Abstract
Pneumococcal conjugate vaccines have been successful, but their use has increased infections by nonvaccine serotypes. Oral streptococci often harbor capsular polysaccharide (PS) synthesis loci (cps). Although this has not been observed in nature, if pneumococcus can replace its cps with oral streptococcal cps, it may increase its serotype repertoire. In the current study, we showed that oral Streptococcus strain SK95 and pneumococcal strain D39 both produce structurally identical capsular PS, and their genetic backgrounds influence the amount of capsule production and shielding from nonspecific killing. SK95 is avirulent in a well-established in vivo mouse model. When acapsular pneumococcus was transformed with SK95 cps, the transformant became virulent and killed all mice. Thus, cps from oral Streptococcus strains can make acapsular pneumococcus virulent, and interspecies cps transfer should be considered a potential mechanism of serotype replacement. Our findings, along with publications from the US Centers for Disease Control and Prevention, highlight potential limitations of the 2013 World Health Organization criterion for studying pneumococcal serotypes carried without isolating bacteria.
We show that an oral streptococcal strain, SK95, and a pneumococcal strain, D39, both produce chemically identical capsular PS. We also show that transferring SK95 cps into noncapsulated, avirulent pneumococcus gave it the capacity for virulence in a mouse model.
Keywords: pneumococcus, vaccine, serotype
(See the Editorial Commentary by Beall, on pages 343–6.)
Streptococcus pneumoniae (the pneumococcus) is often carried in the nasopharynx without clinical symptoms but, in susceptible individuals, it can invade and cause serious infections such as bacteremia, meningitis, and pneumonia. Pneumococcal infections account for a large number of deaths among young children and elderly adults [1, 2]. A critical factor for development of invasive pneumococcal disease is the presence of a polysaccharide (PS) capsule, which protects against opsonophagocytosis. S. pneumoniae has a genetic locus (cps locus) composed of variable combinations of genes that encode approximately 100 antigenically distinct capsule types [3]. Antibodies against the specific PS capsule carried by the bacteria can provide effective protection to the host by opsonizing pneumococci for phagocytes. Current pneumococcal vaccines are designed to elicit antibodies to the capsule types that most commonly cause invasive diseases. Widely used pneumococcal conjugate vaccines (PCVs) contain 10–13 different capsule types, and their use has greatly reduced the occurrence of invasive pneumococcal diseases [4, 5].
Widespread use of PCVs has dramatically altered the pneumococcal serotypes now responsible for invasive pneumococcal diseases by reducing the carriage rates of and conferring resistance against the included vaccine capsule types, but with a concomitant increase in nonvaccine types [6, 7]. This phenomenon is called serotype shift and has been attributed to several mechanisms [8]. One mechanism is unmasking of the serotypes that were present as a minor subpopulation before the disappearance of the vaccine serotype that was dominant. The other is serotype switch as a result of capturing cps from another pneumococcus [9]. A third, but so far unobserved, possibility is that pneumococci may capture in part, or entirety, cps from other species [10]. In the latter circumstance, the most relevant donor species are oral streptococci, which are avirulent, genetically similar to pneumococci, and coexist with pneumococci in the nasopharynx [11].
Current evidence indicates that a majority of oral streptococci contain pneumococcus-like cps loci and actually produce capsular PSs that resemble pneumococcal capsule [12–15]. For instance, SK95 is an oral streptococcal isolate belonging to Streptococcus oralis subsp. dentisani and has a cps that is very similar to pneumococcal capsule type 2 (94% identity in the syntenic region of the capsule locus) [13]. Similarly, capsule type 5 may be among oral streptococci including Streptococcus pseudopneumoniae [16, 17]. Thus, if an interspecies genetic transfer occurs, it could significantly complicate serotype shift that follows the long-term use of PCVs.
Despite general similarity, oral streptococcal cps loci differ from pneumococcal cps by having aliC, aliD, and other genes in addition to random sequence differences [13]. Those small differences may have critical impacts on virulence because a small change in the control of capsule production can starkly reduce the capsule's role in increasing pneumococcal virulence [18, 19]. In addition, even a single nucleotide change can drastically alter the chemical structure of the capsule [3]. Therefore, we have directly investigated the impact of oral streptococcal cps on pneumococcal virulence, by examining whether SK95 produces capsular PS identical to pneumococcal serotype 2, and whether SK95 cps can convert a nonvirulent acapsular pneumococcus into a virulent encapsulated pneumococcus.
