components of the synovial membrane (Firestein, 1996; Ng et al., 2004; Xing et al., 2016). Those synoviocytes, like immune cells, synovial macrophages, produce lots of various pro- and anti-inflammatory cytokines (Lettesjo et al., 1998; Firestein, 2003), leading to synoviocyte invasion (Xing et al., 2016) and joint tissue destruction (Di Giovine et al., 1988; Feldmann and Maini, 2001). RA-FLS is invasive and aggressively migrate into matrix, leading to progressive cartilage destruction (Huber et al., 2006; Xing et al., 2016). EMT markers such as E-cadherin, N-cadherin, Snail, Twist and cadherin-11 are required for FLS migration and invasion (Trollmo et al., 1996; Lee et al., 2007; Agarwal et al., 2008; You et al., 2014; Lauzier et al., 2016; Xing et al., 2016; Zhu et al., 2019). In addition, RA-FLS characteristics could be up-regulated by CCL2 increased in synovial tissue and synovial fluid from RA patients (Nanki et al., 2001; Iwamoto et al., 2008). Excess levels of BAFF are detected in patients with an autoimmune disease, and particularly in the synovial tissue of patients with RA (Nakajima et al., 2007). TNF-α-induced BAFF controls RA angiogenesis by the regulation of VEGF expression in synovial cells (Lee et al., 2013). It has been reported that KR33426 attenuate autoimmune disease symptom (Lee et al., 2011) by interfering the interaction between BAFF and BAFF-R (Moon et al., 2011c). Here, we investigated whether synovial cell migration could be regulated by TNF-α-induced BAFF expression using MH7A cells and KR33426.
BAFF was originally known as a B-cell survival factor for cell proliferation and their maintenance (Mackay and Kalled, 2002). BAFF is not only produced from myeloid cells (Mackay and Browning, 2002; Mackay et al., 2003), but also from various types of non-lymphoid cells (Tan et al., 2003; Ohata et al., 2005; Ittah et al., 2006; Kato et al., 2006; Lee et al., 2013; Woo et al., 2013). However, little is known about the role of BAFF on non-lymphoid synovial cell migration. We observed a significant increase in TNF-α-treated MH7A cell migration compared to control (Fig. 1A, 1B). BAFF expression was also increased by the treatment with TNF-α (Fig. 1C, 1D). As MH7A synovial cell migration was reduced by BAFF-siRNA (Fig. 2A, 2B), we used KR33426 to confirm the role of TNF-α on BAFF expression. Data showed that KR33426 treatment inhibited BAFF expression through JNK and c-Fos (Fig. 3, 4). It suggests that synovial cell migration could be associated with BAFF expression which is decreased by KR33426 and c-Fos-siRNA. In addition, TNF-α–mediated MH7A synovial cell migration was decreased by BAFF-siRNA (Fig. 5A, 5B). Then, CCL2 migration factor increased in TNF-α–treated MH7A cells is inhibited by KR33426 treatment (Fig. 5D). Data demonstrate that TNF-α–mediated synovial cell migration via CCL2 expression might be associated with BAFF expression followed by binding BAFF to BAFF-R receptor.
BAFF transmitted signals through binding to specific BAFF-R in B cells, leading to the inhibition of apoptosis. KR33426 interfere BAFF-BAFF-R interaction and it may inhibit BAFF-mediated anti-apoptosis (Moon et al., 2011c). KR33426 is a novel candidate for anti-autoimmune therapeutics (Lee et al., 2011). Our data showed that KR33426 could be effective on synovial cell responses such as cell migration via BAFF expression. So, it suggests that the effectiveness of KR33426 could be mediated by the regulation of BAFF expression and its role in both immune B cells and non-immune synovial cells in RA.
BAFF expression is regulated by various signaling pathways, including the activation of JAK/STAT (Woo et al., 2013), CREB-binding protein/p300 (Moon and Park, 2007), lipopolysaccharide (LPS)-induced protein kinase A-mediated CREB, Epac1-mediated Rap1 (Moon et al., 2011a, 2011b), nuclear factor-kappa B by LPS-induced production of reactive oxygen species (ROS) (Moon et al., 2006), and ROS-dependent protein kinase C (PKC)/c-Fos pathways. ERK pathways are also involved in TNF-α-induced BAFF expression in FLS (Lee et al., 2017). Our data show that BAFF expression by TNF-α could be associated with the activation of kinase (JNK, p38 and ERK) and transcription factors (p65, CREB, c-Fos and SP1) in FLS. JNK, p38 and c-Fos were inhibited by KR33426. BAFF expression was attenuated by the inhibition of c-Fos with c-Fos-siRNA, effectively. It is required to study further whether what signaling molecules are involved in the regulation of c-Fos in synovial cells.
