Abstract
BCG vaccination has been demonstrated to increase levels of activated CD4+ T cells, thus potentially influencing mother-to-child transmission of human immunodeficiency virus (HIV). To assess the risk of BCG vaccination in HIV infection, we randomly assigned newborn rhesus macaques to receive BCG vaccine or remain unvaccinated and then undergo oral simian immunodeficiency virus (SIV) challenges 3 weeks later. We observed elevated levels of activated peripheral CD4+ T cells (ie, HLA-DR+CD38+CCR5+ CD4+ T cells) by week 3 after vaccination. BCG was also associated with an altered immune gene expression profile, as well as with monocyte activation in both peripheral blood and the draining axillary lymph node, indicating significant BCG vaccine–induced immune activation. Despite these effects, BCG vaccination did not increase the rate of SIV oral transmission or disease progression. Our findings therefore identify patterns of T-cell and monocyte activation that occur after BCG vaccination but do not support the hypothesis that BCG vaccination is a risk factor for postnatal HIV transmission or increased pathogenesis in infants.
Keywords: BCG, infants, HIV, SIV, immune, activation, breastfeeding, oral transmission, macaques
BCG vaccination induces activation of CD4+ T cells, which potentially influences human immunodeficiency virus (HIV) infection in breastfeeding infants. This article demonstrates that BCG vaccine–associated T-cell and monocyte activation in infant macaques is not associated with oral simian immunodeficiency virus (SIV) transmission or pathogenesis.
(See the Editorial Commentary by Kilapandal Venkatraman et al., on pages 1–3.)
Despite reductions in human immunodeficiency virus (HIV) transmission due to early HIV testing and antiretroviral therapy, mother-to-child transmission of HIV remains responsible for nearly 160 000 new infections each year [1]. Consequently, >1 million children aged <5 years continue to die from HIV-associated causes every year [2]. Early in the HIV epidemic, the perinatal period was when infants were at the greatest risk of acquiring HIV. However, the risk of mother-to-child transmission of HIV is now greatest during breastfeeding, owing to transmission through the oral route [2].
Risk factors for HIV acquisition include mucosal inflammation and T-cell activation, both of which can be influenced by immunologic and environmental factors [3–7]. Pediatric vaccinations, which prevent life-threatening infectious illnesses, also have the potential to modulate the activation status of HIV target cells. Vaccination with Bacillus Calmette-Guérin (BCG), which reduces infant morbidity and mortality associated with Mycobacterium tuberculosis infection, is an immunomodulatory, live-attenuated vaccine that has the potential to induce activation of multiple immune cell subsets, including HIV-susceptible T cells [8, 9]. BCG vaccine is currently administered to >120 million infants annually and is given within hours to days after birth in M. tuberculosis–endemic areas. In a recent study, HIV-exposed uninfected South African infants from a periurban settlement outside of Cape Town were randomly assigned to undergo routine BCG vaccination at birth or after an 8-week delay [7]. Elevated levels of HIV target cells (ie, CCR5+CD38+HLA-DR+ CD4+ T cells) were found in blood specimens from BCG-vaccinated infants 6 and 8 weeks after vaccination [7]. Given the wide administration of BCG vaccine, any potential impact on infant HIV acquisition necessitates further exploration and was the impetus for the studies outlined here, which used the infant rhesus macaque model of simian immunodeficiency virus (SIV) infection.
The rhesus macaque SIV infection model is particularly well suited to directly test our hypothesis that BCG vaccination increases susceptibility to HIV infection, since infant macaques respond similarly to humans to standard intradermal BCG vaccination [10–12]. Here we demonstrate that BCG vaccine caused elevated peripheral T-cell activation, increased monocyte activation, and altered transcriptomic profiles of circulating immune cells (ie, peripheral blood mononuclear cells [PBMCs]) during the weeks after receipt. We also observed a relationship between CD83+ monocytes and mycobacteria-specific T-cell responses. However, BCG vaccination did not affect SIV transmission rates or short-term pathogenic outcomes in the first 10–12 weeks after infection in infant macaques.
METHODS
Study Animals
Eighteen infant rhesus macaques were purchased and transported from the Oregon National Primate Center and were housed in the specialized infant primate care wing of the Washington National Primate Research Center (WaNPRC). All infant macaques were either intradermally vaccinated in the right deltoid with 0.05 mL (approximately 1.0 × 106 –4.0 × 106 colony-forming units) of BCG Danish 1331 (Statens Serum Institut, Copenhagen, Denmark, via Aeras, Rockville, MD) at 1–2 weeks of age (n = 9) or left unvaccinated (n = 9). All vaccine recipients exhibited a papule and erythema at the site of injection 1–2 weeks after vaccination and had enlarged draining right axillary lymph nodes 2–4 weeks after vaccination. Three weeks after vaccination or at age-matched time points in unvaccinated infant macaques, up to 8 oral challenges with SIV were administered, ranging from 1000 to 20 000 median tissue culture infectious doses (TCID50) of SIVmac251 (rhesus macaque peripheral blood mononuclear cell [PBMC]–grown supernatant, obtained from Dr Nancy Miller through the National Institutes of Health AIDS Reagent Program). Virus was diluted to 0.25 mL in Roswell Park Memorial Institute 1640 medium and delivered dropwise across the oral mucosa via needleless syringe. Infection was confirmed by assaying for plasma viral loads at the WaNPRC Virology Core. Three infant macaques that remained uninfected after 8 oral challenges (defined as a plasma viral load of ≤30 SIV RNA copies/mL for 2 weeks after each challenge) were infected intravenously with 500 TCID50 of the same challenge stock allowing for the evaluation of disease pathogenesis in all infant macaques; 2 were in the vaccinated group, and 1 was in the unvaccinated group. Infant macaques were monitored for 9–12 weeks after SIV infection before being euthanized. Rhesus major histocompatibility complex (MHC) class 1 typing did not identify any association between MHC genotype and skewed susceptibility to SIV infection in either study group (Table 1).
Table 1.