METHODS
Capsular PS Isolation
Bacteria were grown overnight at 37°C in 1 L of chemically defined medium, as described elsewhere [20]. Bacteria were pelleted, washed, and treated with proteinase K. Capsular PS was released from the bacteria by an overnight incubation at 37°C with 2000 U of mutanolysin and 20 mg of lysozyme. The capsular PS was purified by means of anion exchange chromatography, and the PS-containing fractions were determined through an inhibition enzyme-linked immunosorbent assay (ELISA), which was performed as described elsewhere [21] using Hyp2M2 monoclonal antibody (mAb) [22]. The capsule was further purified by passing the pool through a size-exclusion column (Superose 6; GE Healthcare) and the fractions containing capsule were pooled, dialyzed, and lyophilized. (See Supplementary Material)
Nuclear Magnetic Resonance Spectroscopy
Nuclear magnetic resonance (NMR) data were collected at 50°C on a Bruker Avance II spectrometer 1H, 700.0 MHz) equipped with a cryogenic probe. Complete assignments of 1H and 13C signals were achieved by means of 2-dimensional double-quantum-filtered correlation spectroscopy (COSY), total correlation spectroscopy (TOCSY), nuclear overhauser effect spectroscopy (NOESY), multiplicity-edited 1H-13C heteronuclear single quantum correlation spectroscopy (HSQC), HSQC-TOCSY, and HSQC-NOESY. Characteristic 1H and 13C chemical shifts, described in previous studies [23, 24], were used to identify each sugar residue. MR data were processed using Topspin version 3.6 or NMRPipe software [25] in combination with hmsIST programs to reconstruct non-uniform sampling (NUS) data [26] (see Supplementary Material)
Molecular Weight Determination
Purified capsule or dextran analytical standard samples were analyzed on a 40-mL chromatographic column packed with Sephacryl S 500 resin. The eluted fractions were analyzed for PS content by anthrone assay [27] (see Supplementary Material)
Sandwich Immunoassay
To test linkage between capsular PS and teichoic acid, a sandwich-type assay was performed, as described elsewhere [20]. Briefly, ELISA plate wells were coated with an mAb antiphosphocholine antibody (HPCG2b; 1 μg/mL in phosphate-buffered saline) and tested with various dilutions of D39 capsular PS, SK95 capsular PS, and teichoic acid (CWPS1 [3]; Staten Serum Institute). The bound serotype 2 capsule was detected with a type 2–specific mAb, Hyp2M2 [28]. All reactions were done in triplicate.
Construction of Isogenic Strains
Isogenic strains were constructed using the Janus cassette (JS) [29, 30]. Briefly, the TIGR-JS strain containing the Janus cassette [29, 31] was mixed with D39 or SK95 cell lysates, in competence medium (Todd-Hewitt broth plus 0.5% yeast extract, 0.2% bovine serum albumin, 0.01% calcium chloride, and competence stimulating peptide 1 at 50 ng/μL). Transformants were then selected on Todd Hewitt broth plus 0.5% Yeast extract (THY) agar plates containing streptomycin and were back-crossed 3 times with TIGR-JS. Each transformant was tested for serotype using agglutination with type-specific antiserum [32], surface phenotype by flow cytometry [21] with type 2–specific immunoglobulin M mAb (Hyp2M2) [28], and genetic markers using polymerase chain reaction targeting cpsA/wzg, cpsB/wzh, and cpsD/wze genes.
Nonspecific Killing Assay
The well-characterized University of Alabama at Birmingham opsonophagocytic killing assay [33] (described in detail elsewhere [34]) was adapted to determine nonspecific killing. Briefly, 30 μL of bacteria suspended in opsonization buffer b (OBB; Hanks buffer supplemented with 0.1% gelatin and 5% fetal calf serum) was mixed with 10 μL of baby rabbit serum (BRS) of specified concentration, and 40 μL of differentiated HL60 cells (1.0 × 107 cells/mL) in OBB. To determine whether the bacteria are killed by phagocytosis, some HL60 cells were preincubated with 10 μmol/L cytochalasin D (Sigma-Aldrich) for 30 minutes at room temperature. The mixture was incubated with shaking (700 rpm) for 45 minutes at 37°C with 5% carbon dioxide. Then 10 μL of reaction mixture was plated on THY agar plates, and, after an overnight incubation, the bacterial colonies were counted. Nonspecific killing was calculated by the following formula: nonspecific killing (%) = [1 − (colony-forming units [CFUs] in control B/CFUs in control A)] × 100, where control A contains heat-inactivated BRS and control B, active BRS. All reactions were done in triplicate.
Animal Infection
Female, 5–6 week-old BALB/cJ mice (Jackson Laboratory) were challenged with 1.0 × 103S. pneumoniae (D39), 1.0 × 104S. oralis (SK95), 1.0 × 105 acapsular pneumococcus (TIGR-JS), or isogenic strains (FG34, FG44, FG3, and FG4) in 100 µL of phosphate-buffered saline, by intraperitoneal infection. Bacteremia was assessed with a tail bleed after 6 or 24 hours, and animal status/health was checked every 6 hours by assessing the body score index. When mice were deemed moribund, they were then checked every 30–60 minutes to assess the time of death. All animal experiments were reviewed and approved by the Institutional Animal Care and Use Committee at The University of Alabama at Birmingham (protocol IACUC-20175). Animal care and experimental protocols adhered to public law 89–544 (Animal Welfare Act) and its amendments, Public Health Services guidelines, and the Guide for the Care and Use of Laboratory Animals (US Department of Health and Human Services).
RESULTS
S. oralis SK95 Capsule Structure Is Identical to Pneumococcal Serotype 2 Capsule
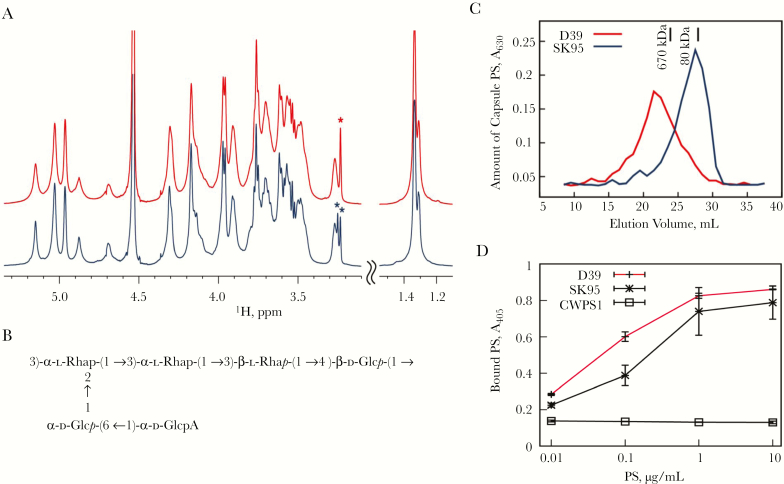
To elucidate the chemical basis for the serologic cross-reactivity to type 2 capsule for SK95 [13], we used MR spectroscopy to determine the structure of SK95 capsular PS. The 1H MR spectra of purified capsular PS from SK95 and D39 were identical (Figure 1A), suggesting that SK95 and D39 produce identical capsular PS. Both spectra showed 1H signals arising from choline (chemical shifts at 3.23 and 3.25 ppm, marked with an asterisk in Figure 1A), suggesting the presence of teichoic acid. Teichoic acid is often present in pneumococcal capsule preparations because both the capsule and teichoic acid are covalently linked to peptidoglycan [35].
