Abstract
Background
How cerebrovascular disease and neurodegeneration affect each other to impact cognition is not yet known. We aimed to test whether Alzheimer’s disease-signature (AD) cortical thickness mediates the association between cholinergic white matter lesion load and change in domain-specific cognition.
Methods
Clinically stroke-free participants from the Northern Manhattan Study with both regional white matter hyperintensity volume (WMHV) and gray matter measurements were included (N = 894). Tract-specific WMHVs were quantified through FSL using the Johns Hopkins University white matter tract atlas. We used Freesurfer 5.1 to estimate regional cortical thickness. We fit structural equation models, including multiple indicator latent change score models, to examine associations between white matter hyperintensity volume (WMHV) in cholinergic tracts, AD-signature region cortical thickness (CT), and domain-specific cognition.
Results
Our sample (N = 894) had a mean (SD) age = 70 (9) years, years of education = 10 (5), 63% women, and 67% Hispanics/Latinos. Greater cholinergic WMHV was significantly related to worse processing speed at baseline (standardized β = −0.17, SE = 0.05, p = .001) and over time (standardized β = −0.28, SE = 0.09, p = .003), with a significant indirect effect of AD-signature region CT (baseline: standardized β = −0.02, SE = 0.01, p = .023; change: standardized β = −0.03, SE = 0.02, p = .040).
Conclusions
Cholinergic tract WMHV is associated with worse processing speed, both directly and indirectly through its effect on AD-signature region CT.
Keywords: Biomarkers, Cognitive aging, Epidemiology, Imaging
Prevention of cerebrovascular injury is a proposed strategy for attentuating the burden of cognitive decline and dementia (1,2). However, the relationship between cerebrovascular and neurodegenerative pathology is uncertain, despite their frequent co-occurrence (3). The “brain reserve hypothesis” posits that neural capital, often represented by cortical thickness, moderates the association between pathology and cognitive decline (4). However, evidence from animal (5–7) and human studies (8,9) indicates that cerebrovascular disease may directly affect neurodegenerative processes, and thus atrophy may mediate the association between cerebrovascular disease and cognitive decline.
The cholinergic system underlies normal cognition and is dysregulated in AD (10). Recent studies suggest that cholinergic tract white matter lesions may contribute to cognitive impairment and decline (11–14). However, the extent to which injury to cholinergic white matter tracts is related to neurodegeneration, and whether this mediates associations with cognitive decline, are largely underexplored.
In this study, we aimed to test the following hypotheses: (i) greater cholinergic white matter tract lesion load is related to worse domain-specific cognition, (ii) AD-signature region cortical thickness partially mediates this association, and (iii) these associations are strongest for episodic memory. We used data from a stroke-free, racially and ethnically diverse, aging sample from Northern Manhattan. By applying a structural equation modeling approach, we were able to employ data reduction techniques to deal with the multiple correlated indicators of brain structure and cognition, explicitly model measurement error, and more robustly estimate direct and indirect effects.
Methods
Analytic Sample
The Northern Manhattan Study (NOMAS) was originally designed as a prospective cohort study of stroke risk among residents of Northern Manhattan, NY. Enrollment occurred between 1993 and 2001, as previously described (15). Eligibility criteria included: (i) being clinically stroke-free, (ii) aged >40 years old (>55 after 1998), and (iii) living in Northern Manhattan for at least 3 months in a household with a telephone. From this original cohort, 1,091 participants were recruited for participation in the NOMAS Magnetic Resonance Imaging (MRI) Sub-Study. In addition, 199 household members were enrolled as previously described (Sub-Study Total N = 1,290) (16). The following eligibility criteria were used: (i) >50 years old, (ii) no contraindications to MRI, and (iii) clinically stroke-free. Brain MRIs were obtained on a 1.5T Philips Intera scanner (Philips, Best, the Netherlands) at Columbia University Medical Center. Participants provided written informed consent, and the institutional review boards at the University of Miami and Columbia University approved the study.
Our analytic sample included participants with both regional white matter hyperintensity volume (WMHV) and cortical thickness data available (N = 894). Of these 894 participants, 710 had neuropsychological data at time 2.
Measurement of Variables
Structural MRI-derived measures of brain aging
All MRI markers were measured at the same time point. Quantification of regional WMHV using the FSL package (http://www.fmrib.ox.ac.uk/fsl) has been previously described (17). Eighty-five participants were excluded from analysis due to lack of FLAIR data, image artifact, failure of the registration method due to large ventricles, tumors, or infarcts, or hyperintense lesions outside the white matter. From T2-weighted FLAIR sequences, regional WMHV were measured using customized protocols from the FSL software package. Skull stripping was performed using the FSL-BET tool (18), and whole brain segmentation was performed with the FSL-FAST algorithm after correction for nonuniformity (19). WMH voxels were defined as voxels with an intensity greater than 3.5 SD above the mean intensity. Tract-specific WMHVs were quantified using the Johns Hopkins University diffusion tensor imaging-based white matter tract atlas as a reference (20). Based on previous pathological and imaging studies of cholinergic white matter lesions (11,14), as well as our confirmatory factor analysis (see below), we defined the following as cholinergic tracts: genu and splenium of corpus callosum, anterior corona radiata, posterior thalamic radiation, superior longitudinal fasciculus, sagittal striatum, and external capsule. These tracts are also relevant as some, especially the superior longitudinal faciculus, partially contribute to the periventricular WM (21), which has been robustly associated with cognition (22). For each tract, we computed the sum of the bilateral WMHV.