Characteristics of Infant Rhesus Macaques in the Study
| Identifier | Sex | MHC Class I Alleles | BCG Vaccinated | Age at SIV Positivity, wk | SIV TCID50 Resulting in Positivity/No. of Challenges | Peak Viral Load | Viral Load at Necropsy |
|---|---|---|---|---|---|---|---|
| A16079-ZZ | F | A1*004, A1*023, B*001, B*012 | No | 12 | 20 000/8 | 2.43E+08 | 2.43E+08 |
| A16080-AA | F | A1*002, B*015 | No | 10 | 12 000/6 | 5.05E+07 | 6.03E+06 |
| A16081-AB | F | A1*002, A1*006, B*012, B*024 | No | 15 | IV | 3.91E+08 | 3.91E+08 |
| A16082-ACa | F | … | No | 8 | 4000/4 | – | – |
| A16083-AD | M | A1*026, A1*002, B*001, B*055 | No | 11 | 15 000/7 | 1.06E+08 | 3.88E+05 |
| A17151-FT | F | … | No | 11 | 12 000/6 | 3.29E+07 | 2.14E+06 |
| A17152-FU | F | A1*004, A1*011, B*001, B*066 | No | 13 | 20 000/8 | 4.04E+08 | 4.04E+08 |
| A17153-FV | F | A1*023, A1*025, B*008, B*028 | No | 6 | 1000/2 | 2.68E+06 | 3.85E+05 |
| A17154-FW | F | A1*002, A1*004, B*001 | No | 10 | 12 000/6 | 4.90E+07 | 1.41E+07 |
| A16185-AT | F | A1*008, A1*032, B*012, B*068 | Yes | 10 | 12 000/6 | 1.07E+07 | 6.18E+06 |
| A16186-AUb | F | … | Yes | … | … | … | … |
| A16187-AV | M | A1*002, A1*001, B*015, B*002 | Yes | 8 | 4000/4 | 7.29E+07 | 7.29E+07 |
| A16188-AW | M | A1*004, B*012 | Yes | 15 | IV | 2.64E+08 | 2.55E+08 |
| A16189-AX | M | A1*012, A1*025, B*008 | Yes | 15 | IV | 2.82E+07 | 5.95E+05 |
| A17097-FA | F | A1*008, A1*011, B*001, B*008 | Yes | 10 | 12 000/6 | 5.74E+07 | 2.23E+06 |
| A17098-FB | M | A1*008, A1*023, B*012, B*015 | Yes | 13 | 20 000/8 | 1.74E+08 | 1.51E+08 |
| A17099-FC | F | A1*011, A1*023, B*012, B*024 | Yes | 10 | 12 000/6 | 3.29E+08 | 1.22E+08 |
| A17100-FD | M | A1*001, A1*023, B*002, B*028 | Yes | 13 | 20 000/8 | 1.09E+06 | 2.63E+06 |
Abbreviations: MHC, major histocompatibility complex; SIV, simian immunodeficiency virus; TCID50, median tissue culture infectious dose.
aInfant died prematurely 1 week after SIV challenge.
bInfant died prematurely 6 weeks after BCG vaccination and remained uninfected.
Phenotypic Analysis of Immune Cell Subsets
Phenotypic analyses of PBMC and lymph node cell populations were performed by multiparametric flow cytometry. Freshly isolated PBMCs were counted and stained as previously described [13]. Briefly, activation of T cells (CD3+CD20−CD14−), classical monocytes (HLA-DR+CD14+CD16−CD20−CD3−), and CD16+ monocytes (HLA-DR+CD14+CD16+CD20−CD3−) was assessed using gating strategies outlined in Supplementary Figures 1 and 2. The following antibodies were used: CD3(SP34-2)-Pacific Blue and APC, CD4(OKT4)-BV650 and APC-Cy7, CD8(SK1)-APC-H7, CD20(2H7)-BV570 and PE, CD14(M5E2)-BV785 and APC-H7, CD16(3G8)-BV605, CD11c(S-HCL-3)-APC, CD123(7G3)-PerCP-Cy5.5, CCR5(3A9)-APC, CXCR3(1C6)-PE-CF594, CD38(AT-1)-FITC, Ki-67(B65)-PE and FITC, HLA-DR(L243)-BV711 and PE, CD80(L307.4)-PE-Cy7, CD83(HB15e)-PE-CF594, and CD86(2331)-BV711 (BD Life Sciences). Stained cells were washed and fixed in 1% paraformaldehyde before analysis on a BD LSR-II flow cytometer. Compensation and analysis were performed using FlowJo, version 10 (Treestar, Woodburn, OR).
Analysis of Plasma Protein and Specific Immunoglobulin G (IgG)/Immunoglobulin A (IgA) Concentrations
Plasma samples were collected after centrifugation of whole-blood specimens collected in ethylenediaminetetraacetic acid–coated tubes at 200 ×g for 15 minutes. Plasma samples collected 1 week before, 4–5 weeks after, and 10–12 weeks after SIV infection were analyzed. Plasma interferon α (IFN-α) concentrations were measured by an enzyme-linked immunosorbent assay (ELISA; human IFN-α ELISAPRO kit [Mabtech]) in accordance with the manufacturer’s instructions. Measurement of plasma anti-SIVgp140 IgG and IgA concentrations was performed using a custom ELISA containing wells coated with rGP130 (catalog no. 12797; National Institutes of Health AIDS Reagent Program). Samples were serially diluted, and absorbance values were fit to standard curves generated using either purified rhesus macaque IgG or IgA.
Measurement of Mycobacteria-Specific T-Cell Cytokine Responses
Production of cytokines in response to stimulation with a BCG peptide pool was assayed by intracellular cytokine staining (ICS). ICS experiments were performed on PBMC samples stored in liquid nitrogen with either a positive control (50 ng/mL phorbol 12-myristate 13-acetate with 1 µg/mL ionomycin) or 2 µg/mL M. tuberculosis–derived MTB300 peptide pool, as described previously [14, 15]. The MTB300 megapool was developed by Lindestam Arlehamn et al [14] and contains 300 peptides ranging from 15 to 20 amino acids in length and representing 125 reactive epitopes identified in M. tuberculosis. Following stimulation for 9 hours (8 hours with brefeldin A; Sigma), cultures were stained with Fixable Live-Dead Green (Thermo-Fisher), CD20(2H7)-PE, CD14(M5E2)-PE, CD4(OKT4)-BV650, tumor necrosis factor α (TNF-α; MAb11)-BV785, IL17(BL168)-BV421 (Biolegend), CD8(SK1)-APC-H7, CD3(SP-342)-APC, and IFN-γ (4S.B3)-BV711 (BD Life Sciences). Before cells were stained for cytokine analysis, they were permeabilized with BD FACS lysis buffer. Stained PBMCs were analyzed as described above.