Figure 1.
Structure of type 2 capsule polysaccharide (PS). A, Overlay of partial (1H) Nuclear magnetic resonance (NMR) spectra of capsular PSs from type 2 Streptococcus pneumoniae (D39) (red, top) and Streptococcus oralis (SK95) (blue, bottom) recorded at 50°C. As shown, spectra are identical, but the signals of D39 are broader than those of SK95. Asterisks mark choline signals arising from teichoic acid. To facilitate presentation, the 2 spectral regions are plotted with different vertical scales. B, Structure of capsular PS from S. oralis (SK95) as determined with NMR spectroscopy. C, Amount of capsular PSs from SK95 (blue) and D39 (red) versus elution volumes of an S-500 column. Elution volumes of dextran molecular weight standards are indicated with vertical bars. D, Amount of type 2 capsule (y-axis) bound to the enzyme-linked immunosorbent assay (ELISA) plate at different amounts of PS (x-axis). ELISA wells were coated with antiphosphocholine antibody and captured PSs were determined with a monoclonal antibody (mAb) specific for type 2 PS (Hyp2M2). The mAb supernatant was diluted at 1:320, 1:160, and 1:80, respectively, for D39, SK95, and CWPS1 (teichoic acid) wells. Error bars at the data points indicate standard deviations (error bars for CWPS1 were too small to be seen). Abbreviations: A405, absorbance at 405 nm; A630, absorbance at 630 nm.
To completely delineate SK95 capsular PS structure, the 1H and 13C chemical shifts of SK95 PS were obtained using a set of 2-dimensional MR studies (Table 1). When all the chemical shifts were identified, the SK95 capsule structure was found to be identical to the published structure of type 2 capsular PS (Figure 1B) [36]. The D39 capsule structure was partially determined because the MR signals of D39 capsule were noticeably broader [37]. The partial structure of D39 was identical to the published structure of serotype 2 [36], and also to the SK95 structure. The broad MR signals suggest that the D39 capsule is longer than the SK95 capsule (ie, consisting of more identical oligosaccharide repeats). Indeed, the molecular weight of the purified D39 capsule was much larger than that from SK95 (Figure 1C).
Table 1.
Proton and Carbon Chemical Shifts of Capsular Polysaccharide From SK95
| Chemical Shift, ppm | ||||||
|---|---|---|---|---|---|---|
| Sugar Residue | H1/C1 | H2/C2 | H3/C3 | H4/C4 | H5/C5 | H6/CH3 |
| →3)-α-l-Rhap-(1→ | 5.13 | 4.29 | 4.09 | 3.69 | 3.89 | 1.32 |
| 2 ↑ | 104.6 [170 Hz]a | 81.1 | 83.1 | 76.3 | 74.1 | 21.2 |
| →3)-α-l-Rhap-(1→ | 5.02 | 4.15 | 3.95 | 3.55 | 3.88 | 1.29 |
| 106.8 [171 Hz] | 74.7 | 83.1 | 76.1 | 73.9 | 21.3 | |
| →6)-α-d-Glcp-(1→ | 5.01 | 3.55 | 3.75 | 3.66 | 4.28 | 4.12, 3.71 |
| 102.4 [171 Hz] | 76.0 | 77.9 | 73.8 | 75.0 | 69.9 | |
| α-d-GlcpA-(1→ | 4.95 [2.4 Hz] | 3.59 | 3.74 | 3.52 | 3.94 | … |
| 102.9 [169 Hz] | 76.2 | 77.7 | 76.8 | 77.0 | 181.1 | |
| →3)-β-l-Rhap-(1→ | 4.86 | 4.15 | 3.64 | 3.46 | 3.43 | 1.32 |
| 105.2 [163 Hz] | 75.2 | 85.1 | 76.1 | 76.7 | 21.4 | |
| →4)-β-d-Glcp-(1→ | 4.67 [6.3 Hz] | 3.25 | 3.60 | 3.59 | 3.48 | 3.96, 3.78 |
| 109.0 [161 Hz] | 78.6 | 80.5 | 81.9 | 79.3 | 65.9 | |
aAnomeric one-bond 13C-1H and resolved three-bond 1H-1H J couplings are given in brackets.
To examine whether the SK95 capsule is linked to teichoic acid, as pneumococcal capsule is, we performed a sandwich-type ELISA, which determined the amount of type 2 capsule captured with antiphosphocholine antibody. The ELISA captured type 2 PS from D39 and SK95 capsules in a dose-dependent manner (Figure 1D). In contrast, our negative control of purified teichoic acid (CWPS1) did not produce any signals. Thus, SK95 capsule is anchored to teichoic acid and to peptidoglycan.
