Abstract
Telmisartan is an angiotensin-II receptor blocker and acts as a selective modulator of peroxisome proliferator-activated receptor gamma (PPARγ). Several studies have demonstrated that telmisartan ameliorates depression and memory dysfunction and reduces brain inflammation. We hypothesized that the beneficial effects of telmisartan on brain could be due to modulation of the blood-brain barrier (BBB) function. Here, we examined the effect of telmisartan on tumor necrosis factor alpha (TNF-α)-induced expression of intercellular adhesion molecule 1 (ICAM-1) which plays an important role in leukocyte transcytosis through the BBB. Telmisartan blocked TNF-α-induced ICAM-1 expression and leukocyte adhesion in U87MG human glioma cells but showed no effect on human brain microvascular endothelial cells. In U87MG cells, a PPAR antagonist, GW9662 did not block the effect of telmisartan on ICAM1 expression but rather potentiated. Moreover, GW9662 caused no change in TNF-α-induced ICAM-1 expression, suggesting no implication of PPARγ in the telmisartan effect. Further studies showed that telmisartan blocked TNF-α-induced activation of c-Jun N-terminal kinase (JNK), extracellular signal-regulated kinase 1/2 (ERK1/2), p38, and nuclear factor-kappa B (NF-κB). In contrast, inhibitors of JNK, ERK1/2 and NF-κB but not p38, blocked ICAM-1 expression induced by TNF-α. Thus, our findings suggest that the beneficial effect of telmisartan is likely due to the reduction of astrocytic ICAM1 expression and leukocytes adhesion to astrocytes, and that this response was mediated by the inhibition of JNK/ERK1/2/NF-κB activation and in the PPAR-independent manner. In conclusion, this study enhances our understanding of the mechanism by which telmisartan exerts the beneficial brain function.
Keywords: Telmisartan, ICAM-1, TNF-α, Inflammation, Astroglial, Blood-brain barrier
INTRODUCTION
The blood-brain barrier (BBB) is a highly specialized structure which is formed by endothelial cells, pericytes, and astrocytes and ensures the export of neurotoxic molecules from the brain to the blood vessels (Sweeney et al., 2019). BBB disruption allows the influx of neurotoxic plasma components such as hematopoietic proteins, leukocytes, and microbial pathogens into the central nervous system. Recently, vascular inflammation and dysregulated immune cell trafficking through the BBB have been implicated in the pathogenesis of brain diseases such as dementia (Erdő et al., 2017), although the importance of leukocyte traffic in these diseases remains unclear. The invasion of neutrophils or monocytes into the brain may cause neuroinflammation, oxidative stress, neuronal toxicity, and neuronal detachment from the supporting extracellular matrix (Bell et al., 2010; Daneman et al., 2010; Winker et al., 2012), leading to progressive neurodegeneration.
Intercellular adhesion molecule-1 (ICAM-1; CD54) is a transmembrane glycoprotein of the Ig superfamily which is involved in cell adhesion. Endothelial ICAM-1 is a ligand for the membrane-bound integrin receptors lymphocyte function-associated antigen 1 (LFA-1) and Mac-1 on leukocytes, and plays an important role in leukocyte-endothelial cell adhesion and migration (Lawson and Wolf, 2009). ICAM-1 is expressed by endothelial cells, leukocytes, fibroblasts, and microglia. Although the function of endothelial ICAM-1 is well-known, the role of ICAM-1 in astrocytes remains unclear. Interestingly, ICAM-1 is aberrantly expressed by astrocytes in multiple sclerosis and Alzheimer’s disease (AD) brains (Verbeek et al., 1994; Ponath et al., 2018) and has been suggested as a blood marker for AD (Janelidze et al., 2018), suggesting the important role of ICAM-1 in the pathogenesis of AD.
Telmisartan is an angiotensin-II type 1 receptor blocker and peroxisome proliferator-activated gamma (PPARγ) agonist used to treat hypertension (Benson et al., 2004). PPAR activation suppresses inflammatory responses in neurons, endothelial cells, astrocytes, and microglial cells and promotes amyloid β clearance (Camacho et al., 2004; Elkahloun et al., 2018). Telmisartan had protective effects on memory impairment, glial inflammation, and amyloid burden in a transgenic mouse model of AD (Tsukuda et al., 2009; Torika et al., 2017). Furthermore, in human prospective cohort and nested case control studies (Davies et al., 2011), AD patients treated with angiotensin-II type 1 receptor blockers showed lower incidence and progression rate for dementia (Li et al., 2010). Therefore, telmisartan is a possible candidate for the treatment of AD, but its molecular target and the mechanism underlying its effect remain unclear.
In the present study, in order to understand the molecular mechanism underlying the neuroprotective role of telmisartan, the effects of telmisartan on tumor necrosis factor alpha (TNF-α)-induced ICAM-1 expression and leukocyte adhesion in microendothelial cells and astrocytes were investigated. In addition, the molecular signaling pathways underlying this effect were monitored. Unexpectedly, telmisartan reduced TNF-α-induced ICAM-1 protein expression in astrocytes but not in endothelial cells. Furthermore, telmisartan regulated ICAM-1 expression through inhibition of the TNF-α-activated extracellular signal-regulated kinase 1/2 (ERK1/2), c-Jun N-terminal kinase (JNK), and nuclear factor-kappa B p65 (NF-κB p65) signaling pathways.
MATERIALS AND METHODS
Materials and reagents
Telmisartan was purchased from Cayman Chemical Co (Ann Arber, MI, USA) and human fibroblast growth factor (bFGF) from Millipore (Temecula, CA, USA). Human TNF-α, U0126, SP600125, SB202190, BAY11-7082 and GW9662 were purchased from Sigma-Aldrich (St. Louis, MO, USA). Monoclonal anti-ICAM-1 and anti-TNFR antibodies were obtained from Santa Cruz Biotechnology (La Jolla, CA, USA). Monoclonal anti-α-tubulin and anti-β-actin antibodies were obtained from Invitrogen (Carlsbad, CA, USA) and Abcam (Cambridge, MA, USA), respectively. Antibodies against PARP and phospho-/total forms of NF-κB p65, JNK, ERK1/2 and p38 MAPK were purchased from Cell Signaling Technology (Danvers, MA, USA). Minimum essential medium alpha (MEM-α), Medium 199, RPMI1640 medium, fetal bovine serum (FBS), heparin, penicillin and streptomycin were obtained from Gibco-BRL (Gaithersburg, MD, USA) and medium 199 from Life Technologies (Carlsbad, CA, USA).