To measure cortical thickness, we used the Freesurfer image analysis suite version 5.1 (https://surfer.nmr.mgh.harvard.edu/), as previously described (16). T1-weighted MRIs underwent motion correction, skull stripping, and transformation into Talairch space before segmentation, identification of gray and white matter boundaries, and automated topology correction and surface deformation4. Labels for regional gray matter parcellations were assigned to each voxel via a probabilistic atlas and Bayesian classification rule5 and was based on the Desikan-Killiany Atlas (23). Based on previous literature (24), we defined the AD-signature region as: entorhinal, parahippocampal, inferior temporal, temporal pole, inferior parietal, superior frontal, superior parietal, supramarginal, precuneus, pars opercularis, pars orbitalis, and pars triangularis (inferior frontal gyrus). For each region, we computed the mean of the bilateral cortical thickness measurements.
Total intracranial volume (TIV) was measured at MRI baseline for all participants. TIV was measured at the University of California, Davis, using morphometric analysis and operator-guided tracing of the dura mater within the cranium as previously described (16). From T1-weighted images, nonbrain elements were removed manually by operator-guided tracing of the dura mater within the cranium (including the middle cranial fossa and excluding the posterior fossa and brainstem) to obtain TIV.
Domain-specific cognitive performance
Participants underwent a neurocognitive battery as previously described (16). Briefly, the baseline neuropsychological battery was administered the same day as the brain MRI, and the second neuropsychological battery was administered an average of 5 years later. Interrelationships between individual neuropsychological test scores were explored using factor analysis and a Scree plot of eigenvalues to determine the number of constructs (ie, domains) (25). Executive function comprised the difference in time to complete the Color Trails Forms 1 and 2 (reverse coded) (26) and the sum of Odd-Man-Out subtests 2 and 4 (27). Episodic memory comprised scores from three subtests from the modified California Verbal Learning Test: list learning total, delayed recall, and delayed recognition (28). Semantic memory comprised the 15-item Boston Naming test (29), category fluency (Animal Naming) (30), and phonemic fluency test (C, F, L in English speakers and F, A, S in Spanish speakers) (30). Processing speed comprised the Grooved Pegboard task in the non-dominant hand (reverse coded) (31) and the Color Trails test Form 1 (reverse coded) (26).
Covariates
As previously published, demographic information (age, gender, race/ethnicity, years of education) was collected during a baseline evaluation with standardized questions adapted from the Behavioral Risk Factor Surveillance System and U.S. Census (15).
Measurement Models and Structural Equation Models
Analyses were conducted with R (https://www.r-project.org/, version 3.5.1) (32) through RStudio, using the lavaan (version 0.6–3) (33) and psych packages (34). We determined “acceptable” model fit by assessing the following fit statistics and their cutoffs comprehensively: X2p > .05, Comparative Fit Index (CFI ≥ 0.95), Tucker-Lewis Index (TLI ≥ 0.90), Root Mean Square Error of Approximation (RMSEA < 0.08), Standardized Root Mean Square Residual (SRMR < 0.08), and normed X2/df statistic (X2/df <3) (35,36). We determined “excellent” model fit by assessing the following fit statistics and their cutoffs comprehensively: X2p > .05, CFI ≥ 0.95, TLI ≥ 0.95, RMSEA < 0.05, SRMR < 0.08, and normed X2/df statistic (X2/df <3) (35,36). All hypothesis testing was two-sided and alpha was a priori set at 0.05.
Exposure of interest: cholinergic tract white matter lesion load
Cholinergic white matter lesion load was represented in the analysis as a single-order latent variable. We hypothesized that the indicators of interest would load onto a single factor. Since WMHV is right-skewed, we applied a natural log-transformation to some of these indicators after addition of a small constant to achieve normality. Some WMHV were zero-inflated and not amenable to log-transformation; thus, we categorized these such that those with no WMHV were grouped as the reference category and those with non-zero values were categorized into the other groups indicating increasing WMHV. We retained indicators that had a communalities and standardized loadings >0.3. We also consulted modification indices to improve model fit and added error covariances that corresponded on an anatomical/theoretical basis. Estimates for this confirmatory factor analysis (CFA) were computed using the weighted least squares means and variance adjusted (WLSMV) estimator with sandwich-type robust standard errors and robust (shift-scaled) test statistics and are shown in Supplementary Table S1 (37).
Mediator of interest: AD-signature region cortical thickness
Alzheimer disease signature region cortical thickness (AD-sig. CT) was represented as a second-order latent variable. We first performed an exploratory factor analysis to examine the underlying factor structure in a random 50% of the sample with cortical thickness data available. First, we performed the exploratory factor analysis with an oblique rotation and examined a scree plot of the observed data to determine the factor structure. The scree plot suggested that these data had a three-factor structure. We then examined the factor loadings for 12 indicators and retained 11 indicators with loadings and communalities >0.3 (38). To confirm this factor structure, we conducted a CFA of a two-order factor model for AD-sig. CT in the remaining random 50% of participants, with three lower order factors representing the frontal, parietal, and temporal regions. We re-fit the model in the entire sample, and estimates for this CFA were computed using the maximum likelihood estimator. Results of this CFA are displayed in Supplementary Table S2.
Outcome of Interest: Domain-specific cognitive performance (baseline and change)
We modeled baseline cognition as a four-factor correlated factors model. Estimates for this CFA were computed using the maximum likelihood estimator. Results of this CFA are displayed in Supplementary Table S3.