Immune Gene Expression Profiling
RNA samples were prepared from PBMC lysates (3 × 106 cells) by using an RNA Nucleospin kit (Takara Bio) and were diluted to a concentration of 20 ng/µL before being analyzed at the WaNPRC Primate Diagnostic Services Laboratory. Transcriptomic profiles using 770 immune genes were acquired from infant rhesus macaque PBMCs collected 0 and 3 weeks after BCG vaccination, using an nCounter MAX platform (Nanostring Technologies). RNA (100 ng) was directly hybridized for 18 hours, using the Non-Human Primate Immunology chipset, version 2.0. Images were acquired from 280 fields of view for each sample, and data were tabulated and differential expression analysis performed with nSolver software (version 4.0) [16].
Tissue Imaging
Tissue specimens were collected at necropsy and transferred immediately to neutral buffered formalin. Fixed tissue specimens were washed in 70% ethanol and embedded in paraffin blocks. Embedded tissue specimens were cut into 5-µm sections and mounted on positively charged glass slides. Immunofluorescent staining was then performed as described previously [17]. Right axillary lymph nodes were stained for acid-fast bacilli to identify mycobacteria or with mouse anti-CD68 and rabbit anti-purified protein derivative (Abcam) for immunofluorescence analysis.
Statistical Analysis
All statistical analyses were performed using either Prism, version 8 (Graph Pad), or R, version 3.5.1. Proportions of immune populations in BCG vaccine recipients and controls were compared using either 2-tailed Mann-Whitney U tests, Wilcoxon matched-pairs signed ranks tests, or t tests when appropriate. For gene expression data, the animal identifier was set as a confounder for analysis of matched samples. Differences in gene expression between weeks 0 and 3 after BCG vaccination were calculated using nSolver analysis software (Nanostring). For these data, either mixed or simplified negative binomial models or log-linear models were used, depending on the data collected for each gene. Adjusted P values were calculated using the Benjamini-Yekutieli correction [18].
RESULTS
BCG Vaccination Increases T-Cell Activation in Blood
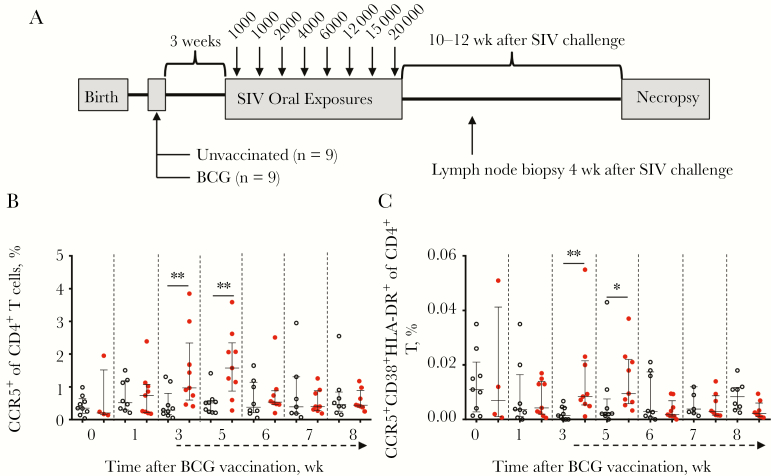
To evaluate the impact of BCG vaccination on immune cells and oral HIV/SIV infection, a longitudinal study was performed by using the rhesus macaque model of oral SIV transmission (Figure 1A and Table 1). Neonatal macaques were randomly assigned at 2 weeks of age to undergo BCG vaccination (n = 9) or remain unvaccinated (n = 9), with peripheral immune activation monitored weekly in both groups. Peripheral CD4+ T-cell activation has been observed in infants 6 and 8 weeks after BCG vaccination [7, 19] and as late as 9 weeks after vaccination in infant macaques [7]. To evaluate the dynamics of BCG vaccine–associated CD4+ T-cell activation, frequencies of cell surface markers of immune activation were measured in peripheral blood specimens by flow cytometry (Supplementary Figure 1). Frequencies of both CCR5+ CD4+ T cells (Figure 1B) and activated CD4+ T cells (defined as triple-positive CD4+ T cells [ie, CCR5+HLA-DR+CD38+]; Figure 1C) were significantly increased 3 and 5 weeks after BCG vaccination. By 6 weeks after BCG vaccination, levels of activated CD4+ T cells were similar between the BCG-vaccinated and control infant macaques. Importantly, this was not due to the presence of SIV infection, as only 1 infant macaque was infected in the unvaccinated group, and none were infected in the vaccinated group. The transient activation of CD4+ T cells was therefore associated with BCG vaccination of these infant macaques.
Figure 1.
Infant macaques that received BCG vaccine had elevated levels of activated CD4+ T cells. A, Infants were assigned to receive BCG vaccine (n = 9) or remain unvaccinated (n = 9) before receiving a series of oral simian immunodeficiency virus (SIV) challenges. Following SIV infection, infants were monitored for 10–12 weeks before necropsy. B and C, T-cell activation was evaluated in peripheral blood mononuclear cells (PBMCs) from unvaccinated (open circles) and BCG-vaccinated (closed circles; red in color version) infants at specified weeks following BCG vaccination. Proportions of CCR5+ (B) and CCR5+HLA-DR+CD38+ (C) CD4+ T cells among PBMCs are shown at the indicated weeks following BCG vaccination. Dashed arrows beneath the x-axis indicate initiation of weekly oral SIV challenges. Horizontal bars represent medians, with error bars representing interquartile ranges. To account for variations across time points in both groups, differences were assessed between BCG-vaccinated and control groups. Comparisons were calculated using the Mann-Whitney U test. *P < .05 and **P < .01.