Cell culture
Human U87MG cells, a human glioma cells, purchased from the American Type Culture Collection (Manassas, VA, USA) were cultured in MEM-α supplemented with 10% FBS, penicillin (100 U/mL), and streptomycin (100 μg/mL). Human brain microvascular endothelial cells (HBMVEC) were purchased from Cell Systems (Kirkland, WA, USA) and cultured in Medium 199 supplement with 20% FBS, 3 ng/mL bFGF, 5 U/mL heparin, penicillin (100 U/mL), and streptomycin (100 μg/mL). HL-60 cells were purchased from the Korean Cell Line Bank (Seoul, Korea) and maintained in RPMI 1640 medium supplemented with 10% FBS, penicillin, and streptomycin. The cells were cultured at 37°C in a 5% CO2 incubator.
Immunoblotting
To prepare the total cell lysate, cells grown in 60 mm plate were washed twice with PBS and solubilized on ice for 30 min with RIPA buffer (25 mM Tris-HCl pH 7.6, 150 mM NaCl, 0.1% SDS, 1% Nonidet P40, 1% sodium deoxycholate; ThermoFisher Scientific, Waltham, MA, USA) containing protease inhibitor cocktail tablets and protein phosphatases inhibitor cocktails (Roche, Indianapolis, IN, USA). Protein concentration was then determined using a BCA assay kit (Pierce, Rockford, IL, USA). Equal amounts of protein were applied and separated on 10 and 12% SDS-PAGE under reducing conditions. The proteins were then transferred to a PVDF membrane and the membrane was blocked. The membrane was then incubated with primary antibodies, and proteins were detected using horseradish peroxidase-coupled secondary antibodies (GE Healthcare Life Science, Piscataway, NJ, USA). Bound antibodies were visualized with ECL Plus or ECL Prime Western Blotting Detection Reagent (GE Healthcare Life Science). Images were acquired using an ImageQuant LAS 4000 system (GE Healthcare Life Science) and densitometric analysis was performed using ImageJ v1.52c (National Institutes of Health, MD, USA). The brightness and contrast of the images were adjusted in ImageJ (National Institutes of Health) and Photoshop v7.0.1 (Adobe, San Jose, CA, USA).
RNA isolation and quantitative PCR
Total RNA was isolated from cells using the RNA NucleoSpin (Macherey-Nagel, Duren, Germany) according to the manufacturer’s instructions. Purified RNA (2 μg) was reverse transcribed in a 20 μL reaction to generate cDNA using the GoScript Reverse Transcription kit (Promega, Madison, WI, USA) according to the manufacturer’s instructions. The 20 μL reaction volume for relative quantitative PCR contained Fast SYBR® Green Master Mix (Applied Biosystems, Foster City, CA, USA), 2 μL cDNA, and 0.2 µM of gene-specific primer mix. The reaction conditions were as follows: 95°C for 10 min, followed by 40 cycles of 95°C for 15 sec, 58°C for 25 sec, and 72°C for 35 sec. At the end of each cycle, the fluorescence was measured and analyzed using a 7500 Fast Real-Time PCR system (Applied Biosystems). Amplification of the sequence of interest was normalized using an endogenous reference gene (glyceraldehyde 3-phosphate dehydrogenase; GAPDH). Fold-change values were calculated using the ∆∆Ct method. RQ Manager software and GraphPad Prism v6.0 (GraphPad Software, La Jolla, CA, USA) were used for data analysis. The following gene-specific primers were used: ICAM-1 (forward, 5’-TGTGACCAGCCCAAGTTGTT-3’; reverse, 5’-AGTCCAGTACACGGTGAGGA-3’) and GAPDH (forward, 5’-CCATGGAGAAGGCTGGGG-3’; reverse, 5’-GGTCATGAGTCCTTCCACGA-3’).
Adhesion assay
Confluent HBMVEC grown in 12-well plates were pretreated with telmisartan (40 µM) for 1 h prior to the treatment with TNF-α (10 ng/mL). After 8 h, HL-60 leukocytes (5×105) in 0.5 mL RPMI 1640 were added to the wells, and the plates were incubated for 1 h to allow cell attachment. In case for the U87MG adhesion assay HL-60 cells labeled with a fluorescent dye were used; HL-60 cells were labeled by incubating HL-60 cells with 5 nM CellTracker Green CMFDA (ThermoFisher Scientific) for 30 min at 37°C and then rinsed three times with the growth media. After incubation, non-adherent HL-60 cells were removed by washing the wells twice with the growth media. The number of adherent HL-60 cells was counted using an inverted microscope at 100 or 200× magnification (CKX41, Olympus, Tokyo, Japan).
Immunocytochemistry and confocal imaging
Cells grown on 2-well chambered coverglass (Lab-TekTM, ThermoFisher Scientific) or 12-well plate were fixed in 4% paraformaldehyde in PBS for 15 min at room temperature, permeabilized with 0.15% (v/v) TritonX-100 in PBS for 15 min, and incubated with 2.5% (w/v) BSA in PBS for 30 min. The fixed cells were incubated with primary antibodies and corresponding secondary antibodies (Alexa Fluor 488/ 594 goat anti-rabbit or mouse IgG, Invitrogen, Waltham, MA, USA) for immunostaining. Images of the cells were captured using an inverted confocal microscope (LSM700, Carl Zeiss, Jena, Germany). ZEN2009 was then used for quantification analysis of the immunofluorescence images. For NF-κB translocation, 6-7 z-sections were collected at 1 µm intervals and three to four fields (over 100 cells/field) per condition were analyzed.
Statistical analysis
Data are presented as mean ± SEM as indicated in the Fig. legends. Data were processed in Microsoft Excel and statistical analyses were performed using GraphPad Prism v6.0 (GraphPad Software). Differences between two groups were analyzed using a two-tailed, unpaired t-test. n.s. represents not significant, *p<0.05, **p<0.01, and ***p<0.001.