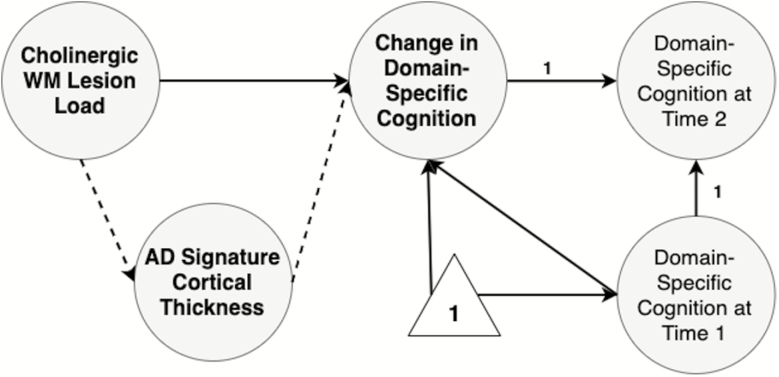
For change in domain-specific cognitive performance, we examined each domain in separate models as multiple indicator univariate latent change score models (39) (Supplementary Figure 1). In these models (39), the change in the latent variable across two time points is modeled as a latent construct and represents the difference in the latent construct between time 2 and time 1. Thus, a negative change represents worse performance at time 2, while a positive change represents better performance at time 2. The latent construct of cognition at time 2 is modeled as an indicator of change in cognition with a fixed path equal to 1. Change in cognition is also a consequence of the latent construct of cognition at time 1. Measurement invariance and correlated residual errors are assumed in this model across both time points. Fixed paths equal to 1 from change in cognition and cognition at time 1, as well as constraining the intercept and variance of cognition at time 2 to equal 0, allow the change score to equal the difference in the latent construct of cognition between time 2 and 1.
Structural equation models
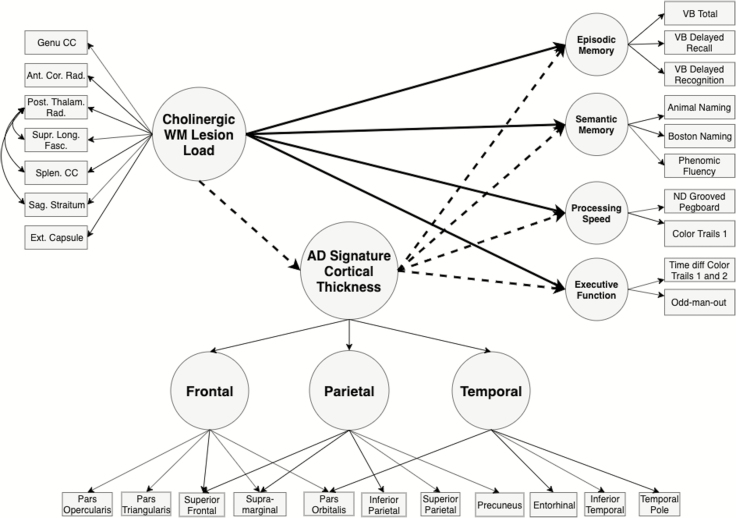
We constructed our structural equation models (SEM) to test the following main hypotheses: (i) cholinergic tract-associated WM lesion load is related to cognition differentially across domains and (ii) AD-sig. CT mediates these associations (Figures 1 and 2). Due to the mix of continuous and categorical (ordinal) indicators in the SEMs, we computed estimates using the WLSMV estimator with sandwich-type robust standard errors and report robust (shift-scaled) test statistics and standardized estimates (37).
Figure 1.
Hypothesized structural equation model for domain-specific cognitive performance at baseline. Residual variances not pictured for clarity.
Figure 2.
Hypothesized structural equation model for change in domain-specific cognitive performance. Indicators and residual variances not pictured for clarity. Change in domain-specific cognitive performance was modeled as a multiple indicator univariate latent change score model (39). Intercept and variance for domain-specific cognition at time 2 fixed at 0. Paths from change in domain-specific cognition and domain-specific cognition at time 1 fixed at 1.
Because of negative variances, we rescaled the following neuropsychological tests by dividing scores by 10: Color Trails test Forms 1 and 2, Grooved Pegboard task (nondominant hand), and category fluency (Animal Naming). Similarly, for AD-sig. CT, indicators were rescaled as z-scores, and the variance of the frontal region factor was constrained to > 0 due to a small, negative, and statistically nonsignificant variance.
The following covariates were included in the models by adding paths to latent constructs of interest: age at baseline, years of education, total intracranial volume, race/ethnicity, and gender. Gender (men vs women) and race/ethnicity (Hispanic/Latino, Black, vs white/other) were dummy-coded. Only significant paths (p < .05) were retained in final models.
We conducted two sensitivity analyses. To examine the potential confounding role of cognitive impairment, we reran the main analyses in those categorized as cognitively unimpaired (defined as having at least one domain-specific z-score ≤ −1.5, computed as done in previous NOMAS studies (16)). To examine the specificity of our regional findings, we reran the main analyses using natural log-transformed total WMHV and total mean cortical thickness as observed variables. We also conducted a post hoc analysis for the most significant associations to explore region-specific mediation (ie, frontal vs temporal vs parietal AD sig. CT), modeling AD-sig. CT as a correlated factors model.
Results
Sample characteristics are displayed in Table 1. Our sample had an average age of 70 (SD = 9) years and average years of education of 10 (SD = 5) and consisted of about 63% women and 67% Hispanics/Latinos. The distribution of the cholinergic WMHV and AD-sig. CT metrics was similar across those with and without neuropsychological data at time 2 (Table 1).
Table 1.