Amount of Capsule Produced by SK95 and D39 cps Loci Is Dependent on Genetic Background
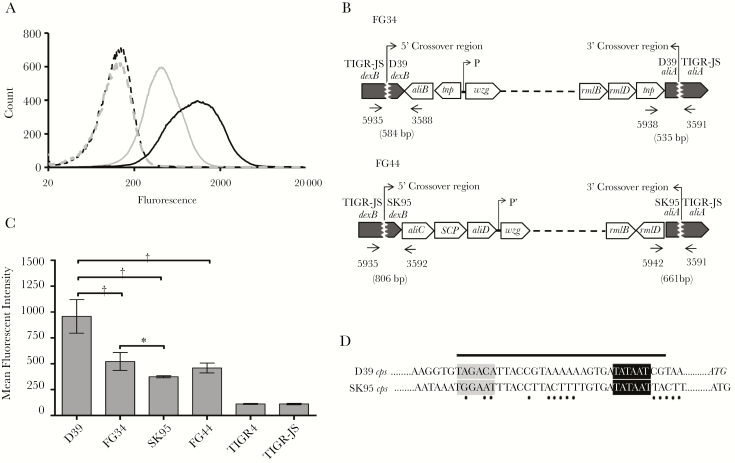
The amount of capsule anchored to SK95 and D39 was then compared by means of flow cytometry. D39 was found to display more capsule on its surface than SK95 in multiple experiments (Figure 2A). To investigate whether the amount of capsule is determined by cps or other genes, we transferred D39 or SK95 cps into TIGR-JS, a noncapsulated variant of TIGR4. Resulting transformants with D39 cps or SK95 cps were named FG34 or FG44, respectively. Genetic mapping showed that the transformants acquired the entire cps flanked by dexB and aliA (Figure 2B and Supplementary Tables 1 and 2). In the case of FG44, it acquired aliC, a gene encoding a SCP domain protein, and aliD genes in addition to the 17 genes needed to produce type 2 capsule. FG34 displayed much less capsule than D39 (P < .001) and FG44 also displayed measurably more capsule than SK95, although the difference was not statistically significant (P = .07) (Figure 2C). In contrast, FG34 and FG44 had similar amounts of capsule on their surface (P = .17). Taken together, within these transformants, the amount of capsule production was more influenced by the genetic background (eg, TIGR-JS background vs D39) than the species origin of cps loci.
Figure 2.
Type 2 capsule expression in wild type and isogenic strains. A, Flow cytometric analysis of capsule expression. Histograms depict binding of monoclonal antibody (mAb) Hyp2M2 to strains D39 (solid black line) and SK95 (solid gray line). The corresponding dashed lines show control binding (secondary antibody alone). B, Genetic map of the cps region of FG34 (TIGR-JS transformant, with D39 cps) and FG44 (TIGR-JS transformant, with SK95 cps). The 5´ crossover points for FG34 and FG44 are between bases 316624–316729 and 316754–316814 of the TIGR4 genome, respectively (see Supplementary Table 2 for details). Arrows and 4-digit numbers indicate binding positions of the polymerase chain reaction (PCR) primers (Supplementary Table 1); numbers in parentheses, expected sizes of the PCR products; and P and P′, promoter locations. C, Mean fluorescent intensity of type 2 capsule expression for indicated strains. Experiments were performed 6 times, and error bars represent standard errors. *P < .01; †P < .001. D, Sequences of the core promoter regions (black bar) of D39 and SK95 (GenBank accession nos. CP000410.2 [41] and AFUB01000057.1 [13]). Gray- and black-shaded regions indicate core promoter motifs at positions −35 and −10, respectively; ATG, translation start codons of cpsA; and black dots, nucleotide mismatch in the core promoter region between D39 and SK95 cps.
S. oralis cps Can Provide Shielding From Nonspecific Phagocytosis to Noncapsulated Pneumococci
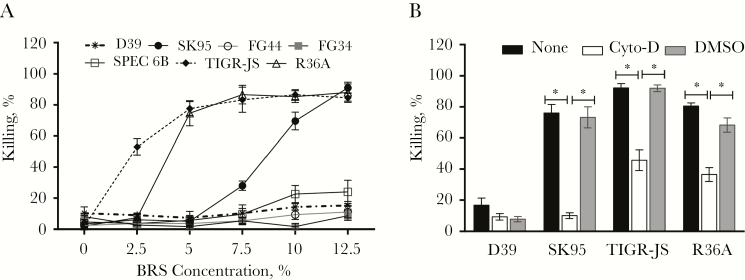
A major function of capsule is to shield bacteria from nonspecific complement deposition and opsonophagocytosis. Indeed, noncapsulated pneumococci (TIGR-JS and R36A) could be killed, essentially completely (about 80% killing), by phagocytes in the presence of 5% of BRS (Figure 3A). In contrast, only 10% of D39 were killed, even when BRS levels were raised to 12.5%. SK95 was more resistant to killing than noncapsulated pneumococci, but about 60% was killed with 10% BRS (Figure 3A). The killing of SK95 is dependent on phagocytosis, because this killing could be almost completely abrogated with 10 μmol/L cytochalasin D (Figure 3B). Heat-inactivated BRS did not kill any bacteria (data not shown). Thus, even though SK95 produces type 2 capsule, similar to D39, SK95 is susceptible to nonspecific complement-mediated phagocytosis. Just like other encapsulated pneumococcal strains, <10% of both FG34 and FG44 were killed, even at 12.5% BRS (Figure 3A). Thus, SK95 cps was as effective as D39 cps in shielding pneumococci from phagocytes.
Figure 3.