RESULTS
Telmisartan reduces TNF-α-induced ICAM-1 expression in U87MG cells but not in HBMVECs
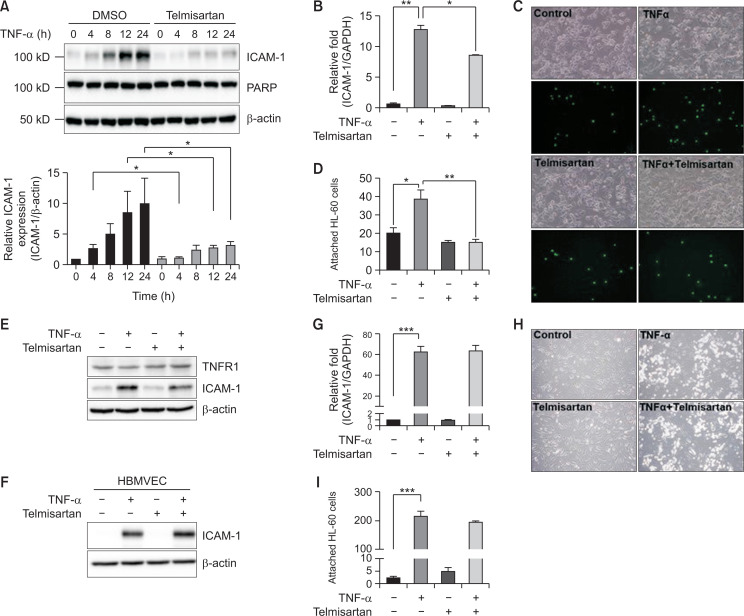
We recently found that TNF-α upregulates ICAM-1 expression in both brain micro-endothelial cells in vitro and micro-vessels in vivo (Choi et al., 2017). Thus, to examine the effect of telmisartan on ICAM-1 expression in BBB-comprising cells, astrocytes and endothelial cells, we measured the ICAM-1 mRNA and protein levels in U87MG cell, a human glioma cell, after treatment with TNF-α. U87MG cells were pre-treated with or without telmisartan (40 µM) for 1 h and then stimulated with 10 ng/mL TNF-α for 4-24 h. Interestingly, U87MG cells treated with TNF-α alone exhibited a significant increase in ICAM-1 protein expression in a time-dependent manner, reaching a maximum at 24 h, but this increase was significantly downregulated following pre-treatment with telmisartan (Fig. 1A). We also examined the ICAM-1 mRNA level using RT-qPCR. As shown in Fig. 1B, telmisartan significantly reduced TNF-α-induced ICAM-1 mRNA expression. Then, the functional alteration of increased ICAM-1 expression was examined by measuring leukocyte adhesion to U87MG cells. As shown in Fig. 1C and 1D, adhesion of HL-60 leukocytes to U87MG cells increased by TNF-α treatment but was blocked by the pre-treatment with telmisartan. Moreover, telmisartan or TNF-α had no effect on TNFR1 expression (Fig. 1E) and no significant cytotoxicity to U87MG cells (data not shown). In contrast, in HBMVECs telmisartan caused no alteration in TNF-α-induced ICAM-1 protein and mRNA levels (Fig. 1F, 1G) and leukocyte adhesion to HBMVECs (Fig. 1H, 1I). Similarly, no significant effect of telmisartan in HUVEC, a human umbilical vein endothelial cell was observed (data not shown). Thus, the effects of telmisartan on ICAM-1 expression are likely astrocyte-specific.
Fig. 1.
Telmisartan attenuates tumor necrosis factor alpha (TNF-α)-induced intercellular adhesion molecule-1 (ICAM-1) expression in U87MG cells but not in human brain microvascular endothelial cells (HBMVECs). (A) Blocking the increase of TNF-α-induced ICAM1 protein level by telmisartan. U87MG cells were incubated with or without telmisartan (40 µM) for 1 h, and further treated with TNF-α (10 ng/mL) for 0, 4, 8, 12, or 24 h. Whole cell lysates were analyzed by Western blotting using anti-ICAM-1, poly (ADP-ribose) polymerase (PARP), and β-actin antibodies. Quantification was performed using ImageJ software (National Institutes of Health) (mean ± SEM; n=3). (B) Blocking the increase of TNF-α-induced ICAM1 mRNA level by telmisartan. U87MG cells were pre-incubated for 1 h with or without telmisartan and then further treated with TNF-α (10 ng/mL) for 8 h. ICAM-1 gene expression was then detected by RT-qPCR (mean ± SEM; n=3). (C) Photographs showing HL-60 cell adhesion to U87MG cells. U87MG cells seeded in a 12-well plate were incubated with or without telmisartan (40 µM) and then treated with TNF-α (10 ng/mL) for 24 h. Adherent HL-60 cells were observed and counted using an inverted microscope (Olympus, Tokyo, Japan) with 200× magnification. (D) Quantitation of HL-60 cell adhesion assay (mean ± SEM; n=5). *p<0.05, **p<0.01. (E) No change in tumor necrosis factor receptor 1 (TNFR1) expression by telmisartan. HL-60 cells were pre-incubated for 1 h with or without telmisartan (40 µM) and then further treated with TNF-α (10 ng/mL) for 24 h. TNFR1 and ICAM-1 expression was then detected by Western blotting. (F, G) No effect of telmisartan on ICAM1 expression in HBMVEC cells. HBMVECs were incubated for 1 h with or without telmisartan and then further treated with TNF-α (10 ng/mL) for 24 h. The ICAM-1 protein levels (F) and mRNA levels (G) were determined by Western blotting and RT-qPCR (mean ± SEM; n=4), respectively. ***p<0.001. (H) Photographs showing HL-60 cell adhesion to HBMVECs. HBMVECs seeded in a 12-well plate were incubated with or without telmisartan (40 µM) and then treated with TNF-α (10 ng/mL) for 24 h. Adherent HL-60 cells were observed and counted using an inverted microscope with 100× magnification. (I) Quantitation of HL-60 cell adhesion assay (mean ± SEM; n=4). ***p<0.001.
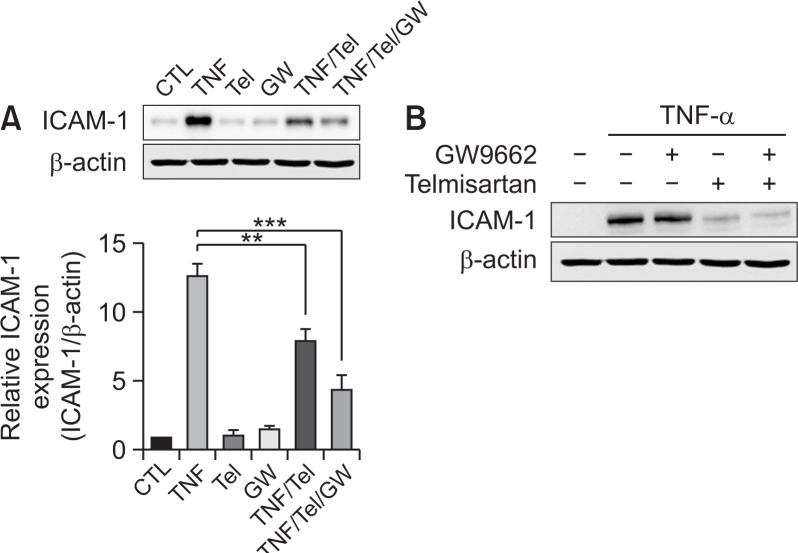
The effect of telmisartan on TNF-α-induced ICAM-1 expression is PPARγ-independent
Telmisartan has been reported to act as a PPARγ agonist. Thus, the implication of PPARγ in TNF-α-induced ICAM-1 expression in U87MG cells was accessed by performing studies using an irreversible PPARγ antagonist, GW9662. Treatment with telmisartan blocked TNF-α-induced ICAM-1 expression (Fig. 2A) as described previously. In contrast, co-treatment with telmisartan and GW9662 did not block the decrease in TNF-α-induced ICAM-1 protein level which is induced by telmisartan. Moreover, GW9662 alone caused no change in TNF-α-induced ICAM-1 expression (Fig. 2B). These results suggest that the effect of telmisartan on ICAM-1 expression is PPARγ-independent.