BCG Vaccination Induces Monocyte Activation in Infant Macaques
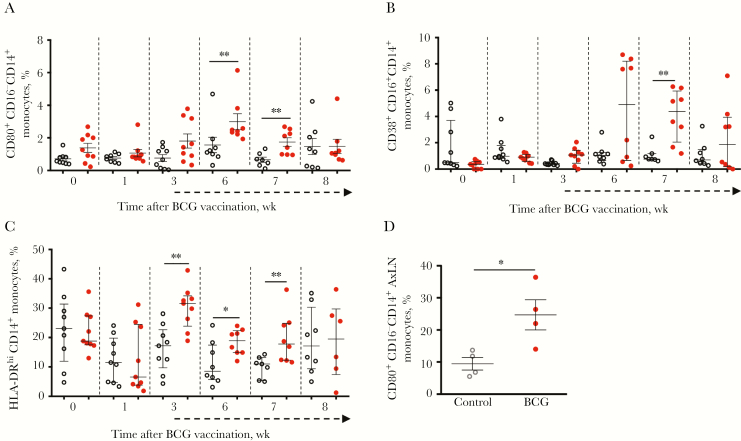
Bacillus Calmette-Guérin replicates in monocytes/macrophages, and therefore we examined this population in peripheral blood specimens from infant macaques (Supplementary Figure 2). Administration of BCG vaccine increased peripheral monocyte activation, characterized by an increase in the percentage of CD80+CD16− monocytes 6 and 7 weeks after BCG vaccination (Figure 2A) and the percentage of CD83+CD16+ monocytes 7 weeks after BCG vaccination, compared with unvaccinated controls (Figure 2B). We also observed a significant increase in the population of monocytes that were HLA-DRhi at weeks 3, 6, and 7 after BCG vaccination (Figure 2C). HLA-DRhi monocytes have been shown previously to have important roles in inflammatory and antibacterial responses [20, 21]. This result suggests that BCG vaccine polarizes monocytes toward phenotypes associated with increased T-cell costimulation and inflammatory signaling in the weeks following vaccination.
Figure 2.
Infant macaques that received BCG vaccine sustained higher levels of activated monocytes. Monocyte activation was monitored in peripheral blood mononuclear cells (PBMCs) (A–C) and axillary lymph nodes (D) from unvaccinated (open circles) and BCG-vaccinated (closed circles; red in color version) infants at specified weeks following BCG vaccination. Proportions of CD80+CD16− (A), CD83+CD16+ (B), and HLA-DRhi (C) CD14+ monocytes among PBMCs are shown. Levels of activated CD16− CD80+CD14+ monocytes were assessed in biopsy specimens from draining lymph nodes collected 4 weeks after simian immunodeficiency virus (SIV) infection (8–12 weeks after BCG vaccination). D, Dashed arrows beneath the x-axes indicate initiation of weekly oral simian immunodeficiency virus challenges. Horizontal bars represent medians, with error bars representing interquartile range. Comparisons between groups were calculated using the Mann-Whitney U test. AxLN, axillary lymph node. *P < .05 and **P < .01.
Bacillus Calmette-Guérin persists in the draining lymph nodes [22, 23], which act as potential sites for monocyte/macrophage activation and sources of systemic BCG infection in the setting of SIV or HIV infection. Therefore, monocyte activation was also evaluated in right axillary lymph node biopsy specimens collected 4 weeks after SIV infection (8–12 weeks after BCG vaccination). BCG-vaccinated infant macaques had a significantly (2.6-fold) higher mean level of classical monocytes (CD80+CD16−) at this site (Figure 2D), compared with unvaccinated infant macaques. Further, the presence of mycobacteria in the vaccine-draining lymph node was observed at necropsy (Supplementary Figure 3), suggesting that BCG persists for weeks to months within the draining lymph node of SIV-infected infant macaques.
BCG-Vaccinated Infant Macaques Develop Mycobacteria-Specific T-Cell Responses
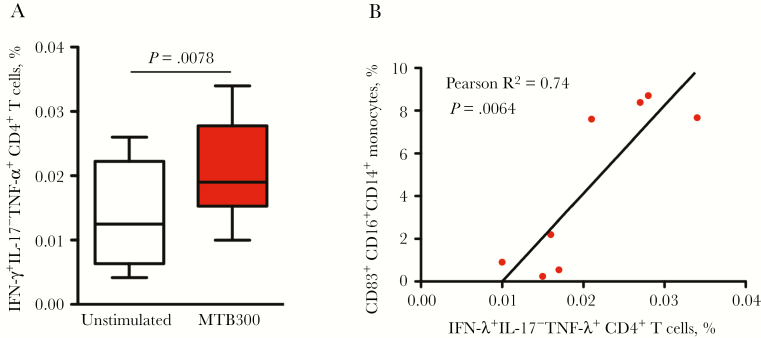
In BCG-vaccinated infant macaques, variations in mycobacteria-specific T-cell responses and protection have been reported within and across vaccinated populations [24–26]. Mycobacteria-specific T-cell responses in peripheral blood specimens were assessed 9 weeks after vaccination, following stimulation with M. tuberculosis–derived T-cell epitopes that cross-react with BCG proteins/peptides [14]. Similar to responses following BCG vaccination in humans, infant macaques that received BCG vaccine developed mycobacteria-specific CD4+ T-cell responses that were polyfunctional, producing both TNF-α and IFN-γ (Figure 3A and Supplementary Figure 4) [27]. Furthermore, the frequency of mycobacteria-specific CD4+ T-cell responses significantly correlated with monocyte activation/maturation (based on the percentage of CD83+CD16+CD14+ cells) in BCG-vaccinated infant macaques (Figure 3B). This correlation suggests a role for this monocyte population in eliciting BCG vaccine–specific CD4+ T-cell responses.
Figure 3.