Sample Characteristics
| Those with Available Regional WMHV and CT Data (N = 894) | Those with Available Regional WMHV and CT Data, and NP Data at Time 2 (N = 710) | |
|---|---|---|
| Covariates at baseline | ||
| Age at MRI (years), mean (SD) | 70 (9) | 69 (8) |
| Education (years), mean (SD) | 10 (5) | 10 (5) |
| Women, n (%) | 565 (63) | 455 (64) |
| Race/ethnicity, n (%) | ||
| Non-Hispanic White/ Other | 150 (17) | 108 (15) |
| Non-Hispanic Black | 149 (17) | 116 (16) |
| Hispanic/Latino | 595 (67) | 486 (68) |
| Total intracranial volume (mL), mean (SD) | 1,147 (119) | 1,148 (120) |
| Continuous Cholinergic WMHV Indicators (mL), median (Q1, Q3) | ||
| Genu of Corpus Callosum | 0.06 (0.03, 0.13) | 0.06 (0.03, 0.12) |
| Splenium of Corpus Callosum | 0.05 (0.02, 0.12) | 0.05 (0.01, 0.11) |
| Anterior Corona Radiata | 0.67 (0.33, 1.33) | 0.63 (0.32, 1.25) |
| Posterior Thalamic Radiation | 0.32 (0.15, 0.70) | 0.30 (0.14, 0.64) |
| Categorical (Ordinal) Cholinergic WMHV Indicators, n (%) | ||
| Superior Longitudinal Fasciculus | ||
| Quartile 1 | 262 (29) | 220 (31) |
| Quartile 2 | 215 (24) | 176 (25) |
| Quartile 3 | 216 (24) | 158 (22) |
| Quartile 4 | 201 (22) | 156 (22) |
| Sagittal Striatum | ||
| Quartile 1 | 237 (27) | 187 (26) |
| Quartile 2 | 229 (26) | 181 (25) |
| Quartile 3 | 213 (24) | 173 (24) |
| Quartile 4 | 215 (24) | 169 (24) |
| External Capsule | ||
| Tertile 1 | 477 (53) | 394 (55) |
| Tertile 2 | 217 (24) | 170 (24) |
| Tertile 3 | 200 (22) | 146 (21) |
| AD-Signature Cortical Thickness Indicators (mm), mean (SD) | ||
| Entorhinal | 2.91 (0.37) | 2.94 (0.36) |
| Inferior Temporal | 2.50 (0.16) | 2.51 (0.16) |
| Temporal Pole | 2.97 (0.38) | 3.00 (0.36) |
| Inferior Parietal | 2.29 (0.13) | 2.31 (0.13) |
| Superior Frontal | 2.56 (0.16) | 2.57 (0.15) |
| Superior Parietal | 2.06 (0.14) | 2.07 (0.13) |
| Supramarginal | 2.36 (0.12) | 2.37 (0.12) |
| Precuneus | 2.18 (0.13) | 2.18 (0.13) |
| Pars Opercularis | 2.32 (0.15) | 2.32 (0.15) |
| Pars Orbitalis | 2.44 (0.24) | 2.46 (0.23) |
| Pars Triangularis | 2.24 (0.16) | 2.26 (0.16) |
Note: MRI = Magnetic resonance imaging; WMHV = White matter hyperintensity volume.
*Had non-zero volume of WMHV in this tract.
Domain-Specific Cognitive Performance at Baseline
Table 2 and Supplementary Figure 2 display the results of the CFA estimating the direct effects between cholinergic tract WMHV and domain-specific cognition at baseline, as well as the indirect effects of AD-sig. CT. The robust model fit statistics for this model are as follows and indicate acceptable fit: X2 = 907.17, df = 469, p < .0001; X2/df = 1.93; CFI = 0.94; TLI = 0.92; RMSEA (90% CI) = 0.035 (0.032, 0.039); SRMR = 0.042. Greater cholinergic tract WMHV was significantly related to worse processing speed at baseline (standardized β = −0.17, SE = 0.05, p = .001). Though not statistically significant, estimates for the other domains were also negative, indicating worse performance with greater cholinergic tract WMHV (Table 2). Greater cholinergic tract WMHV was also significantly associated with smaller AD-sig. CT (standardized β = −0.15, SE = 0.04, p < .0001). Greater AD-sig. CT was significantly related to better performance in episodic memory (standardized β = 0.10, SE = 0.04, p = .012) and processing speed (standardized β = 0.14, SE = 0.05, p = .004). Finally, we observed a significant indirect effect for processing speed (standardized β = −0.02, SE = 0.01, p = .023). Estimates for associations with semantic memory and executive function were null or close to null and did not reach statistical significance (Table 2 and Supplementary Figure 2).
Table 2.
Path Coefficients from the Structural Equation Model of Cholinergic-Associated WMHV, Alzheimer’s Disease-Signature Cortical Thickness (AD-Sig CT), and Baseline Domain-Specific Cognitive Performance (Modeled as a Correlated Factors Model), Adjusted for Covariates*
| Effects | Independent Latent Variable | Dependent Latent Variable | Standardized Loading | SE | p |
|---|---|---|---|---|---|
| Direct Effects | Cholinergic WMHV | Episodic Memory | −0.01 | 0.04 | .831 |
| Semantic Memory | −0.07 | 0.04 | .084 | ||
| Processing Speed | −0.17 | 0.05 | .001 | ||
| Executive Function | −0.06 | 0.05 | .218 | ||
| AD-Sig. CT | −0.15 | 0.04 | .001 | ||
| AD-Sig. CT | Episodic Memory | 0.10 | 0.04 | .012 | |
| Semantic Memory | 0.00 | 0.04 | .994 | ||
| Processing Speed | 0.14 | 0.05 | .004 | ||
| Executive Function | 0.04 | 0.05 | .395 | ||
| Indirect Effects | Chol. WMHV → AD-Sig. CT | Episodic Memory | −0.02 | 0.01 | .051 |
| Semantic Memory | 0.00 | 0.01 | .994 | ||
| Processing Speed | −0.02 | 0.01 | .023 | ||
| Executive Function | −0.01 | 0.01 | .413 |
Note: WMHV = White matter hyperintensity volume.
*SE = standard error. p Value from z-test of standardized coefficient. Model fit statistics: Chi-square test: X2 = 907.17, df = 469, p < .0001; X2/df = 1.93; CFI = 0.94; TLI = 0.92; RMSEA (90% CI) = 0.035 (0.032, 0.039); SRMR = 0.042. Paths from covariates (age at baseline, years of education, total intracranial volume, male gender, Hispanic ethnicity, and Black race) to latent variables were added to the model, and those with statistically significant loadings (p < .05) were retained.