Fig. 2.
Telmisartan mediates its inhibitory effects on the tumor necrosis factor alpha (TNF-α)-induced expression of intercellular adhesion molecule-1 (ICAM-1) by the peroxisome proliferator-activated receptor gamma (PPARγ)-independent pathway. (A) GW9662 does not block the telmisartan-mediated effect. U87MG cells were either untreated, pre-treated with the PPARγ antagonist GW9662 (5 µM) or telmisartan (40 µM), or co-treated with telmisartan and GW9662 for 1 h and then further treated with 10 ng/mL TNF-α for 24 h. Whole cell lysates were analyzed by Western blotting. The error bars represent mean ± SEM (n=4). Statistical differences were determined using the student’s t-test. **p<0.01, ***p<0.001. (B) GW9662 does not alter TNF-α-induced ICAM1expression. U87MG cells were treated as describe in (A).
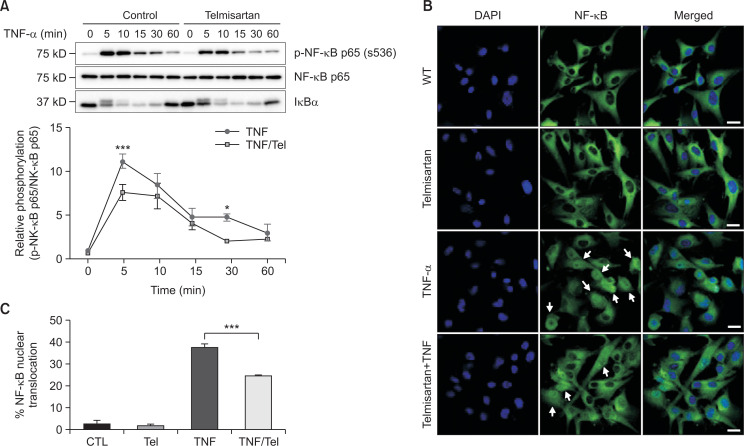
Telmisartan inhibits TNF-α-induced NF-κB activation
It has been reported that one major signaling pathway of ICAM-1 expression is the activation of NF-κB (Roebuck and Finnegan, 1999). NF-κB is an important component in the cellular responses to damage, stress, and inflammation. Various exogenous and endogenous stimuli can activate NF-κB in human cells, including several pro-inflammatory mediators such as TNF-α, interleukin (IL)-1β, and IL-6. Thus, we investigated the involvement of the NF-κB signaling pathway in the telmisartan effect on TNF-α-induced ICAM-1 expression. With regards to this, it has been reported that in response to TNF-α NF-κB p65 is phosphorylated by kinases including IKKα and IKKβ (Hinz and Scheidereit, 2014) and translocated into the nucleus. In addition, IκBα, which normally binds to NF-κB dimers in the cytosol and inhibits the translocation of NF-κB into the nucleus, is released from the complex upon phosphorylation by IKK and subsequently degraded. Thus, the time-course (5-60 min) of NF-κB p65 phosphorylation and the level of IκBα after TNF-α treatment was examined. As seen in Fig. 3A, increased NF-κB p65 phosphorylation was observed in 5 min after TNF-α treatment and then decreased over 60 min (Fig. 3A). However, pre-treatment with telmisartan significantly suppressed TNF-α-induced NF-κB p65 phosphorylation in a time-dependent manner. Then, we traced the translocation of NF-κB p65 into the nucleus using a specific antibody and a confocal scanning microscope. Following stimulation with TNF-α alone, the expression of the NF-κB p65 subunit was significantly increased in the nucleus, while in the untreated control group it was predominantly located in the cytoplasm (Fig. 3B). In contrast, pre-treatment with telmisartan suppressed the TNF-α-induced nuclear translocation of NF-κB p65 (Fig. 3B, 3C). Taken together, these results suggest that telmisartan inhibits TNF-α-induced NF-κB p65 translocation to the nucleus in U87MG cells.
Fig. 3.
Telmisartan inhibits phosphorylation and nuclear translocation of nuclear factor-kappa B (NF-κB). (A) Blocking tumor necrosis factor alpha (TNF-α)-induced NF-κB p65 activation by telmisartan. U87MG cells were incubated with or without telmisartan for 1 h and then treated with tumor necrosis factor alpha (TNF-α) (10 ng/mL) for 0, 5, 10, 15, 30, or 60 min (n=3). The transient phosphorylation of NF-κB p65 in U87MG cells were analyzed by Western blotting with the indicated antibodies. Phospho-NF-κB intensity values were normalized to the corresponding NF-κB value. Statistical differences were determined using the student’s t-test. *p<0.05, ***p<0.001. (B) Blocking TNF-α-induced NF-κB p65 nuclear translocation by telmisartan. U87MG cells were incubated with or without telmisartan (40 µM) for 1 h and then treated with TNF-α (10 ng/mL) for 1 h. Fixed cells were stained with anti-NF-κB p65 antibody and DAPI and further processed for the immunofluorescence staining. White bars, 20 µm. White arrows indicate nuclear localization of NF-B p65. (C) Quantification of endogenous NF-κB p65 nuclear localization in U87MG cells. Over hundred cells were counted in three separate wells. The error bars represent mean ± SEM from three independent experiments. Statistical differences were determined using the student’s t-test. ***p<0.001.