BCG-vaccinated infants exhibit Mycobacterium tuberculosis–specific T-cell responses. BCG-specific CD4+ T-cell responses were determined by peptide stimulation (MTB300), followed by intracellular cytokine staining of peripheral blood mononuclear cells (PBMCs) collected 9 weeks after BCG vaccination. A, Interferon γ (IFN-γ)+, interleukin 17 (IL-17)−, and tumor necrosis factor α (TNF-α)+ CD4+ T cells are shown for unstimulated and MTB300-stimulated PBMCs. B, The relationship between levels of IFNγ +IL-17−TNF-α + CD4+ T cells at week 9 after BCG vaccination and levels of CD83+CD16+ CD14+ monocytes at week 6 after BCG vaccination.
BCG Vaccination Influences Global Gene Expression 3 Weeks After Vaccination
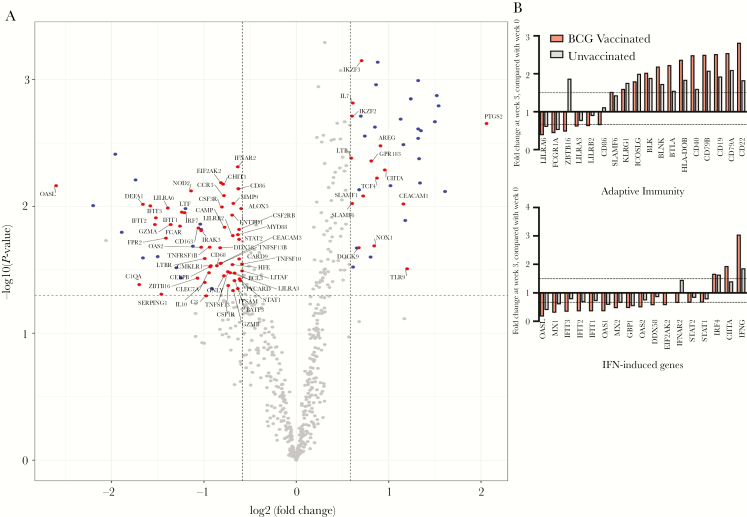
Transcriptomic analysis was performed on RNA that was extracted from freshly isolated, unstimulated PBMCs from BCG-vaccinated and unvaccinated infant macaques. By using the Nanostring platform, 770 rhesus macaque immune genes were assessed to compare gene expression patterns before vaccination to patterns at week 3 after vaccination in both the BCG-vaccinated and unvaccinated macaques. Week 3 was chosen because it corresponds to the specific elevation of the CD4+ target cell count in BCG-vaccinated infant macaques and is the time point at which SIV oral challenges were initiated. While levels of numerous transcripts were increased in both the BCG-vaccinated and unvaccinated groups, our focus was on genes that were specifically upregulated in the BCG-vaccinated macaques. At the 3-week time point, 15 of 39 upregulated genes (ie, those with a >1.5-fold increase in expression; P < .05) were uniquely upregulated in BCG-vaccinated infant macaques, compared with prevaccination levels (Figure 4A and Supplementary Tables 1 and 2). Of the transcripts with elevated levels only in BCG-vaccinated infant macaques, there was a 4.2-fold increase in expression of PTGS2 (COX2), the key inducible enzyme involved in prostaglandin biosynthesis. PTGS2 has previously been associated with inflammatory and immunosuppressive effects associated with BCG vaccination [28–30] (Figure 4A). A majority of the genes with increased transcript levels in both BCG-vaccinated and unvaccinated infant macaques were linked to adaptive immunity and specifically B-cell responses (CD19, CD79A, CD79B, MS4A1, and BLK; Figure 4B and Supplementary Tables 1 and 2). Assessment of IFN signaling pathways identified increased levels of the positive regulator of MHC-II expression CIITA in BCG-vaccinated but not unvaccinated infant macaques (Figure 4C). Levels of transcripts of IFNG (which encodes IFN-γ) were also more elevated in BCG-vaccinated infant macaques, compared with unvaccinated macaques (3.86-fold vs 1.86-fold increases; Figure 4C). Moreover, 55 of 68 genes were uniquely downregulated in BCG-vaccinated infant macaques, including type I IFN–induced genes OASL (6.06-fold decrease) and OAS2 (2.86-fold decrease; Figure 4A and 4C). These results demonstrate transcriptional changes in immune genes within PBMCs that are unique to BCG-vaccinated infant macaques by 3 weeks after vaccination.
Figure 4.
Transcriptional changes in immune genes associated with BCG vaccination. Differential expression of 770 immune genes in infant macaque peripheral blood mononuclear cells (PBMCs) was assessed 0 weeks and 3 weeks after BCG vaccination. A, Changes from baseline (ie, week 0) to week 3 after BCG vaccination are shown in volcano plots for BCG-vaccinated infants (n = 6). Colored points represent genes whose expression significantly increased or decreased by >1.5-fold (P < .05; dashed lines), with blue points indicating genes with levels that were reduced or elevated in all infants and red points indicating genes that were only changed in BCG-vaccinated infants. B and C, Genes labeled in panel A with increased or decreased expression within adaptive immunity (B) and interferon-related (C) signaling pathways are shown with fold changes for BCG-vaccinated (red bars) or unvaccinated (gray) bars. Statistical measurement of differentially expressed genes is described in the Methods. IFN, interferon.
BCG Vaccination Did Not Influence Oral SIV Transmission in Infant Macaques
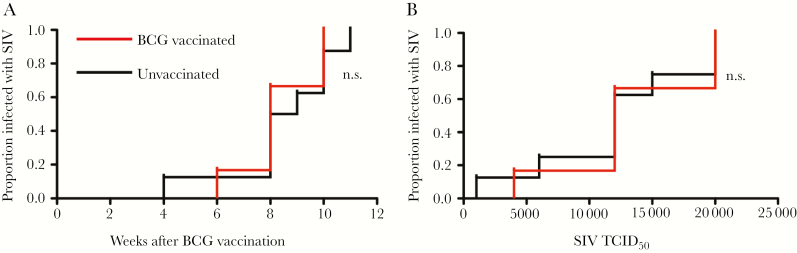
At 3 weeks after vaccination, both groups of infant macaques received weekly, escalating-dose oral challenges from 1000 to 20 000 TCID50 of SIVmac251 until testing positive for plasma SIV RNA. The rate (median, 8 weeks after BCG vaccination) and dose (mean, 12 571 TCID50) when infant macaques acquired SIV was similar in the BCG-vaccinated and unvaccinated groups (Figure 5A and 5B). Our findings, therefore, suggest that despite significant activation of circulating immune cells, BCG vaccination does not influence oral SIV acquisition in infant rhesus macaque macaques.