FLS migration and invasion are critical for RA pathogenesis (Li et al., 2013). EMT is the process whereby cells undergo a switch from an epithelial phenotype with lack of mobility into mesenchymal cells that are non-polarized, motile and produce an extracellular matrix (Zvaifler, 2006). FLS expressed various EMT markers and cadherin-11 (Trollmo et al., 1996; Agarwal et al., 2008; You et al., 2014; Lauzier et al., 2016; Zhu et al., 2019). E-cadherin decreased, while N-cadherin increased in RA-FLS migration (Zhu et al., 2019). Transcription factors, Twist1 and Snail are required for FLS migration and invasion (You et al., 2014; Lauzier et al., 2016). Then, it looks like that FLS migration is very similar to EMT of epithelial cells. However, EMT in FLS has different meaning in EMT of epithelial cells. Our data showed that the changes in EMT markers by KR33426 are opposite to those reported previously. So, it seems to be that EMT was increased by KR33426 treatment, due to that Twist, Snail, cadherin-11, and N-cadherin were increased but E-cadherin was decreased in KR33426-treated cells. So, these indicated that the effect of KR33426 could be independent of EMT markers. Cadherin-11 also plays a role in tissue remodelling, cartilage invasion, and influences FLS during an inflammatory reaction (Lee et al., 2007). IL-17A might contribute to the migration and invasion of RA-FLSs by the upregulation of MMP2 and MMP9 via NF-kB activation (Li et al., 2013). It suggests that TNF-α-induced BAFF expression could promote synovial cell migration through other molecules except EMT markers.
Synovial tissue and fluid from RA patients showed an increase in concentrations of monocyte chemoattractant protein (MCP)-1/CCL2, macrophage inflammatory protein (MIP)-1α/CCL3, MCP-4/CCL13, pulmonary and activation-regulated chemokine (PARC)/CCL18, CXCL9, stromal cell-derived factor 1 (SDF-1)/CXCL12, and Fractalkine/CXC3CL1 (Iwamoto et al., 2008). CCL2, CCL5, and CXCL12 were expressed on RA-FLS. These chemokines not only play a role in inflammatory cell migration, but are also involved in the activation of RA-FLS (Nanki et al., 2001; O’Hayre et al., 2008). Among them, CCL2 has been contributed to the pathophysiology of several inflammatory conditions including arthritis (Liu et al., 2013). It is consistent with our data that CCL2 expression was reduced by KR33426, leading to the inhibition of synovial cell migration. So, it suggests that TNF-α-induced synovial cell migration could be associated with CCL2 expression. It has to be clarified the mechanism of action on CCL2 expression in TNF-α-induced FLS.
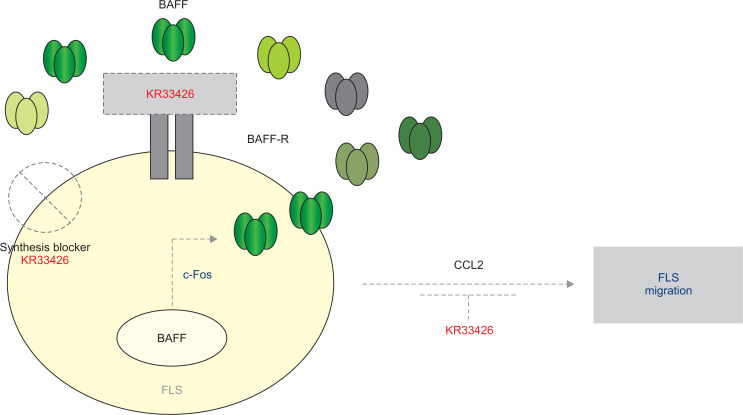
Even though all possible signaling molecules have not been cleared for TNF-α-induced BAFF expression and cell migration, data demonstrate that BAFF expression in synovial cells could be decreased by KR33426 and c-Fos-siRNA. It proposes that synovial cell migration might be mediated by CCL2 and also by BAFF expression through c-Fos activation followed by binding BAFF to BAFF-R receptor. It also suggests that the role of BAFF-siRNA on FLS migration might be matched in the effect of KR33426 on BAFF expression, leading to therapeutic meaning of BAFF control in rheumatic FLS (Fig. 6).