Change in Domain-Specific Cognitive Performance
Table 3 and Supplementary Figure 3 display the results of the CFA estimating the direct effects between cholinergic-associated WMHV and change in domain-specific cognition, as well as the indirect effects of AD-sig. CT. For change in episodic memory, the robust model fit statistics are as follows and indicate acceptable fit: X2 = 638.98, df = 374, p < .0001; X2/df = 1.71; CFI = 0.96; TLI = 0.95; RMSEA (90% CI) = 0.032 (0.28, 0.036); SRMR = 0.056. Neither cholinergic WMHV nor AD-sig. CT were significantly related to change in episodic memory, directly or indirectly (Table 3 and Supplementary Figure 3A).
Table 3.
Path Coefficients from the Structural Equation Model of Cholinergic-Associated WMHV, Alzheimer’s Disease-Signature Cortical Thickness (AD-Sig CT), and Change in Domain-Specific Cognitive Performance (Domains Modeled Separately)*
| Independent Latent Variable | Standardized Loading | SE | p | |
|---|---|---|---|---|
| Change in Episodic Memory | ||||
| Direct Effects | Cholinergic WMHV | −0.05 | 0.07 | .433 |
| AD-Sig. CT | 0.09 | 0.06 | .127 | |
| Indirect Effect | Chol. WMHV → AD-Sig. CT | −0.01 | 0.01 | .130 |
| Change in Semantic Memory | ||||
| Direct Effects | Cholinergic WMHV | −0.24 | 0.09 | .011 |
| AD-Sig. CT | −0.11 | 0.09 | .205 | |
| Indirect Effect | Chol. WMHV → AD-Sig. CT | 0.02 | 0.01 | .244 |
| Change in Processing Speed | ||||
| Direct Effects | Cholinergic WMHV | −0.28 | 0.09 | .003 |
| AD-Sig. CT | 0.21 | 0.09 | .017 | |
| Indirect Effect | Chol. WMHV → AD-Sig. CT | −0.03 | 0.02 | .040 |
| Change in Executive Function | ||||
| Direct Effects | Cholinergic WMHV | −0.15 | 0.40 | .709 |
| AD-Sig. CT | 0.10 | 0.39 | .798 | |
| Indirect Effect | Chol. WMHV → AD-Sig. CT | −0.02 | 0.06 | .798 |
Note: WMHV = White matter hyperintensity volume.
*SE = standard error. Model fit statistics for episodic memory: Chi-square test: X2 = 638.98, df = 374, p < .0001; X2/df = 1.71; CFI = 0.96; TLI = 0.95; RMSEA (90% CI) = 0.032 (0.28, 0.036); SRMR = 0.056. Model fit statistics for semantic memory: Chi-square test: X2 = 774.15, df = 372, p < .0001; X2/df = 2.08; CFI = 0.92; TLI = 0.91; RMSEA (90% CI) = 0.041 (0.037, 0.045); SRMR = 0.056. Model fit statistics for processing speed: Chi-square test: X2 = 595.74, df = 320, p < .0001; X2/df = 1.86; CFI = 0.95; TLI = 0.94; RMSEA (90% CI) = 0.036 (0.031, 0.040); SRMR = 0.048. Model fit statistics for executive function: Chi-square test: X2 = 513.02, df = 320, p < .0001; X2/df = 1.60; CFI = 0.96; TLI = 0.95; RMSEA (90% CI) = 0.032 (0.027, 0.037); SRMR = 0.042. Paths from covariates (age at baseline, years of education, total intracranial volume, male gender, Hispanic ethnicity, and Black race) to latent variables were added to the model, and those with statistically significant loadings (p < .05) were retained.
For semantic memory, the robust model fit statistics are as follows and indicate acceptable fit: X2 = 774.15, df = 372, p < .0001; X2/df = 2.08; CFI = 0.92; TLI = 0.91; RMSEA (90% CI) = 0.041 (0.037, 0.045); SRMR = 0.056. Greater cholinergic WMHV was related to a greater decline in semantic memory performance (standardized β = −0.24, SE = 0.09, p = .011). However, AD-sig. CT was not significantly related, nor was there a significant indirect effect of AD-sig. CT on change in semantic memory (Table 3 and Supplementary Figure 3B).
For processing speed, the robust model fit statistics are as follows and indicate acceptable fit: X2 = 595.74, df = 320, p < .0001; X2/df = 1.86; CFI = 0.95; TLI = 0.94; RMSEA (90% CI) = 0.036 (0.031, 0.040); SRMR = 0.048. Greater cholinergic WMHV was associated with a negative change in processing speed (standardized β = −0.28, SE = 0.09, p = .003). Greater AD-sig. CT was directly related to a positive change in processing speed (standardized β = 0.21, SE = 0.09, p = .017). The indirect effect of AD-sig. CT was significant, such that greater cholinergic WMHV is associated with smaller AD-sig. CT, and in turn, a negative change in processing speed (standardized β = −0.03, SE = 0.002, p = .040) (Table 3 and Supplementary Figure 3C).
For executive function, the robust model fit statistics for executive function indicate acceptable fit: X2 = 513.02, df = 320, p < .0001; X2/df = 1.60; CFI = 0.96; TLI = 0.95; RMSEA (90% CI) = 0.032 (0.027, 0.037); SRMR = 0.042. Neither cholinergic WMHV nor AD-sig. CT were significantly related to change in executive function, directly or indirectly (Table 3 and Supplementary Figure 3D).