Nonspecific killing (NSK) and inhibition of phagocytosis. A, NSK of various bacterial strains in the presence of different baby rabbit serum (BRS) concentrations. TIGR-JS and R36A are nonencapsulated control strains. FG34 and FG44 are TIGR-JS transformants with D39 cps and SK95 cps, respectively. SPEC1, TIGR4, and TREP19A are encapsulated pneumococcal isolates [33], and their killing curves lie between FG34 and SPEC6B, and are not shown for the purpose of clarity. B, NSK of bacterial isolates (labeled on x-axis) with HL60 cells treated with OBB alone (white bar), OBB with 10 µmol/L cytochalasin D (Cyto-D) (black bar), or OBB with 1% dimethyl sulfoxide (DMSO) control (gray bar) at 10% BRS. Error bars indicate means of 3 replicates with standard errors. *P < .001.
S. oralis cps Can Make Nonvirulent Noncapsulated Pneumococci Virulent
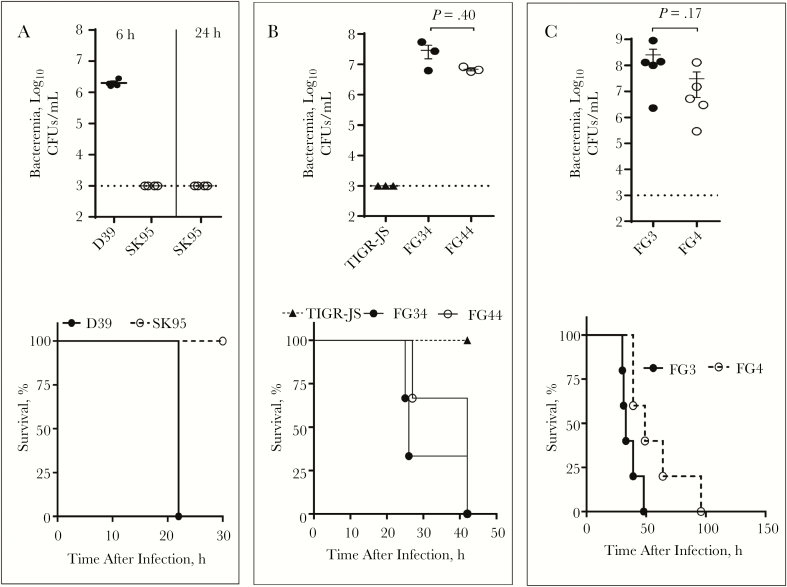
To directly test the virulence of the transformants in vivo, we intraperitoneally infected young female BALB/cJ mice with either 103 CFUs of D39 or 104 CFUs of SK95 per mouse. Mice are highly susceptible to this route of challenge, and this is a long-established model of pneumococcal bacteremia and sepsis. Within 6 hours of infection, SK95 was undetectable (<103 CFUs/mL) in the blood, but D39 caused a high level (approximately 2x106 CFUs/mL) of bacteremia. Within 24 hours, D39 infection killed all mice but SK95 infection had killed none (P = .045) (Figure 4A). The difference in virulence was even more striking when considering that SK95 challenged mice were injected with 10 times more CFUs than D39 challenged animals.
Figure 4.
In vivo virulence study of D39 and SK95 cps. A, Bacteremia at 6 and 24 hours, and survival of BALB/cJ mice that were intraperitoneally infected with either D39 or SK95 strains (4 mice per group). B, Bacteremia and survival of mice after intraperitoneal infection with acapsular pneumococcus (TIGR-JS, 4 mice/group), pneumococcus strain TIGR4-JS with D39 cps (FG34; 3 mice per group), and TIGR4-JS with SK95 cps (FG44; 3 mice per group). C, Bacteremia and survival of mouse after intraperitoneal infection with TIGR-JS with D39 cps (FG3) or with SK95 cps (FG4). Each group has 5 mice. Infection doses per mice were 103 colony-forming units (CFUs) of D39, 104 CFUs of SK95, or 105 CFUs of TIGR-JS, FG34, FG44, FG3, and FG4. B, C, For bacteremia, errors bars represent standard errors of the mean, and t test were used. Survival curves were compared using log-rank (Mantel-Cox) tests: P = .045 for D39 versus SK95 (A); P = .01 for both TIGR-JS versus FG34 and TIGR-JS versus FG44 (B); P = .02 for FG34 versus FG44 (C). h, hours.
To directly compare the impact of SK95 cps on virulence, we infected mice with 105 CFUs of noncapsulated pneumococcus (TIGR-JS), TIGR-JS transformed with D39 cps (FG34), and TIGR-JS transformed with SK95 cps (FG44). As shown in Figure 4B, TIGR-JS killed no mice but both FG34 and FG44 killed all animals within 2 days (P < .01 for both). To confirm that the experimental result was not specific to a single transformation event, this experiment was repeated using clones from a different transformation experiment (FG3 and FG4). Again, the results showed that both FG strains have comparable virulence in causing bacteremia and killing the infected mice (Figure 4C). For both experiments (Figure 4B and 4C), D39 and SK95 cps showed similar bacteremia levels, but D39 cps killed slightly faster than SK95 cps. Nevertheless, it is clear that SK95 cps has the potential to make acapsular pneumococcus become virulent.
DISCUSSION
Although capsules (“coaggregation receptor PSs”) of oral streptococci were described some time ago [12] and have been proposed to be involved in adherence to other bacteria [38], there is now an increasing appreciation of the capsular PSs produced by oral streptococci [13–15]. More recently, genomic studies have shown that >74% of oral Streptococcus isolates have pneumococcuslike cps, except for S. pseudopneumoniae [13]. Serologic similarities between pneumococcal and oral streptococcal capsular PSs have been shown elsewhere [13, 15–17, 39]. However, it was unknown whether oral streptococcal capsule was chemically identical to that of pneumococci. In the current study, we show that SK95 produces capsular PS that is chemically identical to highly virulent, pneumococcal serotype 2 capsule, although it is shorter than the D39 capsule. Although the SK95 capsule is anchored to peptidoglycan, as pneumococcal capsule is, SK95 produces less capsule than D39, consistent with previously reported thin capsules of oral streptococci [40] with inadequate shielding [13]. Studies with FG44 indicate that genetic background (ie, non-cps genes) significantly influences the amount of capsule production.