Figure 5.
BCG vaccination did not influence simian immunodeficiency virus (SIV) transmission in infant macaques. Rates of oral SIV transmission were compared between BCG-vaccinated and unvaccinated groups by week following vaccination (A) or by dose of SIV (B), using Kaplan-Meier plots and statistical tests. NS, not significant; TCID50, median tissue culture infectious dose.
BCG Vaccination Was Not Associated With an Altered Rate of SIV Disease Progression
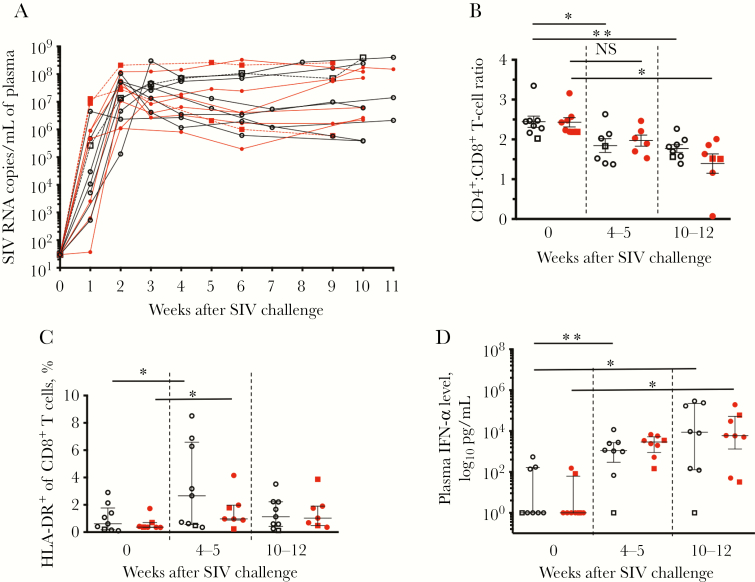
The ability of BCG vaccination to influence key indicators of SIV disease progression was evaluated in the infant macaques infected orally, as well as in 3 other infant macaques (2 vaccinated and 1 unvaccinated) that were infected intravenously with 100 TCID50. SIV-infected infant macaques were monitored, and animals were euthanized 10–12 weeks after SIV infection. Evaluation of SIV-specific antibody (ie, IgG and IgA) responses did not identify any differences between the BCG-vaccinated and unvaccinated infant macaques (Supplementary Figure 5). Peak plasma viral loads occurred 2–3 weeks after SIV infection (Figure 6A) for all infant macaques in the study, with similar average plasma viral loads measured in both groups. While there was a significant reduction in the ratio of CD4+ to CD8+ T cells in all infant macaques, as expected with SIV infection, BCG vaccination had no significant impact on this measure of SIV disease (Figure 6B). Furthermore, while significant increases in levels of HLA-DR+ CD8+ T cells occurred after infection, indicating SIV-mediated immune activation, there were no significant differences between the BCG-vaccinated and unvaccinated groups (Figure 6C). Finally, the plasma IFN-α level was similarly increased in both groups (Figure 6D). Therefore, BCG vaccination did not appear to influence indicators generally associated with disease progression in SIV-infected infant macaques.
Figure 6.
BCG vaccination was not associated with differences in simian immunodeficiency virus (SIV) disease progression. Markers of disease progression were assessed in BCG-vaccinated (red line, closed red circles) or unvaccinated (black line, open black circles) infant macaques. A, Plasma viral loads were monitored at the time of and in the weeks following SIV infection. B, CD4+ T-cell depletion after SIV challenge was assessed by determining the ratio of the percentage of CD3+ CD4+ cells to the percentage of CD3+ CD8+ cells in blood. C and D, Levels of HLA-DR+ CD8+ T cells among peripheral blood mononuclear cells (PBMCs; C) and plasma interferon α (IFN-α; D) were monitored following infection to evaluate
SIV-associated immune activation. The 3 intravenously infected infants are labeled using dashed lines and open or closed squares. Horizontal bars represent medians, with error bars representing interquartile ranges. Comparisons within groups were calculated using the Wilcoxon signed-ranks test. NS, not significant. *P < .05 and **P < .01.
DISCUSSION
Strategies to reduce the impact of HIV infection in infants and children will require a greater understanding of factors influencing transmission and progression to AIDS, including those that have been shown to influence CD4+ T-cell activation. Here, experiments using the infant macaque SIV infection model were performed to assess the impact of BCG vaccine on oral SIV transmission and disease progression. The goal of this study was to provide insight into BCG vaccination and its influence on HIV infection of infants. The experiments evaluated the role of BCG vaccine in immune activation at the cellular and transcriptomic level, rates of oral SIV transmission, BCG vaccine–induced immune responses, and SIV disease progression.
With growing evidence that BCG vaccine influences innate immune development, it is particularly relevant to explore a potential relationship between innate responses to BCG vaccine and the impact of these responses on T-cell activation and HIV transmission [12, 31]. Previous studies assessed bacillus Calmette-Guérin stimulation of cord blood cells and observed an innate response that was characterized by increased IFN-γ, interleukin 10, and TNF-α release and partially mediated by CD14+ monocytes [8]. In addition, other studies demonstrated that BCG-vaccinated infants have greater T-helper type 1 responses to nonmycobacterial infections than unvaccinated infants, thus supporting the trained immunity hypothesis, whereby BCG vaccine induces epigenetic reprogramming of monocytes [32, 33]. Here, evaluation of the immune responses following BCG vaccination identified elevated levels of activated monocyte populations in blood specimens 6–7 weeks after vaccination and in draining lymph node specimens 8–12 weeks after vaccination (4 weeks after SIV infection). These results support the hypothesis that BCG vaccine induces immune changes with long-lasting consequences for the infant immune system. Specifically, elevated levels of HLA-DRhi, CD83+, and CD80+ monocytes were present in the weeks following vaccination. Our findings also identify monocyte activation in BCG-vaccinated infants as a potential contributor to the increased mycobacteria-specific IFN-γ– and TNF-α–expressing CD4+ T-cell responses (Figure 3). Therefore, our results demonstrate elevated baseline monocyte activation in vaccinated infant macaques in blood and lymph nodes both before and following SIV infection.