Fig. 6.
Scheme about action of KR33426 on FLS migration by BAFF expression and by binding BAFF to BAFF-R receptor. BAFF expression in FLS cells is regulated by KR33426 via c-Fos activation. FLS cell migration might be controlled by binding BAFF to BAFF-R receptor via the expression of CCL2 and cadherin-11. Our findings are presented by grey dotted lines and diagrams.
ACKNOWLEDGMENTS
This work was supported by grants from the Mid-career Researcher Program (# 2016-R1A2B400746 and # 2018R1A2A3075602) through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (MEST), Korea.
Footnotes
CONFLICT OF INTEREST
None.
References
- Abraham D. J., Eckes B., Rajkumar V., Krieg T. New developments in fibroblast and myofibroblast biology: implications for fibrosis and scleroderma. Curr. Rheumatol. Rep. 2007;9:136–143. doi: 10.1007/s11926-007-0008-z. [DOI] [PubMed] [Google Scholar]
- Agarwal S. K., Lee D. M., Kiener H. P., Brenner M. B. Coexpression of two mesenchymal cadherins, cadherin 11 and N-cadherin, on murine fibroblast-like synoviocytes. Arthritis Rheum. 2008;58:1044–1054. doi: 10.1002/art.23369. [DOI] [PubMed] [Google Scholar]
- Di Carlo E., D'Antuono T., Pompa P., Giuliani R., Rosini S., Stuppia L., Musiani P., Sorrentino C. The lack of epithelial interleukin-7 and BAFF/BLyS gene expression in prostate cancer as a possible mechanism of tumor escape from immunosurveillance. Clin. Cancer Res. 2009;15:2979–2987. doi: 10.1158/1078-0432.CCR-08-1951. [DOI] [PubMed] [Google Scholar]
- Di Giovine F. S., Nuki G., Duff G. W. Tumour necrosis factor in synovial exudates. Ann. Rheum. Dis. 1988;47:768–772. doi: 10.1136/ard.47.9.768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldmann M., Maini R. N. Anti-TNF alpha therapy of rheumatoid arthritis: what have we learned? Annu. Rev. Immunol. 2001;19:163–196. doi: 10.1146/annurev.immunol.19.1.163. [DOI] [PubMed] [Google Scholar]
- Firestein G. S. Invasive fibroblast-like synoviocytes in rheumatoid arthritis. Passive responders or transformed aggressors? Arthritis Rheum. 1996;39:1781–1790. doi: 10.1002/art.1780391103. [DOI] [PubMed] [Google Scholar]
- Firestein G. S. Evolving concepts of rheumatoid arthritis. Nature. 2003;423:356–361. doi: 10.1038/nature01661. [DOI] [PubMed] [Google Scholar]
- Huber L. C., Distler O., Tarner I., Gay R. E., Gay S., Pap T. Synovial fibroblasts: key players in rheumatoid arthritis. Rheumatology (Oxford) 2006;45:669–675. doi: 10.1093/rheumatology/kel065. [DOI] [PubMed] [Google Scholar]
- Ittah M., Miceli-Richard C., Eric Gottenberg J., Lavie F., Lazure T., Ba N., Sellam J., Lepajolec C., Mariette X. B cell-activating factor of the tumor necrosis factor family (BAFF) is expressed under stimulation by interferon in salivary gland epithelial cells in primary Sjogren's syndrome. Arthritis Res. Ther. 2006;8:R51. doi: 10.1186/ar1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ittah M., Miceli-Richard C., Gottenberg J. E., Sellam J., Eid P., Lebon P., Pallier C., Lepajolec C., Mariette X. Viruses induce high expression of BAFF by salivary gland epithelial cells through TLR- and type-I IFN-dependent and -independent pathways. Eur. J. Immunol. 2008;38:1058–1064. doi: 10.1002/eji.200738013. [DOI] [PubMed] [Google Scholar]
- Iwamoto T., Okamoto H., Toyama Y., Momohara S. Molecular aspects of rheumatoid arthritis: chemokines in the joints of patients. FEBS J. 2008;275:4448–4455. doi: 10.1111/j.1742-4658.2008.06580.x. [DOI] [PubMed] [Google Scholar]