Sensitivity and Post hoc Analyses
Among those cognitively unimpaired, direct effects of cholinergic WMHV and baseline domain-specific cognitive performance were generally similar to the main analyses (Supplementary Table S4). The direct effects of AD-sig. CT and indirect effects on episodic memory and processing speed were attenuated and no longer significant among those cognitively unimpaired (Supplementary Table S4). Direct effects of total WMHV on baseline domain-specific cognitive performance were slightly stronger and statistically significant for processing speed and executive function, but indirect effects were similar to main analyses (Supplementary Table S5).
Among those cognitively unimpaired, direct and indirect effects of cholinergic WMHV were similar as main analyses for change in episodic memory (Supplementary Table S6). When examining total WMHV and mean CT, results were similar for change in episodic memory (Supplementary Table S7). For changes in semantic memory and processing speed, direct effects were stronger in the main analyses, while the indirect effect remained similar in magnitude (Supplementary Table S7). For change in executive function, effect sizes were stronger when using total WMHV and mean CT, though inferences did not change (Supplementary Table S7).
In additional post hoc analyses, we examined whether frontal, parietal, or temporal regions of AD-sig. CT differentially mediated the association between cholinergic WMHV and processing speed, at baseline and over time. Generally, indirect effects were not statistically significant at baseline or for change over time (Supplementary Table S8). However, the strongest effect estimates were observed for frontal region of AD-sig. CT, when compared with effect estimates for temporal or parietal regions (Supplementary Table S8).
Discussion
In this study, we found that greater cholinergic tract WM lesion load was most robustly associated with worse processing speed at baseline and over time, contrary to our original hypothesis. Additionally, cholinergic tract WM lesion load was indirectly associated with processing speed through AD-sig. CT, suggesting possible partial mediation. These associations are consistent with previous studies that examined periventricular WMLs (22). These associations were similar in the subsample of participants who were cognitively unimpaired and were attenuated when examining total WMHV, suggesting a specific regional association. Though not statistically significant, our exploratory analyses for regional associations suggest that the frontal region of the AD-signature may contribute the most to processing speed performance. Though episodic memory is often the first domain affected by AD, impaired processing speed is often an important consequence in AD in addition to being the primary consequence of vascular cognitive impairment (40).
Few population-based studies have used SEM to examine how cortical atrophy may mediate associations between WM lesion load and cognition. Similar to our findings, Knopman et al. showed that associations of WMH and psychomotor speed/executive function were partially mediated by cortical volume in AD-related regions (8). Rizvi et al. and Tuladhar et al. found that global cortical thickness mediated the association between total WMHV and domain-specific cognitive performance, including psychomotor speed (9,41). Rizvi et al. also found significant associations with memory, including indirect effects of entorhinal cortex thickness and hippocampal volume (9). These findings were in contrast to our study, though both samples were recruited from the same geographic location in Northern Manhattan. However, participants in the study by Rizvi et al. were slightly older on average and exhibited a more diverse distribution of cognitive status than participants in our study. Generally, the aforementioned studies modeled brain MRI variables as observed variables, were cross-sectional in nature, and examined global or lobar measures of brain structure. Our study expands upon this literature in three ways: (i) the use of latent variable modeling to represent brain structure, (ii) ability to examine changes in cognitive performance across two time points in addition to cross-sectional associations, and (iii) defining brain structure in AD-related regions (ie, cholinergic tract WMHV and AD-sig. CT).
Our study suggests that WM injury in cholinergic tracts is associated with cortical structure in AD-vulnerable regions, and that both types of changes explain variability in cognitive performance. These data may help explain why cerebrovascular and AD pathologies often co-occur (3,42,43) and are consistent with evidence from animal studies showing how ischemic vascular injury triggers AD-related neurodegenerative pathology, such as increased tau phosphorylation (5), neuronal death via phagocytosis (6), and gray matter atrophy (7). Though we propose that WM lesions in cholinergic tracts could contribute to AD pathology (11,13), evidence also suggests that AD pathology may trigger neurovascular dysfunction (44). Future work should explore these possible causal frameworks in addition to the one proposed in this study. Further, both cholinergic tract WMHV and AD-signature region cortical thinning may be due to pathology other than AD-specific pathways, so further work is needed to examine AD-specific biomarkers. Taken together, these studies imply that preventing white matter injury may attenuate cognitive decline directly, but also indirectly by preserving cortical structure.
The strengths of this study include the racially/ethnically diverse, population-based sample, availability of a rich neuroimaging dataset that includes regional markers of white and gray matter structure, and the application of SEM to model brain structure as latent constructs and robustly estimate direct and indirect effects. Limitations of this study should be noted. First, our longitudinal analysis is limited because only two measurements of cognition across time are available in the NOMAS. Second, we lack diagnoses of dementia and were unable to examine dementia incidence or exclude those with prevalent dementia. Analyses among those deemed cognitively unimpaired (defined using neuropsychological testing only) yielded generally similar results. Third, we lack other markers of AD pathology and microstructural white matter integrity, which are important to examine in future work given the extent of the AD-signature region. Examining these markers in the context of our proposed causal framework will be important to elucidate the relationships between vascular and neurodegenerative pathology. Fourth, selection bias may attenuate our associations. Fifth, true causal mediation cannot be inferred since the mediating variable was measured at the same time as the predictor of interest. Sixth, since some post hoc modifications were made by examining modification indices for the cholinergic WMHV construct, these models may not be generalizable to other datasets. Further work is warranted to replicate our findings. Finally, residual and unmeasured confounding is still likely.
In conclusion, white matter injury in cholinergic tracts is associated with worse processing speed, both directly and indirectly through its association with AD-signature region cortical thickness. Further work is warranted to extend these findings to studies with more repeated measures of cognition as well as with AD-specific neuroimaging markers.
Supplementary Material
Acknowledgments
We would like to thank the participants of the NOMAS and acknowledge our data manager, Janet DeRosa, MPH.
Funding
This work was supported by the National Institutes of Neurological Disease and Stroke (R01 NS29993, F30 NS103462) and the Evelyn F. McKnight Brain Institute.