We were surprised to find that SK95 cps is as effective as is D39 cps in increasing virulence of acapsular pneumococci. Our finding was unexpected because prior studies have demonstrated that a slight difference in the control of capsule production greatly reduced resistance to in vitro phagocytosis or to the in vivo virulence of a pneumococcus strain. For instance, a slight sequence variation in cpsE/wchA [19] or small changes in the cps core promoter [18] starkly reduces in vivo virulence, and the core promoter region is highly conserved among pneumococcal isolates [41]. Although similar, oral streptococcal and pneumococcal cps loci do have significant differences. The 2 species have different core promoter sequences (Figure 2D). In addition, oral streptococcal cps has aliC and aliD genes, and occasionally other genes, such as a gene encoding a SCP domain protein in SK95, which are absent in most pneumococcal cps. Thus, in view of the differences, it is surprising that SK95 cps was as effective as D39 cps in increasing virulence. This finding is important, because it indicates that oral streptococci, even though nonpathogenic, can serve for S. pneumoniae as genetic reservoirs of the cps, the key virulence factor.
The potential of such an interspecies cps transfer, perhaps facilitated by PCV usage, is important for several reasons. First oral streptococci have been considered a genetic reservoir of allelic variants of virulence genes for pneumococci [10], including the antibiotic resistance genes that appeared in pneumococci after long-term use of antibiotics [42, 43]. Even though interspecies cps transfer has not been observed yet, it may occur, because their cps loci share similar genomic architecture in general, and are often syntenic. Second, interspecies cps transfers should be considered in interpreting epidemiologic observations. For instance, serotype 2 infections have appeared in several areas after a long global absence [44, 45]. One should consider whether the recent epidemics are from pneumococcal acquisition of type 2 cps from oral streptococci. Third, oral streptococci are genetically more diverse than pneumococci, and oral streptococci should harbor many unique and distinct cps that can be potentially transferred to pneumococci [10, 13]. We need not only to continue monitoring serotype shift but also to expect the appearance of unusual new serotypes and increase our knowledge of capsule types produced by oral streptococci as a part of our long-term strategy of using PCVs.
Conversely, immunizations with PCVs may reduce the prevalence of oral streptococci expressing the vaccine capsule type and the coaggregating bacteria, thereby leading to changes in the oral microbiome [46]. Several studies reported that adults who were vaccinated with 13-valent PCV were less likely to have pneumococci vaccine-type genes detected in their specimens than those who were unvaccinated [15, 47, 48]. In a response to serotype replacement, many companies are developing PCVs with 15 [49], 20 [50], or more serotypes. As we anticipate deployment of these next-generation PCVs, this converse possibility is a matter of increasing interest. The long-term consequence of oral microbiome change for oral health is unclear and may have important health implications. As such, the impact of PCV usage on the oral microbiome requires study.
Finally, our finding has another important implication for studying nasopharyngeal carriage of pneumococci. The current World Health Organization recommendation for carriage studies states that nasopharyngeal swab samples can be directly examined for certain pneumococcal genes (including capsule genes) without first obtaining bacterial isolates [51]. Based on the recommended criterion, a study found that PCV may reduce pneumococcal carriage of vaccine type strains among adults [47]. However, because oral streptococci can have pneumococcuslike cps, the PCV may have reduced carriage of oral streptococci expressing vaccine serotypes instead of pneumococci. Several studies from the US Centers for Disease Control and Prevention have noted the presence of pneumococcuslike cps among oral streptococci and have cautioned against using the 2013 World Health Organization recommended approach [14–17]. Our findings further support the caution from the Centers for Disease Control and Prevention, and future studies of pneumococcal carriage should consider the possibility that oral streptococci not only can have pneumococcuslike cps but also can produce pneumococcal capsule itself. Moreover, because oral streptococci and pneumococci are so similar in capsule production, the source of a pneumococcal capsule would not be identified without examining the bacterial isolates.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author contributions. M. H. N. supervised the group, devised and executed the project, had full access to all study data, and takes responsibility for the integrity and accuracy of the data analysis, and contributed to drafting and revision of the manuscript. M. K., C. J. O., and J. S. S. helped with the conceptualization, experimental design, and critical revision of the manuscript. T. B., J. V., and F. G. performed the experiments, performed data analysis and interpretation, and contributed to the critical revision of the manuscript.
Financial support. This work was supported by the O'Neal Comprehensive Cancer Center at the University of Alabama at Birmingham (funded by National Cancer Institute grant P30 CA013148) for the high-field-strength magnetic resonance facility, and by the National Institutes of Health (grants AG-050607 and HHSN272201200005C to M. H. N. and AI114800 to C. J. O.).