- Kato A., Truong-Tran A. Q., Scott A. L., Matsumoto K., Schleimer R. P. Airway epithelial cells produce B cell-activating factor of TNF family by an IFN-beta-dependent mechanism. J. Immunol. 2006;177:7164–7172. doi: 10.4049/jimmunol.177.10.7164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiener H. P., Watts G. F., Cui Y., Wright J., Thornhill T. S., Skold M., Behar S. M., Niederreiter B., Lu J., Cernadas M., Coyle A. J., Sims G. P., Smolen J., Warman M. L., Brenner M. B., Lee D. M. Synovial fibroblasts self-direct multicellular lining architecture and synthetic function in three-dimensional organ culture. Arthritis Rheum. 2010;62:742–752. doi: 10.1002/art.27285. [DOI] [PubMed] [Google Scholar]
- Lauzier A., Lavoie R. R., Charbonneau M., Gouin-Boisvert B., Harper K., Dubois C. M. Snail is a critical mediator of invadosome formation and joint degradation in arthritis. Am. J. Pathol. 2016;186:359–374. doi: 10.1016/j.ajpath.2015.10.021. [DOI] [PubMed] [Google Scholar]
- Lee D. M., Kiener H. P., Agarwal S. K., Noss E. H., Watts G. F., Chisaka O., Takeichi M., Brenner M. B. Cadherin-11 in synovial lining formation and pathology in arthritis. Science. 2007;315:1006–1010. doi: 10.1126/science.1137306. [DOI] [PubMed] [Google Scholar]
- Lee G. H., Lee J. W., Lee J. W., Choi W. S., Moon E. Y. B cell activating factor-dependent expression of vascular endothelial growth factor in MH7A human synoviocytes stimulated with tumor necrosis factor-alpha. Int. Immunopharmacol. 2013;17:142–147. doi: 10.1016/j.intimp.2013.04.026. [DOI] [PubMed] [Google Scholar]
- Lee G. H., Lee M. H., Yoon Y. D., Kang J. S., Pyo S., Moon E. Y. Protein kinase C stimulates human B cell activating factor gene expression through reactive oxygen species-dependent c-Fos in THP-1 pro-monocytic cells. Cytokine. 2012;59:115–123. doi: 10.1016/j.cyto.2012.03.017. [DOI] [PubMed] [Google Scholar]
- Lee G. H., Oh J. M., Kim H. S., Yoon W. K., Yi K. Y., Yang Y., Han S. H., Lee S., Moon E. Y. KR33426, [2-(2,5-dichlorophenyl)-5-methyloxazol-4yl]carbonylguanidine, is a novel compound to be effective on mouse systemic lupus erythematosus. Eur. J. Pharmacol. 2011;668:459–466. doi: 10.1016/j.ejphar.2011.07.026. [DOI] [PubMed] [Google Scholar]
- Lee J. W., Lee J., Um S. H., Moon E. Y. Synovial cell death is regulated by TNF-alpha-induced expression of B-cell activating factor through an ERK-dependent increase in hypoxia-inducible factor-1alpha. Cell Death Dis. 2017;8:e2727. doi: 10.1038/cddis.2017.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S., Yi K. Y., Youn S. J., Lee B. H., Yoo S. E. (2-Aryl-5-methylimidazol-4-ylcarbonyl)guanidines and (2-aryl-5-methyloxazol-4-ylcarbonyl)guanidines as NHE-1 inhibitors. Bioorg. Med. Chem. Lett. 2009;19:1329–1331. doi: 10.1016/j.bmcl.2009.01.060. [DOI] [PubMed] [Google Scholar]
- Lettesjo H., Nordstrom E., Strom H., Nilsson B., Glinghammar B., Dahlstedt L., Moller E. Synovial fluid cytokines in patients with rheumatoid arthritis or other arthritic lesions. Scand. J. Immunol. 1998;48:286–292. doi: 10.1046/j.1365-3083.1998.00399.x. [DOI] [PubMed] [Google Scholar]
- Li G., Zhang Y., Qian Y., Zhang H., Guo S., Sunagawa M., Hisamitsu T., Liu Y. Interleukin-17A promotes rheumatoid arthritis synoviocytes migration and invasion under hypoxia by increasing MMP2 and MMP9 expression through NF-kappaB/HIF-1alpha pathway. Mol. Immunol. 2013;53:227–236. doi: 10.1016/j.molimm.2012.08.018. [DOI] [PubMed] [Google Scholar]
- Lipsky P. E. Why does rheumatoid arthritis involve the joints? N. Engl. J. Med. 2007;356:2419–2420. doi: 10.1056/NEJMcibr070846. [DOI] [PubMed] [Google Scholar]