Conflict of Interest
M.R.C. reports no disclosures. K.S. reports no disclosures. Y.K.C. reports no disclosures. N.A. reports no disclosures. S.H.L. reports no disclosures. M.S.V.E. receives research support from the BMS-Pfizer Alliance for Eliquis, and Roche for a clinical trial of stroke prevention; has given expert legal opinions on behalf of Organon (NuvaRing and stroke litigation), Auxilium (testosterone and stroke), and LivaNova (cardiac surgery and stroke); and serves on the National, Founders Affiliate, and New York City Chapter Boards of the American Heart Association/American Stroke Association. He receives royalties from UpToDate for chapters related to stroke. R.L.S. receives federal grant support (R01 NS 29993, CTSA UL1 TR002736), private foundation support (American Heart Association Bugher Center), and pharma research support (Boehringer Ingelheim). C.B.W. receives royalties for two chapters on Vascular Dementia from UpToDate. T.R. reports no disclosures.
References
- 1. Gorelick PB, Furie KL, Iadecola C, et al. ; American Heart Association/American Stroke Association Defining optimal brain health in adults: a presidential advisory from the American Heart Association/American Stroke Association. Stroke. 2017;48:e284–e303. doi: 10.1161/STR.0000000000000148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sweeney MD, Montagne A, Sagare AP, et al. . Vascular dysfunction-The disregarded partner of Alzheimer’s disease. Alzheimers Dement. 2019;15:158–167. doi: 10.1016/j.jalz.2018.07.222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Schneider JA, Arvanitakis Z, Bang W, Bennett DA. Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology. 2007;69:2197–2204. doi: 10.1212/01.wnl.0000271090.28148.24 [DOI] [PubMed] [Google Scholar]
- 4. Jones RN, Manly J, Glymour MM, Rentz DM, Jefferson AL, Stern Y. Conceptual and measurement challenges in research on cognitive reserve. J Int Neuropsychol Soc. 2011;17:593–601. doi: 10.1017/S1355617710001748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Koike MA, Green KN, Blurton-Jones M, Laferla FM. Oligemic hypoperfusion differentially affects tau and amyloid-{beta}. Am J Pathol. 2010;177:300–310. doi: 10.2353/ajpath.2010.090750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Neher JJ, Emmrich JV, Fricker M, Mander PK, Théry C, Brown GC. Phagocytosis executes delayed neuronal death after focal brain ischemia. Proc Natl Acad Sci USA 2013;110:E4098–E4107. doi: 10.1073/pnas.1308679110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tajima A, Hans FJ, Livingstone D, et al. . Smaller local brain volumes and cerebral atrophy in spontaneously hypertensive rats. Hypertens (Dallas, Tex 1979). 1993;21:105–111. doi: 10.1161/01.hyp.21.1.105 [DOI] [PubMed] [Google Scholar]
- 8. Knopman DS, Griswold ME, Lirette ST, et al. . Vascular imaging abnormalities and cognition: mediation by cortical volume in nondemented individuals: atherosclerosis risk in communities-neurocognitive study. Stroke. 2015;46:433–440. doi: 10.1161/STROKEAHA.114.007847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rizvi B, Narkhede A, Last BS, et al. . The effect of white matter hyperintensities on cognition is mediated by cortical atrophy. Neurobiol Aging. 2018;64:25–32. doi: 10.1016/j.neurobiolaging.2017.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hampel H, Mesulam M-M, Cuello AC, et al. . The cholinergic system in the pathophysiology and treatment of Alzheimer’s disease. Brain. 2018;141:1917–1933. doi: 10.1093/brain/awy132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Liu Q, Zhu Z, Teipel SJ, et al. . White matter damage in the cholinergic system contributes to cognitive impairment in subcortical vascular cognitive impairment, no dementia. Front Aging Neurosci. 2017;9:47. doi: 10.3389/fnagi.2017.00047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Santiago C, Herrmann N, Swardfager W, et al. . Subcortical hyperintensities in the cholinergic system are associated with improvements in executive function in older adults with coronary artery disease undergoing cardiac rehabilitation. Int J Geriatr Psychiatry. 2018;33:279–287. doi: 10.1002/gps.4729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kim HJ, Moon WJ, Han SH. Differential cholinergic pathway involvement in Alzheimer’s disease and subcortical ischemic vascular dementia. J Alzheimers Dis. 2013;35:129–136. doi: 10.3233/JAD-122320 [DOI] [PubMed] [Google Scholar]
- 14. Selden NR, Gitelman DR, Salamon-Murayama N, Parrish TB, Mesulam MM. Trajectories of cholinergic pathways within the cerebral hemispheres of the human brain. Brain. 1998;121 (Pt 12):2249–2257. doi: 10.1093/brain/121.12.2249 [DOI] [PubMed] [Google Scholar]
- 15. Sacco RL, Anand K, Lee HS, et al. . Homocysteine and the risk of ischemic stroke in a triethnic cohort: the Northern Manhattan study. Stroke. 2004;35:2263–2269. doi: 10.1161/01.STR.0000142374.33919.92 [DOI] [PubMed] [Google Scholar]
- 16. Dong C, Nabizadeh N, Caunca M, et al. . Cognitive correlates of white matter lesion load and brain atrophy: the Northern Manhattan Study. Neurology. 2015;85:441–449. doi: 10.1212/WNL.0000000000001716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dhamoon MS, Cheung Y-K, Bagci A, et al. . Periventricular white matter hyperintensities and functional decline. J Am Geriatr Soc. 2018;66:113–119. doi: 10.1111/jgs.15149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17:143–155. doi: 10.1002/hbm.10062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhang Y, Brady M, Smith S. Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Trans Med Imaging. 2001;20:45–57. doi: 10.1109/42.906424 [DOI] [PubMed] [Google Scholar]