Our results show that BCG vaccination induced significant monocyte and T-cell activation and a transcriptomic profile associated with immune activation in blood. However, we did not observe any clear influence of these effects on the immune response to and susceptibility to infection with SIV in infant macaques. It is also important to note that infant macaques in our study began receiving oral SIV challenges 3 weeks after BCG vaccination and that infection occurred 4–12 weeks after vaccination. In a previous study, infant macaques began receiving oral SIV 9 weeks after receiving BCG vaccine or remaining unvaccinated [12]. The vaccinated macaques exhibited elevated peak viral loads and required on average fewer challenges to become infected, compared with unvaccinated macaques, although the differences were not statistically significant [12]. This result is in contrast to our observations, which detected no relationship between BCG vaccination and viral acquisition or disease. These results suggest that the timing between BCG vaccination and HIV/SIV exposure may be a key element influencing outcome.
In a South African infant cohort, BCG vaccine recipients had elevated levels of activated CD4+ T cells (CD38+HLA-DR+CCR5+), compared with levels at 6 and 8 weeks of age in a group in which BCG vaccine was delayed by 8 weeks, suggesting that BCG vaccine increases this HIV-susceptible T-cell population [7]. While we observed an increase in this same population of activated CD4+ T cells, immune activation was not associated with SIV transmission or disease progression. The delivery modality of BCG vaccine was an important consideration when designing this study. Indeed, other recent rhesus macaque studies, in which BCG vaccine was delivered through the pulmonary and intravenous routes, consistently induced strong T-cell responses and protected recipients from subsequent M. tuberculosis challenge [10, 11]. Although, the intradermal route is generally not associated with strong T-cell responses in macaques [34], the goal of our study was to recapitulate human intradermal BCG vaccination to most accurately assess any associated risks.
As our vaccination/SIV infection model recapitulates key aspects of bacillus Calmette-Guérin infection and oral HIV infection, these findings have implications for vertical HIV transmission. It is important to note that our model does not completely replicate infection by human breast milk exposure because the SIV doses are administered weekly rather than daily. In addition, our macaques were not exposed to SIV before birth, which influences infant immune development, as demonstrated in a number of studies of HIV-exposed, uninfected children [35, 36]. Overall, from the studies described here, using the infant macaque model of SIV infection, we conclude that BCG vaccination increased immune activation but that this effect did not translate to an increase in the rates of oral SIV transmission or postpartum SIV disease course. These findings provide evidence that BCG vaccination is not a major contributor to HIV transmission when infants in tuberculosis-endemic regions are breastfed by HIV-infected mothers.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. We thank the veterinary and support staff at the Washington National Primate Research Center and LaRene Kuller and Richard Grant, for support with RNA processing for NanoString analysis; Brian Johnson at the University of Washington Histology and Imaging Core, for expertise and technical assistance; and Roger Wiseman and Eileen Maher at the University of Wisconsin Madison and the Wisconsin National Primate Research Center, for assistance with MHC typing.
Financial support. This work was supported by the National Institute of Dental and Cranial Research, National Institutes of Health (NIH; grant R01-DE023047), the University of Washington (Pathobiology Training Grant T32-A1750915. to M. P. W.), the NIH Office of Research Infrastructure Programs (grant P51 OD010425 to the Washington National Primate Center), and the NIH (fellowship F30-ES022535 to L. F. W.).
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Presented in part: 36th Annual Symposium on Nonhuman Primate Models for AIDS, Seattle, Washington, 2–5 October 2018; 2018 Keystone Symposia on HIV and Co-infections: Pathogenesis, Inflammation, and Persistence, Whistler, Canada, 15–19 April 2018.
References
- 1. Joint United Nations Programme on HIV/AIDS (UNAIDS). UNAIDS data 2017. Geneva: UNAIDS, 2017:1–248. [Google Scholar]
- 2. World Health Organization (WHO). Updates on HIV and infant feeding. Geneva: WHO, 2016. [PubMed] [Google Scholar]
- 3. Masson L, Passmore JA, Liebenberg LJ, et al. Genital inflammation and the risk of HIV acquisition in women. Clin Infect Dis 2015; 61:260–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. McKinnon LR, Kaul R. Quality and quantity. Curr Opin HIV AIDS 2012; 7:195–202. [DOI] [PubMed] [Google Scholar]
- 5. Wood LF, Wood MP, Fisher BS, Jaspan HB, Sodora DL. T cell activation in South African HIV-exposed infants correlates with Ochratoxin A exposure. Front Immunol 2017; 8:1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wood LF, Brown BP, Lennard K, et al. Feeding-related gut microbial composition associates with peripheral T-cell activation and mucosal gene expression in African infants. Clin Infect Dis 2018; 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gasper MA, Hesseling AC, Mohar I, et al. BCG vaccination induces HIV target cell activation in HIV-exposed infants in a randomized trial. JCI Insight 2017; 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Watkins ML, Semple PL, Abel B, Hanekom WA, Kaplan G, Ress SR. Exposure of cord blood to Mycobacterium bovis BCG induces an innate response but not a T-cell cytokine response. Clin Vaccine Immunol 2008; 15:1666–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kleinnijenhuis J, Oosting M, Joosten LA, Netea MG, Van Crevel R. Innate immune recognition of mycobacterium tuberculosis. Clin Dev Immunol; 2011:2011:405310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sharpe S, White A, Sarfas C, et al. Alternative BCG delivery strategies improve protection against Mycobacterium tuberculosis in non-human primates: Protection associated with mycobacterial antigen-specific CD4 effector memory T-cell populations. Tuberculosis (Edinb) 2016; 101:174–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Verreck FAW, Tchilian EZ, Vervenne RAW, et al. Variable BCG efficacy in rhesus populations: Pulmonary BCG provides protection where standard intra-dermal vaccination fails. Tuberculosis (Edinb) 2017; 104:46–57. [DOI] [PubMed] [Google Scholar]