- Liu S. C., Hsu C. J., Fong Y. C., Chuang S. M., Tang C. H. CTGF induces monocyte chemoattractant protein-1 expression to enhance monocyte migration in human synovial fibroblasts. Biochim. Biophys. Acta. 2013;1833:1114–1124. doi: 10.1016/j.bbamcr.2012.12.014. [DOI] [PubMed] [Google Scholar]
- Mackay F., Browning J. L. BAFF: a fundamental survival factor for B cells. Nat. Rev. Immunol. 2002;2:465–475. doi: 10.1038/nri844. [DOI] [PubMed] [Google Scholar]
- Mackay F., Kalled S. L. TNF ligands and receptors in autoimmunity: an update. Curr. Opin. Immunol. 2002;14:783–790. doi: 10.1016/S0952-7915(02)00407-7. [DOI] [PubMed] [Google Scholar]
- Mackay F., Schneider P., Rennert P., Browning J. BAFF AND APRIL: a tutorial on B cell survival. Annu. Rev. Immunol. 2003;21:231–264. doi: 10.1146/annurev.immunol.21.120601.141152. [DOI] [PubMed] [Google Scholar]
- Miyazawa K., Mori A., Okudaira H. Establishment and characterization of a novel human rheumatoid fibroblast-like synoviocyte line, MH7A, immortalized with SV40 T antigen. J. Biochem. 1998;124:1153–1162. doi: 10.1093/oxfordjournals.jbchem.a022233. [DOI] [PubMed] [Google Scholar]
- Moon E. Y., Lee J. H., Lee J. W., Song J. H., Pyo S. ROS/Epac1-mediated Rap1/NF-kappaB activation is required for the expression of BAFF in Raw264.7 murine macrophages. Cell. Signal. 2011a;23:1479–1488. doi: 10.1016/j.cellsig.2011.05.001. [DOI] [PubMed] [Google Scholar]
- Moon E. Y., Lee J. H., Oh S. Y., Ryu S. K., Kim H. M., Kwak H. S., Yoon W. K. Reactive oxygen species augment B-cell-activating factor expression. Free Radic. Biol. Med. 2006;40:2103–2111. doi: 10.1016/j.freeradbiomed.2006.02.007. [DOI] [PubMed] [Google Scholar]
- Moon E. Y., Lee Y. S., Choi W. S., Lee M. H. Toll-like receptor 4-mediated cAMP production up-regulates B-cell activating factor expression in Raw264.7 macrophages. Exp. Cell Res. 2011b;317:2447–2455. doi: 10.1016/j.yexcr.2011.07.003. [DOI] [PubMed] [Google Scholar]
- Moon E. Y., Park H. B cell activating factor (BAFF) gene promoter activity depends upon co-activator, p300. Immunobiology. 2007;212:637–645. doi: 10.1016/j.imbio.2007.06.002. [DOI] [PubMed] [Google Scholar]
- Moon E. Y., Yi K. Y., Lee S. An increase in B cell apoptosis by interfering BAFF-BAFF-R interaction with small synthetic molecules. Int. Immunopharmacol. 2011c;11:1523–1533. doi: 10.1016/j.intimp.2011.05.009. [DOI] [PubMed] [Google Scholar]
- Nakajima K., Itoh K., Nagatani K., Okawa-Takatsuji M., Fujii T., Kuroki H., Katsuragawa Y., Aotsuka S., Mimori A. Expression of BAFF and BAFF-R in the synovial tissue of patients with rheumatoid arthritis. Scand. J. Rheumatol. 2007;36:365–372. doi: 10.1080/03009740701286615. [DOI] [PubMed] [Google Scholar]
- Nanki T., Nagasaka K., Hayashida K., Saita Y., Miyasaka N. Chemokines regulate IL-6 and IL-8 production by fibroblast-like synoviocytes from patients with rheumatoid arthritis. J. Immunol. 2001;167:5381–5385. doi: 10.4049/jimmunol.167.9.5381. [DOI] [PubMed] [Google Scholar]
- Ng L. G., Sutherland A. P., Newton R., Qian F., Cachero T. G., Scott M. L., Thompson J. S., Wheway J., Chtanova T., Groom J., Sutton I. J., Xin C., Tangye S. G., Kalled S. L., Mackay F., Mackay C. R. B cell-activating factor belonging to the TNF family (BAFF)-R is the principal BAFF receptor facilitating BAFF costimulation of circulating T and B cells. J. Immunol. 2004;173:807–817. doi: 10.4049/jimmunol.173.2.807. [DOI] [PubMed] [Google Scholar]
- O'Hayre M., Salanga C. L., Handel T. M., Allen S. J. Chemokines and cancer: migration, intracellular signalling and intercellular communication in the microenvironment. Biochem. J. 2008;409:635–649. doi: 10.1042/BJ20071493. [DOI] [PubMed] [Google Scholar]