Potential conflicts of interest. The University of Alabama at Birmingham has intellectual property rights to several opsonophagocytosis assay reagents developed in M. H. N.'s laboratory. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Drijkoningen JJC, Rohde GGU. Pneumococcal infection in adults: burden of disease. Clin Microbiol Infect 2014; 20:45–51. [DOI] [PubMed] [Google Scholar]
- 2. World Health Organization. Pneumococcal conjugate vaccine for childhood immunization—WHO position paper. Wkly Epidemiol Rec 2007; 82:93–104. [PubMed] [Google Scholar]
- 3. Geno KA, Gilbert GL, Song JY, et al. Pneumococcal capsules and their types: past, present, and future. Clin Microbiol Rev 2015; 28:871–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lee LH, Gu XX, Nahm MH. Towards new broader spectrum pneumococcal vaccines: the future of pneumococcal disease prevention. Vaccines (Basel) 2014; 2:112–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Diawara I, Zerouali K, Katfy K, et al. Invasive pneumococcal disease among children younger than 5 years of age before and after introduction of pneumococcal conjugate vaccine in Casablanca, Morocco. Int J Infect Dis 2015; 40:95–101. [DOI] [PubMed] [Google Scholar]
- 6. Hausdorff WP, Hanage WP. Interim results of an ecological experiment—conjugate vaccination against the pneumococcus and serotype replacement. Hum Vaccin Immunother 2016; 12:358–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Park SY, Moore MR, Bruden DL, et al. Impact of conjugate vaccine on transmission of antimicrobial-resistant Streptococcus pneumoniae among Alaskan children. Pediatr Infect Dis J 2008; 27:335–40. [DOI] [PubMed] [Google Scholar]
- 8. Andam CP, Hanage WP. Mechanisms of genome evolution of Streptococcus. Infect Genet Evol 2015; 33:334–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wyres KL, Lambertsen LM, Croucher NJ, et al. Pneumococcal capsular switching: a historical perspective. J Infect Dis 2013; 207:439–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kilian M, Riley DR, Jensen A, Brüggemann H, Tettelin H. Parallel evolution of Streptococcus pneumoniae and Streptococcus mitis to pathogenic and mutualistic lifestyles. MBio 2014; 5:e01490–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Do T, Jolley KA, Maiden MC, et al. Population structure of Streptococcus oralis. Microbiology 2009; 155:2593–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Abeygunawardana C, Bush CA, Cisar JO. Complete structure of the cell surface polysaccharide of Streptococcus oralis C104: a 600-MHz NMR study. Biochemistry 1991; 30:8568–77. [DOI] [PubMed] [Google Scholar]
- 13. Sorensen UB, Yao K, Yang Y, Tettelin H, Kilian M. Capsular polysaccharide expression in commensal Streptococcus species: genetic and antigenic similarities to Streptococcus pneumoniae. mBio 2016; 7: e01844-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Carvalho Mda G, Pimenta FC, Moura I, et al. Non-pneumococcal mitis-group streptococci confound detection of pneumococcal capsular serotype-specific loci in upper respiratory tract. PeerJ 2013; 1:e97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lessa FC, Milucky J, Rouphael NG, et al. Streptococcus mitis expressing pneumococcal serotype 1 capsule. Sci Rep 2018; 8:17959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pimenta F, Gertz RE Jr, Park SH, et al. Streptococcus infantis, Streptococcus mitis, and Streptococcus oralis strains with highly similar cps5 loci and antigenic relatedness to serotype 5 pneumococci. Front Microbiol 2018; 9:3199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Garriss G, Nannapaneni P, Simoes AS, et al. Genomic characterization of the emerging pathogen Streptococcus pseudopneumoniae. mBio 2019; 10: e01286-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shainheit MG, Mule M, Camilli A. The core promoter of the capsule operon of Streptococcus pneumoniae is necessary for colonization and invasive disease. Infect Immun 2014; 82:694–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shainheit MG, Valentino MD, Gilmore MS, Camilli A. Mutations in pneumococcal cpsE generated via in vitro serial passaging reveal a potential mechanism of reduced encapsulation utilized by a conjunctival isolate. J Bacteriol 2015; 197:1781–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Oliver MB, van der Linden MP, Kuntzel SA, Saad JS, Nahm MH. Discovery of Streptococcus pneumoniae serotype 6 variants with glycosyltransferases synthesizing two differing repeating units. J Biol Chem 2013; 288:25976–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bratcher PE, Kim KH, Kang JH, Hong JY, Nahm MH. Identification of natural pneumococcal isolates expressing serotype 6D by genetic, biochemical and serological characterization. Microbiology 2010; 156:555–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yu J, Lin J, Benjamin WH Jr, Waites KB, Lee CH, Nahm MH. Rapid multiplex assay for serotyping pneumococci with monoclonal and polyclonal antibodies. J Clin Microbiol 2005; 43:156–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bock K, Pedersen C. Carbon-13 nuclear magnetic resonance spectroscopy of monosaccharides. In: Tipson RS, Horton D, eds. Advances in carbohydrate chemistry and biochemistry. Vol 41 United States: Academic Press, 1983:27–66. [Google Scholar]
- 24. Jansson PE, Kenne L, Widmalm G. Computer-assisted structural analysis of polysaccharides with an extended version of CASPER using 1H- and 13C-n.m.r. data. Carbohydr Res 1989; 188:169–91. [DOI] [PubMed] [Google Scholar]
- 25. Delaglio F, Grzesiek S, Vuister GW, Zhu G, Pfeifer J, Bax A. NMRPipe: a multidimensional spectral processing system based on UNIX pipes. J Biomol NMR 1995; 6:277–93. [DOI] [PubMed] [Google Scholar]
- 26. Hyberts SG, Milbradt AG, Wagner AB, Arthanari H, Wagner G. Application of iterative soft thresholding for fast reconstruction of NMR data non-uniformly sampled with multidimensional Poisson gap scheduling. J Biomol NMR 2012; 52:315–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ashwell G. Colorimetric analysis of sugars. Meth Enzymol 1957; 3:73–105. [Google Scholar]
- 28. Yu J, Lin J, Kim KH, Benjamin WH Jr, Nahm MH. Development of an automated and multiplexed serotyping assay for Streptococcus pneumoniae. Clin Vaccine Immunol 2011; 18:1900–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Park IH, Park S, Hollingshead SK, Nahm MH. Genetic basis for the new pneumococcal serotype, 6C. Infect Immun 2007; 75:4482–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sung CK, Li H, Claverys JP, Morrison DA. An rpsL cassette, Janus, for gene replacement through negative selection in Streptococcus pneumoniae. Appl Environ Microbiol 2001; 67:5190–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Trzcinski K, Thompson CM, Lipsitch M. Construction of otherwise isogenic serotype 6B, 7F, 14, and 19F capsular variants of Streptococcus pneumoniae strain TIGR4. Appl Environ Microbiol 2003; 69:7364–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Calix JJ, Porambo RJ, Brady AM, et al. Biochemical, genetic, and serological characterization of two capsule subtypes among Streptococcus pneumoniae serotype 20 strains: discovery of a new pneumococcal serotype. J Biol Chem 2012; 287:27885–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Burton RL, Nahm MH. Development and validation of a fourfold multiplexed opsonization assay (MOPA4) for pneumococcal antibodies. Clin Vaccine Immunol 2006; 13:1004–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nahm MH, Burton RL. Protocol for multiplexed opsonophagocytic killing assay (UAB-MOPA) for antibodies against Streptococcus pneumoniae. 2014. https://www.vaccine. uab.edu/uploads/mdocs/UAB-MOPA.pdf. Accessed 12 August 2019.