- 12. Jensen K, Dela Pena-Ponce MG, Piatak M, et al. Balancing trained immunity with persistent immune activation and the risk of simian immunodeficiency virus infection in infant macaques vaccinated with attenuated mycobacterium tuberculosis or mycobacterium bovis BCG vaccine. Clin Vaccine Immunol 2017; 24:1–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gasper MA, Biswas SP, Fisher BS, Ehnert SC, Sherman DR, Sodora DL. Nonpathogenic SIV and pathogenic HIV infections associate with disparate innate cytokine signatures in response to Mycobacterium bovis BCG. PLoS One 2016; 11:e0158149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lindestam Arlehamn CS, McKinney DM, Carpenter C, et al. A quantitative analysis of complexity of human pathogen-specific CD4 T cell responses in healthy M. tuberculosis infected South Africans. PLoS Pathog 2016; 12:1–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mothé BR, Lindestam Arlehamn CS, Dow C, et al. The TB-specific CD4(+) T cell immune repertoire in both cynomolgus and rhesus macaques largely overlap with humans. Tuberculosis (Edinb) 2015; 95:722–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Danaher P, Warren S, Dennis L, et al. Gene expression markers of tumor infiltrating leukocytes. J Immunother Cancer 2017; 5:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fisher BS, Green RR, Brown RR, et al. Liver macrophage-associated inflammation correlates with SIV burden and is substantially reduced following cART. PLoS Pathog 2018; 14:e1006871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Benjamini Y, Yekutieli D. The control of the false discovery rate in multiple testing under dependency. Ann Stat 2001; 29:1165–88. [Google Scholar]
- 19. Fletcher HA, Snowden MA, Landry B, et al. T-cell activation is an immune correlate of risk in BCG vaccinated infants. Nat Commun 2016; 7:11290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Genel F, Atlihan F, Ozsu E, Ozbek E. Monocyte HLA-DR expression as predictor of poor outcome in neonates with late onset neonatal sepsis. J Infect 2010; 60:224–8. [DOI] [PubMed] [Google Scholar]
- 21. Linton L, Karlsson M, Grundström J, et al. HLA-DR(hi) and CCR9 define a pro-inflammatory monocyte subset in IBD. Clin Transl Gastroenterol 2012; 3:e29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Olsen AW, Brandt L, Agger EM, van Pinxteren LA, Andersen P. The influence of remaining live BCG organisms in vaccinated mice on the maintenance of immunity to tuberculosis. Scand J Immunol 2004; 60:273–7. [DOI] [PubMed] [Google Scholar]
- 23. Harris SA, White A, Stockdale L, et al. Development of a non-human primate BCG infection model for the evaluation of candidate tuberculosis vaccines. Tuberculosis (Edinb) 2018; 108:99–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dockrell HM, Smith SG, Lalor MK. Variability between countries in cytokine responses to BCG vaccination: what impact might this have on protection? Expert Rev Vaccines 2012; 11:121–4. [DOI] [PubMed] [Google Scholar]
- 25. Fine PE. Variation in protection by BCG: implications of and for heterologous immunity. Lancet 1995; 346:1339–45. [DOI] [PubMed] [Google Scholar]
- 26. Rodrigues LC, Diwan VK, Wheeler JG. Protective effect of BCG against tuberculous meningitis and miliary tuberculosis: a meta-analysis. Int J Epidemiol 1993; 22:1154–8. [DOI] [PubMed] [Google Scholar]
- 27. Kidzeru EB, Hesseling AC, Passmore JA, et al. In-utero exposure to maternal HIV infection alters T-cell immune responses to vaccination in HIV-uninfected infants. AIDS 2014; 28:1421–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bansal K, Narayana Y, Patil SA, Balaji KN. M. bovis BCG induced expression of COX-2 involves nitric oxide-dependent and -independent signaling pathways. J Leukoc Biol 2009; 85:804–16. [DOI] [PubMed] [Google Scholar]
- 29. Bansal K, Narayana Y, Balaji KN. Inhibition of TNF-alpha-induced cyclooxygenase-2 expression by Mycobacterium bovis BCG in human alveolar epithelial A549 cells. Scand J Immunol 2009; 69:11–9. [DOI] [PubMed] [Google Scholar]
- 30. Dovedi SJ, Kirby JA, Atkins H, Davies BR, Kelly JD. Cyclooxygenase-2 inhibition: a potential mechanism for increasing the efficacy of bacillus calmette-guerin immunotherapy for bladder cancer. J Urol 2005; 174:332–7. [DOI] [PubMed] [Google Scholar]
- 31. van der Meer JW, Joosten LA, Riksen N, Netea MG. Trained immunity: a smart way to enhance innate immune defence. Mol Immunol 2015; 68:40–4. [DOI] [PubMed] [Google Scholar]
- 32. Libraty DH, Zhang L, Woda M, et al. Neonatal BCG vaccination is associated with enhanced T-helper 1 immune responses to heterologous infant vaccines. Trials Vaccinol 2014; 3:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kleinnijenhuis J, Quintin J, Preijers F, Joosten LAB, Ifrim DC, Saeed S. Bacille Calmette-Guérin induces NOD2-dependent nonspecific protection from reinfection via epigenetic reprogramming of monocytes. Proc Natl Acad Sci U S A 2012; 109:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Langermans JA, Andersen P, van Soolingen D, et al. Divergent effect of bacillus Calmette-Guérin (BCG) vaccination on Mycobacterium tuberculosis infection in highly related macaque species: implications for primate models in tuberculosis vaccine research. Proc Natl Acad Sci U S A 2001; 98:11497–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Afran L, Knight MG. HIV-exposed uninfected children: a growing population with a vulnerable immune system? Clin Exp Immunol 2013; 11–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Garcia-Knight MA, Nduati E, Hassan AS, et al. Altered memory T-cell responses to Bacillus Calmette-Guerin and Tetanus Toxoid vaccination and altered cytokine responses to polyclonal stimulation in HIV-exposed uninfected Kenyan infants. PLoS ONE 2015; 10:1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.