- Ohata J., Zvaifler N. J., Nishio M., Boyle D. L., Kalled S. L., Carson D. A., Kipps T. J. Fibroblast-like synoviocytes of mesenchymal origin express functional B cell-activating factor of the TNF family in response to proinflammatory cytokines. J. Immunol. 2005;174:864–870. doi: 10.4049/jimmunol.174.2.864. [DOI] [PubMed] [Google Scholar]
- Ryu Y. K., Lee J. W., Moon E. Y. Thymosin beta-4, actin-sequestering protein regulates vascular endothelial growth factor expression via hypoxia-inducible nitric oxide production in hela cervical cancer cells. Biomol. Ther. (Seoul) 2015;23:19–25. doi: 10.4062/biomolther.2014.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu Y. K., Lee Y. S., Lee G. H., Song K. S., Kim Y. S., Moon E. Y. Regulation of glycogen synthase kinase-3 by thymosin beta-4 is associated with gastric cancer cell migration. Int. J. Cancer. 2012;131:2067–2077. doi: 10.1002/ijc.27490. [DOI] [PubMed] [Google Scholar]
- Suurmond J., Diamond B. Autoantibodies in systemic autoimmune diseases: specificity and pathogenicity. J. Clin. Invest. 2015;125:2194–2202. doi: 10.1172/JCI78084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan S. M., Xu D., Roschke V., Perry J. W., Arkfeld D. G., Ehresmann G. R., Migone T. S., Hilbert D. M., Stohl W. Local production of B lymphocyte stimulator protein and APRIL in arthritic joints of patients with inflammatory arthritis. Arthritis Rheum. 2003;48:982–992. doi: 10.1002/art.10860. [DOI] [PubMed] [Google Scholar]
- Trollmo C., Nilsson I. M., Sollerman C., Tarkowski A. Expression of the mucosal lymphocyte integrin alpha E beta 7 and its ligand E-cadherin in the synovium of patients with rheumatoid arthritis. Scand. J. Immunol. 1996;44:293–298. [PubMed] [Google Scholar]
- Wahren-Herlenius M., Dorner T. Immunopathogenic mechanisms of systemic autoimmune disease. Lancet. 2013;382:819–831. doi: 10.1016/S0140-6736(13)60954-X. [DOI] [PubMed] [Google Scholar]
- Woo S. J., Im J., Jeon J. H., Kang S. S., Lee M. H., Yun C. H., Moon E. Y., Song M. K., Kim H. H., Han S. H. Induction of BAFF expression by IFN-gamma via JAK/STAT signaling pathways in human intestinal epithelial cells. J. Leukoc. Biol. 2013;93:363–368. doi: 10.1189/jlb.0412210. [DOI] [PubMed] [Google Scholar]
- Xing R., Jin Y., Sun L., Yang L., Li C., Li Z., Liu X., Zhao J. Interleukin-21 induces migration and invasion of fibroblast-like synoviocytes from patients with rheumatoid arthritis. Clin. Exp. Immunol. 2016;184:147–158. doi: 10.1111/cei.12751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You S., Yoo S. A., Choi S., Kim J. Y., Park S. J., Ji J. D., Kim T. H., Kim K. J., Cho C. S., Hwang D., Kim W. U. Identification of key regulators for the migration and invasion of rheumatoid synoviocytes through a systems approach. Proc. Natl. Acad. Sci. U.S.A. 2014;111:550–555. doi: 10.1073/pnas.1311239111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu D., Zhao J., Lou A., Huang Q., OuYang Q., Zhu J., Fan M., He Y., Ren H., Yang M. Transforming growth factor beta1 promotes fibroblast-like synoviocytes migration and invasion via TGF-beta1/Smad signaling in rheumatoid arthritis. Mol. Cell. Biochem. 2019;459:141–150. doi: 10.1007/s11010-019-03557-0. [DOI] [PubMed] [Google Scholar]
- Zvaifler N. J. Relevance of the stroma and epithelial-mesenchymal transition (EMT) for the rheumatic diseases. Arthritis Res. Ther. 2006;8:210. doi: 10.1186/ar1963. [DOI] [PMC free article] [PubMed] [Google Scholar]