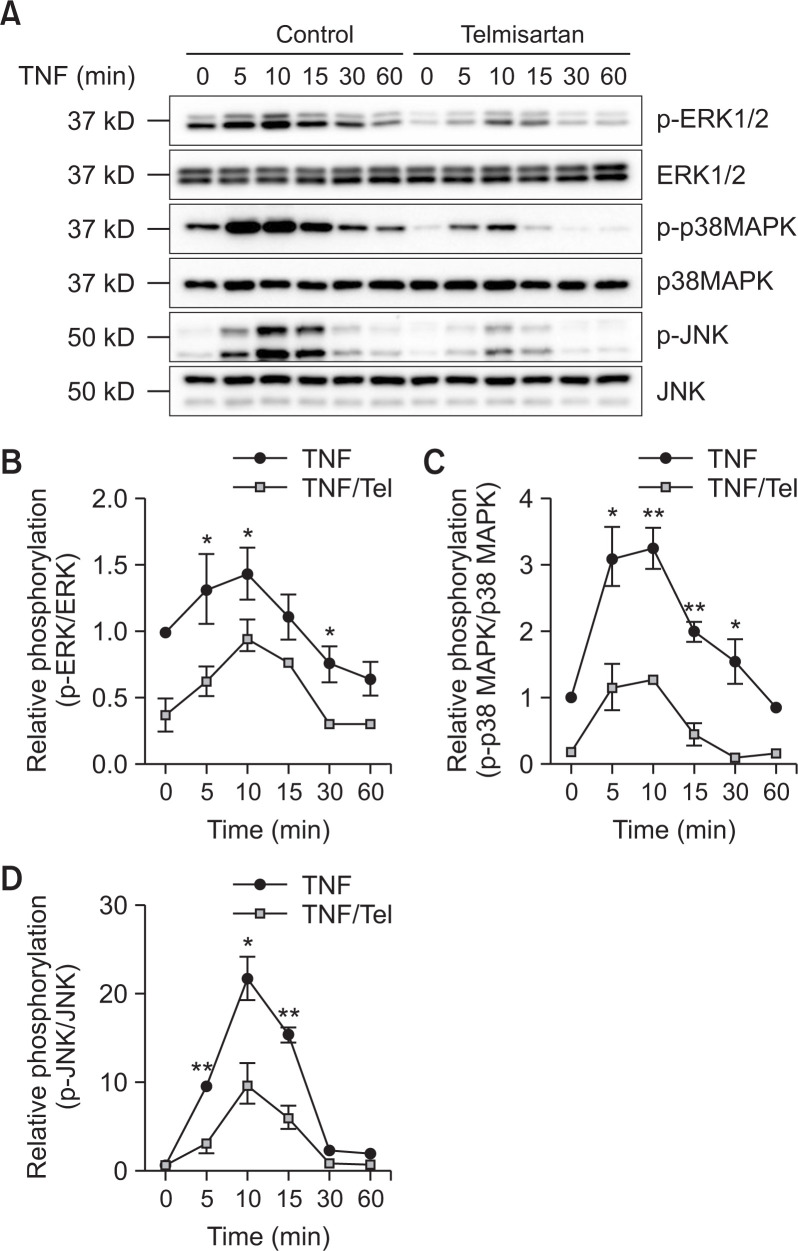
Telmisartan inhibits TNF-α-induced ERK1/2, p38, and JNK activation
It has been reported that TNF-α activates the MAPKs signaling in HUVECs (Wang et al., 2016). To verify whether telmisartan modulates TNF-α-induced MAPK activation, the phosphorylation of ERK1/2, p38, and JNK was examined using Western blotting. TNF-α induced ERK1/2, p38, and JNK phosphorylation in U87MG cells, reaching a maximum after approximately 10 min of exposure and then decreasing (Fig. 4A). Telmisartan significantly inhibited this TNF-α-induced activation of ERK1/2, p38, and JNK in U87MG cells (Fig. 4B-4D).
Fig. 4.
Telmisartan inhibits the tumor necrosis factor alpha (TNF-α)-induced extracellular signal-related kinase 1/2 (ERK1/2), p38 mitogen-activated protein kinase (MAPK), and c-Jun N-terminal kinase (JNK) signaling pathways in U87MG cells. Cells were incubated with or without telmisartan for 1 h and treated with TNF-α (10 ng/mL) for 0, 5, 10, 15, 30, or 60 min. Cell lysates were analyzed by Western blotting (A). The levels of phospho-p42/p44 MAPK (B), phospho-p38 (C), and phospho-JNK1/2 (D) were determined. The error bars represent mean ± SEM (n=3). Statistical differences were determined using the student’s t-test. *p<0.05 and **p<0.01 compared to the cells exposed to TNF-α alone.
Telmisartan regulates ICAM1 expression through the ERK1/2, JNK and NF-κB signaling pathways
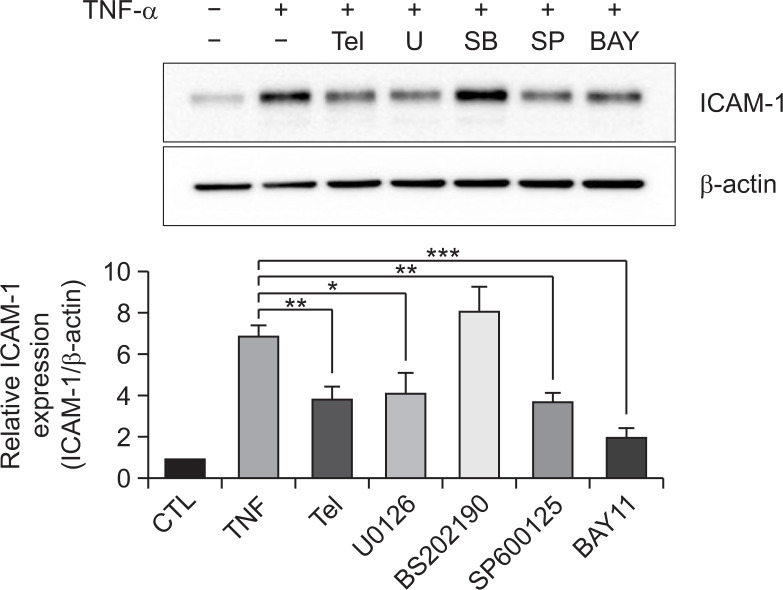
Then, we determined the role of NF-κB and MAPK signaling pathways in ICAM-1 expression. U87MG cells were pre-treated with specific inhibitors of ERK1/2 (U0126, 10 µM), p38 (SB202190, 10 µM), JNK (SP600125, 10 µM), and IKKα (BAY11-7082, 10 µM) for 1 h prior to incubation with TNF-α, and the ICAM-1 protein levels were then evaluated. U0126, SP600125, and BAY11-7082 significantly downregulated TNF-α-induced ICAM-1 expression in U87MG cells (Fig. 5). Inhibition of the NF-κB signaling pathway caused the greatest reduction in ICAM-1 protein level. In contrast, SB201190 treatment did not affect ICAM-1 protein expression. Taken together, these results suggest that telmisartan modulates the NF-κB, JNK, and ERK1/2 signaling pathways to reduce TNF-α-induced ICAM1 protein expression.