- 35. Sorensen UBS, Henrichsen J, Chen HC, Szu SC. Covalent linkage between the capsular polysaccharide and the cell wall peptidoglycan of Streptococcus pneumoniae revealed by immunochemical methods. Microb Pathog 1990; 8:325–34. [DOI] [PubMed] [Google Scholar]
- 36. Jansson PE, Lindberg B, Anderson M, Lindquist U, Henrichsen J. Structural studies of the capsular polysaccharide from Streptococcus pneumoniae type 2, a reinvestigation. Carbohydr Res 1988; 182:111–7. [DOI] [PubMed] [Google Scholar]
- 37. Lanie JA, Ng WL, Kazmierczak KM, et al. Genome sequence of Avery's virulent serotype 2 strain D39 of Streptococcus pneumoniae and comparison with that of unencapsulated laboratory strain R6. J Bacteriol 2007; 189:38–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yang J, Ritchey M, Yoshida Y, Bush CA, Cisar JO. Comparative structural and molecular characterization of ribitol-5-phosphate-containing Streptococcus oralis coaggregation receptor polysaccharides. J Bacteriol 2009; 191:1891–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yang J, Shelat NY, Bush CA, Cisar JO. Structure and molecular characterization of Streptococcus pneumoniae capsular polysaccharide 10F by carbohydrate engineering in Streptococcus oralis. J Biol Chem 2010; 285:24217–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yurchak AM, Austrian R. Serologic and genetic relationships between pneumococci and other respiratory streptococci. Trans Assoc Am Physicians 1966; 79:368–75. [PubMed] [Google Scholar]
- 41. Wen Z, Liu Y, Qu F, Zhang JR. Allelic variation of the capsule promoter diversifies encapsulation and virulence in Streptococcus pneumoniae. Sci Rep 2016; 6:30176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hakenbeck R, Balmelle N, Weber B, Gardès C, Keck W, de Saizieu A. Mosaic genes and mosaic chromosomes: intra- and interspecies genomic variation of Streptococcus pneumoniae. Infect Immun 2001; 69:2477–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Jensen A, Valdórsson O, Frimodt-Møller N, Hollingshead S, Kilian M. Commensal streptococci serve as a reservoir for β-lactam resistance genes in Streptococcus pneumoniae. Antimicrob Agents Chemother 2015; 59:3529–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Saha SK, Al Emran HM, Hossain B, et al. ; Pneumococcal Study Group Streptococcus pneumoniae serotype-2 childhood meningitis in Bangladesh: a newly recognized pneumococcal infection threat. PLoS One 2012; 7:e32134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Gaensbauer JT, Asturias EJ, Soto M, Holt E, Olson D, Halsey NA; Guatemala Pediatric Bacterial Surveillance Working Group Pediatric invasive pneumococcal disease in Guatemala City: importance of serotype 2. Pediatr Infect Dis J 2016; 35:e139–43. [DOI] [PubMed] [Google Scholar]
- 46. Kilian M. The oral microbiome—friend or foe? Eur J Oral Sci 2018; 126 Suppl 1:5–12. [DOI] [PubMed] [Google Scholar]
- 47. van Deursen AMM, van Houten MA, Webber C, et al. The impact of the 13-valent pneumococcal conjugate vaccine on pneumococcal carriage in the Community Acquired Pneumonia Immunization Trial in Adults (CAPiTA) study. Clin Infect Dis 2018; 67:42–9. [DOI] [PubMed] [Google Scholar]
- 48. Branche AR, Yang H, Java J, et al. Effect of prior vaccination on carriage rates of Streptococcus pneumoniae in older adults: a longitudinal surveillance study. Vaccine 2018; 36:4304–10. [DOI] [PubMed] [Google Scholar]
- 49. Skinner JM, Indrawati L, Cannon J, et al. Pre-clinical evaluation of a 15-valent pneumococcal conjugate vaccine (PCV15-CRM197) in an infant-rhesus monkey immunogenicity model. Vaccine 2011; 29:8870–6. [DOI] [PubMed] [Google Scholar]
- 50. Emini EA, Watson WJ, Prasad AK, et al. US patent US20150202309. 2015.
- 51. Satzke C, Turner P, Virolainen-Julkunen A, et al. Standard method for detecting upper respiratory carriage of Streptococcus pneumoniae: updated recommendations from the World Health Organization Pneumococcal Carriage Working Group. Vaccine 2013; 32:165–79. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.