- 20. Wakana S, Caprihan A, Panzenboeck MM, et al. . Reproducibility of quantitative tractography methods applied to cerebral white matter. Neuroimage. 2007;36:630–644. doi: 10.1016/j.neuroimage.2007.02.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kamali A, Flanders AE, Brody J, Hunter JV, Hasan KM. Tracing superior longitudinal fasciculus connectivity in the human brain using high resolution diffusion tensor tractography. Brain Struct Funct. 2014;219:269–281. doi: 10.1007/s00429-012-0498-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Debette S, Bombois S, Bruandet A, et al. . Subcortical hyperintensities are associated with cognitive decline in patients with mild cognitive impairment. Stroke. 2007;38:2924–2930. doi: 10.1161/STROKEAHA.107.488403 [DOI] [PubMed] [Google Scholar]
- 23. Desikan RS, Ségonne F, Fischl B, et al. . An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31:968–980. doi: 10.1016/j.neuroimage.2006.01.021 [DOI] [PubMed] [Google Scholar]
- 24. Dickerson BC, Bakkour A, Salat DH, et al. . The cortical signature of Alzheimer’s disease: regionally specific cortical thinning relates to symptom severity in very mild to mild AD dementia and is detectable in asymptomatic amyloid-positive individuals. Cereb Cortex. 2009;19:497–510. doi: 10.1093/cercor/bhn113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Siedlecki KL, Rundek T, Elkind MS, Sacco RL, Stern Y, Wright CB. Using contextual analyses to examine the meaning of neuropsychological variables across samples of english-speaking and spanish-speaking older adults. J Int Neuropsychol Soc. 2012;18:223–233. doi: 10.1017/S135561771100155X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. D’Elia LF, Satz P, Uchiyama CL, White T.. Color Trails Test Professional Manual. Lutz, FL: PAR; 1996. [Google Scholar]
- 27. Flowers KA, Robertson C. The effect of Parkinson’s disease on the ability to maintain a mental set. J Neurol Neurosurg Psychiatry. 1985;48:517–529. doi: 10.1136/jnnp.48.6.517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Siedlecki KL, Stern Y, Reuben A, Sacco RL, Elkind MS, Wright CB. Construct validity of cognitive reserve in a multiethnic cohort: the Northern Manhattan Study. J Int Neuropsychol Soc. 2009;15:558–569. doi: 10.1017/S1355617709090857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Flanagan JL, Jackson ST. Test-retest reliability of three aphasia tests: performance of non-brain-damaged older adults. J Commun Disord. 1997;30:33–42; quiz 42. doi: 10.1016/s0021-9924(96)00039-1 [DOI] [PubMed] [Google Scholar]
- 30. Harrison JE, Buxton P, Husain M, Wise R. Short test of semantic and phonological fluency: normal performance, validity and test-retest reliability. Br J Clin Psychol. 2000;39:181–191. doi: 10.1348/014466500163202 [DOI] [PubMed] [Google Scholar]
- 31. Matthews CG, Klove H.. Instruction Manual for the Adult Neuropsychology Test Battery. Madison, WI: University of Wisconsin Medical School; 1964. [Google Scholar]
- 32. R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing. Vienna, Austria: https://www.r-project.org/. [Google Scholar]
- 33. Rosseel Y. lavaan: an R package for structural equation modeling. J Stat Softw. 2012;48:1–36. doi: 10.18637/jss.v048.i02 [Google Scholar]
- 34.Revelle W. Psych: Procedures for Personality And Psychological Research, Version = 1.8.10. 2018.
- 35. Kline RB. Principles and Practice of Structural Equation Modeling. 3rd ed.New York City, NY: Guilford Press; 2011:189–228. [Google Scholar]
- 36. Hu L, Bentler PM. Fit indices in covariance structure modeling: sensitivity to underparameterized model misspecification. Psychol Methods. 1998;3:424–453. doi: 10.1037/1082-989X.3.4.424 [Google Scholar]
- 37. Asparouhov T, Muthen B. Simple Second Order Chi-Square Correction; 2010. http://www.statmodel.com/download/WLSMV_new_chi21.pdf.
- 38. Hair JF, Anderson RE, Tatham RL, Black WIC.. Multivariate Data Analysis. New York City, NY: Pearson; 1995. [Google Scholar]
- 39. Kievit RA, Brandmaier AM, Ziegler G, et al. ; NSPN Consortium Developmental cognitive neuroscience using latent change score models: a tutorial and applications. Dev Cogn Neurosci. 2018;33:99–117. doi: 10.1016/j.dcn.2017.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gorus E, De Raedt R, Lambert M, Lemper JC, Mets T. Reaction times and performance variability in normal aging, mild cognitive impairment, and Alzheimer’s disease. J Geriatr Psychiatry Neurol. 2008;21:204–218. doi: 10.1177/0891988708320973 [DOI] [PubMed] [Google Scholar]
- 41. Tuladhar AM, Reid AT, Shumskaya E, et al. . Relationship between white matter hyperintensities, cortical thickness, and cognition. Stroke. 2015;46:425–432. doi: 10.1161/STROKEAHA.114.007146 [DOI] [PubMed] [Google Scholar]
- 42. Habes M, Erus G, Toledo JB, et al. . White matter hyperintensities and imaging patterns of brain ageing in the general population. Brain. 2016;139(Pt 4):1164–1179. doi: 10.1093/brain/aww008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Power MC, Mormino E, Soldan A, et al. . Combined neuropathological pathways account for age-related risk of dementia. Ann Neurol. 2018;84:10–22. doi: 10.1002/ana.25246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Koizumi K, Wang G, Park L. Endothelial dysfunction and amyloid-β-induced neurovascular alterations. Cell Mol Neurobiol. 2016;36:155–165. doi: 10.1007/s10571-015-0256-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.