Fig. 5.
Telmisartan regulates intercellular adhesion molecule-1 (ICAM1) expression through the NF-κB, c-Jun N-terminal kinase (JNK) and extracellular signal-related kinase (ERK) signaling pathways. Telmisartan and JNK, ERK and NF-κB inhibitors attenuated tumor necrosis factor alpha (TNF-α)-induced ICAM-1 expression in U87MG cells. Cells were incubated with telmisartan for 1 h or inhibitors (U0126, SB202190, SP600125, and BAY11-7082; all at 10 µM) for 30 min and were then treated with TNF-α (10 ng/mL) for 10 h. Whole cell lysates were analyzed by immunoblotting. The error bars represent mean ± SEM (n=3). Statistical differences were determined using the student’s t-test. *p<0.05, **p<0.01 and ***p<0.001 compared to the cells exposed to TNF-α alone.
DISCUSSION
Recent studies have suggested that hyperactivation of angiotensin-II type 1 receptor promotes hypertension and vascular inflammation and enhances neuronal loss and neurodegeneration in brain (Saavedra, 2012; Villapol and Saavedra, 2015). In addition, angiotensin-II type 1 receptor inhibition reduced the production of pro-inflammatory mediators by astrocytes and microglia cells (Miyoshi et al., 2008; Lanz et al., 2010; Benicky et al., 2011) and promoted a shift in microglial phenotype to the neuroprotective phenotype (Xu et al., 2015). Indeed, telmisartan or valsartan has the beneficial effects on cognitive function in mouse model of AD (Wang et al., 2007; Tsukuda et al., 2009). Thus, angiotensin-II receptor blockers may alleviate amyloid plaque-related pathologies and cognitive dysfunction by modulating brain inflammation, but the detailed molecular mechanism is unknown. In this study we proposed the implication of ICAM1, an inflammatory adhesion protein induced by various stimuli, in the telmisartan effect and demonstrated that telmisartan reduces TNF-α-induced ICAM1 expression and thereby blocks leukocyte adhesion to astrocyte.
Although the function of ICAM-1 expressed in endothelial cells of various tissues is quite well known, its role in astrocytes has not been examined. Astrocytes in the neurovascular unit surround most portions of the micro-vessels and capillaries and interact with endothelial cells through the end-feet of their processes. Thus, ICAM1 in astrocytes of BBB is likely to be involved in adhesion of leukocytes to astrocytes to facilitate transcytosis or migration into the brain. This explanation is supported from our results that the leukocyte binding to astrocyte is enhanced by TNF-α and this increase is blocked by telmisartan (Fig. 1). Since the leukocyte entry to the brain is considered as an initial step of brain inflammation, blocking this step by telmisartan may be useful to apply telmisartan for the treatment of various brain diseases. In addition, an early study demonstrated that ICAM-1 ligation by a monoclonal antibody to astrocytes induced expression of pro-inflammatory cytokines (IL-1α, IL-1β, IL-6, and TNF-α), causing astrogliosis, the induction of acute phase proteins, and neurodegeneration (Lee et al., 2000). Interestingly, telmisartan modulates glial activation reducing TNF-α, IL-1β and NO release from the glial cells (Torika et al., 2016). Moreover, ICAM-1-positive astrocytes interact with LFA-1 and/or Mac-1-positive cells (infiltrating inflammatory cells and resident microglia) leading to the production of inflammatory cytokines by astrocytes (Lee and Benveniste, 1999). Taken together, aberrant expression of astrocytic ICAM-1 plays an important role in brain inflammation, and the suppression of ICAM-1 expression by telmisartan protects the brain against neuronal damage by reducing the attachment of leukocytes to astrocytes and the release of inflammatory cytokines.
Our results showed that telmisartan blocks TNF-α-induced ICAM-1 expression in astroglioma U87MG cells, but not HBMVECs (Fig. 1). This finding is consistent with a previous study that telmisartan attenuates TNF-α-induced vascular cell adhesion molecule-1 (VCAM1) expression but not ICAM-1 in HUVECs (Cianchetti et al., 2008). Although it is hard to explain why telmisartan does not modulate ICAM-1 expression in endothelial cells, it is quite interesting to find the key molecule(s) of the telmisartan effect which is absent in endothelial cells.
In fact, telmisartan acts as both an angiotensin-II type 1 receptor blocker and a PPARγ agonist (Saavedra, 2012). Angiotensin-II type 1 receptor activation is associated with decreased cerebral blood flow, pathological responses to stress, and inflammation (Saavedra et al., 2006; Ohshima et al., 2013). It has also been reported that PPARγ activation suppresses TNF-α-induced VACM-1 expression in HUVECs, thereby inhibiting the adhesion of monocytes to HUVECs (Wang et al., 2016). Furthermore, telmisartan-induced PPARγ activation prevented cognitive decline in AD mice (Tsukuda et al., 2009). Thus, we attempted to determine the relative contribution of each pathway to the effect of telmisartan and accessed the implication of PPARγ activation. We found that GW9662, an irreversible PPARγ antagonist failed to block the effect of telmisartan on TNF-α-induced ICAM-1 expression and that GW9662 alone did not alter the TNF-α effect (Fig. 2). Taken together, our results suggest that the inhibition of TNF-α-induced ICAM-1 expression by telmisartan is mediated in a PPARγ independent manner and may be exerted through blockade of angiotensin-II type 1 receptor-mediated pathways.
The activation of angiotensin-II type 1 receptor stimulates NF-κB-dependent transcription, induces the transcription of several pro-inflammatory cytokines, and stimulates various kinases involved in the propagation of inflammatory responses and apoptotic pathways (Villapol and Saavedra, 2015). We found that telmisartan blocked TNF-α-induced NF-κB p65 phosphorylation, IκBα degradation, and NF-κB nuclear translocation. Although the detailed molecular mechanism of the telmisartan action is unclear it has been reported that telmisartan attenuates hyperglycemia-exacerbated VCAM-1 expression in HUVECs by inhibiting IKKβ expression (Song et al., 2016). Thus, it is possible in U87MG cells that telmisartan attenuates ICAM1 expression by the NF-κB signaling pathway via the inhibition of IKK expression.
It is known that MAPK (ERK1/2, p38, and JNK) signaling pathways are also involved in TNF-α-induced ICAM-1 and VCAM-1 expression in HUVECs (Kuldo et al., 2005; Wang et al., 2016). Thus, using specific inhibitors of MAPKs, the role of each MAPK pathway in TNF-α-induced ICAM-1 expression in U87MG cells was examined. The activation of all three MAPK pathways by TNF-α was reduced by telmisartan, but only U0126 and SP600125 significantly decreased TNF-α-induced ICAM-1 expression; SB202190 had no effect on or slightly increased TNF-α-induced ICAM-1 expression. These results suggest that blocking ERK1/2 and JNK activation by telmisartan is implicated in reduction of TNF-α-induced ICAM-1 expression while p38 may not be related to this process.
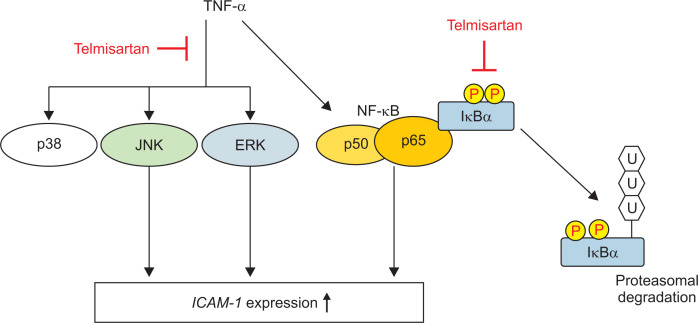
In conclusion, our study demonstrated that in U87MG human glioma cells, telmisartan inhibits TNF-α-induced ICAM-1 expression and leukocyte adhesion by blocking the NF-κB, JNK, and ERK1/2 pathways and in a PPARγ-independent manner (Fig. 6). This study provides an information of the pharmacological target cell and the molecular mechanism of telmisartan.
Fig. 6.
Proposed model of the mechanism by which telmisartan attenuates tumor necrosis factor alpha (TNF-α)-induced intercellular adhesion molecule-1 (ICAM-1) expression in U87MG cells.
ACKNOWLEDGMENTS
This work was supported by the 2016 Research fund of Dankook University (R201601384).
Footnotes
CONFLICT OF INTEREST
The authors declare no financial and non-financial competing interests.
References
- Bell R. D., Winker E. A., Sagare A. P., Singh I., LaRue B., Deane R., Zlokovic B. V. Pericytes control key neurovascular functions and neuronal phenotype in adult brain and during brain aging. Neuron. 2010;68:409–427. doi: 10.1016/j.neuron.2010.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benicky J., Sanchez-Lemus E., Honda M., Pang T., Orecna M., Wang J., Leng Y., Chuang D., Saavedra J. M. Angiotensin II AT1 receptor blockade ameliorates brain inflammation. Neuropsychopharmacology. 2011;36:857–870. doi: 10.1038/npp.2010.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson S. C., Pershadsinggh H. A., Ho C. I., Chittiboyina A., Desai P., Pravenec M., Qi N., Wang J., Avery M. A., Kurtz T. W. Identification of telmisartan as a unique angiotensin II receptor antagonist with selective PPARγ-modulating activity. Hypertension. 2004;43:993–1002. doi: 10.1161/01.HYP.0000123072.34629.57. [DOI] [PubMed] [Google Scholar]
- Camacho I. E., Serneels L., Spittaels K., Merchiers P., Dominguez D., De Strooper B. Peroxiosome proliferator-activated receptor γ induces a clearance mechanism for the amyloid-β peptide. J. Neurosci. 2004;24:10908–10917. doi: 10.1523/JNEUROSCI.3987-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi G. Y., Yoon S. S., Kim S. E., Jo S. A. KDM4B histone demethylase and G9a regulate expression of vascular adhesion proteins in cerebral microvessels. Sci. Rep. 2017;7:45005. doi: 10.1038/srep45005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cianchetti S., Fiorentino A. D., Colognato R., Stefano R. D., Franzoni F., Pedrinelli R. Anti-inflammatory and antioxidant properties of telmisartan in cultured human umbilical vein endothelial cells. Atherosclerosis. 2008;198:22–28. doi: 10.1016/j.atherosclerosis.2007.09.013. [DOI] [PubMed] [Google Scholar]
- Daneman R., Zhou L., Kebede A. A., Barres B. A. Pericytes are required for blood-brain barrier integrity during embryogenesis. Nature. 2010;468:562–566. doi: 10.1038/nature09513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies N. M., Kehoe P. G., Ben-Shiomo Y., Martin R. M. Association of anti-hypertensive treatments with Alzheimer's disease, vascular dementia, and other dementias. J. Alzheimers Dis. 2011;26:699–708. doi: 10.3233/JAD-2011-110347. [DOI] [PubMed] [Google Scholar]
- Elkahloun A. G., Rodriguez Y., Alaiyed S., Wenzel E., Saavedra J. M. Telmisartan protects a microglia cell line from LPS injury beyond AT1 receptor blockade or PPARγ activation. Mol. Neurobiol. 2018;56:3193–3210. doi: 10.1007/s12035-018-1300-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdő F., Denes L., de Lange E. Age-associated physiological and pathological changes at the blood-brain barrier: a review. J. Cereb. Blood Flow Metab. 2017;37:4–24. doi: 10.1177/0271678X16679420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinz M., Scheidereit C. The IκB kinase complex in NF-κB regulation and beyond. EMBO Rep. 2014;15:46–61. doi: 10.1002/embr.201337983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janelidze S., Mattsson N., Stomrud E., Lindberg O., Palmqvist S., Zetterberg H., Blennow K., Hansson O. CSF biomarkers of neuroinflammation and cerebrovascular dysfunction in early Alzheimer disease. Neurology. 2018;91:e867–e877. doi: 10.1212/WNL.0000000000006082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuldo J. M., Westra J., Asgeirsdóttir S. A., Kok R. J., Oosterhuis K. Differential effects of NF-kB and p38 MAPK inhibitors and combinations thereof on TNF-α and IL-1β-induced proinflammatory status of endothelial cells in vitro. Am. J. Physiol. Cell Physiol. 2005;289:C1229–C1239. doi: 10.1152/ajpcell.00620.2004. [DOI] [PubMed] [Google Scholar]
- Lanz T. V., Ding Z., Ho P. P., Luo J., Agrawal A. N., Srinagesh H., Axtell R., Zhang H., Platten M., Wyss-Coray T., Steinman L. Angiotensin II sustains brain inflammation in mice via TGF-beta. J. Clin. Invest. 2010;120:2782–2794. doi: 10.1172/JCI41709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson C., Wolf S. ICAM-1 signaling in endothelial cells. Pharmacol. Rep. 2009;61:22–32. doi: 10.1016/S1734-1140(09)70004-0. [DOI] [PubMed] [Google Scholar]
- Lee S. J., Benveniste E. N. Adhesion molecule expression and regulation on cells of the central nervous system. J. Neuroimmunol. 1999;98:77–88. doi: 10.1016/S0165-5728(99)00084-3. [DOI] [PubMed] [Google Scholar]
- Lee S. J., Drabik K., Van Wagoner N. J., Lee S. J., Choi C., Dong Y., Benveniste E. N. ICAM-1-induced expression of proinflammatory cytokines in astrocytes: involvement of extracellular signal-regulated kinase and p38 mitogen-activated protein kinase pathways. J. Immunol. 2000;165:4658–4666. doi: 10.4049/jimmunol.165.8.4658. [DOI] [PubMed] [Google Scholar]
- Li N. C., Lee A. C., Whitmer R. A., Kivipelto M., Lawler E., Kazis L. E., Wolozin B. Use of angiotensin receptor blockers and risk of dementia in a predominantly male population: prospective cohort analysis. BMJ. 2010;340:b5465. doi: 10.1136/bmj.b5465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyoshi M., Miyano K., Moriyama N., Tangiguchi M., Watanabe T. Angiotensin type 1 receptor antagonist inhibits lipopolysaccharide-induced stimulation of rat microglial cells by suppressing nuclear factor kappaB and activator protein-1 activation. Eur. J. Neurosci. 2008;27:343–351. doi: 10.1111/j.1460-9568.2007.06014.x. [DOI] [PubMed] [Google Scholar]
- Ohshima K., Mogi M., Horiuchi M. Therapeutic approach for neuronal disease by regulating renin-angiotensin system. Curr. Hypertens. Rev. 2013;9:99–107. doi: 10.2174/15734021113099990004. [DOI] [PubMed] [Google Scholar]
- Ponath G., Park C., Pitt D. The role of astrocytes in Multiple sclerosis. Front. Immunol. 2018;9:217. doi: 10.3389/fimmu.2018.00217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roebuck K. A., Finnegan A. Regulation of intercellular adhesion molecules-1 (CD54) gene expression. J. Leukoc. Biol. 1999;66:876–888. doi: 10.1002/jlb.66.6.876. [DOI] [PubMed] [Google Scholar]
- Saavedra J. M. Angiotensin II AT(1) receptor blockers as treatments for inflammatory brain disorders. Clin. Sci. 2012;123:567–590. doi: 10.1042/CS20120078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saavedra J. M., Benicky J., Zhou J. Angiotensin II: multitasking in the brain. J. Hypertens. Suppl. 2006;24:S131–S137. doi: 10.1097/01.hjh.0000220418.09021.ee. [DOI] [PubMed] [Google Scholar]
- Song K. H., Park J. H., Jo I., Park J. Y., Seo J., Kim S. A., Cho D. H. Telmisartan attenuates hyperglycemia-exacerbated VCAM-1 expression and monocytes adhesion in TNF-α-stimulated endothelial cells by inhibiting IKKβ expression. Vasc. Pharmacol. 2016;78:43–52. doi: 10.1016/j.vph.2015.10.001. [DOI] [PubMed] [Google Scholar]
- Sweeney M. D., Zhao Z., Montagne A., Nelson A. R., Zlokovic B. V. Blood-brain barrier: from physiology to disease and back. Physiol. Rev. 2019;99:21–78. doi: 10.1152/physrev.00050.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torika N., Asraf K., Danon A., Apte R. N., Fleisher-Berkovich S. Telmisartan modulates glial activation: in vitro and in vivo studies. PLoS ONE. 2016;11:e0155823. doi: 10.1371/journal.pone.0155823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torika N., Asraf K., Cohen H., Fleisher-Berkovich S. Intranasal telmisartan ameliorates brain pathology in five familial Alzheimer's disease mice. Brain Behav. Immun. 2017;64:80–90. doi: 10.1016/j.bbi.2017.04.001. [DOI] [PubMed] [Google Scholar]
- Tsukuda K., Mogi M., Iwanami J., Min L. J., Sakata A., Jing F., Iawi M., Horiuchi M. Cognitive deficit in amyloid-beta-injected mice was improved by pretreatment with a low dose of telmisartan partly because of peroxisome proliferator-activated receptor-gamma activation. Hypertension. 2009;54:782–787. doi: 10.1161/HYPERTENSIONAHA.109.136879. [DOI] [PubMed] [Google Scholar]
- Verbeek M. M., Otte-Höller I., Westphal J. R., Wesseling P., Ruiter D. J., de Waal R. M. Accumulation of intercellular adhesion molecule-1 in senile plaques in brain tissue of patients with Alzheimer's disease. Am. J. Pathol. 1994;144:104–116. [PMC free article] [PubMed] [Google Scholar]
- Villapol S., Saavedra J. M. Neuroprotective effects of angiotensin receptor blockers. Am. J. Hypertens. 2015b;28:289–299. doi: 10.1093/ajh/hpu197. [DOI] [PubMed] [Google Scholar]
- Wang J., Ho L., Chen L., Zhao Z., Zhao W., Qian X., Humala N., Seror I., Bartholomew S., Rosendorff C., Pasinetti G. M. Valsartan lowers brain beta-amyloid protein levels and improves spatial learning in a mouse model of Alzheimer disease. J. Clin. Invest. 2007;117:3393–3402. doi: 10.1172/JCI31547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Cao J., Fan Y., Xie Y., Xu Z., Yin Z., Gao L., Wang C. Artemisinin inhibits monocyte adhesion to HUVECs through the NF-κB and MAPK pathways in vitro. Int. J. Mol. Med. 2016;37:1567–1575. doi: 10.3892/ijmm.2016.2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winker E. A., Sengillo J. D., Bell R. D., Wang J., Zlokovic B. V. Blood-spinal cord barrier pericyte reductions contribute to increased capillary permeability. J. Cereb. Blood Flow Metab. 2012;32:1841–1852. doi: 10.1038/jcbfm.2012.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y., Xu Y., Wang Y., Wang Y., He L., Jiang Z., Huang Z., Liao H., Li J., Saavedra J. M., Zhang L., Pang T. Telmisartan prevention of LPS-induced microglia activation involves M2 microglia polarization via CaMKKβ-dependent AMPK activation. Brain Behav. Immun. 2015;50:298–313. doi: 10.1016/j.bbi.2015.07.015. [DOI] [PubMed] [Google Scholar]